Abstract
Sleep is characterized by dynamic coupling between central (CNS) and peripheral autonomic (ANS) nervous systems. However, further research is needed to better understand the multiple interactions occurring among electroencephalographic (EEG) features and respiratory and cardiovascular (CV) outputs modulated by the ANS during sleep. Here, we developed new methods to study EEG slow-wave activity (SWA) during non-rapid eye movement (NREM) sleep with respect to the phases of peripheral oscillations. EEG, respiration, and continuous blood pressure signals recorded from 20 participants were analyzed. Digital filters, designed to decompose the signals into different frequency bands, and the Hilbert transform were applied to estimate the instantaneous phases and frequencies of the peripheral oscillations. The peripheral oscillations were categorized into four phases representing up and down states. EEG delta power (synchronized SWA) was computed and compared across these phases during NREM sleep. Results show that EEG delta power is higher during down phases of slow and respiratory frequency components of blood pressure and during up phases of respiration, suggestive of CNS-ANS coupling during NREM sleep. The developed techniques provide the preliminary framework to further analyze and interpret complex interactions between cortical and cardiac oscillations and their synchrony.
I. INTRODUCTION
Sleep is essential for human physical and mental health and is a time for restoration across multiple physiological systems. While sleep is mainly defined based on the state of cortical activation, it can broadly be viewed as a coordinated cascade of events occurring across several bio-domains. Changes in autonomic nervous system (ANS) function during sleep are notable, with an overall reduction in breathing rate, heart rate, and blood pressure (BP) during non-rapid eye movement (NREM) sleep relative to wakefulness [1]. Recently, attention has been paid to the dynamic coordination between these ANS-modulated changes and oscillations in the central nervous system (CNS) and their interplay across the night [2–4]. The specific neural pathways that couple CNS to ANS during sleep are still unclear, however, it has been suggested that CNS-ANS coupling reflects the common dependence of electroencephalogram (EEG) and ANS variables on the activity of specific hypothalamic and brainstem circuits, rather than reflecting direct causal links from EEG to ANS [5, 6].
During sleep, CNS cortical activity gradually synchronizes resulting in high-voltage, slow-frequency EEG waves, pronounced in the deepest stage of NREM sleep, slow-wave sleep.
Growing attention has been given to EEG slow-wave activity (SWA), mainly for its role in sleep homeostasis, cardio-metabolic and immune functions, and cognitive functioning (e.g., memory consolidation) [7, 8]. Insufficient or altered nocturnal distribution patterns of SWA are associated with detrimental consequences, including increased insulin resistance and altered ANS function, and several conditions are linked with compromised SWA (e.g., insomnia, major depressive disorder, Alzheimer’s disease and related disorders). Over recent years, several methods have been proposed to enhance SWA, such as non-invasive auditory stimulation (e.g., pulses of pink noises) [8, 9], in an attempt to harness the benefits of SWA. Taking these methods a step further, it has been shown that phase-locking the timing of acoustic stimuli to the phases of slow oscillations (< 1 Hz) is crucial for SWA enhancement and the related enhancement of sleep dependent memory consolidation [10]. Existing methods are aimed at enhancing SWA based on EEG only (see [11], for an example). Further understanding of the CNS-ANS interactions during sleep can lead to more advanced SWA optimization techniques based on features of dominant peripheral nervous system oscillations [12]. Advanced signal processing techniques are required to further understand this relationship during sleep and according to time of night.
In this paper, we studied EEG SWA with respect to specific phases of the peripheral oscillations extracted from respiration and BP signals. Peripheral oscillation frequencies can dynamically change over time [13], and therefore we chose two main frequency bands containing the respiratory (0.15–0.4 Hz) and BP slow oscillations (0.01–0.15 Hz). Digital bandpass filters were designed to extract the respiratory and slow oscillations of the peripheral signals and Hilbert transform was applied to estimate the instantaneous phases and frequencies of each component. Peripheral oscillations were divided into four phase regions and EEG delta power (SWA) was calculated and compared within each phase. We hypothesized that SWA would vary according to different states of peripheral oscillations.
The paper is organized as follows. In Section II, the experimental design and our developed framework for characterization of peripheral oscillations and EEG SWA are described. In Section III, the experimental results obtained on a sleep dataset collected from 20 participants are summarized and discussed, and in Section IV, the paper is concluded.
II. Methodology
A. Experimental Design
Twenty healthy adults (10 female) (age 46.2±8.4 years) were recruited from the San Francisco Bay Area and underwent standard polysomnography (PSG) recordings at the SRI Human Sleep Research Laboratory [14]. Participants slept in temperature-controlled and sound-attenuated bedrooms. Participants had no current mental or medical conditions and were not taking medication affecting sleep or cardiovascular functioning. The study was reviewed and approved by the SRI International Institutional Review Board, and all participants provided written informed consent.
Standard PSG was performed using EEG (F3, F4, C3, C4, 01, 02, referenced to the contralateral mastoid; 256 Hz sampled), bilateral EOG and submental EMG recordings using Compumedics amplifiers (Compumedics, Abbotsford, Victoria, Australia), following the American Academy of Sleep Medicine (AASM) guidelines [15]. Sleep stages (wake, N1, N2, N3, and REM sleep) were visually scored in 30-s epochs by experts. Brief arousals (≥3s, <15s) were marked according to AASM rules [15].
BP raw waveforms were obtained using Portapres technology (Model-2; TNO TPD Biomedical Instrumentation, Amsterdam, NL), a validated method allowing prolonged non-invasive BP measurements [16]. The BP signal was obtained from the index and middle fingers of the non-dominant hand using photo-plethysmography cuffs, which inflate and deflate continuously. Measurements alternated between fingers every 30 min to minimize discomfort. The respiratory waveform was recorded via standard Compumedics piezo-electric respiratory effort belts applied to the participants’ abdomen.
B. Preprocessing
To mitigate the distortion caused by aliasing, all the signals were digitally low-passed filtered using a 4th-order Butterworth filter with cutoff frequency of 32 Hz and then downsampled to 64 Hz. The sampling rate was chosen based on the maximum frequency of interest which was 30 Hz in the EEG signal.
The continuous BP signal was then processed to estimate the beat-to-beat systolic blood pressure (SBP). Automatic algorithms were developed to identify and exclude artifacts corresponding to calibration and cuff switching, and to detect systolic peaks, from which SBP (mmHg) was derived. The SBP signal was then linearly interpolated to 64 Hz.
C. Characterization of Peripheral Oscillations
A digital 4th-order Butterworth bandpass filter was designed to extract the peripheral oscillations from the respiration and BP signals. The respiration signal was filtered using lower and upper cutoff frequencies of 0.15 Hz and 0.3 Hz, respectively. The BP signal was filtered out into two components: the respiratory component by bandpass filtering it between 0.15 Hz to 0.3 Hz, and the slow component by bandpass filtering it between 0.01 Hz to 0.15 Hz (see Figure 1). To estimate the instantaneous phases and frequencies of the peripheral oscillations, Hilbert transform was applied to each extracted component, and an analytic signal was formed on the complex plane. If the real-valued signal is expressed by x(t), the analytic signal is defined as [17]:
| (1) |
where and is the Hilbert transform of . The instantaneous phases and requencies f(t) were computed as follows:
| (2) |
| (3) |
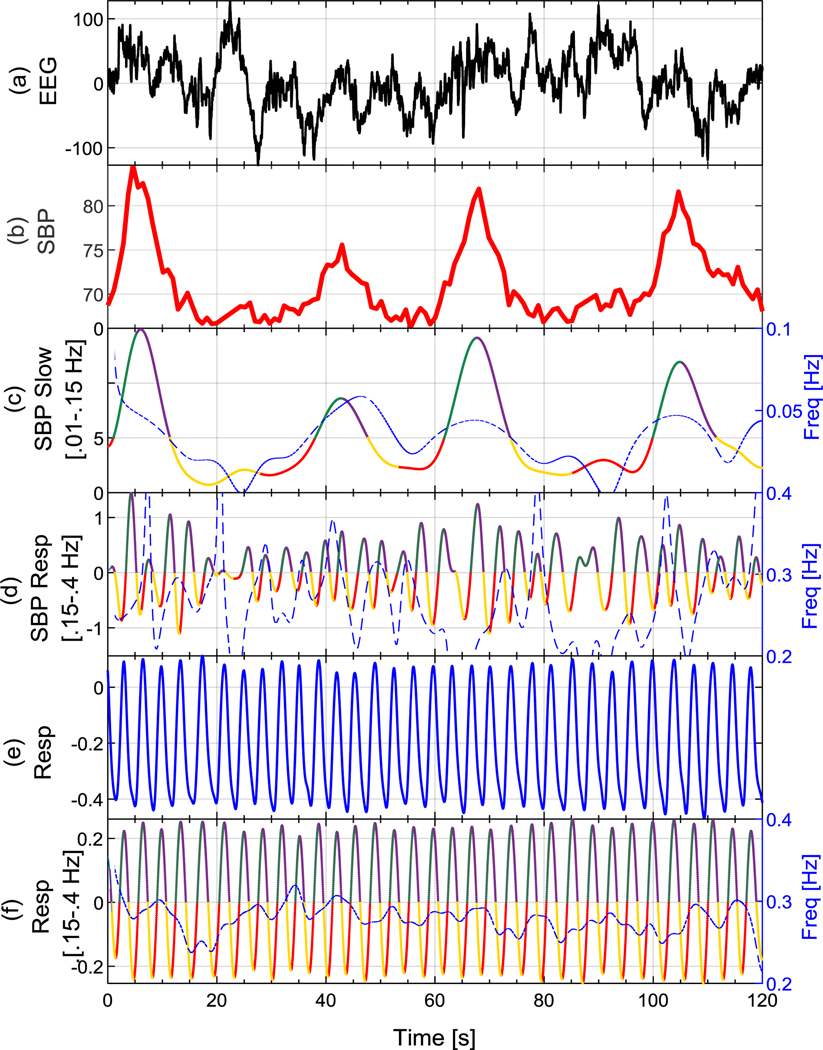
Figure 1.
Examples of (a) EEG, (b) SBP, (c) SBP slow component, (d) SBP respiratory component, (e) raw respiration, and (f) filtered respiration signals. The extracted phases of peripheral oscillations using Hilbert transform are shown in Fig1(c), (d), and (f), where phases 1 through 4 are shown in red, green, purple, and yellow, respectively. The instantaneous frequency of each oscillation is plotted on its top in blue dashed lines.
Figure 1(b) shows an example of the SBP signal with its slow and respiratory components plotted in Figs. 1(c) and (d), respectively. Figure 1(e) and (f) show the respiration signal obtained from the respiration belt and its filtered version, respectively. The instantaneous frequencies estimated using the Hilbert transform are plotted on top of Figs. 1(c), (d), and (f) using blue dashed lines.
In order to study EEG SWA with respect to different phases of the peripheral oscillations, each oscillation was decomposed into four different phases defined as follows:
Phase1:
Phase2:
Phase3:
Phase4:
where Phase1 and Phase2 roughly represent the starting and ending halves of an ascending peripheral oscillation pattern, and Phase3 and Phase4 represent the starting and ending halves of a descending oscillation pattern. In other words, Phase1 and Phase4 represent the down state and Phase2 and Phase3 represent the up state of peripheral oscillations. Examples of the extracted phases from SBP slow oscillations, SBP respiratory oscillations, and respiration signal are shown in Figs. 1(c), (d), and (f), where Phase1 through Phase4 are shown in red, green, purple, and yellow, respectively.
D. EEG SWA
Within each extracted phase of peripheral oscillations, the EEG Welch’s power spectral density (PSD) [17] was computed using a Hamming window of the same length as the input signal. The EEG absolute delta power (SWA) was then computed by integrating the PSD within frequency ranges of 0.4 Hz to 4 Hz. The total EEG power was computed similarly within frequency ranges of 0.4 Hz to 30 Hz. The EEG delta relative power was then computed as the ratio of absolute delta power over total power.
E. Data Analysis
Data were analyzed and segmented into 120-second bins of sleep stage N2 or N3 with no arousals within, in the past and following 30-s intervals, similar to previous work [18]. Every phase segment was separately processed (according to Section II–C), and the derived EEG powers per each peripheral oscillation phase (according to Section II–D) were averaged over the bin. The bins were then averaged over each individual. EEG relative delta power was compared across phases via repeated measures ANOVA (four within levels) and post-hoc analysis on the significant models; p < 0.05 was considered as significant.
All the algorithms were developed in MATLAB R2018a (MathWorks, INC., Natick, MA).
III. Results
A total of 2128 120-s NREM (N2 and N3) sleep stage bins were analyzed out of which 1632 bins belonged to N2 and 496 belonged to N3. On average, each subject contributed 82 bins of N2 and 25 bins of N3 sleep.
The average estimated frequency of SBP slow oscillations across subjects was about 0.034 Hz. Over 91% of the bins had SBP slow oscillation frequency of between 0.03 Hz to 0.05 Hz. The average estimated frequency of the SBP respiratory component was 0.22 Hz, which matched closely with that of the respiration signal (0.23 Hz).
Table I lists the relative delta powers at different phases of peripheral oscillations in N2 and N3 sleep stages, and NREM sleep (N2+N3). Figure 2 shows the distribution of relative delta powers for NREM in different phases.
TABLE I.
Relative Delta Power (SWA) (Mean (SD)) During Sleep in According to Peripheral Oscillation Phases Averaged Across 20 Participants.
| Relative Delta Power (Absolute/Total) (%) | Phase1 | Phase2 | Phase3 | Phase4 | |
|---|---|---|---|---|---|
| SBP Slow Component | NREM | 76.00 (6.90) | 72.45 (6.41) | 71.08 (6.91) | 72.46 (6.42) |
| N2 | 72.93 (6.90) | 68.34 (6.07) | 66.85 (6.59) | 68.65 (6.29) | |
| N3 | 86.40 (5.80) | 86.28 (5.22) | 84.97 (5.84) | 84.43 (6.00) | |
| SBP Respiratory Component | NREM | 65.10 (6.39) | 64.49 (6.39) | 64.73 (6.55) | 64.96 (6.40) |
| N2 | 60.58 (5.89) | 60.09 (6.01) | 60.08 (6.08) | 60.46 (5.84) | |
| N3 | 76.91 (6.24) | 76.24 (6.94) | 76.19 (6.99) | 76.95 (6.03) | |
| Respiration | NREM | 65.00 (6.68) | 65.09 (6.65) | 65.60 (6.45) | 64.76 (6.73) |
| N2 | 60.89 (6.14) | 61.09 (6.04) | 61.68 (6.20) | 60.63 (6.31) | |
| N3 | 78.43 (6.48) | 78.11 (6.53) | 78.36 (5.96) | 78.22 (6.12) | |
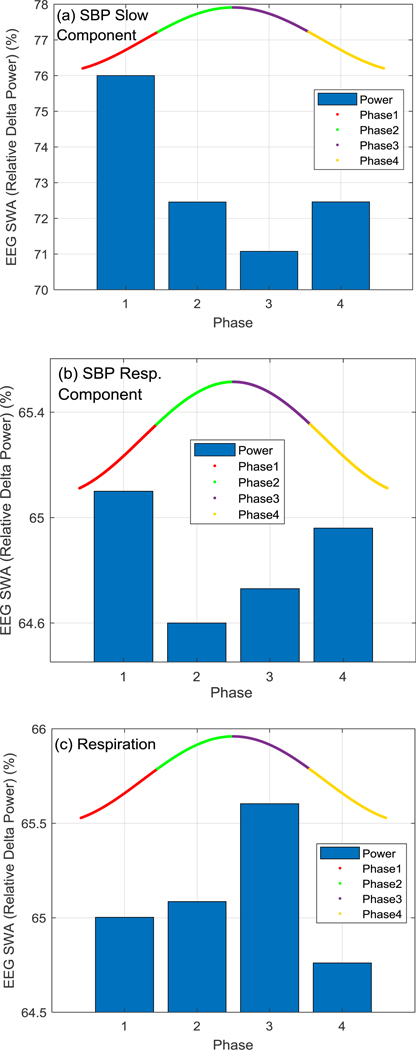
Figure 2.
EEG relative delta power distribution in different phases of (a) SBP slow oscillation, (b) SBP respiratory component, and (c) respiration signal. Phases 1 through 4 are shown in red, green, purple, and yellow, respectively.
A phase main effect (F1,19 = 35.8, p < 0.001) indicated that EEG SWA (relative delta power) during NREM sleep was significantly higher in phase 1 of the SBP slow oscillation compared to the other phases (p < 0.001). SWA in phase 4 and phase 2 were also significantly higher than phase 3 (p = 0.007). The highest EEG SWA occurred in phases 1 and 4 of SBP slow oscillation which are both the down phases of the oscillation. This pattern also existed for the SBP respiratory component (phase main effect, F1,17 = 3.24, p = 0.030) where the highest EEG delta powers happened in phases 1 and 4 that were both significantly higher than phase 2 (p = 0.006 and 0.030, respectively). For the respiration signal (phase main effect, F1,19 = 6.46, p < 0.001), the highest EEG SWA occurred in phase 3 that was significantly higher than phases 1 (p = 0.004), 2 (p = 0.011) and 4 (p < 0.001). Given that the SBP respiratory component and the respiration signal are about 180 degrees phase shifted during normal breathing, i.e. SBP decreases during inspiration and increases during expiration [19] (see Fig.1(d)–(f)), it is expected that the pattern of SWA-phase relationships will be opposite for these signals.
IV. Conclusions
The CNS and ANS are considered to interact and work together as a feedback–feed-forward system. Further study is required to understand this interaction over different sleep stages and time. Here, we developed new methods for characterizing EEG SWA with respect to peripheral oscillations observed in breathing and BP signals, which are under regulatory control of the ANS. We showed that the EEG delta power level increases during certain phases of the peripheral oscillations in NREM sleep, suggesting an underlying coupling between the CNS and ANS. Some results suggest that phase relationships may be stronger in N2 sleep that N3 sleep, however, there were more bins available for N2 sleep and further study of any potential sleep stage difference is required.
Recent studies have shown that deep sleep characterized by EEG SWA can be improved by applying stimuli at specific phases of the EEG slow wave [8–11]. Other work has shown that such SWA enhancement is accompanied by increased high frequency heart rate variability, reflecting increased parasympathetic activity, supporting an interaction between the CNS and ANS [3]. Our results extend these studies to suggest that it might be plausible to enhance SWA with precisely timed stimuli that not only consider phase of the EEG slow wave but also phases of peripheral signals. However, whether the operationalization of the coupling between spontaneous EEG SWA and peripheral oscillations shown here can be extended to a paradigm of neuromodulation (under evoked conditions) needs to be investigated.
In this preliminary study, we only studied EEG delta power variations at specific phases of the respiration and SBP signals. Studying other physiological measures (e.g. heart rate and pulse pressure), frequency components, and other features of the oscillation (e.g. amplitude) can lead to better understanding of CNS-ANS coupling.
This preliminary study provided new insights and framework for a more in-depth analysis and understanding of the interplay between the brain EEG and cardiovascular oscillations.
Acknowledgments
*Research supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA, R21AA024841) (to I.M.C. and M.dZ.), and National Heart, Lung, and Blood Institute (NHLBI, R01HL139652) (to M.dZ.).
Contributor Information
Mohamad Forouzanfar, Center for Health Sciences, SRI International, Menlo Park, CA 94025, USA.
Fiona C. Baker, Center for Health Sciences, SRI International, Menlo Park, CA 94025, USA
Ian M. Colrain, Center for Health Sciences, SRI International, Menlo Park, CA 94025, USA
Massimiliano de Zambotti, Center for Health Sciences, SRI International, Menlo Park, CA 94025, USA.
References
- [1].Mancia G, “Autonomic modulation of the cardiovascular system during sleep,” N. Engl. J. Med, vol. 328, no. 5, pp. 347–9, February 4 1993. [DOI] [PubMed] [Google Scholar]
- [2].de Zambotti M, Trinder J, Silvani A, Colrain I, and Baker FC, “Dynamic coupling between the central and autonomic nervous systems during sleep: a review,” Neurosci Biobehav Rev, vol. 90, pp. 84–103, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grimaldi D et al. , “Strengthening sleep-autonomic interaction via acoustic enhancement of slow oscillations,” Sleep, February 6 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lechinger J, Heib DP, Gruber W, Schabus M, and Klimesch W, “Heartbeat-related EEG amplitude and phase modulations from wakefulness to deep sleep: Interactions with sleep spindles and slow oscillations,” Psychophysiology, vol. 52, no. 11, pp. 1441–50, November 2015. [DOI] [PubMed] [Google Scholar]
- [5].Silvani A and Dampney RA, “Central control of cardiovascular function during sleep,” Am. J. Physiol. Heart Circ. Physiol, vol. 305, no. 12, pp. H1683–92, December 2013. [DOI] [PubMed] [Google Scholar]
- [6].Silvani A, Calandra-Buonaura G, Dampney RA, and Cortelli P, “Brain-heart interactions: physiology and clinical implications,” Philos Trans A Math Phys Eng Sci, vol. 374, no. 2067, May 13 2016. [DOI] [PubMed] [Google Scholar]
- [7].Diekelmann S and Born J, “The memory function of sleep,” Nat Rev Neurosci, vol. 11, no. 2, pp. 114–126, 2010. [DOI] [PubMed] [Google Scholar]
- [8].Wilckens KA, Ferrarelli F, Walker MP, and Buysse DJ, “Slow-Wave Activity Enhancement to Improve Cognition,” Trends Neurosci, vol. 41, no. 7, pp. 470–482, July 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Santostasi G et al. , “Phase-locked loop for precisely timed acoustic stimulation during sleep,” J. Neurosci. Methods, vol. 259, pp. 101–114, February 1 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ngo HV, Martinetz T, Born J, and Molle M, “Auditory closed-loop stimulation of the sleep slow oscillation enhances memory,” Neuron, vol. 78, no. 3, pp. 545–53, May 8 2013. [DOI] [PubMed] [Google Scholar]
- [11].Santostasi G and Zee P, “Phase-locked loop to enhance slow wave sleep,” US Patent Appl. US20170304587A1, 2017. [Google Scholar]
- [12].de Zambotti M, Baker FC, Colrain IM, Forouzanfar M, Goldstone A, and Willoughby A, “Slow wave activity optimization based on dominant peripheral nervous system oscillations,” WO Patent Appl. PCT/US2018/042493, 2019. [Google Scholar]
- [13].Stefanovska A, “Coupled oscillators: complex but not complicated cardiovascular and brain interactions,” IEEE Eng. Med. Biol. Mag, vol. 26, no. 6, pp. 25–29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Forouzanfar M, Baker FC, Goldstone A, Colrain IM, and de Zambotti M, “Automatic analysis of pre‐ejection period during sleep using impedance cardiogram,” Psychophysiology, vol. e13355, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification, 2007.
- [16].Castiglioni P et al. , “Broad-band spectral analysis of 24 h continuous finger blood pressure: comparison with intra-arterial recordings,” Clin. Sci. (Lond.), vol. 97, no. 2, pp. 129–39, August 1999. [PubMed] [Google Scholar]
- [17].Rangayyan RM, Biomedical Signal Analysis: A Case-Study Approach. Wiley-IEEE Press, 2002. [Google Scholar]
- [18].de Zambotti M et al. , “Sex- and Age-Dependent Differences in Autonomic Nervous System Functioning in Adolescents,” J. Adolesc. Health, vol. 62, no. 2, pp. 184–190, February 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dornhorst AC, Howard P, and Leathart GL, “Respiratory variations in blood pressure,” Circulation, vol. 6, no. 4, pp. 553–8, October 1952. [DOI] [PubMed] [Google Scholar]