Significance
A molecular clock controls circadian rhythmicity in mammals. The interplay of the clock with cellular growth and development is of interest regarding possible connections between cancer and clock disruption. The c-MYC oncoprotein is a transcriptional activator that has been reported to control and be controlled by the clock. We have used knockout mice mutated in genes controlling either the negative or positive arm of the molecular clock to clarify the role of the clock in c-MYC regulation. Results with both types of mutant mice consistently show that the clock controls c-MYC protein levels by controlling expression of the c-Myc oncogene. These findings will aid in understanding the interplay of the clock and clock disruption on cell growth control and cancer.
Keywords: circadian clock, cryptochromes, transcription regulation, BMAL1, c-MYC
Abstract
The circadian clock is a global regulatory mechanism that controls the expression of 50 to 80% of transcripts in mammals. Some of the genes controlled by the circadian clock are oncogenes or tumor suppressors. Among these Myc has been the focus of several studies which have investigated the effect of clock genes and proteins on Myc transcription and MYC protein stability. Other studies have focused on effects of Myc mutation or overproduction on the circadian clock in comparison to their effects on cell cycle progression and tumorigenesis. Here we have used mice with mutations in the essential clock genes Bmal1, Cry1, and Cry2 to gain further insight into the effect of the circadian clock on this important oncogene/oncoprotein and tumorigenesis. We find that mutation of both Cry1 and Cry2, which abolishes the negative arm of the clock transcription–translation feedback loop (TTFL), causes down-regulation of c-MYC, and mutation of Bmal1, which abolishes the positive arm of TTFL, causes up-regulation of the c-MYC protein level in mouse spleen. These findings must be taken into account in models of the clock disruption–cancer connection.
The circadian clock is a molecular clock that regulates biochemical, physiologic, and behavioral functions with about 24 h periodicity. In mammals the core molecular clock is made up of CLOCK (or NPAS2) and BMAL1 transcriptional activators and Cryptochrome (CRY1 and CRY2) and Period (PER1 and PER2) transcriptional repressors. CLOCK and BMAL1 activate transcription of CRYs and PERs, and these proteins, after a time delay, enter the nucleus and inhibit their own transcription (transcription–translation feedback loop [TTFL]) with a period of about 24 h. This primary period is consolidated by the nuclear receptors NR1D1 and NR1D2 which control the expression of the Bmal1 gene (1–8). Downstream clock-controlled genes are regulated by promoter binding by CLOCK-BMAL1, which is integrated with gene-specific regulatory factors, and clock control manifests as gene expression levels that either do not oscillate or do oscillate with peak/nadir expression times characteristic of each circadian gene (9–12). Fifty to 80% of all protein-encoding genes in mice and 10 to 20% of protein-encoding genes in a given tissue are clock controlled. Naturally, such a global regulatory system interfaces with nearly all cellular metabolic systems and signaling pathways. Among these, the link between the circadian clock and cell proliferation and thus between the clock and oncogenes and tumor suppressors has been the focus of numerous studies. Of these investigations the ones on the CLOCK/c-MYC interconnection are of special interest for several reasons (13–16).
First, like the CLOCK-BMAL1 heterodimer, the MYC-MAX heterodimer binds to the E-box element (CACGTG) in cognate promoters for gene regulation (17–19). Second, there is ∼30% overlap of BMAL1 binding sites with c-MYC binding sites (18, 20). Third, Myc is mutated or amplified in a significant fraction of all cancers, in particular in leukemias and lymphomas (16, 17, 21). Fourth, there are reports that there is an inverse correlation between MYC levels in lymphomas and clock gene expression and that high MYC expression in a variety of tumors is associated with down-regulation of BMAL1 and poor clinical outcomes (20, 22). Finally, there are reports that in addition to a BMAL1-MYC connection, MYC is also controlled by the PER and CRY proteins that make up the negative arm of the TTFL (13–16). To gain further insights into this important subject, we decided to investigate the effects of Bmal1 and Cry knockouts in mice on c-Myc expression in spleen with regard to both circadian pattern and protein level. Our data show that Bmal1 KO (knockout) causes up-regulation of c-Myc over the entire circadian cycle. Cry1 or Cry2 knockouts do not significantly affect c-Myc level. However, Cry1/2 double knockout causes significant depression of the c-Myc level over the entire circadian cycle.
Results
To investigate the effect of the circadian clock on c-MYC expression and activity, we analyzed the effects of mutations in the positive and negative arms of the circadian TTFL on the level of c-MYC and its target genes in mouse spleen. Spleen has been used in prior studies of c-MYC since leukemias and lymphomas are often associated with c-Myc dysregulation, and spleen is a major lymphoid organ in which c-MYC expression is relatively high. Under the conditions of our experiments, wild-type (WT) mice exhibited normal circadian rhythmicity; results showing rhythmic expression of PER2 in spleen with a peak at ZT20 (zeitgeber time) are shown in SI Appendix, Fig. S1.
Regulation of c-MYC by the Positive Arm of the Circadian Clock.
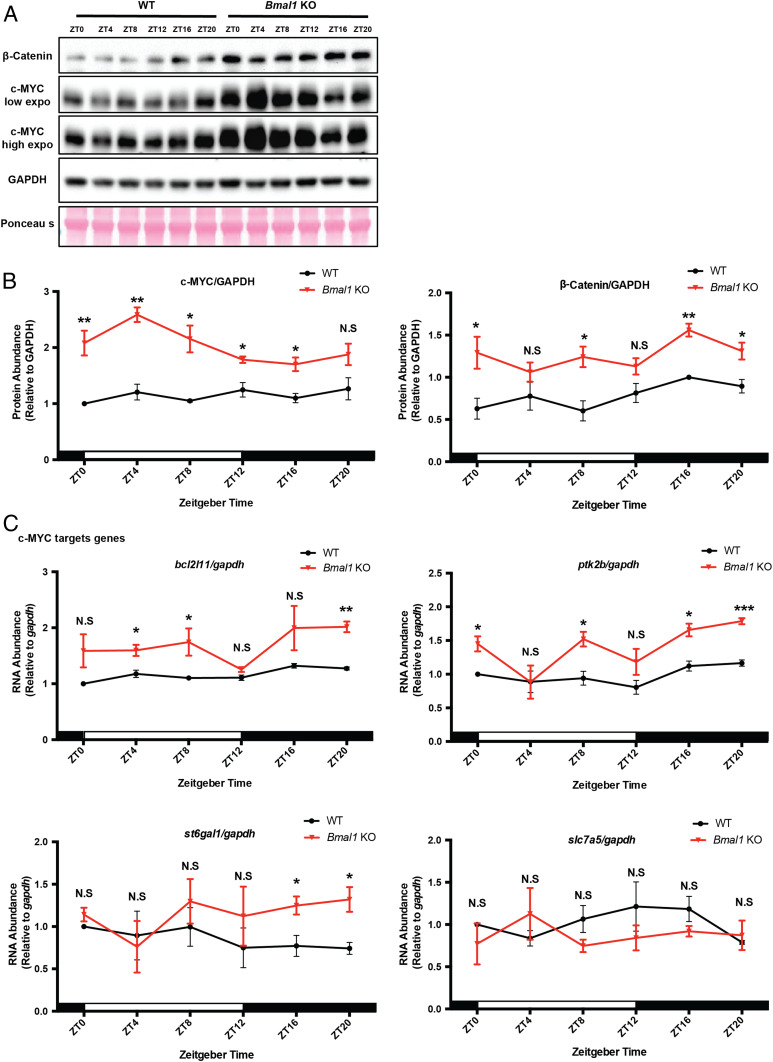
Previous work has shown that c-MYC inhibits Bmal1 transcription either directly by binding to the Bmal1 promoter in the form of MYC-MIZ1 and repressing its transcription (20) or by binding to the E-box in Nr1d1/2 promoters in the form of MYC-MAX and up-regulating NR1D1/2, which in turn binds to the RER elements in the Bmal1 promoter (22). Here we wished to investigate the converse regulation to find out how the positive arm of the circadian clock affects c-Myc expression by comparing c-MYC protein levels over a circadian cycle in the spleen of WT and Bmal1 KO (knockout) mice. Fig. 1 A and B shows that in WT spleen c-MYC is expressed at a constant level over the circadian cycle. In Bmal1 KO spleen the c-MYC level is elevated 1.5- to 2.0-fold over most of the circadian cycle (Fig. 1 B, Left) (9–12). To confirm these changes in c-MYC level as a consequence of Bmal1 KO, we measured the mRNA levels of four c-MYC regulated genes as sentinels over a circadian cycle (18, 19, 23). Fig. 1C shows that three out of four of these genes exhibit elevated transcription over most or part of the circadian cycle. These sentinel genes exhibit no consistent, rhythmic pattern. These data taken in totality suggest that c-MYC expression is controlled negatively by the positive arm of the circadian clock.
Fig. 1.
Bmal1 deletion increases c-MYC expression in vivo. (A) β-Catenin and c-MYC expression were measured by Western blot of spleen extracts from WT and Bmal1 KO mice using GAPDH and Ponceau S as loading controls. Samples were collected at the indicated time points (ZT = Zeitgeber time). (B) Quantification of c-MYC and β-catenin protein levels. For each genotype and time point, three mice were used for quantification. White and black bars indicate lights on and lights off, respectively. Error bars correspond to SEM (standard error of the mean). For c-MYC, data were normalized to a value of 1 for WT at ZT0; for β-catenin, data were normalized to a value of 1 for WT at ZT16. N.S, not significant, *P < 0.05, **P < 0.01, as determined by t test. (C) c-MYC target gene mRNA levels in spleen of WT and Bmal1 KO mice. For each genotype and time point, three mice were used for quantification after normalizing to gapdh RNA. White and black bars indicate lights on and lights off, respectively. Error bars correspond to SEM. Data were normalized to a value of 1 for WT at ZT0. N.S, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, as determined by t test.
BMAL1 Regulates c-Myc Level by Transcriptional Control.
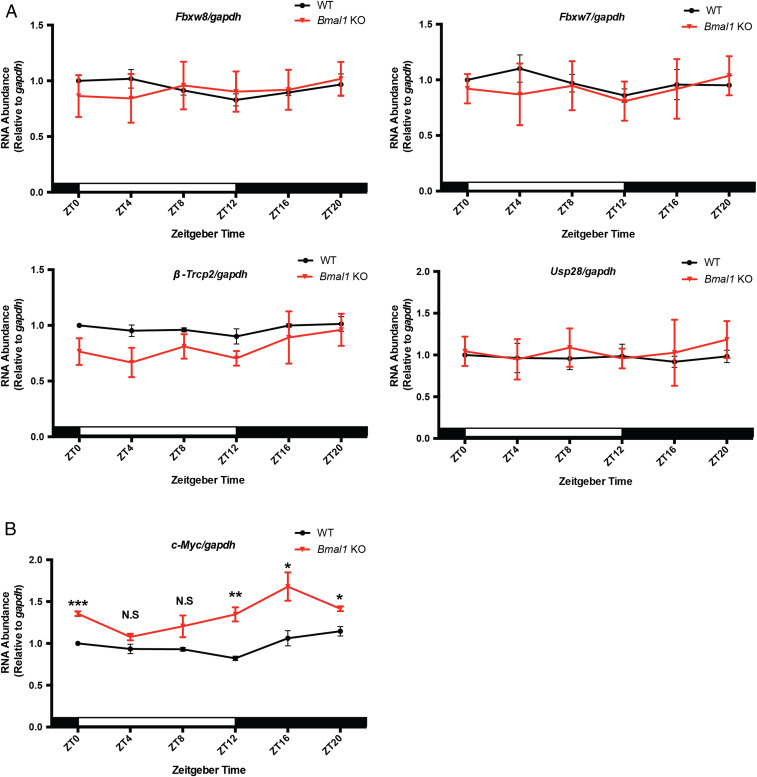
In our Western blots, we also measured levels of β-catenin, which was recently shown to be an important transcriptional activator of c-Myc (24–27). Fig. 1 A and B, Right shows that in Bmal1 KO mice (28), β-catenin levels are uniformly elevated over the circadian cycle compared to WT mice. A corresponding increase in c-Myc RNA in Bmal1 KO mice compared to WT, shown in Fig. 2B, suggests that transcriptional control of c-Myc is an important mechanism of clock control of c-MYC levels.
Fig. 2.
BMAL1 regulates c-Myc at the transcriptional level. (A) Expression of genes that regulate c-MYC degradation was measured by reverse transcription-qPCR using spleens of WT and Bmal1 KO mice and using gapdh as an internal control. Samples were collected at the indicated time points. Error bars correspond to SEM. Data were normalized to a value of 1 for WT at ZT0. (B) c-Myc mRNA levels in spleen samples detected by reverse transcription-qPCR. Three biological repeats were used for quantification. White and black bars indicate lights on and lights off, respectively. Error bars correspond to SEM. Data were normalized to a value of 1 for WT at ZT0. N.S, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, as determined by t test.
c-MYC is an extremely unstable protein that is degraded with a half-life of 15 to 20 min through the ubiquitin–proteosome system mediated by several E3 ligases, including β-TrCP2, FBXW7, FBXW8, and USP28 (29–32). To examine whether the clock affected c-MYC expression by a posttranscriptional mechanism by altering E3 ligase levels, we analyzed the transcription of some key E3 ligases that have been implicated in c-MYC degradation. As seen in Fig. 2A the expression of these genes is not affected by Bmal1 KO, while the transcription of c-Myc is elevated over most of the cycle (Fig. 2B), consistent with the notion that the elevation of c-MYC in Bmal1 KO mice is caused by elevated transcription of c-Myc mediated by clock-controlled β-catenin.
Regulation of c-MYC by the Negative Arm of the Circadian Clock.
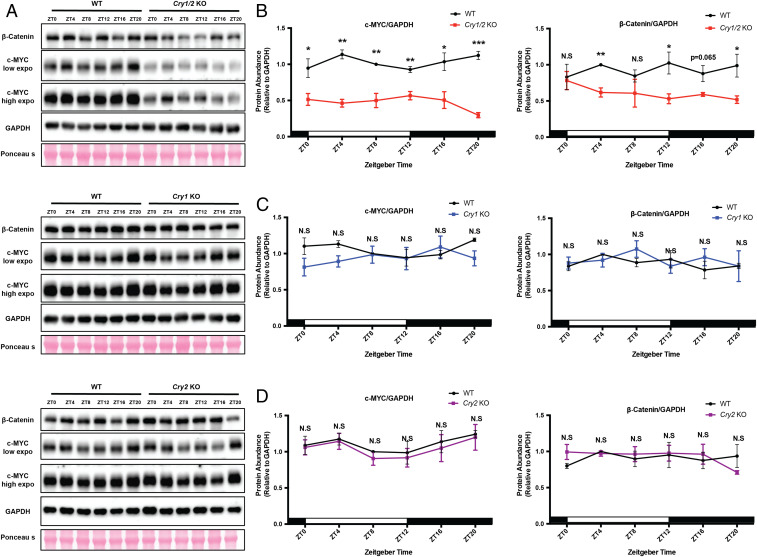
The data for Bmal1 KO mice indicate that c-Myc is a second-order clock-controlled gene. To further test this model, we analyzed the effect of the negative arm of the TTFL by investigating c-MYC expression in Cry mutant mouse strains (33, 34). The Western blot results in Fig. 3 show c-MYC protein levels in spleens from WT, Cry1 KO, Cry2 KO, and Cry1/2 KO mice. As is apparent, Cry1 KO and Cry2 KO single mutants have no effect on c-MYC level over the entire circadian cycle, while Cry1/2 KO mutant mice exhibit uniform down-regulation of c-MYC over virtually the entire cycle (Fig. 3 A–D). Thus, c-MYC expression is controlled positively by the negative arm of the circadian clock.
Fig. 3.
Cry1/2 double knockout decreases c-MYC expression in vivo. (A) Endogenous proteins c-MYC and β-catenin were detected by WB (Western blotting) in spleens of WT and three Cry mutants using GAPDH and Ponceau S as loading controls. Samples were collected at the indicated time points. (B–D) Quantification of c-MYC and β-catenin protein levels in the indicated genotypes. For each genotype and time point, three mice were used for quantification. White and black bars indicate lights on and lights off, respectively. Error bars correspond to SEM. For c-MYC, data were normalized to a value of 1 for WT at ZT8; for β-catenin, data were normalized to a value of 1 for WT at ZT4. N.S, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, as determined by t test.
CRYs Regulate c-Myc Level by Transcriptional Control.
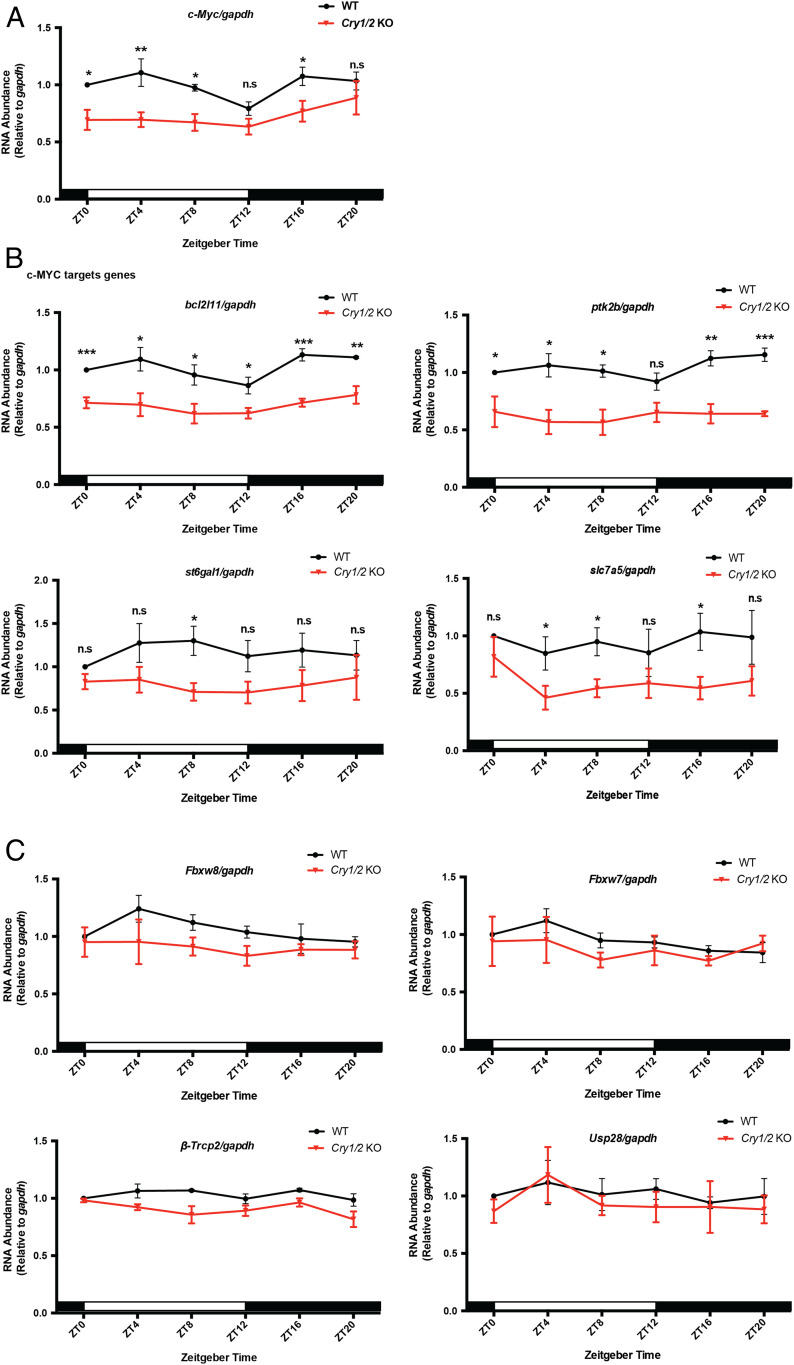
Experiments were then done to see whether the negative and positive arms of the clock control c-MYC by a common mechanism. We found that c-MYC and β-catenin protein levels are depressed exclusively in the Cry1/2 KO mice (Fig. 3), and c-Myc expression is also depressed at most time points exclusively in Cry1/2 KO mice (Fig. 4A). Fig. 4B shows that depressed c-MYC levels in Cry1/2 KO mice are associated with depressed transcription of c-MYC target genes, while there are no significant changes in the transcription levels of the c-MYC-acting E3 ligases (Fig. 4C). In addition, previous studies indicated that GSK-3β participates in phosphorylation and degradation of c-MYC (35–37). However, we did not observe differences in the amounts of either active form (p-Tyr216-GSK-3β) or total GSK-3β between wild-type and mutant (Cry1 KO, Cry2 KO, and Cry1/2 KO) mice (SI Appendix, Fig. S2). Thus, the evidence derived from both positive and negative arms of the clock indicate that clock regulation of c-Myc is via transcriptional control.
Fig. 4.
CRYs modulate c-MYC expression through transcriptional regulation. (A) c-Myc levels in the spleen samples of WT and Cry1/2 KO mice detected by reverse transcription-qPCR. Three biological repeats were used for quantification. White and black bars indicate lights on and lights off, respectively. (B) mRNA levels of c-MYC target genes in the spleens of WT and Cry1/2 KO. For each genotype and time point, four mice were used for quantification. (C) Expression of genes that regulate c-MYC degradation were measured by reverse transcription-qPCR in spleens of WT and Cry1/2 KO mice using gapdh as an internal control. Samples were collected at the indicated time points. Error bars correspond to SEM. Data were normalized to a value of 1 for WT at ZT0. n.s, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, as determined by the t test.
BMAL1 Regulates c-Myc Level by Repressing Ctnnb1 Transcription.
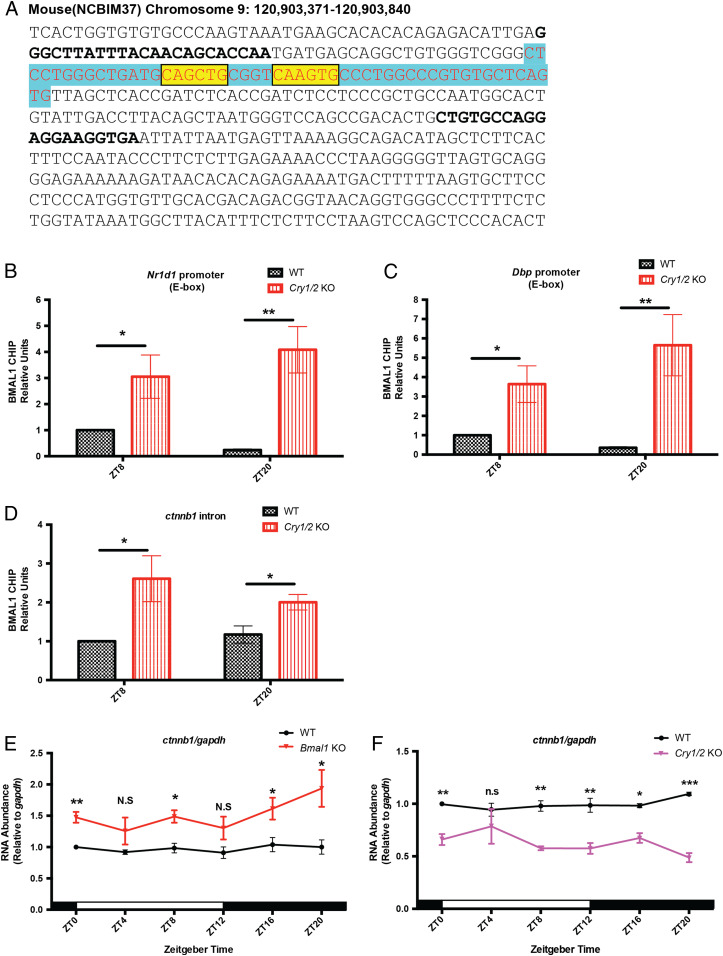
In light of the involvement of β-catenin in clock control of c-Myc, we were interested in the control of the Ctnnb1 gene that expresses β-catenin. Previous studies showed that BMAL1 can rhythmically bind to the Ctnnb1 intron region illustrated in Fig. 5A (10, 12, 38). To address how BMAL1 regulates Ctnnb1, we performed chromatin immunoprecipitation (ChIP) qPCR experiments using the BMAL1 antibody to clarify CLOCK-BMAL1 binding to this intron region in WT and Cry1/2 KO spleen. Two time points were examined, ZT8 and ZT20, which are close to the peak and trough times of BMAL1 binding. Positive control results in Fig. 5 B and C show that binding of BMAL1 to Nr1d1 and Dbp promoters in wild-type spleen is higher at ZT8, consistent with previous mouse liver ChIP-seq results (10, 12, 38). In Cry1/2 KO spleen, the absence of the negative arm of the clock is associated with significant enrichment of BMAL1 binding at both ZT8 and ZT20 compared to WT (Fig. 5 B and C). Interestingly, Fig. 5D shows that in WT mice, the binding of BMAL1 to the intron region is constitutive; it does not exhibit circadian oscillation as it does in the Nr1d1 and Dbp promoters. Importantly, there is dramatically higher BMAL1 binding in Cry1/2 KO spleen at both ZT8 and ZT20 compared to WT (Fig. 5D). Measurements of overall BMAL1 expression at all circadian time points in WT and Cry1/2 KO spleen showed that BMAL1 was lower in the Cry1/2 KO at all time points; however, the phosphorylated, active form of BMAL1 was more highly expressed at all time points in the Cry1/2 KO (SI Appendix, Fig. S3), as reported previously (39, 40). Consistent with these results, and our findings in Fig. 5 E and F showing that Ctnnb1 transcription is elevated in Bmal1 KO mice and depressed in Cry1/2 KO mice over the circadian cycle, is a model in which the binding of BMAL1 to the Ctnnb1 intron region represses Ctnnb1. While CLOCK and BMAL1 are generally recognized as transcriptional activators, inhibition of gene expression by CLOCK-BMAL1 binding has also been reported (5).
Fig. 5.
Transcription of Ctnnb1 is controlled by circadian clock proteins. (A) Sequence of the BMAL1 binding site in the Ctnnb1 intron region is in blue (10, 12, 38). Nucleotides conserved in the E-box-like (CANNTG) sequences are colored in yellow. Nucleotides targeted by qPCR primers are marked by bold font. (B–D) Levels of BMAL1 binding to the Nr1d1 E-box, Dbp E-box, and Ctnnb1 intron region in the spleens of WT and Cry1/2 KO mice. For each genotype and time point, at least three mice were used for quantification. Error bars correspond to SEM. Data were normalized to a value of 1 for WT at ZT8. *P < 0.05, **P < 0.01, as determined by the t test. (E and F) Ctnnb1 mRNA levels in spleen samples from WT, Bmal1 KO, and Cry1/2 KO mice detected by reverse transcription-qPCR. Three biological repeats were used for quantification. White and black bars indicate lights on and lights off, respectively. Error bars correspond to SEM. Data were normalized to a value of 1 for WT at ZT0. N.S/n.s, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, as determined by the t test.
Discussion
c-Myc is an extensively investigated oncogene that is either mutated or amplified in a significant number of cancers. Recent research has implicated Myc mutation or overproduction in circadian clock disruption (9, 20). Conversely, it has been reported that CRY2, which participates in the negative arm of the circadian TTFL, binds to phosphorylated c-MYC and targets it to ubiquitylation by FBXL3 and ultimate degradation by the proteasome (23). This study showed that in Cry2 KO mice, c-MYC was overproduced and these mice had increased incidence of lymphosarcomas.
The mechanisms by which c-MYC affects the clock are a matter of some debate but in essence are in agreement on the fact that c-MYC overproduction disrupts the circadian clock. Here we have approached the c-MYC–circadian clock connection from the clock perspective, namely, the effects of various clock mutations on c-MYC levels. We find that Bmal1 and Cry1/2 knockout mutations have opposite effects on c-MYC transcription and protein levels: In Bmal1 KO c-MYC transcription and protein levels are elevated, whereas in Cry1/2 KO c-MYC transcription and protein levels are depressed. Furthermore, we have obtained data showing that these outcomes are the consequence of c-Myc being a second-order clock-controlled gene through β-catenin, which is controlled by the core circadian clock (Fig. 6). Our results are in apparent contrast to a report that CRY2 stimulates ubiquitylation of c-MYC by SCF (FBXL3) and its degradation by the proteasome and, as a consequence, Cry2 KO mice have higher levels of c-MYC and higher levels of lymphosarcomas compared to WT controls (23). However, these experiments were in mice with Eμ-Myc+/− background in which the translocation of c-Myc and IgH element mimics the translocation in human Burkitt lymphoma (23, 41). Under our experimental conditions in which c-Myc is expressed under the control of its native promoter at physiologically relevant levels the Cry2 KO mutant has no measurable effect on c-MYC level, nor does the Cry1 KO mutant (Fig. 3 and SI Appendix, Fig. S4). Only in the Cry1/2 KO mutant do we observe an effect of CRY on c-MYC manifested as consistently down-regulated c-MYC levels over the entire circadian cycle. Further studies are need to reconcile these seemingly contradictory results.
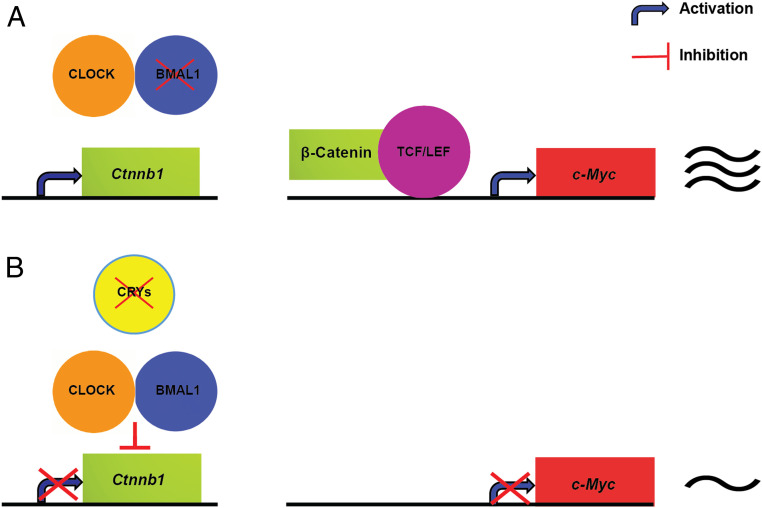
Fig. 6.
BMAL1 and CRY control of c-MYC expression in mouse spleen (model of circadian regulation of c-MYC). (A) BMAL1 deficiency induces the transcription of Ctnnb1 (β-catenin), which binds the TCF/LEF family of transcription factors resulting in high expression of c-Myc. (B) In the Cry1/2 KO, CLOCK-BMAL1 at its binding site in the Ctnnb1 intron suppresses the transcription of Ctnnb1 (β-catenin), which results in low expression of c-Myc.
Experimental Procedures
Mice.
Bmal1 KO (28) mice were bred by intercrossing of heterozygotes. Littermates were genotyped by PCR as previously described. The Cry1 KO, Cry2 KO, and Cry1/2 KO mice (33, 34, 42) were bred as homozygotes and were genotyped by Transnetyx. Bmal1 KO and WT were obtained from The Jackson Laboratory. Bmal1 KO and all three Cry KO mice (33, 34) are in the C57BL/6J background. All mice were synchronized to a standard light:dark 12 h:12 h schedule at a constant temperature of 21 °C to 23 °C, with food and water ad libitum; both males and females were used interchangeably in this study. Traditionally, ZT0 is the time of lights on, and ZT12 is the time of lights off. Mice were handled according to the guidelines of the NIH and the University of North Carolina School of Medicine (Institutional Animal Care and Use Committee).
Western Blotting Assay.
Spleens were collected at the indicated circadian time points from 3- to 6-month-old, light:dark 12 h:12 h-synchronized mice and homogenized with 800 µL ice-cold protein lysis buffer: 20 mM Tris⋅HCl, 150 mM NaCl, 1 mM Na2EDTA (disodium ethylenediaminetetraacetic acid), 1 mM EGTA (ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid), 1% Nonidet P-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na2VO4, 1× tablet protease inhibitor (Roche, catalog no. 42364500), and 1 mM PMSF (phenylmethylsulfonyl fluoride) by 10 strokes of a Teflon homogenizer. Homogenates were then incubated 5 min on ice and then further homogenized with 65 strokes in an ice-cold Dounce homogenizer with a tight pestle. The supernatants were collected after centrifugation for 8 min at 15,000 rpm at 4 °C in a centrifuge (Model 5425, Eppendorf). Proteins were quantified using Bio-Rad Protein Assay (Bio-Rad, catalog no. 5000006). For the immunoblot procedure, proteins resolved by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) were transferred to 0.45 μM nitrocellulose membranes (Bio-Rad, catalog no. 1620115) by the Trans-Blot Turbo transfer system (Bio-Rad). Then, membranes were blocked with 5% nonfat dry milk diluted in 1XPBST (0.1% TWEEN-20 in phosphate buffer solution) to block for ∼2 h at room temperature and then incubated with primary antibodies at 4 °C overnight. Rabbit anti-c-MYC (Abcam: ab32072), mouse anti-β-catenin (Novus: 12F7), rabbit anti-GSK-3β [p Tyr216] (Novus: NB100-81946), rabbit anti-GSK-3β (Cell Signaling: 27C10), rabbit anti-PER2 (Alpha Diagnostic: PER21-A) were all diluted 1:1,000. Mouse Anti-β-ACTIN (Sigma: A1978) and rabbit anti-GAPDH (EMD Millipore: ABS16) were both diluted 1:10,000. After three washes with PBST (10 min each), membranes were incubated with the corresponding secondary antibody for ∼1 h at room temperature. The membranes were imaged using western ECL (enhanced chemiluminescence) substrate (Bio-Rad, catalog no. 170-5061) after three washes with PBST (10 min each). For quantification, band intensity was analyzed with Image J. Relative signals for test proteins were obtained by normalizing the test protein signal to the GAPDH or ACTIN loading control signal.
Reverse Transcription-qPCR.
Spleens were harvested at the indicated time points and homogenized with 200 µL TRIzol (Ambion, catalog no. 260706) using RNase-Free Disposable Pellet Pestles (Fisher Scientific, catalog no. 12-141-364). Eight hundred milliliters of TRIzol were added to homogenates, mixed, and then incubated at room temperature for 5 min. Chloroform (200 µL) was added with mixing, and the supernatants were collected after centrifugation for 20 min at 15,000 rpm at 4 °C in a centrifuge (Model 5425, Eppendorf). Samples went through all steps of a RNA Mini Kit (Ambion, catalog no. 12183018A) to obtain total RNA. A 1-µg quantity of each RNA was reverse transcribed with a cDNA Synthesis Kit (Bio-Rad, catalog no. 1708891). For reverse transcription-qPCR of c-Myc, bcl2l11, Ptk2b, st6gal1, slc7a5, Fbxw8, Fbxw7, β-Trcp2, Usp28, Ctnnb1, and gapdh, we used a SYBR qPCR detection kit (Bio-Rad, catalog no. 1725121). The specific primer sequences for qPCR are described in Table 1. At least three biological replicates were performed using the QuantStudio Real-Time PCR System (Life Technologies).
Table 1.
Primers used in this study
| Primers | Sequence (5′ → 3′) | Reference |
| c-Myc fwd | GCGACTCTGAAGAAGAGCAAG | This study |
| c-Myc rev | GCCTCGGGATGGAGATGAG | This study |
| Ctnnb1 fwd | ACAGCACCAATGATGAGCAG | This study |
| Ctnnb1 rev | GGCTGGACCCATTAGCTGTA | This study |
| Bcl2l11 fwd | CCCGGAGATACGGATTGCAC | Huber et al. (23) |
| Bcl2l11 rev | GCCTCGCGGTAATCATTTGC | Huber et al. (23) |
| Slc7a5 fwd | ATATCACGCTGCTCAACGGTG | Huber et al. (23) |
| Slc7a5 rev | CTCCAGCATGTAGGCGTAGTC | Huber et al. (23) |
| Ptk2b fwd | TGAGCCCTTGAGCCGTGTA | Huber et al. (23) |
| Ptk2b rev | AGCTTGAAGTTCTTCCCTGGG | Huber et al. (23) |
| St6gal1 fwd | CTCCTGTTTGCCATCATCTGC | Huber et al. (23) |
| St6gal1 rev | GGGTCTTGTTTGCTGTTTGAGA | Huber et al. (23) |
| TrCP2 fwd | TGGCGCCTATGATGGGAA | Huber et al. (23) |
| TrCP2 rev | GTCAAGAGCAGCCTGCAAGTC | Huber et al. (23) |
| Fbxw7 fwd | GAGACTTCATCTCCTTGCTTCCTAAA | Huber et al. (23) |
| Fbxw7 rev | CGCTTGCAGCAGGTCTTTG | Huber et al. (23) |
| Fbxw8 fwd | GCCAGGTTGCCTTTGGAGT | Huber et al. (23) |
| Fbxw8 rev | TCCCGGATGTTGACACAGGTA | Huber et al. (23) |
| Usp28 fwd | GGGTCCGAGAAGGAAAGCC | Huber et al. (23) |
| Usp28 rev | CACGGAACGATCCGAAGGAAG | Huber et al. (23) |
| gapdh fwd | CATCACTGCCACCCAGAAGACTG | Chiou et al. (5) |
| gapdh rev | ATGCCAGTGAGCTTCCCGTTCAG | Chiou et al. (5) |
| Ctnnb1 intron F | GGGCTTATTTACAACAGCACCAA | This study |
| Ctnnb1 intron R | TCACCTTCCTCCTGGCACAG | This study |
| Nr1d1 E-box F | GCTGCTGGAAAAGTGTGTCA | Annayev et al. (43) |
| Nr1d1 E-box R | ATGGAGAAATGAGGCACCAG | Annayev et al. (43) |
| Dbp E-box F | TGTGAACACTCGGCTCCTTT | Annayev et al. (43) |
| Dbp E-box R | ATATTTGGCCAATGGGAGGA | Annayev et al. (43) |
ChIP-qPCR.
ChIP-qPCR was performed using published protocols with minor modifications (4, 5). Spleens were collected from mice at the indicated circadian time points, placed in 2.5 mL per spleen of 1% formaldehyde in PBS, and then homogenized by 10 strokes of a Teflon homogenizer and then 15 strokes of a glass homogenizer (WHEATON, catalog no. 357544) using the loose “A” pestle. Homogenates were then incubated for 10 min at room temperature. Twenty-one milliliters of ice-cold nuclei buffer (2.2 M sucrose, 125 mM glycine, 10 mM Hepes [pH 7.6], 15 mM KCl, 2 mM EDTA, 0.1 mM spermine, 0.5 mM spermidine, 0.5 DTT, 1× tablet protease inhibitor, and 0.5 mM PMSF) were add to stop the cross-linking reactions. Samples were carefully layered on top of 10 mL of ice-cold nuclei buffer in ultraclear tubes (Beckman Coulter, catalog no. Z00302SCA) and then centrifuged for 1.5 h at 24,000 rpm and 4 °C in a SW27 rotor (Beckman). The nuclei were washed two times with nuclei wash buffer (20 mM Tris [pH 7.5], 150 mM NaCl, and 2 mM EDTA), suspended in 400 µL RIPA (radioimmunoprecipitation assay) ChIP buffer containing 0.6% SDS (50 mM Tris [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, and 1× tablet protease inhibitor), and then sonicated using an ultrasonicator (Qsonica) for 7 min with a setting of 30% AMPL (amplitude) 3 s on and 5 s off. Samples were centrifuged at 14,000 rpm for 10 min at 4 °C. Supernatants were added to 2 mL of SDS-free RIPA ChIP buffer to dilute the SDS concentration from 0.6 to 0.1% and then divided into 1.1 mL for IP (immunoprecipitation) and 11 μL for input. For each IP, 10 μL of Dynabeads Protein G slurry (Invitrogen, catalog no. 00787116) was prepared by incubating with 1 μg of rabbit anti-BMAL1 (Bethyl, catalog no. A302-616A) for 6 h at 4 °C with rotation. After overnight incubation of supernatants with resins, resins were then washed, and bound material was eluted using elution buffer (0.1 M NaHCO3 and 1% SDS). Eluates were digested by Protease K, purified by a PCR purification kit (QIAGEN, catalog no. 163037455), and subjected to qPCR. At least two technical replicates of qPCR were performed for each biological ChIP replicate, and three biological replicates were performed for BMAL1 assays. The specific primers used for qPCR are described in Table 1. The error bars represent SEM among biological replicates; t tests were used to measure statistical differences.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant GM118102 (to A.S.) and funding from the Azerbaijan National Academy of Sciences (to K.E.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011225117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Reppert S. M., Weaver D. R., Coordination of circadian timing in mammals. Nature 418, 935–941 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Hastings M. H., Reddy A. B., Maywood E. S., A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4, 649–661 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Partch C. L., Green C. B., Takahashi J. S., Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 24, 90–99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye R. et al., Dual modes of CLOCK:BMAL1 inhibition mediated by Cryptochrome and Period proteins in the mammalian circadian clock. Genes Dev. 28, 1989–1998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiou Y. Y. et al., Mammalian Period represses and de-represses transcription by displacing CLOCK-BMAL1 from promoters in a Cryptochrome-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 113, E6072–E6079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sancar A., Mechanisms of DNA repair by photolyase and excision nuclease (Nobel lecture). Angew. Chem. Int. Ed. Engl. 55, 8502–8527 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Cederroth C. R. et al., Medicine in the fourth dimension. Cell Metab. 30, 238–250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patke A., Young M. W., Axelrod S., Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Mange F. et al.; CycliX Consortium , Diurnal regulation of RNA polymerase III transcription is under the control of both the feeding-fasting response and the circadian clock. Genome Res. 27, 973–984 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike N. et al., Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Martelot G. et al.; CycliX Consortium , Genome-wide RNA polymerase II profiles and RNA accumulation reveal kinetics of transcription and associated epigenetic changes during diurnal cycles. PLoS Biol. 10, e1001442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trott A. J., Menet J. S., Regulation of circadian clock transcriptional output by CLOCK:BMAL1. PLoS Genet. 14, e1007156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman B. J., Cancer clocks out for lunch: Disruption of circadian rhythm and metabolic oscillation in cancer. Front. Cell Dev. Biol. 4, 62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamia K. A., Ticking time bombs: Connections between circadian clocks and cancer. F1000 Res. 6, 1910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masri S., Sassone-Corsi P., The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 24, 1795–1803 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walton Z. E., Altman B. J., Brooks R. C., Dang C. V., Circadian clock’s cancer connections. Annu. Rev. Cancer Biol. 2, 133–153 (2018). [Google Scholar]
- 17.Larsson L. G., Henriksson M. A., The Yin and Yang functions of the Myc oncoprotein in cancer development and as targets for therapy. Exp. Cell Res. 316, 1429–1437 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Walz S. et al., Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature 511, 483–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabò A. et al., Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature 511, 488–492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shostak A. et al.; ICGC MMML-Seq Project , MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation. Nat. Commun. 7, 11807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rana S. et al., Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Mol. Biol. Rep. 41, 95–103 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Altman B. J. et al., MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 22, 1009–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber A. L. et al., CRY2 and FBXL3 cooperatively degrade c-MYC. Mol. Cell 64, 774–789 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He T. C. et al., Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998). [DOI] [PubMed] [Google Scholar]
- 25.van de Wetering M. et al., The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Cadigan K. M., Waterman M. L., TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 4, a007906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgs M. R., Lerat H., Pawlotsky J. M., Hepatitis C virus-induced activation of β-catenin promotes c-Myc expression and a cascade of pro-carcinogenetic events. Oncogene 32, 4683–4693 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Bunger M. K. et al., Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yada M. et al., Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popov N. et al., The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 9, 765–774 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Popov N., Schülein C., Jaenicke L. A., Eilers M., Ubiquitylation of the amino terminus of Myc by SCF(β-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nat. Cell Biol. 12, 973–981 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Farrell A. S., Sears R. C., MYC degradation. Cold Spring Harb. Perspect. Med. 4, a014365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thresher R. J. et al., Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Vitaterna M. H. et al., Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 96, 12114–12119 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sears R. C., The life cycle of C-myc: From synthesis to degradation. Cell Cycle 3, 1133–1137 (2004). [PubMed] [Google Scholar]
- 36.Kim S. H., Song Y., Seo H. R., GSK-3β regulates the endothelial-to-mesenchymal transition via reciprocal crosstalk between NSCLC cells and HUVECs in multicellular tumor spheroid models. J. Exp. Clin. Cancer Res. 38, 46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears R. et al., Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14, 2501–2514 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rey G. et al., Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 9, e1000595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauger M. A., Sancar A., Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 65, 6828–6834 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Tamaru T. et al., CRY drives cyclic CK2-mediated BMAL1 phosphorylation to control the mammalian circadian clock. PLoS Biol. 13, e1002293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams J. M. et al., The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 (1985). [DOI] [PubMed] [Google Scholar]
- 42.Ozturk N., Lee J. H., Gaddameedhi S., Sancar A., Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 106, 2841–2846 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annayev Y. et al., Gene model 129 (Gm129) encodes a novel transcriptional repressor that modulates circadian gene expression. J. Biol. Chem. 289, 5013–5024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.