Significance
Photosystem II (PSII) harbors the water-splitting activity underlying oxygenic photosynthesis. The PSII reaction center protein D1 is subject to photodamage and must be replaced with nascent D1 to maintain photosynthetic activity. How new D1 synthesis is coordinated with D1 damage has been a long-standing question. Our results clarify the nature of the light-induced signal that activates D1 synthesis for PSII repair in plants, and suggest an autoregulatory mechanism in which degradation of damaged D1 relieves a repressive interaction between D1 and translational activators in a complex that functions in PSII assembly and repair. This proposed mechanism comprises a responsive switch that couples D1 synthesis to need for D1 during PSII biogenesis and repair.
Keywords: psbA, translational control, chloroplast, photosystem II
Abstract
The D1 reaction center protein of photosystem II (PSII) is subject to light-induced damage. Degradation of damaged D1 and its replacement by nascent D1 are at the heart of a PSII repair cycle, without which photosynthesis is inhibited. In mature plant chloroplasts, light stimulates the recruitment of ribosomes specifically to psbA mRNA to provide nascent D1 for PSII repair and also triggers a global increase in translation elongation rate. The light-induced signals that initiate these responses are unclear. We present action spectrum and genetic data indicating that the light-induced recruitment of ribosomes to psbA mRNA is triggered by D1 photodamage, whereas the global stimulation of translation elongation is triggered by photosynthetic electron transport. Furthermore, mutants lacking HCF136, which mediates an early step in D1 assembly, exhibit constitutively high psbA ribosome occupancy in the dark and differ in this way from mutants lacking PSII for other reasons. These results, together with the recent elucidation of a thylakoid membrane complex that functions in PSII assembly, PSII repair, and psbA translation, suggest an autoregulatory mechanism in which the light-induced degradation of D1 relieves repressive interactions between D1 and translational activators in the complex. We suggest that the presence of D1 in this complex coordinates D1 synthesis with the need for nascent D1 during both PSII biogenesis and PSII repair in plant chloroplasts.
Oxygenic photosynthesis shapes earth’s ecosystems by generating molecular oxygen, consuming atmospheric CO2, and producing the carbohydrates that fuel life. Oxygenic photosynthesis is mediated by a set of protein–prosthetic group complexes embedded in the thylakoid membranes of cyanobacteria and chloroplasts: photosystem I (PSI), photosystem II (PSII), the cytochrome b6f complex (cyt b6f), and ATP synthase (1, 2). PSII drives this process by using the energy of sunlight to extract electrons from water, producing molecular oxygen as a byproduct (3, 4). However, exposure to light also damages the PSII reaction center. An elaborate repair cycle involves the partial disassembly of PSII, selective proteolysis of D1, its replacement with newly synthesized D1, and reassembly of PSII (5–7). D1 is damaged even at low light intensities, but the rate of damage increases with light intensity (8–10). D1 synthesis keeps pace with this damage up to moderate light intensities; beyond this point, D1 synthesis plateaus or even decreases, and photosynthesis is inhibited (8, 9, 11). In chloroplasts, the PSII repair cycle is promoted by membrane rearrangements that expose the damaged core to proteases that degrade D1 and to the ribosomes that synthesize new D1 (6, 7, 12).
The PSII repair cycle has come under scrutiny due to its importance for maintaining photosynthesis. Considerable progress has been made in understanding the source of D1 damage, the architectural reorganization of thylakoid membranes, the disassembly of damaged PSII, the proteases that degrade damaged D1, and factors that promote PSII reassembly (5–7, 12). However, mechanisms underlying the selective translation of psbA mRNA to provide D1 for PSII repair remain obscure (13, 14). The consensus view in recent years has been that psbA translation for PSII repair is regulated at the elongation step (7, 15–17), a view that arises primarily from experiments with the green alga Chlamydomonas reinhardtii (Chlamydomonas) (18). However, we showed recently that regulated translation initiation makes a large contribution in plants (19). These experiments used ribosome profiling (ribo-seq) to monitor ribosome occupancy on chloroplast open reading frames (ORFs) in maize and Arabidopsis upon shifting seedlings harboring mature chloroplasts between light and dark. The results showed that ribosome occupancy on psbA mRNA increases dramatically within 15 min of shifting plants from the dark to the light and drops rapidly after shifting plants from light to dark. Furthermore, psbA mRNA is the only chloroplast mRNA to exhibit a substantive change in ribosome occupancy following short-term light–dark shifts, and this results in an “overproduction” of D1 (with respect to other PSII subunits) in the light. These observations imply that the light-induced recruitment of ribosomes to psbA RNA in mature plant chloroplasts serves the purpose of PSII repair. The same study revealed a plastome-wide increase or decrease in translation elongation rate following a shift to light or dark, respectively. Thus, although the rate of translation elongation on psbA mRNA decreased in the dark, it remains unclear whether a psbA-specific change in translation elongation rate contributes to the control of D1 synthesis for PSII repair in plants.
A related issue concerns the nature of the light-induced signal that triggers D1 synthesis for PSII repair. The prevailing view in recent years is that light induces D1 synthesis via its effects on products of photosynthetic electron transport (7, 15–17). Proposed signals include ATP, the trans-thylakoid proton gradient, reduced plastoquinone, and dithiol reduction (20–26). However, the experiments that led to this view did not distinguish plastome-wide changes in translation rate from the psbA-specific activation that provides D1 for PSII repair. These two phenomena may involve distinct signals, so the parsing of global effects from psbA-specific effects is essential for elucidating the signals.
In this study, we sought to clarify the light-induced signal that triggers the recruitment of ribosomes to psbA mRNA in mature plant chloroplasts. For this purpose, we analyzed light-induced D1 synthesis and psbA ribosome occupancy in response to different wave lengths of light and in mutants lacking various components of the photosynthetic apparatus. Our results show that the light-activated recruitment of ribosomes to psbA mRNA is not mediated by the activation of photosynthetic electron transport. Instead, our results strongly suggest that light triggers this response by causing D1 damage, a hypothesis that had been proposed in early studies (27, 28) but that has lost prominence. We show further that the absence of the PSII assembly factor HCF136, which promotes a very early step in D1 assembly (29–32), results in constitutively high psbA ribosome occupancy in the dark. These results reveal an intimate connection between a specific early PSII assembly intermediate, light-induced D1 damage, and light-regulated psbA ribosome occupancy. We propose that the recently elucidated HCF244/OHP1/OHP2 complex, which functions in both PSII assembly and psbA translational activation (32–35), serves as the hub of an autoregulatory mechanism that links D1 synthesis to need for nascent D1 during PSII repair and biogenesis.
Results
Effects of Monochromatic Light Sources on D1 Synthesis Support D1 Damage as the Light-Induced Trigger for psbA Translation in Mature Chloroplasts.
It is widely believed that light induces psbA translation via its effects on intermediates or products of photosynthesis (reviewed in refs. 7, 15–17, and 36). However, early reports proposed instead that D1 synthesis is activated by light-induced D1 degradation (27, 28). Furthermore, the existence of both plastome-wide and psbA-specific effects of light on chloroplast translation (19) complicates interpretation of much of the prior research on this topic, as these two effects may involve distinct signals.
To distinguish between the two proposed signaling modes, we exploited the fact that the action spectrum for PSII damage differs from that for photosynthesis (11, 37–40). In particular, ultraviolet A (UV-A) is particularly potent at inducing D1 damage but is an inefficient driver of photosynthesis. This effect of UV-A light has been attributed to its absorption by the manganese cluster associated with the PSII reaction center (reviewed in ref. 41). If light-induced D1 damage is the triggering event for psbA ribosome recruitment, then the action spectrum for psbA ribosome recruitment would match that for D1 damage and not that of photosynthesis.
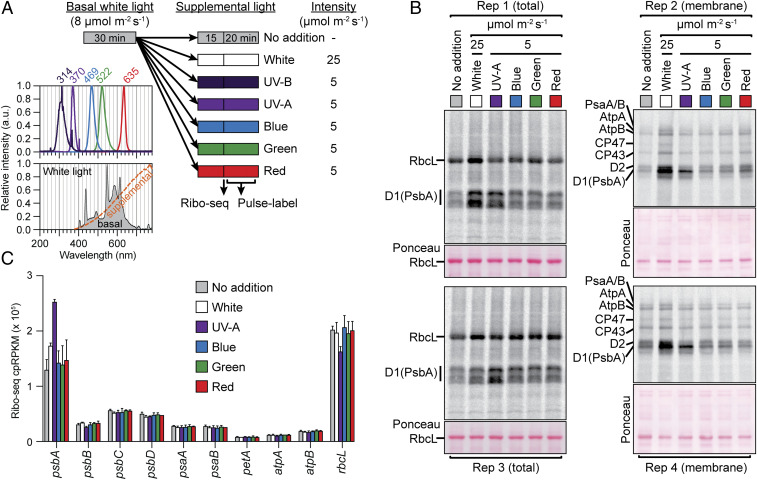
To address this possibility, we used the scheme shown in Fig. 1A. Arabidopsis seedlings were grown in moderate light under diurnal cycles for several weeks. They were then acclimated to very low intensity light (8 µmol m−2 s−1) for 30 min to minimize photodamage. Supplemental low intensity illumination was then provided with either white light (25 µmol m−2 s−1) or with monochromatic UV-A, blue, green, or red light (5 µmol m−2 s−1). The red light (635 nm) is in the peak of the photosynthetically active region of the spectrum; the green and blue lights (522 nm and 469 nm, respectively) are roughly twofold less efficient at driving photosynthesis; and the UV-A light (370 nm) is an additional severalfold less efficient (37, 40). Plants were harvested 15 min after exposure to the supplemental light for analysis by ribosome profiling or were subjected to in vivo pulse labeling during an additional 20 min under the same supplemented light condition. The pulse-labeling assays were performed in the presence of cycloheximide to inhibit cytosolic protein synthesis.
Fig. 1.
Action spectrum analysis of light-induced psbA translation in Arabidopsis. (A) Light treatment regime and spectra of each monochromatic light source. Plants were acclimated to low intensity (8 µmol m−2 s−1) white light for 30 min prior to transfer to the indicated supplemental light (spectra shown to the Left). Fifteen minutes later, plants were harvested for ribo-seq or used for pulse labeling. Samples were loaded on the basis of equal chlorophyll. (B) Pulse-labeling analysis of plants exposed to light regimes diagrammed in A. Excised aerial portions of seedlings were radiolabeled for 20 min in the presence of cycloheximide. Total leaf lysates were analyzed in replicates 1 and 3 (Left), and membrane fractions were analyzed in replicates 2 and 4 (Right). Samples were resolved by SDS/PAGE, transferred to nitrocellulose, and radiolabeled proteins were detected by phosphorimaging. The gels used to resolve membrane samples included urea, which collapses the D1 signal into one band. The chloroplast-encoded proteins RbcL, D1 (PsbA), D2 (PsbD), CP47 (PsbB), CP43 (PsbC), AtpA, AtpB, and PsaA/B are marked. The nitrocellulose filters with the bound radiolabeled proteins were stained with Ponceau S (below) to illustrate relative sample loading. Minor variation among samples in overall labeling efficiency is inevitable. Therefore, the ratios of signal among proteins in the same lane were used to draw conclusions. (C) Normalized ribosome footprint abundance for chloroplast genes following the indicated light treatment. The mean ± SD (from two replicates) is shown. The values for all chloroplast genes are available in Dataset S1.
The pulse-labeling data showed that supplemental UV-A light caused a greater increase in the rate of D1 synthesis than did any of the other single wavelengths sampled (Fig. 1B). For example, analysis of total leaf lysates (Left panels) showed that the labeling of D1 in comparison to the soluble protein RbcL is elevated by the UV-A treatment, but not by the other treatments. Similarly, analysis of the membrane fraction (Right panels) showed that the labeling of D1 in comparison to other membrane proteins was most strongly increased by UV-A supplementation. Analysis of the same material by ribosome profiling (Fig. 1C and Dataset S1) showed that the supplemental UV-A light caused a specific increase in ribosomes on psbA mRNA (roughly twofold), whereas the green, blue, and red lights had no significant effect. The fact that UV-A more effectively stimulated a specific increase in D1 synthesis and psbA ribosome recruitment than did photosynthetically active wavelengths argues against models that invoke products of photosynthesis as triggers for the psbA-specific response and is consistent with the possibility that the trigger is light-induced D1 damage.
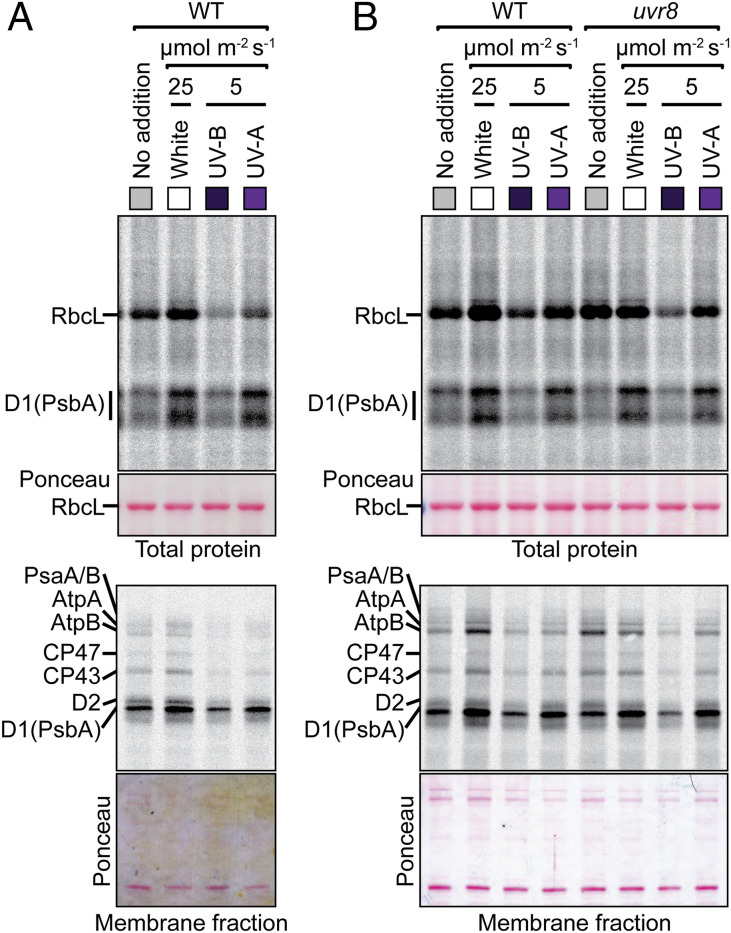
Activation by UV-A could potentially be mediated by one of the photoreceptors known to absorb UV-A: cryptochromes, phototropins, or UVR8 (42). However, this possibility seems unlikely for several reasons. First, peak absorption for phototropins and crytochromes is in the blue region of the spectrum (43, 44), which did not trigger psbA translation. Second, these photoreceptors do not localize to chloroplasts, making them unlikely transducers of the very rapid psbA translation response. Nonetheless, we investigated the potential involvement of UVR8, whose peak response is in the UV-B range (280 to 315 nm) (45). Supplementation of low intensity white light with a low dose of UV-B inhibited synthesis of all detected proteins in wild-type plants (Fig. 2A), implying a global inhibition of chloroplast translation. Furthermore, pulse-labeling analysis of a uvr8 mutant showed normal light-induced D1 synthesis (Fig. 2B).
Fig. 2.
Pulse-labeling analysis of the effects of UV-B on chloroplast translation. The experiment was performed as in Fig. 1A, with supplemental light provided by either UV-B or UV-A as indicated. Excised aerial portions of seedlings were radiolabeled for 20 min in the presence of cycloheximide. Total leaf lysates (Top) and membrane fractions (Bottom) were resolved by SDS/PAGE and transferred to nitrocellulose, and radiolabeled proteins were detected by phosphorimaging. The gels used to resolve membrane samples included urea, which collapses the D1 signal into one band. Samples were loaded on the basis of equal chlorophyll. The nitrocellulose filters with the bound radiolabeled proteins were stained with Ponceau S (below) to illustrate relative sample loading. (A) Analysis of 14-d-old wild-type (WT) plants. (B) Analysis of young rosette leaves from 55-d-old wild-type or uvr8 mutant plants.
The findings above strongly suggest that the psbA-specific light response—whether assayed by pulse labeling or ribosome profiling—is triggered by light-induced D1 damage and not by products or intermediates of photosynthetic electron transport. In addition, two aspects of the data revealed that a different signal triggers the plastome-wide effects on translation elongation rate. First, the supplemental white light increased synthesis of all proteins detected by pulse labeling (Fig. 1B) with no corresponding change in ribosome footprint abundance (except psbA) (Fig. 1C). Second, the supplemental white light resulted in greater D1 synthesis but less increase in psbA ribosome footprints than did the weaker UV-A light (Fig. 1 B and C). These results suggest that the white light increased synthesis of many (if not all) chloroplast-encoded proteins primarily through a concerted increase in elongation and initiation rate that did not change ribosome occupancy, whereas the UV-A treatment acted specifically on psbA at the level of ribosome recruitment. Thus, light-induced recruitment of ribosomes to psbA mRNA is likely triggered by D1 damage, whereas the plastome-wide increase in translation elongation rate may be triggered by a product of photosynthesis.
Normal Light-Induced D1 Synthesis in Mutants Lacking the Chloroplast ATP Synthase, PSI, or the STN7/STN8 Protein Kinases.
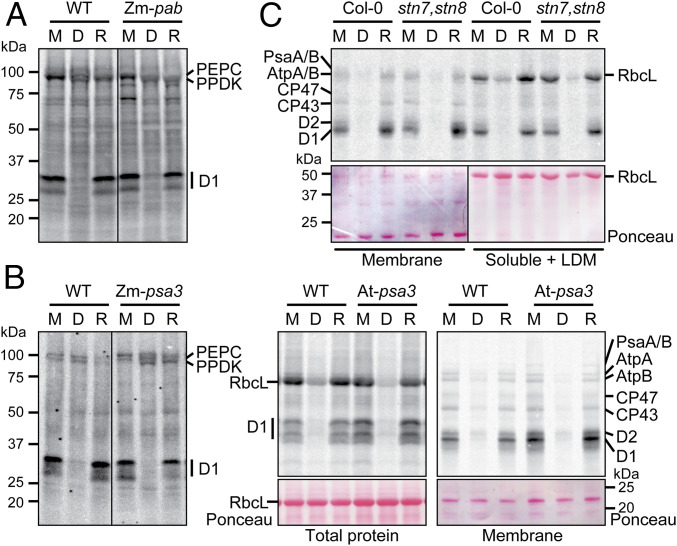
In a complementary approach, we addressed the role of photosynthesis in light-activated chloroplast translation by using maize and Arabidopsis mutants lacking specific components of the photosynthetic apparatus. To minimize energy deficits resulting from the photosynthetic defects, Arabidopsis mutants were grown on sucrose-containing medium, and maize mutants were analyzed prior to the depletion of seed reserves. Nonphotosynthetic maize mutants are similar in size and morphology to their normal siblings at this stage (see, e.g., SI Appendix, Fig. S1A and ref. 33), whereas nonphotosynthetic Arabidopsis mutants show varying degrees of developmental delay despite the sucrose supplementation (see, e.g., refs. 46–48). Plants were grown in diurnal cycles, and translation was assayed by pulse labeling at midday, after 1 h in the dark, or following 15 min of reillumination (Fig. 3).
Fig. 3.
Analysis of light-regulated D1 synthesis in mutants lacking ATP synthase, PSI, or the STN7 and STN8 kinases. Plants were pulse labeled at midday (M), after 1 h in the dark (D), or after a 15-min reillumination (R). Maize mutants (Zm-pab and Zm-psa3) were radiolabeled for 15 min in the absence of cycloheximide, and total leaf lysates were analyzed by SDS/PAGE with sample loading normalized based on labeling of two cytosolic proteins at ∼100 kDa (presumed to be PEPC and PPDK). Arabidopsis mutants (At-psa3 and stn7,stn8) were radiolabeled for 20 min in the presence of cycloheximide, and samples were loaded on the basis of equal chlorophyll. (A) Analysis of a maize Zm-pab mutant, lacking the thylakoid ATP synthase. The mutant is described in SI Appendix, Fig. S1A. Total leaf lysates are shown. The line separates noncontiguous lanes from the same exposure of the same gel. (B) Analysis of mutants lacking PSI in maize (Zm-psa3) and Arabidopsis (At-psa3). Arabidopsis samples were analyzed both as total leaf lysates (Left) and as membrane fractions (Right). The nitrocellulose filters harboring the radiolabeled proteins were stained with Ponceau S to illustrate equal sample loading. RbcL, large subunit of Rubisco. The line in the Zm-psa3 panel separates noncontiguous lanes from the same exposure of the same gel. (C) Analysis of an Arabidopsis stn7,stn8 double mutant. The experiment was performed as for At-psa3 except that radiolabeled RbcL was visualized by analysis of supernatants remaining after membranes were pelleted by centrifugation. This supernatant is designated soluble + LDM because it includes low-density membranes (LDMs), which harbor some of the radiolabeled D1. See SI Appendix, Materials and Methods for details. A single Ponceau S-stained blot was imaged with different contrast (marked by a line) to improve visibility of the bands.
We examined the role of the chloroplast ATP synthase by analysis of a maize mutant lacking the ortholog of Arabidopsis PAB, which is required for ATP synthase assembly (49). Immunoblot analyses of the maize mutant (Zm-pab) showed a roughly 20-fold decrease in the abundance of the AtpB subunit of the ATP synthase, with no impact on the abundance of core subunits of other photosynthetic complexes (SI Appendix, Fig. S1A). The Zm-pab mutant showed robust light-induced D1 synthesis (Fig. 3A). These results suggest that light-induced ATP synthesis is not required to trigger D1 synthesis when sufficient ATP is available from other sources to support the energy demands of translation.
We examined the need for PSI by analysis of psa3 mutants, which lack PSI due to a defect in PSI assembly (50) (SI Appendix, Fig. S1B). The psa3 mutants in both maize and Arabidopsis showed robust light-induced D1 synthesis (Fig. 3B), suggesting that processes that require PSI are not required to trigger D1 synthesis. In fact, the Arabidopsis mutant showed elevated D1 synthesis under moderate light intensity, an effect that was reduced under very low intensity light (SI Appendix, Fig. S1C). This correlates with the increased susceptibility of PSII to light-induced damage in Arabidopsis mutants lacking PSI (51, 52), consistent with the hypothesis that D1 damage triggers the psbA-specific response.
Two paralogous protein kinases, STN7 and STN8, are involved in the adaptation of the photosynthetic apparatus to unbalanced photosystem excitation or excess light energy (reviewed in ref. 53). STN7 phosphorylates light-harvesting chlorophyll a/b binding proteins and is required for state transitions (54), whereas STN8 phosphorylates PSII core proteins and promotes PSII repair (53). To address whether STN7 and STN8 contribute to the regulation of D1 synthesis in response to light, we pulse labeled an Arabidopsis stn7-stn8 double mutant (55) in light and dark (Fig. 3C). Light-induced D1 synthesis was not altered in the double mutant, indicating that the STN7 and STN8 kinases are not necessary for the signal transduction cascade that triggers light-induced D1 synthesis in mature chloroplasts.
Pharmacological and Genetic Data Are Consistent with Prior Evidence That Light Induces the Plastome-Wide Translation Response Via Electron Transport-Induced Effects on pH.
Experiments involving pharmacological treatments of lysed barley chloroplasts suggested that light induces a general increase in translation elongation rate via electron-transport-induced changes in pH (21). In accord with that view, the PSII inhibitor DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea] caused a general inhibition of translation in isolated Chlamydomonas chloroplasts (20), in Chlamydomonas cells (56), and in cyanobacteria (57). Those prior observations suggest that photosynthetic electron transport underlies the plastome-wide effects of light we detected by ribo-seq (19), a possibility that is also supported by the differential effects of monochromatic light sources presented above.
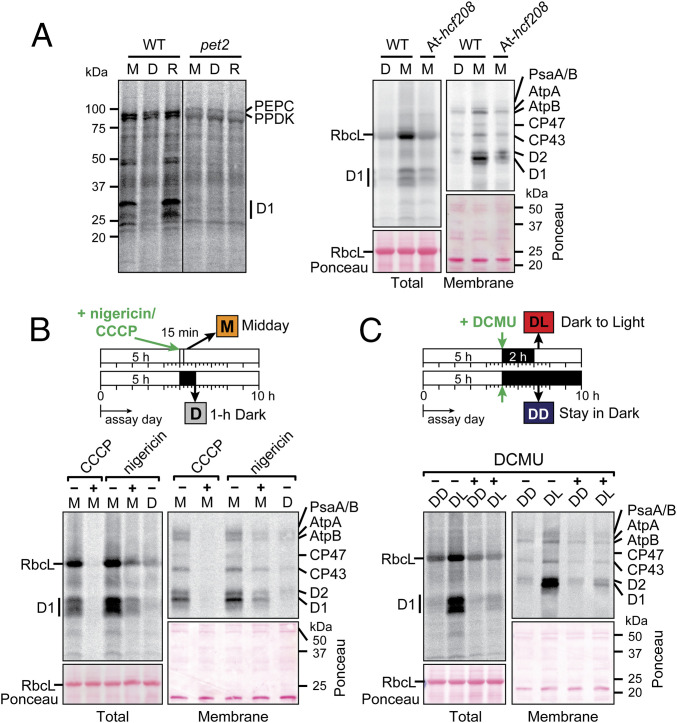
To examine this issue further, we used pulse labeling to analyze chloroplast protein synthesis in mutants lacking the cyt b6f complex (Fig. 4A). The cyt b6f complex transports electrons from PSII to PSI and pumps protons from the stroma to the thylakoid lumen. We analyzed orthologous mutants in maize and Arabidopsis, pet2 and hcf208, respectively, which lack the cyt b6f complex due to a defect in heme attachment (47, 58). Light-induced D1 synthesis in the maize pet2 mutant was undetectable (Fig. 4 A, Left). However, D1 was the only chloroplast-encoded protein whose synthesis we could reliably detect in maize, so we could not discern whether this was a psbA-specific or plastome-wide defect. The Arabidopsis hcf208 mutant also showed a considerable reduction in D1 synthesis in the light (Fig. 4 A, Right). This was particularly apparent when the membrane fractions were analyzed on gels containing urea (Right), which resolve the closely migrating D1 and D2 bands. Furthermore, synthesis of all of the detected membrane proteins was reduced in the hcf208 mutant, as was synthesis of the soluble protein RbcL. This behavior suggests that the cyt b6f complex is required for the plastome-wide activation of translation elongation by light.
Fig. 4.
Analysis of light-regulated chloroplast protein synthesis in mutants lacking the cytochrome b6f complex or after treatment with nigericin, CCCP, or DCMU. (A) Analysis of cytochrome b6f mutants. Pulse-labeling was performed at midday (M), after 1-h in the dark (D), or after 15-min reillumination (R). The Left shows total leaf lysates of the maize pet2 mutant labeled in the absence of cycloheximide. The line separates noncontiguous lanes on the same gel. The two rapidly synthesized proteins at ∼100 kDa are likely to be the nuclear gene products PEP carboxylase and PPDK. The Right shows total leaf lysates and membrane fractions of Arabidopsis At-hcf208 mutants labeled in the presence of cycloheximide. The Ponceau S-stained blots show relative sample loading. Samples were loaded on the basis of equal total protein. (B) Arabidopsis seedlings (Col-0) were treated with 25 µM nigericin or 100 µM CCCP for 15 min before pulse labeling (20 min) in the presence of cycloheximide in the light at midday (M). Mock-treated plants were radiolabeled after 1 h in the dark (D) for comparison. Samples were loaded on the basis of equal chlorophyll. (C) Arabidopsis seedlings (Col-0) were treated with 20 µM DCMU in darkness for 2 h before shifting to light for 15 min (DL) or remaining in the dark (DD), followed by 20-min labeling in the presence of cycloheximide. Samples were loaded on the basis of equal chlorophyll.
Loss of the cyt b6f complex would affect numerous potential signals for the plastome-wide translational response, including ATP, reduced thioredoxin, plastoquinone redox state, and the trans-thylakoid proton gradient. However, the fact that light-induced chloroplast protein synthesis was normal in PSI mutants (Fig. 3) suggests that reduced thioredoxin is not relevant. Furthermore, light-induced D1 synthesis was normal in a mutant lacking ATP synthase (Fig. 3), suggesting that ATP is not an activating signal for the plastome-wide response; however, the inability to detect synthesis of other plastid-encoded proteins in that experiment precludes firm conclusions. To explore these issues further, we analyzed effects of several pharmacological inhibitors of photosynthesis. Treatment of Arabidopsis with the uncouplers nigericin or CCCP (carbonyl cyanide 3-chlorophenylhydrazone) inhibited the synthesis of all detected plastid-encoded proteins in the light (Fig. 4B), consistent with a role for light-induced changes in pH or the trans-thylakoid proton motive force in the signaling pathway. Treatment with DCMU in the dark phase also inhibited light-induced chloroplast translation (Fig. 4C), similar to effects reported previously in Chlamydomonas (20, 56). The fact that DCMU and the absence of the cyt b6f complex had similar inhibitory effects on translation in the light but have opposite effects on the redox state of the plastoquinone pool suggest that plastoquinone redox state is not relevant to the plastome-wide translational induction by light. Taken together, our inhibitor and genetic data are consistent with the prior conclusion from the lysed chloroplast system (21) that light-induced effects on pH trigger a global increase in chloroplast translation elongation rate.
Constitutive psbA Ribosome Occupancy in hcf136 Mutants Implicates an Early PSII Assembly Intermediate as a Repressor of psbA Translation in the Dark.
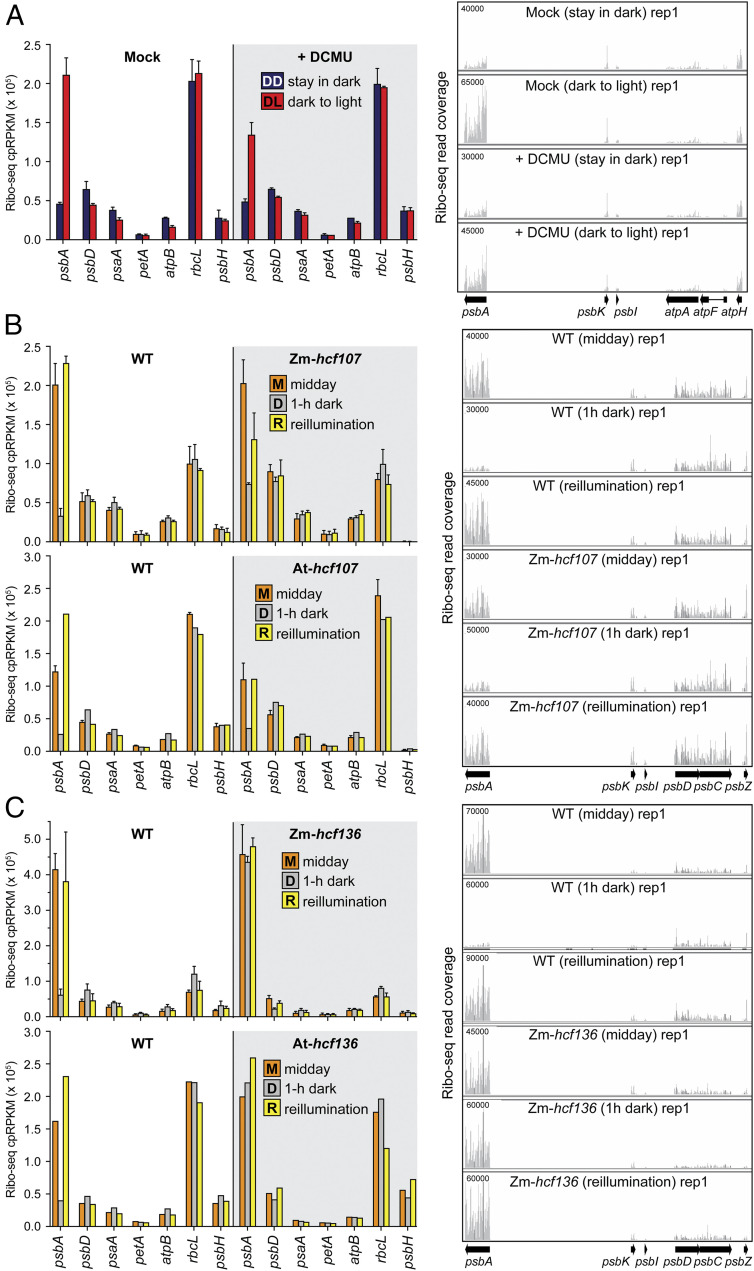
We next used ribo-seq to examine the role of PSII in the light-induced recruitment of ribosomes specifically to psbA mRNA. In one experiment, Arabidopsis seedlings were treated with DCMU using the same regime employed for the pulse-labeling assay (Fig. 4C) and were then harvested for ribosome profiling during the dark phase or after 15 min of reillumination (Fig. 5A). DCMU reduced the recruitment of ribosomes to psbA mRNA in response to light and did not affect ribosome occupancy on other ORFs (Fig. 5A and SI Appendix, Fig. S2). Notably, however, the degree to which DCMU inhibited light-induced psbA ribosome recruitment was modest in comparison with its strong effect on D1 synthesis in the pulse-labeling assay (Fig. 4C). These results suggest that DCMU inhibits light-induced D1 synthesis in two ways: 1) by interfering with the light-induced recruitment of ribosomes specifically to psbA mRNA, and 2) by reducing translation elongation rate on psbA and other chloroplast mRNAs.
Fig. 5.
Ribo-seq analysis of effects of PSII disruptions on chloroplast ribosome occupancies in dark and light. Left shows ribosome footprint abundance for psbA and several other chloroplast genes. The values for all chloroplast genes are available in Dataset S1. Mean ± SD is shown for experiments that were performed with replicates. Several of the Arabidopsis mutant analyses lacked replicates, but results from the orthologous maize mutants demonstrate replicability. Right shows screen captures from the Integrated Genome Viewer (IGV) that display ribosome footprint coverage along psbA and adjacent ORFs. The y axis values indicate the number of reads at each position (not normalized). The maximum y axis values (shown in Upper Left) were chosen such that the magnitude for ORFs adjacent to psbA were similar among the conditions being compared. (A) Analysis of Arabidopsis (Col-0) after DCMU treatment. The treatments were as described in Fig. 4C and were performed in two replicates. IGV screen captures of the second replicate and data showing that psbA mRNA level is not affected by DCMU are shown in SI Appendix, Fig. S2. (B) Analysis of Zm-hcf107 and At-hcf107 mutants. The data show the known loss of psbH expression in these mutants (60, 81). Two replicates were performed for Zm-hcf107. One of the replicates of the midday At-hcf107 samples was published previously (81). The IGV images are from maize data. IGV screen captures of replicates and data showing that psbA mRNA level is not affected in the mutants are shown in SI Appendix, Fig. S3. (C) Ribo-seq analysis of Zm-hcf136 and At-hcf136 mutants. The midday data were published previously (33) and are shown here again in the context of the complete experiment. The Zm-hcf136 experiment was performed with two replicates. The IGV images are from maize data. IGV captures of replicates and data showing that psbA mRNA level is not affected in the mutants are shown in SI Appendix, Fig. S4.
In a second approach, we examined light-induced psbA ribosome recruitment in two mutants lacking PSII: hcf107, which lacks PSII due to a defect in expressing the chloroplast psbH gene (59–61), and hcf136, which lacks PSII due to a defect at an early step in PSII assembly (29–31). In both cases, we analyzed orthologous mutants in maize and Arabidopsis, with leaves harvested at midday, after 1 h in the dark, and after 15 min of reillumination. Light-induced changes in psbA ribosome occupancy were fairly normal in the hcf107 mutants (Fig. 5B and SI Appendix, Fig. S3), although the response to reillumination may be muted. However, the response in hcf136 mutants was dramatically altered (Fig. 5C and SI Appendix, Fig. S4): psbA ribosome occupancy was normal (or perhaps elevated) in the light and did not decrease in the dark. This striking behavior indicates that HCF136 is required to repress the recruitment of ribosomes to psbA mRNA in the dark, thereby linking PSII assembly to light-regulated psbA translation.
This effect of HCF136 is particularly intriguing in light of the functional relationship between HCF136 and a recently elucidated complex harboring proteins denoted HCF244, OHP1, and OHP2 (32, 34, 35, 62, 63). This complex and its cyanobacterial ortholog (Ycf39/HliD/HliC) binds nascent D1 and is required to assemble D1 with other reaction center proteins (32, 34, 63). Current data suggest that HCF136 and its cyanobacterial ortholog (Ycf48) act upstream of the HCF244 complex during PSII assembly, and are required to incorporate nascent D1 into the HCF244 complex (29–31, 64, 65). HCF136 and the HCF244/OHP1/OHP2 complex are involved in both PSII assembly and PSII repair (30, 32, 63). Furthermore, the HCF244 complex in plants is also required for psbA translation initiation (33, 46), providing a molecular link between D1 assembly status and psbA translation.
These recent insights into the HCF244 complex, in conjunction with evidence that unassembled D1 represses psbA translation in Chlamydomonas (18, 66), underlie our recent proposal that nascent D1 negatively autoregulates psbA translation through inhibitory interactions with HCF244/OHP1/OHP2. The constitutively high psbA ribosome occupancy in hcf136 mutants is consistent with this scheme: hcf136 mutants cannot incorporate D1 into the HCF244 complex, leaving the translation activation function of the complex constitutively “on.” These hcf136 data allow us to expand the scope of the HCF244/OHP1/OHP2-centered translational autoregulatory model to incorporate light-regulated psbA translation and PSII repair, as discussed below.
Discussion
The experiments described here address two long-standing questions in plant biology: how the rate of D1 synthesis is coupled to need for nascent D1 following PSII photodamage, and how psbA translation is activated in response to light. Our results show that these two phenomena are intimately connected. Our data strongly suggest that light induces psbA translation in mature chloroplasts by triggering D1 damage, and not by affecting metabolites of photosynthesis as has often been assumed. Our findings also link high psbA ribosome occupancy with the absence of D1 from a complex that is required for PSII assembly, PSII repair, and psbA translation. These results, together with prior literature, suggest an autoregulatory mechanism in which the presence of D1 in the assembly/repair complex negatively regulates D1 synthesis via inhibitory interactions with psbA translational activators in the complex. According to this model, light-induced D1 degradation relieves this repression, thereby triggering the recruitment of ribosomes to psbA mRNA. This model and the evidence for it are elaborated below.
Distinct Light-Induced Signals Trigger the Plastome-Wide and psbA-Specific Translation Response.
When leaves harboring mature chloroplasts are shifted from dark to moderate light, chloroplast translation is rapidly activated in two ways: the recruitment of ribosomes specifically to psbA mRNA is superimposed on a plastome-wide increase in elongation rate that does not change ribosome occupancy on other ORFs (19). Results presented here demonstrate that light triggers these two responses via different signals. Our data show that the psbA-specific response does not require photosynthetic electron transport. For example, UV-A was more effective than photosynthetically active wavelengths at triggering D1 synthesis and psbA ribosome recruitment, and these effects were specific for D1/psbA. Furthermore, light-induced D1 synthesis was not disrupted in mutants with severe PSI or ATP synthase deficiencies. By contrast, the synthesis of all detected chloroplast-encoded proteins was reduced by inhibitors of PSII or photophosphorylation and in a mutant lacking the cyt b6f complex. Taken together, these data indicate that the plastome-wide response is triggered by a product of photosynthetic electron transport, whereas the psbA-specific response is not.
A study with lysed barley chloroplasts concluded that light-induced changes in pH globally activate translation elongation (21), and our in vivo data support that view. However, our findings run counter to the widespread view that light specifically activates D1 synthesis via its effects on photosynthetic electron transport (reviewed by refs. 7 and 15–17). The evidence for this view comes primarily from the use of chemical inhibitors. Although those treatments inhibited D1 synthesis, our results and prior studies (21, 56) indicate that such treatments globally repress chloroplast translation. Misdirection on this issue may have arisen from the fact that some studies only examined D1 synthesis, and others highlighted effects on D1 even though general inhibition was apparent (e.g., refs. 20 and 22). Limiting ATP has also been proposed to be a key control point for D1 repair synthesis (36). Although ATP is a critical resource for translation, our results show that photophosphorylation is not an activating signal for psbA ribosome recruitment and D1 repair synthesis in mature chloroplasts.
Light-Induced D1 Damage as the Trigger for psbA Translation in Mature Chloroplasts.
D1 damage occurs at all light intensities, but photoinhibition occurs only when the rate of damage exceeds the rate of PSII repair (8–11). Thus, light-induced D1 synthesis for PSII biogenesis versus PSII repair cannot be distinguished simply on the basis of light intensity, as is often assumed in discussions of this topic. Although we used moderate “growth” light intensities, we infer that the bulk of the light-induced D1 synthesis in our experiments represents repair synthesis because 1) we analyzed leaf tissue harboring mature chloroplasts that had completed the biogenesis phase, and 2) D1 is overproduced with respect to other PSII subunits at this stage, whereas D1 is produced stoichiometrically with other PSII subunits in immature chloroplasts (67).
The most parsimonious explanation of our data is that light induces D1 synthesis in mature chloroplasts by triggering PSII damage. This view harkens back to mechanisms that had been suggested in early studies (27, 28, 68), but that had lost visibility in recent years (7, 15, 16, 69). A key piece of evidence we present for this conclusion is that UV-A light more efficiently stimulated D1 synthesis and psbA ribosome recruitment than did red, green, or blue light (Fig. 1), correlating with the action spectrum of PSII damage and not with that of photosynthesis (11, 37–40). An alternative scenario could involve a UV-absorbing photoreceptor, but this is unlikely for several reasons. First, light induces D1 translation in isolated chloroplasts (reviewed in ref. 14) but receptors known to absorb in the UV are not found inside chloroplasts (reviewed in ref. 42). Second, the photoreceptors that are known to absorb in the UV-A can be excluded: cryptochromes and phototropins can be excluded based on the lack of response to blue light, and the UV-B receptor UVR8 can be excluded based on the fact that UV-B did not elicit the response and a uvr8 mutant exhibited normal light-induced D1 synthesis. Furthermore, our conclusion that PSII damage is the trigger for D1 repair synthesis is similar to that from studies in cyanobacteria, although the response in cyanobacteria is believed to be primarily at the transcriptional level (70, 71).
Coupling of psbA Translation to D1 Damage and Assembly via the HCF244/OHP1/OHP2 Complex: A Working Model.
Our finding that psbA ribosome occupancy does not decrease in the dark in hcf136 mutants revealed a molecular link between light-regulated psbA translation and PSII assembly/repair. HCF136 (Ycf48 in cyanobacteria) collaborates with the HCF244/OHP1/OHP2 complex (Ycf39, HliD, HliC in cyanobacteria) to mediate early steps in assembly of the PSII reaction center (32, 34, 35, 62, 63, 72, 73). HCF244 and the OHPs are core subunits that interact directly with one another, whereas HCF136/Ycf48 copurified with the complex in some cases but not others (32, 34, 35, 62, 63). The HCF244/Ycf39 complex is required for both PSII assembly and repair, and has been proposed to deliver chlorophyll to nascent D1, scavenge chlorophyll from degraded D1, or protect nascent D1 from photodamage (32, 34, 35, 63). In addition, the HCF244 complex in plants is required for psbA translation initiation (33, 46), evoking our recent proposal that D1 autoregulates psbA translation via allosteric interactions within the complex (33). Results presented here allow us to expand and elaborate on this model.
The Ycf39 complex in cyanobacteria is found in two complexes that represent consecutive stages in PSII assembly: one form, called the D1 module, includes Ycf39/HliD/HliC/Ycf48 together with the PSII proteins D1 and PsbI; the second, denoted RCII*, includes, in addition, the PSII subunits D2, PsbE, and PsbF (63, 74, 75). A complex similar to RCII* has been detected in plants (32, 34). There is evidence that HCF136/Ycf48 acts upstream of the HCF244/Ycf39 complex during PSII assembly and facilitates the incorporation of D1 into the complex (Fig. 6, Bottom) (30, 31, 63, 76).
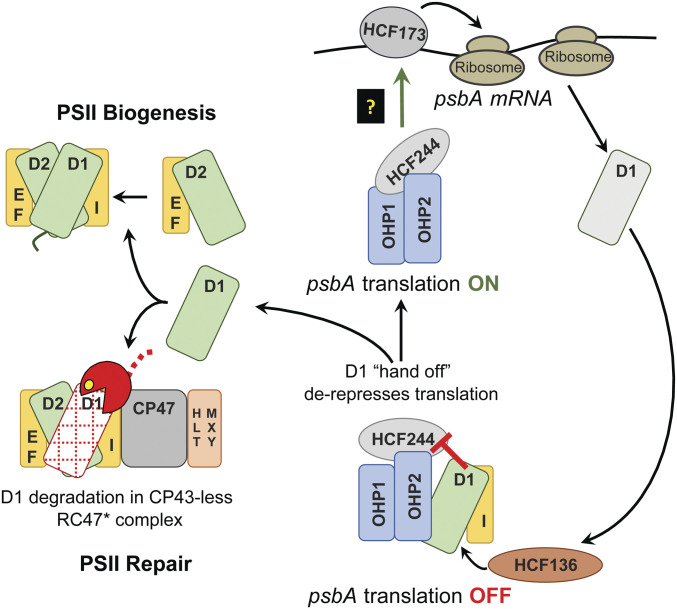
Fig. 6.
Model for the coupling of psbA translation to light-induced D1 damage. The HCF244/OHP1/OHP2 complex is diagrammed at Bottom with bound D1 and PsbI. This form of the complex is referred to as the D1 module in Synechocystis (63). OHP1 and OHP2 are integral thylakoid proteins, whereas HCF244 is bound to the stromal face of the membrane (32, 34, 35, 63). Current data suggest that HCF136 (on the luminal face of the membrane) is required to insert nascent D1 into the complex, possibly in conjunction with the insertion of chlorophyll (29–31, 64). We posit that the presence of D1 in this complex inhibits the ability of the stroma-exposed components (HCF244 and OHP2) to activate psbA translation. Repression is relieved when D1 is transferred out of the complex, either to a repair intermediate from which damaged D1 had been removed (Bottom) or to the “D2 module” during PSII biogenesis (Top). We show the canonical RC47* complex (6, 7) as the repair intermediate that accepts nascent D1, but the mechanism would be similar with the alternative repair intermediate detected recently in Synechocystis (77). We propose that the D1-less HCF244 complex communicates with HCF173 in the stroma, which is known to bind the psbA 5′ UTR and to activate translation initiation (48, 79, 81).
Consideration of our results in light of these findings leads us to propose the working model illustrated in Fig. 6. According to this scheme, the presence of D1 in the HCF244/OHP1/OHP2 complex interferes with its ability to activate psbA translation. We propose that this inhibitory interaction is relieved by light-induced proteolysis of D1, which produces an “empty” repair intermediate to which HCF244/OHP1/OHP2 can hand off nascent D1 (Fig. 6, Bottom Left). This acceptor for nascent D1 could be the CP43-less reaction center complex that is generally believed to be the site of D1 degradation during PSII repair (Fig. 6, Bottom Left) (6, 7), or it could be akin to the recently detected RC* repair intermediate detected in Synechocystis (77).
This model inserts a step into current PSII repair scenarios, which posit the direct incorporation of nascent D1 into a CP43-less intermediate from which damaged D1 had been removed (6, 7, 14). Instead, we posit that HCF244/OHP1/OHP2, in cooperation with HCF136, are the first to engage nascent D1, and that the HCF244 complex then hands D1 off to the D1-less repair intermediate. According to this view, the HCF244 complex and HCF136 perform the same biochemical functions during both PSII repair and de novo PSII biogenesis. Thus, this model can readily be extended to de novo PSII biogenesis by positing that the transfer of D1 to the next assembly intermediate (the D2 module) will also derepress psbA translation (Fig. 6, Top Left). This mechanism might underlie the assembly-linked regulation of D1 synthesis observed in Chlamydomonas (18), where it was proposed that translational efficiency is set to the rate of D1 assembly within a complex along the PSII biogenesis pathway. HCF244, OHP1, and OHP2 are conserved (but unstudied) in Chlamydomonas and are excellent candidates for the hub of this mechanism.
In this study, we present two key pieces of evidence for this model. First, we observed that psbA ribosome occupancy remains high in the dark in hcf136 mutants. Given that HCF136 acts upstream of the HCF244 complex during D1 assembly (29–31, 64, 65), the constitutively high psbA ribosome occupancy in hcf136 mutants correlates with a predicted D1-less HCF244 complex under all light conditions. Second, our data strongly suggest that light-induced psbA ribosome recruitment and D1 synthesis are triggered by D1 damage. This strengthens evidence for the early model positing that D1 synthesis is coupled to degradation of photodamaged D1 (27, 28, 68). Adir et al. (27) observed that the rate of light-induced D1 synthesis in Chlamydomonas correlates with the rate of D1 degradation and suggested that the damaged PSII reaction center couples light-dependent damage with D1 synthesis. Our data support this general scheme, and our model, which incorporates many advances made since that time, elaborates on the nature of the regulatory complexes and interactions.
An important aspect that is not addressed by our data concerns how the HCF244/OHP1/OHP2 complex stimulates the recruitment of ribosomes to psbA mRNA. Ribosome-free psbA mRNA is found primarily in the stroma, and psbA mRNA undergoing translation becomes tethered to the membrane only after D1’s first transmembrane segment emerges from the ribosome (78–80). Therefore, our model demands a means to communicate changes in the HCF244/OHP1/OHP2 complex at the thylakoid membrane to the pool of untranslated psbA mRNA in the stroma (black box in Fig. 6). A protein called HCF173 is a prime candidate for being involved in this membrane–stroma communication. HCF173 is required specifically for the recruitment of ribosomes to psbA mRNA (48, 81), it partitions between the stroma and the stromal face of the thylakoid membrane (48, 82), and it was pulled down with tagged OHP1 from solubilized thylakoid membranes (34). Furthermore, HCF173 associates with the 5′ untranslated region (UTR) of psbA mRNA (48, 79, 82), and it is the only protein known to bind the psbA mRNA and also to be important for psbA translation in plants. As such, our working model posits that the HCF244/OHP1/OHP2 complex impacts psbA translation by affecting the activity of HCF173, which in turn, regulates psbA ribosome recruitment by binding the 5′ UTR of the psbA mRNA. Clarifying the nature of the interactions among these components, and how those interactions change in response to light, D1 damage, and PSII assembly are important areas for future investigation.
Materials and Methods
Additional information is provided in SI Appendix, Materials and Methods.
Plant Material.
The maize Zm-psa3 (Zm-psa3-1), pet2 (pet2-1/-3), Zm-hcf107, and Zm-hcf136 mutants were described previously (33, 50, 58, 59). The maize PAB ortholog (Zm-PAB) is encoded by Zm00001d048524 (B73 RefGen_v4). Evidence for its orthology with Arabidopsis pab (AT4G34090) (49) can be found at cas-pogs.uoregon.edu/#/pog/14960. Two Zm-pab transposon insertion alleles were recovered from the Photosynthetic Mutant Library collection (83) (described in SI Appendix, Fig. S1A); the heteroallelic progeny of a complementation test cross were used for the experiments here.
The Arabidopsis psa3 (SAIL_503_B01) and hcf107-3 (SALK_079285C) mutants were described previously (50, 81). The Arabidopsis stn7-1-stn8-1 double mutant was a gift from Sacha Baginsky, Martin Luther University, Halle (Saale), Germany, and was described in ref. 55. Arabidopsis hcf136 was obtained from Jörg Meurer, Ludwig-Maximilians University, Munich, Germany, and from Susanne Paradies and Peter Westhoff, Heinrich Heine University, Düsseldorf, Germany, and is the same allele studied previously (29, 33). Arabidopsis hcf208 was obtained from Peter Westhoff and was described in ref. 47. Arabidopsis uvr8-6 was obtained from Roman Ulm and Emilie DeMarsy, University of Geneva, Geneva, Switzerland, and was described in ref. 84. See SI Appendix, Materials and Methods for additional information.
Monochromatic Light Treatments.
Arabidopsis Col-0 seedlings were grown for 18 to 21 d in diurnal cycles on MS (Murashige and Skoog) medium and were then acclimated to the basal white light (8 μmol m−2 s−1 fluorescent bulb Sylvania F40/D41/SS) for 30 min at midday. Supplemental lighting was then provided for 15 min, with the lids of the plates removed. Leaves were then harvested for ribo-seq or excised and used for in vivo pulse labeling in the same light condition. See SI Appendix, Materials and Methods for additional information.
DCMU, Nigericin, and CCCP Treatments.
Arabidopsis Col-0 seedlings were grown on MS plates as described for the action spectrum experiment. Wounds were created by excising cotyledons. Each seedling with attached roots was transferred to 0.5 mL MS medium (2% [wt/vol] sucrose, pH 5.7) containing the drug in a well of a clear 24-well plate. DCMU (10 mM stock in 50% ethanol), nigericin (5 mM stock in 95% ethanol), and CCCP (100 mM stock in DMSO [dimethylsulfoxide]) were diluted in MS medium to final concentrations of 20 µM, 25 µM, and 100 µM, respectively. Mock treatments with the solvent were used as controls.
The DCMU treatments used 14-d-old Col-0 seedlings grown under long day conditions (see above). The seedlings were incubated in the DCMU solution in darkness for 2 h. One set of seedlings was then shifted to light (8 μmol m−2 s−1) and another was maintained in darkness. After 15 min in light, the tissues were harvested for ribo-seq or the leaves were excised and used for in vivo pulse labeling in buffer containing DCMU. The nigericin and CCCP experiments used 23-d-old Col-0 seedlings grown in short days. The seedlings were incubated in nigericin or CCCP solutions for 15 min in the light (8 μmol m−2 s−1). The leaves were then excised for in vivo pulse labeling in buffer containing nigericin or CCCP.
In Vivo Pulse Labeling.
In vivo labeling of maize and Arabidopsis seedlings was performed as described previously (19), with minor modifications. Unless otherwise indicated in figures, seedlings were acclimated to the assay light for 30 min prior to the experiment. Briefly, maize seedlings were radiolabeled for 15 min by applying [35S]methionine/cysteine to a leaf abrasion, whereas excised Arabidopsis leaves were submerged in labeling mix for 20 min. The 1-h dark labeling was performed from 40 to 60 min or 45 to 60 min after the shift to dark in Arabidopsi and maize, respectively. The reillumination labeling was performed starting 15 min after the shift back to light. Leaf extracts were separated by SDS (sodium dodecyl sulfate) polyacrylamide gel electrophoresis, transferred to nitrocellulose, and imaged with a Storm PhosphorImager (GE Healthcare). A total of 6 M urea was included in gels used to resolve membrane fractions. Gel loading was normalized based on radiolabeled cytosolic proteins in maize and based on either chlorophyll or total leaf protein mass in Arabidopsis (as stated in figure legends). Additional details are provided in SI Appendix, Materials and Methods.
Ribo-Seq and RNA-Seq.
Maize ribosome footprints were prepared from the apical portion of the second and third leaves to emerge, excising above the ligule of leaf 1. One seedling was used per replicate, except for the first replicate of Zm-hcf107, which used two pooled seedlings. Arabidopsis ribosome footprints were prepared from the aerial portions of pools of three to five seedlings. Tissue was flash frozen in liquid N2 and stored at −80 °C until used. Ribosome footprint preparation, total RNA extraction, sequencing library construction, and data analysis were performed as described previously (19), with minor modifications described in SI Appendix, Materials and Methods.
Immunoblot Analysis and Antibodies.
Immunoblots were performed as described previously (85). Antibodies to AtpB, D1, PsaD, and PetD were generated by our group and were described previously (86). The antibody to D2 was obtained from Agrisera.
Supplementary Material
Acknowledgments
We are grateful to Susan Belcher and Roz Williams-Carrier for expert technical assistance; Helmut Kirchhoff, Ryouichi Tanaka, Josef Komenda, and Roman Sobotka for helpful discussions; Amy Turner for loaning spectrometers; and Nick Stiffler for assistance with data deposition. We also appreciate the generous gifts of mutant Arabidopsis lines from Sacha Baginsky, Jörg Meurer, Susanne Paradies, Peter Westhoff, Emilie Demarsy, and Roman Ulm. This research was funded by NSF Grants MCB-1616016 and IOS-1339130.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2007833117/-/DCSupplemental.
Data Availability.
All data obtained for this study are presented within the main text and Dataset S1. Illumina sequencing data have been deposited in the NCBI Sequence Read Archive under accession PRJNA612079 (87).
References
- 1.Johnson M. P., Photosynthesis. Essays Biochem. 60, 255–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson N., Ben-Shem A., The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 5, 971–982 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Shen J. R., The structure of photosystem II and the mechanism of water oxidation in photosynthesis. Annu. Rev. Plant Biol. 66, 23–48 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Barber J., Photosystem II: The water-splitting enzyme of photosynthesis. Cold Spring Harb. Symp. Quant. Biol. 77, 295–307 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Nishimura K., Kato Y., Sakamoto W., Chloroplast proteases: Updates on proteolysis within and across suborganellar compartments. Plant Physiol. 171, 2280–2293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theis J., Schroda M., Revisiting the photosystem II repair cycle. Plant Signal. Behav. 11, e1218587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Järvi S., Suorsa M., Aro E. M., Photosystem II repair in plant chloroplasts–Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta 1847, 900–909 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Tyystjärvi E., Aro E. M., The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc. Natl. Acad. Sci. U.S.A. 93, 2213–2218 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundby C., McCaffery S., Anderson J. M., Turnover of the photosystem II D1 protein in higher plants under photoinhibitory and nonphotoinhibitory irradiance. J. Biol. Chem. 268, 25476–25482 (1993). [PubMed] [Google Scholar]
- 10.Park Y., Anderson J., Chow W., Photoinactivation of functional photosystem II and D1-protein synthesis in vivo are independent of the modulation of the photosynthetic apparatus by growth irradience. Planta 198, 300–309 (1996). [Google Scholar]
- 11.Nishiyama Y., Murata N., Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 98, 8777–8796 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Kirchhoff H., Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y., Quality control of photosystem II: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front Plant Sci 7, 1136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y., Zerges W., Translational regulation in chloroplasts for development and homeostasis. Biochim. Biophys. Acta 1847, 809–820 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Nickelsen J., Bohne A., Westhoff P., “Chloroplast gene expression - Translation plastid biology, advances” in Plant Biology, Theg S., Wollman F., Eds. (Springer, NY, 2014), pp. 49–78. [Google Scholar]
- 16.Zoschke R., Bock R., Chloroplast translation: Structural and functional organization, operational control, and regulation. Plant Cell 30, 745–770 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L., Aro E. M., Millar A. H., Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 23, 667–676 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Minai L., Wostrikoff K., Wollman F. A., Choquet Y., Chloroplast biogenesis of photosystem II cores involves a series of assembly-controlled steps that regulate translation. Plant Cell 18, 159–175 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chotewutmontri P., Barkan A., Multilevel effects of light on ribosome dynamics in chloroplasts program genome-wide and psbA-specific changes in translation. PLoS Genet. 14, e1007555 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trebitsh T., Danon A., Translation of chloroplast psbA mRNA is regulated by signals initiated by both photosystems II and I. Proc. Natl. Acad. Sci. U.S.A. 98, 12289–12294 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mühlbauer S. K., Eichacker L. A., Light-dependent formation of the photosynthetic proton gradient regulates translation elongation in chloroplasts. J. Biol. Chem. 273, 20935–20940 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Zhang L., Paakkarinen V., van Wijk K. J., Aro E. M., Biogenesis of the chloroplast-encoded D1 protein: Regulation of translation elongation, insertion, and assembly into photosystem II. Plant Cell 12, 1769–1782 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M., Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc. Natl. Acad. Sci. U.S.A. 81, 1380–1384 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda H., Inagaki N., Satoh K., The level of stromal ATP regulates translation of the D1 protein in isolated chloroplasts. Plant Cell Physiol. 33, 33–39 (1992). [Google Scholar]
- 25.Kuroda H., Kobashi K., Kaseyama H., Satoh K., Possible involvement of a low redox potential component(s) downstream of photosystem I in the translational regulation of the D1 subunit of the photosystem II reaction center in isolated pea chloroplasts. Plant Cell Physiol. 37, 754–761 (1996). [Google Scholar]
- 26.Allakhverdiev S. I. et al., Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol. 137, 263–273 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adir N., Shochat S., Ohad I., Light-dependent D1 protein synthesis and translocation is regulated by reaction center II. Reaction center II serves as an acceptor for the D1 precursor. J. Biol. Chem. 265, 12563–12568 (1990). [PubMed] [Google Scholar]
- 28.Zer H., Prasil O., Ohad I., Role of plastoquinol oxidoreduction in regulation of photochemical reaction center IID1 protein turnover in vivo. J. Biol. Chem. 269, 17670–17676 (1994). [PubMed] [Google Scholar]
- 29.Meurer J., Plücken H., Kowallik K. V., Westhoff P., A nuclear-encoded protein of prokaryotic origin is essential for the stability of photosystem II in Arabidopsis thaliana. EMBO J. 17, 5286–5297 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komenda J. et al., The cyanobacterial homologue of HCF136/YCF48 is a component of an early photosystem II assembly complex and is important for both the efficient assembly and repair of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 283, 22390–22399 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Plücken H., Müller B., Grohmann D., Westhoff P., Eichacker L. A., The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett. 532, 85–90 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Li Y. et al., OHP1, OHP2, and HCF244 form a transient functional complex with the photosystem II reaction center. Plant Physiol. 179, 195–208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chotewutmontri P., Williams-Carrier R., Barkan A., Exploring the link between photosystem II assembly and translation of the chloroplast psbA mRNA. Plants (Basel) 9, E152 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myouga F. et al., Stable Accumulation of photosystem II requires ONE-HELIX PROTEIN1 (OHP1) of the light harvesting-like family. Plant Physiol. 176, 2277–2291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hey D., Grimm B., ONE-HELIX PROTEIN2 (OHP2) is required for the stability of OHP1 and assembly factor HCF244 and is functionally linked to PSII biogenesis. Plant Physiol. 177, 1453–1472 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata N., Nishiyama Y., ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 41, 285–299 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S. et al., The solar action spectrum of photosystem II damage. Plant Physiol. 153, 988–993 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarvikas P., Hakala M., Pätsikkä E., Tyystjärvi T., Tyystjärvi E., Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 47, 391–400 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Zavafer A., Chow W. S., Cheah M. H., The action spectrum of Photosystem II photoinactivation in visible light. J. Photochem. Photobiol. B 152, 247–260 (2015). [DOI] [PubMed] [Google Scholar]
- 40.McCree K., The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 9, 191–216 (1972). [Google Scholar]
- 41.Tyystjärvi E., Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 300, 243–303 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Casal J. J., Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Briggs W. R., Christie J. M., Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 7, 204–210 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Ahmad M. et al., Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol. 129, 774–785 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heijde M., Ulm R., UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 17, 230–237 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Link S., Engelmann K., Meierhoff K., Westhoff P., The atypical short-chain dehydrogenases HCF173 and HCF244 are jointly involved in translational initiation of the psbA mRNA of Arabidopsis. Plant Physiol. 160, 2202–2218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyska D., Paradies S., Meierhoff K., Westhoff P., HCF208, a homolog of Chlamydomonas CCB2, is required for accumulation of native cytochrome b6 in Arabidopsis thaliana. Plant Cell Physiol. 48, 1737–1746 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Schult K. et al., The nuclear-encoded factor HCF173 is involved in the initiation of translation of the psbA mRNA in Arabidopsis thaliana. Plant Cell 19, 1329–1346 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao J. et al., PAB is an assembly chaperone that functions downstream of chaperonin 60 in the assembly of chloroplast ATP synthase coupling factor 1. Proc. Natl. Acad. Sci. U.S.A. 112, 4152–4157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen J., Williams-Carrier R., Barkan A., PSA3, a protein on the stromal face of the thylakoid membrane, promotes photosystem I accumulation in cooperation with the assembly factor PYG7. Plant Physiol. 174, 1850–1862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J. et al., PsbP-domain protein1, a nuclear-encoded thylakoid lumenal protein, is essential for photosystem I assembly in Arabidopsis. Plant Cell 24, 4992–5006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fristedt R., Williams-Carrier R., Merchant S. S., Barkan A., A thylakoid membrane protein harboring a DnaJ-type zinc finger domain is required for photosystem I accumulation in plants. J. Biol. Chem. 289, 30657–30667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rochaix J. D., Redox regulation of thylakoid protein kinases and photosynthetic gene expression. Antioxid. Redox Signal. 18, 2184–2201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellafiore S., Barneche F., Peltier G., Rochaix J. D., State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Schönberg A. et al., Identification of STN7/STN8 kinase targets reveals connections between electron transport, metabolism and gene expression. Plant J. 90, 1176–1186 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Michaels A., Herrin D. L., Translational regulation of chloroplast gene expression during the light-dark cell cycle of Chlamydomonas: Evidence for control by ATP/energy supply. Biochem. Biophys. Res. Commun. 170, 1082–1088 (1990). [DOI] [PubMed] [Google Scholar]
- 57.Schmitz O., Tsinoremas N., Schaefer M., Anandan S., Golden S., General effect of photosynthetic electron transport inhibitors on translation precludes their use for investigating regulation of D1 biosynthesis in Synechococcus sp. strain PCC 7942. Photosynth. Res. 62, 261–271 (1999). [Google Scholar]
- 58.Williams-Carrier R. et al., Use of Illumina sequencing to identify transposon insertions underlying mutant phenotypes in high-copy Mutator lines of maize. Plant J. 63, 167–177 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Hammani K., Cook W. B., Barkan A., RNA binding and RNA remodeling activities of the half-a-tetratricopeptide (HAT) protein HCF107 underlie its effects on gene expression. Proc. Natl. Acad. Sci. U.S.A. 109, 5651–5656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Felder S. et al., The nucleus-encoded HCF107 gene of Arabidopsis provides a link between intercistronic RNA processing and the accumulation of translation-competent psbH transcripts in chloroplasts. Plant Cell 13, 2127–2141 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levey T., Westhoff P., Meierhoff K., Expression of a nuclear-encoded psbH gene complements the plastidic RNA processing defect in the PSII mutant hcf107 in Arabidopsis thaliana. Plant J. 80, 292–304 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Hey D., Grimm B., ONE-HELIX PROTEIN1 and 2 forms heterodimers to bind chlorophyll in Photosystem II biogenesis. Plant Physiol. 183, 179–193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knoppová J. et al., Discovery of a chlorophyll binding protein complex involved in the early steps of photosystem II assembly in Synechocystis. Plant Cell 26, 1200–1212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plöchinger M., Schwenkert S., von Sydow L., Schröder W. P., Meurer J., Functional update of the auxiliary proteins PsbW, PsbY, HCF136, PsbN, TerC and ALB3 in maintenance and assembly of PSII. Front Plant Sci 7, 423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu J. et al., Ycf48 involved in the biogenesis of the oxygen-evolving photosystem II complex is a seven-bladed beta-propeller protein. Proc. Natl. Acad. Sci. U.S.A. 115, E7824–E7833 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasala B. A., Muto M., Sullivan J., Mayfield S. P., Improved heterologous protein expression in the chloroplast of Chlamydomonas reinhardtii through promoter and 5′ untranslated region optimization. Plant Biotechnol. J. 9, 674–683 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Chotewutmontri P., Barkan A., Dynamics of chloroplast translation during chloroplast differentiation in maize. PLoS Genet. 12, e1006106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kettunen R., Pursiheimo S., Rintamäki E., Van Wijk K. J., Aro E. M., Transcriptional and translational adjustments of psbA gene expression in mature chloroplasts during photoinhibition and subsequent repair of photosystem II. Eur. J. Biochem. 247, 441–448 (1997). [DOI] [PubMed] [Google Scholar]
- 69.Barnes D., Mayfield S. P., Redox control of posttranscriptional processes in the chloroplast. Antioxid. Redox Signal. 5, 89–94 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Tyystjärvi T., Tuominen I., Herranen M., Aro E. M., Tyystjärvi E., Action spectrum of psbA gene transcription is similar to that of photoinhibition in Synechocystis sp. PCC 6803. FEBS Lett. 516, 167–171 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Tyystjärvi T., Mulo P., Mäenpää P., Aro E. M., D1 polypeptide degradation may regulate psbA gene expression at transcriptional and translational levels in Synechocystis sp. PCC 6803. Photosynth. Res. 47, 111–120 (1996). [DOI] [PubMed] [Google Scholar]
- 72.Hey D., Grimm B., Requirement of ONE-HELIX PROTEIN 1 (OHP1) in early Arabidopsis seedling development and under high light intensity. Plant Signal. Behav. 13, e1550317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chidgey J. W. et al., A cyanobacterial chlorophyll synthase-HliD complex associates with the Ycf39 protein and the YidC/Alb3 insertase. Plant Cell 26, 1267–1279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Komenda J., Sobotka R., Cyanobacterial high-light-inducible proteins–Protectors of chlorophyll-protein synthesis and assembly. Biochim. Biophys. Acta 1857, 288–295 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Kiss É. et al., A photosynthesis-specific rubredoxin-like protein is required for efficient association of the D1 and D2 proteins during the initial steps of photosystem II assembly. Plant Cell 31, 2241–2258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knoppová J., Komenda J., Sequential deletions of photosystem II assembly factors Ycf48, Ycf39 and Pam68 result in progressive loss of autotrophy in the cyanobacterium Synechocystis PCC 6803. Folia Microbiol. (Praha) 64, 683–689 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Weisz D. A. et al., A novel chlorophyll protein complex in the repair cycle of photosystem II. Proc. Natl. Acad. Sci. U.S.A. 116, 21907–21913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zoschke R., Barkan A., Genome-wide analysis of thylakoid-bound ribosomes in maize reveals principles of cotranslational targeting to the thylakoid membrane. Proc. Natl. Acad. Sci. U.S.A. 112, E1678–E1687 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McDermott J. J., Watkins K. P., Williams-Carrier R., Barkan A., Ribonucleoprotein capture by in vivo expression of a designer pentatricopeptide repeat protein in Arabidopsis. Plant Cell 31, 1723–1733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Legen J., Schmitz-Linneweber C., Stable membrane-association of mRNAs in etiolated, greening and mature plastids. Int. J. Mol. Sci. 18, 1881 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams-Carrier R. et al., The Arabidopsis pentatricopeptide repeat protein LPE1 and its maize ortholog are required for translation of the chloroplast psbJ RNA. Plant J. 99, 56–66 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Watkins K. P. et al., Exploring the proteome associated with the mRNA encoding the D1 reaction center protein of Photosystem II in plant chloroplasts. Plant J. 102, 369–382 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Belcher S., Williams-Carrier R., Stiffler N., Barkan A., Large-scale genetic analysis of chloroplast biogenesis in maize. Biochim. Biophys. Acta 1847, 1004–1016 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Favory J. J. et al., Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 28, 591–601 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barkan A., Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 297, 38–57 (1998). [Google Scholar]
- 86.Pfalz J., Bayraktar O. A., Prikryl J., Barkan A., Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J. 28, 2042–2052 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chotewutmontri P., Barkan A., Action spectrum analysis of translation in Arabidopsis and translation in photosynthetic mutants of maize and Arabidopsis. Sequence Read Archive. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA612079. Deposited 11 March 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data obtained for this study are presented within the main text and Dataset S1. Illumina sequencing data have been deposited in the NCBI Sequence Read Archive under accession PRJNA612079 (87).