Significance
G protein-coupled receptors (GPCRs) are targeted by a large fraction of approved drugs and regulate many important cellular processes. Association of GPCRs with heterotrimeric G proteins in response to agonist activation is thought to invariably lead to G protein activation. We find instead that G12 heterotrimers can associate with agonist-bound receptors in a manner that does not lead to activation. These unproductive agonist–receptor-G protein ternary complexes sequester G12 heterotrimers and thus inhibit rather than support G12 signaling. These findings reveal a mechanism whereby agonist activation of GPCRs can inhibit as well as promote G protein signaling.
Keywords: GPCR, ternary complex, G protein-coupled receptor, arrestin
Abstract
G proteins are activated when they associate with G protein-coupled receptors (GPCRs), often in response to agonist-mediated receptor activation. It is generally thought that agonist-induced receptor-G protein association necessarily promotes G protein activation and, conversely, that activated GPCRs do not interact with G proteins that they do not activate. Here we show that GPCRs can form agonist-dependent complexes with G proteins that they do not activate. Using cell-based bioluminescence resonance energy transfer (BRET) and luminescence assays we find that vasopressin V2 receptors (V2R) associate with both Gs and G12 heterotrimers when stimulated with the agonist arginine vasopressin (AVP). However, unlike V2R-Gs complexes, V2R-G12 complexes are not destabilized by guanine nucleotides and do not promote G12 activation. Activating V2R does not lead to signaling responses downstream of G12 activation, but instead inhibits basal G12-mediated signaling, presumably by sequestering G12 heterotrimers. Overexpressing G12 inhibits G protein receptor kinase (GRK) and arrestin recruitment to V2R and receptor internalization. Formyl peptide (FPR1 and FPR2) and Smoothened (Smo) receptors also form complexes with G12 that are insensitive to nucleotides, suggesting that unproductive GPCR-G12 complexes are not unique to V2R. These results indicate that agonist-dependent receptor-G protein association does not always lead to G protein activation and may in fact inhibit G protein activation.
G protein-coupled receptors (GPCRs) mediate important physiological activities and exert most of their effects through activation of G proteins. In the conventional model of coupling, unliganded receptors are poor recruiters and activators of G proteins, whereas agonist-bound GPCRs take on more active conformations that effectively recruit G protein heterotrimers (1, 2). Productive receptor-G protein association promotes GDP release by stabilizing the nucleotide-free state of the Gα subunit, which in turn allows GTP binding, G protein activation, and downstream signaling (3, 4). According to this model, agonist-dependent GPCR-G protein complex formation is essentially synonymous with G protein activation. Four families of G proteins (Gs, Gi/o, Gq/11, and G12/13) can be activated, and each leads to a distinct set of downstream signaling outcomes. It is generally thought that selection of G protein subtypes by GPCRs occurs at the receptor-G protein association step, such that receptors interact with and activate cognate G protein subtypes and do not interact with noncognate G protein subtypes. Here we find that agonist-dependent GPCR-G protein association can occur without promoting subsequent G protein activation, thus, whether a G protein subtype is activated can be determined after initial receptor-G protein engagement. Moreover, noncognate G proteins can impede downstream events, perhaps by competing with other intracellular transducers for access to activated receptors. These findings revise the standard model of G protein coupling by indicating that agonist-induced GPCR-G protein association does not always promote G protein activation and may in some circumstances inhibit downstream signaling.
Results
V2R Interacts with G12 Heterotrimers.
Conventional GPCR-G protein coupling is understood as an allosteric interaction where an agonist-bound active receptor mediates GDP release by stabilizing the nucleotide-free state of an associated Gα subunit (3–5). Receptor complexes with nucleotide-free G proteins are quite transient when guanine nucleotides are present at concentrations similar to those found in cells, but are stabilized when guanine nucleotides are absent. In order to observe allosteric coupling we monitored receptor-G protein association under conditions that allowed us to control both ligand binding to the receptor and nucleotide binding to the G protein. We used bioluminescence resonance energy transfer (BRET) between GPCRs fused to Renilla luciferase (Rluc8) and Gβ1 and Gγ2 subunits fused to complementary fragments of Venus fluorescent protein (6–8) to monitor receptor-G protein association. These components and unlabeled Gα subunits were transfected into HEK 293 cells in which most of the endogenous G proteins had been deleted using CRISPR/Cas9-mediated gene editing (9, 10). In order to control nucleotide binding to G proteins, cells were permeabilized and either supplemented with nucleotides or treated with apyrase to remove residual nucleotides.
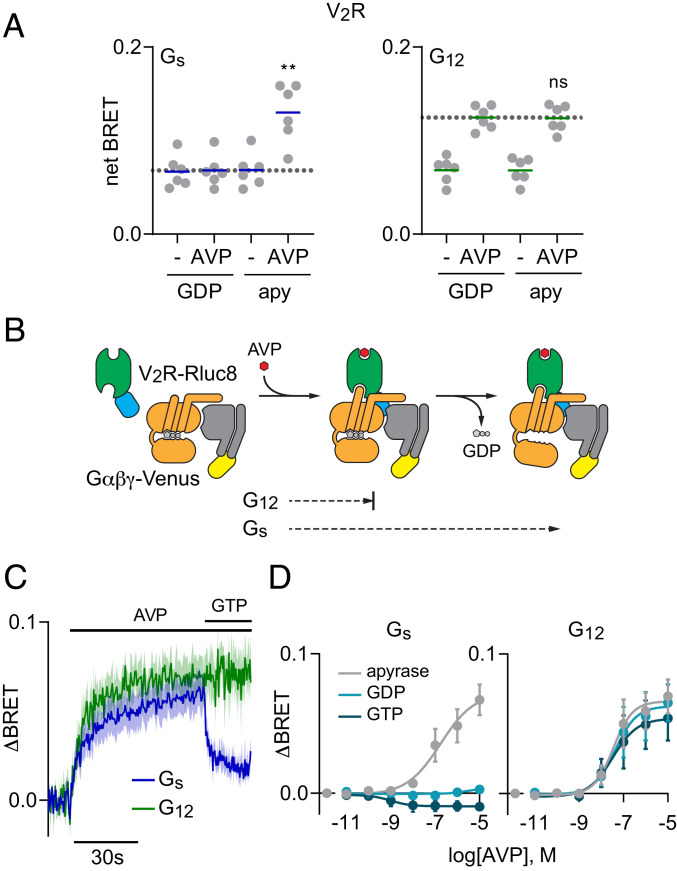
Because receptor-G protein complexes are transient it can be difficult to detect agonist-induced BRET signals between receptors and G proteins when guanine nucleotides are present (6). For example, arginine vasopressin (AVP) did not detectably increase BRET between vasopressin V2 receptors (V2R) and Gs heterotrimers in the presence of GDP (Fig. 1A). In contrast, AVP produced large BRET increases in the absence of nucleotides (Fig. 1A). Stabilization of agonist–receptor-G protein complexes when Gα subunits are nucleotide-free indicates conventional “productive” allosteric coupling and predicts that agonist-bound receptors will promote GDP release and G protein activation under physiological conditions. Nucleotide-free conditions also enhanced AVP-induced BRET between V2R and Gi1 or Gq heterotrimers (SI Appendix, Fig. S1). These results are consistent with cognate V2R activation of Gs and Gq heterotrimers (11, 12) and predict some ability to activate Gi1 heterotrimers. In contrast, we observed surprisingly robust agonist-induced BRET between V2R and G12 heterotrimers in the presence of GDP that was not enhanced by nucleotide depletion (Fig. 1A). These results suggest that AVP-bound V2R can form complexes with GDP-bound G12 heterotrimers that do not progress to the nucleotide-free state and therefore are not stabilized when GDP is removed (Fig. 1B). As an index of allosteric coupling we divide the increase in BRET produced by agonist in the presence of GDP (ΔBRETag) by the increase in BRET produced by the combined effect of agonist and nucleotide depletion (ΔBRETag+apy), and refer to this index as the GDP-resistance ratio, or RGDP. RGDP values that are less than 1 indicate conventional productive coupling, whereas an RGDP value of 1 indicates nucleotide-resistant or unproductive coupling. RGDP for V2R and Gs was 0.08 ± 0.12 (mean ± SD; n = 6), whereas RGDP for V2R and G12 was 1.01 ± 0.06 (n = 6). In contrast to V2R, we observed more conventional productive coupling of both endothelin A (ETA) and thromboxane A2 (TP) receptors with G12 heterotrimers, with RGDP values of 0.66 ± 0.09 (n = 3) and 0.55 ± 0.09 (n = 6), respectively. Both of these receptors also coupled productively with Gq heterotrimers, with RGDP values of 0.29 ± 0.08 (n = 3) and 0.11 ± 0.07 (n = 6), respectively (SI Appendix, Fig. S2).
Fig. 1.
V2R forms GDP-resistant agonist-induced complexes with G12 heterotrimers. (A) BRET between V2R-Rluc8 and Gαβγ-Venus in the presence or absence of AVP (1 μM), and the presence or absence of GDP. When GDP was absent, apyrase (apy) was added to remove residual nucleotides. AVP-induced BRET to Gs (Left) but not G12 (Right) heterotrimers was enhanced when GDP was absent; **P < 0.005; n.s., not significant (P = 0.58); one-way ANOVA (Sidak’s test) compared to GDP+AVP; n = 6. (B) Cartoon representation of two steps of V2R-G protein coupling: agonist-induced formation of receptor-G protein complexes, and GDP release. (C) Time course of BRET between V2R-Rluc8 and Gαβγ-Venus in response to injection of 1 μM AVP, followed by injection of 100 μM GTP in permeabilized cells treated with apyrase (mean ± SEM; n = 4–6). (D) BRET between V2R-Rluc8 and Gαβγ-Venus as a function of AVP concentration in permeabilized cells expressing either Gs (Left) or G12 (Right) heterotrimers treated with apyrase, GDP (100 μM), or GTP (100 μM). The logEC50 for association with Gs was −6.8 ± 0.5 in apyrase-treated cells, and the logEC50s for association with G12 were −7.5 ± 0.3, −7.4 ± 0.4, and −7.5 ± 0.5 in the presence of apyrase, GDP, and GTP, respectively. Data points represent the change in BRET (ΔBRET) in response to AVP (mean ± SEM; n = 3).
Receptors that couple to one member of a Gα subunit family can usually couple to other members of the same family. Therefore, we examined V2R coupling to G13 heterotrimers, the other member of the G12/13 family (13). We found that stimulation with AVP increased BRET between V2R and G13 heterotrimers in the presence of GDP (SI Appendix, Fig. S3). However, unlike what we observed with G12, these responses were enhanced by nucleotide depletion (RGDP = 0.67 ± 0.08; n = 4), indicating productive coupling, consistent with weak V2R-mediated activation of G13 (14). Similar results were obtained with G13 heterotrimers and ETA (RGDP = 0.21 ± 0.03; n = 4) and TP (RGDP = 0.18 ± 0.01; n = 4) receptors (SI Appendix, Fig. S3).
Because nucleotide-resistant V2R-G12 association was unexpected we performed additional experiments to rule out the possibility that our standard BRET assay was simply detecting an agonist-induced change in V2R-Rluc8 conformation. We reasoned that if both ETA and V2R receptors were able to associate with G12 the two receptors should compete for a common pool of heterotrimers. Indeed, we found that stimulation of unlabeled ETA receptors inhibited AVP-induced BRET between V2R-Rluc8 and G12 heterotrimers in intact cells (SI Appendix, Fig. S4A). Conversely, stimulation of unlabeled V2R receptors inhibited endothelin-1–induced BRET between ETA-Rluc8 and G12 heterotrimers (SI Appendix, Fig. S4B). Second, we found that stimulation of unlabeled V2R receptors increased BRET between Gα12-Rluc8 and Gβγ-Venus in intact cells (SI Appendix, Fig. S5 A and B). This increase persisted in permeabilized cells that were treated with apyrase and supplemented with GDPβS to prevent the possibility of heterotrimer activation by residual GTP (SI Appendix, Fig. S5B). This suggests that active V2R receptors may impose a conformational change in G12 heterotrimers that does not require GTP binding or G12 activation. In contrast, stimulation of unlabeled ETA and TP receptors decreased BRET between Gα12-Rluc8 and Gβγ-Venus, and these decreases were largely blocked in permeabilized cells when only GDPβS was present (SI Appendix, Fig. S5C). Finally, we found that AVP increased luciferase complementation when a small fragment (SmBit) of Nanoluc was fused to V2R, and a large fragment of Nanoluc was fused to Gγ2, and these proteins were coexpressed with unlabeled Gα12 and Gβ1 (SI Appendix, Fig. S6). These results are consistent with AVP-induced association of V2R receptors and G12 heterotrimers.
Additional experiments revealed that V2R recruited Gs and G12 heterotrimers at similar rates (Fig. 1C) and that agonist-induced V2R-G12 complexes were equally stable in the presence of GDP or GTP (Fig. 1D). Stimulation of V2R with the agonist oxytocin produced similar responses to AVP, indicating that nucleotide-insensitive V2R-G12 interactions are not restricted to AVP (SI Appendix, Fig. S7A), and AVP-induced responses were inhibited by the antagonist mozavaptan (SI Appendix, Fig. S7B).
V2R Does Not Activate G12 Heterotrimers.
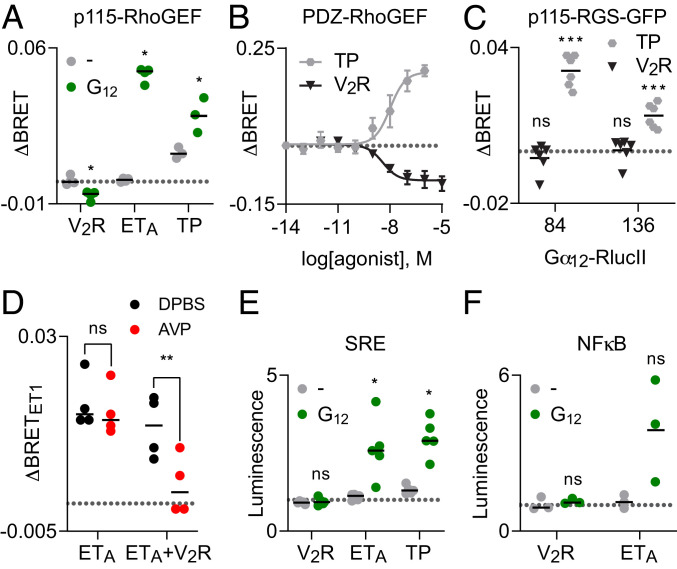
The above results suggested that AVP-stimulated V2R should not activate G12 heterotrimers. To test this prediction we turned to sensitive assays that monitor signaling downstream of G12 activation. We first monitored translocation of full-length p115-RhoGEF and a fragment (amino acids 281–483) of PDZ-RhoGEF from the cytosol to the plasma membrane using bystander BRET assays (15, 16). These proteins bind to activated Gα12 subunits at the plasma membrane to regulate Rho GTPase activity and actin fiber formation (17, 18). ETA and TP receptors robustly recruited p115-RhoGEF to the plasma membrane in a G12-dependent manner (Fig. 2A). In contrast, stimulation of V2R receptors failed to recruit p115-RhoGEF and instead decreased the baseline abundance of this reporter at the plasma membrane (Fig. 2A). Similar results were obtained with V2R and TP receptors and PDZ-RhoGEF recruitment to the plasma membrane (Fig. 2B) and direct recruitment of the RGS homology domain (amino acids 1–246) of p115-RhoGEF, p115-RGS-GFP, to Gα12-RlucII (Fig. 2C). The AVP-induced decrease in p115-and PDZ-RhoGEF at the plasma membrane suggests that active V2R may sequester G12 heterotrimers, preventing activation by endogenous receptors. Consistent with this suggestion, we found that activation of V2R could significantly reduce p115-RhoGEF recruitment mediated by activation of ETA receptors (Fig. 2D). A second sensitive assay of G12 activity is gene transcription driven by activation of the serum response element (SRE) (19). Stimulation of V2R receptors failed to activate SRE-dependent gene transcription, whereas stimulation of both ETA and TP receptors could activate SRE in a G12-dependent manner (Fig. 2E). A similar trend was observed with transcription driven by nuclear factor kappa-light-chain enhancer of activated B cells (NFκB; Fig. 2F). These results demonstrate that active V2R receptors do not detectably activate G12 heterotrimers, even though the two proteins interact in an agonist-dependent manner.
Fig. 2.
V2R does not activate G12 heterotrimers. (A) Activation of V2R decreases bystander BRET between p115RhoGEF-Rluc8 and the plasma membrane marker Venus-Kras when G12 is expressed, whereas activation of ETA and TP receptors increases this signal, indicating association of p115RhoGEF-Rluc8 and active Gα12 at the plasma membrane; *P < 0.05; paired t test compared to mock-transfected control (−); n = 3–4. (B) V2R activation decreases BRET between PDZ-RhoGEF-RlucII and the plasma membrane marker rGFP-CAAX, whereas TP activation increases this signal (mean ± SEM; n = 3). (C) TP activation increases BRET between two different Gα12-RlucII constructs and p115-RGS-GFP, whereas V2R activation has no effect on this signal; ***P < 0.0005; n.s., not significant; one-sample t test compared to zero; n = 6. RlucII was fused to Gα12 after amino acids 84 and 136 in the two different probes. Data points in A–C represent the change in BRET (ΔBRET) in response to agonist, and the broken gray line represents zero. (D) Activation of V2R reduces p115-RhoGEF recruitment mediated by activation of ETA. Activation of ETA (ET1; 100 nM) increases BRET between p115RhoGEF-Rluc8 and Venus-Kras, and this response is significantly inhibited when V2R receptors are coexpressed and activated (AVP; 1 μM); n.s., not significant (P = 0.43); **P < 0.005; one-way ANOVA (Sidak’s test); n = 4. Data points represent the change in bystander BRET (ΔBRET) in response to ET1, and the broken gray line represents zero. (E) Activation of V2R fails to activate the SRE when G12 is expressed, whereas activation of ETA and TP receptors increases SRE-driven gene expression; *P < 0.05; n.s., not significant; paired t test compared to (−); n = 5. (F) V2R receptors fail to activate NFκB-driven gene expression when G12 is expressed; n.s., not significant; paired t test compared to (−); n = 3. Data points in E and F represent luminescence normalized to vehicle-treated controls, and the broken gray line represents one (no change). Agonists were AVP (1 μM), ET1 (100 nM), and U-46619 (10 μM).
V2R Activates G12 Chimeras and Mutants.
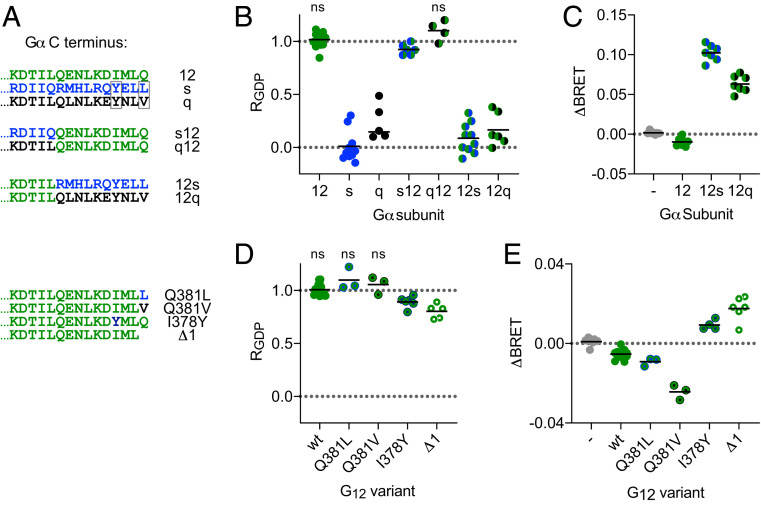
Canonical GPCR-mediated activation of G proteins involves extension of the Gα subunit C terminus (helix 5; H5) into the active receptor core (20, 21). This region of Gα is necessary for productive coupling and is also a key determinant of receptor-G protein selectivity. Therefore, we hypothesized that exchanging the Gα12 C terminus with C-terminal peptides from other Gα subunits might allow productive coupling with V2R. Indeed, we found that Gα12 chimeras bearing the last 10 amino acids of either Gαs or Gαq (Fig. 3A) interacted with AVP-activated V2R in a GDP-sensitive manner; RGDP values were significantly less than 1 for G12s and G12q heterotrimers and were similar to RGDP for Gs and Gq heterotrimers (Fig. 3B). G12s and G12q chimeras also supported V2R-mediated translocation of p115-RhoGEF to the plasma membrane, consistent with productive coupling to these heterotrimers and activation of G12 signaling pathways (Fig. 3C). Conversely, we found that replacing the C-terminal peptides of either Gαs or Gαq with that of Gα12 (Fig. 3A) dramatically increased RGDP, indicating much less productive coupling to Gs12 and unproductive coupling to Gq12 (Fig. 3B). We next made point mutations in the Gα12 C terminus to introduce residues with properties shared by the corresponding residues in Gαs and Gαq (Fig. 3A). We found that Gα12 mutants with a hydrophobic residue in the −1 position (Q381L and Q381V) still coupled unproductively with V2R (Fig. 3 D and E). In contrast, Gα12 mutants with a tyrosine in the −4 position (I378Y) coupled productively with V2R; RGDP was less than 1 (Fig. 3D), and I378Y supported V2R-mediated translocation of p115-RhoGEF (Fig. 3E). Similar weak but productive coupling to V2R was observed when Gα12 was simply truncated by a single amino acid (Δ1; Fig. 3 D and E). These results indicate that the Gα12 C terminus is required for unproductive coupling to active V2R. Together with the observation that subtle modifications of the Gα12 C terminus overcome the barrier to productive coupling, this result suggests that G12 heterotrimers are likely to interact with active V2R in a manner that is structurally similar to canonical GPCR-G protein complexes.
Fig. 3.
Role of the Gα12 C terminus in unproductive coupling with V2R. (A) Amino acid sequences of the distal C terminus of Gα12 (green), Gαs (blue), Gαq (black), and the chimeras and mutants used in B–E. Boxes indicate residues whose properties are shared between Gαs and Gαq but not Gα12. (B) GDP resistance (RGDP) of interactions between V2R-Rluc8 and heterotrimers incorporating the indicated Gα subunits; n.s., not significant; all other groups significantly different from one; P < 0.05; one-sample t test; n = 4–10. (C) Activation of V2R decreases bystander BRET between p115RhoGEF-Rluc8 and Venus-Kras when G12 is expressed, but increases bystander BRET when G12s or G12q are expressed, indicating receptor-mediated activation of these chimeras; all groups were significantly different from mock-transfected controls (−); P < 0.05; one-way ANOVA (Dunnett’s test); n = 7. (D) GDP resistance of interactions between V2R-Rluc8 and G12 heterotrimers bearing the indicated point mutations; n.s., not significant; all other groups significantly different from one; P < 0.05; one-sample t test; n = 3–14. (E) Activation of V2R decreases p115RhoGEF-Rluc8 translocation when G12 wt, Q381L, or Q381V are expressed, but increases translocation when G12 I378Y or Δ1 are expressed; all groups were significantly different from mock-transfected controls (−); P < 0.05; one-way ANOVA (Dunnett’s test); n = 3–13.
The V2R-G12 Interaction Interferes with Other Transducers.
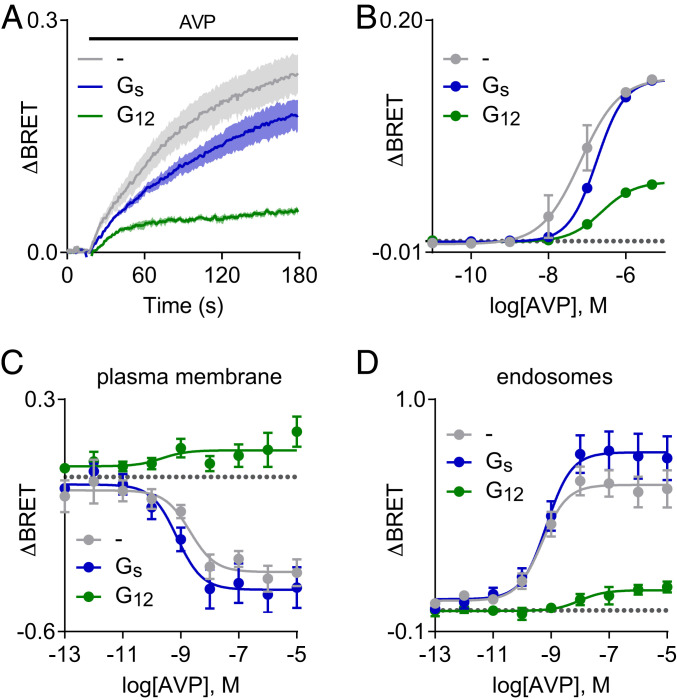
The robust agonist-induced BRET signal between V2R receptors and G12 heterotrimers in the presence of nucleotides suggested that this interaction might be stable enough to interfere with recruitment of other intracellular transducer molecules to V2R. As V2R receptors canonically activate Gs heterotrimers (11), we first asked how overexpressing G12 would influence activation of adenylyl cyclase and cAMP accumulation. We found that overexpressing G12 resulted in modest inhibition of Gs activation, as indicated by a Gs biosensor (SI Appendix, Fig. S8A). Surprisingly, this did not lead to detectable inhibition of V2R-mediated cAMP accumulation, as indicated by two different cAMP sensors (SI Appendix, Fig. S8 B and C). Active V2R receptors are phosphorylated by G protein receptor kinases (GRKs), and phosphorylated V2R bind tightly to β-arrestins (22). Remarkably, we found that overexpressing G12 significantly reduced AVP-induced BRET between V2R-Rluc8 and β-arrestin2–Venus (Fig. 4 A and B). A much smaller but still significant reduction was observed after overexpressing Gs heterotrimers (Fig. 4 A and B). In contrast, overexpressing G12 did not significantly reduce β-arrestin2 recruitment to ETA, β2-adrenergic, or angiotensin AT1 receptors (SI Appendix, Fig. S9). Because V2R–β-arrestin interactions are very stable and because phosphorylated V2R can accommodate G protein and arrestin binding simultaneously (23), we suspected that G12 overexpression was acting upstream of arrestin binding to inhibit V2R interactions with GRKs. Consistent with this hypothesis, we found that G12 overexpression greatly reduced the AVP-induced interaction of V2R and GRK2 (SI Appendix, Fig. S10). Because arrestin binding is critical for agonist-dependent V2R internalization (22) we then asked if G12 overexpression would inhibit receptor endocytosis. Indeed, overexpression of G12 but not Gs heterotrimers inhibited V2R trafficking from the plasma membrane to the endosomal compartment as assessed by enhanced bystander BRET (ebBRET; Fig. 4 C and D). Conversely, there was a small but significant enhancement of V2R internalization in cells lacking Gα12 and Gα13 subunits (SI Appendix, Fig. S11).
Fig. 4.
Overexpression of G12 inhibits arrestin recruitment to V2R and receptor internalization. (A and B) Time course and concentration-dependence of BRET between V2R-Rluc8 and β-arrestin2–Venus in response to AVP (1 μM in A; mean ± SEM; n = 3). Overexpression of G12 but not Gs heterotrimers inhibits arrestin recruitment. (C and D) AVP-induced changes in BRET between V2R-RlucII and the plasma membrane marker rGFP-CAAX (C) and the endosome marker rGFP-FYVE (D), indicating trafficking of V2R-RlucII from the plasma membrane to endosomes (mean ± SEM; n = 6–8). Overexpression of G12 but not Gs heterotrimers inhibits AVP-induced internalization of V2R-RlucII.
Other Receptors Also Form Unproductive Complexes with G12.
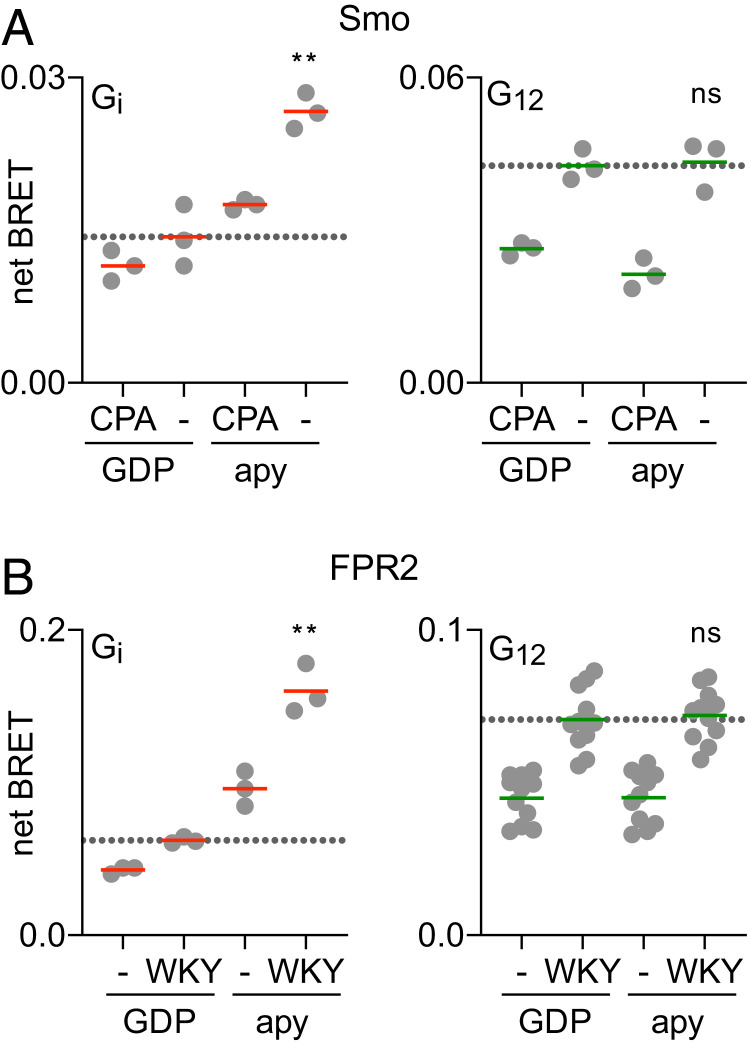
In the course of experiments examining coupling of multiple different GPCRs to G proteins we encountered three additional examples of receptors that interact with G12 heterotrimers in a nucleotide-resistant, unproductive manner. Smoothened (Smo) displays constitutive activity when the sterol transporter Patched is inhibited by Hedgehog or is not present, as is the case in HEK 293 cells. Smo is known to couple to and activate Gi heterotrimers (24). We found that unliganded Smo-Rluc8 did indeed interact with Gi heterotrimers in BRET assays, and this interaction was inhibited by either the inverse agonist cyclopamine or GDP, indicative of productive coupling (RGDP = 0.19 ± 0.10; n = 3). In contrast, BRET between Smo-Rluc8 and G12 heterotrimers was inhibited by cyclopamine but not GDP, indicative of unproductive coupling (RGDP = 1.01 ± 0.22; n = 3; Fig. 5A). Similarly, activation of formyl peptide 2 receptors (FPR2) with the agonist peptide WKYMVm (WKY) promoted productive coupling with Gi heterotrimers (RGDP = 0.30 ± 0.07; n = 4), but unproductive coupling with G12 heterotrimers (RGDP = 0.92 ± 0.04; n = 4; Fig. 5B). Although neither of these two receptors is known to activate G12 we directly assessed activation of downstream G12 signaling pathways by FPR2. As was the case with V2R, activation of FPR2 failed to recruit p115-RhoGEF to the plasma membrane and failed to activate SRE-dependent gene transcription (SI Appendix, Fig. S12). Formyl peptide 1 receptors (FPR1) are highly homologous with FPR2 (68% identical), and we found that FPR1 also coupled productively with Gi heterotrimers (RGDP = 0.26 ± 0.01; n = 3), but unproductively with G12 heterotrimers (RGDP = 0.96 ± 0.02; n = 3).
Fig. 5.
Smo and FPR2 form GDP-resistant complexes with G12 heterotrimers. (A) BRET between Gαβγ-Venus heterotrimers and Smo-Rluc8 in the presence and absence of the inverse agonist cyclopamine (CPA; 10 μM), in the presence and absence of GDP. Smo is constitutively active in the absence of CPA. (B) BRET between Gαβγ-Venus heterotrimers and FPR2-Rluc8 in the presence and absence of the agonist WKYMVm (WKY, 0.5 μM), in the presence and absence of GDP. Both receptors coupled productively to Gi1 heterotrimers, but unproductively to G12 heterotrimers; **P < 0.005; n.s., not significant (P > 0.75); one-way ANOVA (Sidak’s test) compared to GDP-CPA (A) and GDP+WKY (B); n = 3–12.
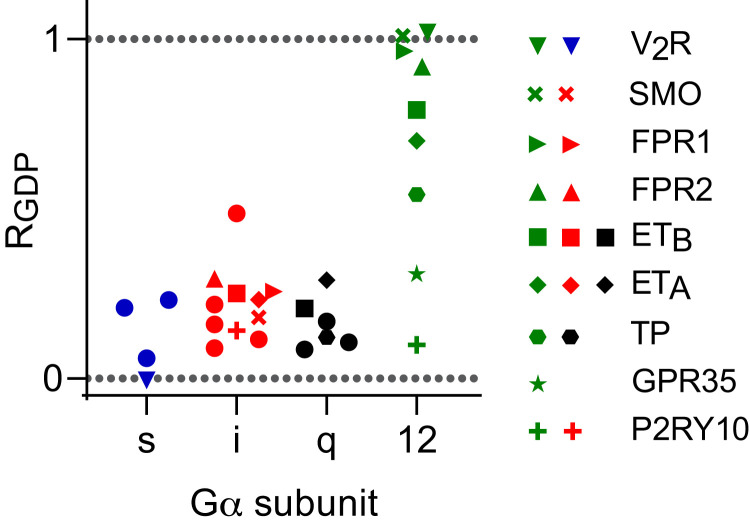
When we examined association of GPCRs with G proteins from all four Gα subtype families, we found that highly GDP-resistant interactions (RGDP > 0.5) were restricted to G12 heterotrimers (Fig. 6). For 19 of the 20 interactions that were studied with Gs, Gi, and Gq heterotrimers, RGDP was <0.3, whereas this was the case for only two of the nine interactions we studied with G12 heterotrimers. These results suggest that receptor-G12 complexes may generally be more stable than other receptor-G protein complexes when G proteins are bound to GDP.
Fig. 6.
Receptor interactions with G12 heterotrimers demonstrate a wide range of sensitivity to GDP. GDP resistance (RGDP) of interactions between a panel of GPCRs fused to Rluc8 and the indicated Gαβγ-Venus heterotrimers (mean; n = 3–6). Receptors that interact in an agonist-dependent manner with G12 heterotrimers are individually identified. Additional receptors are indicated by closed circles, and include: β2AR, H2R, D1R (with Gs), α2AR, M4R, A1R, D2R, MOR (with Gi), AT1R, M3R, H1R (with Gq). A full listing of receptor genes and ligands is provided in SI Appendix, Table S1.
Discussion
Taken together, our results suggest that several GPCRs bind to G12 heterotrimers in an activation-dependent manner, but the resulting GPCR-G12 complexes are insensitive to guanine nucleotides. These interactions do not activate G12 signaling, but may instead have a negative effect on RhoGEF recruitment and signaling by sequestering G12, thus preventing activation by other receptors. These interactions may also interfere with recruitment of other intracellular transducers and thus change signaling or trafficking of receptors that recruit but fail to activate G12 heterotrimers. Whether or not these inhibitory effects occur under physiological conditions will depend on several factors, most notably the local abundance of G12 heterotrimers and the stoichiometry of receptors and intracellular transducers. The normal physiological role of the V2R is to enhance water reabsorption in the kidney by stimulating Gs, which ultimately leads to incorporation of aquaporin-2 water channels to the luminal surface of collecting duct cells (25). An inhibitory effect of V2R activation on G12 signaling could conceivably contribute to the physiological activity of this receptor, as Rho activity has been reported to act as an inhibitor of aquaporin transport (26). An inhibitory effect of the V2R-G12 interaction on arrestin recruitment could also play a regulatory role to limit receptor internalization. Although we found that G12 overexpression weakly inhibited V2R-mediated Gs activation when assessed using a direct Gs activation assay, we were surprised to find that this did not lead to a detectable decrease in cAMP accumulation. It is possible that GRK and arrestin recruitment are more sensitive to competition with G12 than cAMP accumulation because cAMP signals are amplified downstream of Gs. Similar observations have been made after expression of some intrabodies that recognize the active state of β2-adrenergic receptors (27). Further studies with native systems will be required to determine if unproductive GPCR-G12 association has physiological significance.
At present, our findings significantly change the current model of GPCR coupling by demonstrating robust agonist-induced receptor-G protein interactions that do not lead to nucleotide exchange and G protein activation. GPCRs are thought to have access to all G protein subtypes expressed in a given cell, but possible interactions with noncognate heterotrimers (defined as G proteins that cannot be activated by a given GPCR) have, with a few exceptions (28), been overlooked. It is commonly assumed that stable agonist-induced GPCR-G protein interactions are restricted to cognate G proteins and are associated with G protein activation. One implication of this idea is that the conventional selection process whereby receptors reject noncognate G proteins occurs at an early stage of receptor-G protein association, such that complexes with noncognate G proteins do not progress past weak and transient encounter complexes. This seems to be true in the majority of cases, as several previous studies using sensitive methods have shown that interactions between GPCRs and noncognate G proteins are usually undetectable (7, 29). In contrast, our results suggest that some receptors functionally reject G12 heterotrimers despite forming relatively stable GPCR-G12 complexes. It is thought that GPCR-G protein complexes evolve through multiple intermediate conformations prior to receptor-stimulated nucleotide release (30–34). It is possible that receptors such as V2R and FPR2 form similar intermediate complexes with G12 heterotrimers that are unusually stable (Fig. 1B) and are unable to promote the changes in G12 that lead to GDP release. Spontaneous GDP release from G12 heterotrimers is particularly slow (35), and it may be that receptor-mediated GDP release requires relatively stable complexes with G12-GDP, even for receptors that do activate G12. G protein chimeras and mutants revealed that the Gα12 C terminus is necessary for unproductive complexes with V2R, implying that these complexes share some structural features with conventional productive ternary complexes. Our results with intramolecular G12 BRET sensors suggest that V2R and FPR2 may promote conformational changes in G12 that do not lead to activation. However, the resolution of such probes is insufficient to determine if the heterotrimer itself changes conformation or, alternatively, if only the attached BRET donor and acceptor labels are rearranged. In either case, these results suggest that changes in G protein conformation reported by sensors similar to those used here do not necessarily indicate G protein activation.
In summary, our results reveal a mode of GPCR-G protein interaction wherein agonist-activated receptors bind to G12 heterotrimers but do not promote nucleotide exchange and activation. These findings show that receptors can inhibit as well as activate G proteins, adding to the complexity of GPCR-mediated signaling.
Materials and Methods
Materials.
Trypsin, DPBS, PBS, HBSS, FBS, MEM, DMEM, penicillin/streptomycin, and l-glutamine were from Gibco (ThermoFisher Scientific). Polyethyleneimine MAX (PEI MAX) was purchased from Polysciences, Inc. Some receptor ligands, luciferin-d, and forskolin were purchased from Cayman Chemical. The remaining receptor ligands, digitonin, apyrase, GDP, GTP, GDPβS, and GTPγS were purchased from MilliporeSigma. Coelenterazine h and coelenterazine 400a were purchased from Nanolight Technologies. NanoGlo luciferase substrate was purchased from Promega.
Plasmid DNA Constructs.
GPCR plasmids were purchased from cdna.org (Bloomsburg University) or were provided by Bryan Roth (PRESTO-Tango Kit - #1000000068, Addgene). The V2R-Rluc8 plasmid was received as a gift from Kevin Pfleger (Harry Perkins Institute of Medical Research, Nedlands, Western Australia). A plasmid encoding β-arrestin2–Venus was a gift from Vsevolod Gurevich (Vanderbilt University, Nashville, TN). V2R-SmBit and ETAR-SmBit were generated by replacing the GPCR coding sequence in β2AR-SmBit digested with EcoRI and NotI, which appended the SmBit peptide to the C terminus of each receptor behind a GGRGGGGSG linker. Plasmids encoding Gα subunits, Gβ1, and Gγ2 were purchased from cdna.org. Gα12-Rluc8 was generated by inserting Rluc8 (flanked by GGSG linkers) between a residues N136 and K137 of Gα12 using Quikchange mutagenesis. GRK2-Venus-Kras and GRK2-Venus-Kras R587Q were generated by appending Venus fused to the last 25 amino acids of Kras to the C terminus of bovine GRK2 or GRK2 R587Q using Quikchange mutagenesis. Plasmids encoding the S1 subunit of pertussis toxin (PTX-S1) and LgBit-Gγ2 were kindly provided by Stephen R. Ikeda (NIAAA, Rockville, MD), and the Nluc-EPAC-VV plasmid was provided by Kirill Martemyanov (Scripps Research Institute, Jupiter, FL). The Glosensor-22F cAMP plasmid (E2301) was obtained from Promega. Plasmids encoding GαsΔ10, Venus-Kras, Venus-1–155-Gγ1, and Venus-155–239-Gβ1 GPCR-luciferase constructs, and p115RhoGEF-Rluc8 have been described previously (6, 15, 36). Plasmids encoding rGFP-CAAX, rGFP-FYVE, and V2R-RlucII have been described previously (16). PDZ-RhoGEF-RlucII was generated by amplifying the cytosolic G12/13 interacting domain of PDZ-RhoGEF (aa 281–483) with linkerD (GIRLREALKLPAT) on its C terminus which was then subcloned onto the N terminus of RlucII in pcDNA3.1/Zeo(+) by Gibson assembly. GRK2-RlucII D110A was generated by digesting hGRK2-GFP10 D110A and pcDNA3.1/Hygro(+) GFP10-RlucII db v.2 with NheI and HindIII to excise hGRK2 from the former and GFP10 from the latter. hGRK2 was subsequently ligated in frame with pcDNA3.1/Hygro(+) RlucII db v.2 to produce a C-terminal RlucII construct. All plasmid constructs were verified by Sanger sequencing.
Cell Culture and Transfection.
HEK 293 cells (ATCC) were propagated in plastic flasks and on 6-well plates according to the supplier’s protocol. HEK 293 cells with targeted deletion of GNAS and GNAL (Gs knockouts; GSKO), targeted deletion of GNAS, GNAL, GNAQ, GNA11, GNA12, and GNA13 (G protein three family knockouts; 3GKO), and HEK 293 cells with additional targeted deletions to the 3GKO cells of GNAI1, GNAI2, GNAI3, GNAT1, GNAT2, GNAZ, and GNAO1 (G protein four family knockouts; 4GKO) were derived, authenticated, and propagated as previously described (9, 10). HEK 293 cells with additional targeted deletion of ARRB1 and ARRB2 (beta-arrestin knockouts; ARRBKO) were derived, authenticated, and propagated as previously described (37, 38). Cells were transfected in growth medium using linear PEI MAX (MW 40,000) at a nitrogen/phosphate ratio of 20 and were used for experiments 12–48 h later. Up to 3.0 μg of plasmid DNA was transfected in each well of a 6-well plate. For ebBRET experiments, up to 1.0 μg of plasmid DNA was transfected in suspension to a cell density of 350,000 cells/mL in white 96-well plates.
BRET and Luminescence Assays.
Measurement of coupling between receptor and G protein in nucleotide-depleted cells.
Cells were transfected with a GPCR-Rluc8 and Gα subunit pair, Venus-1–155-Gγ2, Venus-155–239-Gβ1, and pcDNA3.1(+) or PTX-S1 in a (1:3:1:1:1) ratio. Experiments with Gαi were conducted in 4GKO cells for Gαi cognate receptors and in 3GKO cells for all other receptors. Experiments with Gαi were conducted without PTX-S1; all other Gα subunits were cotransfected with PTX-S1. After a 48-h incubation, cells were washed twice with permeabilization buffer (KPS) containing 140 mM KCl, 10 mM NaCl, 1 mM MgCl2, 0.1 mM KEGTA, 20 mM NaHEPES (pH 7.2); harvested by trituration; permeabilized in KPS buffer containing 10 μg mL−1 high-purity digitonin; and transferred to opaque black 96-well plate. Measurements were made from permeabilized cells supplemented either with 100 μM GDP or 2U mL−1 apyrase, in both cases with or without agonist (SI Appendix, Table S1).
Luciferase complementation.
Cells were transfected with a GPCR-SmBit, Gα, LgBit-Gγ2, Gβ1, and pcDNA3.1(+) or PTX-S1 in a (1.5:4:1:2.5:3) ratio. After a 24-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque white 96-well plates.
GPCR competition assays.
Cells were transfected with an untagged GPCR or pcDNA3.1(+), GPCR-Rluc8, Gα, Venus-1–155-Gγ2, Venus-155–239-Gβ1, and PTX-S1 in a (10:1:2:2:4) ratio. After a 48-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque black 96-well plates.
G protein BRET conformational biosensor.
HEK 293 cells were transfected with an untagged GPCR, Gα12-Rluc8, Venus-1–155-Gγ2 and Venus-155–239-Gβ1 in a (15:1:4:4) ratio. After a 48-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque black 96-well plates.
Gs activation nanoBiT sensor.
NanoBiT-Gs protein (39) consisting of Gαs subunit fused with a large fragment (LgBiT) at the alpha-helical domain and an N-terminally small fragment (SmBiT)-fused Gγ2 subunit along with untagged Gβ1 subunit was expressed in the presence or absence of Gα12 subunit. HEK 293 cells were seeded in a 6-well culture plate at a concentration of 2 × 105 cells mL−1 (2 mL per well in DMEM; Nissui) supplemented with 10% fetal bovine serum (Gibco), glutamine, penicillin, and streptomycin) 1 d before transfection. Transfection solution was prepared by combining 5 μL (per well hereafter) of PEI MAX solution (1 mg mL−1), 200 μL of Opti-MEM (ThermoFisher Scientific), and a plasmid mixture consisting of 100 ng LgBiT-containing Gαs subunit, 500 ng Gβ1, 500 ng SmBiT-fused Gγ2, 100 ng RIC8b, 200 ng untagged V2R (pCAGGS plasmid) with or without 20 ng Gα12 subunit (pCAGGS plasmid; gene-synthesized with codon optimization). After incubation for 1 d, transfected cells were harvested with 0.5 mM EDTA-containing DPBS, centrifuged and suspended in 4 mL of HBSS containing 0.01% BSA (fatty acid–free grade; SERVA) and 5 mM Hepes (pH 7.4) (assay buffer). The cell suspension was dispensed in a white 96-well plate at a volume of 80 µL per well and loaded with 20 µL of 50 µM coelenterazine (Carbosynth) diluted in the assay buffer. After 2 h incubation at room temperature, the plate was measured for baseline luminescence (Spectramax L, Molecular Devices), and 20 µL of titrated ligand (AVP) were manually added. The plate was immediately read at room temperature for the following 10 min at a measurement interval of 20 s with an accumulation time of 0.17 s per read. The luminescence counts over 5–10 min after ligand addition were averaged and normalized to the initial count. The fold-change values were further normalized to that of vehicle-treated samples.
Translocation of p115RhoGEF.
Cells were transfected with an untagged GPCR, Gα, Gγ2, Gβ1, p115RhoGEF-Rluc8, Venus-Kras, and PTX-S1 in a (2:12:4:4:1:6:2) ratio. After a 48-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque black 96-well plates.
Translocation of PDZ-RhoGEF.
ΔβARR1/2 HEK 293 cells were transfected with either FLAG-V2R or HA-TPα, Gα12, PDZ-RhoGEF-RlucII and rGFP-CAAX in a (8:4:1:12) ratio. After a 48-h incubation, cells were washed once with Tyrode’s buffer (140 mM NaCl, 2.7 mM KCl, 1 mM CaCl2, 12 mM NaHCO3, 5.6 mM d-glucose, 0.5 mM MgCl2, 0.37 mM NaH2PO4, 25 mM Hepes [pH 7.4]) and maintained in the same buffer. Cells were stimulated for 5 min with agonist before BRET measurements.
p115-RGS-GFP biosensor to monitor Gα12 activity.
A BRET-based biosensor composed of RGS homology (RH) domain (amino acids 1–246) of p115RhoGEF fused to GFP10 (p115-RGS-GFP) and one of two Gα12-RlucII fusions (RlucII inserted after amino acid 84 or 136) was used to measure Gα12 activity (40). HEK 293 cells were transfected with 40 ng of Gα12-RlucII, 500 ng of p115-RGS-GFP, and 300 ng of receptor per row of a 96-well plate. BRET was monitored 2 min after agonist addition.
SRE transcriptional reporter assay.
Cells were transfected with a GPCR, Gα subunit, SRE-Luc, and PTX-S1 in a (10:1:100:25) ratio. Medium was exchanged to serum-free 2 h after transfection. After a 24-h incubation, cells were treated with or without agonist for 5 h. Cells were washed twice with DPBS, harvested by trituration, centrifuged at 500 × g for 3 min, and resuspended in equilibration buffer (1× HBSS, 20 mM NaHEPES; pH 7.5) supplemented with 10% FBS by volume, and 2 mM d‐luciferin. Cells equilibrated in this solution at room temperature for 30 min and were transferred to opaque white 96-well plates.
NFκB transcriptional reporter assays.
Cells were transfected with a GPCR, Gα subunit, NFκB-Luc, and empty vector in a (300:1:300:199) ratio. After a 24-h incubation, cells were treated with or without agonist for 5 h. Cells were washed twice with DPBS, harvested by enzyme-free, centrifuged at 500 × g for 3 min, and resuspended in equilibration buffer (1× HBSS, 20 mM NaHEPES; 0.1% wt/vol BSA, pH 7.5) and transferred into 96-well black/white Isoplates (Perkin-Elmer). Cells were incubated with 2 mM D‐luciferin for 30 min before reading luminescence emission at 525 nm after 30 of incubation using a PHERAstar FS (BMG LABTECH).
Nluc-EPAC-VV cAMP assay.
Cells were transfected with a pcDNA3.1(+), a GPCR, Gα subunit or pcDNA3.1(+), Gγ2, Gβ1, and Nluc-EPAC-VV in a (59:15:5:15:10:10:1) ratio. After a 24-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque black 96-well plates.
Glosensor cAMP assay.
GSKO cells were transfected with a GPCR, Gα subunit, Gγ2, Gβ1, Glosensor 22F, and either pcDNA3.1(+) or PTX‐S1 in a (1:1:1:1:4:1) ratio. After a 24‐h incubation, cells were washed twice with DPBS and treated with trypsin‐EDTA (0.05%). Detached cells were harvested and centrifuged at 250 × g for 5 min, and the cell pellet was resuspended in equilibration buffer supplemented with 10% FBS by volume and 2 mM d‐luciferin. Cells were incubated at room temperature for 1 h and then distributed to opaque white 96‐well plates. Luminescence measurements were made from cells treated with vehicle, agonist, or 100 μM forskolin.
Arrestin recruitment.
HEK 293 cells were transfected with a GPCR-Rluc8, Gα, Gγ2, Gβ1, and β-arrestin2–Venus in a (1:2:1:1:1) ratio. After a 24-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque black 96-well plates.
Bystander BRET V2R trafficking.
HEK 293 cells were transfected with V2R-RlucII, Gα, and either rGFP-CAAX or rGFP-FYVE in a (1:20:60) ratio. After a 48-h incubation, cells were washed once with Tyrode’s buffer and maintained in the same buffer. Cells were stimulated for 30 min with agonist before BRET measurements.
HiBiT-based V2R internalization.
Parental HEK 293 cells, G12/13-deficient HEK 293 cells (39), or β-arrestin1/2-deficient HEK 293 cells (37) in growth phase were seeded in a 6-well culture plate at a concentration of 2 × 105 cells mL−1. Cells were transfected with 100 ng of HiBiT-V2R, which contained an Interleukin 6-derived signal sequence followed by a HiBiT sequence and a linker at the N terminus (MNSFSTSAFGPVAFSLGLLLVLPAAFPAPVSGWRLFKKISGGSGGGGSG; gene synthesized with codon optimization) and an unintended SmBiT tag at the C terminus. After 1 d, cells were harvested, suspended in 1 mL of assay buffer, dispensed in a white 96-well half-area plate at a volume of 25 µL per well, and mixed with 25 µL of 2× substrate buffer consisting of 1:200 of a LgBiT stock solution (Promega) and 20 µM furimazine in the assay buffer. After 40 min at room temperature, the plate was measured for baseline luminescence, and a titrated ligand (10 µL) diluted in the 1× substrate buffer was manually added. The plate was immediately read at room temperature for the following 30 min at a measurement interval of 30 s with an accumulation time of 0.4 s per read. The luminescence counts over 27–30 min after ligand addition were averaged and normalized to the initial count.
GRK2 recruitment.
For the experiments shown in SI Appendix, Fig. S10A, ΔβARR1/2 HEK 293 cells were transfected with FLAG-V2R, GRK2-RlucII D110A, Gα, and GFP-CAAX in a (2:1:2:6) ratio in suspension and distributed into white 96-well plates. After a 48-h incubation, cells were washed once with Tyrode’s buffer and maintained in the same buffer. Cells were stimulated with agonist immediately after addition of coelenterazine 400a. For the experiments shown in SI Appendix, Fig. S10B, ARRBKO cells were transfected with a GPCR-Rluc8, Gα subunit, Gγ2, Gβ1, and GRK2-Venus in a (1:3:1:1:3) ratio. After a 48-h incubation, cells were washed twice with DPBS, harvested by trituration, and transferred to opaque black 96-well plates.
BRET, luminescence measurements.
Steady-state BRET and luminescence measurements were made using a Mithras LB940 photon-counting plate reader (Berthold Technologies GmbH). Kinetic BRET and luminescence time course measurements were made using a Polarstar Optima plate reader (BMG Labtech). Coelenterazine h (5 μM; Nanolight) or furimazine (NanoGlo; 1:1,000, Promega) were added to all wells immediately prior to making measurements with Rluc8 and Nluc, respectively. Raw BRET signals were calculated as the emission intensity at 520–545 nm divided by the emission intensity at 475–495 nm. Net BRET signals were calculated as the raw BRET signal minus the raw BRET signal measured from cells expressing only the Rluc8 donor.
Supplementary Material
Acknowledgments
We thank Steve Ikeda, Kevin Pfleger, Philip Wedegaertner, and Bryan Roth for providing plasmid DNA used in this study. We thank Kayo Sato, Shigeko Nakano, and Ayumi Inoue (Tohoku University) for their assistance with plasmid preparation, maintenance of cell culture and cell-based GPCR assays. This work was supported by grants from the NIH (GM130142 [to N.A.L.], MH54137 [to J.A.J.]) and a Ruth L. Kirschstein National Research Service Award Individual Fellowship (GM131672 [to N.O.]). A.I. was funded by the PRIME JP17gm5910013 and the LEAP JP17gm0010004 from the Japan Agency for Medical Research and Development, and Grants-in-aid for Scientific Research (KAKENHI) 17K08264 from the Japan Society for the Promotion of Science (JSPS). K.K. is supported by JSPS Fellows 19J11256. S.C.W. is supported by a fellowship from the Swedish Society for Medical Research (P18-0098). M.B. is funded by the Canadian Institutes of Health Research (FDN-148431) and holds a Canada Research Chair in Signal Transduction and Molecular Pharmacology.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003787117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Weis W. I., Kobilka B. K., The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87, 897–919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce K. L., Premont R. T., Lefkowitz R. J., Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 (2002). [DOI] [PubMed] [Google Scholar]
- 3.De Lean A., Stadel J. M., Lefkowitz R. J., A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J. Biol. Chem. 255, 7108–7117 (1980). [PubMed] [Google Scholar]
- 4.Maguire M. E., Van Arsdale P. M., Gilman A. G., An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol. Pharmacol. 12, 335–339 (1976). [PubMed] [Google Scholar]
- 5.Yao X. J. et al., The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc. Natl. Acad. Sci. U.S.A. 106, 9501–9506 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okashah N. et al., Variable G protein determinants of GPCR coupling selectivity. Proc. Natl. Acad. Sci. U.S.A. 116, 12054–12059 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galés C. et al., Real-time monitoring of receptor and G-protein interactions in living cells. Nat. Methods 2, 177–184 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Héroux M., Hogue M., Lemieux S., Bouvier M., Functional calcitonin gene-related peptide receptors are formed by the asymmetric assembly of a calcitonin receptor-like receptor homo-oligomer and a monomer of receptor activity-modifying protein-1. J. Biol. Chem. 282, 31610–31620 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Hisano Y. et al., Lysolipid receptor cross-talk regulates lymphatic endothelial junctions in lymph nodes. J. Exp. Med. 216, 1582–1598 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundmann M. et al., Lack of beta-arrestin signaling in the absence of active G proteins. Nat. Commun. 9, 341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong J. F., et al. , The IUPHAR/BPS Guide to PHARMACOLOGY in 2020: Extending immunopharmacology content and introducing the IUPHAR/MMV Guide to MALARIA PHARMACOLOGY. Nucleic Acids Res. 48, D1006–D1021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong S. P., Seeber R. M., Ayoub M. A., Feldman B. J., Pfleger K. D. G., Characterization of three vasopressin receptor 2 variants: An apparent polymorphism (V266A) and two loss-of-function mutations (R181C and M311V). PLoS One 8, e65885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strathmann M. P., Simon M. I., G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc. Natl. Acad. Sci. U.S.A. 88, 5582–5586 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avet C., et al. , Selectivity landscape of 100 therapeutically relevant GPCR profiled by an effector translocation-based BRET platform. bioRxiv:2020.04.20.052027 (24 April 2020).
- 15.Lan T.-H., Liu Q., Li C., Wu G., Lambert N. A., Sensitive and high resolution localization and tracking of membrane proteins in live cells with BRET. Traffic 13, 1450–1456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namkung Y. et al., Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. Nat. Commun. 7, 12178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharyya R., Wedegaertner P. B., Characterization of G alpha 13-dependent plasma membrane recruitment of p115RhoGEF. Biochem. J. 371, 709–720 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart M. J. et al., Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by G13. Science 280, 2112–2114 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Liu B., Wu D., “Analysis of the coupling of G12/13 to G protein-coupled receptors using a luciferase reporter assay” in G Protein Signaling, Smrcka A. V., Ed. (Humana Press, 2004), pp. 145–150. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen S. G. F. et al., Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahoney J. P., Sunahara R. K., Mechanistic insights into GPCR-G protein interactions. Curr. Opin. Struct. Biol. 41, 247–254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G., Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 274, 32248–32257 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Thomsen A. R. B. et al., GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166, 907–919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden S. K. et al., G protein Gαi functions immediately downstream of Smoothened in Hedgehog signalling. Nature 456, 967–970 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juul K. V., Bichet D. G., Nielsen S., Nørgaard J. P., The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am. J. Physiol. Ren. Physiol. 306, F931–F940 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Klussmann E. et al., An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J. Biol. Chem. 276, 20451–20457 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Staus D. P. et al., Regulation of β2-adrenergic receptor function by conformationally selective single-domain intrabodies. Mol. Pharmacol. 85, 472–481 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupte T. M., Malik R. U., Sommese R. F., Ritt M., Sivaramakrishnan S., Priming GPCR signaling through the synergistic effect of two G proteins. Proc. Natl. Acad. Sci. U.S.A. 114, 3756–3761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hein P., Frank M., Hoffmann C., Lohse M. J., Bünemann M., Dynamics of receptor/G protein coupling in living cells. EMBO J. 24, 4106–4114 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Y. et al., Assembly of a GPCR-G protein complex. Cell 177, 1232–1242.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato H. E. et al., Conformational transitions of a neurotensin receptor 1-Gi1 complex. Nature 572, 80–85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X. et al., Structural insights into the process of GPCR-G protein Complex Formation. Cell 177, 1243–1251.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregorio G. G. et al., Single-molecule analysis of ligand efficacy in β2AR-G-protein activation. Nature 547, 68–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hausdorff W. P., Hnatowich M., O’Dowd B. F., Caron M. G., Lefkowitz R. J., A mutation of the β2-adrenergic receptor impairs agonist activation of adenylyl cyclase without affecting high affinity agonist binding. Distinct molecular determinants of the receptor are involved in physical coupling to and functional activation of Gs. J. Biol. Chem. 265, 1388–1393 (1990). [PubMed] [Google Scholar]
- 35.Kozasa T., Gilman A. G., Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of alpha 12 and inhibition of adenylyl cyclase by alpha z. J. Biol. Chem. 270, 1734–1741 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Hollins B., Kuravi S., Digby G. J., Lambert N. A., The c-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell. Signal. 21, 1015–1021 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Hayre M. et al., Genetic evidence that β -arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci. Signal. 10, eaal3395 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luttrell L. M. et al., Manifold roles of β -arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci. Signal. 11, eaat7650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue A. et al., Illuminating G-protein-coupling selectivity of GPCRs. Cell 177, 1933–1947.e25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukasheva V. et al., Signal profiling of the β1AR reveals coupling to novel signalling pathways and distinct phenotypic responses mediated by β1AR and β2AR. Sci. Rep. 10, 8779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.