Significance
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS). B cells play a key role in MS immunopathology, as demonstrated by the success of B cell-directed therapies; however, the target antigen of MS remains unknown. Using a combination of ELISpot-based prescreening of peripheral blood mononuclear cells followed by investigation of antibody specificity with a CNS antigen array, we identified a population of MS patients characterized by a highly active B cell response. These individuals with MS, who have active B cell responses, exhibited heterogeneous interindividual anti-CNS antibody responses, although the antigenic specificity of the antibodies remained stable over time for each individual.
Keywords: multiple sclerosis, antibody, ELISpot, B cells, myelin
Abstract
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS), with characteristic inflammatory lesions and demyelination. The clinical benefit of cell-depleting therapies targeting CD20 has emphasized the role of B cells and autoantibodies in MS pathogenesis. We previously introduced an enzyme-linked immunospot spot (ELISpot)-based assay to measure CNS antigen-specific B cells in the blood of MS patients and demonstrated its usefulness as a predictive biomarker for disease activity in measuring the successful outcome of disease-modifying therapies (DMTs). Here we used a planar protein array to investigate CNS-reactive antibodies in the serum of MS patients as well as in B cell culture supernatants after polyclonal stimulation. Anti-CNS antibody reactivity was evident in the sera of the MS cohort, and the antibodies bound a heterogeneous set of molecules, including myelin, axonal cytoskeleton, and ion channel antigens, in individual patients. Immunoglobulin reactivity in supernatants of stimulated B cells was directed against a broad range of CNS antigens. A group of MS patients with a highly active B cell component was identified by the ELISpot assay. Those antibody reactivities remained stable over time. These assays with protein arrays identify MS patients with a highly active B cell population with antibodies directed against a swathe of CNS proteins.
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS). While it was traditionally considered to be a T cell-mediated disease, recent successful clinical trials with B cell-depleting anti-CD20 antibodies have demonstrated the critical role of B cells in MS pathology. The exact mechanism of how B cells promote neuroinflammation is incompletely understood but likely comprises autoantibody secretion, immunomodulation, and autoantigen-specific antigen presentation (1). B cell depletion reduces annual relapse rates by 46% but does not completely suppress disease activity (2). In addition, long-term treatment has been associated with undesirable side effects, including hypogammaglobulinemia (3), neutropenia (4), and increased risk of malignancies (5).
Anti-CD20 antibodies spare the majority of plasmablasts and plasma cells, which are the main antibody-secreting cells and pathogenic effector cells in MS, but express low levels of CD20 (6). In addition, while the number of circulating B cells in the blood is significantly diminished by anti-CD20 therapies, depletion rates in secondary lymphoid organs are low, and both the meninges as well as the brain parenchyma are unaffected (7). After B cell depletion, cells typically reemerge within 6 mo of treatment with high intraindividual and interindividual variability (8), which emphasizes the need for personalized monitoring and adjustment of treatments. These caveats call for a better understanding of B cell pathology and biomarkers that identify patients with a B cell-driven disease, predicting a high likelihood of treatment success. To improve B cell-targeting treatments, it is crucial to understand the origin of a highly active B cell component, its contribution to neuroinflammation via autoantibody secretion and antigen presentation, and in particular B cell antigen specificity.
To date, there is no biomarker that predicts treatment outcome in MS patients; however, there is evidence for a B cell-driven subtype of MS. Demyelinating lesions can be classified into four distinct patterns, demonstrating interindividual heterogeneity in MS patients. The most frequent lesion, pattern II, which accounts for approximately 60% of patients, is characterized by depositions of antibodies and complement (9). Plasmapheresis is most successful in this subset of MS patients (10–12). We have recently introduced an assay for monitoring the CNS-specific B cell response in the blood of MS patients that enables identification of a subset of MS patients with a highly active B cell component, and we hypothesize that this subset overlaps with pattern II patients (13). For our B cell assay, peripheral blood mononuclear cells (PBMCs) are seeded onto CNS antigen-coated plates and stimulated with interleukin (IL)-2 and Toll-like receptor (TLR) ligands. Secretion of anti-CNS antibodies by activated B cells is measured by an enzyme-linked immunospot (ELISpot) assay. The prevalence of patients that tested positive in this B cell assay (ELISpot-pos) was comparable to that of patients with pattern II lesions in previous studies (9, 13, 14); however, the antigen specificity and clonality of the in vitro restimulated B cell response have remained elusive.
The search for specific pathogenic autoantibodies and their antigens has been notoriously challenging in MS. Most studies have suggested major myelin antigens as potential targets, including myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), as well as lipids (15–24). Recently, the potassium channel KIR4.1 was proposed as a dominant autoantigenic target in MS (25), but this could not be confirmed in larger follow-up trials (26). Antibodies to anoctamin-2 (ANO2) were found in 14.2% of MS patients, likely originating from a B cell response to Epstein–Barr virus (EBV) (27). Unlike the related neuroimmunologic disease neuromyelitis optica, in which antibodies against the water channel aquaporin-4 (AQP4) are clearly disease-specific and pathogenic (28, 29), the current research in MS reveals a variable set of different antibodies targeting a wide group of proteins that contribute to pathology, likely differing in their specificity between patients.
In the present study, we combined a well-characterized B cell assay with CNS proteins attached to a planar surface with peptide microarrays. The combination of these two methods differs from previous approaches in which solely sera and/or cerebrospinal fluid (CSF) specimens were screened for antibody reactivity to CNS proteins (20, 30, 31). Using these two platforms has several advantages. First, the capacity to stratify patient samples according to their ELISpot results allows us to choose an ELISpot-pos group in which we expect high antibody reactivity and an ELISpot-negative (ELISpot-neg) group with antibody reactivity similar to healthy controls (HC) and patients with other neurologic diseases (OND). Second, experimental knowledge based on previous work (14) indicates that an individual can switch from an ELISpot-neg to an ELISpot-pos classification. This enables sampling individuals at a time point with high B cell activity. Third, the approach enables assaying supernatants from restimulated B cells, allowing the narrowing of the B cell pool to those B cells present in a patient’s circulation. This approach excludes bone marrow resident plasma cells, which produce antibodies against a wide variety of infectious antigens. Fourth, autoreactive antibodies can be absorbed in the target organ, where their antigen-binding sites may be occupied by resident antigens (32). Restimulation in vitro and testing of B cell supernatants enables a possible circumvention of this issue.
Here we demonstrate that MS patients can be subdivided into patients with a highly active vs. less active B cell component (ELISpot-pos vs. ELISpot-neg), as reflected by significantly different levels of anti-myelin antibody reactivity. The antibody response was mainly restricted to CNS antigens but remained relatively variable within the patient cohorts; therefore, dominant antigens could not be determined. However, time-course measurements demonstrated that the intraindividual antibody response remained relatively constant in the ELISpot-pos group, but not in the ELISpot-neg group.
Results
Serum Antibody Reactivity against Myelin Proteins and Peptides.
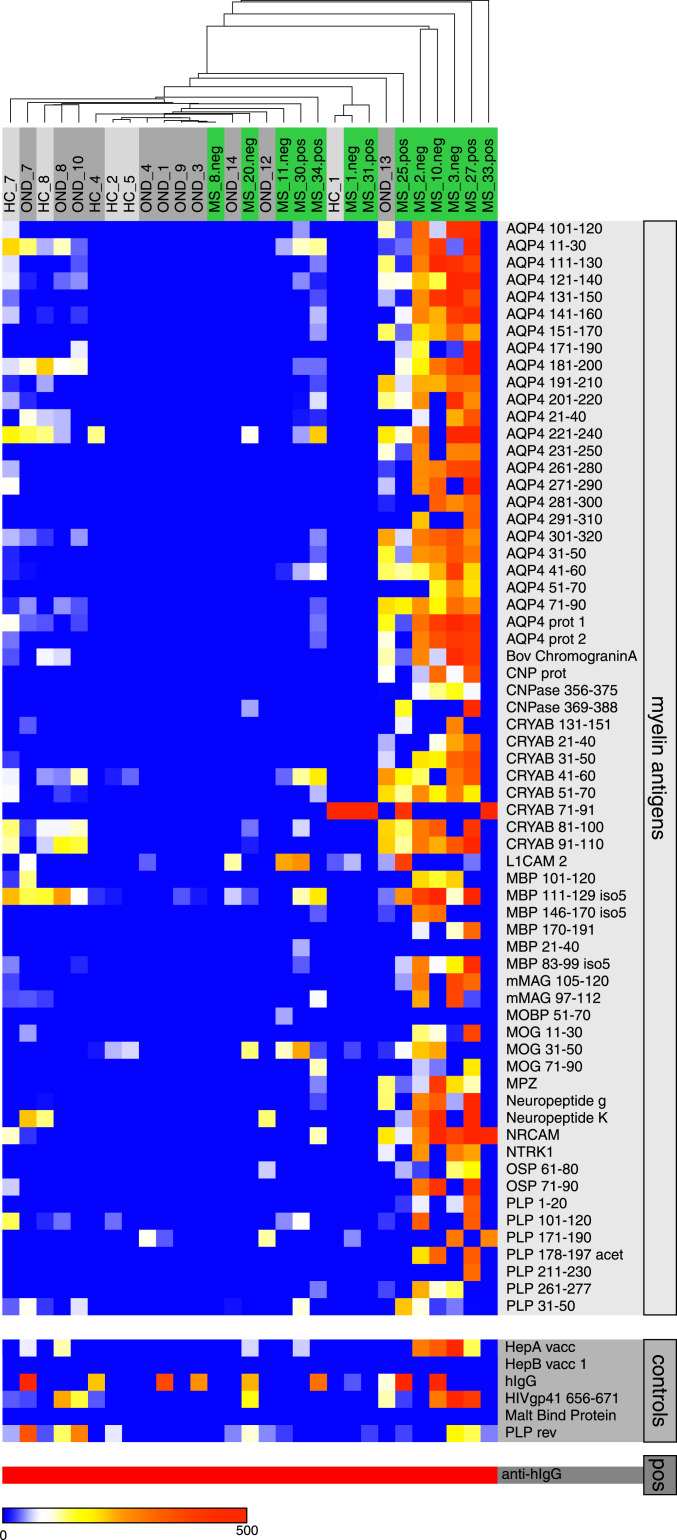
Previous studies that tested serum and plasma samples from MS patients on protein/peptide microarrays showed elevated antibody reactivities in MS vs. HC/OND against a broad spectrum of myelin antigens (20, 24, 30). This is reflective of a polyclonal and polyspecific humoral immune response against myelin and neuronal antigens in MS patients. However, the statistical power of these previous analyses was modest: (relapsing remitting multiple sclerosis [RRMS]) vs. HC: positive predictive value, 0.69 to 0.85; negative predictive value, 0.58 to 0.80 (30); MS vs. acute disseminated encephalomyelitis: false discovery rate (FDR), 16.7 to 33.3% (20). Here we tested plasma from a cohort of 13 MS patients on a microarray representing 205 selected well-described myelin antigens (SI Appendix, Table S1). Significance analysis of microarrays (SAM) (33) identified a subset of 64 myelin antigens for which differential antibody reactivities could be detected in MS vs. HC samples. The FDR of 10.67% was comparable to values reported in the aforementioned studies (Fig. 1). While the data confirm broadly elevated anti-myelin autoantibody levels in plasma of MS patients, the levels of significance were not high enough to serve as a clinical biomarker for MS.
Fig. 1.
Serum antibody reactivity against myelin antigens. Heatmaps of plasma IgG reactivity, mean fluorescence intensity (MFI), for 64 myelin antigens identified as significantly different between HC/OND and MS patients in SAM analysis (Top), nonmyelin controls (Middle), and positive control (Bottom).
In Vitro B Cell Stimulation and ELISpot Testing Defines a Subset of Broadly Myelin-Reactive Patients.
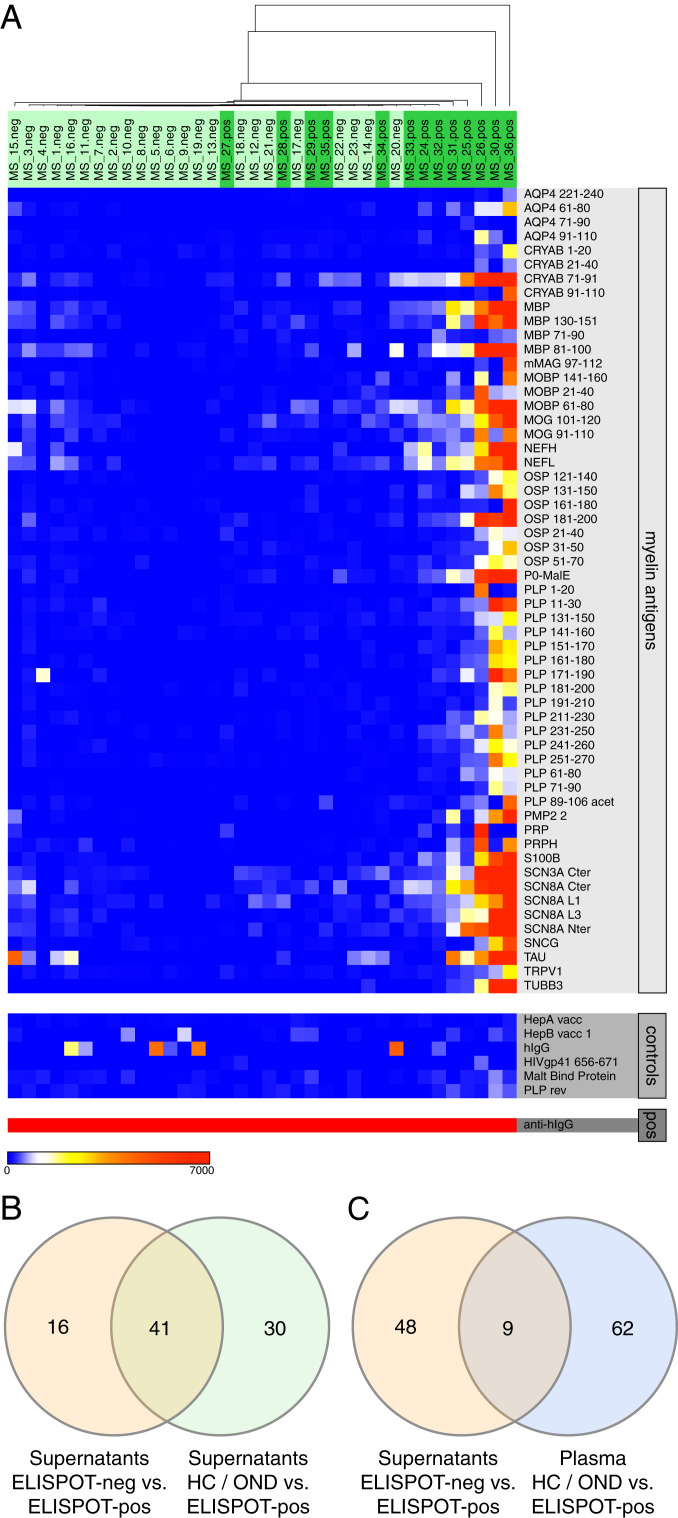
In our previously described ELISpot assay, we restimulated PBMCs in vitro and detected CNS-reactive IgG, thereby defining a subset of MS patients with a highly active B cell component (9, 13, 14). However, detailed specificities of the activated B cells have been elusive so far, and whether the restimulated B cells were representative of the patient’s antibody repertoire was unclear. Microarray testing of B cell supernatants obtained from ELISpot cultures showed high reactivity against a broad set of myelin protein and peptide antigens in the ELISpot-pos group. A subset of 57 antigens separated ELISpot-pos and ELISpot-neg supernatants with an FDR of 0.94% (Fig. 2A), an order of magnitude superior to the aforementioned separation between MS and HC based on serum samples (13, 14). ELISpot reactivity is restricted to a subset of MS patients, and no HC/OND patients were found to be ELISpot-pos in any previous assays (9, 13, 14). Not surprisingly, a similar set of 71 antigens separated HC and OND from ELISpot-pos B cell supernatants (FDR, 3.79%), and 41 of these antigens overlapped with those antigens identified by the comparison of ELISpot-neg and ELISpot-pos supernatants (Fig. 2B and SI Appendix, Fig. S1). Strikingly, no differential antigen reactivity was identified when comparing HC and OND with ELISpot-neg supernatants.
Fig. 2.
IgG reactivity in B cell supernatants, comparing ELISpot-neg and ELISpot-pos samples. (A) Heatmap of antibody reactivity (in mean fluorescence intensity [MFI]) against 57 myelin antigens identified as significantly different between ELISpot-neg and ELISpot-pos B cell supernatants in SAM analysis (Top), nonmyelin controls (Middle), and positive control (Bottom). (B) Venn diagram indicating the number of overlapping significant antigens between the comparisons of ELISpot-neg vs. ELISpot-pos (supernatants) (orange) and HC/OND vs. ELISpot-pos (supernatants) (green). (C) Venn diagram indicating the number of overlapping significant antigens between the comparisons ELISpot-neg vs. ELISpot-pos (supernatants) (orange) and HC/OND vs. MS (plasma) (blue).
A smaller set of antigens (9 of 57) overlapped with the set of antigens that separated HC/OND plasma samples from MS samples (Fig. 2C and SI Appendix, Fig. S2), indicating considerable variability in antimyelin reactivity.
Antimyelin reactivity was assessed in a separate validation cohort (cohort 2) of MS patients, where again ELISpot-pos and ELISpot-neg groups were compared. In this smaller cohort (6 ELISpot-neg samples and 8 ELISpot-pos samples), a set of 40 antigens separated the two groups (FDR, 3.4%; Fig. 3A). However, only 10 significant antigens overlapped in the two cohorts, and only 3 antigens overlapped in both supernatant cohorts and plasma reactivity (HC/OND vs. MS), comprising one alpha-B crystallin peptide (CRYAB 21 to 40) and two proteolipid protein (PLP) peptides (PLP 1 to 20 and PLP 211 to 230) (Fig. 3B and SI Appendix, Fig. S3). PLP in particular has been implicated as a potential MS antigen in previous studies (30). We conclude that MS patients have elevated antimyelin immunoglobulin reactivity, and that B cell restimulation improves sensitivity and group separation. However, the antimyelin reactivity is broad, and dominant antigens cannot be discerned.
Fig. 3.
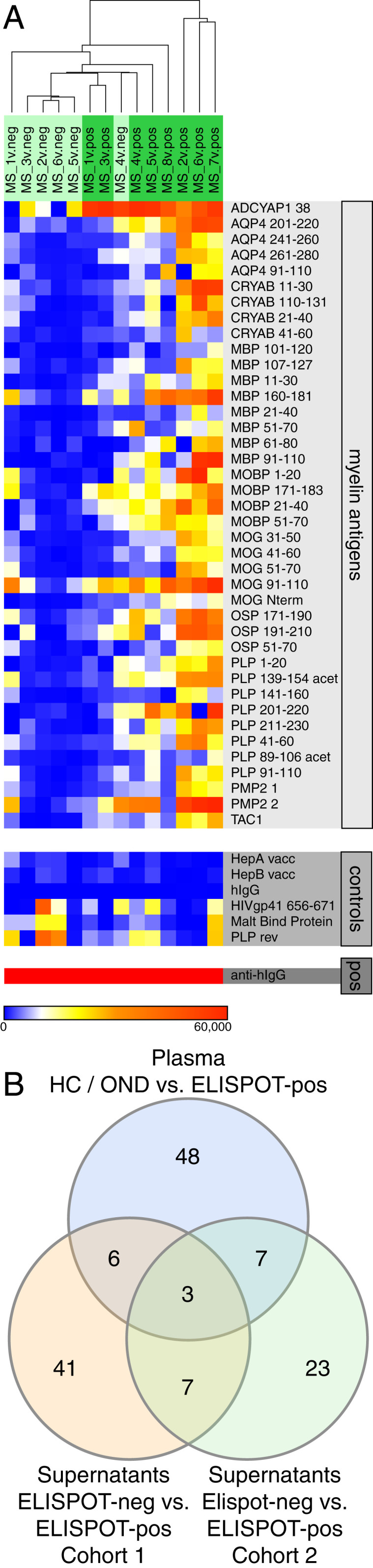
IgG reactivity in B cell supernatants, second cohort. (A) Heatmap of antibody reactivity (in MFI) against 40 myelin antigens identified as significantly different between ELISpot-neg vs. ELISpot-pos B cell supernatants in SAM analysis (Top), nonmyelin controls (Middle), and positive control (Bottom). (B) Venn diagram indicating the number of overlapping significant antigens among the comparisons of ELISpot-neg vs. ELISpot-pos (supernatants, cohort 1) (orange), ELISpot-neg vs. ELISpot-pos (supernatants, cohort 2) (green), and HC/OND vs. MS (plasma) (blue).
Antimyelin Immunoglobulin Reactivity in ELISpot-Pos Patients Is Stable over Time.
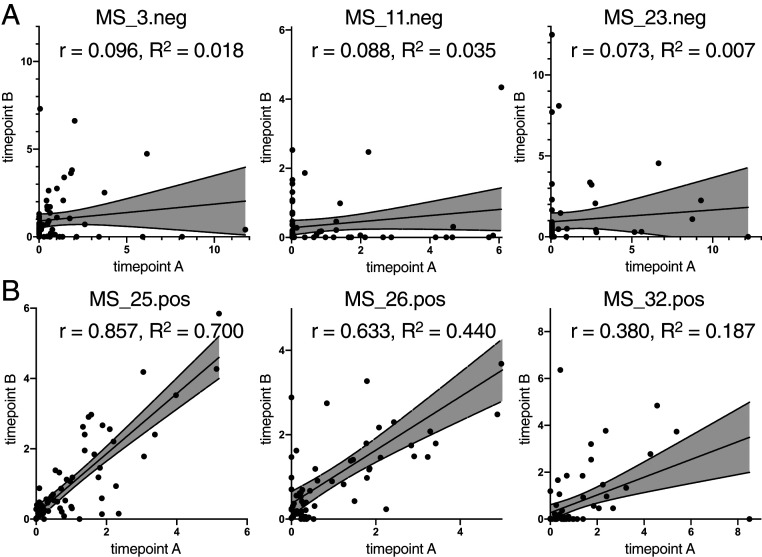
To test whether antimyelin reactivity remains constant in an individual MS patient over time or if intraindividual variability adds to the observed interindividual variability, we retested B cell supernatants of three ELISpot-neg and three ELISpot-pos patients after 3 mo. While ELISpot-neg B cells were overall less reactive than ELISpot-pos B cells, as expected (SI Appendix, Fig. S4), variability between time points was higher in ELISpot-neg patients, while ELISpot-pos reactivities remained more constant (Fig. 4). This demonstrates that the amount of B cells used for restimulation was a large enough sample to represent the patient’s antibody repertoire.
Fig. 4.
Antigen reactivity in B cell supernatants of individual MS patients over time. Scatterplots depict antibody reactivities (in MFI) of time point A (x-axes) and time point B (y-axes) 3 mo later in ELISpot-neg samples (A) and ELISpot-pos samples (B). The correlation coefficient, r, and coefficient of determination, R2, for each patient are indicated in each plot.
Discussion
MS is an inflammatory disease of the CNS directed in part against myelin, implying that the disease is autoimmune. Several target antigens of the myelin sheath have been suggested and investigated in MS patients, including MBP (19, 34–36), myelin-associated glycoprotein (35), MOG (19, 37–39), PLP (36), CRYAB (40, 41), and oligodendrocyte-specific protein (42). In addition, neuronal and axonal antigens are being increasingly acknowledged as target structures in MS, as it has been shown that pathogenic features of MS include axonal damage (43). The cytoskeleton molecule neurofilament light chain has emerged as a potential biomarker for disease activity in MS patients (44). Further neuronal antigenic targets of the adaptive immunity include contactin-2 (45) and the chloride channel protein ANO2 (27). Several of these antigens have structural or sequence similarities with epitopes of bacterial and viral antigens. Thus, molecular mimicry is an appealing hypothesis for the origin of autoreactivities, with EBV and human herpes virus 6 proposed as viral candidates that mimic myelin proteins (27, 46, 47). New bacterial candidates have been identified as molecular mimics relevant to MS from in-depth studies of the microbiome (48). The immune response in MS is diverse, with no single antigen emerging as the dominant target of B and T cells. This is in contrast to other neuroimmunologic diseases for which dominant pathogenic autoantigens are well described, such as the acetylcholine receptor in myasthenia gravis (49) and AQP4 in neuromyelitis optica (29).
Our results are concordant with the concept that a diverse set of antigenic targets can be identified in MS patients. As shown here, the antibody specificities illuminated on the myelin antigen array on plasma samples enable the segregation of patients with MS from HC/OND patients, similar to what has been described in previous publications (19, 29).
We show here that preselecting MS patients based on our well-described ELISpot-based B cell assay focuses the analysis on patients with a highly active B cell component. Combining the B cell assay with protein arrays and assessing antibody reactivity in B cell supernatants instead of assessing the immune response in plasma enabled us to separate ELISpot-pos from ELISpot-neg patients as well as ELISpot-pos from HC/OND with a high degree of discrimination. Repeating the analysis in two separate patient cohorts confirmed the elevated reactivity in ELISpot-pos over ELISpot-neg patients and further supports the concept of a broad antimyelin response without major dominant antigens; only 17.5% of the significant antigens from the first cohort overlapped with the second cohort. Not only did we find higher antimyelin reactivity in ELISpot-pos patients, but the intraindividual B cell response remained constant over time, in contrast to the lack of correlation between timepoints in ELISpot-neg patients. The relative consistency of these measurements over time provides further validation of the assay strategy used here. In addition, our findings validate our strategy of using whole-brain lysate instead of recombinant antigens as a coating for the ELISpot assay, to cover the broad range of possible CNS antigens (19, 29).
We recently showed that the ELISpot assay is suitable for use as a biomarker for predicting treatment success in RRMS patients. Specifically, we were able to show that ELISpot-pos patients were more likely to respond to glatiramer acetate treatment compared with IFN-β treatment (50). In addition, the majority of RRMS patients (∼70%) harbored brain-specific B cells in the blood, and reactivation of such B cells was associated with subsequent clinical relapses, emphasizing the importance of B cell-mediated autoimmunity in MS (14). The confirmation that ELISpot-pos B cells are reactive to myelin antigens suggests that the ELISpot-pos group of patients may respond particularly well to B cell-depleting therapies.
The combination of the ELISpot assay with antigen arrays or other proteomics methods could be an effective approach for antigen identification, superior to solely probing for antigen reactivity in CSF and plasma. As shown here, preselection of ELISpot-pos patients focuses the patient collective on a subset with a highly reactive B cell component. In addition, by using B cell supernatants, we were able to circumvent the dilemma posed by the fact that antigen-binding sites of antibodies secreted into the CSF or blood are often occupied by their cognate antigens, or antibodies are absorbed in target organs (32).
This two-tiered approach for determining B cell and antibody reactivity against antigens of the CNS in MS patients indicates that there is a diverse adaptive immune response in MS. Experimental results from this combined approach with ELISpot and planar arrays emphasize the interindividual heterogeneity of the adaptive immune response in MS. Even in individuals with a strong antibody response in MS, a single dominant antigen does not emerge from explorations of adaptive immunity. The identification of individuals with active antibody responses may help stratify those who may benefit most from B cell-targeted therapeutics.
Materials and Methods
Study Design and Human Subjects.
In cohort 1, PBMCs were obtained from 36 MS patients (23 ELISpot-neg and 13 ELISpot-pos), 14 patients with OND, and 10 HCs (Table 1, and SI Appendix, Tables S2 to S4). Cohort 2 included 6 ELISpot-neg and 8 ELISpot-pos MS patients (Table 2). Patients in cohort 1 as well as OND patients were recruited from the Departments of Neurology at the University Hospitals of Cologne and Würzburg, as well as from the Department of Neurology at the Caritas-Krankenhaus in Bad Mergentheim, Germany. Patients in cohort 2 were recruited from the NeuroTransData (NTD) network of physicians. NTD is a Germany-wide network founded in 2008 and run since then by physicians in the fields of neurology and psychiatry. Currently, 153 neurologists work in 78 NTD practices that serve approximately 600,000 outpatients per year. MS was diagnosed according to the 2010 McDonald criteria (51). Patients with a history of other autoimmune diseases and severe accompanying systemic or psychiatric disorders were excluded from the study, as were patients who had undergone plasmapheresis, B cell depletion therapy, i.v. immunoglobulin or immunosuppressive treatment within 12 mo before study initiation. All experimental protocols were approved by the Ethical Review Boards of the University Hospitals of Cologne (file no. 10–221), Würzburg (file nos. 65/10 and 149/11), and the Carl Gustav Carus University in Dresden (file no. EK 523122016). Written informed consent was obtained from each patient. Disability was graded according to the Expanded Disability Status Scale (EDSS) (52).
Table 1.
Demographic data of participants in cohort 1
| Variable | Value |
| HC | |
| Total n | 10 |
| Female patients, n (%) | 9 (90) |
| Age, y, median (range) | 32 (24 to 58) |
| OND | |
| Total n | 14 |
| Female patients, n (%) | 9 (64.29) |
| Age, y, median (range) | 66.5 (26 to 78) |
| MS ELISpot-neg (MS_neg) | |
| Total n | 23 |
| Female patients, n (%) | 18 (78.26) |
| Age, y, median (range) | 36 (23 to 58) |
| Time since diagnosis, y, median (range) | 2.5 (0 to 18) |
| EDSS score, median (range) | 2 (0 to 7) |
| MS ELISpot-pos (MS_pos) | |
| n | 13 |
| patients, n (%) | 8 (61.54) |
| Age, y, median (range) | 36 (18 to 63) |
| Time since diagnosis, y, median (range) | 5 (2 to 15) |
| EDSS score, median (range) | 2.5 (0 to 6.5) |
Table 2.
Demographic data of participants in cohort 2
| Variable | Value |
| MS ELISpot-neg | |
| Total n | 6 |
| Female patients, n (%) | 5 (83.3) |
| Age, y, median (range) | 42 (31 to 67) |
| Time since diagnosis, y, median (range) | 9 (8 to 13) |
| EDSS score, median (range) | 1.5 (1 to 2) |
| MS ELISpot-pos | |
| Total n | 8 |
| Female patients, n (%) | 6 (75) |
| Age, y, median (range) | 46.5 (34 to 51) |
| Time since diagnosis, y, median (range) | 7.5 (2.5 to 26) |
| EDSS score, median (range) | 1 (0 to 4) |
Polyclonal Stimulation of B Cells.
PBMCs and plasma were separated from heparinized blood by density gradient centrifugation. Plasma samples were stored at –80 °C. PBMCs were cultured at a concentration of 3 × 106 cells/mL in complete RPMI-1640 supplemented with IL-2 at 15 ng/mL (Peprotech), the TLR7 and TLR8 agonist R-848 at 2.5 μg/mL (Enzo Life Sciences), and β-mercaptoethanol at 1 mM (Sigma-Aldrich) for 96 h at 37 °C and 7% CO2, according to the protocol described by Pinna et al. (53). Culture supernatants were collected for subsequent array analysis, and polyclonally stimulated B cells were further processed for ELISpot analysis.
ELISpot Assay.
Here 96-well PVDF ELISpot plates (MultiScreen HTS; Millipore) were coated overnight with whole human brain lysate (30 μg/mL; Novus Biologicals). Coating with anti-human Igκ (Southern Biotech) served as a positive control at a concentration of 10 μg/mL, and 10% FBS served as negative control. Plates were blocked with 10% FBS for 2 h at room temperature. Each sample was plated in triplicate with 1 × 106 cells/well and incubated at 37 °C and 7% CO2 for 26 h. After culture, the plates were incubated with biotinylated anti-human IgG (clone MT78/145; Mabtech) at 0.2 μg/mL in 1% BSA. Subsequently, all plates were developed with AP-KIT III substrate (Vector Blue; Vector Laboratories). Spots were counted on an ImmunoSpot Series 6 Analyzer (Cellular Technology Limited).
Array Production and Probing.
Myelin antigen protein/peptide arrays were printed on SuperEpoxy slides (ArrayIt) (54). Between 4 and 12 replicates of each compound were printed. A list of all antigens included is provided in SI Appendix, Table S1. Arrays were circumscribed with a hydrophobic marker, blocked overnight at 4 °C in PBS containing 3% FCS and 0.1% Tween-20, incubated with B cell culture supernatants at 1:3 dilution or plasma samples at 1:125 dilution in blocking buffer for 1 h at 4 °C, and then washed twice for 20 min in blocking buffer on a rotating shaker. Arrays were incubated with cyanin-3 dye-conjugated goat anti-human IgG + IgM (Jackson ImmunoResearch) at a concentration of 0.8 µg/mL for 1 h at 4 °C, then washed twice for 30 min in blocking buffer, twice for 30 min in PBS, and twice for 15 s in water. Arrays were spun dry and scanned with a GenePix 4000B scanner (Axon Instruments). The protocol has been described in detail previously (54) and is available at https://web.stanford.edu/group/antigenarrays/.
Array Data Analysis.
GenePix Pro-3.0 software (Axon Instruments) was used to determine the net median pixel intensities for individual features. Normalized median net digital fluorescence units represent median values from 4 to 12 identical antigen features on each array normalized to the median intensity of 8 anti-IgG features, so that the normalized anti-IgG reactivity was 25,000 for all arrays. SAM analysis for microarrays was used to identify antigens with significantly different antibody reactivities between individual groups (samr package in R6.1; https://statweb.stanford.edu/∼tibs/SAM/) (33, 55). SAM was run with “two class unpaired” settings, using the Mann–Whitney–Wilcoxon test, a delta value of 0.25, and a minimum fold change of 2.5 and (12.5 for comparison of supernatants ELISpot-neg vs. ELISpot-pos, cohort 1). Heatmaps were generated with Morpheus software (The Broad Institute; https://software.broadinstitute.org/morpheus). Heatmap colors were adjusted for batch-dependent differences in intensities, as described in the figure legends. Euclidian distance with single linkage was used for hierarchical clustering. For time point analyses, data for each time point were normalized by division with the mean of all the data points for that time point. Linear regression analysis was performed using the least-squares method in GraphPad Prism 8.0.2, and the correlation coefficient, r, as well as the coefficient of determination, R2, are reported.
Supplementary Material
Acknowledgments
We thank Christopher Hohmann, Bianca Milles, Jolanta Kozlowski, Damiano M. Rovituso, and Sabine Tacke for help with the ELISpot analysis and Samir Jabari for help with array probing. We also thank Alexey Y. Karulin, Meik Kunz, Sandra Andorf, and Amin Zia for discussion on the statistical analysis, as well as Paul V. Lehmann for scientific input. Funding for this project was provided by the Bavaria California Technology Center (to S.K.) and the German Research Foundation (LA3657/1, to T.V.L.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011249117/-/DCSupplemental.
Data Availability.
All study data are included in the main text and SI Appendix.
References
- 1.Pröbstel A.-K., Sanderson N. S. R., Derfuss T., B cells and autoantibodies in multiple sclerosis. Int. J. Mol. Sci. 16, 16576–16592 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser S. L. et al.; OPERA I and OPERA II Clinical Investigators , Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Kim S.-H. et al., Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: Influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol. 72, 989–995 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Rigal J., Ciron J., Lépine Z., Biotti D., Late-onset neutropenia after rituximab therapy for multiple sclerosis, neuromyelitis optica spectrum disorders, and MOG-antibody-associated diseases. Mult. Scler. Relat. Disord. 41, 102019 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Mulero P., Midaglia L., Montalban X., Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 11, 1756286418773025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cepok S. et al., Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 128, 1667–1676 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Greenfield A. L., Hauser S. L., B-cell therapy for multiple sclerosis: Entering an era. Ann. Neurol. 83, 13–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellwardt E., Ellwardt L., Bittner S., Zipp F., Monitoring B-cell repopulation after depletion therapy in neurologic patients. Neurol. Neuroimmunol. Neuroinflamm. 5, e463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucchinetti C. et al., Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707–717 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Stork L. et al., Differences in the responses to apheresis therapy of patients with 3 histopathologically classified immunopathological patterns of multiple sclerosis. JAMA Neurol. 75, 428–435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortese I. et al., Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 76, 294–300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehler J. et al., Response to therapeutic plasma exchange as a rescue treatment in clinically isolated syndromes and acute worsening of multiple sclerosis: A retrospective analysis of 90 patients. PLoS One 10, e0134583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hohmann C. et al., Categorization of multiple sclerosis relapse subtypes by B cell profiling in the blood. Acta Neuropathol. Commun. 2, 138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuerten S. et al., Identification of a B cell-dependent subpopulation of multiple sclerosis by measurements of brain-reactive B cells in the blood. Clin. Immunol. 152, 20–24 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Weber M. S., Hemmer B., Cepok S., The role of antibodies in multiple sclerosis. Biochim. Biophys. Acta 1812, 239–245 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Hohlfeld R., Dornmair K., Meinl E., Wekerle H., The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 15, 317–331 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Egg R., Reindl M., Deisenhammer F., Linington C., Berger T., Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult. Scler. 7, 285–289 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Marti Fernandez I. et al., The glycosylation site of myelin oligodendrocyte glycoprotein affects autoantibody recognition in a large proportion of patients. Front. Immunol. 10, 1189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reindl M. et al., Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: A comparative study. Brain 122, 2047–2056 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Van Haren K. et al., Serum autoantibodies to myelin peptides distinguish acute disseminated encephalomyelitis from relapsing-remitting multiple sclerosis. Mult. Scler. 19, 1726–1733 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson W. H. et al., Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Ho P. P.-K. et al., Identification of naturally occurring fatty acids of the myelin sheath that resolve neuroinflammation. Sci. Transl. Med. 4, 137ra73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spadaro M. et al., Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann. Neurol. 84, 315–328 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Hecker M. et al., High-density peptide microarray analysis of IgG autoantibody reactivities in serum and cerebrospinal fluid of multiple sclerosis patients. Mol. Cell. Proteomics 15, 1360–1380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava R. et al., Potassium channel KIR4.1 as an immune target in multiple sclerosis. N. Engl. J. Med. 367, 115–123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brickshawana A. et al., Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: A comparative study. Lancet Neurol. 13, 795–806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tengvall K. et al., Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. U.S.A. 116, 16955–16960 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennon V. A. et al., A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 364, 2106–2112 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R., IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana F. J. et al., Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 105, 18889–18894 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prineas J. W., Parratt J. D. E., Multiple sclerosis: Serum anti-CNS autoantibodies. Mult. Scler. 24, 610–622 (2018). [DOI] [PubMed] [Google Scholar]
- 32.O’Connor K. C. et al., Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J. Immunol. 175, 1974–1982 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tusher V. G., Tibshirani R., Chu G., Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 98, 5116–5121 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPherson T. A., Gilpin A., Seland T. P., Radioimmunoassay of CSF for encephalitogenic basic protein: A diagnostic test for MS? Can. Med. Assoc. J. 107, 856–859 (1972). [PMC free article] [PubMed] [Google Scholar]
- 35.Wajgt A., Górny M., CSF antibodies to myelin basic protein and to myelin-associated glycoprotein in multiple sclerosis. Evidence of the intrathecal production of antibodies. Acta Neurol. Scand. 68, 337–343 (1983). [DOI] [PubMed] [Google Scholar]
- 36.Sellebjerg F., Christiansen M., Garred P., MBP, anti-MBP and anti-PLP antibodies, and intrathecal complement activation in multiple sclerosis. Mult. Scler. 4, 127–131 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Mazzucco S. et al., A synthetic glycopeptide of human myelin oligodendrocyte glycoprotein to detect antibody responses in multiple sclerosis and other neurological diseases. Bioorg. Med. Chem. Lett. 9, 167–172 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Xiao B. G., Linington C., Link H., Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J. Neuroimmunol. 31, 91–96 (1991). [DOI] [PubMed] [Google Scholar]
- 39.Berger T. et al., Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. 349, 139–145 (2009). [DOI] [PubMed] [Google Scholar]
- 40.van Noort J. M. et al., The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature 375, 798–801 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Rothbard J. B. et al., Chaperone activity of α B-crystallin is responsible for its incorrect assignment as an autoantigen in multiple sclerosis. J. Immunol. 186, 4263–4268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bronstein J. M., Lallone R. L., Seitz R. S., Ellison G. W., Myers L. W., A humoral response to oligodendrocyte-specific protein in MS: A potential molecular mimic. Neurology 53, 154–161 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Trapp B. D. et al., Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Varhaug K. N., Torkildsen Ø., Myhr K.-M., Vedeler C. A., Neurofilament light chain as a biomarker in multiple sclerosis. Front. Neurol. 10, 338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derfuss T. et al., Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl. Acad. Sci. U.S.A. 106, 8302–8307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jog N. R. et al., Epstein–Barr virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J. Autoimmun. 106, 102332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engdahl E. et al., Increased serological response against human herpesvirus 6A is associated with risk for multiple sclerosis. Front. Immunol. 10, 2715 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berer K. et al., Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479, 538–541 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Lennon V. A., Griesmann G. E., Evidence against acetylcholine receptor having a main immunogenic region as target for autoantibodies in myasthenia gravis. Neurology 39, 1069–1076 (1989). [DOI] [PubMed] [Google Scholar]
- 50.Rovituso D. M. et al., The brain antigen-specific B cell response correlates with glatiramer acetate responsiveness in relapsing-remitting multiple sclerosis patients. Sci. Rep. 5, 14265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polman C. H. et al., Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurtzke J. F., Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 33, 1444–1452 (1983). [DOI] [PubMed] [Google Scholar]
- 53.Pinna D., Corti D., Jarrossay D., Sallusto F., Lanzavecchia A., Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur. J. Immunol. 39, 1260–1270 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson W. H. et al., Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 8, 295–301 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Tibshirani R., A simple method for assessing sample sizes in microarray experiments. BMC Bioinformatics 7, 106 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.