Abstract
We herein report a case of fulminant Legionnaires' disease with autopsy findings in a patient on maintenance hemodialysis (HD). Chronic kidney disease is a strong risk factor for Legionnaires' disease, although there have been only a few reports in HD patients. Because most patients on HD are anuric, the use of rapid assay kits to detect antigens in urine samples for the diagnosis of Legionnaires' disease is not always feasible. We suggest the use of clinical predictive tools or the loop-mediated isothermal amplification (LAMP) method, which can be applied for anuric patients, such as those on HD, with pneumonia.
Keywords: atypical pneumonia, hemodialysis patients, immunocompromised hosts, Legionella pneumophila
Introduction
Legionnaires' disease was first identified in 1976 as a human epidemic pneumonia in Philadelphia (PA, USA). With the spread of rapid assay kits for detecting antigens in urine samples, the incidence of Legionnaires' disease has been increasing in cases of community-acquired pneumonia. However, the use of these kits for the diagnosis of Legionnaires' disease in most patients on hemodialysis (HD) who are anuric is difficult. Furthermore, only a few reports of Legionnaires' disease among HD patients have been published.
We herein report a very rare case of fulminant and fatal Legionnaires' pneumonia in a patient on maintenance HD. Severe lobar pneumonia in HD patients should be promptly diagnosed and managed using a clinical prediction tool because of the increasing incidence of Legionnaires' disease, which is difficult to diagnose in HD patients and might be fatal in this population.
Case Report
A 50-year-old man was hospitalized because of a dry cough, chills, and fever for 4 days. Four days before admission, he experienced chills, back pain, and cough and visited another clinic. He had no gastrointestinal symptoms, such as diarrhea. Acetaminophen and carbocysteine were prescribed for 4 days, but the symptoms did not improve. Therefore, he was referred to our hospital after HD. He was a previous 20-pack-year smoker but did not drink alcohol.
He lived in an over 40-year-old house, so contaminated shower use was presumed. He worked in a bar. During the rainy season, he did not use a humidifier or an air conditioner. He had no recent history of travel to a spa or other countries. He had been on maintenance HD for three years because of diabetic kidney disease. In addition, he had undergone percutaneous coronary intervention of his right coronary artery for angina pectoris eight years earlier. He had been diagnosed with congestive heart failure and was taking beta blockers and angiotensin receptor blockers.
Echocardiography had shown an ejection fraction of 33% and diffuse hypokinesis 4 months earlier. Echocardiography on admission revealed an ejection fraction of 30%, which was not a significant change from the previous examination. He had no history of gastrointestinal disorders, such as peptic ulcers. There was no family history of diabetes, including HD or immunodeficiency.
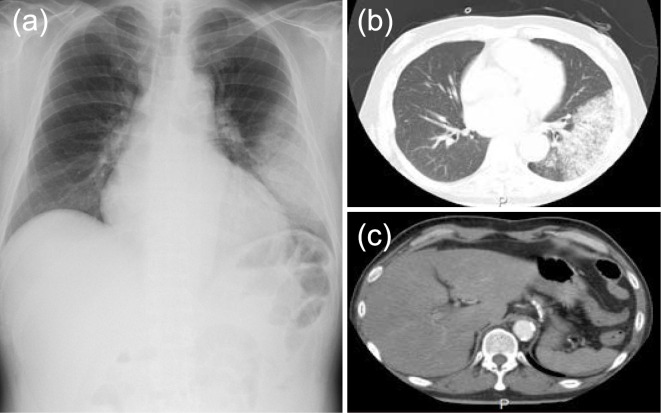
Vital signs showed body temperature of 38.9 °C, blood pressure of 131/97 mmHg, heart rate of 117 per minute, and respiratory rate of 18 per minute. The arterial oxygen saturation on pulse oximetry was 96%, and his consciousness was intact. A physical examination revealed left pulmonary rales and bilateral lower leg edema. He did not have xerostomia. Clinical laboratory tests showed an elevated leukocyte count, markedly elevated C-reactive protein levels, severe abnormalities in the liver function and creatine kinase value, and moderate hypoxemia (Table). An electrocardiogram showed tachycardia, but there was no specific ST elevation to indicate ischemic heart disease. The chest radiograph showed lobular infiltrates in the left lower lung field. There was mild heart enlargement without a significant amount of pleural effusion (Fig. 1a). Computed tomography (CT) showed lobar consolidation with areas of air bronchogram in the left lower lobe, chronic liver injury, and fatty liver (Fig. 1b, c).
Table.
Laboratory Findings on Admission.
| Arterial Blood Gas (room air) | Biochemical Data | |||||||
| pH | 7.6 | Albumin | 3.3 | g/dL | ||||
| Partial pressure of carbon dioxide (PCO2) | 29 | mmHg | Lactate dehydrogenase | 9,905 | U/L | |||
| Partial pressure of oxygen (PO2) | 74 | mmHg | Asparete amino transferase | 8,524 | U/L | |||
| Bicarbonate (HCO3) | 27.6 | mmol/L | Alanine amino transferase | 3,011 | U/L | |||
| Lactate | 3.2 | mmol/L | Total bilirubin | 1.0 | mg/dL | |||
| Complete Blood Count | Alkaline phosphatase | 160 | U/L | |||||
| White blood cell | 19,630 | /μL | Creatine kinase | 1,037 | U/L | |||
| Hemoglobin | 12.7 | g/dL | Creatine kinase MB | 5 | U/L | |||
| Platelet | 107,000 | /μL | C-reactive protein | 28.89 | mg/dL | |||
| Blood Coagulation Test | Brain Natriuretic Protein | 6,399 | pg/mL | |||||
| Prothrombin time-international normalized ratio | 1.67 | Troponin T | 4.46 | pg/mL | ||||
| Activated partial thromboplastin time | 38 | sec | Blood Urea Nitrogen | 32.3 | mg/dL | |||
| Infectious Screening | Creatinine | 5.96 | mg/dL | |||||
| Human immunodeficiency virus Antigen/Antibody | - | Sodium | 137 | mEq/L | ||||
| Hepatitis B surface antigen | - | Potassium | 4.7 | mEq/L | ||||
| Hepatitis C virus-antibody | - | Chloride | 95 | mEq/L | ||||
| Syphlis antibody | - | Calcium | 8.3 | mg/dL | ||||
| Inorganic Phosphorus | 4.3 | mg/dL | ||||||
Figure 1.
Chest X-ray and CT findings on admission. (a) The chest radiograph shows left-sided lobular pneumonia. (b, c) A CT scan of the chest shows consolidation, chronic liver injury, and fatty liver. CT: computed tomography
Given these findings, bacterial pneumonia with severe liver dysfunction was diagnosed on admission. However, his general condition was actually good, without dyspnea necessitating oxygen inhalation. The A-DROP (age, dehydration, respiratory failure, orientation disturbance, low blood pressure) score was <1, and the quick Sequential Organ Failure Assessment score was 0.
Piperacillin/Tazobactam (2.25 g every 8 hours) was started because he was expected to be immunodeficient as an HD patient. Despite treatment, the laboratory test results progressively worsened the next day. Blood cultures were negative, and sputum culture only showed oral microflora. The patients' sputum had less purulent and more serous components. Considering the possibility of Legionnaires' disease, as CT showed lobar pneumonia, we started oral azithromycin (500 mg daily) on day 1. Ten hours later, however, his consciousness level and systolic blood pressure had gradually deteriorated. We diagnosed the patient with septic shock and started intravenous infusion of noradrenaline.
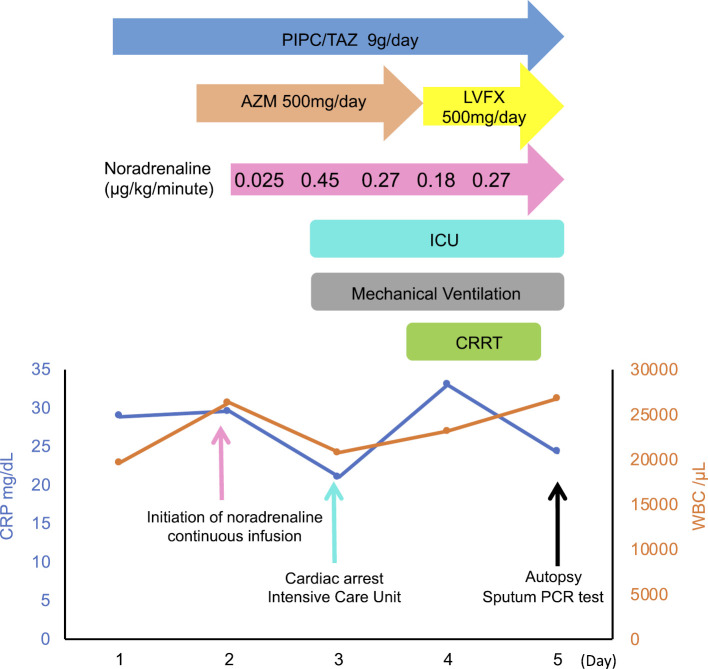
On day 2, he gradually developed bradycardia and went into cardiac arrest, for which we promptly started cardiopulmonary resuscitation for 40 minutes, with 2 defibrillation attempts and 7 adrenaline bolus doses. After the return of spontaneous circulation, he was transferred to the intensive-care unit and was started on hydrocortisone phosphate 200 mg for severe pneumonia and 5.0 g of gamma globulin for severe infection (Fig. 2). On day 3, we started continuous renal replacement therapy (CRRT) and were able to reduce the noradrenaline dose because his blood pressure gradually improved. Although no definitive diagnosis was made at this time, we changed oral azithromycin to levofloxacin (500 mg daily) to enhance the treatment for Legionnaires' disease.
Figure 2.
The clinical course. WBC: white blood cell count, CRP: C-reactive protein, LVFX: levofloxacin, AZM: azithromycin, PIPC/TAZ: piperacillin/tazobactam, ICU: intensive care unit, CRRT: continuous renal replacement therapy, PCR: polymerase chain reaction
On day 5 of hospitalization, his blood pressure suddenly dropped. We promptly discontinued CRRT, but he eventually died. Legionella pneumophila serotype 1 was detected on sputum multiplex polymerase chain reaction (PCR) after his death. An autopsy showed lobar pneumonia and hepatic lobular central congestion and shock liver (Fig. 3). Therefore, we definitively concluded Legionnaires' disease as the cause of death.
Figure 3.
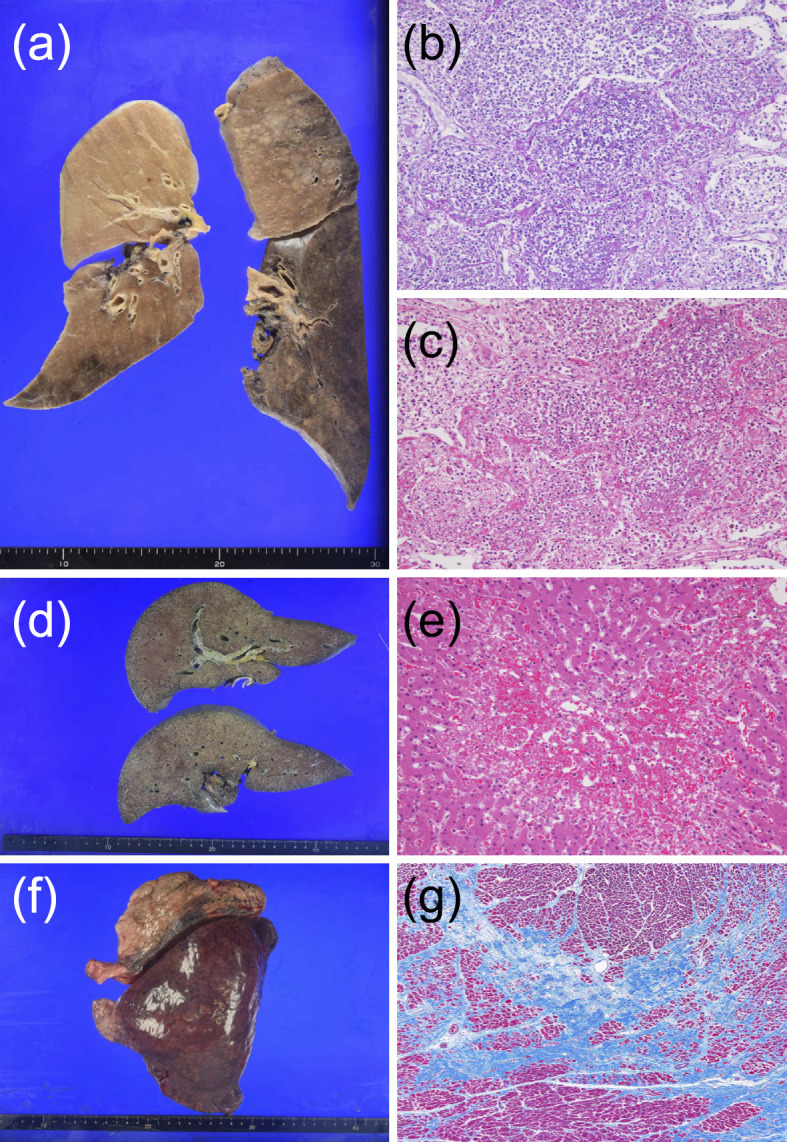
Autopsy findings. (a-c) Histologically, there was strong lobar pneumonia in the lower lobe and lingular segment of the left lung. The left lower lobe contained macrophages, and neutrophils were filling the alveolus. Bleeding could be seen in the lingular segment. The tracheal contents of the left lung showed brown sputum. We concluded that these findings indicated lobar pneumonia caused by Legionella. Yellow pleural effusion (100 mL) was detected in both lungs, and there was no pleural adhesion. (d, e) The liver showed centrilobular congestion and necrosis without an inflammatory response, which was compatible with shock liver. (f, g) There were no new heart infarctions. Afferent cardiac hypertrophy due to hypertension was shown. The absence of heart infarction indicated that the cause of creatine kinase elevation was lower limb ischemia.
Discussion
The incidence of Legionnaires' disease has been increasing annually, with the spread of rapid assay kits for detecting antigens in urine samples. According to a recent report from the Japanese Ministry of Health, Labour and Welfare, 96% of patients with Legionnaires' disease were diagnosed by the presence of urinary antigens (1). In the United States, 95% of Legionnaires' disease cases were diagnosed by the presence of urinary antigens (2), and Legionnaires' disease was observed in only 2% to 9% of community-acquired pneumonia cases (2). However, there have been only a few reports (3,4) on Legionnaires' disease in patients on maintenance HD, probably because most HD patients are anuric. Furthermore, since 1994, there have been no studies on the prevalence of Legionnaires' disease in patients with end-stage renal disease (ESRD) (5). Notably, the rate of ESRD was reported to be 21 times higher than that of other cases and has the third-highest prevalence rate, following acquired immune deficiency syndrome and hematologic malignancies (5). The present case had several risk factors, such as male sex, a smoking history, diabetes, and ESRD. We reported this case to the health center covering his residential area, but no Legionella species were detected in his house or in its surrounding areas. In addition, there have been no other cases of Legionnaires' disease at the clinic where he had been receiving HD, and no source of Legionella infection could be identified.
Several clinical prediction tools for legionellosis have been developed. The Winthrop University Hospital criteria are complicated and difficult to use for HD patients, as acute kidney injury and hematuria are included in the evaluation items (6). The Community-Based Pneumonia Incidence Study Group Scoring System would also not be useful for HD patients because it contains an evaluation item for creatinine elevation (7). In a study on possible clinical predictors for Legionella, a multivariate analysis found 6 independent diagnostic factors, including a high body temperature, absence of sputum production, low serum sodium concentration, high level of lactate dehydrogenase, high level of C-reactive protein, and low platelet count; a Legionella diagnosis was 66% likely if ≥4 factors were present and 3% likely if ≤1 was present (8). In the present case, more than four of these factors were present. When these 6 factors were applied to cases of community-acquired pneumonia, the negative predictive value for Legionella was 99.4% if ≤1 factor was present (9). This predictive tool was thus considered useful and reliable for making an exclusion diagnosis (9).
Another clinical prediction tool for Legionnaires' disease was created using a multivariate analysis in Japan (10). Other studies have suggested that Legionnaires' disease should be considered when there are elevated levels of aspartate aminotransferase and alanine transferase, which indicate hepatocyte damage (11), or creatine kinase, which indicates rhabdomyolysis (12). Therefore, in patients with pneumonia and anuria, a clinical prediction tool should be used to improve the pretest probability of Legionnaires' disease for the further performance of appropriate examinations.
The urinary antigen test is suitable only for legionellosis serotype 1, with a sensitivity of about 70% to 90% (13). Furthermore, the test cannot be performed in patients with pneumonia who have no urine output. The PCR method for sputum is the gold standard for the diagnosis of Legionnaires' disease, but the procedure is complicated (14). In contrast, compared with the conventional PCR method, the loop-mediated isothermal amplification (LAMP) method was suggested to be more accurate, simpler, and faster (15). Therefore, the LAMP method, which can be performed quickly and easily, should be considered to precisely diagnose Legionnaires' disease. This approach uses serum in place of a urine antigen test kit. In Japan, there have been two reported cases (16) of Legionnaires' disease that were confirmed by the LAMP method. At present, the PCR method is not covered by Japanese national health insurance for the diagnosis of legionellosis. The LAMP method, by contrast, has been covered since 2011. Both the LAMP and PCR approaches should be made available in general hospitals.
Although the severity of community-acquired pneumonia has been evaluated by a few severity scores (i.e. CURB-65 system and A-DROP system), it may be underestimated (17,18). Conventionally, levofloxacin, which is a fluoroquinolone antibiotic, is bactericidal and has been considered the first-line drug for Legionnaires' disease (19-21). However, the latest research showed that the effects were not markedly different between levofloxacin and intravenous azithromycin, which is a macrolide antibacterial agent (22). Ciprofloxacin, pazufloxacin, levofloxacin, garenoxacin, moxifloxacin, clarithromycin, azithromycin, and rifampicin were all shown to have relatively good antibacterial activity against Legionella with no resistant strains (23). Although a previous study showed that the efficacy of moxifloxacin was not inferior to that of conventional intravenous drugs or oral drugs, that study enrolled only a small number of cases (24). Furthermore, there has been a report on the oral administration of moxifloxacin in dialysis patients (25). Among patients with Legionnaires' disease, moxifloxacin may be a good choice for those who have no gastrointestinal symptoms and need strict fluid control.
The present case likely had accompanying heart failure during maintenance HD. Therefore, we were cautious about exacerbating the heart failure due to the water load from the administration of a new quinolone injection. In addition, because he had no history of gastrointestinal symptoms or disease and was in a good condition and able to take medications orally, we decided to start treatment with oral azithromycin. Of note, no intestinal edema or gastrointestinal bleeding was found at the autopsy. Legionnaires' disease is often associated with multiple gastrointestinal symptoms, including nausea, vomiting, and secretory diarrhea. Therefore, intravenous drugs may be the better choice in patients with gastrointestinal symptoms. For intravenous drugs, the volume to be administered is 750 mL/day for azithromycin and 150 mL/day for levofloxacin.
In summary, HD patients with lobar pneumonia should be treated using a clinical prediction tool, keeping in mind the possibility of Legionnaires' disease, which should be promptly treated. The LAMP method, which can be quickly and easily performed, should be considered in order to diagnose Legionnaires' disease, if necessary.
Akira Kawashima, a clinical trainee, received the encouragement award for his presentation of this case at the 655th Kanto Regional Assembly of The Japanese Society of Internal Medicine.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Legionellosis, January 2008-December 2012. IASR 34: 155-157, 2013. [Google Scholar]
- 2. Cunha BA, Burillo A, Bouza E. Legionnaires' disease. Lancet 23: 376-385, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Bernat A, Garrigos E, Martin J, Moll R, Perez A. Legionnaires' disease outbreak in a haemodialysis unit. Nephrol Dial Transplant 9: 217-218, 1994. [PubMed] [Google Scholar]
- 4. Shimizu Y, Nagase K, Kadono KN, et al. The haemodialysis patient who developed acute respiratory distress syndrome after a trip to a hot spring spa. Nephrol Dial Transplant 14: 455-457, 1999. [DOI] [PubMed] [Google Scholar]
- 5. Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch Intern Med 154: 2417-2422, 1994. [PubMed] [Google Scholar]
- 6. Gupta SK, Imperiale TF, Sarosi GA. Evaluation of the Winthrop-University Hospital criteria to identify Legionella pneumonia. Chest 120: 1064-1071, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Fernández-Sabé N, Rosón B, Carratalà J, Dorca J, Manresa F, Gudiol F. Clinical diagnosis of Legionella pneumonia revisited: evaluation of the Community-Based Pneumonia Incidence Study Group scoring system. Clin Infect Dis 37: 483-489, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Fiumefreddo R, Zaborsky R, Haeuptle J, et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med 9: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolliger R, Neeser O, Merker M, et al. Validation of a prediction rule for Legionella pneumonia in emergency department patients. Open Forum Infect Dis. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyashita N, Horita N, Higa F, et al. Validation of a diagnostic score model for the prediction of Legionella pneumophila pneumonia. J Infect Chemother 25: 407-412, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Cunha A, Jensen L, Calubrian O, Rosenbaum GS, Klein NC. Cytomegalovirus and legionella species as the cause of liver enzyme elevations in haemodialysis patients. J Hosp Infect 14: 95-98, 1989. [DOI] [PubMed] [Google Scholar]
- 12. Sopena N, Sabrià-Leal M, Pedro-Botet ML, et al. Comparative study of the clinical presentation of Legionella pneumonia and other community-acquired pneumonias. Chest 113: 1195-1200, 1998. [DOI] [PubMed] [Google Scholar]
- 13. Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev 28: 95-133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avni T, Bieber A, Green H, et al. Diagnostic accuracy of PCR alone and compared to urinary antigen testing for detection of Legionella spp.: a systematic review. J Clin Micro 54: 401-411, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu X, Mo ZY, Zhao HB, et al. LAMP-based method for a rapid identification of Legionella spp. and Legionella pneumophila. Appl Microbial Biotechnol 92: 179-187, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Kimura M, Kawaguchi T, Kadomura M, et al. Diagnosis of Legionella pneumonia based on Legionella urinary antigen testing of serum instead of urine in a hemodialysis patient. J Jpn Soc Dial Ther 50: 641-646, 2017. [Google Scholar]
- 17. Simonsen Ø, Ringstad J. Severity of illness scoring systems in community-acquired Legionella pneumonia. Crit Care 14(Suppl 2): 25, 2010. [Google Scholar]
- 18. Haranaga S, Higa F, Tateyama M, et al. [A-DROP system might result in underestimation of severity of cases with Legionnaires' disease]. Nihon Kokyuki Gakkai Zasshi 46: 351-355, 2008(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 19. Mykietiuk A, Carratalà J, Fernández-Sabé N, et al. Clincal outcomes for hospitalized patients with Legionella pneumonia in the antigenuria era: the influ ence of levofloxacin therapy. Clin Infect Dis 40: 794-799, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Sebrià M, Pedro-Botet ML, Gómez J, et al. Fluoroquinolones vs macrolides in the treatment of Legion naires disease. Chest 128: 1401-1405, 2005. [DOI] [PubMed] [Google Scholar]
- 21. Blázquez Garrido RM, Espinosa Parra FJ, Alemany Francés L, et al. Antimicrobial chemotherapy for Legionnaires disease: levofloxacin versus macrolides. Clin Infect Dis 40: 800-806, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Vidal C, Sanchez-Rodriguez I, Simonetti AF, et al. Levofloxacin versus azithromycin for treating legionella pneumonia: a propensity score analysis. Clin Microbiol Infect 23: 653-658, 2017. [DOI] [PubMed] [Google Scholar]
- 23. Miyashita N, Kobayashi I, Higa F, et al. In vitro activity of various antibiotics against clinical strains of Legionella species isolated in Japan. J Infect Chemother 24: 325-329, 2018. [DOI] [PubMed] [Google Scholar]
- 24. Garau J, Fritsch A, Arvis P, Read RC. Clinical efficacy of moxifloxacin versus comparator therapies for community-acquired pneumonia caused by Legionella spp. J Chemother 22: 264-266, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Takakuwa O, Yamamoto T, Kawai A, Torii M, Nakamura A. [Successful treatment of Legionella pneumonia with moxifloxacin in a hemodialysis patient]. Nihon Kokyuki Gakkai Zasshi 47: 625-630, 2009(in Japanese, Abstract in English). [PubMed] [Google Scholar]