Abstract
Background: In a previous clinical study, the authors evaluated the potential of antitenascin C monoclonal antibody (mAb) 81C6 labeled with 211At via the prosthetic agent N-succinimidyl 3-[211At]astatobenzoate (SAB) for the treatment of primary brain tumors. Although encouraging results were obtained, labeling chemistry failed while attempting to escalate the dose to 370 MBq. The goal of the current study was to develop a revised procedure less susceptible to radiolysis-mediated effects on 211At labeling that would be suitable for use at higher activity levels of this α-emitter.
Materials and Methods: Addition of N-chlorosuccinimide to the methanol used to remove the 211At from the cryotrap after bismuth target distillation was done to thwart radiolytic decomposition of reactive 211At and the tin precursor. A series of 11 reactions were performed to produce SAB at initial 211At activity levels of 0.31–2.74 GBq from 50 μg of N-succinimidyl 3-trimethylstannylbenzoate (Me-STB), which was then reacted with murine 81C6 mAb without purification of the SAB intermediate. Radiochemical purity, immunoreactive fraction, sterility, and apyrogenicity of the 211At-labeled 81C6 preparations were evaluated.
Results: Murine 81C6 mAb was successfully labeled with 211At using these revised procedures with improved radiochemical yields and decreased overall synthesis time compared with the original clinical labeling procedure.
Conclusions: With 2.74 GBq of 211At, it was possible to produce 1.0 GBq of 211At-labeled 81C6 with an immunoreactive fraction of 92%. These revised procedures permit production of 211At-labeled mAbs suitable for use at clinically relevant activity levels.
Keywords: α-particles, Astatine-211, monoclonal antibodies, radioimmunotherapy, targeted radiotherapy
Introduction
Targeted α-particle radiotherapy is an emerging modality for cancer treatment, particularly in the settings of minimal residual disease and micrometastases.1,2 One of the most attractive α-emitters for this purpose is the 7.2-h half-life radiohalogen 211At, which emits one α-particle per decay as well as polonium K x-rays that permit convenient monitoring of therapy doses in patients.
Astatine-211 labeled monoclonal antibodies (mAbs) and fragments thereof have advanced to clinical trial, for example, in patients with ovarian carcinoma, in which the therapeutic potential of 211At-labeled MX-35 F(ab′)2 has been evaluated.3,4 The authors' own efforts in this regard involved 211At-labeled chimeric 81C6 (ch-81C6), an antitenascin mAb, which was used to treat recurrent brain tumor patients by direct injection into their surgically created resection cavity.5 The results were very encouraging, with a median survival of 52 weeks observed in patients with high-grade glioblastoma (GBM) compared with 31 weeks for the best alternative treatment at that time. Moreover, 2 of 14 recurrent GBM patients survived for nearly 3 years.
Despite the promising results obtained with 211At-labeled ch-81C6, the trial was discontinued because of difficulties in being able to reliably perform the labeling chemistry once the 370 MBq dose level was reached. In the clinical labeling procedure, the prosthetic agent N-succinimidyl 3-[211At]astatobenzoate (SAB) was synthesized by reaction of 211At with 2 mg (4 μmol) of N-succinimidyl 3-(n-butyl)stannyl benzoate (Bu-STB) using tert-butyl hydroperoxide as the oxidant and chloroform as the solvent.6
When higher activity levels of labeled mAb were needed, both the radiochemical yield for the synthesis of SAB and the efficiency of conjugation of SAB to the mAb decreased substantially. In addition, after reaction of the SAB with the mAb, 40%–60% of the 211At activity was retained on the walls of the reaction vessel. Finally, the immunoreactivity of the 211At-labeled mAb became unacceptably low, necessitating an evaluation of the potential causes for the unfortunate activity-dependent decline in radiochemical tractability.
A series of four studies were performed to investigate the potential effects of α-particle-mediated radiolysis on 211At radiochemistry, which the authors hypothesized were responsible for the problems noted above. In the first, it was discovered that increasing activity levels of 211At in chloroform, the solvent used in their clinical procedure, led to the generation of chorine radicals, which consumed the Bu-STB precursor, such that under the reaction conditions required to produce 370 MBq of 211At-labeled mAb, <0.5% of the tin precursor would not be consumed by radiolytically generated chlorine radicals and be available for reaction with 211At.7 This led to the selection of methanol as a preferable solvent for performing astatodestannylation reactions.7
However, a subsequent investigation on the effects of variables, including pH and oxidant on SAB synthesis, demonstrated that even in methanol, radiation dose-dependent decreases in SAB synthesis occurred beginning at about 3500 Gy due to the generation of reducing species.8 Having discovered that this was related to in situ generation of [211At]astatide,9 a species not suitable for electrophilic astatination, they developed a 211At stabilization strategy that consists of introduction of an oxidant into the methanol solution used to trap the 211At during distillation of the cyclotron target. Using N-chlorosuccinimide (NCS) for this purpose, astatodestannylation reactions could be performed in >80% yield even after exposure to doses >100,000 Gy.10 In contrast, in parallel studies performed without NCS stabilization in methanol, SAB yields were <20% after exposure to doses >5000 Gy.10
The goal of the current study was to incorporate the lessons learned above about the effects of α-particle-mediated radiolysis on 211At radiochemistry into the synthesis of a 211At-labeled mAb at activity levels and with specifications compatible with the exigencies of clinical application. The steps involved, from 211At production to final product, are outlined in Figure 1. In addition, the authors note changes in cyclotron and distillation hardware that have led to more robust 211At production and purification capabilities, respectively.
FIG. 1.
Two-step procedure for labeling 81C6 mAb with 211At involving synthesis of SAB from tin precursor followed by reaction of SAB with mAb. mAb, monoclonal antibody; SAB, N-succinimidyl 3-[211At]astatobenzoate.
Materials and Methods
General
All chemicals and reagents used in the work were obtained from Sigma-Aldrich unless stated otherwise. N-succinimidyl 3-trimethylstannylbenzoate (Me-STB) was synthesized as described previously.11 High-performance liquid chromatography (HPLC) was performed using the following three systems: (1) a Beckman Gold HPLC system equipped with a Model 126 programmable solvent module, a Model 166 NM variable wavelength detector, and a Model 170 radioisotope detector and a Beckman System Gold remote interface module SS420X; data were acquired using the 32 Karat® software (Beckman Coulter, Inc., Brea, CA). Radioactivity was monitored using a ScanRam RadioTLC scanner/HPLC detector combination (LabLogic, Brandon, FL). (2) A Beckman System Gold HPLC equipped with a Model 126 programmable solvent module, a Model 168 diode array detector, a Model 170 radioisotope detector, and a Model 406 analog interface module. (3) A Beckman System Gold HPLC equipped with a pump module 126, a UV/visible detector module 168, a Bioscan radioactivity detector module 106 (Bioscan, Inc., Washington, DC), and 32 Karat software.
Analytical normal phase HPLC was performed on the first system using a Partisil 5 μ, 4.6 mm × 260 mm silica column (Grace, Columbia, MD), which was eluted in isocratic mode at 1 mL/min with 25% ethyl acetate in hexanes containing 0.1% acetic acid. Under these conditions, N-succinimidyl 3-iodobenzoate (SIB) elutes with a tR of ∼17 min. Reversed-phase HPLC was performed on the second system using a Waters Xterra 4.6 mm × 250 mm (10 μm) column, which was eluted at a flow rate of 1 mL/min using mobile phase consisted of solvent A (acetonitrile/water/acetic acid [5/95/0.1]) and solvent B (0.1% acetic acid in acetonitrile). The proportion of B was held at 10% for 10 min, then linearly increased to 28% over 10 min, then held at this for 10 min, and then linearly increased to 100% over next 20 min; under these conditions, SAB eluted with a tR of 46.8 min.
Size-exclusion chromatography was performed on the third system using a Super SW3000 TSK gel 4.6 mm × 30 cm column and a 4.6 mm × 3.5 cm guard column (Tosoh Bioscience, Montgomeryville, PA), which were equilibrated and eluted with TSK buffer (70 mM Na2HPO4, 62.5 mM NaH2PO4, and 100 mM Na2SO4), pH 6.7 at a flow rate of 0.25 mL/min with a run time of 30 min. TLC of SAB was performed using 50:50 ethyl acetate:hexanes as the mobile phase; under these conditions SIB eluted with an Rf of 0.6. The TLC plate was analyzed either by the radio-TLC scanner (ScanRAM noted above) or by cutting into small strips and counting them for 211At activity in a γ-counter (LKB 1282; Wallac, Finland or Perkin Elmer Wizard II, Shelton, CT). Astatine-211 activity was measured using a CRC-7 dose calibrator (Capintec, Pittsburgh, PA) at the 133Xe setting for higher doses, multiplying the values by a correction factor of 2.3. Lower levels of 211At activity were counted using the automated γ-counters described above.
81C6 antibody
The characteristics of murine antitenascin mAb 81C6 (mu-81C6) and its use in clinical targeted radioimmunotherapy trials have been described previously.12,13 For some experiments, clinical grade antibody (Product Codes: BV1/PDS002, Lot No. FIL066G01), produced under cGMP conditions for Bradmer Pharmaceuticals for its planned Phase 3 131I-labeled 81C6 trial, was used.
Astatine-211 production and purification
Astatine-211 was produced on the Duke University CS-30 cyclotron via the 209Bi(α, 2n)211At reaction by bombarding natural bismuth metal targets with 28 MeV α-particles essentially as described previously,6,9 except for the following changes that are now part of their 211At production and purification standard operating procedures: To provide more α-particle beam current capability at lower ion source currents as well as a cleaner ion source operation, the original ion source power supply in the cyclotron was replaced with an Magna-Power power supply (Model TS3000-4.8; Flemington, NJ). The new supply utilizes solid state components rather than obsolete, hard to find components and obviates the need for water cooling.
In addition, the amount of bismuth target material was reduced from 1.25 to 0.01–0.20 g. In the distillation process, 6 ft of 0.0625′′ ID × 1/8′′ OD, clinical grade, clear PTFE tubing was used for trapping 211At instead of 0.040′′ ID × 1/16′′ OD opaque PEEK tubing of same length. The argon flow rate was increased from 50 to 110 mL/min at the onset of distillation. In addition, the distillation temperature was reduced from 715°C to 700°C. Based on previous studies evaluating the effects of dose and dose rate on 211At chemical tractability,10 the 211At was eluted from the PTFE tubing using a solution of N-chlorosuccinimide in methanol (200 μg in 0.5 mL) and kept at 20°C until needed.
Synthesis of SAB at high 211At activity levels
These studies were performed with 211At produced and purified using the modifications described above. Me-STB was dissolved in chloroform, and an aliquot containing 50 μg (130 nmol) was transferred to the bottom of a one-dram vial, and chloroform was evaporated with a gentle stream of argon. A solution of 211At in methanol containing 0.2 mg/mL of NCS (500 μL; 0.3–2.7 GBq) was added to the above vial, and the mixture was left at 20°C for 20 min with occasional swirling. A solution of NCS in methanol (4 μL of 44.3 mg/mL; 1.3 μmol) was added, and the reaction was allowed to continue for another minute to convert any unconsumed Me-STB to its chloro derivative. In some cases, an aliquot of the reaction mixture at this stage was analyzed by normal phase HPLC or TLC.
Production of 211At-labeled mu-81C6 mAb
Methanol from the above reaction mixture was evaporated with a gentle stream of argon. The pH of the mu-81C6 in phosphate buffer was adjusted to 8.5 by the addition of an equivalent volume of saturated sodium borate. The resultant solution (1.0–1.3 mL) containing 10 mg of mu-81C6 was added to the vial containing the SAB and the mixture was incubated at 20°C for 15 min. An equivalent volume (1.0–1.3 mL) of 0.2 M glycine in saturated borate was added, and the reaction mixture was incubated for another 3 min at 20°C.
Astatine-211-labeled mu-81C6 was isolated by gel filtration over a PD10 column (GE Healthcare, Piscataway, NJ) or a Sephadex column prepared in house6 using 0.05 M phosphate buffer as mobile phase. In both cases, the columns were preconditioned with human serum albumin as reported6 to minimize nonspecific binding. In the case of the three preparations performed under asceptic conditions to evaluate the sterility and pyrogenicity of the final product produced with these procedures, the fractions containing the 211At-labeled mu-81C6 were pooled and passed through a 0.22-μm sterile filter into a sterile, pyrogen-free glass vial.
Evaluation of 211At-labeled mu-81C6 radiochemical purity
Four methods were used to determine radiochemical purity of the 211At-labeled mu-81C6 preparations. ITLC was performed using glass microfiber sheet impregnated silica gel strips (Varian, Lake Forest, CA) developed with phosphate-buffered saline (PBS), pH 7.4. Under these conditions, the labeled mAb remains at the origin (Rf = 0), and SAB elutes with an Rf = 0.7–0.8. Protein-associated 211At activity was determined by methanol precipitation. For this, 0.1 mL of 10% bovine serum albumin and 0.5 mL of methanol were added to an aliquot (∼5 ng) of 211At-labeled mu-81C6 in triplicate, and incubated at 4°C for 10 min. After centrifugation, the pellets and supernatants were counted and the percent of total radioactivity in the pellet was calculated as the protein-associated radioactivity.
Sodium dodecyl sulfate-polyacrylamide gel elcetrophoresis (SDS-PAGE) of 211At-labeled mu-81C6 was performed under nonreducing conditions. A 3.7 kBq aliquot in 10 μL of PBS was mixed with 20 μL of Laemmli buffer (BioRad, Hercules, CA) and incubated at 95°C for 10 min. The incubates were cooled to 20°C, spun, and 10 μL of the supernatant was added to wells of Mini-PROTEAN TGX Any kD gels (BioRad) and electrophoresed for 40 min at 140 V. Radioactivity on the dried gels was visualized using a Storage Phosphor System Cyclone Plus phosphor imager (Perkin-Elmer Life and Analytical Sciences, Downers Grove, IL) and analyzed using version 5.0 software provided by the manufacturer. Finally, size-exclusion HPLC was performed by injecting an aliquot (∼3.7 MBq) of 211At-labeled mu-81C6 under conditions described above.
Determination of immunoreactive fraction
Immunoreactive fraction of SAB-mu-81C6 was determined by Lindmo method using a previously described assay.6 In brief, streptavidin-coated MPG magnetic beads (PureBiotech, Middlesex, NJ, Controlled Pore Glass; Lincoln Park, NJ) were reacted with biotinylated recombinant tenascin CD fragment containing the 81C6 binding epitope or for nonspecific binding determination, with biotinylated human serum albumin. Three doubling concentrations of each type of beads were incubated with 211At-labeled mu-81C6 (∼5 ng) in triplicate in 115 mM phosphate buffer, pH 7.4, containing 0.05% bovine serum albumin, and 0.05% Brij 35 for 30 min at 20°C. The beads were isolated using a magnetic separator, and radioactivity in both the beads and the supernatants was counted using an automated gamma counter. The immunoreactive fraction was calculated using the Lindmo method.14
Sterility and pyrogenicity assays
These assays were performed on three preparations of 211At-labeled mu-81C6 by the Duke University Medical Center Clinical Radiopharmacy. The standard USP sterility test was used. The level of pyrogens was determined using the USP Limulus amebocyte lysate endotoxin test using Endosafe PTS cartridges of 0.005 eu/mL sensitivity (Charles River Laboratories, Charleston, SC).
Analysis of tin content in the final formulations
After allowing for decay, aliquots of selected 211At-labeled mu-81C6 preparations were analyzed for tin content by ICP-MS using a VG Plasma Quad-3 spectrometer, equipped with the S-option pump for increased sensitivity and a UV laser-ablation microprobe for spot analyses, located at the Duke University Nicholas School of Environment.
Biodistribution in athymic mice bearing subcutaneous D-54 MG glioma xenografts
Animal studies were performed following guidelines established by the Duke University Institutional Animal Care and Use Committee. Athymic mice bearing subcutaneous D-54 MG glioma xenografts were generated as described before.15,16 Groups of five mice were injected via the tail vein with 222 kBq (∼20 μg) of 211At-labeled mu-81C6 in PBS. At 2, 4, 16, and 24 h after administration, blood and urine samples were collected from each mouse. Mice were killed with an overdose of isoflurane and dissected. Blood and urine as well as blot-dried tumor and other solid organs were isolated, weighed, and counted in an automated gamma counter along with 211At activity injection standards. From these, percentage-injected dose (%ID) per organ and %ID per gram of tissue were calculated.
Results
211At production and purification
With the modifications in cyclotron hardware and reduction in the amount of bismuth deposited on the aluminum back plate constituting the cyclotron target, 1-h irradiations at a beam current of 100 μA resulted in the production of up to 2.8 GBq of 211At. Use of the thinner bismuth target along with the modification in several distillation parameters noted above resulted in an average decay-corrected distillation yield of 61.8 ± 8.2% (n = 12) with about 15% of the radioactivity remaining in the target. In more recent runs including some of those described herein, the argon carrier gas flow had been optimized for the increased diameter of the cryotrap tubing, which resulted in about 65%–75% recovery of the 211At activity. Using optimized conditions, about 1.65 GBq of distilled 211At, from a target that was irradiated for 1 h, could be obtained.
Synthesis of 211At-labeled mu-81C6
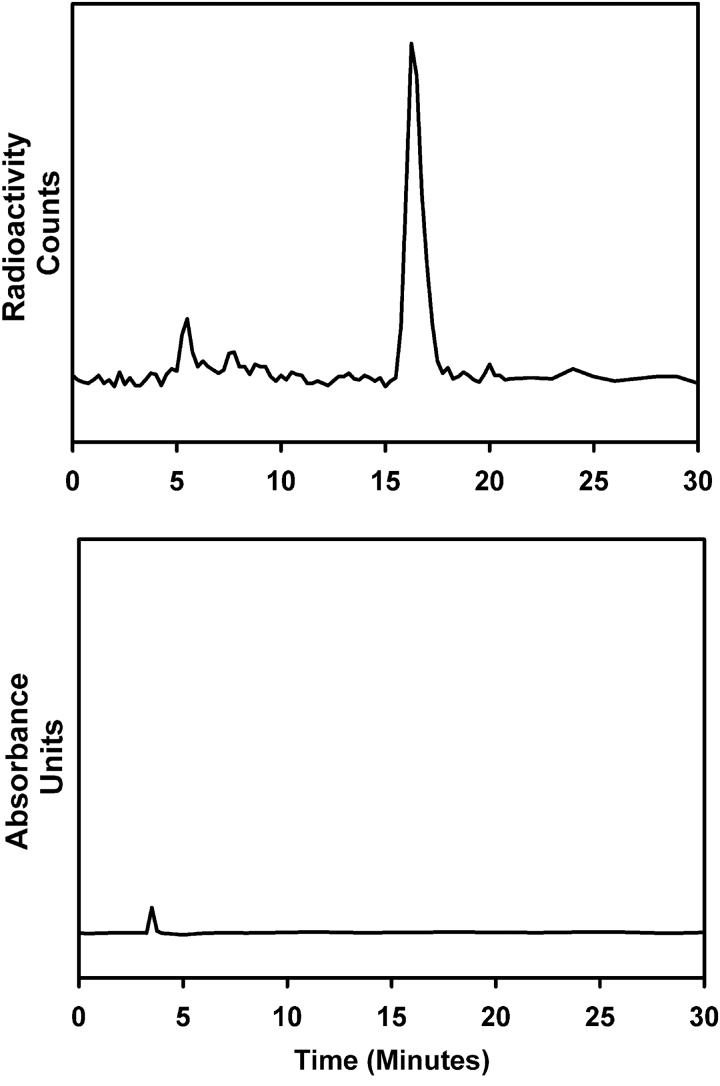
A total of 11 batches of labeled mu-81C6 were produced starting from 333 MBq to 2.7 GBq (∼9–74 mCi) of 211At activity (Table 1). Of these, batches 5–11 were prepared using the clinical grade mu-81C6 obtained from Bradmer. In the authors' revised procedure, SAB was not purified before reaction with the mu-81C6 mAb. HPLC analysis of the SAB intermediate was done for some batches and a typical chromatogram is shown in Figure 2, which indicates that 211At activity was predominantly (>95%) associated with a single radioactive species, corresponding to the retention time of SIB, with no UV peaks observed. TLC of the reaction mixture indicated a radiochemical purity of 86.2 ± 12.3% (n = 6).
Table 1.
Summary of 211At-Labeled Mu-81C6 Antibody 81C6 Production Runs
| Radiochemical purity (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp # | Initial activity (MBq) | To SEC column (MBq) | Labeled mAb (MBq) | mAb conjugation yielda(%) | Overall yieldb(%) | ITLC | MeOH precipitation | SEC | SDS-PAGE | IRF (%) |
| 1 | 337.8 | 166.1 | 70.7 | 44 | 25 | 95.0 | 98.3 | ND | ND | ND |
| 2 | 408.5 | 263.8 | 112.9 | 44 | 32 | 100.0 | 99.1 | ND | 94.5 | ND |
| 3 | 314.9 | 178.7 | 81.8 | 47 | 30 | 98.5 | 99.4 | ND | 96.0 | ND |
| 4 | 429.9 | 246.8 | 99.2 | 41 | 27 | 99.4 | 99.5 | ND | 97.0 | ND |
| 5 | 583.1 | 437.3 | 213.9 | 50 | 42 | 99.5 | 98.2 | ND | 100.0 | 92 |
| 6 | 822.1 | 552.4 | 210.2 | 39 | 30 | 97.8 | 98.4 | ND | 100.0 | 98 |
| 7 | 2033.1 | 1239.5 | 694.5 | 58 | 38 | ND | 84.5 | ND | ND | 93 |
| 8 | 523.6 | 437.3 | 303.4 | 71 | 65 | ND | 95.6 | 100.0 | ND | 85 |
| 9 | 1678.3 | 1169.2 | 704.1 | 62 | 47 | ND | 97.6 | 98.6 | ND | 80 |
| 10 | 2740.2 | 1793.8 | 1024.5 | 58 | 42 | ND | 91.6 | 98.9 | ND | 92 |
| 11 | 425.5 | 255.3 | 254.9 | 100 | 69 | ND | ND | ND | ND | ND |
Calculated as fraction of activity added to the size-exclusion column eluted as labeled mAb; decay corrected.
Calculated as fraction of initial activity isolated as labeled mAb; decay corrected.
mAb, monoclonal antibody; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
FIG. 2.
Reversed-phase HPLC chromatogram for SAB synthesis reaction; radioactivity trace (top) and UV trace (bottom). HPLC, high-performance liquid chromatography.
The average decay-corrected radiochemical yield for the conjugation of SAB to mu-81C6 was 56 ± 18% and the overall yield of 211At-labeled mu-81C6 based on the initial 211At activity was 41 ± 15%. Because of the potential for radiolytic effects to influence the labeling chemistry, the correlation between initial 211At activity levels and the overall yield for the 211At-labeled mAb was investigated. As shown in Figure 3, there was no correlation between 211At-labeled mu-81C6 yield and the level of 211At activity used for SAB synthesis. The total duration of synthesis was about 75 min starting from the time the distilled 211At was extracted into NCS/methanol solution. Thus, with these procedures, 364 MBq of 211At-labeled mu-81C6 can be obtained starting from 1 GBq of 211At. To date, the authors have produced a maximum of 1 GBq (28 mCi) of 211At-labeled mu-81C6 with a specific activity of 100 MBq/mg, which is more than adequate for the requirements of the clinical protocol.
FIG. 3.
Plot of overall decay corrected yield of 211At-labeled mu-81C6 as a function of initial 211At activity added to the SAB reaction mixture. No significant correlation was observed (r2 = 0.05).
Quality control
With the exception of the last production run, radiochemical purity was determined by ITLC, methanol precipitation,17 size-exclusion HPLC, and/or SDS-PAGE. Typical results for size-exclusion HPLC and SDS-PAGE are presented in Figures 4 and 5, respectively. In most cases, the radiochemical purity of the labeled mAb was >95%. The immunoreactive fraction of six batches of 211At-labeled mu-81C6 prepared from a clinical grade lot of mAb was 89.9 ± 6.2%. After allowing for radioactive decay, 10 batches of 211At-labeled mu-81C6 were analyzed for tin content by ICP-MS. There was an outlier batch which had a tin content of 1087 ppm. The average tin content of the remaining nine batches was 194 ± 81 ppm. Three batches of 211At-labeled mu-81C6 were subjected to sterility and pyrogen testing and found to be sterile and pyrogen free (<0.125 EU/mL).
FIG. 4.

Size-exclusion HPLC profile of 211At-labeled mu-81C6 mAb.
FIG. 5.

Nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis phosphor image profile of 211At-labeled mu-81C6 mAb with molecular weight standards provided for comparison.
Tissue distribution of 211At-labeled mu-81C6
A tissue distribution experiment was performed to evaluate the in vivo behavior of 211At-labeled mu-81C6 to confirm that changes in the labeling procedures did not have an adverse effect on the tumor targeting capacity or in vivo stability of the labeled mAb. As summarized in Table 2, uptake in D-54 MG subcutaneous human glioma xenografts reached 19.89 ± 2.97% ID/g at 16 h postinjection with no significant decline in tumor localization observed at the 24 h time point. Uptake in blood and normal organs was consistent with the behavior of intact mAbs during this time interval (2–24 h), selected to cover the time, during which more than 90% of 211At atoms would decay.
Table 2.
Uptake of 211At Activity After Intravenous Administration of 211At-Labeled Murine 81C6 in Athymic Mice Bearing D54-MG Subcutaneous Xenografts
| % Injected dose/grama |
||||
|---|---|---|---|---|
| Tissue | 2 h | 4 h | 16 h | 24 h |
| Liver | 8.95 ± 1.91 | 9.55 ± 1.89 | 5.79 ± 2.28 | 5.62 ± 1.47 |
| Spleen | 6.69 ± 1.12 | 7.83 ± 1.89 | 7.18 ± 1.87 | 7.30 ± 1.37 |
| Lung | 14.94 ± 1.79 | 11.28 ± 2.09 | 11.17 ± 2.57 | 10.69 ± 1.52 |
| Heart | 8.51 ± 1.98 | 7.00 ± 1.28 | 5.49 ± 1.43 | 5.42 ± 0.80 |
| Kidney | 8.15 ± 0.58 | 7.12 ± 0.66 | 5.78 ± 1.03 | 5.70 ± 1.16 |
| Stomach | 3.97 ± 0.48 | 6.60 ± 1.57 | 5.32 ± 0.60 | 8.15 ± 3.52 |
| Sm. Int. | 2.82 ± 0.11 | 2.71 ± 0.20 | 2. 35 ± 0.43 | 2.86 ± 0.42 |
| Lg. Int. | 1.39 ± 0.15 | 1.5 ± 0.25 | 1.46 ± 0.25 | 1.98 ± 0.35 |
| Thyroidb | 1.18 ± 0.34 | 0.86 ± 0.35 | 0.56 ± 0.14 | 0.80 ± 0.25 |
| Muscle | 0.96 ± 0.19 | 0.86 ± 0.20 | 0.98 ± 0.43 | 1.31 ± 0.21 |
| Blood | 28.84 ± 1.93 | 21.11 ± 2.69 | 14.28 ± 3.11 | 15.65 ± 3.67 |
| Bone | 3.10 ± 0.51 | 3.18 ± 0.57 | 3.22 ± 1.19 | 3.16 ± 0.53 |
| Brain | 1.18 ± 0.26 | 0.79 ± 0.19 | 0.72 ± 0.16 | 0.75 ± 0.11 |
| Tumor | 8.30 ± 1.72 | 10.78 ± 0.80 | 19.89 ± 2.97 | 19.40 ± 4.12 |
Mean ± SD (n = 5).
Injected dose per organ.
Discussion
Earlier, the authors conducted a Phase I study investigating the feasibility, safety, and efficacy of administering 211At-labeled chimeric antitenascin mAb 81C6 into surgically created resection cavities of patients with recurrent primary brain tumors.5 Encouraging responses were observed with two patients with recurrent GBM surviving for almost 3 years. The maximum tolerated dose was never reached because once the 370-MBq dose level was reached, the labeling chemistry became unreliable both in terms of yields and the quality (immunoreactive fraction) of the product. For this reason, the trial was halted, and efforts were directed at understanding the potential effects of radiolysis derived from increasing activity levels of 211At on its labeling chemistry under the conditions encountered for the production of clinically relevant levels of 211At-labeled radiopharmaceuticals.7–10
Key findings that were incorporated into the revised procedures described herein were (1) the unsuitability of chloroform as a solvent for destannylation reactions at high activity levels of 211At due to the consumption of the tin precursor; (2) acetic acid can (and should) be omitted from the reaction mixture to avoid generating reducing species in methanol at high 211At activity levels; (3) radiolytic conversion of 211At to an unreactive species for electrophilic destannylation; and (4) that this conversion can be circumvented by “preconditioning” the 211At by adding NCS to the methanol used to trap the 211At during the distillation procedure.
Having determined that chloroform was not a good solvent at high 211At activity levels because radiolytically generated chorine radicals consumed the tin precursor making it unavailable for SAB formation,7 the solvent was switched to methanol, which permitted reduction of the level of tin precursor from the 4 μmol required in high activity level runs performed in chloroform5,6 to 132 nmol in their current procedure. As a consequence of this 30-fold reduction in tin precursor, it was possible to omit the silica cartridge-based SAB purification step used previously, which decreased handling and overall synthesis time.
Moreover, without the necessity of separating SAB from the tin precursor, it was possible to replace the tributyltin precursor (Bu-STB) that was used in earlier clinical studies5,6 with the trimethyl tin analog (Me-STB), which offers two potential advantages. First, it has been reported that trimethyltin compounds are considerably less toxic than their tributyltin analogs with differences >250-fold observed in some cases.18 Second, with the larger halogen atoms iodine and astatine, reactivity of the trimethyl analog should be higher than the tributyl analog.19,20
A potential disadvantage of omitting the SAB purification step present in the original procedure is the possibility of conjugating highly lipophilic trialkylstannyl groups to mu-81C6, thereby compromising its specificity. To minimize this possibility, after the 20-min astatination reaction, the mixture was treated with a 10-fold molar excess of NCS to convert any Me-STB that might remain to its chloro derivative. Reverse-phase HPLC analyses of selected SAB production runs (Fig. 1) showed no UV absorbing peaks at the retention time corresponding to N-succinimyldyl 3-chlorobenzoate in the chromatogram, suggesting that little if any of the chlorinated active ester had been produced.
Importantly, the immunoreactive fraction of 211At-labeled mu-81C6 for tenascin in the magnetic bead assay was excellent, 89.9 ± 6.2%, which compares favorably with results obtained previously for clinical batches of 211At-labeled ch-81C66 as well as preclinical preparations of 211At-labeled mu-81C6 (80%–90%).21 Likewise, the tissue distribution of a representative batch of 211At-labeled mu-81C6 prepared without SAB purification displayed excellent tumor localizing capability and normal tissue distribution that was essentially the same as that observed for 211At-labeled ch-81C6 prepared with considerably lower 211At activity and purification of the SAB intermediate by HPLC.16
An alternate approach for labeling mAbs with 211At via trialkylstannyl precursors involves preconjugation of the precursor to the mAb and labeling the mAb in a single step.22 This method is attractive because of its simplicity, high radiochemical yields and adaptability to automated synthesis.23 Although the authors considered adapting this procedure for 211At labeling of mu-81C6, they decided to optimize the two-step procedure instead for several reasons.
Because trialkylstannyl compounds can cause neurotoxicity24 and their clinical protocol involves injection of the labeled mAb directly into GBM resection cavities in the brain,5 minimizing the potential presence of trimethyl tin in the final preparation is of critical importance. Unfortunately, in the single-step procedures, 6–7 tin groups are conjugated per mAb molecule and ∼5%–16.8%—corresponding to up to about one trimethyl tin per mAb molecule remain after the N-iodosuccinimide treatment step designed to remove unreacted trimethyl tin groups.22,25 Although not observed with MX35 and/or trastuzumab by these authors,22,25 with many mAbs including mu-81C6 (data not shown), immunoreactivity and affinity tend to deteriorate with increasing substitution level.
Experience with the antibody-drug conjugate trastuzumab-emtansine (T-DM1) illustrates some of the issues that become relevant with increasing substitutions per mAb molecule. The Food and Drug Administration (FDA)-approved version of T-DM1 involves an average of 3–3.6 DM1 moieties per mAb molecule.26 Higher DM1 substitution levels were found to destabilize the molecule and alter pharmacokinetics,27,28 making this strategy for potentially enhancing efficacy of the T-DM1conjugate unfavorable.
An additional consideration is the heterogeneity of the drug preparation. The heterogeneity of a clinical preparation of T-DM1 has been evaluated by enzymatic digestion followed by liquid chromatography/mass spectroscopy (LC/MS) analysis of the peptide fragments.29 LC/MS analysis confirmed 29 different lysine DM1 conjugation sites (9 light chain, 20 heavy chain) while showing that an additional 5 lysines also could potentially be modified. While T-DM1 was approved for human use by the FDA in 2013, defining drug heterogeneity and understanding its potential impact on efficacy and toxicity is becoming increasingly important, particularly in the context of attempting to obtain regulatory approval. For these reasons, they utilized conditions where one lysine modification per mAb was most likely.
From a practical standpoint, particularly for reagents intended for use in clinical trials, consideration of reagent shelf life is important. Although radiochemical yields obtained with trastuzumab-trimethyl tin conjugates remained constant over the ∼110-d observation period, there was some evidence for loss of tin from the mAb conjugate after about 21 d.30 In contrast, the clinical batch of mu-81C6 as well as the Me-STB precursor used in these studies were several years old, yet, provided 211At-labeled mu-81C6 in good yield and with excellent quality control characteristics.
The authors also note several changes that have been made in cyclotron hardware as well as 211At production and distillation have increased the reliability and convenience of performing clinical-level production runs. By replacing the ion source power supply with a solid-state version, α-particle beam current capability could be improved from 40 to 120 μA (electrical) at lower ion source currents (1.5–2.0 Å vs. 3.5–4.5 Å). This enabled reduction of ion source cathode deterioration and hence the likelihood of a cyclotron failure.
Melting of the bismuth target material was a frequent occurrence with their cyclotron at alpha beam currents as low as 40 μA (electrical), which often compromised distillation yields and 211At reactivity. By reducing the amount of bismuth plated on the aluminum backing by more than a factor of five, it was possible to prevent melting even after 1 h runs with beam currents (electrical) as high as 120 μA (60 μA particle). With these changes, it was possible to consistently produce up to 2.8 GBq of 211At on the target after 1 h irradiation using a beam current of 100 μA. In contrast, before these modifications, a 1.5-h irradiation at 52 μA provided 2.1 GBq of 211At.6
With the goal of increasing reliability and ease of handling, the inner diameter of the tubing used for trapping 211At during the distillation was increased mainly to prevent clogging and possible flow restriction. Moreover, because PTFE is flexible, it can be submerged in the ethanol/dry ice cold bath to a greater degree than the rigid PEEK tubing. PTFE tubing also allowed decreasing the cold bath temperature to −77°C without concomitant clogging. Another advantage is that PTFE tubing is translucent, allowing visualization of the extractant flow, facilitating monitoring of 211At recovery. The argon flow rate was increased to accommodate the increased tubing dead volume and to efficiently purge 211At from the quartz still into the cold trap. This also minimized the deposition of 211At on the still walls and the transition fitting connecting the still to the cold trap. Once argon flow was optimized, distillation yields were comparable to those reported previously6; however, the above alterations made the 211At distillation procedure more convenient and easier to monitor.
Conclusions
Using the previous work evaluating the effects of radiolysis of 211At as a guide, the authors developed a revised two-step procedure for labeling mAbs with 211At. The maximum level of 211At-labeled mu-81C6 that was prepared was about 1 GBq, more than twice the level that was possible with the previous procedure.5 Radiochemical yields were independent of initial 211At activity up to 2.74 GBq, the highest level studied, and the total synthesis time, starting from delivery of the 211At to the clinical labeling laboratory, was reduced to 75 min. Batches of 211At-labeled mu-81C6 prepared using these procedures were shown to be sterile and pyrogen free, and retain excellent immunoreactivity and tumor localizing capacity. Based on these encouraging results, it should now be possible to restart the dose escalation study that was stopped previously due to radiochemistry failure at high 211At activity levels.
Acknowledgments
The authors wish to thank Steven Clayton and Darryl McDougald for their excellent technical assistance and David Soule, Duke Clinical Radiopharmacy, for performing the sterility and pyrogenicity assays.
Author Confirmation Statement
The corresponding author (M.R.Z.) confirms that all coauthors have reviewed and approved the article before submission.
Authors' Contributions
Conception and design: G.V., O.R.P., and M.R.Z.; Development of methodology: O.R.P. and S.M.; acquisition of data: J.C., X.-G.Z., and S.M.; analysis and interpretation of data: G.V., O.R.P., and M.R.Z.; writing, review, and revision of article: G.V., J.C., and M.R.Z..; administrative, technical, and material support: G.V., J.C., X.-G.Z., and S.M.; study supervision: G.V. and M.R.Z.
Disclosure Statement
There are no existing financial conflicts.
Funding Information
This work was supported by Grant CA42324 from the National Institutes of Health.
References
- 1. Aghevlian S, Boyle AJ, Reilly RM. Radioimmunotherapy of cancer with high linear energy transfer (LET) radiation delivered by radionuclides emitting alpha-particles or Auger electrons. Adv Drug Deliv Rev 2017;109:102. [DOI] [PubMed] [Google Scholar]
- 2. Vaidyanathan G, Zalutsky MR. Applications of 211At and 223Ra in targeted alpha-particle radiotherapy. Curr Radiopharm 2011;4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersson H, Cederkrantz E, Back T, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: Pharmacokinetics and dosimetry of 211At-MX35 F(ab′)2—a phase I study. J Nucl Med 2009;50:1153. [DOI] [PubMed] [Google Scholar]
- 4. Cederkrantz E, Andersson H, Bernhardt P, et al. Absorbed doses and risk estimates of 211At-MX35 F(ab′)2 in intraperitoneal therapy of ovarian cancer patients. Int J Radiat Oncol Biol Phys 2015;93:569. [DOI] [PubMed] [Google Scholar]
- 5. Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med 2008;49:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zalutsky MR, Zhao XG, Alston KL, et al. High-level production of alpha-particle-emitting 211At and preparation of 211At-labeled antibodies for clinical use. J Nucl Med 2001;42:1508. [PubMed] [Google Scholar]
- 7. Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 1: Effects of solvent on the degradation of radiohalogenation precursors by 211At alpha-particles. J Nucl Med 2005;46:700. [PubMed] [Google Scholar]
- 8. Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 2: Radiolytic effects of 211At alpha-particles influence N-succinimidyl 3-211At-astatobenzoate synthesis. J Nucl Med 2005;46:1393. [PubMed] [Google Scholar]
- 9. Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 3: Alpha-particle-induced radiolytic effects on the chemical behavior of 211At. J Nucl Med 2007;48:1190. [DOI] [PubMed] [Google Scholar]
- 10. Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 4: Strategies for 211At labeling at high activities and radiation doses of 211At alpha-particles. Nucl Med Biol 2017;46:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaidyanathan G, Zalutsky MR. Preparation of N-succinimidyl 3-[*I]iodobenzoate: An agent for the indirect radioiodination of proteins. Nat Protoc 2006;1:707. [DOI] [PubMed] [Google Scholar]
- 12. Bourdon MA, Coleman RE, Blasberg RG, et al. Monoclonal antibody localization in subcutaneous and intracranial human glioma xenografts: Paired-label and imaging analysis. Anticancer Res 1984;4:133. [PubMed] [Google Scholar]
- 13. Reardon DA, Zalutsky MR, Akabani G, et al. A pilot study: 131I-antitenascin monoclonal antibody 81C6 to deliver a 44-Gy resection cavity boost. Neuro Oncol 2008;10:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindmo T, Boven E, Cuttitta F, et al. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77. [DOI] [PubMed] [Google Scholar]
- 15. Schold SC Jr., Bullard DE, Bigner SH, et al. Growth, morphology, and serial transplantation of anaplastic human gliomas in athymic mice. J Neurooncol 1983;1:5. [DOI] [PubMed] [Google Scholar]
- 16. Zalutsky MR, Stabin MG, Larsen RH, et al. Tissue distribution and radiation dosimetry of astatine-211-labeled chimeric 81C6, an alpha-particle-emitting immunoconjugate. Nucl Med Biol 1997;24:255. [DOI] [PubMed] [Google Scholar]
- 17. Reist CJ, Foulon CF, Alston K, et al. Astatine-211 labeling of internalizing anti-EGFRvIII monoclonal antibody using N-succinimidyl 5-[211At]astato-3-pyridinecarboxylate. Nucl Med Biol 1999;26:405. [DOI] [PubMed] [Google Scholar]
- 18. Rohl C, Gulden M, Seibert H. Toxicity of organotin compounds in primary cultures of rat cortical astrocytes. Cell Biol Toxicol 2001;17:23. [DOI] [PubMed] [Google Scholar]
- 19. Garg PK, Archer GE Jr., Bigner DD, et al. Synthesis of radioiodinated N-succinimidyl iodobenzoate: Optimization for use in antibody labelling. Int J Rad Appl Instrum A 1989;40:485. [DOI] [PubMed] [Google Scholar]
- 20. Zalutsky MR, Garg PK, Friedman HS, et al. Labeling monoclonal antibodies and F(ab′)2 fragments with the alpha-particle-emitting nuclide astatine-211: Preservation of immunoreactivity and in vivo localizing capacity. Proc Natl Acad Sci U S A 1989;86:7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zalutsky MR, McLendon RE, Garg PK, et al. Radioimmunotherapy of neoplastic meningitis in rats using an alpha-particle-emitting immunoconjugate. Cancer Res 1994;54:4719. [PubMed] [Google Scholar]
- 22. Lindegren S, Frost S, Back T, et al. Direct procedure for the production of 211At-labeled antibodies with an ɛ-lysyl-3-(trimethylstannyl)benzamide immunoconjugate. J Nucl Med 2008;49:1537. [DOI] [PubMed] [Google Scholar]
- 23. Aneheim E, Albertsson P, Back T, et al. Automated astatination of biomolecules—a stepping stone towards multicenter clinical trials. Sci Rep 2015;5:12025 (DOI: 10.1038/srep12025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferraz da Silva I, Freitas-Lima LC, Graceli JB, et al. Organotins in neuronal damage, brain function, and behavior: A short review. Front Endocrinol (Lausanne) 2017;8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aneheim E, Gustafsson A, Albertsson P, et al. Synthesis and evaluation of astatinated N-[2-(maleimido)ethyl]-3-(trimethylstannyl)benzamide Immuno-conjugates. Bioconjug Chem 2016;27:688. [DOI] [PubMed] [Google Scholar]
- 26. Peddi PF, Hurvitz SA. Trastuzumab emtansine: The first targeted chemotherapy for the treatment of breast cancer. Future Oncol 2013;9:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bender B, Leipold DD, Xu K, et al. A mechanistic pharmacokinetic model elucidating the disposition of trastuzumab emtanasine (T-DM1), an antibody-drug conjugate for treatment of metastatic breast cancer. AAPS J 2014;16:994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barok M, Joensuu H, Isola J. Trastuzumab emansine: Mechanisms of action and drug resistance. Breast Cancer Res 2014;16:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen J, Zhu A.. Mapping the drug conjugation sites of an antibody-drug conjugate. Agilent Technologies Application Note 2015:5991-6389EN [Google Scholar]
- 30. Aneheim E, Hallerod J, Albertsson P, et al. Shelf-life of ɛ-lysyl-3-(trimethylstannyl)benzamide immunoconjugates, precursors for 211At labeling of antibodies. Cancer Biother Radiopharm 2015;30:41. [DOI] [PMC free article] [PubMed] [Google Scholar]