Abstract
Cholestatic liver diseases (CLD) begin to develop after an impairment of bile flow start to affect the biliary tree. Cholangiocytes actively participate in the liver response to injury and repair and the intensity of this reaction is a determinant factor for the development of CLD. Progressive cholangiopathies may ultimately lead to end-stage liver disease requiring at the end orthotopic liver transplantation. This narrative review will discuss cholangiocyte biology and pathogenesis mechanisms involved in four intrahepatic CLD: Primary biliary cholangitis, primary sclerosing cholangitis, cystic fibrosis involving the liver, and polycystic liver disease.
Keywords: Cholestasis, Cholangitis, Epigenomics, Immunogenetics, Pathogenesis, Bile acid
Core tip: Several factors can condition bile flow derangements including environmental triggering factors, bile transport obstruction and conditions that alter bile concentration. Sustained pro inflammatory signaling associated with genetic and/or epigenetic dysregulation can condition a chronic dysfunctional state that can lead to a fibrogenic state with loss of homeostasis and sometimes malignant transformation.
INTRODUCTION
Cholestatic liver diseases (CLD) encompasses progressive cholangiopathies, which may evolve to end-stage liver disease. In the United States from 1988 to 2018, this group of illness corresponded to 14.2% of all liver transplants[1]. Thus far, their high morbidity and mortality are an economic burden that evolved from the lack of effective treatments. Moreover, 10% to 40% of these patients will have a recurrence of the primary disease after liver transplantation (LT)[2].
New prospective therapeutic targets are an unmet necessity, a number of which are under preclinical development. To evaluate these potential therapies, it is essential to understand the primary target of these pathologies, the cholangiocytes. This review will reinforce the current understanding of the core concepts of CLD pathogenesis in the light of the last translational advancements that may impact clinical management.
CLD: COMMON PATHOGENIC MECHANISMS
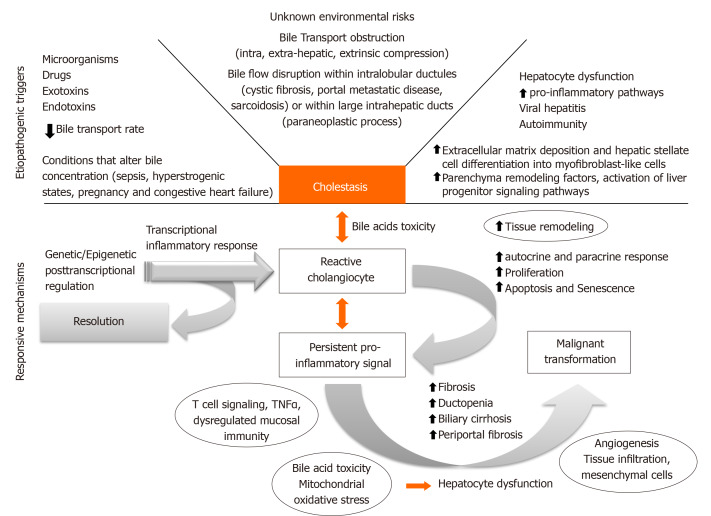
Several factors can condition bile flow derangements (Figure 1). Although environmental triggering factors are mostly unknown, antigenic stimuli, exotoxins, endotoxins, xenobiotics, and microorganisms can promote cholangiocyte reaction that will evolve into a cholestatic state[3]. Bile transport obstruction is another predisposing factor. Intrahepatic and extrahepatic obstruction can take place due to extrinsic benign compression (cystic diseases), malignant mass effect (cholangiocarcinomas), and also as a consequence of cholelithiasis formation or migration throughout the biliary tree. Moreover, conditions that slow biliary flow promote a cholestatic state with increased bile acid (BA) concentration. Sepsis, hyperestrogenic states (pregnancy), congestive heart failure, and dysfunction of BA transporter genes may alter the main characteristics of BA, conditioning a more cytotoxic BA component.
Figure 1.
Core pathogenic mechanism of cholestatic liver diseases.
Early cholangiocyte response may allow resolution of injury, however, sustained pro-inflammatory signaling associated with disragulation of genetic and/or epigenetic regulatory mechanisms could condition late dysfunctional permanent state. Eventually fibrogenic state with biliary and periportal fibrosis, loss of tissue homeostasis and autocrine and paracrine remodeling would be achieved. Ultimately, proliferation may lead to cell-cycle alteration, senescence, apoptosis, ductopenia, mesenchymal infiltration and sometimes malignant transformation. To date, new therapeutic targets are being developed for each CLD considering the core of this pathogenic process. The main framework will be analyzed along with the foundation for potential clinical development.
Ductular reaction: First core concept
Intra and extra-hepatic bile ductules of different sizes are lined by cholangiocytes, which are epithelial cells that regulate and modify bile volume and composition[3]. These vary in size, metabolic rate as well as proliferative and plasticity capabilities. Biliary differentiation pathways are being more thoroughly understood and so it is now known that hepatocytes and cholangiocytes have a common stem cell precursor, and trans differentiation may occur in massive parenchymal loss from one to another, although the exact mechanisms are not well understood[4].
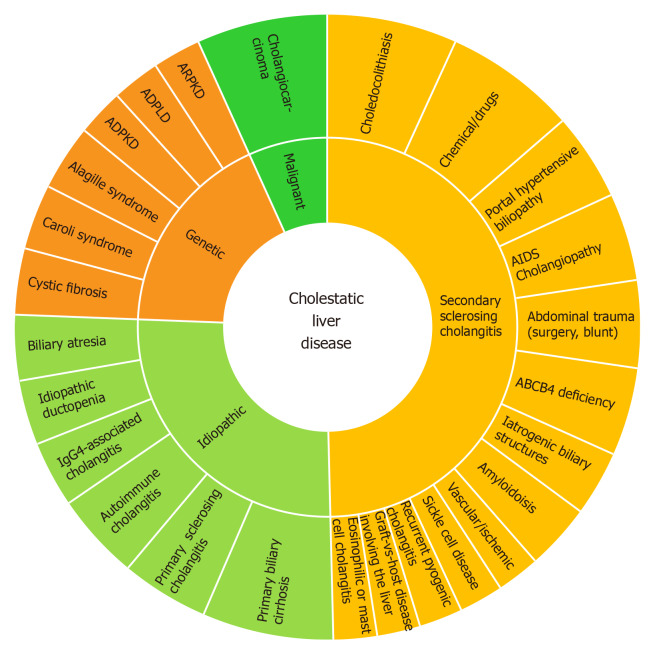
Ductular reaction (DR) is part of the injury response. It is triggered by cholestasis which activates the hepatic progenitor cells in CLD[5]. The sonic-hedgehog pathway promotes both cholangiocyte maturation and deposition of fibronectin in ductular-reactive cells[6]. DR may induce injury resolution, or, biliary fibrosis in the presence of perpetuating transcriptional inflammatory addiction. The cytokine panel for this transcriptional impairment depends on the disease phenotype and ultimately will condition different histological classifications beyond the scope of this review[7]. Figure 2 lists the dominant spectrum of CLD.
Figure 2.
Cholestatic liver disease clinical spectrum.
Bile acid toxicity and mitochondrial dysfunction
The second core fundamental framework of CLD pathogenesis is BA cytotoxicity and mitochondrial dysfunction. Besides its functional role of converting lipid bilayers into mixed micelles, BA are endogenous ligands that activate a network of receptors including nuclear receptor farnesoid X (FXR), vitamin D3 receptor (VDR), pregnane X receptor (PXR), constitutive androstane receptor (CAR), membrane G protein-coupled bile acid receptor-1, and Takeda-G-protein receptor5 (TGR5). Indeed, FXR and TGR5 provide an anti-inflammatory liver response in mouse models[8]. In fact, FXR mutations have been considered a cause of progressive familial intrahepatic cholestasis. Intestinal activation of FXR increases FGF15, a bile synthesis repressor through CYP7A1, a main regulatory enzyme, which reduces the pool size of BA and protects against escalating pro-inflammatory signaling in mouse models[9].
Likewise, BA hepatobiliary transport dysfunction may lead to several phenotypes of cholestatic diseases. Although transcellular BA transport details are mostly unknown, a number of apical and basolateral transporters have been identified. After synthesis of BA in the liver by CYP7A1 and hydroxylation by CYP8B1, bile acids and phospholipids are excreted and secreted across the canalicular membrane of hepatocytes into the biliary tree by BSEP (bile salt export pump/ABCB11) and ABCB4 (ATP binding cassette subfamily B member 4), respectively. BA are then re-uptaken in the terminal ileum by ASBT (apical sodium-dependent bile acid transporter/ SCL10A2), and released into the portal system by a basolateral transporter (OSTα/β) and may later be re-uptaken by the liver via NTCP (Na+/taurocholate cotransporting polypeptide) or OATP (organic anion transporting polypeptides) transporters. Intrahepatic BA can further be processed by hydroxylation, glucuronidation or sulfation, and excreted back into sinusoidal and systemic circulation by OSTα/β and MRP3/4 bile acid transporters. Critical steps in the enterohepatic circulation are regulated by the BA receptor FXR, which limits BA uptake and synthesis by enhancing biliary and basolateral BA export. FGF19, a gut-derived FXR-dependent enterocrine hormone, suppresses hepatic bile acid synthesis and induces gallbladder filling when it is activated by high intestinal BA concentrations[10].
Recently, AMP-activated protein kinase (AMPK) signaling pathways have been implicated in the pathogenesis of drug-induced cholestasis[11]. An example of this pathway is metformin. An older study reported that after 2-3 wk of metformin usage, several patients developed portal inflammation and ductular proliferation[12].
Moreover, it is well-known that the hydrophilic profiles in BA spectrum protects against apoptosis (TCA and UDCA), while those in the hydrophobic range induce hepatic apoptosis and liver injury (TLCA and GCDCA). Additionally, accumulation of cytotoxic BA activates NF-κB-mediated inflammatory cytokines. This pathway is significant in intrahepatic cholestasis of pregnancy as it may arrest placental inflammation[13].
Several studies have described BA toxicities and established commonalities between this toxicity and mitochondrial dysfunction in extra-hepatic cholestasis[14]. In vitro studies demonstrated BA effect in normal liver cell line LO2. Glycochenodeoxycholic acid (GCDCA) stimulated cytotoxicity, disrupted the mitochondrial membrane potential, increasing production of reactive oxygen species (ROS), and leading to decreased mitochondrial mass and mitochondrial DNA content[14]. This feature can be fundamentally related to the development of anti-mitochondrial antibodies (AMA) in primary biliary cholangitis (PBC), consequence of infiltration by both CD4+ and CD8+ T cells reactive to conserved mitochondrial and nuclear antigens, particularly the E2 component of the pyruvate dehydrogenase complex — the principal target of circulating AMA[15]. Moreover, one study pointed deacetylation of the gene PGC-1α, peroxisome proliferator-activated receptor gamma, coactivator one alpha. PGC-1 α acts as an enzyme in mitochondria biogenesis[14]. In chronic intrahepatic cholestasis, the lipid peroxidation activates extracellular matrix cells, ROS, and aldehydes; which may exert direct fibrogenic effects on activated hepatic stellate cells[16].
Immunogenetic and epigenetic setpoints
The third fundamental aspect of the core framework is the influence of immunogenetics and epigenetics on immunoinflammatory response. Patients with CLD exhibit a variety of genetic alterations that account for the different elements of each CLD. However, some of those genes may be directly implicated in the progression rate of the cholestatic phenotype. Recently one study screened some of the progression-related candidate genes for primary biliary cholangitis[17]. They evaluated 315 DNA samples from patients for single nucleotide polymorphisms (SNPs) of 11 candidate genes involved in regulation of bile acid synthesis. Interestingly, genetic variants of CYP7A1, as well as its transcriptional activators (HNF4A and PPARGC1A), may activate bile acid synthesis in an escalating fashion leading to the progressing cholestasis in PBC[17]. It is significant that this gene could become a potential target for new therapeutics, or indirectly their transcriptional activators could serve as modulatory targets. This modulation is a type of epigenetic control of gene expression as a pathogenic mechanism.
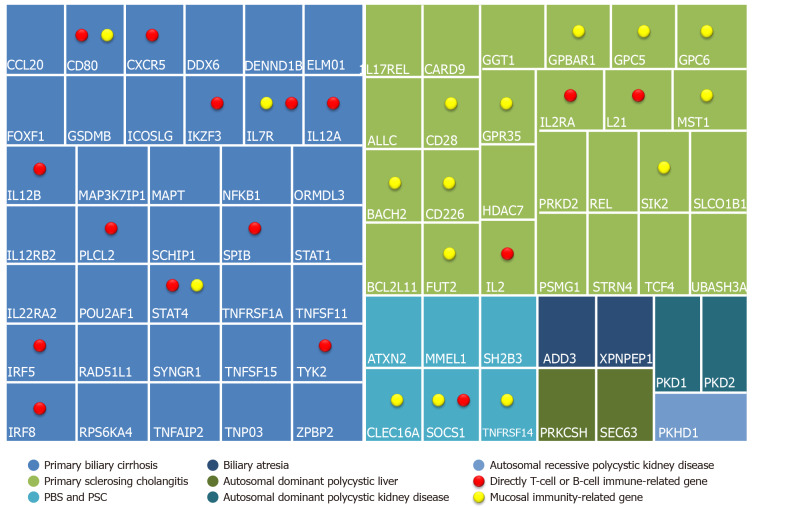
Another study highlighted the central role of the IL-12-STAT4-Th1 pathway, a pro-inflammatory pathway in the progression of PBC, as well as the HLA associations and epigenetic effects[18,19]. Figure 3 shows a panel of immunogenetic genes, where those directly related to the T-cell function or the B-cells or the IL12-STAT4-Th1 are highlighted with a red dot. Additionally, genes associated with loss of immune-tolerance and epithelial permeability are marked with a yellow dot[20,21].
Figure 3.
Immunogenetics related to the core of cholestatic liver diseases. PSC: Primary sclerosing cholangitis.
Dysfunctional matrix re-arrangements and fibrogenesis
To complete the core framework of CLD, dysfunctional matrix rearrangements and fibrogenesis are the fourth concept. Fibrogenesis is a dynamic process that appears intricate to immunoinflammatory mechanisms, secretion of tissue metalloproteinases, cytokine networks and derangements of mesenchymal cells infiltration with ultimate loss of tissue maintenance homeostasis[16]. The pattern of extra cellular matrix (ECM) accumulation in some CLD such as PBC is characterized by increased expression of mRNA encoding collagen type I, III, and IV, which in mesenchymal cells promotes the expansion of portal tracts, leading to deposition of excessive fibrillar ECM. In this way the fibrogenic processes involve damaged and non-damaged bile ducts as well as the periportal sinusoidal system, resulting in progressive cholestasis[16]. In contrast in patients with primary sclerosing cholangitis (PSC), the fibrogenic process has been compared to atherosclerosis onion-like concentric recruitment of pro-fibrogenic cells. Also animal models have reported vascular injury with ischemia of the bile duct epithelial cells during development of PSC lesion[22].
Hepatic stellate cells (HSC) are the primary source of myofibroblast during liver injury, however mesenchymal cells also give rise to myofibroblasts (portal myofibroblasts (PMF) as these cells are located in the portal tract)[23]. Studies in animal models of biliary cirrhosis (rat) reported that PMF use vascular endothelial growth factor A-containing microparticles signaling for newly formed vessels, driving scar progression, while acting as mural cells[24]. This type of fibrosis progression originating from the portal tract is crucial in cystic fibrosis-related liver fibrosis[25]. In PBC epigenetic influence has been observed in the discordance of monozygotic twins. The role of the CD40-CD40L interaction in T-cell and B-cell mechanisms has been reported in the decreased methylation of CD40L promoter regions amongst PBC patients compared with controls[18]. Similarly, X chromosome monosomy has been found on peripheral cells of PBC patients[26]. Recently the Milan PBC epigenetic Study Group reported demethylation of the CXCR3 promoter, which is negatively correlated with peripheral blood receptor expression in CD4+ T-cells[27]. The epigenetic role of demethylation is considered as CXCL9-11 is up-regulated in damaged bile ducts and it is a co-ligand for CXCR3, which is highly expressed in Th1 and Th17[28]. Another group evaluated the role of microRNA (miR), that can also promote downregulation of protein-coding gene expression. Down-Regulation of miR-122a and miR-26a was reported, as well as an increased expression of miR-328 and miR-299-5p. These microRNAs are known to affect cell proliferation, inflammation, oxidative stress metabolism, and apoptosis[29].
PRE-CLINICAL THERAPEUTIC DEVELOPMENTS
From a pathogenic standpoint, a number of therapeutic genetic and epigenetic targets can be considered. Some pathways already have one or more target drugs available. Table 1.
Table 1.
Potential pathways as targets for existing antibodies
| Drug | Primary role of the pathway in specific cholestatic liver disease | Previous disease of drug-testing | Ref. |
| Anti-CD40 (dacetuzumab/lucatumumab) | T-cell-B-cell interactions in primary biliary cholangitis | Multiple sclerosis (pre-clinical) | [53] |
| Anti-CXCL10 (MDX-1100) | CXCR3-CXCL9/10/11 CXCR3 is upregulated on liver-infiltrating Th1 and Th17 in primary biliargy cholangitis | Rheumatoid arthritis | [54] |
| Anti-CXCL13 (Mab 5261) | T- and B-cell migration to germinal centers in primary biliary cholangitis | Preclinical development | [55] |
| Anti-CCR6 | Recruitment of Th17 cells around inflamed biliary epithelial cells in primary biliary cholangitis | Preclinical development | [56] |
| Anti-GRP35 | Activation of GPR35 reduces IL-4 release from natural killer T cells in primary sclerosing cholangitis | Antibody recently developed | [57] |
| Anti-PRKD2 | SIK2 pathway in PSC, AMPK-related kinase PRKD2 polymorphism are seen in early inflammatory bowel disease in primary sclerosing cholangitis | Preclinical development | [58] |
PSC: Primary sclerosing cholangitis.
A number of preclinical studies may pave the way to new clinical advancements. A few of them are listed in Table 2, where we highlight the main pathogenic framework as described before.
Table 2.
Preclinical research cholestatic liver diseases
| Area of concern | Findings | Approach | Ref. |
| Mitochondrial damage by GCDCA | Mitofusin 2 protects hepatocyte mitochondrial function | In vitro (LO2 cell lines) | [59] |
| Immunomodulation in primary biliary cholangitis with CTLA-4-Ig (immunoglobulin) as an immunotherapeutic agent | Signaling by CTLA-4 can modulate costimulation and induce inhibitory signals | In vivo (murine models) | [60] |
| Immunomodulation in primary biliary cholangitis with anti-CD40L | Reduced liver inflammation significantly initial lowering of anti-mitochondrial antibodies was observed but non-sustained. | In vivo (murine models) | [61] |
| Action of nuclear bile acid receptor FXR in cholestasis | Hepatoprotection from cholestasis by inducing FGF-15 | In vivo (murine model) | [9] |
| Immunomodulation Anti-CCR5/CCR2 in combination with all-trans-retinoic acid | Significant reduction in plasma liver enzymes, bilirubin, liver fibrosis, bile duct proliferation and hepatic infiltration of neutrophils and T cells and expression of cytokines | In vivo (murine model) | [62] |
| Curcumin acts through FXR signaling | Protection against alpha-naphthylisothiocyanate ANIT-induced cholestasis | In vitro and in vivo (murine model) | [63] |
| Modulation of bile duct proliferation, with Melatonin | GnRH stimulated fibrosis gene expression in Hepatic stellate cells; melatonin may improve outcomes of cholestasis by suppressing GnRH. | In vivo (murine model) | [64] |
| Apamin, an apitoxin (bee venom) derivate prevented tetrachloride-induced liver fibrosis | Apamin suppressed the deposition of collagen, the proliferation of BECs and expression of fibrogenic genes | In vivo (murine model) | [65] |
| Toxic bile acids induce mitochondrial fragmentation. Preventing fragmentation improved outcome | Decreasing mitochondrial fission substantially diminished ROS levels, liver injury, and fibrosis under cholestatic conditions | In vivo Knockout mouse models | [66] |
| Epigenetic approach Histone deacetylase 4 (HDAC4) restores prohibitin-1 (PHB1) | Genomic reprogramming, with regression of the fibrotic phenotype | In vivo Knockout mouse models | [67] |
| Anti-γ-glutamyl transpeptidase antibody for osteodystrophy in cholestatic liver disease | GGT inhibited mineral nodule formation and expression of alkaline phosphatase and bone sialoprotein in osteoblastic cells. | In vivo (murine model) | [68] |
| EGFR signaling protects from cholestatic liver injury and fibrosis. | STAT3 is a negative regulator of bile acids synthesis and protects from bile acid-induced apoptosis. Additionally, it regulates EGFR expression | In vivo Knockout mouse models | [69] |
| Necroptosis pathway in primary biliary cholangitis | Necroinflammatory pathways regulated by receptor-interacting protein 3 (RIP3), with deleterious progress in cholestatic diseases. RIP3 deficiency blocked bile-duct-ligation-induced (BDL) necroinflammation at 3 and 14 d post-BDL | In vivo Knockout mouse models | [70] |
| Tauroursodeoxycholic acid modulates apoptosis in mice | Significant reduction of liver fibrosis, accompanied by a slight decrease of liver damage | In vivo (murine model) | [71] |
CLINICAL TRIALS AND TRANSLATIONAL RESEARCH
The core fundamental concepts and pathogenic framework are platforms to build new models of clinical interventions for specific CLD. This section addresses the main cholestatic diseases individually.
Primary biliary cholangitis
PBC is characterized histologically by intralobular nonsuppurative bile duct destruction by lymphocytic cholangitis[30]. Patients with PBC often have a decreased quality of life as the disease progresses to hepatic fibrosis and end-stage liver disease. To date, one-third of the patients do not have a biochemical response to ursodeoxycholic acid (UDCA), which is primarily defined by bilirubin and alkaline phosphatase levels after one year of UDCA.
PBC inflammatory disarrays present with increased cholangiocyte chemokines released mainly CXCL10, CXCL9, CX3CL1, and CCL20, which involve the IL-12/IL23 pathways[31]. A number of novel therapeutics in immunomodulation such as fibrates and budesonide had promising results as an alternative to UDCA nonresponders, and recently obeticholic acid was approved by the FDA for UDCA non responders[32-34]. Advancements for PBC patients also include agonists for peroxisome proliferator-activated receptor alpha (PPARα), FXR, GR/PXR most often in combination with UDCA, fibrates, obeticholic acid (OCA) and budesonide, respectively[35]. Some of these translational therapeutics are mentioned in Table 3 and can also be used in PSC as discussed as follows.
Table 3.
Clinical trials and translational research
| Area of concern and specific cholestatic liver disease | Findings | Phase, study description | Clinical trial number | Ref. |
| IL12/IL23 Inflammatory pathway and loss of self-tolerance (Primary biliary cholangitis) | After 28 wk of treatment modest decreases in alkaline phosphatase | Phase 2, open-label proof of concept using Ustekimunab for ursodeoxycholic acid non-responsive patients | NCT01389973 | [72] |
| Ileal bile acid transporter (IBAT) (Primary biliary cholangiti, Alagille syndrome, progressive familial intrahepatic cholestasis) | Bile acid transporter inhibitor A4250 interrupts enterohepatic bile acid circulation at the terminal ileum | Phase 1 (40 individuals) completed Bile acids A4250 either as monotherapy or in combination with colonic release cholestyramine | NCT02963077 | [73] |
| Modified bile acid and FXR agonist derived from chenodeoxycholic acid Obeticholic acid (OCA) (Primary biliary cholangitis) | Durable treatment response; the drug was approved by FDA in May 2017 for non-UDCA responders | Phase 4, double-blind, randomized, placebo-controlled, multicenter (428 patients) estimated completion by 2025 (COBALT study) | NCT02308111 | [34] |
| IBAT inhibition by GSK2330672 | After 14 d, GSK2330672 demonstrated to be safe, well tolerated and reduced pruritus severity | Phase 2 double-blind, randomized, placebo-controlled | NCT01899703 | [74] |
| Bile acids | Significantly reduced ALT and the bile acid intermediate C4 | Phase I: Combination of UDCA and ATRA | NCT01456468 | [75] |
| Bile acids Obeticholic acid monotherapy (Primary biliary cholangitis) | With ursodiol or as monotherapy for 12 mo decreases from baseline in alkaline phosphatase and total bilirubin levels that differed significantly from the placebo. observed changes | Phase 3, double-blind, placebo-controlled trial and long-term safety extension of obeticholic acid (217 patients) (POISE study) | NCT01473524 | [76] |
| Bezafibrate 400 mg alternative | PBC patients with inadequate response to ursodeoxycholic acid alone, treatment with bezafibrate in addition to ursodeoxycholic acid resulted in a rate of complete biochemical response that was significantly higher than the rate with placebo and ursodeoxycholic acid therapy | Phase 3 multi-center, randomized, placebo-controlled, parallel-group (100 patients) (BEZURSO study) | NCT01654731 | [77] |
| Different doses of UDCA in primary sclerosing cholangitis | Significantly reduced ALP values dose-dependently | Phase 2 double-blind, randomized, multi-center, placebo-controlled (159 patients) (NUC3) | NCT01755507 | [78] |
| Pentoxifylline as immunomodulator for primary biliary cholangitis | The study is small, and results were in clinicaltrials.gov, but due to study size no conclusion can be safely achieved | Phase 2, pilot study, open-label Pentoxifylline 400 mg TID for six months (20 participants) | NCT01249092 | Results at clinicaltrials.gov |
| Umbilical cord-derived mesenchymal cells (UC-MSC) | A significant decrease in alkaline phosphatase | Phase1/2 study, randomized, parallel group (100 participants) 12 wk of treatment | NCT01662973 | [79] |
| Mitomycin C in primary sclerosing cholangitis | Final results awaited | Phase 2, double-blind, randomized, parallel group (130 participants) | NCT01688024 | - |
| Curcumin in primary sclerosing cholangitis | Final results awaited | Phase1/2 open-label pilot study Evaluating the safety and efficacy of curcumin (15 participants) | NCT02978339 | - |
| Human monoclonal antibody (BTT1023) that targets the vascular adhesion protein (VAP-1) in primary sclerosing cholangitis | Recruiting | Phase 2, a single arm, two-stage, multicenter, open-label (41 participants) | NCT02239211 | [80] |
| Cenicriviroc a CCR2/CCR5 inhibitor proof of concept in primary sclerosing cholangitis | Results awaited | Phase 2, proof of concept, open-label (24 participants) (PERSEUS study) | NCT02653625 | - |
| Bile acids Maralixibat Apical bile acids transporter inhibition (ASBTi) in primary sclerosing cholangitis | Although results are online, complete information is still awaited | Phase 2, pilot, open-label | NCT02061540 | Results available at clinicaltrial.gov |
| Immunomodulation Simtuzumab in primary sclerosing cholangitis Monoclonal antibody against lysyl oxidase-like 2 (LOXL2) | Results awaited | Phase 2b, dose-ranging, randomized, double-blind, placebo-controlled (235 participants) | NCT01672853 | - |
| Bile acids Obethicolic acid in primary biliary cholangitis | Treatment with OCA 5-10 mg reduced serum ALP in patients with PSC. Mild to moderate dose-related pruritus was the most common adverse event | Phase 2, double-blind, placebo-controlled trial. Dose-Finding (AESOP) | NCT02177136 | [80] |
PSC: Primary sclerosing cholangitis.
Primary sclerosing cholangitis
There are currently no approved therapies for PSC. The disease causes a significant economic burden, and patients have high hospitalization and malignancy rates, often progressing to end-stage liver disease, requiring eventually liver transplantation. Table 3 summarizes the main translational research in the field. Novel approaches for PSC include transcriptional modifiers of bile formation, such as the agonists of FXR, PXR, GR and activation of PPARα. This activation can be promoted by fibrates as they decrease expression of inflammatory cytokines, also reducing hepatocyte BA synthesis. Another approach is the use of agonists of Takeda-G-protein 5 (TGR5), a BA membrane receptor expressed in various tissues as it can lower the levels of proinflammatory cytokines in bile ducts[36]. Other approaches include inhibitors of the ileal apical sodium BA transporter, derivatives of the FXR-induced fibroblast growth factor 19 (FXR-induced FGF19) from the ileum that suppress hepatic BA synthesis, and norursodesoxicholic acid (norUDCA), a side chain shortened UDCA derivative.
Cystic fibrosis involving the liver – hepatobiliary spectrum
The frequency of biliary manifestations in cystic fibrosis (CF) is still unclear. Clinical phenotypes range from gallbladder dyskinesia, symptomatic cholelithiasis to sclerosing obstructive cholangitis. Early diagnosis can be challanging. Tools like the Aspartate Aminotransferase-to-Platelet Ratio Index (APRI) are reliable at predicting severe fibrosis, but not for differentiating fibrosis in early stages. Therefore, serum biomarkers are an unmet necessity thus far. Promising research areas include further investigating the role of intestinal bile salt malabsorption such as the plasma fibroblast growth factor 19 (FGF19) and the intermediate of CYP7A activity and the 4-cholesten-3-one (C4)[37]. Transient elastography may be useful as well, however appropriate validation in mild-to-moderate fibrosis is still pending[38]. Clinical trials for CF cholestasis, using the new generation of therapeutic targets beyond UDCA, would also provide benefits to patients. Some agents discussed previously had good results in preclinical research, such as NorUDCA, tested in mice[39].
Recent CF animal model investigations uncovered the underpinning relationship of the CF transmembrane conductance regulator and the control of biliary epithelial inflammation and permeability mediated by TLR4-NF-κB[40]. Moreover, a number of studies have identified a dysfunctional PPAR-gamma (peroxisome proliferator-activated receptor gamma), that was partially recovered with PPAR-gamma ligands, as rosiglitazone, particularly attenuating biliary fibrosis in CF[41]. Another study, also in murine model, linked those PPAR-gamma as a limiting factor for NF-κB-dependent inflammation[42]. These findings can possibly be further studied as possible target for future therapies.
Polycystic liver disease
Polycystic liver diseases are autosomal dominant disorders that result from a mutation of PRKCSH or Sec63 genes; genes that are mainly expressed in cholangiocytes[43]. Cystogenesis in this scenario is due to benign cholangiocyte proliferation, with cell-cycle dysregulation and increased level of cAMP in cholangiocytes leading to cyst progression and abnormal fluid transport[44]. Over time, the cyst growth may compress the biliary tree impairing bile flow as well. Liver volume is a prognostic marker as complications may occur as the disease progresses, such as hepatic cyst infection, rupture, hemorrhage and hepatic venous outflow obstruction[45]. Therapeutic developments have focused in preclinical studies in lowering cAMP and stopping or reversing progression, usually evaluated by the organ size and hepatic cystic volume. Octreotide became an option for treatment via decrease in cAMP levels[46,47]. Recently an open-label clinical trial tested UDCA effect in cystic liver diseases and reported a reduction of liver cyst volume growth after 24 wk of treatment[48,49]. This effect was expected as UDCA decreases the concentration of cytotoxic BA and therefore diminishes proliferation stimuli[50]. Additionally, more than 50% of patients may have fibrosis[51].
CONCLUSION
Although CLD pathogenic features are becoming unveiled, and translational research is achieving success, some findings still challenge what we know about the basic molecular developments in CLD, such as the relationship of FXR agonists, synthesis of FGF19 and metabolism expression and cell survival[52], and ultimately possible carcinogenesis. To date, inhibitors of the FGF19/FGFR4 pathway are in development for the treatment of hepatocellular malignancies. This acknowledgment for the regular hepatology practice is essential, as for a number of cases, hepatologists and oncologist specialized in hepatobiliary tumors do not often work on the same cases at the same point in time. However, the same patient may experience interactions with these professionals on different occasions in the course of disease progression. For the current therapeutics of cholestatic disease, FXR agonists may represent a novel approach for PBC, and trigger experimentational use for PSC. In the long run, however, the aberrant expression of FGF19 in its oncogenic driver is not entirely presumed. The landscape of modulation of the fibroblast growth factor family, as well as its signal through the transmembrane tyrosine kinase receptors, needs an operable spotlight in cholestatic diseases.
Moreover, in pre-carcinogenic sclerosing conditions such as PSC, the agonistic effect of cell proliferation, differentiation, and tissue repair through a potential oncogenic signaling pathway demands further scrutiny. Besides, a possible role in therapeutic resistance for advanced metastatic hepatocellular carcinomas, once the pathway is wired up, is also concerning. Epigenetic modulation in the core of the CLD and the hepatostat growth activation through FGF19/FGFR4 may interface with the Hippo-Yap signaling and play an essential role in liver carcinogenesis.
It is expected that the current understanding of the multifactorial pathogenic process and the potential substantial role of epigenetics will drive further much needed basic research and introduce new concepts and prospective therapeutic targets to the world of CLD.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association; and American Society for Gastrointestinal Endoscopy.
Peer-review started: May 6, 2020
First decision: May 24, 2020
Article in press: August 1, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang XQ S-Editor: Gong ZM L-Editor: A P-Editor: Li JH
Contributor Information
Raquel T Yokoda, Department of Anatomic and Clinical Pathology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, United States.
Eduardo A Rodriguez, Department of Gastroenterology, Hepatology and Nutrition, University of Utah, Salt Lake City, UT 84132, United States. eduardo.rodriguez@hsc.utah.edu.
References
- 1. United Network for Organ Sharing - UNOS [Internet]. 2018. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ [Google Scholar]
- 2.Khungar V, Goldberg DS. Liver Transplantation for Cholestatic Liver Diseases in Adults. Clin Liver Dis. 2016;20:191–203. doi: 10.1016/j.cld.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazaridis KN, LaRusso NF. The Cholangiopathies. Mayo Clin Proc. 2015;90:791–800. doi: 10.1016/j.mayocp.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Assuncao TM, Sun Y, Jalan-Sakrikar N, Drinane MC, Huang BQ, Li Y, Davila JI, Wang R, O'Hara SP, Lomberk GA, Urrutia RA, Ikeda Y, Huebert RC. Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes. Lab Invest. 2015;95:684–696. doi: 10.1038/labinvest.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sclair SN, Fiel MI, Wu HS, Doucette J, Aloman C, Schiano TD. Increased hepatic progenitor cell response and ductular reaction in patients with severe recurrent HCV post-liver transplantation. Clin Transplant. 2016;30:722–730. doi: 10.1111/ctr.12740. [DOI] [PubMed] [Google Scholar]
- 6.Jalan-Sakrikar N, De Assuncao TM, Lu J, Almada LL, Lomberk G, Fernandez-Zapico ME, Urrutia R, Huebert RC. Hedgehog Signaling Overcomes an EZH2-Dependent Epigenetic Barrier to Promote Cholangiocyte Expansion. PLoS One. 2016;11:e0168266. doi: 10.1371/journal.pone.0168266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Ayata G, Baker SP, Banner BF. Cholangitis: a histologic classification based on patterns of injury in liver biopsies. Pathol Res Pract. 2005;201:565–572. doi: 10.1016/j.prp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Chiang JYL, Ferrell JM. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18:71–87. doi: 10.3727/105221618X15156018385515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modica S, Petruzzelli M, Bellafante E, Murzilli S, Salvatore L, Celli N, Di Tullio G, Palasciano G, Moustafa T, Halilbasic E, Trauner M, Moschetta A. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–65.e1-4. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury - What is the link? J Hepatol. 2017;67:619–631. doi: 10.1016/j.jhep.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Liu R, Zhang L, Jiang Z. The emerging role of AMP-activated protein kinase in cholestatic liver diseases. Pharmacol Res. 2017;125:105–113. doi: 10.1016/j.phrs.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Nammour FE, Fayad NF, Peikin SR. Metformin-induced cholestatic hepatitis. Endocr Pract. 2003;9:307–309. doi: 10.4158/EP.9.4.307. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Pan Y, Lin C, Zheng Y, Sun H, Zhang H, Wang J, Yuan M, Duan T, Du Q, Chen J. Bile acids evoke placental inflammation by activating Gpbar1/NF-κB pathway in intrahepatic cholestasis of pregnancy. J Mol Cell Biol. 2016;8:530–541. doi: 10.1093/jmcb/mjw025. [DOI] [PubMed] [Google Scholar]
- 14.Tan M, Tang C, Zhang Y, Cheng Y, Cai L, Chen X, Gao Y, Deng Y, Pan M. SIRT1/PGC-1α signaling protects hepatocytes against mitochondrial oxidative stress induced by bile acids. Free Radic Res. 2015;49:935–945. doi: 10.3109/10715762.2015.1016020. [DOI] [PubMed] [Google Scholar]
- 15.Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303–330. doi: 10.1146/annurev-pathol-020712-164014. [DOI] [PubMed] [Google Scholar]
- 16.Pinzani M, Luong TV. Pathogenesis of biliary fibrosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1279–1283. doi: 10.1016/j.bbadis.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Inamine T, Higa S, Noguchi F, Kondo S, Omagari K, Yatsuhashi H, Tsukamoto K, Nakamura M. Association of genes involved in bile acid synthesis with the progression of primary biliary cirrhosis in Japanese patients. J Gastroenterol. 2013;48:1160–1170. doi: 10.1007/s00535-012-0730-9. [DOI] [PubMed] [Google Scholar]
- 18.Webb GJ, Siminovitch KA, Hirschfield GM. The immunogenetics of primary biliary cirrhosis: A comprehensive review. J Autoimmun. 2015;64:42–52. doi: 10.1016/j.jaut.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato K, Hall C, Glaser S, Francis H, Meng F, Alpini G. Pathogenesis of Kupffer Cells in Cholestatic Liver Injury. Am J Pathol. 2016;186:2238–2247. doi: 10.1016/j.ajpath.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi PJ, Hirschfield GM. The Immunogenetics of Autoimmune Cholestasis. Clin Liver Dis. 2016;20:15–31. doi: 10.1016/j.cld.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfield GM, Chapman RW, Karlsen TH, Lammert F, Lazaridis KN, Mason AL. The genetics of complex cholestatic disorders. Gastroenterology. 2013;144:1357–1374. doi: 10.1053/j.gastro.2013.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fickert P, Pollheimer MJ, Beuers U, Lackner C, Hirschfield G, Housset C, Keitel V, Schramm C, Marschall HU, Karlsen TH, Melum E, Kaser A, Eksteen B, Strazzabosco M, Manns M, Trauner M International PSC Study Group (IPSCSG) Characterization of animal models for primary sclerosing cholangitis (PSC) J Hepatol. 2014;60:1290–1303. doi: 10.1016/j.jhep.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 24.Lemoinne S, Cadoret A, Rautou PE, El Mourabit H, Ratziu V, Corpechot C, Rey C, Bosselut N, Barbu V, Wendum D, Feldmann G, Boulanger C, Henegar C, Housset C, Thabut D. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology. 2015;61:1041–1055. doi: 10.1002/hep.27318. [DOI] [PubMed] [Google Scholar]
- 25.Debray D, Narkewicz MR, Bodewes FAJA, Colombo C, Housset C, de Jonge HR, Jonker JW, Kelly DA, Ling SC, Poynard T, Sogni P, Trauner M, Witters P, Baumann U, Wilschanski M, Verkade HJ. Cystic Fibrosis-related Liver Disease: Research Challenges and Future Perspectives. J Pediatr Gastroenterol Nutr. 2017;65:443–448. doi: 10.1097/MPG.0000000000001676. [DOI] [PubMed] [Google Scholar]
- 26.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–535. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 27.Lleo A, Zhang W, Zhao M, Tan Y, Bernuzzi F, Zhu B, Liu Q, Tan Q, Malinverno F, Valenti L, Jiang T, Tan L, Liao W, Coppel R, Invernizzi P, Lu Q, Adams DH, Gershwin ME PBC Epigenetic Study Group. DNA methylation profiling of the X chromosome reveals an aberrant demethylation on CXCR3 promoter in primary biliary cirrhosis. Clin Epigenetics. 2015;7:61. doi: 10.1186/s13148-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang YH, Lian ZX, Cheng CM, Lan RY, Yang GX, Moritoki Y, Chiang BL, Ansari AA, Tsuneyama K, Coppel RL, Gershwin ME. Increased levels of chemokine receptor CXCR3 and chemokines IP-10 and MIG in patients with primary biliary cirrhosis and their first degree relatives. J Autoimmun. 2005;25:126–132. doi: 10.1016/j.jaut.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Padgett KA, Lan RY, Leung PC, Lleo A, Dawson K, Pfeiff J, Mao TK, Coppel RL, Ansari AA, Gershwin ME. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Autoimmun. 2009;32:246–253. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabibian JH, Lindor KD. Primary biliary cirrhosis: safety and benefits of established and emerging therapies. Expert Opin Drug Saf. 2015;14:1435–1444. doi: 10.1517/14740338.2015.1073260. [DOI] [PubMed] [Google Scholar]
- 31.Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, Bowlus CL, Yang GX, Leung PS, Ansari AA, Wu L, Coppel RL, Gershwin ME. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944–1953. doi: 10.1002/hep.26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mousa HS, Lleo A, Invernizzi P, Bowlus CL, Gershwin ME. Advances in pharmacotherapy for primary biliary cirrhosis. Expert Opin Pharmacother. 2015;16:633–643. doi: 10.1517/14656566.2015.998650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali AH, Lindor KD. Obeticholic acid for the treatment of primary biliary cholangitis. Expert Opin Pharmacother. 2016;17:1809–1815. doi: 10.1080/14656566.2016.1218471. [DOI] [PubMed] [Google Scholar]
- 34.Kowdley KV, Luketic V, Chapman R, Hirschfield GM, Poupon R, Schramm C, Vincent C, Rust C, Parés A, Mason A, Marschall HU, Shapiro D, Adorini L, Sciacca C, Beecher-Jones T, Böhm O, Pencek R, Jones D Obeticholic Acid PBC Monotherapy Study Group. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–1902. doi: 10.1002/hep.29569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chazouillères O. Novel Aspects in the Management of Cholestatic Liver Diseases. Dig Dis. 2016;34:340–346. doi: 10.1159/000444544. [DOI] [PubMed] [Google Scholar]
- 36.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodewes FA, Verkade HJ, Taminiau JA, Borowitz D, Wilschanski M Working group C​ystic Fibrosis and Pancreatic Disease of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Cystic fibrosis and the role of gastrointestinal outcome measures in the new era of therapeutic CFTR modulation. J Cyst Fibros. 2015;14:169–177. doi: 10.1016/j.jcf.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Sadler MD, Crotty P, Fatovich L, Wilson S, Rabin HR, Myers RP. Noninvasive methods, including transient elastography, for the detection of liver disease in adults with cystic fibrosis. Can J Gastroenterol Hepatol. 2015;29:139–144. doi: 10.1155/2015/138530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trauner M, Halilbasic E, Claudel T, Steinacher D, Fuchs C, Moustafa T, Pollheimer M, Krones E, Kienbacher C, Traussnigg S, Kazemi-Shirazi L, Munda P, Hofer H, Fickert P, Paumgartner G. Potential of nor-Ursodeoxycholic Acid in Cholestatic and Metabolic Disorders. Dig Dis. 2015;33:433–439. doi: 10.1159/000371904. [DOI] [PubMed] [Google Scholar]
- 40.Fiorotto R, Villani A, Kourtidis A, Scirpo R, Amenduni M, Geibel PJ, Cadamuro M, Spirli C, Anastasiadis PZ, Strazzabosco M. The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity. Hepatology. 2016;64:2118–2134. doi: 10.1002/hep.28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, Glass CK. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nat Med. 2010;16:313–318. doi: 10.1038/nm.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015;62:1551–1562. doi: 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25:265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Aerts RMM, van de Laarschot LFM, Banales JM, Drenth JPH. Clinical management of polycystic liver disease. J Hepatol. 2018;68:827–837. doi: 10.1016/j.jhep.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, 3rd, Rossetti S, Harris PC, LaRusso NF, Torres VE. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, Irazabal MV, Kim B, King BF, Glockner JF, Larusso NF, Torres VE. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27:3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lammert F, Méndez-Sánchez N. The effect of ursodeoxycholic acid in cystic cholangiopathies. Ann Hepatol. 2016;15:949–950. doi: 10.5604/16652681.1222121. [DOI] [PubMed] [Google Scholar]
- 49.D'Agnolo HM, Kievit W, Takkenberg RB, Riaño I, Bujanda L, Neijenhuis MK, Brunenberg EJ, Beuers U, Banales JM, Drenth JP. Ursodeoxycholic acid in advanced polycystic liver disease: A phase 2 multicenter randomized controlled trial. J Hepatol. 2016;65:601–607. doi: 10.1016/j.jhep.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Perugorria MJ, Labiano I, Esparza-Baquer A, Marzioni M, Marin JJ, Bujanda L, Banales JM. Bile Acids in Polycystic Liver Diseases: Triggers of Disease Progression and Potential Solution for Treatment. Dig Dis. 2017;35:275–281. doi: 10.1159/000450989. [DOI] [PubMed] [Google Scholar]
- 51.Barbier L, Ronot M, Aussilhou B, Cauchy F, Francoz C, Vilgrain V, Soubrane O, Paradis V, Belghiti J. Polycystic liver disease: Hepatic venous outflow obstruction lesions of the noncystic parenchyma have major consequences. Hepatology. 2018;68:652–662. doi: 10.1002/hep.29582. [DOI] [PubMed] [Google Scholar]
- 52.Massafra V, Milona A, Vos HR, Burgering BM, van Mil SW. Quantitative liver proteomics identifies FGF19 targets that couple metabolism and proliferation. PLoS One. 2017;12:e0171185. doi: 10.1371/journal.pone.0171185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.'t Hart BA, Hintzen RQ, Laman JD. Preclinical assessment of therapeutic antibodies against human CD40 and human interleukin-12/23p40 in a nonhuman primate model of multiple sclerosis. Neurodegener Dis. 2008;5:38–52. doi: 10.1159/000109937. [DOI] [PubMed] [Google Scholar]
- 54.Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, Tao X, Cardarelli PM, Leblanc H, Nichol G, Ancuta C, Chirieac R, Luo A. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:1730–1739. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 55.Klimatcheva E, Pandina T, Reilly C, Torno S, Bussler H, Scrivens M, Jonason A, Mallow C, Doherty M, Paris M, Smith ES, Zauderer M. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16:6. doi: 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liston A, Kohler RE, Townley S, Haylock-Jacobs S, Comerford I, Caon AC, Webster J, Harrison JM, Swann J, Clark-Lewis I, Korner H, McColl SR. Inhibition of CCR6 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol. 2009;182:3121–3130. doi: 10.4049/jimmunol.0713169. [DOI] [PubMed] [Google Scholar]
- 57.Jenkins L, Harries N, Lappin JE, MacKenzie AE, Neetoo-Isseljee Z, Southern C, McIver EG, Nicklin SA, Taylor DL, Milligan G. Antagonists of GPR35 display high species ortholog selectivity and varying modes of action. J Pharmacol Exp Ther. 2012;343:683–695. doi: 10.1124/jpet.112.198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, Kelland L, Jamieson S, Sutherland R, Raynham T, Charles M, Bagherzadeh A, Foxton C, Boakes A, Farooq M, Maru D, Diagaradjane P, Matsuo Y, Sinnett-Smith J, Gelovani J, Krishnan S, Aggarwal BB, Rozengurt E, Ireson CR, Guha S. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2010;9:1136–1146. doi: 10.1158/1535-7163.MCT-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Lv L, Jiang Z, Yang H, Li S, Jiang Y. Mitofusin 2 protects hepatocyte mitochondrial function from damage induced by GCDCA. PLoS One. 2013;8:e65455. doi: 10.1371/journal.pone.0065455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dhirapong A, Yang GX, Nadler S, Zhang W, Tsuneyama K, Leung P, Knechtle S, Ansari AA, Coppel RL, Liu FT, He XS, Gershwin ME. Therapeutic effect of cytotoxic T lymphocyte antigen 4/immunoglobulin on a murine model of primary biliary cirrhosis. Hepatology. 2013;57:708–715. doi: 10.1002/hep.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka H, Yang GX, Iwakoshi N, Knechtle SJ, Kawata K, Tsuneyama K, Leung P, Coppel RL, Ansari AA, Joh T, Bowlus C, Gershwin ME. Anti-CD40 ligand monoclonal antibody delays the progression of murine autoimmune cholangitis. Clin Exp Immunol. 2013;174:364–371. doi: 10.1111/cei.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu D, Cai SY, Mennone A, Vig P, Boyer JL. Cenicriviroc, a cytokine receptor antagonist, potentiates all-trans retinoic acid in reducing liver injury in cholestatic rodents. Liver Int. 2018;38:1128–1138. doi: 10.1111/liv.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang F, Tang X, Ding L, Zhou Y, Yang Q, Gong J, Wang G, Wang Z, Yang L. Curcumin protects ANIT-induced cholestasis through signaling pathway of FXR-regulated bile acid and inflammation. Sci Rep. 2016;6:33052. doi: 10.1038/srep33052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMillin M, DeMorrow S, Glaser S, Venter J, Kyritsi K, Zhou T, Grant S, Giang T, Greene JF, Jr, Wu N, Jefferson B, Meng F, Alpini G. Melatonin inhibits hypothalamic gonadotropin-releasing hormone release and reduces biliary hyperplasia and fibrosis in cholestatic rats. Am J Physiol Gastrointest Liver Physiol. 2017;313:G410–G418. doi: 10.1152/ajpgi.00421.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JY, An HJ, Kim WH, Park YY, Park KD, Park KK. Apamin suppresses biliary fibrosis and activation of hepatic stellate cells. Int J Mol Med. 2017;39:1188–1194. doi: 10.3892/ijmm.2017.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu T, Wang L, Lee H, O'Brien DK, Bronk SF, Gores GJ, Yoon Y. Decreasing mitochondrial fission prevents cholestatic liver injury. J Biol Chem. 2014;289:34074–34088. doi: 10.1074/jbc.M114.588616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbier-Torres L, Beraza N, Fernández-Tussy P, Lopitz-Otsoa F, Fernández-Ramos D, Zubiete-Franco I, Varela-Rey M, Delgado TC, Gutiérrez V, Anguita J, Pares A, Banales JM, Villa E, Caballería J, Alvarez L, Lu SC, Mato JM, Martínez-Chantar ML. Histone deacetylase 4 promotes cholestatic liver injury in the absence of prohibitin-1. Hepatology. 2015;62:1237–1248. doi: 10.1002/hep.27959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawazoe Y, Miyauchi M, Nagasaki A, Furusho H, Yanagisawa S, Chanbora C, Inubushi T, Hyogo H, Nakamoto T, Suzuki K, Moriwaki S, Tazuma S, Niida S, Takata T. Osteodystrophy in Cholestatic Liver Diseases Is Attenuated by Anti-γ-Glutamyl Transpeptidase Antibody. PLoS One. 2015;10:e0139620. doi: 10.1371/journal.pone.0139620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Svinka J, Pflügler S, Mair M, Marschall HU, Hengstler JG, Stiedl P, Poli V, Casanova E, Timelthaler G, Sibilia M, Eferl R. Epidermal growth factor signaling protects from cholestatic liver injury and fibrosis. J Mol Med (Berl) 2017;95:109–117. doi: 10.1007/s00109-016-1462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Afonso MB, Rodrigues PM, Simão AL, Ofengeim D, Carvalho T, Amaral JD, Gaspar MM, Cortez-Pinto H, Castro RE, Yuan J, Rodrigues CM. Activation of necroptosis in human and experimental cholestasis. Cell Death Dis. 2016;7:e2390. doi: 10.1038/cddis.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paridaens A, Raevens S, Devisscher L, Bogaerts E, Verhelst X, Hoorens A, Van Vlierberghe H, van Grunsven LA, Geerts A, Colle I. Modulation of the Unfolded Protein Response by Tauroursodeoxycholic Acid Counteracts Apoptotic Cell Death and Fibrosis in a Mouse Model for Secondary Biliary Liver Fibrosis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirschfield GM, Gershwin ME, Strauss R, Mayo MJ, Levy C, Zou B, Johanns J, Nnane IP, Dasgupta B, Li K, Selmi C, Marschall HU, Jones D, Lindor K PURIFI Study Group. Ustekinumab for patients with primary biliary cholangitis who have an inadequate response to ursodeoxycholic acid: A proof-of-concept study. Hepatology. 2016;64:189–199. doi: 10.1002/hep.28359. [DOI] [PubMed] [Google Scholar]
- 73.Graffner H, Gillberg PG, Rikner L, Marschall HU. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther. 2016;43:303–310. doi: 10.1111/apt.13457. [DOI] [PubMed] [Google Scholar]
- 74.Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D, Storey J, Dukes GE, Corrigan M, Oude Elferink RP, Beuers U, Hirschfield GM, Jones DE. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 75.Assis DN, Abdelghany O, Cai SY, Gossard AA, Eaton JE, Keach JC, Deng Y, Setchell KD, Ciarleglio M, Lindor KD, Boyer JL. Combination Therapy of All-Trans Retinoic Acid With Ursodeoxycholic Acid in Patients With Primary Sclerosing Cholangitis: A Human Pilot Study. J Clin Gastroenterol. 2017;51:e11–e16. doi: 10.1097/MCG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 77.Patel A, Seetharam A. Primary Biliary Cholangitis: Disease Pathogenesis and Implications for Established and Novel Therapeutics. J Clin Exp Hepatol. 2016;6:311–318. doi: 10.1016/j.jceh.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, Schramm C, Spengler U, Chapman R, Bergquist A, Schrumpf E, Nevens F, Trivedi P, Reiter FP, Tornai I, Halilbasic E, Greinwald R, Pröls M, Manns MP, Trauner M European PSC norUDCA Study Group. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, Chen L, Zou Z, Li B, Shi M, Zhang Z, Wang FS. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85–92. doi: 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 80.Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, Shrestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Schiano T, Jin Y, Pencek R, MacConell L, Shapiro D, Bowlus CL AESOP Study Investigators. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]