Abstract
Chloroquine (CQ) is an antimalarial drug widely used in rheumatic, immunological and infectious diseases. CQ is also a well-known autophagy inhibitor. It slows the progression of renal injury in patients with rheumatology diseases. Long-term CQ treatment could also damage podocytes which are highly differentiated cells wrapping the glomerular capillary to maintain renal filtration. However, the related underlying mechanism remains unclear. The effects of CQ treatment on podocytes need to be elucidated. Our results showed that CQ diminished cell motility and disrupted actin cytoskeleton in human podocytes in vitro. Totally 210 up-regulated and 67 down-regulated differentially expressed proteins (DEPs) were identified after CQ treatment in podocytes by using tandem mass tag (TMT)-labeled quantitative proteomics analysis. Gene Ontology (GO) analysis revealed that proteins mainly functioned in cell motility, cell adhesion, localization of cells and response to external stimulus. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment showed that DEPs were predominantly associated with lysosome, cell adhesion molecules (CAMs) and cytokine-cytokine receptor interaction. Protein-protein interaction (PPI) analysis revealed that syndecan-4 was the core protein in regulating podocyte adhesion among differentially expressed CAMs. Moreover, activated RhoA, Cdc42 and Rac1 decreased after CQ treatment. Taken together, our findings suggested that CQ could alter the stability of podocyte cytoskeleton. Proteomic analysis revealed important molecules for understanding the effects of CQ on human podocytes.
Keywords: Podocyte, chloroquine, autophagy, actin cytoskeleton
Introduction
Podocytes are highly differentiated mesenchymal-like cells which have three different morphological and functional parts: cell body, major process and foot process. Foot processes of adjacent podocytes interdigitate and form the outside layer of renal filtration barrier [1]. The cell body and major process of podocyte rely on microtubules and intermediate filaments for keeping normal morphology, while foot process is supported by the actin cytoskeleton [2]. Foot process contains highly ordered parallel and contractile filamentous actin network. This actin cytoskeleton is cross-linked and bundled by several proteins such as alpha-actinin-4 and synaptopodin [3,4]. The normal morphology and function of foot process bases on intact actin cytoskeleton. Instability of actin cytoskeleton contributes to proteinuria.
Proteinuria is the most common symptom if podocytes undergo detachment or foot processes effacement which is marked with depolymerized actin cytoskeleton [2,5]. For instance, minimal change nephrotic syndrome is featured by heavy proteinuria due to foot process effacement [6]. To emphasize the importance of podocyte in glomerular diseases, the term of podocytopathy has been created for describing the podocyte-injury related diseases [7]. Podocytopathy could result from medication, gene mutation and immunological disorders [8-10].
CQ is an anti-malarial drug that has multiple properties, such as anti-inflammation, anti-thrombus and anti-infection. Hence, it is widely used in rheumatic, immunological and infectious diseases [11]. CQ and its analogues show beneficial effects in patients with lupus nephritis as they slow the progression of renal injury and decrease the complications of infection, hypertension and thrombotic events [12]. Good safety profile was noted as only a small portion of patients receiving long-term CQ treatment has mild or moderate side effects including ocular toxicity and gastrointestinal manifestations [13]. Toll-like receptor 3 (TLR3)/IFN-β signaling could be attenuated by CQ in human mesangial cells [14]. It may partially explain the efficacy of CQ in lupus nephritis. However, little is known about the effects of CQ treatment on human podocytes. To date, only one case report showed that CQ treatment induces phospholipidosis in podocytes mimicking Fabry disease as plenty of zebra bodies could be visualized [15]. In addition, CQ is a well-known autophagy inhibitor. It has been reported that autophagic gene deficient cell presents with depolymerization of actin cytoskeleton [16]. It remains unclear whether CQ disrupts the stability of podocyte cytoskeleton.
In this study, our data showed that CQ could diminish cell motility and disrupt actin cytoskeleton of human podocytes. We performed TMT-based quantitative proteomic analysis to identify differentially expressed proteins (DEPs) in CQ-treated human podocytes. This study firstly revealed the impact of CQ treatment on the stability of podocyte cytoskeleton. It not only helps to elucidate the pathogenesis of podocytopathy, but may also explain the efficacy and side effects of CQ in treating rheumatic and immunological diseases.
Materials and methods
Cell culture
Conditionally immortalized human podocytes AB8/13 were cultured in RPMI 1640+10% fetal bovine serum (Thermo Fisher Scientific) as described previously [17]. Cells proliferated at non-permissive temperature (33°C) with the addition of Insulin-Transferrin-Selenium (Thermo Fisher Scientific) and differentiated in permissive temperature (37°C) for 12 days. Fully differentiated human podocytes were treated with Chloroquine (CQ, 25 μM) (Sigma-Aldrich). The optimum concentration of CQ has been titrated in our previous studies [18,19].
Immunofluorescence analysis
Fully differentiated podocytes with 80% confluency in 24-well plates were treated with CQ (25 μM) for 24 h and 48 h. 4% paraformaldehyde was used for fixing podocytes. After blocking with PBS +2% BSA, F-actin and nucleus were stained with Alexa Fluor 594 Phalloidin (Thermofisher Scientific) and ProLong Gold Antifade Reagent with DAPI (Cell signaling Technology) respectively. Podocytes were visualized under confocal microscopy (EZ-C1, Nikon Instrument, Japan). The percentage of podocytes with disrupted cytoskeleton was quantified as the previous study described [18]. At least 100 cells were scored in each of six independent experiments.
Migration assay
Fully differentiated podocytes in 6-well plates were scratched by two strokes with a sterile 0.4 mm 200 μl Gilson style extension length tip. Then, cells were treated with CQ (25 μM) for 8 h. Two researchers counted the number of podocytes migrating into the gap independently as described before [19].
TMT-based quantitative proteomic analysis
Fully differentiated podocytes with 80% confluency were treated with CQ (25 μM) for 24 h. Podocytes were lysed in SDT buffer (4% SDS, 100 mM DTT and 150 mM Tris-HCl pH 8.0). The concentration of supernatant was measured with BCA Protein Assay Kit (Bio-Rad, USA) after centrifuging at 14000 g for 40 minutes. Proteins were purified after repeated ultrafiltration (Microcon units, 10 kD) with UA buffer (8M Urea, 150 mM Tris-HCl pH 8.0). 100 ul iodoacetamide was used to block reduced cysteine residues. Then, samples were put in darkness for 30 min incubation. The filters were washed with UA buffer three times and TEAB buffer (100 mM) twice. Eventually, protein suspensions were digested overnight at 37°C by using 4 μg trypsin (Promega) in 40 μl TEAB buffer. Peptides were collected by filtration. Peptide content was estimated by UV light spectral density at 280 nm. Peptide mixture of each sample (100 μg) was labeled with TMT reagent according to the manufacturer’s instructions (Thermo Fisher Scientific). TMT-labeled digest samples were fractionated by Pierce high pH reversed-phase fractionation kit (Thermo scientific) based on an increasing acetonitrile step-gradient elution as described before. Peptide mixture was added to a reverse phase trap column (Thermo Scientific Acclaim PepMap100, 100 μm*2 cm, nanoViper C18) connected to the C18-reversed phase analytical column (Thermo Scientific Easy Column, 10 cm long, 75 μm inner diameter, 3 μm resin) in buffer A (0.1% Formic acid). It was separated with a 60 min gradient of 0-50% buffer B (50 min), 50-100% buffer B (5 min), 100% buffer B (5 min) at a flow rate of 300 nl/min which was controlled by IntelliFlow technology. LC-MS/MS analysis was carried out on a Q Exactive mass spectrometer (Thermo Scientific) that was linked to Easy nLC (Proxeon Biosystems, now Thermo Fisher Scientific). TMT-based quantitative proteomic analysis was performed in Shanghai Applied Protein Technology Company (Shanghai, China).
Quantitative real-time PCR
Podocytes were treated with CQ (25 μM) for 12 hours. Total RNA was extracted from podocytes by using Trizol reagent (Invitrogen, Carlsbad, CA) and reversely transcribed to single-stranded cDNA with PrimeScript RT Reagent Kit (Takara Bio, Kyoto, Japan) according to manufacture’s instruction. Quantitative real-time PCR was performed in Step One Plus real-time PCR system (ABI, Foster, CA, USA) with 2X SG Fast qPCR Master Mix (High Rox, B639273, BBI). Detection was done in triplicate. Reaction was performed at 95°C for 3 min followed by 45 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was set as an internal reference. The real-time PCR primers are shown in Table 1. Relative mRNA expression was calculated by using the 2-ΔΔct method.
Table 1.
The real-time PCR primers of 6 key cell adhesion molecules (CAMs)
| Gene name | Protein IDs | Protein name | Sequence | Size (bp) |
|---|---|---|---|---|
| ITGB8 | P26012 | Integrin beta-8 | Forward: 5’ CCAGGAGCCGAAGCTAATT 3’ | 190 |
| Reverse: 5’ CGTATGAGCCAAATCCAAGAC 3’ | ||||
| CLDN1 | O95832 | Claudin-1 | Forward: 5’ GAGGATGGCTGTCATTGGG 3’ | 240 |
| Reverse: 5’ GGTGTTGGGTAAGAGGTTGTTT 3’ | ||||
| SDC4 | P31431 | Syndecan-4 | Forward: 5’ AGCCAAGTCCCCACCGA 3’ | 193 |
| Reverse: 5’ CAAAGAGGATGCCCACGAT 3’ | ||||
| CLDN4 | O14493 | Claudin-4 | Forward: 5’ CCTCGTCATCATCAGCATCA 3’ | 165 |
| Reverse: 5’ GACACCGGCACTATCACCAT 3’ | ||||
| VCAM1 | P19320 | Vascular cell adhesion protein 1 | Forward: 5’ TACAACCGTCTTGGTCAGCC 3’ | 206 |
| Reverse: 5’ TTCCTTCACATAAATAAACCCCA 3’ | ||||
| L1CAM | P32004 | Neural cell adhesion molecule L1 | Forward: 5’ TGCGGACAATCAGACGTACA 3’ | 147 |
| Reverse: 5’ TTGGCATAGGGGAAGAAGC 3’ | ||||
| GAPDH | P04406 | GAPDH | Forward: 5’ TGGGTGTGAACCATGAGAAGT 3’ | 126 |
| Reverse: 5’ TGAGTCCTTCCACGATACCAA 3’ |
RhoA, Cdc42 and Rac1 G-LISA assay
G-LISA assay kit (Cytoskeleton) was used for measuring GTP bound (activated) RhoA, Cdc42 and Rac1 in human podocytes following the manufacture’s protocols. Fully differentiated podocytes with 80% confluency were treated with CQ (25 μM) for 24 h. Cells were harvested using the lysis buffer containing protein inhibitors and the final protein concentration was adjusted to 0.8 mg/L. OD values were measured with the absorbance of 595 nm and represented the expression of activated RhoA, Rac1 and Cdc42.
Statistical analysis and bioinformatics
Data were expressed as mean ± SEM. Non parametric T-test was used for two group’s comparison and using the Prism 5.0 Software (GraphPad, San Diego, CA, USA). A p value <0.05 was considered to be statistically significant.
As for bioinformatics in proteome, differential expressed proteins were defined as the ones with 1.2 folds up-regulation or 0.83 folds down-regulation changes (P value <0.05). The protein sequences were aligned to Gene Ontology (GO) Consortium database for GO assignment. The FASTA protein sequences were blasted against the online Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://geneontology.org/) and subsequently mapped to KEGG pathways. Protein-protein interaction (PPI) was retrieved from STRING database (http://string-db.org/). The results were visualized and analyzed by using Cytoscape software (http://www.cytoscape.org/, version 3.2.1). Bioinformatics was performed by Shanghai Applied Protein Technology Company (Shanghai, China).
Results
CQ decreased cell motility and disrupted actin cytoskeleton of human podocytes
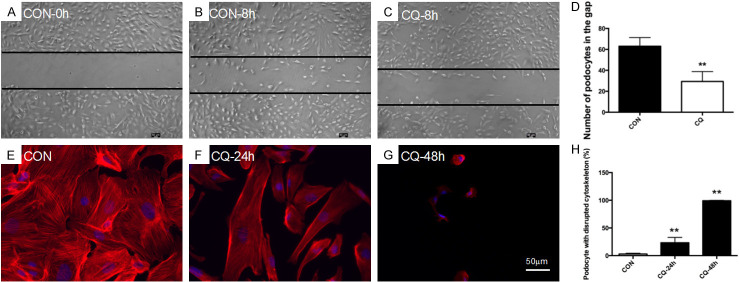
In glomerulus, podocyte motility is kept at a considerable level [20]. It is an essential process for cleaning proteins deposit in subpodocyte space which covers around 60% of GBM [21,22]. Thus, podocyte motility serves as a self-cleaning mechanism to ensure the normal filtration. To test whether CQ affects podocyte motility, migration assay was performed in the present study. As shown in Figure 1A-D, the number of podocytes migrating into the gap after 8 h CQ treatment decreased significantly.
Figure 1.
CQ decreased cell motility and disrupted actin cytoskeleton of human podocyte. (A-D) The number of podocytes migrating into the gap was calculated for evaluating podocyte mobility. Podocytes were treated with CQ (25 μM) for 8 hours. The representative images were taken under inverted microscope (50×). It showed that podocyte mobility decreased significantly after CQ treatment. **P<0.01 versus CON, n=6. (E-H) CQ disrupted actin cytoskeleton of podocytes. The cortical cytoskeleton was observed in podocytes after treating with CQ (25 μM) for 24 h and orderly arranged actin-stress fiber disappeared at 48 h. Data of statistical analysis showed that the percentage of podocytes with disrupted actin cytoskeleton increased significantly. **P<0.01 versus CON, n=6.
In our previous study, we found that depolymerization of podocyte actin cytoskeleton caused by puromycin aminonucleoside was deteriorated after autophagy inhibition by CQ at 24 h [18]. For verifying the impact of CQ uniquely on actin cytoskeleton, we treated podocytes with CQ for 24 h and 48 h. As shown in Figure 1E-H, the cortical cytoskeleton was formed in podocytes at 24 h and orderly actin-stress fiber organization was lost at 48 h. The percentage of podocytes with disrupted actin cytoskeleton increased significantly after 24 h CQ treatment.
Proteomic analysis identified DEPs in human podocytes after CQ treatment
Totally 5247 proteins were identified based on 34969 unique peptides by TMT-based proteomic analysis. There were 277 DEPs (210 up-regulated and 67 down-regulated) after CQ treatment in podocytes (Supplementary Material-1). DEPs were categorized into biological process, molecular function and cellular component according to GO annotation. As showed in Figure 2, response to external stimulus, cell motility, localization of cells, biological adhesion and cell migration were the top 5 biological processes. In the aspect of molecular function, most of DEPs were involving in signaling receptor binding. Additionally, majority of proteins were distributed in cell membrane parts.
Figure 2.
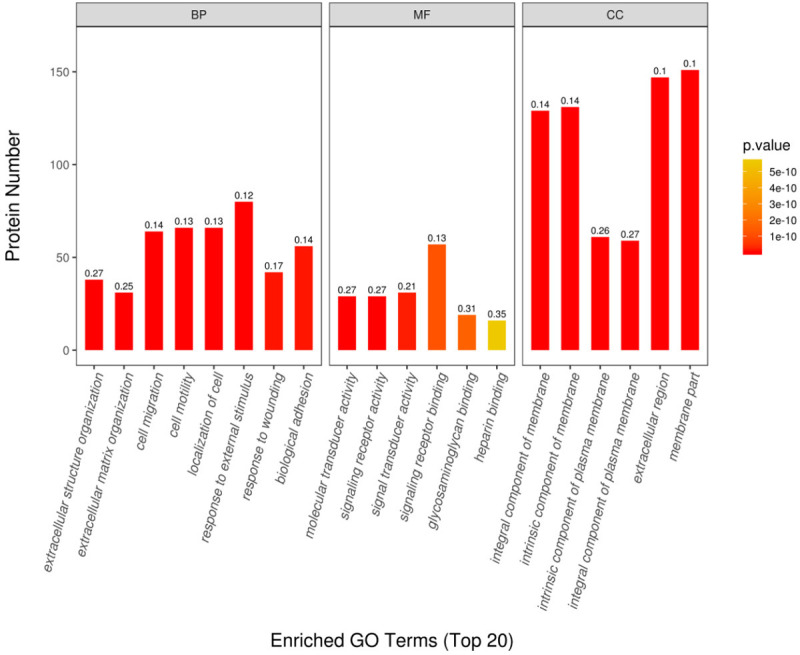
GO annotation of differentially expressed proteins (DEPs) in CQ-treated podocytes. Totally 277 DEPs were identified after CQ treatment in podocytes. They were categorized into biological process (BP), molecular function (MF) and cellular component (CC) according to GO annotation. The magnitude of P value was indicated by color gradient. The number on the top of column was the rich factor (rich factor ≤1). Top 5 biological processes are response to external stimulus, cell motility, localization of cells, biological adhesion and cell migration. In the aspect of molecular function, most of DEPs were involving in signaling receptor binding. As for cellular component, majority of proteins were distributed in cell membrane parts.
To identify the involved signalling pathways, the DEPs were mapped to KEGG pathways. As showed in Figure 3 and Table 2, the top 20 enriched KEGG pathways were listed. The top 5 KEGG pathways basing on the number of involved DEPs were lysosome, proteoglycans in cancer, cytokine-cytokine receptor interaction, cell adhesion molecules (CAMs), complement and coagulation cascades. Since podocyte detachment plays a crucial role in proteinuria diseases, studying the differentially expressed CAMs helps to understand the efficacy and side effects of CQ in renal diseases. Twelve CAMs after CQ treatment are identified as follows: Integrin beta-8 (1.61 folds), Claudin-1 (1.46 folds), Myelin protein zero-like protein 1 (1.42 folds), Receptor-type tyrosine-protein phosphatase F (1.37 folds), Syndecan-4 (1.34 folds), Claudin-4 (1.28 folds), Intercellular adhesion molecule 1 (1.25 folds), Receptor-type tyrosine-protein phosphatase mu (1.24 folds), Vascular cell adhesion protein 1 (1.23 folds), Tumor necrosis factor receptor superfamily member 5 (1.21 folds), Neural cell adhesion molecule L1 (1.2 folds) and CD99 antigen (0.79 folds).
Figure 3.
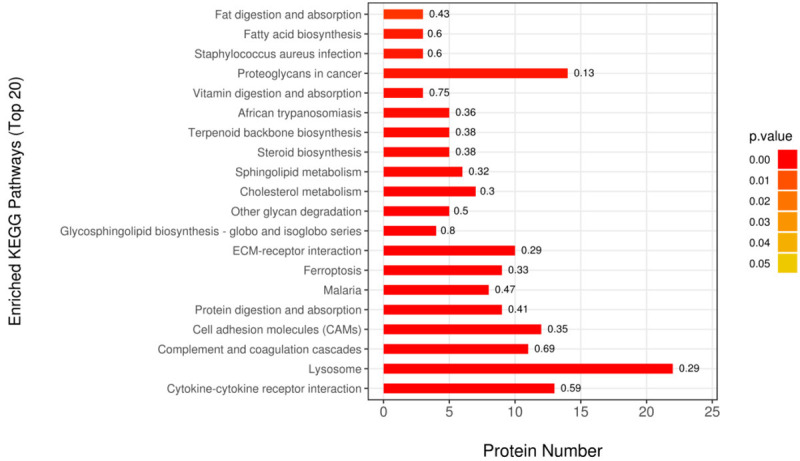
Functional enrichment analysis of DEPs. Top 20 enriched KEGG pathways were identified based on the DEPs in CQ-treated podocytes. The magnitude of P value was indicated by color gradient. The number on the top of column was the rich factor (rich factor ≤1).
Table 2.
The top 20 KEGG pathways enriched from differentially expressed proteins (DEPs) in CQ-treated podocytes
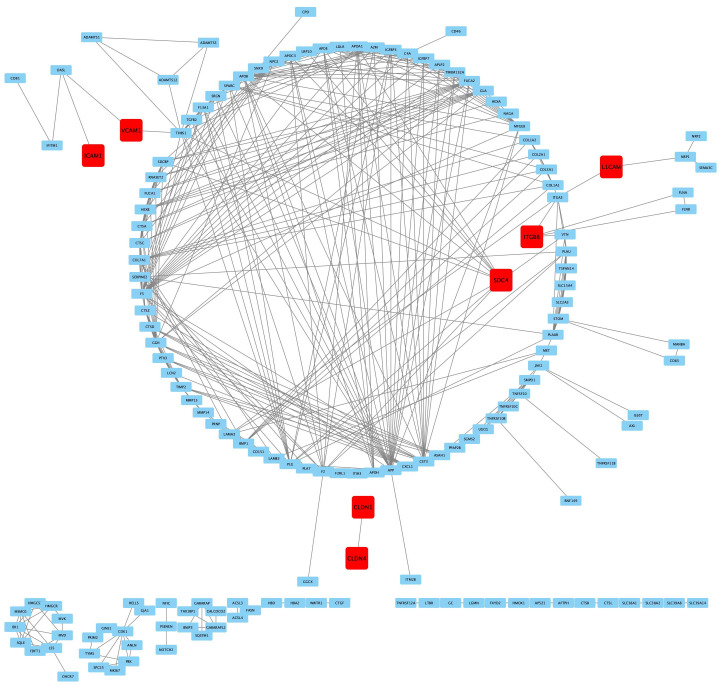
To explore the relationship of DEPs in CQ-treated human podocytes, proteins interaction was constructed by PPI analysis. There were totally 155 nodes and 862 edges in PPI network. Totally 6 CAMs were founded in PPI network: Syndecan-4, Integrin beta-8, Vascular cell adhesion protein 1, Neural cell adhesion molecule L1, Claudin-1 and Claudin-4. Among these CAMs, Syndecan-4 was the core protein in regulating podocyte adhesion (Figure 4).
Figure 4.
PPI analysis of DEPs in CQ-treated human podocytes. Totally 156 nodes and 862 edges were established in PPI network. Six cell adhesion molecules (CAMs) were founded in PPI network and highlighted in red. They are Syndecan-4, Integrin beta-8, Vascular cell adhesion protein 1, Neural cell adhesion molecule L1, Claudin-1 and Claudin-4. The node and edge represented DEP, the interaction between two DEP respectively. More edges indicated more protein interactions existed. Abbreviations: Syndecan-4, SDC4. Integrin beta-8, ITGB8. Vascular cell adhesion protein 1, VCAM1. Neural cell adhesion molecule L1, L1CAM. Claudin-1, CLDN1. Claudin-4, CLDN4.
The expression of 6 key CAMs was verified by qPCR
For verifying the expression of 6 CAMs (Syndecan-4, Integrin beta-8, Vascular cell adhesion protein 1, Neural cell adhesion molecule L1, Claudin-1 and Claudin-4) which were illustrated in PPI, their mRNA expression level was measured by qPCR. The data were consistent with that from TMT assay as all of them increased (Figure 5).
Figure 5.
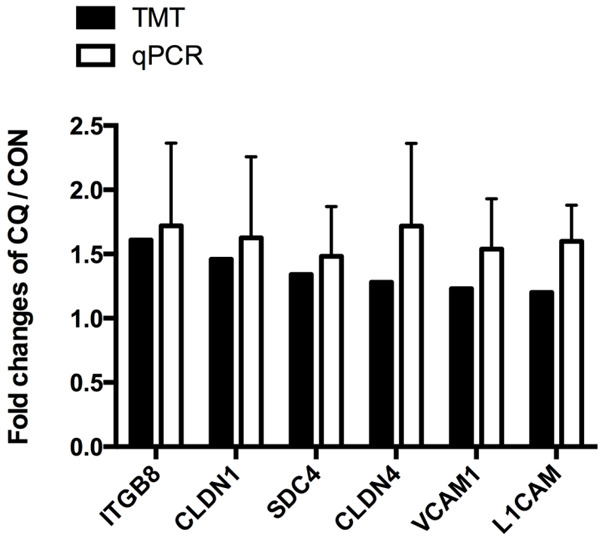
The mRNA expression of 6 key CAMs in human podocytes after CQ treatment. Podocytes were treated with CQ (25 μM) for 12 hours. The mRNA expressions of 6 key CAMs in podocytes were measured by real-time PCR. It showed that mRNA expressions of SDC4, ITGB8, VCAM1, L1CAM, CLDN1 and CLDN4 were consistent with results from TMT-based proteomic analysis. n=4.
Representative active small GTPases decreased in CQ-treated human podocytes
The RhoA family of small GTPases regulates many cellular processes. RhoA, Rac1 and CDC42 are the representative ones and responsible for maintaining the stability of actin cytoskeleton. It has been known that RhoA participates in the formation of actin stress fibers [23]. Rac1 and Cdc42 are responsible for the formation of lamellipodia and filopodia respectively [24]. To verify whether the actin cytoskeleton disassembly is subject to the changes of these small Rho-GTPases, the active form of RhoA, Rac1 and CDC42 were measured after CQ treatment. As shown in Figure 6, active RhoA, Rac1 and CDC42 decreased significantly in podocytes.
Figure 6.
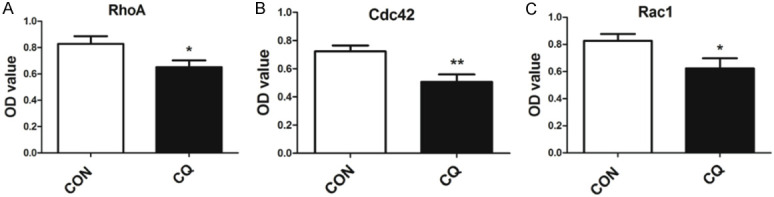
The active form of RhoA, Rac1 and CDC42 decreased in CQ-treated human podocytes. GTP bound (activated) RhoA, Cdc42 and Rac1 in human podocytes were measured with G-LISA assay. Theses three representative active small GTPases decreased after 24 h CQ (25 μM) treatment. *P<0.05, **P<0.01 versus CON, n=6.
Discussion
Podocytes form the outside layer of renal filtration barrier. The stability of actin cytoskeleton architecture in podocyte was crucial for maintaining normal renal filtration. Proteinuria will occur if podocytes detach or actin cytoskeleton undergoes depolymerization. Here we showed the effects of CQ on human podocytes in vitro. In addition, TMT-labeled quantitative proteomics approach was used to explore the underlying mechanisms.
Our results showed that cell motility decreased in CQ-treated human podocytes. It is well known that increased podocyte motility under harmful stimuli disrupts the normal function of renal filtration barrier [1]. Hence, CQ treatment may alleviate podocyte injury by normalizing cell motility. It may explain why CQ and its analogues show therapeutic effects in lupus nephritis [25]. Moreover, CQ might also strengthen cell adhesion. TMT-labeled quantitative proteomics analysis showed that 12 differentially expressed CAMs were involved in cell adhesion. PPI analysis found that 6 of them had at least one protein-protein interaction. They are Syndecan-4, Integrin beta-8, Vascular cell adhesion protein 1, Neural cell adhesion molecule L1, Claudin-1 and Claudin-4. qPCR data of these 6 proteins were consistent with proteomic results, suggesting that their increased protein expression might be regulated at the transcriptional level. Syndecan-4 is a proteoglycan receptor and plays a key role in regulating podocyte adhesion and motility. Overexpression of Syndecan-4 in podocytes reduces RhoA activity which could decrease the formation of actin stress fibre and podocyte motility [26]. Syndecan-4 increased after CQ treatment, suggesting that it may enhance the adhesion between podocytes and glomerular basement membrane, but diminished podocyte motility. Similarly, the other increased 5 CAMs (Integrin beta-8, Vascular cell adhesion protein 1, Neural cell adhesion molecule L1, Claudin-1 and Claudin-4) could also strengthen cell adhesion [27-29]. Taken together, the effects of CQ in podocyte motility and adhesion might be helpful in maintaining normal renal filtration.
Actin cytoskeleton of podocytes could be depolymerized after CQ treatment. It may be attributed to autophagy inhibition [18,19]. CQ is a widely used autophagy inhibitor as it suppresses autolysosomal degradation by increasing lysosomal pH [18]. Our data reveals that lysosome is an important target of CQ treatment in podocytes. There are totally 22 DEPs in which majority of them participate in intracellular degradation and turnover of proteins. Furthermore, we previously demonstrated that CQ could inhibit podocyte autophagy after 24 h treatment [18]. It is well known that autophagy is a highly conserved catabolic process which degrades unwanted cellular components to keep cell homeostasis [30]. Actin cytoskeleton is required for autophagy induction in starved cells, as it not only serves as motors for transporting required components but also engages in membrane invagination to form autophagosomes [31]. On the other hand, autophagy may affect the structure of actin cytoskeleton. Autophagy gene Atg7 deficient fibroblast cells present with depolymerization of actin cytoskeleton [16]. Atg7 or Atg5 mutation in kidney epithelium results in focal segmental glomeruloscerosis (FSGS) which presents with foot process enfacement in rodent models [32]. Thus, it suggested that autophagy not only protects against cell apoptosis but also may safeguard cell structure.
As for the dynamic regulators of actin cytoskeleton, three representative Rho-GTPases (RhoA, Cdc42 and Rac1) have been extensively studied in recent years [33]. For instance, podocyte-specific deletion of Cdc42 leads to severe proteinuria due to foot process effacement which was characterized by actin cytoskeleton depolymerization [34]. Similarly, foot process effacement was also presented in STZ-induced diabetic mice with podocyte-specific deletion of Rac1 [35]. Our data showed that active RhoA, Rac1 and Cdc42 decreased after CQ treatment, suggesting that they might participate in the disruption of actin cytoskeleton. In addition, our finding was also consistent with the migration assay results, as podocyte mobility is regulated by Rho-GTPases. It is known that activation of RhoA could promote podocyte motility, and over-activation or dominant negative RhoA leads to foot process effacement in rodent model [36]. Meanwhile, inactivated Cdc42 and Rac1 reduce cell motility [33]. Thus, decreased active RhoA, Rac1 and Cdc42 might be associated with actin cytoskeleton depolymerization and diminished cell motility in CQ-treated podocytes.
In summary, CQ could alter the stability of podocyte cytoskeleton. It might alleviate podocyte injury in podocytopathy by enhancing cell adhesion and diminishing podocyte motility. On the other hand, it could depolymerize actin cytoskeleton. Additionally, DEPs after CQ treatment were predominantly associated with lysosome, cell adhesion and cytokine-cytokine receptor interaction. These identified DEPs may provide new insights into the effects of CQ on human podocytes. Our findings may explain the efficacy and side effects of CQ and its analogues in treating rheumatic diseases such as lupus nephritis. Even though CQ is a recognized autophagy inhibitor, it is worth verifying whether it damages actin cytoskeleton by inhibiting autophagy. Future studies may investigate the crosstalk between autophagic proteins and actin cytoskeleton. We will explore the signaling pathways controlling podocyte motility and adhesion after CQ treatment. Further investigation on the role of identified DEPs will allow better understanding of the molecular pathways involved in CQ-treated podocytes.
Acknowledgements
We gratefully acknowledge the expert assistance of Dr. Jun-mei Zhou, Dr. Zhu-Ying Li and Dr. Yu-Jie Hu from Shanghai Children’s Hospital, Shanghai Jiao Tong University in performing research experiments. This study was supported by fundings from National Natural Science Foundation of China (#81400723) and Medical excellent youth training program of Shanghai Municipal Health Bureau (#2017YQ073).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Welsh GI, Saleem MA. The podocyte cytoskeleton--key to a functioning glomerulus in health and disease. Nat Rev Nephrol. 2011;8:14–21. doi: 10.1038/nrneph.2011.151. [DOI] [PubMed] [Google Scholar]
- 2.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Feng D, DuMontier C, Pollak MR. The role of alpha-actinin-4 in human kidney disease. Cell Biosci. 2015;5:44. doi: 10.1186/s13578-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud JL, Hosseini-Abardeh M, Farah K, Kennedy CR. Modulating alpha-actinin-4 dynamics in podocytes. Cell Motil Cytoskeleton. 2009;66:166–178. doi: 10.1002/cm.20339. [DOI] [PubMed] [Google Scholar]
- 5.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89:1221–1230. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, Wetzels JF. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int. 2008;74:1568–1576. doi: 10.1038/ki.2008.413. [DOI] [PubMed] [Google Scholar]
- 7.Singh L, Singh G, Dinda AK. Understanding podocytopathy and its relevance to clinical nephrology. Indian J Nephrol. 2015;25:1–7. doi: 10.4103/0971-4065.134531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belingheri M, Moroni G, Messa P. Available and incoming therapies for idiopathic focal and segmental glomerulosclerosis in adults. J Nephrol. 2018;31:37–45. doi: 10.1007/s40620-017-0402-1. [DOI] [PubMed] [Google Scholar]
- 9.Pilania RK, Jindal AK, Sandesh G, Vignesh P, Suri D, Nada R, Singh S. Chylous ascites and podocytopathy as the presentation of childhood lupus-an unusual occurrence. Lupus. 2019;28:244–248. doi: 10.1177/0961203318817831. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava T, Sharma M, Yew KH, Sharma R, Duncan RS, Saleem MA, McCarthy ET, Kats A, Cudmore PA, Alon US, Harrison CJ. LPS and PAN-induced podocyte injury in an in vitro model of minimal change disease: changes in TLR profile. J Cell Commun Signal. 2013;7:49–60. doi: 10.1007/s12079-012-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siso A, Ramos-Casals M, Bove A, Brito-Zeron P, Soria N, Munoz S, Testi A, Plaza J, Sentis J, Coca A. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus. 2008;17:281–288. doi: 10.1177/0961203307086503. [DOI] [PubMed] [Google Scholar]
- 13.Spinelli FR, Moscarelli E, Ceccarelli F, Miranda F, Perricone C, Truglia S, Garufi C, Massaro L, Morello F, Alessandri C, Valesini G, Conti F. Treating lupus patients with antimalarials: analysis of safety profile in a single-center cohort. Lupus. 2018;27:1616–1623. doi: 10.1177/0961203318781008. [DOI] [PubMed] [Google Scholar]
- 14.Imaizumi T, Hayakari R, Matsumiya T, Yoshida H, Tsuruga K, Watanabe S, Kawaguchi S, Tanaka H. Chloroquine attenuates TLR3/IFN-beta signaling in cultured normal human mesangial cells: a possible protective effect against renal damage in lupus nephritis. Mod Rheumatol. 2017;27:1004–1009. doi: 10.1080/14397595.2017.1289646. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Hocker J, Schmid H, Weiss M, Dendorfer U, Braun GS. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry’s disease: case report and review of the literature. Hum Pathol. 2003;34:285–289. doi: 10.1053/hupa.2003.36. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo C, Ji Y, Chen Z, Kitazato K, Xiang Y, Zhong M, Wang Q, Pei Y, Ju H, Wang Y. Proteomics analysis of autophagy-deficient Atg7-/-MEFs reveals a close relationship between F-actin and autophagy. Biochem Biophys Res Commun. 2013;437:482–488. doi: 10.1016/j.bbrc.2013.06.111. [DOI] [PubMed] [Google Scholar]
- 17.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 18.Kang YL, Saleem MA, Chan KW, Yung BY, Law HK. The cytoprotective role of autophagy in puromycin aminonucleoside treated human podocytes. Biochem Biophys Res Commun. 2014;443:628–634. doi: 10.1016/j.bbrc.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Kang YL, Saleem MA, Chan KW, Yung BY, Law HK. Trehalose, an mTOR independent autophagy inducer, alleviates human podocyte injury after puromycin aminonucleoside treatment. PLoS One. 2014;9:e113520. doi: 10.1371/journal.pone.0113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835–1841. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neal CR, Crook H, Bell E, Harper SJ, Bates DO. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol. 2005;16:1223–1235. doi: 10.1681/ASN.2004100822. [DOI] [PubMed] [Google Scholar]
- 22.Neal CR, Muston PR, Njegovan D, Verrill R, Harper SJ, Deen WM, Bates DO. Glomerular filtration into the subpodocyte space is highly restricted under physiological perfusion conditions. Am J Physiol Renal Physiol. 2007;293:F1787–1798. doi: 10.1152/ajprenal.00157.2007. [DOI] [PubMed] [Google Scholar]
- 23.Saito K, Shiino T, Kurihara H, Harita Y, Hattori S, Ohta Y. Afadin regulates RhoA/Rho-associated protein kinase signaling to control formation of actin stress fibers in kidney podocytes. Cytoskeleton (Hoboken) 2015;72:146–156. doi: 10.1002/cm.21211. [DOI] [PubMed] [Google Scholar]
- 24.Mouawad F, Tsui H, Takano T. Role of Rho-GTPases and their regulatory proteins in glomerular podocyte function. Can J Physiol Pharmacol. 2013;91:773–782. doi: 10.1139/cjpp-2013-0135. [DOI] [PubMed] [Google Scholar]
- 25.Norgaard JC, Stengaard-Pedersen K, Norgaard M, de Thurah A. Antimalarials in the treatment of systemic lupus erythematosus: a registry-based cohort study in Denmark. Lupus. 2015;24:299–306. doi: 10.1177/0961203314555351. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Echtermeyer F, Thilo F, Theilmeier G, Schmidt A, Schulein R, Jensen BL, Loddenkemper C, Jankowski V, Marcussen N, Gollasch M, Arendshorst WJ, Tepel M. The proteoglycan syndecan 4 regulates transient receptor potential canonical 6 channels via RhoA/Rho-associated protein kinase signaling. Arterioscler Thromb Vasc Biol. 2012;32:378–385. doi: 10.1161/ATVBAHA.111.241018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elangbam CS, Qualls CW Jr, Dahlgren RR. Cell adhesion molecules--update. Vet Pathol. 1997;34:61–73. doi: 10.1177/030098589703400113. [DOI] [PubMed] [Google Scholar]
- 28.Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann N Y Acad Sci. 2000;915:129–135. doi: 10.1111/j.1749-6632.2000.tb05235.x. [DOI] [PubMed] [Google Scholar]
- 29.Liddelow S, Hoyer D. Astrocytes: adhesion molecules and immunomodulation. Curr Drug Targets. 2016;17:1871–1881. doi: 10.2174/1389450117666160101120703. [DOI] [PubMed] [Google Scholar]
- 30.De Rechter S, Decuypere JP, Ivanova E, van den Heuvel LP, De Smedt H, Levtchenko E, Mekahli D. Autophagy in renal diseases. Pediatr Nephrol. 2016;31:737–752. doi: 10.1007/s00467-015-3134-2. [DOI] [PubMed] [Google Scholar]
- 31.Zientara-Rytter K, Subramani S. Role of actin in shaping autophagosomes. Autophagy. 2016;12:2512–2515. doi: 10.1080/15548627.2016.1236877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakami T, Gomez IG, Ren S, Hudkins K, Roach A, Alpers CE, Shankland SJ, D’Agati VD, Duffield JS. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol. 2015;26:1040–1052. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding WY, Saleem MA. Current concepts of the podocyte in nephrotic syndrome. Kidney Res Clin Pract. 2012;31:87–93. doi: 10.1016/j.krcp.2012.04.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, Vining C, Randolph A, Herbach N, Wanke R, Atkins KB, Gyung Kang H, Henger A, Brakebusch C, Holzman LB, Kretzler M. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int. 2013;84:920–930. doi: 10.1038/ki.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishizaka M, Gohda T, Takagi M, Omote K, Sonoda Y, Oliva Trejo JA, Asao R, Hidaka T, Asanuma K, Horikoshi S, Tomino Y. Podocyte-specific deletion of Rac1 leads to aggravation of renal injury in STZ-induced diabetic mice. Biochem Biophys Res Commun. 2015;467:549–555. doi: 10.1016/j.bbrc.2015.09.158. [DOI] [PubMed] [Google Scholar]
- 36.Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.