Abstract
Acquired tracheal stenosis is a common disease occurring after endotracheal intubation or tracheotomy. Currently, surgery is the main option to treat the stenosis. This study investigated therapeutic effect and possible mechanism of nintedanib on tracheal stenosis. The rabbit models of tracheal stenosis were established and were administered with nintedanib and budesonide. The damage and repair of the tracheal tissue were determined using hematoxylin and eosin (HE) staining. The expression of histone deacetylase 2 (HDAC2), interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF) was detected by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR), Western Blot and immunofluorescence assay. The expression of collagens I and III was assayed immunohistochemically. Remarkable tracheal stenosis was observed after the trachea was brushed in the rabbit model. Compared with control, the stenosis was improved after nintedanib treatment. The mRNA of HDAC2 was increased and that of IL-8 and VEGF was decreased significantly in the tracheal tissue following nintedanib treatment. Western blot analysis showed that HDAC2 increased to the level similar to that of control while VEGF remained unchanged following nintedanib treatment. Budesonide treatment also resulted in increased HDAC2 expression and decreased IL-8 and VEGF expression. Immunofluorescence assays also showed an increased HDAC2 expression following nintedanib treatment. Collagens I and III decreased significantly after nintedanib treatment in the tracheal tissues of models. Therefore, it is concluded that nintedanib alleviates the acquired tracheal stenosis by activating HDAC2 expression and suppressing IL-8 and VEGF expression, and may offer new option to medical treatment for the disease.
Keywords: Tracheal stenosis, nintedanib, HDAC2, VEGF, IL-8
Introduction
Acquired tracheal stenosis is one of the most important late complication after endotracheal intubation or tracheotomy [1]. It is a life-threatening disease that causes different levels of dyspnea, asphyxia or even death. Currently, the pathogenesis of disease is largely known. Tracheal stenosis is mainly caused by granulation tissue formation, fibrosis or scar formation. Due to the pressing and friction of endotracheal tube or tube cannula on the tracheal wall during endotracheal intubation, stress response may be induced in the tracheal mucosa to the release of transforming growth factors (TGFs) and endothelin, leading to increased synthesis of collagen fiber and deposition of extracellular matrix (ECM) [2,3]. In addition, during intubation or tracheotomy, incomplete sterilization of the oropharynx may result in infection of lower respiratory tract [4], leading to increased incidence of tracheal stenosis. For example, it was showed that tracheal stenosis could occur even stenosis animal models were infected with a small amount of staphylococcus [5].
A number of options are available to treat tracheal stenosis and surgical resection is the most effective, particularly in the youths and children [6]. However, the recurrence rate of surgery treatment is still high [7,8] and the therapeutic effect decreases and the recurrent risk increases in multiple surgery [9]. For elder or severe patients, medication treatment for tracheal stenosis has less the side effects [7]. Therefore, medical treatment with drugs such as budesonide is becoming increasingly popular [10]. Budesonide is a glucocorticoid with strong local anti-inflammatory effect, which inhibits the inflammatory response in the airway, reduces local tissue edema and mucus secretion. After atomization, inhaled budesonide forms drug particles with proper diameter that deposit on the respiratory mucosa, increasing the local drug concentration with enhanced anti-inflammatory effect that reduces the edema of the local respiratory mucosa [10]. Nintedanib, a receptor tyrosine kinase (RTK) inhibitor has been developed as therapeutics for idiopathic pulmonary fibrosis, non-small lung cancer and is shown to alleviate dermatitis symptom in OXA-induced animal model [11,12].
To broaden the drugs that may be used to treat tracheal stenosis, we tested the therapeutic effect of nintedanib using rabbit models of acquired tracheal stenosis, examined the pathological changes in the lesion area and analyzed the expression of inflammation-related factors. The findings would help identify new therapeutic drugs for tracheal stenosis.
Materials and methods
Animal model
Thirty adult New Zealand white rabbits, both male and female, aged 13 to 15 weeks and weighing 2.0 to 2.5 kg were purchased from Longping Rabbits, Nanchang, China and used in this study. The animals were housed individually under controlled temperature and lighting, with free access to filtered water and diet. After a week of acclimatization, the animals were used for experiments. All animal experiment protocols were approval by the ethical committee of Guangxi Medical University and carried out according to the guidelines for care and use of laboratory animals and as well as to the principles of laboratory animal care and protection.
Rabbit models of tracheal stenosis was made as previously described [13]. Briefly, the animals were fasted 8 h prior to surgery and anaesthetized by injecting intravenously with 10% chloral hydrate (1 mL/kg). An incision of 4-5 cm long was made on the skin in the anterior region of neck and the subcutaneous tissue was separated until the trachea was exposed. The trachea was incised transversely along the tracheal cartilage between the third and fourth tracheal rings with an incision two-thirds of the circumference of the trachea long. To generate trachea damage, a nylon brush was inserted into the trachea distal to the heart and brushed back and forward for 20 times on the front, side and back walls of the trachea. The brushed trachea was then sutured.
Treatment
The animals were randomly divided into 5 groups (n = 6): the control group (control), where rabbits did not received any treatment, the model group, where the rabbits underwent tracheal brushing but did not receive any drug, normal saline group (model+NS), where the models were aerosolly inhaled with 15 mL saline twice a day, nintedanib group (model+ nintedanib), in which the models were aerosolly inhaled with 10 mg/kg nintedanib twice a day and budesonide group (model+ budesonide, which served as positive control), in which the models were aerosolly inhaled with 0.05 mg/kg budesonide twice a day. All drug treatments last 10 days after the surgery and tracheal brushing. The animals were scarified 2 days after drug treatment by CO2 asphyxiation and used for subsequent experiments.
Hematoxylin and eosin (HE) staining
HE staining was performed as described [14]. Briefly, the tracheas was separated and embedded in paraffin, sectioned and stained with hematoxylin and eosin and examined under microscopy.
Assessment of tracheal stenosis
Trachea was isolated and the length (r1) and width (r2) of endotracheal airway and the length (R1) and width (R2) of the ring surrounded by the ring of trachea cartilage were measured. The tracheal stenosis (S%) was calculated as S = [1-(r1+r2)/(R1+R2)] × 100%.
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was used to quantify the expression of genes at mRNA level. Total RNA was extracted from the tracheal tissues using a RNA Extraction Kit (Takara, Japan) according to manufacturer’s instructions, quantified using a Nanodrop spectrophotometer (NanoDrop Technologies, USA) and reversely transcripted into cDNA using the High Capacity cDNA Transcriptase Reverse kit (Applied Biosystems by Life Technologies, Carlsbad, California, USA) according to manufacturer’s recommendations. qRT-PCR was run with SYBR Premix Ex TaqTM (Takara, Japan) on an Applied Bio-Rad CFX96 instrument using primers listed in Table 1. The relative mRNA levels of vascular endothelial growth factor (VEGF), interleukin (IL-8) and histone deacetylase 2 (HDAC2) were determined using the 2-ΔΔCt method after normalization with rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal reference [15]. The PCR was carried out in a total volume of 10 μl containing 1 μl of diluted and pre-amplified cDNA, 10 μl of TaqMan Gene Expression Master Mix and 1.5 μl of each fluorescence TaqMan probe. The cycling conditions were 50°C for 2 min, 95°C for 10 min followed by 40 cycles, each one consisting of 15 s at 95°C and 1 min at 57°C. Samples were run in triplicate and the mean value was calculated for each case.
Table 1.
Primers for VEGF, IL-8, HDAC2 and GAPDH
| Primer | Sequences | Length of primer (bp) | Length of product (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| VEGF F | CGAGTACATATTCAAGCCTTCC | 22 | 207 | 57.1 |
| VEGF R | CTTGCTCTGTCTTTCTTTGGTC | 22 | ||
| IL-8 F | CTGTTGGTCAGGCCATGAGT | 20 | 125 | 60.1 |
| IL-8 R | AAAGTGCTTCCATGTGCCCT | 20 | ||
| HDAC2 F | AGGAGACTTGAGGGATATTGG | 21 | 228 | 56 |
| HDAC2 R | CATTTAGCATGACCTTTGACTG | 22 | ||
| GAPDH F | GCCGCCCAGAACATCAT | 17 | 192 | 58.2 |
| GAPDH R | TGCCTGCTTCACCACCTT | 18 |
Western blot
Proteins were extracted from the tracheal tissue using a total Protein Extraction Kit from (Beyotime, Beijing) according to manufacturer’s instructions. Approximately 35 μg of protein was separated on 12% SDS-polyacrylamide gel (SDS-PAGE). The protein was transferred to polyvinylidene difluoride membrane (Millipore, USA), blocked with 5% nonfat milk in tris-buffered saline in 0.1% Tween-20 (pH 7.4) for 1 h at room temperature and incubated overnight with primary rabbit anti-human antibodies against HDAC2 (OmnimAbs, OM105905, 1:1000), IL-8 (Abcam, 1:1000) and VEGF (Bioss, 1:1000). A horseradish peroxidase-conjugated antibody against rabbit IgG (1:5000, Abcam, USA) was used as a secondary antibody. Blots were incubated with the ECL reagents (Beyetime, Beijing) for five min and imaged and the intensity of blot signals was quantitated using ultrasensitive chemiluminescence imaging system (ChemiDocXRS+). Three independent assays were performed.
Immunofluorescence assay
Immunofluorescence assay for HDAC2 expression was carried out as previously described [16]. Briefly, tracheal tissue samples were isolated, rinsed three times in phosphate buffer solution (PBS), fixed in fixed with 4% paraformaldehyde and sectioned. After cleared with 0.5% Triton X-100 at room temperature for 20 min, the slices were blocked in 5% BSA at 37°C for 30 min, dropped with rabbit-anti HDAC2 antibody (Omnim Abs, 1:1000), incubated at 37°C for 3 h. Then fluorescein-labelled secondary antibody (1:200) was added and slides were incubated at 37°C for 30 min under the dark. 4’, 6-diamidino-2phenylidole (DAPI) was drop-added and the slides were incubated at room temperature in the dark for 5 min to stain the nuclei. Fluorescence images were captured using a fluorescence microscope (Olympus, Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry assay for collagens was performed as described [16]. Briefly, tracheal tissue samples were embedded in paraffin, sectioned, and hydrated by going through an ethanol series from 100% to 0% for 5 min each. The sections incubated with antibodies against collagens I and III (Bioss, 1:1000) for 2 hours at room temperature followed by incubation with appropriate secondary antibodies for 1 hour before microscopy study.
Statistical analysis
Data were expressed as means ± standard deviation (SD) obtained from at least three independent experiments and analyzed by SPSS version 11.5 for Windows (SPSS Inc., Chicago, IL, USA). For normally distributed continuous variables, means were compared using the student’s t-test or two-way ANOVA with the corresponding post-test. A p-value ≤ 0.05 was considered statistically significant.
Results
Nintedanib reduced tracheal stenosis and inflammation in tracheal tissue
Assessments showed that after brushing the trachea, the stenosis was increased significantly in model and model+NS groups (P < 0.01), while nintedanib and budesonide treatments alleviated the stenosis (P < 0.01, Figure 1A). HE staining showed that mucosa, submucosa, goblet cells and basal cells in the tracheal tissue of control rabbit were compact and intact with loose fibrous connective tissue in the submucosa. After brushing, fibroblast hyperplasia and thicken collagen fiber layers with disordered arrangements were observed in model and model+NS groups, where large amount of inflammatory cells infiltrated into the tracheal tissue. After treatment with nintedanib and budesonide, fibroblast hyperplasia and infiltration of inflammatory cells were significantly reduced (P < 0.01, Figure 1B).
Figure 1.
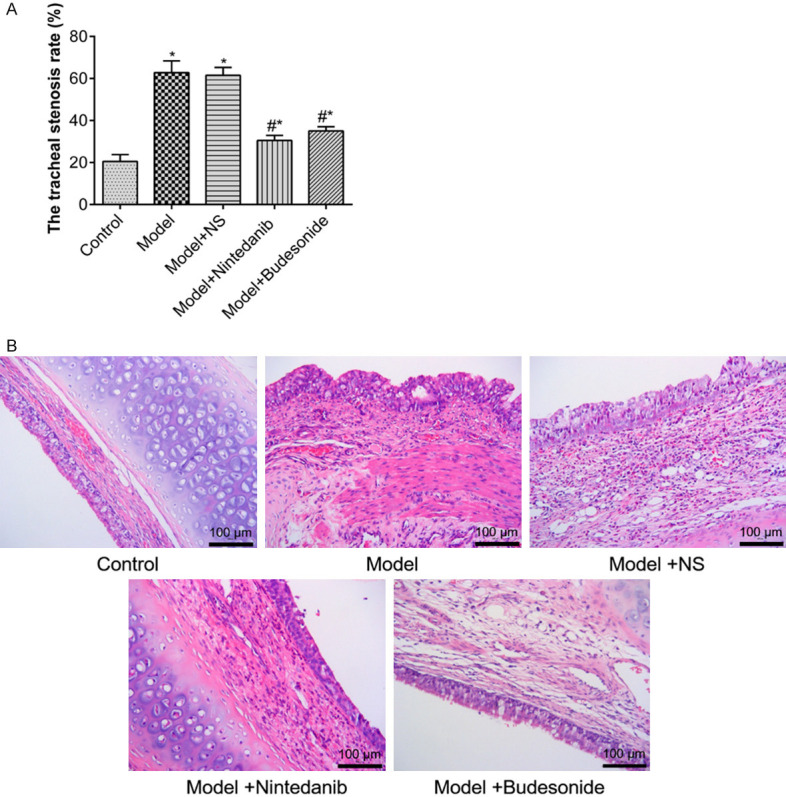
Tracheal stenosis (A) and HE staining (B) of tracheal tissues from rabbits after tracheal brushing and nintedanib treatments 2 day after treatments. Arrows indicated inflammatory cells. * and # denote P < 0.01 vs control and model, respectively.
Nintedanib up-regulated HDAC2 and down-regulated IL-8 and VEGF expression
qRT-PCR analysis showed that at mRNA level, HDAC2 expression was significantly reduced and IL-8 and VEGF expressions were significantly increased in the model and model+NS groups as compared to untreated animals; nintedanib significantly increased HDAC2 expression and decreased IL-8 and VEGF expressions in brushed tracheal tissue (P < 0.01). Similar results were obtained with budesonide (Figure 2). Western blot showed similar effect at protein levels (Figure 3, Supplemetary Figure 1). We further analyzed the expression of HDAC2 in tracheal tissues using immunofluorescence assay. The results showed that red fluorescence from Cy3 coupled to HDAC2 were significantly enhanced after treating the model with nintedanib and budesonide. On the other hand, NS did not generated such increment (Figure 4A). Also, the up-regulation was significantly greater in nintedanib-treated and budesonide-treated rabbits (Figure 4B).
Figure 2.
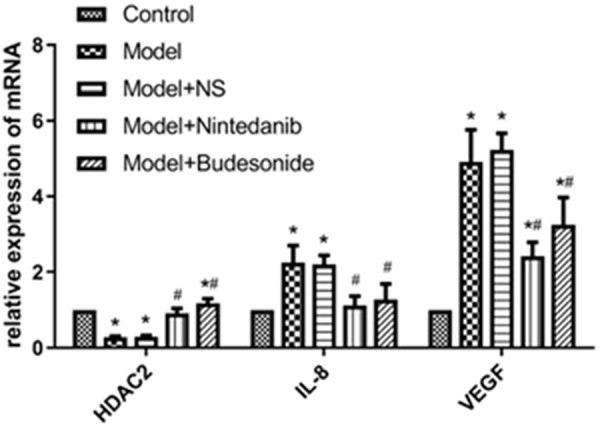
Relative mRNA level of HDAC2, IL-8 and VEGF in tracheal tissues from rabbits after tracheal brushing and nintedanib treatments 2 day after treatments. * and # denote P < 0.01 vs control and model, respectively.
Figure 3.
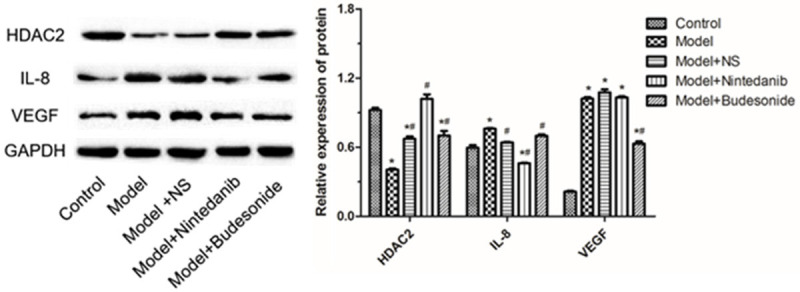
The expression of HDAC2, IL-8 and VEGF proteins in tracheal tissues from rabbits after tracheal brushing and nintedanib treatments 2 day after treatments. Left panel: representative Western blots, right panel: relative protein level. * and # denote P < 0.01 vs control and model, respectively.
Figure 4.
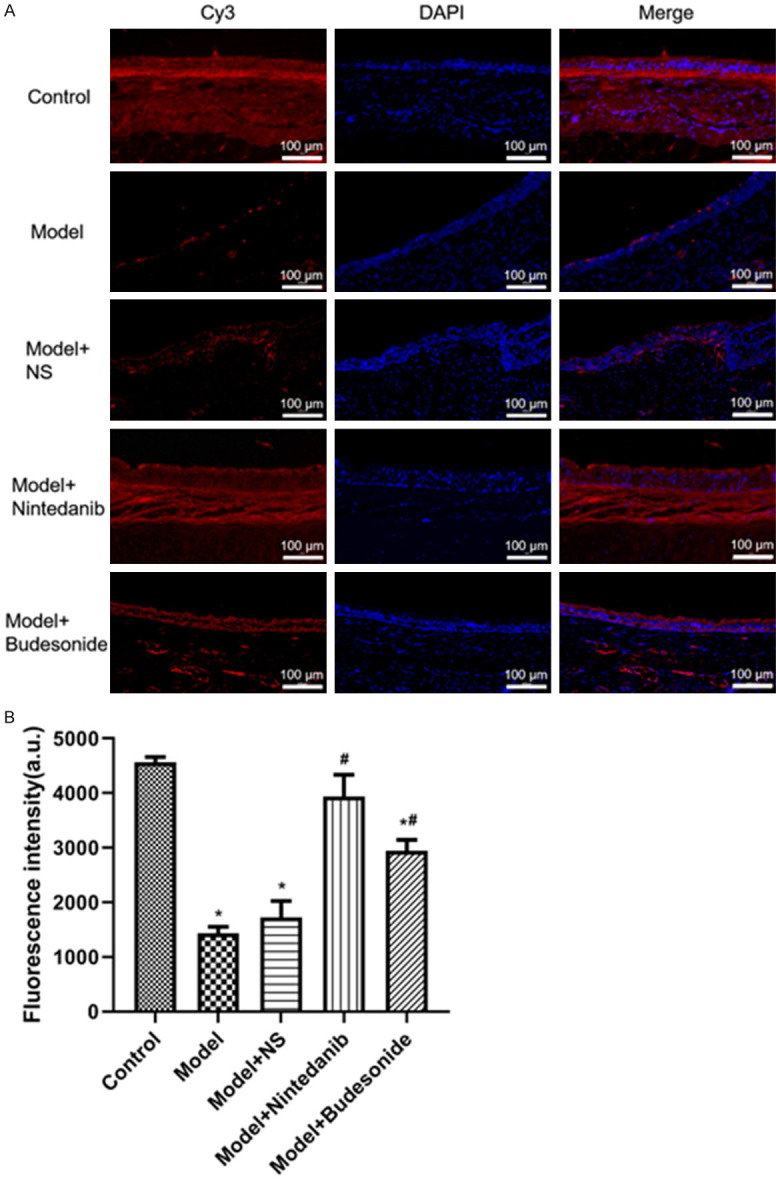
Immunofluorescence assessment of HDAC2 in tracheal tissues from rabbits after tracheal brushing and nintedanib treatments 2 day after treatments. (A) Representative immunofluorescence photos, (B) Immunofluorescence intensity. * and # denote P < 0.01 vs control and model, respectively.
Nintedanib reduced fibrosis in tracheal tissue
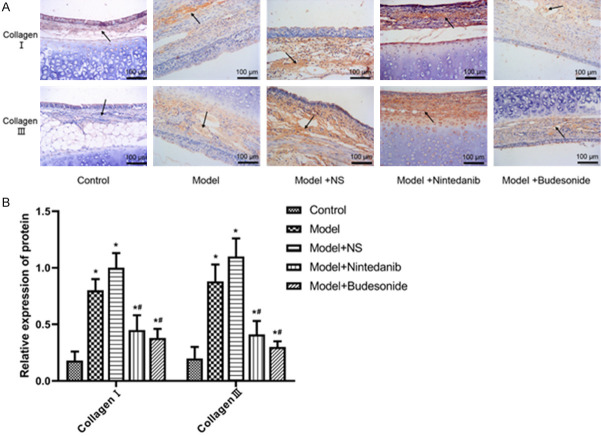
To assess fibrosis in the tracheal tissues, the expression of collagens I and III was detected immunohistochemistrially. Fewer collagens I- and III-expressing cells were detected in normal rabbit and the numbers of these cells increased significantly after the tissue damage in the model group (Figure 5A). On other hand, both nintedanib and budesonide significantly reduced the number of collagen I- and III-expression cells as indicated by reduced protein expression (Figure 5B).
Figure 5.
Assessment of collagens I and III expression in tracheal tissues from rabbits after tracheal brushing and nintedanib treatments 2 day after treatments. A. Immunohistochemistry staining; B. Relative cell numbers. * and # denote P < 0.01 vs control and model, respectively.
Discussion
Our results showed that treatment of nintedanib is effective in alleviating the tracheal stenosis induced by mechanic damage in rabbit models. This alleviation is likely mediated by decreasing HDAC2 down-regulation and -IL-8 and VEGF up-regulation due to brushing-induced damage to the tracheal tissues and would offer new potential medication-based therapy for acquired tracheal stenosis.
To provide insight into the therapeutic effect on the stenosis by nintedanib, we assessed the expression of several inflammation-related factors. HDAC2 is a protease which could play an important role in the structural modification of chromosomes and the regulation of gene expression. HDAC2 could inhibit the expression of inflammatory factors [17]. The expression level of HDAC2 was up-regulated by small dosage of antibiotics such as erythrocin and increased HDAC2 was shown to inhibit the activity of NK-κB and reduce the secretion of TNF-α and IL-8, leading to reduced inflammation [18,19]. VEGF is an important regulator of angiogenesis and is also involved in fibrosis-related diseases [20]. In the animals model, pulmonary fibrosis is shown to result in remarkable structural changes in blood vessels [21]. Currently, the role of VEGF in the tracheal stenosis is largely unknown. However, since it is an effective inducer of vascular permeability and can induce the expression of metalloproteinase (MMP), it may play role in ECM remodeling, wound healing and angiogenesis [22,23]. Stress response due to pressing and friction of endotracheal tube on tracheal wall may induce overexpression of transforming growth factor beta 1 (TGF-β1), IL-8 and VEGF, promoting granulation tissue formation, fibrosis and scar formation that narrow the airway [13,24].
Nintedanib is a triple tyrosine kinase inhibitor and an antagonist to TGF, which can simultaneously block the expression of vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFR) and fibroblast growth factor receptors (FGFR) [25,26]. There are also reports showing that nintedanib could continuously and remarkably inhibit the expression of IL-1β and MMP-1 [27,28]. In our study, HDAC2 expression was upregulated and expression of inflammatory factor IL-8 and VEGF was downregulated significantly by nintedanib in wounded tracheal tissues, suggesting that nintedanib may inhibit granulation tissue hyperplasia, leading to less tracheal stenosis. This is further confirmed with HE staining, which showed that less fibroblast hyperplasia and inflammatory cells when the rabbit models were given nintedanib. Nintedanib is reported to target on the VEGFR to inhibit the angiogenesis and formulation of scar [27]. Our results showed that it is likely that nintedanib may inhibit the function of inflammatory factors by up-regulating the expression of HDAC2, resulting in reduced granulation tissue hyperplasia in the damaged tracheal tissue. Our results also showed that nintedanib has similar therapeutic effect on tracheal stenosis as compared with budesonide (positive control). In addition, at mRNA and protein levels, nintedanib appears to have stronger effect than budesonide.
Since fibrosis often occurs in the tracheal tissue after incubation, we also compared the expression of collagens I and III in the tracheal tissue. Although the numbers of collagen-positive cells were greatly increased after the tissue wounding as compared with unwounded tissue, the numbers were significantly reduced after nintedanib treatment, suggesting that nintedanib may prevent fibrosis in the damaged tissue. The results are consistent with previous investigation, in which nintedanib was found to inhibit the proliferation of fibroblast, differentiation and the secretion of collagen [29]. Also therapeutic effect has been observed for nintedanib on acquired tracheal stenosis in the animal models, more studies are needed to further optimize the dose and duration of medication, to investigate other factors that might contribute to the wounding healing process and possible side effect. Furthermore, the work needs to be validated in other animal models and human for clinical use.
Conclusion
We demonstrate for the first time that nintedanib can alleviate acquired tracheal stenosis in rabbit models by reducing fibrosis and tissue hyperplasia. Nintedanib differentially regulates the expression of HDAC2, IL-8 and VEGF, leading to reduced stenosis. These findings provide cue to develop new medicine for treatment of tracheal stenosis and insight into the mechanism underlying the therapeutic effect.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 81760001).
Disclosure of conflict of interest
None.
Abbreviations
- HE
hematoxylin and eosin
- HDAC2
histone deacetylase 2
- IL-8
interleukin-8
- VEGF
vascular endothelial growth factor
- qRT-PCR
real-time quantitative reverse transcription polymerase chain reaction
- TGF
transforming growth factors
- ECM
extracellular matrix
- RTK
receptor tyrosine kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- SDS-PAGE
SDS-polyacrylamide gel
- PBS
phosphate buffer solution
- DAPI
4’, 6-diamidino-2phenylidole
- SD
standard deviation
- MMP
metalloproteinase
- VEGFR
vascular endothelial growth factor receptor
- PDGFR
platelet-derived growth factor receptors
- FGFR
fibroblast growth factor receptors
Supporting Information
References
- 1.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125:1137–1143. doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ressler B, Lee RT, Randell SH, Drazen JM, Kamm RD. Molecular responses of rat tracheal epithelial cells to transmembrane pressure. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1264–1272. doi: 10.1152/ajplung.2000.278.6.L1264. [DOI] [PubMed] [Google Scholar]
- 3.Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol. 2003;28:142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann S, Sid H, Rautenschlein S. Avian metapneumovirus infection of chicken and turkey tracheal organ cultures: comparison of virus-host interactions. Avian Pathol. 2015;44:480–489. doi: 10.1080/03079457.2015.1086974. [DOI] [PubMed] [Google Scholar]
- 5.Squire R, Brodsky L, Rossman J. The role of infection in the pathogenesis of acquired tracheal stenosis. Laryngoscope. 1990;100:765–770. doi: 10.1288/00005537-199007000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Li S. The research progress of laryngotracheal stenosis in children. J Clin Olorhinolaryngol Head Neck Surg (China) 2018;32:1684–1687. doi: 10.13201/j.issn.1001-1781.2018.21.019. [DOI] [PubMed] [Google Scholar]
- 7.Wray J, Ryde M, Butler CR, Hewitt RJ. Quality of life can be good after slide tracheoplasty for long-segment tracheal stenosis. Interact Cardiovasc Thorac Surg. 2019;29:876–882. doi: 10.1093/icvts/ivz194. [DOI] [PubMed] [Google Scholar]
- 8.Nouraei SA, Ghufoor K, Patel A, Ferguson T, Howard DJ, Sandhu GS. Outcome of endoscopic treatment of adult postintubation tracheal stenosis. Laryngoscope. 2007;117:1073–1079. doi: 10.1097/MLG.0b013e318050ca12. [DOI] [PubMed] [Google Scholar]
- 9.Lewis S, Earley M, Rosenfeld R, Silverman J. Systematic review for surgical treatment of adult and adolescent laryngotracheal stenosis. Laryngoscope. 2017;127:191–198. doi: 10.1002/lary.26151. [DOI] [PubMed] [Google Scholar]
- 10.Yokoi A, Nakao M, Bitoh Y, Arai H, Oshima Y, Nishijima E. Treatment of postoperative tracheal granulation tissue with inhaled budesonide in congenital tracheal stenosis. J Pediatr Surg. 2014;49:293–295. doi: 10.1016/j.jpedsurg.2013.11.041. discussion 295. [DOI] [PubMed] [Google Scholar]
- 11.Heo MJ, Lee C, Choi SY, Choi YM, An IS, Bae S, An S, Jung JH. Nintedanib ameliorates animal model of dermatitis. Sci Rep. 2020;10:4493. doi: 10.1038/s41598-020-61424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grohe C, Gleiber W, Haas S, Mueller-Huesmann H, Schulze M, Atz J, Kaiser R. Efficacy and safety of nintedanib + docetaxel in lung adenocarcinoma patients (pts) following treatment with immune checkpoint inhibitors (ICIs): First results of the ongoing non-interventional study (NIS) VARGADO. Ann Oncol. 2019;30(Suppl 2):ii48. [Google Scholar]
- 13.Nakagishi Y, Morimoto Y, Fujita M, Ozeki Y, Maehara T, Kikuchi M. Rabbit model of airway stenosis induced by scraping of the tracheal mucosa. Laryngoscope. 2005;115:1087–1092. doi: 10.1097/01.MLG.0000163105.86513.6D. [DOI] [PubMed] [Google Scholar]
- 14.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4986. pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Mori H, Cardiff RD. Methods of immunohistochemistry and immunofluorescence: converting invisible to visible. Methods Mol Biol. 2016;1458:1–12. doi: 10.1007/978-1-4939-3801-8_1. [DOI] [PubMed] [Google Scholar]
- 17.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol. 2009;40:464–473. doi: 10.1165/rcmb.2008-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Zhong X, He Z, Wen M, Li J, Peng X, Liu G, Deng J, Zhang J, Bai J. Effect of erythromycin on cigarette-induced histone deacetylase protein expression and nuclear factor-kappaB activity in human macrophages in vitro. Int Immunopharmacol. 2012;12:643–650. doi: 10.1016/j.intimp.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim Biophys Acta. 2013;1832:897–904. doi: 10.1016/j.bbadis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Farkas L, Kolb M. Pulmonary microcirculation in interstitial lung disease. Proc Am Thorac Soc. 2011;8:516–521. doi: 10.1513/pats.201101-007MW. [DOI] [PubMed] [Google Scholar]
- 22.Zhong J, Gencay MM, Bubendorf L, Burgess JK, Parson H, Robinson BW, Tamm M, Black JL, Roth M. ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action and affect cell migration: a comparison between mesothelioma and mesothelial cells. J Cell Physiol. 2006;207:540–552. doi: 10.1002/jcp.20605. [DOI] [PubMed] [Google Scholar]
- 23.Zucker S, Hymowitz M, Conner C, DeClerck Y, Cao J. TIMP-2 is released as an intact molecule following binding to MT1-MMP on the cell surface. Exp Cell Res. 2004;293:164–174. doi: 10.1016/j.yexcr.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Cai Z, Li H, Zhang H, Han S, An R, Yan X. Novel insights into the role of hypoxia-inducible factor-1 in the pathogenesis of human post-intubation tracheal stenosis. Mol Med Rep. 2013;8:903–908. doi: 10.3892/mmr.2013.1595. [DOI] [PubMed] [Google Scholar]
- 25.King CS, Nathan SD. POINT: should all patients with idiopathic pulmonary fibrosis, even those with more than moderate impairment, be treated with nintedanib or pirfenidone? yes. Chest. 2016;150:273–5. doi: 10.1016/j.chest.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Ogura T, Taniguchi H, Azuma A, Inoue Y, Kondoh Y, Hasegawa Y, Bando M, Abe S, Mochizuki Y, Chida K, Kluglich M, Fujimoto T, Okazaki K, Tadayasu Y, Sakamoto W, Sugiyama Y. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1382–1392. doi: 10.1183/09031936.00198013. [DOI] [PubMed] [Google Scholar]
- 27.Inomata M, Nishioka Y, Azuma A. Nintedanib: evidence for its therapeutic potential in idiopathic pulmonary fibrosis. Core Evid. 2015;10:89–98. doi: 10.2147/CE.S82905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangarajan S, Kurundkar A, Kurundkar D, Bernard K, Sanders YY, Ding Q, Antony VB, Zhang J, Zmijewski J, Thannickal VJ. Novel mechanisms for the antifibrotic action of nintedanib. Am J Respir Cell Mol Biol. 2016;54:51–59. doi: 10.1165/rcmb.2014-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.