Abstract
Objective
While metabolic dysfunction occurs in several pulmonary arterial hypertension (PAH) animal models, its role in the human hypertensive right ventricle (RV) and lung is not well characterised. We investigated whether circulating metabolite concentrations differ across the hypertensive RV and/or the pulmonary circulation, and correlate with invasive haemodynamic/echocardiographic variables in patients with PAH.
Methods
Prospective EDTA blood collection during cardiac catheterisation from the superior vena cava (SVC), pulmonary artery (PA) and ascending aorta (AAO) in children with PAH (no shunt) and non-PAH controls (Con), followed by unbiased screens of 427 metabolites and 836 lipid species and fatty acids (FAs) in blood plasma (Metabolon and Lipidyzer platforms). Metabolite concentrations were correlated with echocardiographic and invasive haemodynamic variables.
Results
Metabolomics/lipidomics analysis of differential concentrations (false discovery rate<0.15) revealed several metabolite gradients in the trans-RV (PA vs SVC) setting. Notably, dicarboxylic acids (eg, octadecanedioate: fold change (FC)_Control=0.77, FC_PAH=1.09, p value=0.044) and acylcarnitines (eg, stearoylcarnitine: FC_Control=0.74, FC_PAH=1.21, p value=0.058). Differentially regulated metabolites were also found in the transpulmonary (AAO vs PA) setting and between-group comparisons, that is, in the SVC (PAH-SVC vs Con-SVC), PA and AAO. Importantly, the differential PAH-metabolite concentrations correlated with numerous outcome-relevant variables (e.g., tricuspid annular plane systolic excursion, pulmonary vascular resistance).
Conclusions
In PAH, trans-RV and transpulmonary metabolite gradients exist and correlate with haemodynamic determinants of clinical outcome. The most pronounced differential trans-RV gradients are known to be involved in lipid metabolism/lipotoxicity, that is, accumulation of long chain FAs. The identified accumulation of dicarboxylic acids and acylcarnitines likely indicates impaired β-oxidation in the hypertensive RV and represents emerging biomarkers and therapeutic targets in PAH.
Keywords: metabolites, trans-RV gradient, transpulmonary gradient, β-oxidation, hemodynamics, PAH, human, omics, biomarker, metabolomics, lipidomics
Introduction
Pulmonary arterial hypertension (PAH) is a progressive, fatal disease characterised by obliteration of pulmonary arterioles leading to a vicious cycle of increased pulmonary arterial pressure (PAP), pulmonary vascular resistance (PVR) and right ventricular (RV) pressure overload. The sequelae are RV hypertrophy (RVH), dilation and dysfunction, left ventricular (LV) compression/underfilling, RV capillary rarefication, decreased coronary perfusion (ischaemia, fibrosis) and ultimately RV failure.1–3 The pathobiological processes in PAH include altered gene expression, epigenetic changes, metabolic dysfunction and inflammation in the heart and pulmonary vasculature, but also organs and tissues outside the chest.2 4–6 To date, no universally effective therapies are available, and ≈25%–50% of PAH patients die within 5 years after diagnosis.1
Previous work by others attempted to address possible transpulmonary biomarker gradients of circulating cyclic guanosine monophosphate,7 peptides (endothelin-18 9 and B-type natriuretic peptide7) and proteins (IL-6, Platelet-Derived Growth Factor BB, Transforming Growth Factor beta1, Vascular Endothelial Growth Factor)10 in PAH. However, despite its limitations, sole right heart catheterisation is frequently performed at PH centres, so that, most of the above studies used either peripheral arterial blood10 or even pulmonary arterial wedge specimen7 8 11 as ‘post lung samples’. Most of the very few previously identified molecules with ‘differential transpulmonary concentrations’ were not associated with prognostic haemodynamic variables in these studies.9 10 Although RV size and function are the major determinants of clinical outcome in PAH1 and heart failure with preserved ejection fraction,12 trans-RV biomarker gradients have not been studied at all in children or adults with PAH, or any other cardiovascular disease (CVD), so far.
A few studies identified venous metabolites as potential biomarkers for PAH13 14 or CVD-related mortality.15 Circulating metabolites, particularly fatty acids (FAs), have been shown to reflect cardiac metabolic defects, and deteriorating ventricular function in left heart failure in mice.16 However, to our knowledge, no unbiased screens of trans-RV or transpulmonary metabolite gradients have been pursued in human cardiovascular disease so far. We hypothesised there is differential release or uptake of certain metabolites across both the hypertensive RV and lung, in the circulation of PAH versus non-PAH patients. We further hypothesised that such metabolic alterations are most likely due to high RV pressure afterload, pulmonary arterial shear stress (high PVR), RV-PA uncoupling/decreased RV efficiency, or other pathophysiological changes characteristic for PAH, and thus correlate with clinically relevant variables obtained by cardiac catheterisation and echocardiography.
Methods
Study population and study design
This was a prospective study enrolling consecutively eight PAH patients and eight non-PAH control subjects, all strictly meeting entry criteria, from August 2013 to July 2015. Paediatric PAH was defined as per 2015/2016 international guidelines.1 3 Non-PAH control patients had mild to moderate LV outflow tract obstruction (n=6), benign mediastinal teratoma (n=1), or portal vein stenosis (n=1), see table 1 and online supplementary table S1. We excluded subjects with any intracardiac or extracardiac shunt, end-stage PAH, and/or any recent clinical instability or infection. EDTA blood was collected nearly simultaneously at three sites (superior vena cava (SVC); pulmonary artery (PA); ascending aorta (AAO)). First, we used the Metabolon platform to profile 427 metabolites. Subsequently, we performed lipidomics analysis (using the Lipidyzer platform) in a cohort of eight PAH patients and nine controls (online supplementary tables S2 and S3), which largely overlapped with the subjects used for the Metabolon study. The analysis included 836 lipid species and their corresponding FAs; the latter are reported in the following format throughout the text: Lipid (FA carbon atoms: unsaturated double bonds). We maintained platform-specific abbreviations for (glycero-)phosphatidyl and (glycero-)phosphoethanolamine lipids as PC and PE, respectively, for lipids identified by Lipidyzer and GPC and GPE for lipids identified by Metabolon.
Table 1.
Characteristics of control subjects and PAH patients studied
| Control (non-PAH) (n=8) |
PAH (n=8) |
P value | |
| Demographics | |||
| Age, years (mean, range) | 6.4 (0.4–17) | 7.9 (0.6–18) | n.s. (0.5627) |
| Male sex, n (%) | 4 (50) | 3 (38) | |
| Height (m) | 113.5±16.0 | 118.9±15.6 | n.s. (0.7209) |
| Weight (kg) | 24.1±6.3 | 29.1±8.2 | n.s. (0.9163) |
| BSA (m2) | 0.86±0.18 | 0.95±0.20 | n.s. (0.8330) |
| Disease subtypes (n) | Mild to moderate LVOTO (6), mediastinal teratoma (1), portal vein stenosis (1) | IPAH (3), PAH (2) PAH-repaired CHD (2), portopulmonary PH (1) | |
| Key haemodynamics | |||
| Cardiac catheterisation | |||
| sPAP (mm Hg) | 23.4±2.5 | 68.8±11.4 | 0.0024 |
| mPAP (mm Hg) | 17.3±2.2 | 60.4±8.9 | 0.0009 |
| dPAP (mm Hg) | 12.3±2.1 | 38.4±5.3 | 0.0052 |
| mPAP/mSAP | 0.28±0.03 | 0.82±0.12 | 0.0003 |
| mTPG (mm Hg) | 6.4±1.1 | 51.5±9.2 | 0.0014 |
| dTPG (mm Hg) | 1.6±0.6 | 35.5±8.0 | 0.0033 |
| PVRi (WU·m2) | 1.69±0.30 | 16.3±3.6 | 0.0003 |
| PVR/SVR | 0.11±0.02 | 0.82±0.14 | 0.0012 |
| Qpi | 4.08±0.36 | 3.64±0.52 | n.s. (0.5054) |
| Qsi | 4.46±0.42 | 3.78±0.60 | n.s.(0.3823) |
| Qp/Qs | 0.92±0.04 | 0.99±0.04 | n.s. (0.3823) |
| mRAP (mm Hg) | 5.5±0.6 | 6.6±1.2 | n.s. (0.1605) |
| Echocardiography | |||
| RVAWD (cm) | 0.34±0.03 | 0.72±0.09 | 0.0022 |
| RVEDD (cm) | 1.26±0.21 | 2.27±0.38 | n.s. (0.1098) |
| TAPSE (cm) | 1.95±0.09 | 1.62±0.12 | n.s. (0.1111) |
| LVEF (%) | 74.7±2.2 | 67.7±3.6 | n.s. (0.1419) |
Values are presented as mean±SEM. A Mann-Whitney U test was applied. P<0.05 was considered significant. All PAH patients with repaired CHD (PAH-CHD) had the repair >12 months prior to cardiac catheterisation. Two of the PAH patients had trisomy 21 (all with PAH-repaired CHD; patient ID #7 and #8 in online supplementary table S1).
BSA, body surface area; CHD, congenital heart disease; dPAP, diastolic pulmonary arterial pressure; dTPG, diastolic transpulmonary pressure gradient; IPAH, idiopathic PAH; LVEF, left ventricular ejection fraction; LVOTO, left ventricular outflow tract obstruction; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; mSAP, mean systemic arterial pressure; mTPG, mean transpulmonary pressure gradient; PAH, pulmonary arterial hypertension; PFO, patent foramen ovale; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; PVRi, PVR index; Qp, pulmonary blood flow; Qpi, pulmonary flow index; Qs, systemic blood flow; Qsi, systemic flow index; RVAWD, right ventricular anterior wall diameter; RVEDD, right ventricular end-diastolic diameter; sPAP, systolic pulmonary arterial pressure; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; WU, Wood units.
heartjnl-2019-315900supp001.pdf (1.5MB, pdf)
Materials and methods
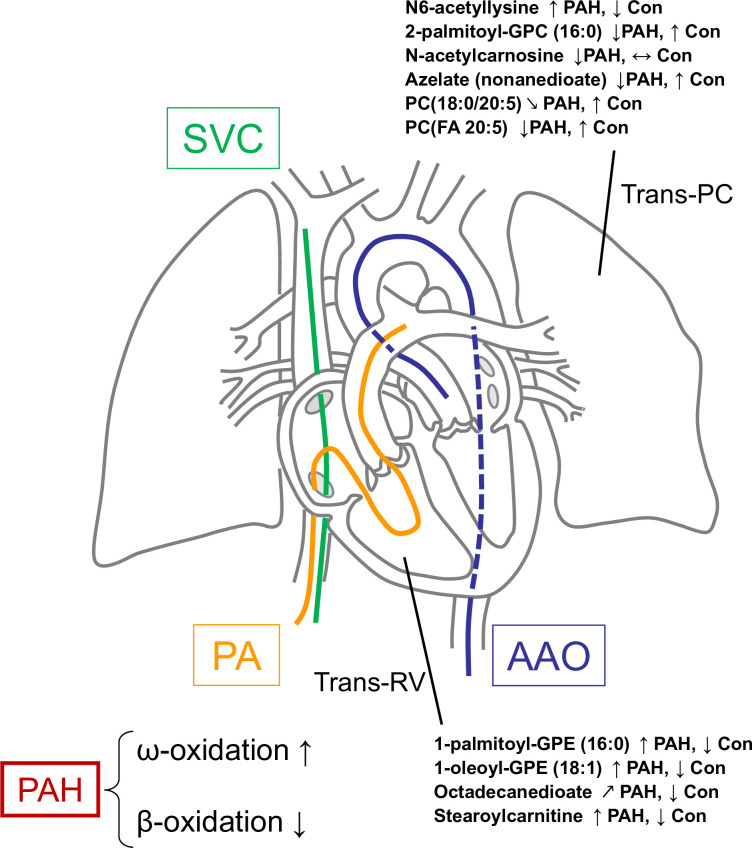
A detailed description of the study design, sample collection, methods and statistical analysis can be found in the online supplement. See also figure 1.
Figure 1.
Catheter positions during cardiac catheterisation used in this study. Selected metabolites in the trans-RV and transpulmonary gradients and the direction of their change are shown. The changes in octadecanedioate suggest disruption of peroxisomal β-oxidation and a compensatory shift to ω-oxidation of fatty acids in the hypertensive RV of PAH patients. Accumulation of stearoylcarnitine in PAH RV indicates incomplete mitochondrial fatty acid oxidation. Accumulation of both octadecanedioate and stearoylcarnitine contribute to lipotoxicity in PAH. A more complete overview of the trans-RV metabolic changes and their likely effects in PAH is presented in figure 6. AAO, ascending aorta; Con, control; GPE, glycerophosphoethanolamine; GPC, glycerophosphocholine; PA, pulmonary artery (right or left); PAH, pulmonary arterial hypertension; PC, phosphatidylcholine; RV, right ventricle; SVC, superior vena cava; Trans-PC, transpulmonary circulation.
Ethics statement
All cardiac catheterisations were clinically indicated. Informed consent for study participation was obtained from the legal caregivers.
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination of our research.
Results
Demographic and clinical characteristics
Demographic and clinical characteristics of subjects for the Metabolon study are shown in table 1. The same information for the Lipidyzer (lipidomics) study is shown in online supplementary table S2. Detailed information on each individual in the study including age, weight, body surface area (BSA), WHO functional class, and medication is provided in online supplementary table S1. PAH and non-PAH study groups were well-matched with respect to age, gender, height, weight and BSA (table 1).
Phenotype discrimination in metabolite profiles of each experimental setting (principal component analysis)
To assess the impact of metabolite variability on phenotype discrimination in each experimental setting (trans-RV and transpulmonary) and per individual catheterisation sites (SVC, PA, AAO) we performed principal component analysis as described in the online supplement. The results indicate adequate phenotype separation in each setting (online supplementary figures S1 and S3), which was better for the trans-RV than the transpulmonary fold changes.
Differential trans-RV gradients (PAH vs control) are dominated by ‘lipid metabolites’ and correlate with invasive haemodynamic and echocardiographic variables
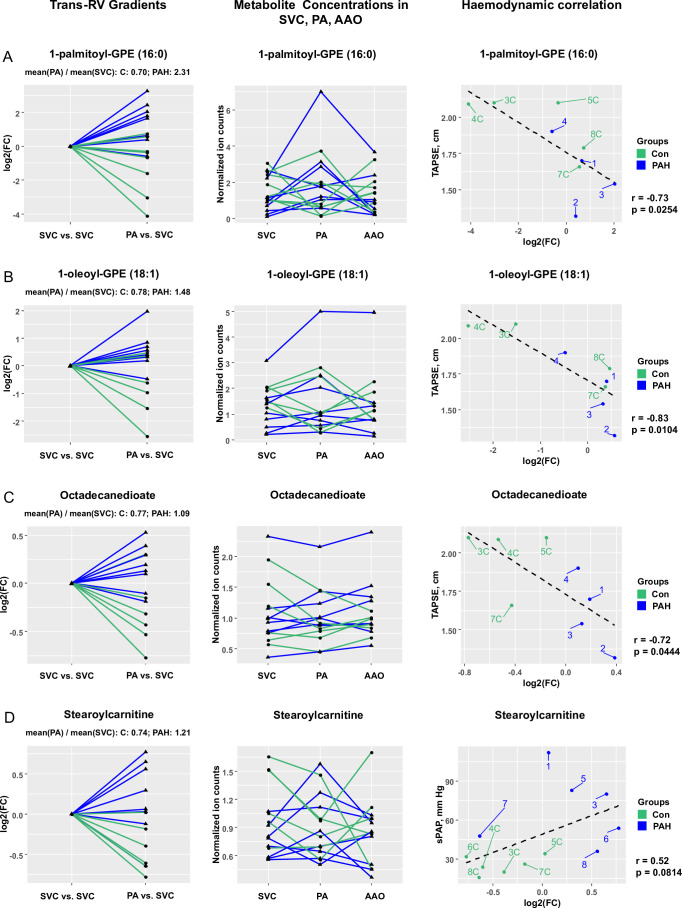
We identified 12 metabolites with significantly different trans-RV (PA vs SVC) plasma concentration gradients (PAH vs control; false discovery rate (FDR)<0.15). Four of these metabolites with the most pronounced differential gradients and probable biological relevance are shown in figures 1 and 2. These are glycerophosphoethanolamines (GPE) (1-palmitoyl-GPE (16:0) and 1-oleoyl-GPE (18:1)), a dicarboxylic acid (octadecanedioate) and an acylcarnitine (stearoylcarnitine), all of which are involved in lipid and phospholipid metabolism. All four of the above metabolites showed a step-up from SVC to PA in PAH patients, and/or the levels dropped from SVC to PA in controls only (step-down in controls; figure 1).
Figure 2.
Several metabolites have differential trans-RV (PA vs SVC) gradients in paediatric PAH (vs non-PAH control). Statistical test: Wald χ2 test (linear mixed effects models). Data filtration: Metabolites with a missing value in at least one of the three catheterisation sites were removed from the gradient analysis. Outliers were removed using a recursive removal procedure for the outliers identified using Grubb’s test as described in the statistics section of the online supplement. (A) Step-up (FC=2.31) in PAH and step-down (FC=0.70) in controls for 1-palmitoyl-GPE (16:0) levels (FDR-adjusted p value=0.0133); raw data (normalised ion counts) for 1-palmitoyl-GPE (16:0) levels in all three catheterisation sites; correlation of 1-palmitoyl-GPE (16:0) levels with haemodynamics (TAPSE). (B) Step-up (FC=1.48) in PAH and step-down (FC=0.78) in controls for 1-oleoyl-GPE (18:1) levels (FDR-adjusted p value=0.0767); raw data (normalised ion counts) for 1-oleoyl-GPE (18:1) levels in all three catheterisation sites; correlation of 1-oleoyl-GPE (18:1) levels with haemodynamics (TAPSE). (C) No change (FC=1.09) in PAH and step-down (FC=0.77) in controls for octadecanedioate levels (FDR-adjusted p value=0.0439); raw data (normalised ion counts) for octadecanedioate levels in all three catheterisation sites; correlation of 11-oleoyl-GPE (18:1) levels with haemodynamics (TAPSE). (D) Step-up (FC=1.21) in PAH and step-down (FC=0.74) in controls for stearoylcarnitine levels (FDR-adjusted p value=0.0582); raw data (normalised ion counts) for stearoylcarnitine levels in all three catheterisation sites; correlation of stearoylcarnitine levels with haemodynamics (sPAP). AAO, ascending aorta; Con, control; FC, fold change; FDR, false discovery rate GPE, glycerophosphoethanolamine; PA, pulmonary artery; PAH, pulmonary arterial hypertension; RV, right ventricle; sPAP, systolic pulmonary arterial pressure; SVC, superior vena cava; TAPSE, tricuspid annular plane systolic excursion.
Comparison of the trans-RV log2 fold changes (for the four metabolites presented in figure 2) with key haemodynamic and echographical variables (defined in table 1) revealed several important correlations, the strongest of which are shown in table 2 and online supplementary table S4, and include tricuspid annular plane systolic excursion (TAPSE), right ventricular anterior wall diameter (RVAWD), systolic pulmonary arterial pressure (sPAP), mean pulmonary arterial pressure (mPAP), PVR index (PVRi), etc. Overall, plasma levels of the aforementioned four ‘lipid metabolites’ (1-palmitoyl-GPE (16:0), 1-oleoyl-GPE (18:1), octadecanedioate, stearoylcarnitine), positively correlated with haemodynamic indicators of RV pressure afterload and PVD severity (sPAP, mPAP, PVRi), and negatively with systolic longitudinal RV function (TAPSE).
Table 2.
Differential trans-RV metabolite concentration gradients and their correlation with invasive haemodynamic/echocardiographic variables
| Metabolite or metabolite ratio | Fold change Control |
Fold change PAH | FDR-adjusted p value | Selected haemodynamic or echocardiographic variables | r | P value |
| Subclass: GPE Role: Phospholipid biosynthesis, glycerophospholipid metabolism, lipid metabolism | ||||||
| 1-palmitoyl-GPE (16:0) | 0.70 | 2.31 | 0.0134 | TAPSE, cm | −0.73 | 0.0254 |
| sPAP, mm Hg | 0.55 | 0.0424 | ||||
| Subclass: GPE Role: Phospholipid metabolism, lipid transport, lipid metabolism, fatty acid metabolism | ||||||
| 1-oleoyl-GPE (18:1) |
0.78 | 1.48 | 0.0767 | TAPSE, cm | −0.83 | 0.0104 |
| sPAP | −0.52 | 0.0533 | ||||
| Subclass: Fatty acids and conjugates Role: Lipid transport, lipid metabolism, fatty acid metabolism | ||||||
| Octadecanedioate | 0.77 | 1.09 | 0.0157 | TAPSE, cm | −0.72 | 0.0444 |
| RVAWD, cm | 0.52 | 0.0560 | ||||
| Tricuspid valve E/A | −0.71 | 0.0226 | ||||
| mPAP, mm Hg | 0.56 | 0.0371 | ||||
| PVRi, WU·m2 | 0.54 | 0.0565 | ||||
| Subclass: Fatty acid esters Role: Lipid transport, Lipid metabolism, Fatty acid metabolism | ||||||
| Stearoylcarnitine | 0.74 | 1.21 | 0.0582 | sPAP, mm Hg | 0.52 | 0.0814 |
For gradient analysis, the p values were generated using Wald χ2 test (FDR<0.15). A more comprehensive data set, including metabolite ratios, is shown in online supplementary table S4.
FDR, false discovery rate; GPE, glycerophosphoethanolamine; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PVRi, pulmonary vascular resistance index; RV, right ventricle; RVAWD, right ventricular anterior wall diameter; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; WU, Wood units.
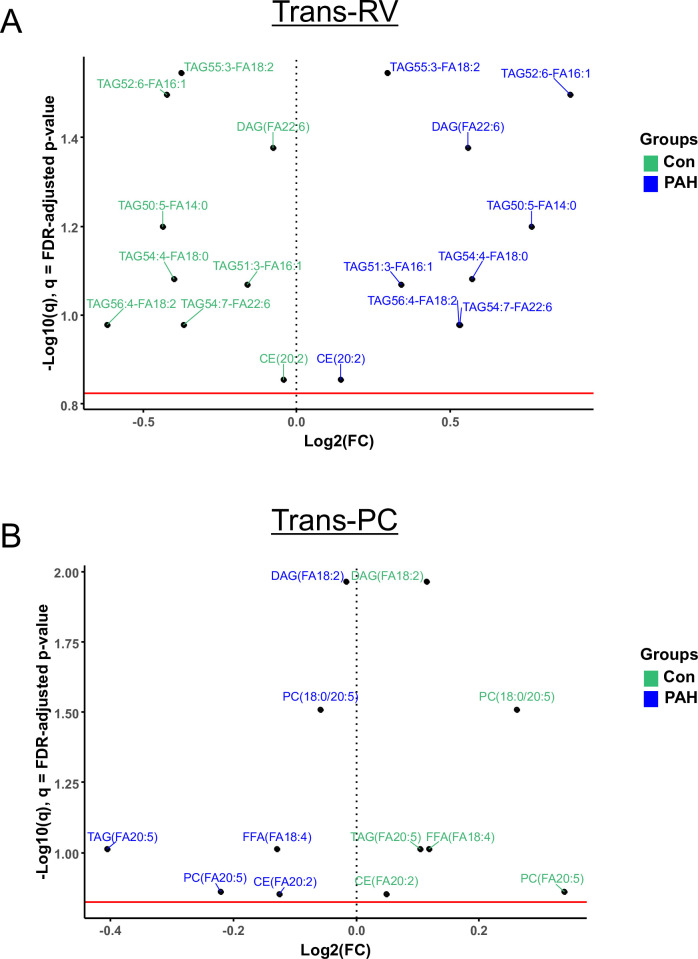
The lipidomics analysis of the trans-RV gradients revealed that seven triacylglycerols (TAGs), one diacylglycerol (DAG(FA22:6)) and one cholesterol ester (CE20:2) had a step-up in PAH and a step-down in controls (figure 3A). The carbon chain lengths of their FA groups ranged between 14 and 20.
Figure 3.
Several lipids have differential trans-RV (PA vs SVC) and transpulmonary (AAO vs PA) gradients in paediatric PAH (vs non-PAH control). Volcano plots (A and B) show pairs of lipids with statistically significant fold change differences. Interestingly, all such lipids had a step-up in PAH and a step-down in controls trans-RV (A), while the fold change direction was reversed (PAH down, non-PAH controls up) for the lipids with significantly different gradients transpulmonary (B). Statistical test: Wald χ2 test (linear mixed effects models). Data filtration: see legend for figure 1. AAO, ascending aorta; CE, cholesterol ester; DAG, diacylglycerol; FA, fatty acid; FFA, free FA; FDR, false discovery rate; FC, fold change; PAH, pulmonary arterial hypertension; PC, phosphatidylcholine; RV, right ventricle; SVC, superior vena cava; TAG, triacylglycerol; Trans-PC, transpulmonary circulation.
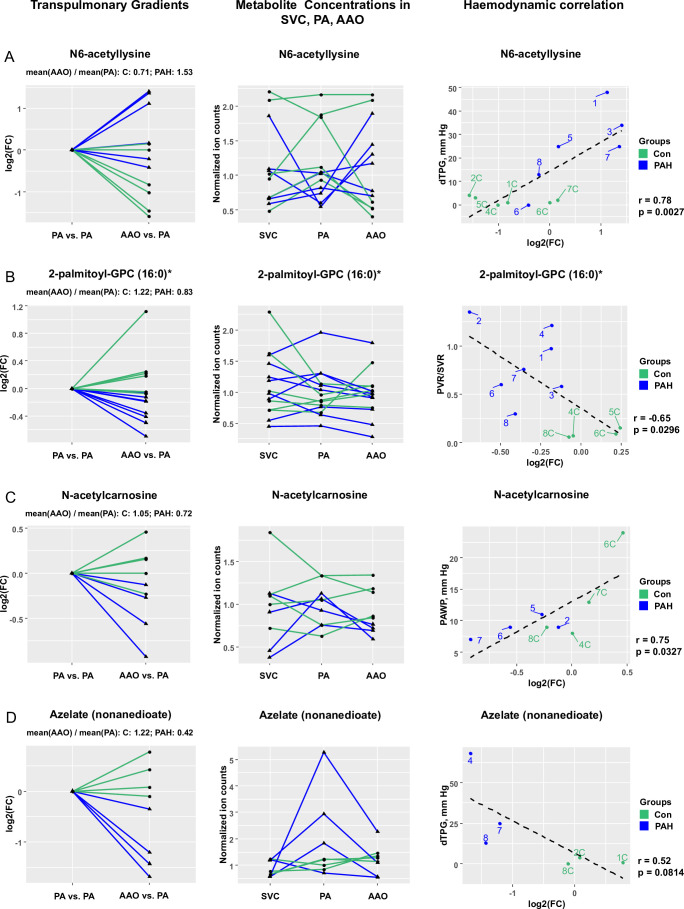
Differential transpulmonary gradients (PAH vs control) of several circulating metabolites exist and correlate with invasive haemodynamic and echocardiographic variables
We identified seven metabolites with significantly (FDR<0.15) different levels (PAH vs control) across the pulmonary circulation (AAO vs PA). The transpulmonary plasma concentration gradients of four of these metabolites, that is, N6-acetyllysine (step up in PAH), 2-palmitoyl-glycerophosphocholine (16:0) (step down in PAH), N-acetylcarnosine (step down in PAH) and azelate (nonanedioate) (step down in PAH) along with their correlation to haemodynamic and echocardiographic variables are shown in figure 4 (presented metabolites were selected based on function and effect size). Further discussion of metabolite and lipid transpulmonary gradients and their correlation with haemodynamics is provided in the online supplement (supplementary results).
Figure 4.
Several metabolites have differential transpulmonary (AAO vs PA) gradients in paediatric PAH (vs non-PAH control). Statistical test: Wald χ2 test (linear mixed effects models). Data filtration: see legend for figure 1. (A) Step-up (FC=1.53) in PAH and step-down (FC=0.71) in controls for N6-acetyllysine levels (FDR-adjusted p value=0.0476); raw data (normalised ion counts) for N6-acetyllysine levels in all three catheterisation sites; correlation of N6-acetyllysine levels with haemodynamics (dTPG). (B) Step-down (FC=0.83) in PAH and step-up (FC=1.22) in controls for 2-palmitoyl-GPC (16:0) levels (FDR-adjusted p value=0.0184); raw data (normalised ion counts) for 2-palmitoyl-GPC (16:0) levels in all three catheterisation sites; correlation of 2-palmitoyl-GPC (16:0) levels with haemodynamics (PVR/SVR). (C) Step-down (FC=0.72) in PAH and no change (FC=1.05) in controls for N-acetylcarnosine levels (FDR-adjusted p value=0.0683); raw data (normalised ion counts) for N-acetylcarnosine levels in all three catheterisation sites; correlation of N-acetylcarnosine levels with haemodynamics (PAWP). (D) Step-down (FC=0.42) in PAH and step-up (FC=1.22) in controls for azelate (nonanedioate) levels (FDR-adjusted p value=0.0006); raw data (normalised ion counts) for azelate (nonanedioate) levels in all three catheterisation sites; correlation of azelate (nonanedioate) levels with haemodynamics (dTPG). AAO, ascending aorta; Con, control; dTPG, diastolic transpulmonary pressure gradient; FC, fold change; FDR, false discovery rate; GPC, glycerophosphocholine; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; SVC, superior vena cava.
Metabolite plasma levels of FAs, bile acids, glycerophosphoinositols and amino acids are altered in PAH at the individual SVC and PA catheterisation sites and correlate with invasive haemodynamic and echocardiographic variables
In order to identify global differences in levels of plasma metabolites for each site, we performed comparisons between groups (PAH vs controls; that is, here subjects did not serve as their own controls). Five metabolites were significantly (FDR<0.15) higher in the SVC and three were differentially concentrated in the PA of PAH patients vs controls (figure 5, online supplement file 1). These metabolites are further discussed in the online supplement (supplementary results).
Figure 5.
Several metabolites have differential levels in paediatric PAH (vs non-PAH control) in SVC and PA. Mean±SEM statistical test: Mann-Whitney U test with FDR correction for multiple testing. Significance levels: *p<0.15, **p<0.05 for FDR-adjusted p values. (A) Plasma levels of heptanoate (7:0) in SVC (significant upregulation), PA and AAO. (B) Plasma levels of caproate (6:0) in SVC (significant upregulation), PA and AAO. (C) Plasma levels of glycocholenate sulfate in SVC (significant upregulation), PA and AAO. (D) Plasma levels of 1-stearoyl-GPI (18:0) in SVC, PA (significant upregulation) and AAO. (E) Plasma levels of N6-acetyllysine in SVC, PA (significant downregulation) and AAO. AAO, ascending aorta; Con, control; FC, fold change; GPI, glycosylphosphatidylinositol; mPAP, mean pulmonary arterial pressure; mAAO, mean ascending aorta pressure; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PVRi, pulmonary vascular resistance index; RVAW, right ventricular anterior wall diameter; RVEDD, right ventricular end-diastolic diameter; SVC, superior vena cava; WU, Wood units.
heartjnl-2019-315900supp002.xlsx (25.8KB, xlsx)
Using metabolite ratios did not improve correlations of metabolites with haemodynamic/echocardiographic variables
We explored the metabolite ratios that were identified based on significantly differentially concentrated metabolites (FDR<0.15) (numerator) and the corresponding metabolites located in a comprehensive network of human metabolites with a distance ≤2 (denominator), as described in the online supplement. Using the ratios did not produce significantly better correlations of metabolites with haemodynamic/echocardiographic variables.
Arachidonate is a hub in metabolite network of second-order correlations
Finally, we performed metabolite network analysis to identify ‘regulatory hub’ metabolites with the largest impact on the identified, differentially concentrated metabolites in each experimental setting (trans-RV, transpulmonary, or between-group comparisons in a single catheterisation site). Among the transpulmonary gradients, arachidonate (20:4n6) stood out as a ‘hub’ node with second-order correlations (r≥0.40) to five other metabolites, however the correlations were only moderate (0.40≤r ≤ 0.49). The detailed results of the metabolite network analysis are presented in the online supplementary figure S2. A complete list of metabolites, metabolite ratios, lipid species and FAs with significantly differential concentration profiles in all experimental settings is in online supplementary file 1. Metabolite annotation and identifiers are presented in online supplementary file 2.
heartjnl-2019-315900supp003.xlsx (62.6KB, xlsx)
Discussion
Here, we first present a translational metabolomics PAH study and demonstrate differential gradients of a number of metabolites across the hypertensive RV and across the pulmonary circulation in blood plasma of PAH versus non-PAH patients (FDR<0.15; online supplementary file 1). We provide the first evidence for a likely major disruption of peroxisomal β-FAO in the hypertensive RV in human PAH, by linking trans-RV accumulation of octadecanedioate to clinically relevant indicators of RV function. Additionally, the detrimental sequelae of dysfunctional peroxisomal β-fatty acid oxidation (FAO) extends to disrupted metabolism of very-long-chain FAs (including the subsequent mitochondrial β-FAO of the corresponding chain-shortened Acyl-CoAs), long-chain dicarboxylic acids, eicosanoids, docosahexaenoic acid (DHA), among other alterations.17
The mechanisms involved in these changes are, most likely, either altered uptake into the organ (lung or heart), or differential release from the organ. The most pronounced differential trans-RV and transpulmonary gradients are presented in the figures 2 and 4. The sections on: (1) accumulation of lysophospholipids across the right ventricle (trans-RV), (2) transpulmonary circulation metabolite gradients, (3) differential same-site metabolite concentration levels in paediatric PAH and (4) trans-RV PAH-specific lipids associated with CVD are presented in the online supplement. The results of our subsequent lipidomics study (Lipidyzer platform) further supported our interpretations and conclusions (online supplementary figures S4 and S6).
Evidence for a block of peroxisomal β-oxidation in the hypertensive RV
In homeostasis, FAs are primarily oxidised by β-oxidation in mitochondria (long-chain, medium-chain and short-chain FAs) and by α-oxidation and β-oxidation in peroxisomes (very-long-chain FAs (VLCFAs), long-chain dicarboxylic FAs, eicosanoids). Additionally, 5%–10% of FAO occurs through ω-oxidation in the smooth endoplasmic reticulum (long-chain and very-long-chain FAs), thus producing long-chain dicarboxylic FAs.18 Acetyl-CoAs, required for delivery of acetyl groups into the tricarboxylic acid (TCA) cycle, are directly produced by peroxisomal and/or mitochondrial β-oxidation of FAs, but not by ω-oxidation.17 18 However, when β-oxidation is disrupted, ω-oxidation provides an alternative to prevent accumulation of the long-chain FAs (LCFAs) and the detrimental effects such FA accumulation may cause in pathological conditions,18 e.g., in PAH.19
Octadecanedioate belongs to the group of dicarboxylic FAs, which are generated by conversion of the terminal methyl group of a FA into a carboxyl group by ω-oxidation; dicarboxylic FAs are then further β-oxidised into shorter dicarboxylic acids (figure 6). As a long-chain dicarboxylic fatty acid, octadecanedioate is β-oxidised in peroxisomes rather than mitochondria.17 Intriguingly, in our translational study, octadecanedioate trans-RV gradients were present and had a step-up in PAH and a prominent step-down in the non-PAH controls (Con) (figure 2C), indicating dysfunctional peroxisomal β-oxidation in PAH versus controls. The latter conclusion is also supported by the observed accumulation of other dicarboxylic acids (dodecanedioate and tetradecanedioate) trans-RV (PAH vs Con), though not reaching the significance threshold (online supplementary table S7). Additionally, the trans-RV Lipidyzer (lipidomics) analysis revealed an accumulation of VLCFAs (carbon chain length ≥22) that are oxidised exclusively by peroxisomal β-oxidation (or much less efficiently by ω-oxidation). In particular, lipid species TAG54:7–FA22:6 with the C22:6 acyl group (DHA) had a significant step-up in PAH and a step-down in controls (online supplementary table S7). In addition, the DAG component of DHA, that is, DAG(FA22:6) also had a step-up in PAH and a small step-down in controls (online supplementary table S7). This cumulative evidence further supports our conclusion about a likely peroxisomal β-oxidation block in the hypertensive RV (online supplementary figure S4 and S6).
Figure 6.
Disruption of β-oxidation in the right ventricle in human PAH indicates major disturbance of lipid and energy metabolism (model). Our results in this article are shown in blue font. Conversion of carboxylic acids to dicarboxylic acids is facilitated by ω-oxidation, which results in conversion of the methyl group (CH3) at the ω-end into a carboxyl group (COOH). The produced dicarboxylic acids (eg, octadecanedioate) are further metabolised by β-oxidation. Unlike β-oxidation, ω-oxidation does not require acyl-CoAs and provides an alternative for disrupted β-oxidation. The accumulation of octadecanedioate (a dicarboxylic acid) in PAH that we demonstrated as trans-RV blood plasma gradient, strongly suggests disruption of peroxisomal β-oxidation in the hypertensive RV, because very long chain and long chain dicarboxylyl-CoAs (such as the end-product of octadecanedioate ω-oxidation) can only be metabolised by peroxisomal β-oxidation. The accumulation of stearoylcarnitine and other acylcarnitines suggests incomplete mitochondrial fatty acid oxidation (FAO), probably resulting from FAO flux outpacing TCA flux. The lipotoxic effects of the molecules with detected accumulation in the PAH RV plasma concentration gradients include: cardiomyocyte apoptosis for octadecanedioate and activation of pro-inflammatory signalling for stearoylcarnitine. Both of these effects are hallmarks of heart failure. Additionally, disruption of β-oxidation results in lower ATP production and thereby impaired cardiac performance. CPT1, carnitine palmitoyltransferase I; ETC, electron transport chain; ER, endoplasmic reticulum; LCFA, long-chain fatty acid; PAH, pulmonary arterial hypertension; RV, right ventricle; TCA, tricarboxylic acid cycle; VLCFA, very-LCFA.
Importantly, octadecanedioate trans-RV gradients correlated with clinically relevant variables of RV systolic function and PAH severity, such as TAPSE (figure 2C), RVAWD, mPAP and PVRi (table 2). Consistently, octadecanedioate was previously reported to be upregulated in both peripheral venous blood13 and explant lung tissue from PAH patients.19
Trans-RV accumulation of metabolites known to drive lipotoxicity in the heart
Aside from the peroxisomal β-oxidation block discussed above, accumulation of octadecanedioate can be attributed to increased LCFA concentrations (including octadecanoic acid (18:0)) due to impaired mitochondrial β-oxidation (manifested via accumulation of acylcarnitines in our dataset). In the Lipidyzer experiments LCFAs accumulated trans-RV (figures 3A and online supplementary table S4). Importantly, accumulated LCFAs, particularly in their saturated form, are considered to be a potent driver of cardiac lipotoxicity,20 for example in neonatal rat ventricular myocytes.21 Additionally, cardiomyopathy is associated with elevated serum levels of TAGs (triglycerides) and non-esterified fatty acids in obesity and type 2 diabetes, that is, diseases which are characterised by accumulation of free fatty acids and neutral lipids within cardiomyocytes.22 It has been reported that lipid overload causes cardiac lipotoxicity leading to cellular dysfunction, cell death and organ dysfunction.22 Cardiomyocyte apoptosis not only decreases RV contractility in PAH, but is a hallmark of heart failure in general,23 besides fibrosis and inflammation. Indeed, several studies provided strong evidence for cardiac lipotoxicity as a metabolic component of PAH-related RV dysfunction in animal models5 24 and PAH patients.25
Incomplete mitochondrial β-oxidation in the hypertensive RV in PAH
In the current study, we found a step-up in trans-RV stearoylcarnitine gradients in PAH and a step-down in controls (figure 2E), suggesting incomplete mitochondrial β-oxidation (ie, lack of reconversion of stearoylcarnitine into Acyl-CoAs) in PAH. The net result is an accumulation of long-chain acylcarnitines due to incomplete β-oxidation still outpacing the TCA cycle flux—a biochemical condition also found in insulin resistance.26 Thus, we conclude that the trans-RV accumulation of stearoylcarnitine in our study is due to incomplete mitochondrial β-oxidation and decreased TCA flux in response to the metabolic switch from glucose and lipid oxidation toward glycolysis.5 Several other acylcarnitines accumulated trans-RV, shown in grey in online supplementary figure S4, however not reaching the pre-selected FDR significance cut-off.
In human PAH-cardiomyocytes, glucose uptake and glycolysis are increased, mitochondrial β-oxidation is decreased (resulting in the smaller net amount of acylcarnitines vs control25), and TCA flux is suppressed even more (probably due to the simultaneous reduction in glucose oxidation). We propose that accumulating, unused acylcarnitines (online supplementary figure S4), such as stearoylcarnitine, are then gradually exported into the blood stream of PAH patients. Moreover, long-chain acylcarnitines have been linked to activation of pro-inflammatory pathways in a macrophage cell line27 thereby potentially contributing to the inflammatory component of heart failure.
Importantly, our lipidomics experiments (Lipidyzer platform) provided additional evidence for incomplete mitochondrial β-oxidation in the hypertensive RV. While Lipidyzer cannot measure accumulation of acylcarnitines that would directly prove this point, we still observed accumulation of LCFAs. In animals LCFAs are preferentially oxidised via mitochondrial β-oxidation, while peroxisomes preferentially β-oxidise FAs not meeting the substrate range of the mitochondria, that is, VLCFAs and branched-chain FAs.28 Therefore, the accumulation of LCFAs observed in our lipidomics experiments further supports our conclusion about compromised mitochondrial β-oxidation in the hypertensive RV (online supplementary figure S4).
Cardiomyocyte FAO in PAH
We previously linked insulin resistance and dyslipidemia to PAH in mice and adult PAH patients.29 30 More recently, we demonstrated that the peroxisome proliferator-activated receptor gamma (PPARγ) agonist pioglitazone reverses PAH and prevents RV failure in the Sugen-hypoxia (SuHx) rat model, by boosting FAO in cardiomyocytes.5 Our current results on disturbed β-FAO in the RV of children with PAH are in line with our previous findings on (1) impaired FAO and mitochondrial disarray in RV tissue from end-stage PAH patients, (2) impaired FAO in the hypertensive RV of SuHx rats and (3) the normotensive RV of mice with targeted deletion of PPARγ in cardiomyocytes.5 Impaired β-FAO in mitochondria and peroxisomes will result in decreased ATP production and thus decreased contractile performance in stressed cardiomyocytes (figure 6).5 However, decreased β-FAO (and induced glycolysis) goes along with decreased oxygen consumption; the latter could be a useful rescue mechanism in end-stage PAH with severe RVH, largely elevated right-ventricular end-diastolic pressure (RVEDP), decreased coronary perfusion, and consequently limited myocardial oxygen supply.
Metabolite gradients correlate with haemodynamic and echocardiographic variables in PAH
Importantly, the alterations of novel circulating metabolite biomarkers for PAH identified in our study correlated with several invasive haemodynamic and echocardiographic variables that are essential to diagnosis, prognosis and outcome in PAH (table 2, online supplementary tables S4–S6), as outlined in the new paediatric PAH risk score.3
Strengths and limitations
We followed strict enrollment criteria for paediatric patients undergoing invasive catheterisation (see methods). As a result, we have relatively small sample sizes. To increase robustness of our data, we excluded measurements with a missing value in at least one of the three catheterisation sites (due to very low concentrations or technical issues). Therefore, we could only unravel the metabolites with large enough concentration gradient difference (figures 2–4) or concentration difference (eg, PAH SVC vs Con SVC, figure 5) (ie, sufficient effect size) to still produce significantly low p values. See the online supplement for more details.
Conclusions
Taken together, we identified for the first time in human disease (PAH) trans-RV and transpulmonary metabolite concentration gradients in blood plasma, which may be involved in (anti-)remodelling processes in the RV and pulmonary vessels. The identified alterations in trans-RV gradients likely indicate (1) a major block in peroxisomal/mitochondrial β-FAO and (2) emergence of lipotoxicity in the hypertensive RV of PAH patients as potential cause for subsequent RV failure. The clinical importance of the differential metabolite levels presented here is supported by their correlations with key haemodynamic and echocardiographic variables used for diagnosis, risk assessment and selection of therapy in clinical PAH. By doing so, we unravelled new metabolic biomarkers and emerging targets for future PAH therapy.
Key questions.
What is already known on this subject?
Metabolic dysfunction occurs in several PAH animal models, however, its role in human disease is not well characterised. We hypothesised that trans-right ventricle (RV) and transpulmonary metabolite concentration gradients exist in PAH, and might correlate with prognostic invasive haemodynamic and echocardiographic variables.
What might this study add?
We performed combined right and left heart catheterisation in PAH patients without shunt, and identified—for the first time—such trans-RV and transpulmonary metabolite gradients. We demonstrate accumulation of dicarboxylic acids (eg, octadecanedioate) and acylcarnitines (eg, stearoylcarnitine), among other metabolites, indicating a block in β-fatty acid oxidation (β-FAO) in the hypertensive right ventricle.
How might this impact on clinical practice?
The identified trans-RV and transpulmonary metabolite gradients not only indicate a major block in β-FAO trans-RV, but also correlate with haemodynamic determinants of clinical outcome, and can become emerging biomarkers and therapeutic targets in PAH.
Acknowledgments
We thank Dr. Ekaterina Legchenko for preparing EDTA plasma samples and Evelyne Steenvoorden for Lipidyzer measurements.
Footnotes
Twitter: @Hansmann_Lab and @PVD_Network
Correction notice: Since this paper was first published online, the ORCID IDs have been added to authors Martin Giera, Harald Bertram and Georg Hansmann
Contributors: PC performed data analysis and wrote the manuscript. MG supervised lipidomics analysis and edited the manuscript for important intellectual content. GK performed initial data analysis and edited the manuscript for important intellectual content. AA performed the metabolomics measurements, quality control, prepared data for metabolite annotation and edited the manuscript for important intellectual content. JA edited the manuscript for important intellectual content. HB performed cardiac catheterisations, obtained blood samples and edited the manuscript for important intellectual content. GH designed the study, obtained IRB approval, blood samples, written consent and funding, performed cardiac catheterisations and data analysis and wrote the manuscript.
Funding: This study was supported by the German Research Foundation (DFG; HA4348/2-2 and HA4348/6-2 KFO311), Kinderherzen (W-H-001-2014), Stiftung KinderHerz (2511-6-13-011) and the European Pediatric Pulmonary Vascular Disease Network (all grants to GH).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Human EDTA plasma samples were handled anonymously, according to the principles expressed in the Declaration of Helsinki. The study was approved by the ethics committee of Hannover Medical School (IRB #2200).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Hansmann G. Pulmonary hypertension in infants, children, and young adults. J Am Coll Cardiol 2017;69:2551–69. 10.1016/j.jacc.2017.03.575 [DOI] [PubMed] [Google Scholar]
- 2. Humbert M, Guignabert C, Bonnet S, et al. . Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019;53:1801887 10.1183/13993003.01887-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansmann G, Koestenberger M, Alastalo TP, et al. . Updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European pediatric pulmonary vascular disease network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019;2019:879–901. [DOI] [PubMed] [Google Scholar]
- 4. Calvier L, Chouvarine P, Legchenko E, et al. . Pparγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab 2017;25:1118–34. 10.1016/j.cmet.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 5. Legchenko E, Chouvarine P, Borchert P, et al. . Pparγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med 2018;10:eaao0303 10.1126/scitranslmed.aao0303 [DOI] [PubMed] [Google Scholar]
- 6. Hong Z, Chen K-H, DasGupta A, et al. . Microrna-138 and MicroRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am J Respir Crit Care Med 2017;195:515–29. 10.1164/rccm.201604-0814OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melenovsky V, Al-Hiti H, Kazdova L, et al. . Transpulmonary B-type natriuretic peptide uptake and cyclic guanosine monophosphate release in heart failure and pulmonary hypertension: the effects of sildenafil. J Am Coll Cardiol 2009;54:595–600. 10.1016/j.jacc.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 8. Meoli DF, Su YR, Brittain EL, et al. . The transpulmonary ratio of endothelin 1 is elevated in patients with preserved left ventricular ejection fraction and combined pre- and post-capillary pulmonary hypertension. Pulm Circ 2018;8:2045893217745019 10.1177/2045893217745019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latus H, Karanatsios G, Basan U, et al. . Clinical and prognostic value of endothelin-1 and big endothelin-1 expression in children with pulmonary hypertension. Heart 2016;102:1052–8. 10.1136/heartjnl-2015-308743 [DOI] [PubMed] [Google Scholar]
- 10. Selimovic N, Bergh C-H, Andersson B, et al. . Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 2009;34:662–8. 10.1183/09031936.00174908 [DOI] [PubMed] [Google Scholar]
- 11. Fares WH, Ford HJ, Ghio AJ, et al. . Safety and feasibility of obtaining wedged pulmonary artery samples and differential distribution of biomarkers in pulmonary hypertension. Pulm Circ 2012;2:477–82. 10.4103/2045-8932.105036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melenovsky V, Hwang S-J, Lin G, et al. . Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–62. 10.1093/eurheartj/ehu193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhodes CJ, Ghataorhe P, Wharton J, et al. . Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017;135:460–75. 10.1161/CIRCULATIONAHA.116.024602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis GD, Ngo D, Hemnes AR, et al. . Metabolic profiling of right Ventricular-Pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol 2016;67:174–89. 10.1016/j.jacc.2015.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang J, Weinstein SJ, Moore SC, et al. . Serum metabolomic profiling of all-cause mortality: a prospective analysis in the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study cohort. Am J Epidemiol 2018;187:1721–32. 10.1093/aje/kwy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müller OJ, Heckmann MB, Ding L, et al. . Comprehensive plasma and tissue profiling reveals systemic metabolic alterations in cardiac hypertrophy and failure. Cardiovasc Res 2019;115:1296–305. 10.1093/cvr/cvy274 [DOI] [PubMed] [Google Scholar]
- 17. Reddy JK. Nonalcoholic steatosis and steatohepatitis. III. peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2001;281:G1333–9. [DOI] [PubMed] [Google Scholar]
- 18. Wanders RJA, Komen J, Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. Febs J 2011;278:182–94. 10.1111/j.1742-4658.2010.07947.x [DOI] [PubMed] [Google Scholar]
- 19. Zhao Y, Peng J, Lu C, et al. . Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One 2014;9:e88727 10.1371/journal.pone.0088727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drosatos K, Schulze PC. Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 2013;10:109–21. 10.1007/s11897-013-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Vries JE, Vork MM, Roemen TH, et al. . Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 1997;38:1384–94. [PubMed] [Google Scholar]
- 22. Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep 2005;7:412–7. 10.1007/s11906-005-0035-y [DOI] [PubMed] [Google Scholar]
- 23. Vonk Noordegraaf A, Chin KM, Haddad F, et al. . Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019;53:1801900 10.1183/13993003.01900-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talati MH, Brittain EL, Fessel JP, et al. . Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med 2016;194:719–28. 10.1164/rccm.201507-1444OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brittain EL, Talati M, Fessel JP, et al. . Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016;133:1936–44. 10.1161/CIRCULATIONAHA.115.019351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schooneman MG, Vaz FM, Houten SM, et al. . Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rutkowsky JM, Knotts TA, Ono-Moore KD, et al. . Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab 2014;306:E1378–87. 10.1152/ajpendo.00656.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schrader M, Costello J, Godinho LF, et al. . Peroxisome-mitochondria interplay and disease. J Inherit Metab Dis 2015;38:681–702. 10.1007/s10545-015-9819-7 [DOI] [PubMed] [Google Scholar]
- 29. Hansmann G, Wagner RA, Schellong S, et al. . Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome Proliferator–Activated receptor-γ activation. Circulation 2007;115:1275–84. 10.1161/CIRCULATIONAHA.106.663120 [DOI] [PubMed] [Google Scholar]
- 30. Zamanian RT, Hansmann G, Snook S, et al. . Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009;33:318–24. 10.1183/09031936.00000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2019-315900supp001.pdf (1.5MB, pdf)
heartjnl-2019-315900supp002.xlsx (25.8KB, xlsx)
heartjnl-2019-315900supp003.xlsx (62.6KB, xlsx)