Abstract
Objectives
We sought to determine whether the relationship between a history of vaginal douching and pelvic inflammatory disease (PID) is mediated by endometrial infection with one or more novel bacterial vaginosis (BV)-associated organisms among Atopobium vaginae, the BV-associated bacterium 1 (BVAB1), neathia (Leptotrichia) amnionii and Sneathia sanguinegens.
Methods
We first conducted log-binomial regression analyses to identify risk factors for endometrial infection in 535 adolescent and adult women with clinically suspected PID in the PID Evaluation and Clinical Health (PEACH) study. We then examined whether endometrial infection by the BV-associated organisms mediated the association between a history of vaginal douching and histologically confirmed PID using inverse probability weighted marginal structural models.
Results
Vaginal douching was significantly associated with endometrial infection with one or more of the targeted BV-associated organisms (relative risk (RR) 1.21, 95% CI: 1.08 to 1.35). The total effect estimate suggested that vaginal douching increased the risk of endometritis by 24% (RR 1.24, 95% CI: 1.03 to 1.49). The controlled direct effect of this association was attenuated with endometrial infection by one or more BV-associated organisms (adjusted RR (aRR) 1.00, 95% CI: 0.57 to 1.74) and endometrial infection by all four BV-associated organisms (aRR 0.95, 95% CI: 0.53 to 1.70) as intermediate variables.
Conclusions
Endometrial infection with one or more of the novel BV-associated organisms partially mediated the relationship between vaginal douching and histologically confirmed endometritis in the PEACH study. Frequent vaginal douching may confer risk for endometritis through increasing the risk of endometrial infection by novel-BV-associated organisms. Other potential pathways should be explored.
Keywords: pelvic inflammatory disease, bacterial vaginosis, epidemiology (general)
Introduction
Pelvic inflammatory disease (PID), the infection and inflammation of a woman’s fallopian tubes (salpingitis) and uterine lining (endometritis), is a frequent and morbid condition among young women. The estimated prevalence of PID among sexually experienced women aged 18–44 years is 4.4%, suggesting that approximately 2.5 million reproductive-aged women have ever been diagnosed with PID.1 Major reproductive and gynecologic sequelae result from PID, including infertility, ectopic pregnancy, recurrent PID and chronic pelvic pain.2
Ness et al previously identified an association between vaginal douching and PID.3 Among women in the PID Evaluation and Clinical Health (PEACH) study, those who reported douching more than once per month were 60% more likely to have histologically confirmed endometritis or upper genital tract gonorrheal or chlamydial infection.3 Another study in a similar cohort, the Gynecologic Infections Follow-Through (GIFT) study found that douching for symptoms or hygiene at least once per month was associated with bacterial vaginosis (BV), diagnosed using Nugent’s criteria.4 BV is a common condition among women, with prevalence estimates ranging from 5% to 36%,5 and is characterised by a shift from a vaginal flora with predominant lactobacilli to one with high concentrations of a diverse collection/array of other aerobic and anaerobic bacteria. These findings support the notion that douching disrupts the normal vaginal flora, which, in turn, promotes colonisation with BV-associated bacteria. Women with BV also have an increased risk of acquiring sexually transmitted infections (STIs), particularly Neisseria gonorrhoeae and Chlamydia trachomatis, which if left untreated can ascend to the upper genital tract and cause PID.6 Although PID is often associated with N. gonorrhoeae and C. trachomatis infection, up to 70% of PID cases are of unknown microbial aetiology, and BV has been directly associated with PID, independent of chlamydial and gonococcal infection.7
Using traditional bacterial cultivation methods, the vaginal flora of women with BV is frequently defined by overgrowth of Gardnerella vaginalis, Ureaplasma species, Mycoplasma hominis, Mobiluncus species, Prevotella species and several other bacteria, including anaerobes.8 The role of recently identified fastidious vaginal microbes in gynecological and reproductive morbidity has not been widely researched. Fredericks et al conducted a broad range PCR sequencing study among women aged 18–42 years to identify novel bacteria in vaginal fluid obtained from 27 women with BV and 46 women without BV.8 They found a strong association between BV and Atopobium vaginae, Sneathia (Leptotrichia) sanguinegens, Sneathia (Leptotrichia) amnionii, and three newly recognised bacteria designated Bacterial Vaginosis-associated bacteria (BVAB): BVAB1, BVAB2 and BVAB3. Furthermore, these bacteria were highly prevalent among women with BV compared with women without BV.
We previously demonstrated that vaginal douching is associated with an increased likelihood of cervical and/or endometrial infection with S. sanguinegens and S. amnionii in the PEACH study, and that endometrial infection by the microorganisms is associated with an increased likelihood for endometritis.9 We suspected that endometrial infection by BV-associated organisms is an intermediate between vaginal douching and endometritis (figure 1). Our objective was to assess the mediation effect of endometrial infection by BV-associated organisms in the relationship between a history of vaginal douching and histologically confirmed endometritis using data collected from the PEACH cohort.
Figure 1.
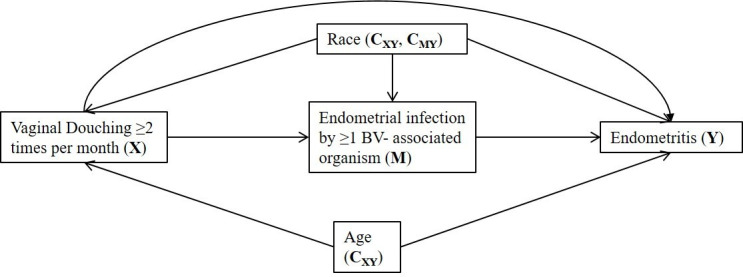
Illustration of the hypothesised associated pathways in the relationship between a history of vaginal douching twice or more in the past month and endometritis in the PEACH study. PEACH, PID Evaluation and Clinical Health; BV, bacterial vaginosis.
Methods
Study population
We conducted this study using baseline data from the PEACH study. Details of subject recruitment and data collection have previously been described.10 In brief, the primary aim of the PEACH study was to compare the effectiveness of inpatient versus outpatient treatment for preventing long-term complications of mild-to-moderate PID. In all, 831 women, aged 14–37 years with clinically suspected PID, were recruited from emergency departments, OB/GYN clinics, STI clinics and private practices in 13 sites throughout the Eastern, Southern and Central regions of the USA. Recruitment for the study took place between March 1996 and February 1999, and the average follow-up time was 84 months by the conclusion of the study in 2004 (69% participant retention). The University of Pittsburgh Institutional Review Board approved this study.
Data collection and laboratory analyses
Baseline data were collected by trained research staff at each study centre using standardised interviews, examinations and specimen collection techniques.10 Information was collected on demographic characteristics, sexual history and behavioural characteristics. Demographic characteristics evaluated as potential risk factors for BV-associated bacterial infection included age, race/ethnicity, marital status, employment, health insurance, and level of education. In addition, we assessed reproductive history, sexual activity in the past 4 weeks, number of lifetime sexual partners, new sexual partner in the past 4 weeks, history of STIs, history of BV, history of PID, recent hormonal contraceptive use, condom use, oral sex, anal sex, age at sexual debut, history of vaginal douching practices, drug and alcohol use, and tobacco use. Vaginal douching at baseline was assessed through questions of douching frequency in the past 4 weeks, type of products used and reasons for douching. This analysis, consistent with prior studies, assessed vaginal douching as frequency two times or more in the past month (Yes or No).
At baseline and at 30 days after enrolment in the parent PEACH study, cervical swabs and endometrial biopsies were obtained for histology, chlamydial PCR (Roche Diagnostics) and gonococcal culture. Endometrial biopsies were obtained using a sterile, single use, disposable endometrial suction curette after first swabbing the exocervix twice with betadine and the endocervix once with betadine. Histological examination was performed on hematoxylin and eosin-stained and methyl green pyronine stained biopsy tissue specimens, read separately by two reference pathologists who reviewed the slide together to reach consensus on disagreement. A modification of the criteria proposed by Kiviat et al was used to diagnose endometritis, defined by at least five neutrophils per ×400 in the endometrial surface epithelium in the absence of menstrual endometrium and/or at least two plasma cells per ×120 in the endometrial stroma. For this current substudy, archived endometrial biopsy and cervical swab specimens were thawed, purified using the MasterPure DNA purification kit for patient specimens (Epicentre) and then tested by species-specific PCR assays: S. sanguinegens, S. amnionii, A. vaginae and BVAB1, as previously described.9
Statistical analysis
We restricted analysis to women in the PEACH cohort who had complete endometrial biopsy and PCR assay baseline data (n=535). Study participants who did not have complete endometrial biopsy and PCR assay baseline data were more likely to be African-American (36.6% vs 21.8% Caucasian/Hispanic/Other race, p=0.0001) and report being a current tobacco smoker (36.5% vs 29.6% non-smokers, p=0.0385). In previous analysis of this cohort, endometrial detection of these four BV-associated organisms, A. vaginae, BVAB1, S. amnionii and S. sanguinegens, were highly correlated with each other, and this microbial community was predictive of subsequent infertility.9 Therefore, we similarly combined PCR results for these four BV-associated bacteria into one variable that indicated endometrial infection with one or more organism, and a variable that indicated infection with all four bacteria. We then conducted univariate analyses to explore a range of characteristics and risk factors for endometrial infection with one or more BV-associated organisms. Lastly, we assessed the unadjusted risk of endometritis in women infected with one or more BV-associated organisms and women infected with all four organisms, stratified by douching frequency.
Figure 1 depicts the pathways of the proposed mediation. We used the counterfactual outcomes framework to assess the mediation effect of the BV-associated organisms (M) in the relationship between a history of vaginal douching and endometritis using inverse probability weighted marginal structural models (online supplementary appendix).11 This allowed for estimation of the controlled direct effect of a history of douching on endometritis.11 The controlled direct effect is an estimate of the relative risk of endometritis among women who douched twice or more a month if all women were not infected with the BV-associated organisms at baseline (M=0), compared with those who did not, and adjusting for exposure-outcome (CXY) and mediator-outcome confounders (CMY) and accounting for interaction between the exposure (douching) and mediator (BV-associated organisms). Confounders were assessed by whether they changed the estimate by >10% when added to the bivariate model predicting the outcome. Based on this method, age (<25 years vs ≥25 years) and race (African-American vs Caucasian/Hispanic/Other) were identified as confounders between a history of douching and endometritis, and race was also a confounder between endometrial infection with the BV-associated organisms and endometritis.
sextrans-2019-054191supp001.pdf (434.8KB, pdf)
The outcome variable was dichotomised into ‘Positive’ or ‘Negative’ for histologically confirmed endometritis at baseline. Log-binomial regression models were used to predict the exposure (douching) and mediator (BV-associated organisms). The inverse of the predicted probabilities from the fitted model was used as weights in subsequent mediation analyses. The inverse probability weighted log-binomial regression models provided adjusted relative risks (aRR) and 95% CI. In addition, we conducted a sensitivity analysis to assess the controlled direct effect of a history of vaginal douching on endometritis independent of endometrial infection with the BV-associated organisms in women who tested negative for N. gonorrhoeae and C. trachomatis infection at baseline (n=273). All analyses were conducted using SAS V.9.4 (SAS Institute, Cary, NC, USA) and STATA 15.
Results
Of the 535 women included in the analysis, 47.5% had histologically confirmed endometritis at study baseline. Vaginal douching twice or more a month was reported by 100 (18.8%) of these participants. The majority of participants had endometrial infection with one or more of these fastidious bacteria (358/535, 66.9%). In univariate analysis, demographic factors significantly associated with endometrial infection with one or more BV-associated organisms included African-American race (RR 1.45, 95% CI: 1.23 to 1.71), being unmarried (RR 1.46, 95% CI: 1.11 to 1.92) and having less than a high school education (RR 1.23, 95% CI: 1.09 to 1.38). In addition, report of having a new sexual partner in the last month (RR 1.18, 95% CI: 1.01 to 1.37), history of anal sex (RR 1.26, 95% CI: 1.01 to 1.56) and having more than seven alcoholic drinks in the past week (RR 1.19, 95% CI: 1.03 to 1.39) were associated with an increased risk of endometrial infection (table 1).
Table 1.
Characteristics of study participants and association with endometrial infection with one or more novel BV-associated organisms in the PEACH study
| Characteristic | Total n=535 N (%) |
Positive * n=358 % |
Negative n=177 % |
RR (95% CI) |
| Demographic | ||||
| Age | ||||
| <25 years | 350 (65.4) | 65.9 | 64.4 | 1.02 (0.90 to 1.16) |
| ≥25 years | 185 (34.6) | 34.1 | 35.6 | Ref. |
| Race/ethnicity | ||||
| African-American | 374 (69.9) | 77.1 | 55.4 | 1.45 (1.23 to 1.71)† |
| Caucasian/Hispanic/Other | 161 (30.1) | 22.9 | 44.6 | Ref. |
| Marital status | ||||
| Unmarried | 439 (88.2) | 91.6 | 81.3 | 1.46 (1.11 to 1.92)† |
| Married | 59 (11.8) | 8.4 | 18.7 | Ref. |
| Education | ||||
| <High school | 205 (38.3) | 43.3 | 28.2 | 1.23 (1.09 to 1.38)† |
| ≥High school | 330 (61.7) | 56.7 | 71.8 | Ref. |
| Sexual health | ||||
| Sexually active in past 4 weeks | ||||
| Yes | 449 (83.9) | 83.2 | 85.3 | 0.95 (0.82 to 1.11) |
| No | 86 (16.1) | 16.8 | 14.7 | Ref. |
| ≥2 Life time sexual partners | ||||
| Yes | 45 (8.4) | 8.7 | 7.9 | 1.03 (0.84 to 1.27) |
| No | 490 (91.6) | 91.3 | 92.1 | Ref. |
| New sexual partner in last month | ||||
| Yes | 57 (10.7) | 12.3 | 7.3 | 1.18 (1.01 to 1.37)† |
| No | 478 (89.3) | 87.7 | 92.7 | Ref. |
| History of STI ‡ | ||||
| Yes | 318 (60.5) | 60.1 | 61.1 | 0.99 (0.87 to 1.11) |
| No | 208 (39.5) | 39.9 | 38.9 | Ref. |
| History of BV | ||||
| Yes | 125 (24.0) | 24.4 | 23.1 | 1.02 (0.89 to 1.18) |
| No | 396 (76.0) | 75.6 | 76.9 | Ref. |
| History of PID | ||||
| Yes | 163 (30.7) | 30.4 | 31.4 | 0.98 (0.86 to 1.12) |
| No | 367 (69.3) | 69.6 | 68.6 | Ref. |
| Recent hormonal contraception use | ||||
| Yes | 98 (21.8) | 20.5 | 24.5 | 0.92 (0.78 to 1.09) |
| No | 351 (78.2) | 79.5 | 75.5 | Ref. |
| Rare/occasional condom use§ | ||||
| Yes | 318 (70.8) | 71.5 | 69.5 | 1.03 (0.89 to 1.20) |
| No | 131 (29.2) | 28.5 | 30.5 | Ref. |
| Consistent condom use¶ | ||||
| Yes | 58 (12.9) | 12.4 | 13.9 | 0.96 (0.78 to 1.17) |
| No | 391 (87.1) | 87.6 | 86.1 | Ref. |
| History of oral sex | ||||
| Yes | 121 (24.2) | 21.9 | 28.6 | 0.88 (0.75 to 1.03) |
| No | 380 (75.8) | 78.1 | 71.4 | Ref. |
| History of anal sex | ||||
| Yes | 18 (3.4) | 4.2 | 1.7 | 1.26 (1.01 to 1.56)† |
| No | 517 (96.6) | 95.8 | 98.3 | Ref. |
| Age at sexual debut | ||||
| ≤15 years | 277 (52.0) | 53.1 | 49.7 | Ref. |
| >15 years | 256 (48.0) | 46.9 | 50.3 | 0.96 (0.85 to 1.08) |
| Risky sexual behaviour** | ||||
| Yes | 404 (90.0) | 90.6 | 88.7 | 1.07 (0.85 to 1.36) |
| No | 45 (10.0) | 9.4 | 11.3 | Ref. |
| Behavioural | ||||
| Vaginal douche ≥2 times in past month | ||||
| Yes | 100 (18.8) | 21.9 | 12.4 | 1.21 (1.07 to 1.38)† |
| No | 433 (81.2) | 78.1 | 87.6 | Ref. |
| Illicit drug use | ||||
| Yes | 143 (26.9 | 28.2 | 24.3 | 1.07 (0.94 to 1.21) |
| No | 389 (73.1) | 71.8 | 75.7 | Ref. |
| Current tobacco smoker | ||||
| Yes | 228 (42.8) | 45.2 | 37.8 | 1.10 (0.98 to 1.24) |
| No | 305 (57.2) | 54.8 | 62.2 | Ref. |
| Alcohol use | ||||
| Yes | 290 (54.4) | 52.5 | 58.2 | 0.93 (0.82 to 1.04) |
| No | 243 (45.6) | 47.5 | 41.8 | Ref. |
| Alcohol drinks per week | ||||
| >7 drinks | 59 (11.1) | 12.9 | 7.3 | 1.19 (1.03 to 1.39)† |
| ≤7 drinks | 474 (88.9) | 87.1 | 92.7 | Ref. |
| Risky social behaviour†† | ||||
| Yes | 270 (50.7) | 52.8 | 46.3 | 1.09 (0.97 to 1.23) |
| No | 263 (49.3) | 47.2 | 53.7 | Ref. |
Missing observations: marital status, n=37; history of STI, n=9; history of BV, n=14; history of PID, n=5; hormonal contraception use, n=86; condom use, n=86; oral sex, n=34; age at sexual debut, n=2; risky sexual behaviour, n=86; vaginal douching, n=2; drug use, n=3; smoking, n=2, alcohol use, n=2; risky social behaviour, n=2.
*Tested positive for Atopobium vaginae, BVAB1, Sneathia amnionii, or Sneathia sanguinegens.
†P value<0.05.
‡History of N. gonorrhoeae, C. trachomatis, or Trichonomas vaginalis.
§Condoms used 0 to 5 out of 10 sexual encounters.
¶Condoms used 10 out of 10 sexual encounters.
**≥2 sexual partners, new sexual partner in the last month, inconsistent condom use.
††Current tobacco smoker, >7 alcohol drinks per week, illicit drug use.
BV, bacterial vaginosis; PEACH, PID Evaluation and Clinical Health; PID, pelvic inflammatory disease; STI, sexually transmitted infection.
History of douching was also associated with an increased risk of endometrial infection with one or more of the BV-associated organisms (RR 1.21, 95% CI: 1.08 to 1.35) and endometrial infection with all four organisms combined (RR 1.75, 95% CI: 1.05 to 2.93) (table 2). In strata of douching frequency, the risk of endometritis was almost double in those who reported douching twice or more a month and who had endometrial infection with one or more or with all of the BV-associated organisms, although the estimates were not statistically significant (RR 1.93, 95% CI: 0.97 to 3.82). Conversely, in strata of endometrial infection with BV-associated organisms, there was a decreased magnitude for risk of endometritis among women with no endometrial infection who reported douching twice or more in a month (RR 0.97, 95% CI 0.54 to 1.75) (online supplementary table 1).
Table 2.
Associations between vaginal douching, endometrial infection with BV-associated organisms1 and endometritis in the PEACH study
| Histologically confirmed endometritis | ||
| Douching <2 times /month n=433 RR (95% CI) |
Douching ≥2 times/month n=100 RR (95% CI) |
|
| Endometrial infection with one or more BV-associated organisms* | ||
| Yes | 1.36 (1.07 to 1.71) | 1.622 (0.91 to 2.61) |
| No | Ref. | Ref. |
| Endometrial infection with all four BV-associated organisms* | ||
| Yes | 1.53 (1.07 to 2.17) | 1.93 (0.97 to 3.82) |
| No | Ref. | Ref. |
*BV-associated organisms: Atopobium vaginae, BVAB1, Sneathia amnionii, or Sneathia sanguinegens.
BV, bacterial vaginosis; PEACH, PID Evaluation and Clinical Health; RR, relative risk.
sextrans-2019-054191supp002.pdf (100.4KB, pdf)
Report of douching twice or more per month at baseline was associated with a 24% increased risk of histologically confirmed endometritis in crude analysis (RR 1.24, 95% CI: 1.03 to 1.49) (table 3). The controlled direct effect of vaginal douching on endometritis was nullified after removing the intermediate effect of endometrial infection with one or more of the bacteria (aRR 1.00, 95% CI: 0.57 to 1.74). The findings were similar after removing the intermediate effect of endometrial infection with all bacteria (aRR 0.95, 95% CI: 0.53 to 1.70).
Table 3.
Crude and controlled-direct associations between vaginal douching and endometritis in the PEACH study
| Total effect RR (95% CI) |
Controlled direct effect accounting for endometrial infection with one or more BV-associated organisms* aRR† (95% CI) |
Controlled direct effect accounting for Endometrial infection with all four BV-associated organisms* aRR† (95% CI) |
|
| Vaginal douche ≥2 times in past month | |||
| Yes | 1.24 (1.03 to 1.49) | 1.00 (0.57 to 1.74) | 0.95 (0.53 to 1.70) |
| No | Ref. | Ref. | Ref. |
*BV-associated organisms: Atopobium vaginae, BVAB1, Sneathia amnionii, or Sneathia sanguinegens.
†Adjusted for race and age.
BV, bacterial vaginosis; PEACH, PID Evaluation and Clinical Health.
In the sensitivity analysis among 273 women who tested negative for N. gonorrhoeae and C. trachomatis infection at baseline, women who reported douching twice or more per month at baseline had an approximately 60% increased risk of histologically confirmed endometritis (RR 1.59, 95% CI: 1.06 to 2.37) (data not shown). This association was nullified after removing the intermediate effect of endometrial infection by one or more of the BV-associated organisms (aRR 0.91, 95% CI: 0.38 to 2.17).
Discussion
In our study of 535 women with clinically suspected PID, we found that infection of the endometrium by A. vaginae, BVAB1, and S. amnionii and/or S. sanguinegens is an intermediate in the relationship between vaginal douching and endometritis. We also identified participant characteristics and risk factors that are correlates of infection by A. vaginae, BVAB1, S. amnionii and/or S. sanguinegens. In particular, endometrial infection by one or more of the BV-associated organisms is associated with race, marital status, level of education, a new sexual partner in the last month, history of anal sex and frequent alcohol consumption. To our knowledge, no previous studies have assessed the mediating effects of novel BV-associated organisms on PID.
Only a handful of studies have examined the risk factors for novel bacteria associated with BV. No previous studies have assessed the risk factors specifically for the bacteria designated as BVAB. In an Australian study assessing 342 women with a range of sexual exposures from two cohorts, A. vaginae was detected in sexually naïve women, but was not found to be significantly associated with high sexual exposure.12 On the other hand, Sneathia (formerly Leptotrichia) spp. was significantly associated with women who engage in sex with women (WSW), and had a strong relationship with increased sexual exposure. In contrast to the findings of this study, none of the sexual exposure predictors were statistically significant in univariate and multivariate analyses for endometrial infection with one or more BV-associated organism. In addition, the PEACH study did not assess the prevalence of WSW in this cohort; therefore, we could not evaluate this as a risk factor in our analysis.
A history of vaginal douching has been previously associated with development of PID in the PEACH study,3 but not in the GIFT study, which was a prospective cohort study.6 The current study suggests that the observed relationship in the PEACH study could in part be mediated by endometrial infection by novel BV-associated organisms. Vaginal douching is a custom in the USA and around the world that is associated with cultural beliefs about cleanliness commonly inherited by women from their own mothers.13–15 In addition to vaginal douching, it has been found that women in the USA partake in vaginal insertion practices with various other products, such as petroleum jelly, for hygiene and lubrication, and these insertions practices also increase the risk of BV.16 The rate of vaginal douching in the USA is estimated at 32.2% in women aged 15–44 years, and is highest among African-American women, followed by Hispanic women, with Caucasian women reporting the lowest rates.17 A randomised, controlled trial in the southeastern USA showed that behavioural interventions, individualised counselling sessions, reduced the practice of vaginal douching by almost 50% among young women who had no intention to change their behaviour at baseline.18 Similarly, a literature review of research on vaginal douching found that healthcare providers have the most influence in discouraging vaginal douching practices since they can relay the associated health risks.19 This was evident in interviews of two generations of African-American mothers and daughters in another randomised clinical trial which found that vaginal douching was less frequent among daughters than among mothers, in part due to healthcare provider messages, which suggests that with increased education and awareness, the trend of vaginal douching acceptance is changing and can potentially be eliminated.17 Our findings suggest that behaviour modification interventions are warranted to discourage the uptake of vaginal douching to reduce the risk of acquiring endometrial infection by BV-associated organisms, and subsequently developing PID. In the present study, it is not clear whether practising vaginal douching leads to a change in the vaginal microbial composition, or whether women are douching to alleviate symptoms associated with changes in the microbial composition. Further research is needed to assess the effect of douching cessation and vaginal bacterial outcomes.
Strengths of our study include the large study sample size and the objective measure of the mediator and outcome using laboratory techniques, which reduce the chance of misclassification error. In addition, we were able to apply counterfactual mediation methods to determine controlled direct effects and adjust for mediator-outcome confounders.11 Our study is limited by the cross-sectional design of our analysis, which did not allow us to prove a causal association. Prospective studies are needed to determine the temporal relationship between vaginal douching, BV-associated organisms and PID. Furthermore, as vaginal douching is measured by self-report, it is possible that women may have under-reported this behaviour, thus resulting in underestimation of the total effect of vaginal douching on endometritis. In addition, given that the PEACH study concluded in 2004, it is possible that the microbial aetiology of PID may have evolved. Lastly, although women in the PEACH study are generally representative of all women with PID in terms of risk profile, all PEACH study participants had clinically suspected PID at enrolment, which may limit the generalisability of our findings.
Our study sought to determine whether novel BV-associated organisms are mediators in the relationship between vaginal douching and histologically confirmed endometritis in the PEACH study. To our knowledge, this is the first study to examine the potential mediation effect of novel BV-associated microorganisms on vaginal douching and endometritis. This effect is important to define to better target PID prevention efforts. In particular, vaginal douching cessation would have a major impact on reduction of PID based on the premise that endometrial infection with one or more BV-associated organism results from vaginal douching. Additional research should be conducted to elucidate other demographic and behavioural risk factors for infection with novel BV-associated organisms in women. Other than vaginal douching and sexual exposure, little else is known about the aetiology of these novel organisms. Furthermore, optimal treatment options should be investigated in light of drug resistance to commonly used therapies. For instance, the A. vaginae strains tested to date have been resistant to metronidazole, the current first line of treatment for uncomplicated BV infection.20 Recurrent BV infection has also been attributed to the ineffectiveness of metronidazole, confirming the urgency for new and more effective therapies.20 In conclusion, whether endometrial infection by novel BV-associated organisms is on the causal pathway between vaginal douching and endometritis warrants further research to provide impetus on the part of healthcare providers to (1) strongly discourage the initiation of vaginal douching, particularly among women of reproductive age and especially adolescents and (2) optimise screening for and treatment of novel BV-associated organisms to prevent the adverse sequelae of PID.
Key messages.
While vaginal douching has been found to be associated with pelvic inflammatory disease, it is unknown whether infection with novel bacterial vaginosis-associated organisms is on the causal pathway.
Using inverse probability weighted marginal structural models allowed for determination of the controlled direct effect of vaginal douching on endometritis after adjusting for confounders.
Endometrial infection with either Atopobium vaginae, BVAB1, Sneathia amnionii and/or Sneathia sanguinegens was found to partially mediate the association between vaginal douching and endometritis.
Acknowledgments
The authors acknowledge Dr Michael Ferris and Johana Norori for their contribution to the PEACH study.
Footnotes
Handling editor: Anna Maria Geretti
Contributors: All authors have seen and approved the manuscript, have contributed significantly to the work, and do not have conflicts of interest to report.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R01AI073940 to C.L.H.]
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: University of Pittsburgh Institutional Review Board (IRB number: 0608052).
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement: Data are available upon reasonable request with approval from the corresponding author at the contact provided.
References
- 1. Kreisel K, Torrone E, Bernstein K, et al. Prevalence of Pelvic Inflammatory Disease in Sexually Experienced Women of Reproductive Age - United States, 2013-2014. MMWR Morb Mortal Wkly Rep 2017;66:80–3. 10.15585/mmwr.mm6603a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992;19:185–92. [PubMed] [Google Scholar]
- 3. Ness RB, Soper DE, Holley RL, et al. Douching and endometritis: results from the PID evaluation and clinical health (peach) study. Sex Transm Dis 2001;28:240–5. 10.1097/00007435-200104000-00010 [DOI] [PubMed] [Google Scholar]
- 4. Ness RB, Hillier SL, Richter HE, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol 2002;100:765 10.1016/s0029-7844(02)02184-1 [DOI] [PubMed] [Google Scholar]
- 5. Morris M, Nicoll A, Simms I, et al. Bacterial vaginosis: a public health review. BJOG 2001;108:439–50. 10.1111/j.1471-0528.2001.00124.x [DOI] [PubMed] [Google Scholar]
- 6. Ness RB, Hillier SL, Kip KE, et al. Douching, pelvic inflammatory disease, and incident gonococcal and chlamydial genital infection in a cohort of high-risk women. Am J Epidemiol 2005;161:186–95. 10.1093/aje/kwi025 [DOI] [PubMed] [Google Scholar]
- 7. Haggerty CL, Hillier SL, Bass DC, et al. Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis 2004;39:990–5. 10.1086/423963 [DOI] [PubMed] [Google Scholar]
- 8. Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 2005;353:1899–911. 10.1056/NEJMoa043802 [DOI] [PubMed] [Google Scholar]
- 9. Haggerty CL, Totten PA, Tang G, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect 2016;92:441–6. 10.1136/sextrans-2015-052285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ness RB, Soper DE, Peipert J, et al. Design of the PID evaluation and clinical health (peach) study. Control Clin Trials 1998;19:499–514. 10.1016/S0197-2456(98)00022-1 [DOI] [PubMed] [Google Scholar]
- 11. VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology 2009;20:18–26. 10.1097/EDE.0b013e31818f69ce [DOI] [PubMed] [Google Scholar]
- 12. Fethers K, Twin J, Fairley CK, et al. Bacterial vaginosis (bv) candidate bacteria: associations with bv and behavioural practices in sexually-experienced and inexperienced women. PLoS One 2012;7:e30633 10.1371/journal.pone.0030633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rupp R, Short MB, Head-Carroll Y, et al. Intergenerational transfer of douching information. J Pediatr Adolesc Gynecol 2006;19:69–73. 10.1016/j.jpag.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 14. Ness RB, Hillier SL, Richter HE, et al. Why women douche and why they may or may not stop. Sex Transm Dis 2003;30:71–4. 10.1097/00007435-200301000-00014 [DOI] [PubMed] [Google Scholar]
- 15. Rajamanoharan S, Low N, Jones SB, et al. Bacterial vaginosis, ethnicity, and the use of genital cleaning agents: a case control study. Sex Transm Dis 1999;26:404–9. 10.1097/00007435-199908000-00008 [DOI] [PubMed] [Google Scholar]
- 16. Brown JM, Hess KL, Brown S, et al. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol 2013;121:773–80. 10.1097/AOG.0b013e31828786f8 [DOI] [PubMed] [Google Scholar]
- 17. Mark H, Sherman SG, Nanda J, et al. What has changed about vaginal douching among African American mothers and daughters? Public Health Nurs 2010;27:418–24. 10.1111/j.1525-1446.2010.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimley DM, Oh MK, Desmond RA, et al. An intervention to reduce vaginal douching among adolescent and young adult women: a randomized, controlled trial. Sex Transm Dis 2005;32:752–8. 10.1097/01.olq.0000190018.58079.05 [DOI] [PubMed] [Google Scholar]
- 19. Cottrell BH. An updated review of of evidence to discourage douching. MCN Am J Matern Child Nurs 2010;35:102–7. 10.1097/NMC.0b013e3181cae9da [DOI] [PubMed] [Google Scholar]
- 20. Sobel R, Sobel JD. Metronidazole for the treatment of vaginal infections. Expert Opin Pharmacother 2015;16:1109–15. 10.1517/14656566.2015.1035255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
sextrans-2019-054191supp001.pdf (434.8KB, pdf)
sextrans-2019-054191supp002.pdf (100.4KB, pdf)


