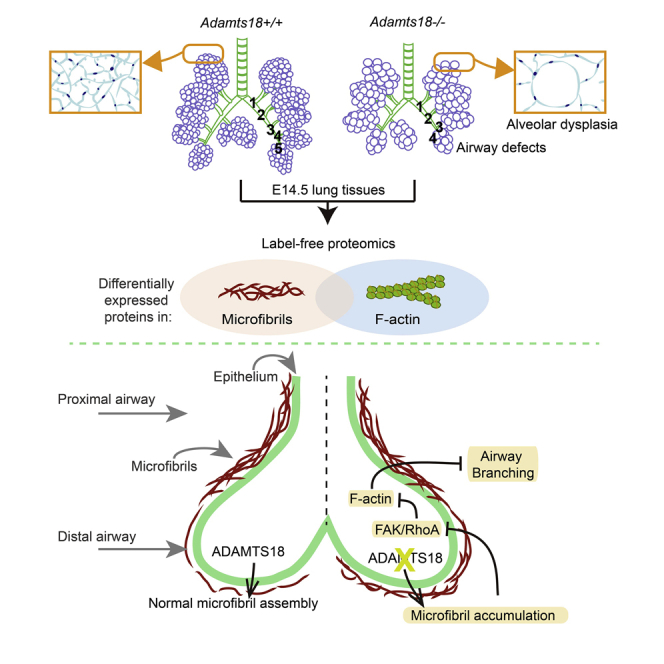
Summary
ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) are secreted metalloproteinases that play a major role in the assembly and degradation of the extracellular matrix (ECM). In this study, we show that ADAMTS18, produced by the epithelial cells of distal airways and mesenchymal cells in lung apex at early embryonic stages, serves as a morphogen in lung development. ADAMTS18 deficiency leads to reduced number and length of bronchi, tipped lung apexes, and dilated alveoli. These developmental defects worsen lipopolysaccharide-induced acute lung injury and bleomycin-induced lung fibrosis in adult Adamts18-deficient mice. ADAMTS18 deficiency also causes increased levels of fibrillin1 and fibrillin2, bronchial microfibril accumulation, decreased focal adhesion kinase signaling, and disruption of F-actin organization. Our findings indicate that ECM homeostasis mediated by ADAMTS18 is pivotal in airway branching morphogenesis.
Subject Areas: Developmental Genetics, Molecular Biology
Graphical Abstract
Highlights
-
•
ADAMTS18 serves as a morphogen in early lung development
-
•
ADAMTS18 deficiency increases lung susceptibility to injuries
-
•
ADAMTS18 affects airway branching by regulating bronchial microfibril abundance
Developmental Genetics; Molecular Biology
Introduction
The lungs provide two vital physiological functions including passive gas exchange (alveolar respiration) and innate immune defense against microbial infections. Early lung development has a lifelong effect on respiratory health and disease (Stocks et al., 2013). Factors that adversely affect lung development may accelerate lung function decline and worsen respiratory morbidity in adulthood. Therefore, identification of key cellular and molecular mechanisms involved in early lung development is important for the development of novel strategies to prevent lung diseases.
ADAMTSs (a disintegrin and metalloproteinase with thrombospondin motifs) are a group of 19 secreted metalloproteinases with major roles in the assembly and degradation of the extracellular matrix (ECM). Previous studies have shown that some of these enzymes are produced by lung cells and are involved in lung pathophysiology. Among them, ADAMTS1 is secreted by developing lung epithelial cells at embryonic stages (Thai and Iruela-Arispe, 2002). Adamts9 mRNAs are expressed in interstitial cells at E14.5 (Jungers et al., 2005). Adamts10 mRNAs are present in the cells surrounding the bronchial tree and blood vessels at E14.5 to E17.5 (Somerville et al., 2004). ADAMTS1, 4, 9, 12, and 15 have been implicated in asthma (Di Valentin et al., 2009; Kurz et al., 2006; Paulissen et al., 2006).
In humans, ADAMTS18 mutations have been linked to tumorigenesis (Jin et al., 2007), developmental eye disorders (Aldahmesh et al., 2011, Aldahmesh et al., 2013; Peluso et al., 2013), reduced bone mineral density (Xiong et al., 2009), and decreased white matter integrity of the brain (Lopez et al., 2012). To further study the role of ADAMTS18 in vivo, we developed an Adamts18 knockout (Adamts18−/−) mouse strain (Lu et al., 2017) and found that Adamts18−/− mice exhibited severely dilated alveoli. This novel finding prompted further studies on the role of ADAMTS18 in lung pathophysiology.
In this study, we demonstrated that Adamts18 is spatiotemporally expressed in the branching epithelium of distal airways and mesenchymal cells in lung apex at early development stages. We also found that ADAMTS18 deficiency leads to reduced number and length of bronchi, tipped lung apex, dilated alveoli, and increased susceptibility to lung injuries. In addition, ADAMTS18 was found to affect bronchus branching partly by interacting with fibrillin1 (FBN1) and regulating the abundance of microfibrils.
Results
Expression of Adamts18 in Mouse Lungs
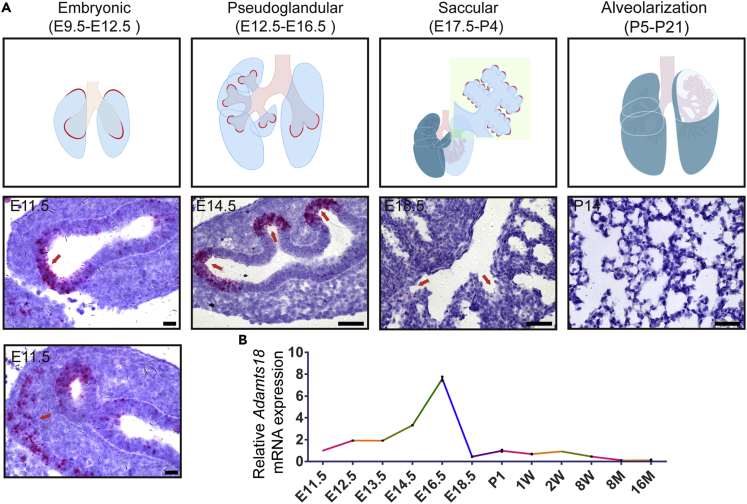
To investigate the role of ADAMTS18 in lung development, Adamts18 mRNA distribution at different developmental stages was determined by in situ hybridization. In embryonic lungs (E9.5–E12.5), Adamts18 mRNAs were detected in the epithelium of branching tips and mesenchymal cells in lung apex. At pseudoglandular stage (E12.5–E16.5), Adamts18 mRNAs were most abundant in distal epithelium. At saccular stage (E17.5–P4), the levels of Adamts18 mRNAs were very low in the epithelium of the distal part of bronchiole. At alveolarization stage (P5–P21), Adamts18 mRNAs were barely detectable in both airway and alveolar cells (Figure 1A). Determination of Adamts18 mRNA levels by quantitative RT-PCR (qRT-PCR) in lung tissues from E11.5 to 16-month-old mice demonstrated that Adamts18 is a phase-specific gene and is expressed only in the early embryonic stages (Figure 1B).
Figure 1.
Spatiotemporal Expression of Adamts18 mRNAs in Mouse Lungs
(A) Upper panels: Cartoon picture illustrates the spatiotemporal expression pattern of Adamts18 mRNAs at different stages of lung development in mice. Lower panels: In situ hybridization (ISH) of Adamts18 mRNA in wild-type mouse lungs (consecutive transverse sections of lung tissue). ISH-positive signals are shown as pink dots in cells (red arrows). Scale bar, 50 μm.
(B) qRT-PCR analysis of Adamts18 mRNA from E11.5 to 16-month-old mouse lungs (n = 3/time point). The relative quantity of Adamts18 mRNA was normalized to that of the housekeeping gene Gapdh using the ΔΔCt method. Data are expressed as mean ± SEM.
See also Table S5.
Decreased Number and Length of Bronchi due to ADAMTS18 Deficiency
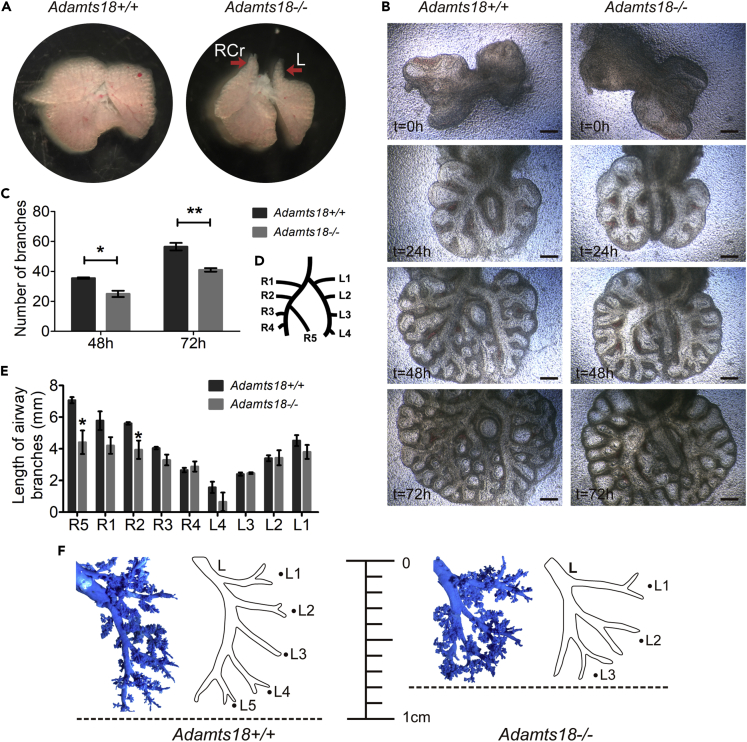
At E14.5, the lung apexes of Adamts18−/− mice were bilaterally tipped, whereas those of Adamts18+/+ mice had a smooth contour (Figure 2A). To characterize branching morphogenesis, E11.5 lungs of both Adamts18+/+ and Adamts18−/− mice were in vitro cultured and examined every 24 h for 72 h (Figure 2B). No macroscopic differences between the two groups of lung explants were observed at the time of dissection. At 24 h of culture, Adamts18−/− lungs exhibited an abnormal branching pattern at the distal part of the right lung. At 48 and 72 h of culture, the number of branches in Adamts18−/− lungs was significantly decreased compared with that of Adamts18+/+ lungs (48 h, 35.5 ± 0.5 versus 25 ± 2.9, p = 0.031; 72 h, 56.5 ± 2.5 versus 41 ± 1.6, p = 0.007) (Figure 2C). In addition, the lengths of R5 and R2 secondary bronchi (Figure 2D) in cultured Adamts18−/− lung explants were significantly shorter than those of Adamts18+/+ lung explants (R5, 7.07 ± 0.14 mm versus 4.41 ± 0.61 mm, p = 0.018; R2, 5.61 ± 0.06 mm versus 3.94 ± 0.47 mm, p = 0.031) (Figure 2E), suggesting a role of ADAMTS18 in epithelial mobility. At 12 weeks old, left lung airway casting showed a decreased bronchus number in Adamts18−/− mice compared with Adamts18+/+ controls. The length from apical to the bottom was shorter in Adamts18−/− lungs than in normal Adamts18+/+ lungs (Figure 2F).
Figure 2.
Bronchial Tree in Mouse Lungs
(A) Representative images of embryonic lungs at E14.5. Red arrows indicate tipped apexes of Adamts18−/− lungs.
(B) E11.5 lungs were cultured in vitro and photographed at different time points (0–72 h). Scale bar, 200 μm.
(C) Branch number of each explant at 48 and 72 h of culture.
(D) Cartoon illustration of bronchial branches in lungs. L1-L4 and R1-R5 represent bronchial branches in left and right lungs, respectively.
(E) Lengths of L1-L4 and R1-R5 at 48 h of culture. Scale bar, 50 μm.
(F) Left lobe lung cast of 12-week-old Adamts18+/+ and Adamts18−/− mice.
Results in (C and E) are expressed as mean ± SD. (n = 4; ∗p < 0.05; ∗∗p < 0.01; Student's t test). These experiments were repeated independently at least three times. RCr, right cranial; L, left.
See also Figures S2 and S5.
Abnormal Lung Morphology in Adamts18−/− Mice
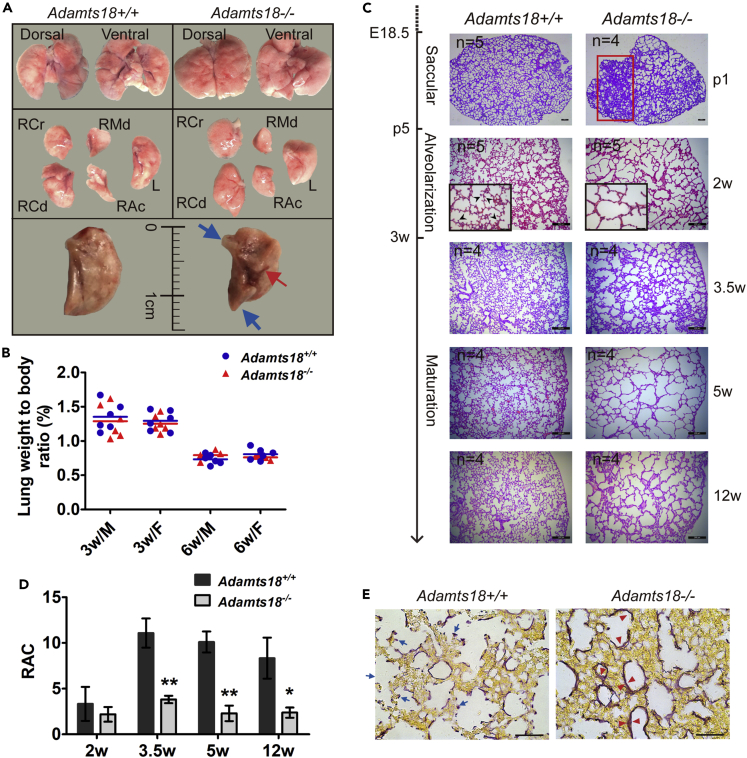
Adult Adamts18−/− lungs exhibited several morphologic features that are different from those of Adamts18+/+ lungs, including tipped distal part, bulged center part in the ventral side of left lobes, and shorter axis length (Figure 3A). There was no significant difference in the ratio of lung weight to body weight between Adamts18−/− and Adamts18+/+ mice (Figure 3B). Histological analyses of lungs showed linear atelectasis at postnatal day 1 and dilated alveoli with decreased number of radical alveolar counts (RACs) in Adamts18−/− mice after alveolar maturation (Figures 3C and 3D). Elastin and collagen are the two main ECM proteins during alveolar septation. With Hart's staining, a thicker elastin layer was observed on the alveolar walls of 2-week-old Adamts18−/− mice compared with their Adamts18+/+ littermates (Figure 3E). Determination of mRNA levels by qRT-PCR of key proteins involved in elastic fiber synthesis, assembly, and degradation revealed that only the expression of Tropoelastin and elastin degradation protease Mmp2 was significantly increased in Adamts18−/− lungs; no significant change in the expression of other molecules was observed (Figure S1A). In addition, lung collagen of the two genotypes of mice showed no significant difference at mRNA levels by qRT-PCR (Figure S1B) and protein levels by Sirius red staining (Figure S1C).
Figure 3.
Abnormal Lung Morphogenesis in Adamts18−/− Mice
(A) Gross morphology of 12-week-old adult lungs. Dorsal and ventral views are displayed in upper panels. Separated right cranial (RCr), right middle (RMd), right accessory (RAc), right caudal (RCd), and left (L) lobes are shown in middle panels. In lower panels, blue arrows indicate tipped distal parts of the left lobe in Adamts18−/− mice. Red arrow denotes a ventral central bulge.
(B) Lung weight to body weight ratio (%) at 3 and 6 weeks (w). M, male; F, female. Each dot or triangle represents one individual; data means are shown by solid horizontal lines.
(C) Representative images of hematoxylin and eosin (H&E)-stained lung sections of Adamts18+/+ and Adamts18−/− mice at indicated development stages. Red box indicates an area with linear atelectasis in Adamts18−/− lung at postnatal day 1 (p1). Black arrowheads in boxed area indicate sprouting secondary crests in Adamts18+/+ lungs. Scale bar, 200 μm.
(D) Quantification of radial alveolar count (RAC). Results are expressed as mean ± SD (n = 4 or 5/group; ∗p < 0.05; ∗∗p < 0.01; Student's t test).
(E) Representative Hart's staining of lung elastin fibers in 2-week-old mice. Blue arrows indicate thin elastin distribution in secondary crests and on alveolar walls in Adamts18+/+ mice. Red arrowheads indicate thicker elastin deposition on alveolar walls in Adamts18−/− mice. Scale bar, 50 μm.
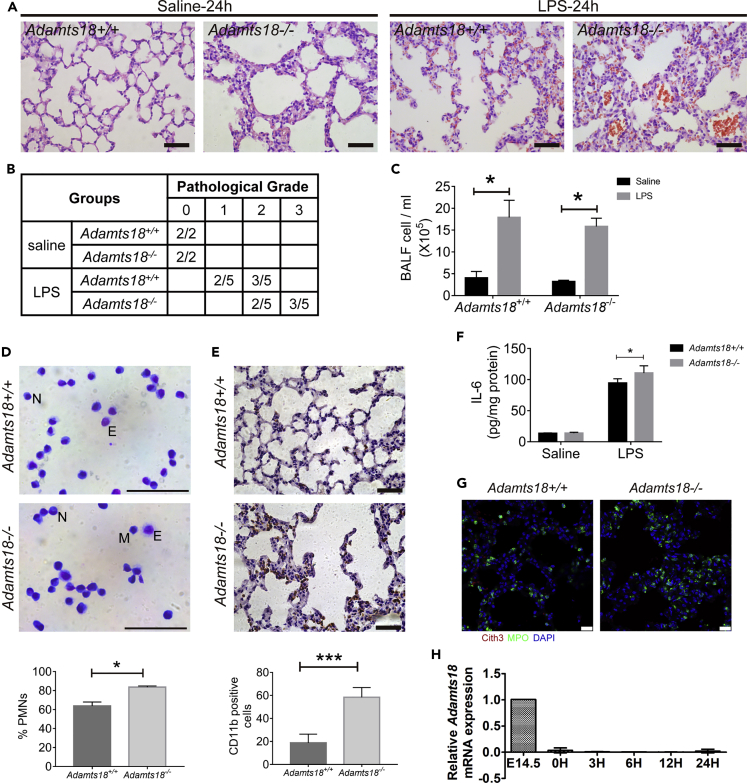
Increased Susceptibility to LPS-Induced Acute Lung Injury and Bleomycin-Induced Lung Fibrosis due to ADAMTS18 Deficiency
There were no differences in basic lung functions between Adamts18+/+ and Adamts18−/− mice (Table S1). However, Adamts18−/− mice (8 weeks old) showed more severe pathological injury (e.g., inflammation and bleeding) with a higher pathological score than Adamts18+/+ littermates (8 weeks old) after intraperitoneal injection of lipopolysaccharide (LPS) (Figures 4A and 4B). Bronchoalveolar lavage fluid (BALF) cell counts were significantly increased 24 h after LPS injection in both genotypes of mice compared with saline-injected control mice (Figure 4C). However, Adamts18−/− lungs had a higher percentage of polymorphonuclear neutrophil (PMN) in BALF than Adamts18+/+ lungs (Figure 4D). Adamts18−/− lungs also showed a significant increase in CD11b+ neutrophil infiltration and interleukin (IL)-6 expression in injured lung tissues (Figures 4E and 4F). The release of neutrophil extracellular traps (NETs) was barely detectable in both genotypes of mice (Figure 4G).
Figure 4.
Increased Susceptibility of Adamts18−/− Mice to Lipopolysaccharide (LPS)-Induced Acute Lung Injury
(A) Representative images of H&E-stained lung sections of saline-treated (left panels) or LPS-treated (right panels) mice. Scale bar, 50 μm.
(B) Pathological grade of lung injury (n = 5/group).
(C) Total number of cells in bronchoalveolar lavage fluid (BALF) collected 24 h after LPS treatment. Results are expressed as mean ± SD (n = 3/group, ∗p < 0.05, Student's t test).
(D) Diff-quick staining of BALF cells for quantification of neutrophils (% polymorphonuclear neutrophil). N, neutrophil, E, eosinophil, M, monocyte. Scale bar, 50 μm. Results are expressed as mean ± SD (n = 3/group, ∗p < 0.05, Student's t test).
(E) Immunostaining of CD11b+ neutrophils in lung sections of LPS-treated mice. Quantification of CD11b+ cells in each microscopic field was performed with ImageJ. Each lung section was analyzed for 5 fields. Results are expressed as mean ± SD (N = 5/group, ∗∗∗p < 0.001, Student's t test). Scale bar, 50 μm.
(F) IL-6 expression in lung tissues of saline- or LPS-treated mice was analyzed by ELISA. Results are expressed as mean ± SD (n = 5/group, ∗p < 0.05, Student's t test).
(G) Confocal microscopy of Cit-H3+MPO+ neutrophil extracellular traps (NETs) in mouse lung sections. Scale bar, 100 μm.
(H) Relative mRNA levels of Adamts18 at different time points of LPS-treated Adamts18+/+ mice determined by quantitative real-time RT-PCR (n = 3/time point). The quantity of Adamts18 mRNA was normalized to that of the housekeeping gene Gapdh using the ΔΔCt method. Data are expressed as mean ± SEM.
See also Table S4.
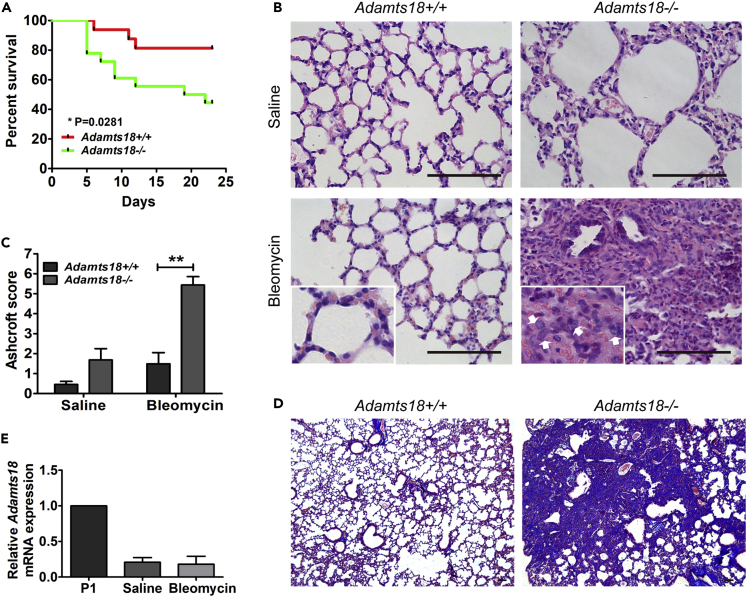
Adamts18−/− mice also exhibited a higher mortality rate than Adamts18+/+ mice (Figure 5A) and more severe lung inflammation and fibrosis after intratracheal injection of bleomycin (Figures 5B–5D). To determine whether LPS or bleomycin induced Adamts18 expression, Adamts18 mRNAs levels in the lungs of LPS- or bleomycin-treated Adamts18+/+ mice were measured by qRT-PCR. Results showed that LPS or bleomycin treatment did not result in increased transcription of Adamts18 mRNAs in the lungs of these mice at various time points after the treatment (Figures 4H and 5E).
Figure 5.
Increased Susceptibility of Adamts18−/− Mice to Bleomycin-Induced Lung Fibrosis
(A) Log rank curves of 8-week-old Adamts18+/+ and Adamts18−/− male mice challenged with bleomycin (n = 19/group, ∗p < 0.05).
(B) Representative images of lung sections of saline-treated (upper panels) or bleomycin-treated (lower panels) mice. Increased inflammation in Adamts18−/− lung sections is revealed by H&E staining (lower panel, white arrows). Scale bar, 100 μm.
(C) Ashcroft scoring of lung fibrosis in mice 23 days after treatment with bleomycin or saline. Results are expressed as mean ± SD (n = 4/group, ∗∗p < 0.01, Student's t test).
(D) Masson's trichrome staining for aggregated collagen deposition in lung sections of bleomycin-treated Adamts18−/− mice. Scale bar, 100 μm.
(E) Relative mRNA levels of Adamts18 in lungs of saline or bleomycin-treated mice determined by quantitative real-time RT-PCR. P1 mouse lung serves as the negative control. Data are expressed as mean ± SEM (n = 3).
Branching-Related Signaling Molecules in Adamts18−/− Lungs
Airway branching is controlled by growth factors and matrix proteins in the epithelium and mesenchyme (Stocks et al., 2013). To determine whether the aberrant bronchus structure in Adamts18−/− lungs is related to altered expressions of these factors, mRNA levels of several critical signaling transducers were determined at E14.5. The mRNA levels of Fgf10, Wnt2, and Bmp4 in lung tissues were similar between the two genotypes of mice. However, mRNA levels of Fgfr2 and Shh were significantly increased in Adamts18−/− lungs compared with Adamts18+/+ lungs (Figure S2A). Hhip and Ptch1 genes are direct targets of SHH signaling (Kugler et al., 2015), and Ext1 has been shown to control SHH-FGF10 signaling (He et al., 2017). Results showed that mRNA levels of Hhip, Ptch1, and Ext1 were comparable between Adamts18−/− and Adamts18+/+ lungs (Figure S2B). Immunohistochemistry (IHC) analysis of the distribution of FGF10 and FGFR2 in E14.5 lungs also showed no significant difference between the two genotypes of mice (Figure S2C).
Embryonic Lung Proteomes
To investigate the role of ADAMTS18 in embryonic bronchus branching, proteins in E14.5 lungs from Adamts18−/− and Adamts18+/+ mice were analyzed by label-free mass spectrometry. A total of 5,797 proteins were identified (data not show). The abundance of 203 lung proteins (3.5%) was significantly different between Adamts18−/− and Adamts18+/+ lungs. Gene ontology term and pathway analyses of significantly changed proteins by Metascape revealed enrichment of proteins of several pathways related to ribosome, supramolecular fiber organization, and protein folding (Figures S3A and S3B). Forty-three proteins were enriched in the category of supramolecular fiber organization, suggesting disarrangements in actin fiber and ECM (Table S2). Among them, the abundance of two major components of microfibrils, fibrillin1 (FBN1) and FBN2, was increased in Adamts18−/− lungs.
Increased Levels of FBN1 and FBN2 and Accumulation of Microfibrils in Adamts18−/− Bronchi
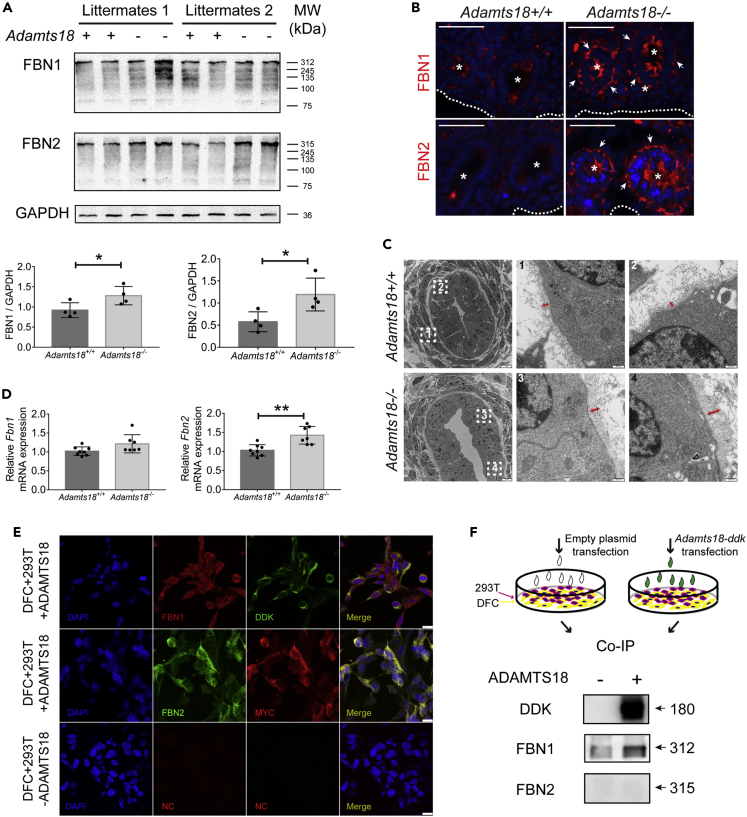
Precise spatiotemporal regulation of ECM proteins is essential for lung development (Zhou et al., 2018). Western blotting confirmed that the levels of FBN1 and FBN2 were significantly higher in Adamts18−/− mice than in Adamts18+/+ mice (Figure 6A). FBN1 and FBN2 proteins were barely detectable in the distal airway of E14.5 Adamts18+/+ mice by immunofluorescence staining. However, deposition of FBN1 and FBN2 was seen around the distal airway epithelium of E14.5 Adamts18−/− lungs (Figure 6B). These two fibrillin proteins were also found in the proximal airway, but there was no significant difference in their abundance in Adamts18+/+ and Adamts18−/− lungs (Figure S4). Transmission electron microscopic images showed that Adamts18−/− lungs had a thicker layer of microfibrils in the basement membrane surrounding epithelial tubes (Figure 6C). Results of qRT-PCR analysis showed that Fbn2 mRNA levels were 1.4-fold higher in Adamts18−/− lungs than in Adamts18+/+ lungs, whereas Fbn1 mRNA levels in Adamts18−/− lungs were comparable to those in Adamts18+/+ lungs (Figure 6D). These observations suggest that the increase in FBN1 protein level in Adamts18−/− lungs was not due to an increase in Fbn1 mRNA transcription.
Figure 6.
Interactions between ADAMTS18 and Fibrillins
(A) Western blotting results of FBN1 and FBN2 in E14.5 lungs of Adamts18+/+ and Adamts18−/− mice. The relative quantity of FBN1 and FBN2 proteins is normalized to that of GAPDH and expressed as mean ± SD (n = 4).
(B) Representative immunohistochemical images of FBN1 and FBN2 in E14.5 lung sections. ∗ denote distal airways, and white arrows indicate FBN1 or FBN2 distribution around distal airways. White dotted curves mark visceral pleura. Scale bar, 100 μm.
(C) Transmission electron microscopic images of E14.5 lungs. 1, 2 and 3, 4 are selected fields of the basement membrane of Adamts18+/+ and Adamts18−/− lungs. The lengths of red arrows indicate the thickness of the microfibril layer outside the lamina dense.
(D) Relative mRNA levels of Fbn1 and Fbn2 determined by real-time RT-PCR. The levels of Fbn1 and Fbn2 mRNAs are normalized to those of the housekeeping gene Gapdh using the ΔΔCt method. Data are expressed as mean ± SEM (n = 7).
(E) Colocalization of ADAMTS18 and fibrillins. Adamts18-myc-ddk transiently transfected HEK293T cells were co-cultured with or without mouse dermal fibroblasts (DFCs) and stained with DAPI (blue), anti-FBN1 (red), anti-FBN2 (green), and antibodies against DDK (green) or MYC (red) to label ADAMTS18. Merged yellow sites showed co-localization of ADAMTS18 and FBN1 or FBN2. Scale bar, 100 μm.
(F) Western blotting results of co-IP. ∗p < 0.05, ∗∗p < 0.01. These experiments were repeated independently at least three times.
See also Figures S3 and S4, Tables S3 and S4.
Interaction between ADAMTS18 and FBN Proteins
To investigate whether ADAMTS18 binds to fibrillins, HEK293T cells transiently transfected with Adamts18-myc-ddk were seeded on mouse dermal fibroblast cells (DFCs), which provided FBN1 and FBN2 proteins in vitro (Figure 6E). ADAMTS18 was found to co-localize with both exogenous FBN1 and FBN2 proteins. Co-immunoprecipitation (co-IP) was then performed to confirm the interaction between ADAMTS18 and fibrillins. Cell lysates of co-cultures of Adamts18-myc-ddk transfected 293T cells and DFCs were incubated overnight with anti-DDK (FLAG tag) agarose beads. A 180-kDa band of ADAMTS18-MYC-DDK was observed by western blotting, and no band in the sample of untransfected cells was seen. FBN1 was pulled down by ADAMTS18 and detected with anti-FBN1-C-terminal antibody, whereas there was no co-IP of FBN2 with ADAMTS18 (Figure 6F). This result suggests that ADAMTS18 binds to FBN1.
Recovery of Adamts18−/− Lung Morphogenesis by Inhibiting FBN Expression
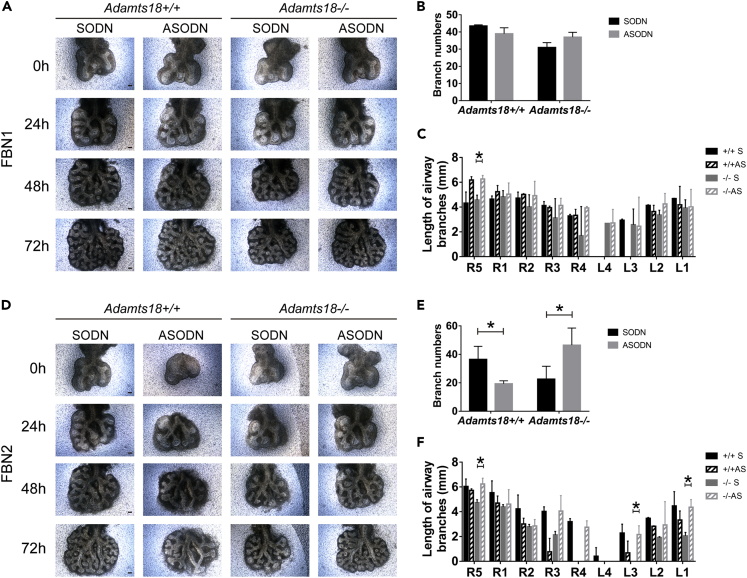
To investigate whether decreasing the abundance of FBN1 or FBN2 in mutant lungs could rescue their branching defects, Fbn1 or Fbn2 antisense (AS)-phosphorothioated oligodeoxynucleotides (ODNs) were added to lung explant cultures (Figure 7). For lung explants of Adamts18+/+ mice, Fbn1 ASODN treatment had little effect on airway branching; however, Fbn2 ASODN induced dysmorphogenesis of the lung explants, including reduced number of branches and decreased length of secondary bronchi, as previously reported (Yang et al., 1999). For lung explants of Adamts18−/− mice, Fbn1 ASODN treatment partially restored the length of secondary bronchi resulting in a profound increase in the length of R5 secondary bronchi. Treatment with Fbn2 ASODN resulted in increased number of branches and lengths of R5, L3, and L1 secondary bronchi.
Figure 7.
Restoration of Adamts18−/− Lung Branching Morphogenesis by Fbn1 or Fbn2 Sense (Control) and Antisense Oligonucleotides (ASODN) in Lung Explants
(A and D) E11.5 lungs were cultured in vitro and photographed at 0, 24, 48, and 72 h. 0.5 μM Fbn1 ODN or 1 μM Fbn2 ODN was added to the cultures every 24 h. Scale bar, 100 μm.
(B and E) Branch number of lungs cultured for 72 h.
(C and F) Lengths of L1-L5 and R1-R5 bronchi of 72 h-cultured explants. SODN, sense oligonucleotides. ASODN, antisense oligonucleotides. Results are expressed as mean ± SD, n = 3 (∗p < 0.05). These experiments were repeated independently at least three times.
Altered Cytoskeleton Signaling in Adamts18−/− Lungs
In addition to fibrillins, other ECM proteins involved in branching were also examined (Table S3). Some of these ECM proteins, such as Col1a2, Col3a1, Lama1, Lama3, Lamb1, Lamc1, dystroglycan, nidogen1, and Ctgf, showed significant difference in mRNA or protein levels between the two genotypes of mice.
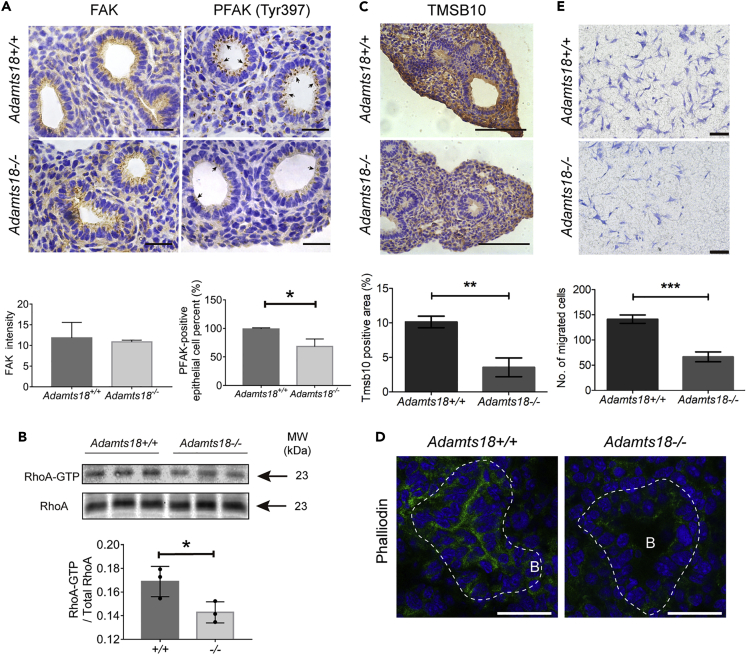
ECM provides mechanical strength to the epithelium and induces new branches in the lungs. The mechanical signal is transduced in part by the focal adhesion kinase (FAK) in lung epithelial cells (Gjorevski and Nelson, 2010). By IHC analyses, total FAK levels in Adamts18+/+ and Adamts18−/− lungs were found to be similar, but the levels of Tyr297-phosphorylated FAK were lower in lung epithelial cells of Adamts18−/− mice (Figure 8A). The RhoA GTPase, which regulates cell cytoskeleton arrangement in conjunction with FAK (Provenzano and Keely, 2011), also showed a lower activation level in Adamts18−/− lungs (Figure 8B). In proteomic study, 43 proteins involved in supramolecular fiber organization were found to be differentially expressed, most of which were involved in F-actin filament assembly (Table S2). The level of the cytoskeleton protein Tmsb10, which binds and stabilizes G-actin (Fanni et al., 2011), was found to be significantly decreased (0.3-fold) in Adamts18−/− lungs. This result was confirmed by IHC (Figure 8C). Phalloidin staining showed less F-actin distribution on the apical surface of the epithelial cells facing the lumen in Adamts18−/− lungs (Figure 8D). These data indicate that ADAMTS18 deficiency caused F-actin disorganization, which may result in reduced cell mobility. In vitro transwell assays showed a significant decrease in the migration of E14.5 Adamts18−/− mouse embryonic fibroblasts (MEFs) compared with Adamts18+/+ lung MEFs (Figure 8E).
Figure 8.
Reduced Amount of phosphorylated FAK (PFAK) and Altered Cytoskeleton in Adamts18−/− Lungs
(A) IHC results of Tyr397-PFAK and total FAK of E14.5 lungs. Scale bar, 50 μm. Determination of the intensity of FAK staining and the percentage of Tyr397-PFAK-positive epithelial cell was performed with Image Pro Plus. Scale bar, 100 μm.
(B) GTP-bound RhoA in E15.5 lungs was precipitated with Rhotekin and analyzed by western blotting for RhoA. RhoA-GTP was quantified relative to total RhoA protein.
(C) Representative images of anti-TMSB10 IHC at E14.5. Quantification of TMSB10-positive areas was performed with Image Pro Plus. Scale bar, 100 μm.
(D) Representative images of phalloidin-stained E14.5 lung sections. White dotted lines indicate the boundary of distal epithelium. B, bronchiole. Scale bar, 20 μm.
(E) Transwell analysis of mouse embryonic fibroblasts (MEFs) from Adamts18+/+ or Adamts18−/− mice. Data are expressed as mean ± SEM (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
See also Table S2.
Discussion
ADAMTS18 is a poorly characterized member of the ADAMTS family of metalloproteinases. Previously, Ataca et al. created Adamts18−/− C57Bl6/Ola mice in which exons 8–9 of the Adamts18 gene was deleted and found that these mice have a higher percentage of adjacent bronchioles and larger airspaces with thinner walls (Ataca et al., 2016). Recently, Rudge et al. generated another strain of Adamts18−/− mice (VG12442) by deleting a 3,616-bp fragment encompassing the region between the ATG codon in exon 1 and the end of exon 3 (Rutledge et al., 2019). They found that these Adamts18−/− mice have shorter primary branches but maintain the ability to form secondary lateral branches in E12.5 lungs. These findings indicate that ADAMTS18 is crucial for early lung development. However, the mechanisms by which ADAMTS18 affects lung morphogenesis and the effect of ADAMTS18 deficiency on lung function remain largely unknown. In this study, we performed experiments using another Adamts18−/− mouse strain with the C57BL6/126SV background. In this mouse strain, exons 5–6 of the Adamts18 gene are deleted (Lu et al., 2017). These Adamts18−/− mice exhibit reduced numbers and lengths of bronchi, tipped lung apexes, and dilated alveoli. These developmental defects worsen LPS-induced acute lung injury and bleomycin-induced lung fibrosis in adult Adamts18−/− mice. By examining the bronchial ECM of these mice, we revealed a novel function of ADAMTS18 in modulating fibrillin microfibril formation.
Mouse microfibril is mainly composed of FBN1 and FBN2. Our results showed that Adamts18−/− lungs had more microfibrils in the basement membrane surrounding the distal airway at E14.5 (Figures 6A–6C). The accumulation of microfibrils in the bronchial wall of Adamts18−/− mice was mainly due to increased FBN2 expression (Figures 6A and 6B). Inhibition of FBN1 or FBN2 expression by ASODNs revealed that FBN2 plays a more important role than FBN1 in early bronchial development (Figure 7). Similar to our findings, Hubmacher et al. found that Adamtsl2 deletion results in bronchial fibrillin microfibril accumulation due to increased FBN2 deposition on the bronchial wall (Hubmacher et al., 2015). Adamtsl2 deletion increases bronchial FBN2 expression only at protein level, and Adamtsl2 is shown to bind directly to FBN2. However, our data showed that FBN2 levels were increased at both protein and mRNA levels in the lungs of Adamts18−/− mice (Figures 6A, 6B, and 6D), suggesting that the increased FBN2 expression is due to enhanced Fbn2 mRNA transcription. It is possible that ADAMTS18 directly processes certain ECMs of microfibril networks, such as FBN1, thus altering tissue stiffness and mechano-signaling and resulting in secondary transcription of other ECM protein genes. In addition to altered Fbn2 mRNA levels, we have previously observed increased laminin transcription in Adamts18−/− adipose tissue and embryonic brains affecting early adipocyte differentiation and neurite formation (Zhu et al., 2018, Zhu et al., 2019). This possibility was further indicated by the finding that ADAMTS18 regulates mammary stem cell niche by cleaving fibronectin. This action may lead to changes in the abundance of collagen I, collagen IV, laminin, and collagen XVIII (Ataca et al., 2020).
In this study, we found that ADAMTS18 co-localizes with both FBN1 and FBN2 in the ECM of cultured fibroblasts (Figure 6E). Co-IP results showed that FBN1, but not FBN2, was pulled down by ADAMTS18 (Figure 6F). These data suggest that ADAMTS18 binds to FBN1. Similar to our findings, previous studies showed that some ADAMTS and ADAMTSL proteases can bind to FBN1 or FBN2 or both. Among them, ADAMTS10 has two FBN1 binding sites and binds to both the N (exons 8–11) and C termini of FBN1 (Hubmacher and Apte, 2011; Kutz et al., 2011). ADAMTS6 has been shown to bind to an N-terminal region of FBN1 (exons 8–11) (Cain et al., 2016). ADAMTS17 binds to both FBN1 and FBN2 (Hubmacher et al., 2017). ADAMTSL2, ADAMTSL4, and ADAMTSL6 are known to bind FBN1 (Gabriel et al., 2012; Le Goff et al., 2011; Tsutsui et al., 2010). ADAMTSL5 has been shown to bind both FBN1 and FBN2 (Bader et al., 2012). We speculate that ADAMTS18 forms a complex with FBN1 and regulates the activity of FBN2 in the fibrillin microfibril scaffold. Because of technical difficulties (Mead and Apte, 2018), we have yet to purify full-length ADAMTS18 proteins for further affinity analysis.
Fibrillin microfibrils represent pivotal ECM signaling platforms integrating the functions of transforming growth factor β, bone morphogenetic protein (BMP), and mechano-signaling (Ramirez and Sakai, 2010). ECM mechanical properties are affected by elastic fibers, fibrillar collagens, glycosaminoglycans, and related proteoglycans. Fibrillar collagens provide tissue stiffness and strength, whereas microfibril-containing elastic fibers are associated with extensibility and resilience (Humphrey et al., 2014). Thus, increased microfibril composition in ECM results in a compliant matrix. Surrounding cells sense the mechanics of ECM through integrins, focal adhesions proteins, and actomyosin cytoskeleton. It has been demonstrated that the phosphorylation level of FAK increases in response to changes in the stiffness of ECM (Du et al., 2016), and FAK signaling is suppressed in compliant ECM (Humphrey et al., 2014). Therefore, ADAMTS18 deficiency increases the levels of fibrillin and tissue compliance, resulting in down regulation of FAK signaling.
Epithelial-mesenchymal transition (EMT) also plays key roles in lung development. BMP, WNT, and FGF signaling induce EMT during branching morphogenesis (Nieto et al., 2016). We found that mRNA levels of Bmp4, Wnt2, and Fgf10 in lung tissues of Adamts18+/+ and Adamts18−/− mice were similar (Figure S2) and that those of Fgfr2 were significantly increased in Adamts18−/− lungs compared with Adamts18+/+ lungs (Figure S2). These pathways can activate one or more EMT-driving transcription factors such as SNAIL1 and SNAIL2 (Nieto et al., 2016). However, Snail1 and Snail2 mRNA levels showed no difference in Adamts18+/+ and Adamts18−/− lung tissues (Figure S5A). The hallmark of EMT is loss of epithelial cell-cell adhesion molecule E-cadherin and/or concomitant expression of mesenchymal markers such as N-cadherin, vimentin, and alpha-smooth muscle actin (Nieto et al., 2016). The expression levels of E-cadherin, N-cadherin, and Vimentin also showed no difference in lung tissues of Adamts18+/+ and Adamts18−/− mice (Figure S5B). Reorganization of the actin cytoskeleton and activation of the RhoA GTPase equip epithelial cells with the mesenchymal traits of migration. Although major EMT biomarkers detected were not changed in Adamts18−/− lungs, Adamts18−/− distal epithelial cells showed fewer F-actin bundles and reduced activation of RhoA GTPase during branching morphogenesis, suggesting diminished migratory property of terminal epithelial cells and EMT involvement.
The lungs of Adamts18−/− mice exhibited several structural defects, including linear atelectasis and dilated alveoli with decreased number of RACs (Figure 3D). As Adamts18 mRNA is not expressed at the alveolization stage, these lung defects are likely the secondary effect of bronchodysplasia. We observed a thicker elastin layer on alveolar walls of Adamts18−/− lungs than those of Adamts18+/+ lungs (Figures 3E and S1). Normally, elastin is distributed in alveolar tips and guides the formation of alveoli (Zhou et al., 2018). It is likely that excessive fibrillin accumulation in mice with ADAMTS18 deficiency promotes elastic fiber synthesis, leading to increased elastin production and ectopic elastin deposition on alveolar walls.
In the study of LPS-induced acute lung injury and bleomycin-induced lung fibrosis, adult Adamts18−/− mice demonstrated a high susceptibility to lung inflammation and fibrosis (Figures 4 and 5). LPS treatment may induce production and release of proinflammatory cytokines IL-1, tumor necrosis factor-α, IL-6, and chemokines (IL-8 and macrophage inflammatory protein-2), leading to recruitment of neutrophils and acute lung injury (Moreland et al., 2002). Although release of NETs was not observed in both Adamts18+/+ and Adamts18−/− mice 24 h after LPS injection, increased capillary permeability, interstitial edema, and more serious tissue damages were clearly observed in Adamts18−/− lungs. These symptoms may be due to elevated levels of the proinflammatory cytokine IL-6 and infiltration of CD11b+ neutrophils.
Taken together, results of this study indicate that ADAMTS18 is secreted by bronchial epithelial cells and binds to FBN1. In vivo, ADAMTS18 deficiency causes increased levels of FBN1 and FBN2 and accumulation of microfibrils in bronchi. Accumulation of microfibrils causes a weakened FAK signaling and abnormal F-actin organizations. In vitro, ADAMTS18 deficiency causes a reduction in the migration of embryonic fibroblasts.
Limitations of the Study
In the present study, we demonstrated that ADAMTS18 regulates early lung development in a microfibril-dependent manner by binding to fibrillin1 protein. However, the binding site of ADAMTS18 on fibrillin1 and how it affects fibrillin abundance remain to be investigated.
Resource Availability
Lead Contact
Further information and requests for resources and reagents will be fulfilled by the Lead Contact, Wei Zhang (wzhang@sat.ecnu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The raw data of this article are available from the leading contact upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (No. 81770139, 81570389 and 81170481 to W.Z.) and the Shanghai Municipal Natural Science Foundation (16ZR1423700 to S.D.). We thank Dr. Chao-Hung Lee for editing the manuscript and providing valuable advices.
Author Contributions
T.L., S.D., and W.Z. conceived the study and designed the experiments. T.L., X.L., and C.W. performed experiments and analyzed data. S.Y., Q.Z., and T.Z. genotyped mice and maintained mouse colonies. R.Z., Y.-H.P., T.M.W., Z.C., and B.-S.D. provided valuable advices. T.L. and W.Z. wrote the manuscript.
Declaration of Interests
The authors declare that they have no conflict of interest.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101472.
Contributor Information
Suying Dang, Email: suyingdang@shsmu.edu.cn.
Wei Zhang, Email: wzhang@sat.ecnu.edu.cn.
Supplemental Information
References
- Aldahmesh M.A., Khan A.O., Mohamed J.Y., Alkuraya H., Ahmed H., Bobis S., Al-Mesfer S., Alkuraya F.S. Identification of ADAMTS18 as a gene mutated in Knobloch syndrome. J. Med. Genet. 2011;48:597–601. doi: 10.1136/jmedgenet-2011-100306. [DOI] [PubMed] [Google Scholar]
- Aldahmesh M.A., Alshammari M.J., Khan A.O., Mohamed J.Y., Alhabib F.A., Alkuraya F.S. The syndrome of microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) is caused by mutations in ADAMTS18. Hum. Mutat. 2013;34:1195–1199. doi: 10.1002/humu.22374. [DOI] [PubMed] [Google Scholar]
- Ataca D., Aouad P., Constantin C., Laszlo C., Beleut M., Shamseddin M., Rajaram R.D., Jeitziner R., Mead T.J., Caikovski M. The secreted protease Adamts18 links hormone action to activation of the mammary stem cell niche. Nat. Commun. 2020;11:1571. doi: 10.1038/s41467-020-15357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataca D., Caikovski M., Piersigilli A., Moulin A., Benarafa C., Earp S.E., Guri Y., Kostic C., Arsenijevic Y., Soininen R. Adamts18 deletion results in distinct developmental defects and provides a model for congenital disorders of lens, lung, and female reproductive tract development. Biol. Open. 2016;5:1585–1594. doi: 10.1242/bio.019711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader H.L., Wang L.W., Ho J.C., Tran T., Holden P., Fitzgerald J., Atit R.P., Reinhardt D.P., Apte S.S. A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) is a novel fibrillin-1-, fibrillin-2-, and heparin-binding member of the ADAMTS superfamily containing a netrin-like module. Matrix Biol. 2012;31:398–411. doi: 10.1016/j.matbio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain S.A., Mularczyk E.J., Singh M., Massam-Wu T., Kielty C.M. ADAMTS-10 and -6 differentially regulate cell-cell junctions and focal adhesions. Sci. Rep. 2016;6:35956. doi: 10.1038/srep35956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Valentin E., Crahay C., Garbacki N., Hennuy B., Gueders M., Noel A., Foidart J.M., Grooten J., Colige A., Piette J. New asthma biomarkers: lessons from murine models of acute and chronic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L185–L197. doi: 10.1152/ajplung.90367.2008. [DOI] [PubMed] [Google Scholar]
- Du J., Zu Y., Li J., Du S., Xu Y., Zhang L., Jiang L., Wang Z., Chien S., Yang C. Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci. Rep. 2016;6:20395. doi: 10.1038/srep20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanni D., Gerosa C., Nemolato S., Locci A., Marinelli V., Cabras T., Messana I., Fanos V., Castagnola M., Faa G. Thymosin beta 10 expression in developing human salivary glands. Early Hum. Dev. 2011;87:779–783. doi: 10.1016/j.earlhumdev.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Gabriel L.A., Wang L.W., Bader H., Ho J.C., Majors A.K., Hollyfield J.G., Traboulsi E.I., Apte S.S. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest. Ophthalmol. Vis. Sci. 2012;53:461–469. doi: 10.1167/iovs.10-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N., Nelson C.M. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr. Biol. 2010;2:424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Huang M., Sun S., Wu Y., Lin X. Epithelial heparan sulfate regulates Sonic Hedgehog signaling in lung development. PLoS Genet. 2017;13:e1006992. doi: 10.1371/journal.pgen.1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmacher D., Apte S.S. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: a novel mechanism influencing microfibril assembly and function. Cell. Mol. Life Sci. 2011;68:3137–3148. doi: 10.1007/s00018-011-0780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmacher D., Schneider M., Berardinelli S.J., Takeuchi H., Willard B., Reinhardt D.P., Haltiwanger R.S., Apte S.S. Unusual life cycle and impact on microfibril assembly of ADAMTS17, a secreted metalloprotease mutated in genetic eye disease. Sci. Rep. 2017;7:41871. doi: 10.1038/srep41871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmacher D., Wang L.W., Mecham R.P., Reinhardt D.P., Apte S.S. Adamtsl2 deletion results in bronchial fibrillin microfibril accumulation and bronchial epithelial dysplasia – anovel mouse model providing insights into geleophysic dysplasia. Disease Models & Mechanisms. 2015;8:487–499. doi: 10.1242/dmm.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Wang X., Ying J., Wong A.H., Li H., Lee K.Y., Srivastava G., Chan A.T., Yeo W., Ma B.B. Epigenetic identification of ADAMTS18 as a novel 16q23.1 tumor suppressor frequently silenced in esophageal, nasopharyngeal and multiple other carcinomas. Oncogene. 2007;26:7490–7498. doi: 10.1038/sj.onc.1210559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungers K.A., Le Goff C., Somerville R.P., Apte S.S. Adamts9 is widely expressed during mouse embryo development. Gene Expr. Patterns. 2005;5:609–617. doi: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kugler M.C., Joyner A.L., Loomis C.A., Munger J.S. Sonic hedgehog signaling in the lung. From development to disease. Am. J. Respir. Cell Mol. Biol. 2015;52:1–13. doi: 10.1165/rcmb.2014-0132TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz T., Hoffjan S., Hayes M.G., Schneider D., Nicolae R., Heinzmann A., Jerkic S.P., Parry R., Cox N.J., Deichmann K.A. Fine mapping and positional candidate studies on chromosome 5p13 identify multiple asthma susceptibility loci. J. Allergy Clin. Immunol. 2006;118:396–402. doi: 10.1016/j.jaci.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Kutz W.E., Wang L.W., Bader H.L., Majors A.K., Iwata K., Traboulsi E.I., Sakai L.Y., Keene D.R., Apte S.S. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 2011;286:17156–17167. doi: 10.1074/jbc.M111.231571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C., Mahaut C., Wang L.W., Allali S., Abhyankar A., Jensen S., Zylberberg L., Collod-Beroud G., Bonnet D., Alanay Y. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am. J. Hum. Genet. 2011;89:7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L.M., Bastin M.E., Maniega S.M., Penke L., Davies G., Christoforou A., Valdes Hernandez M.C., Royle N.A., Tenesa A., Starr J.M. A genome-wide search for genetic influences and biological pathways related to the brain's white matter integrity. Neurobiol. Aging. 2012;33:1847.e1–1847.e14. doi: 10.1016/j.neurobiolaging.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Lu T., Dang S., Zhu R., Wang Y., Nie Z., Hong T., Zhang W. Adamts18 deficiency promotes colon carcinogenesis by enhancing beta-catenin and p38MAPK/ERK1/2 signaling in the mouse model of AOM/DSS-induced colitis-associated colorectal cancer. Oncotarget. 2017;8:18979–18990. doi: 10.18632/oncotarget.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead T.J., Apte S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018;71-72:225–239. doi: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland J.G., Fuhrman R.M., Pruessner J.A., Schwartz D.A. CD11b and intercellular adhesion molecule-1 are involved in pulmonary neutrophil recruitment in lipopolysaccharide-induced airway disease. Am. J. Respir. Cell Mol. Biol. 2002;27:474–480. doi: 10.1165/rcmb.4694. [DOI] [PubMed] [Google Scholar]
- Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Paulissen G., Rocks N., Quesada-Calvo F., Gosset P., Foidart J.M., Noel A., Louis R., Cataldo D.D. Expression of ADAMs and their inhibitors in sputum from patients with asthma. Mol. Med. 2006;12:171–179. doi: 10.2119/2006-00028.Paulissen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso I., Conte I., Testa F., Dharmalingam G., Pizzo M., Collin R.W., Meola N., Barbato S., Mutarelli M., Ziviello C. The ADAMTS18 gene is responsible for autosomal recessive early onset severe retinal dystrophy. Orphanet J. Rare Dis. 2013;8:16. doi: 10.1186/1750-1172-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P.P., Keely P.J. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J. Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F., Sakai L.Y. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010;339:71–82. doi: 10.1007/s00441-009-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge E.A., Parvez R.K., Short K.M., Smyth I.M., McMahon A.P. Morphogenesis of the kidney and lung requires branch-tip directed activity of the Adamts18 metalloprotease. Dev. Biol. 2019;454:156–169. doi: 10.1016/j.ydbio.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville R.P., Jungers K.A., Apte S.S. Discovery and characterization of a novel, widely expressed metalloprotease, ADAMTS10, and its proteolytic activation. J. Biol. Chem. 2004;279:51208–51217. doi: 10.1074/jbc.M409036200. [DOI] [PubMed] [Google Scholar]
- Stocks J., Hislop A., Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir. Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- Thai S.N., Iruela-Arispe M.L. Expression of ADAMTS1 during murine development. Mech. Dev. 2002;115:181–185. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Manabe R., Yamada T., Nakano I., Oguri Y., Keene D.R., Sengle G., Sakai L.Y., Sekiguchi K. ADAMTSL-6 is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J. Biol. Chem. 2010;285:4870–4882. doi: 10.1074/jbc.M109.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D.H., Liu X.G., Guo Y.F., Tan L.J., Wang L., Sha B.Y., Tang Z.H., Pan F., Yang T.L., Chen X.D. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am. J. Hum. Genet. 2009;84:388–398. doi: 10.1016/j.ajhg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Ota K., Tian Y., Kumar A., Wada J., Kashihara N., Wallner E., Kanwar Y.S. Cloning of rat fibrillin-2 cDNA and its role in branching morphogenesis of embryonic lung. Dev. Biol. 1999;212:229–242. doi: 10.1006/dbio.1999.9331. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Horowitz J.C., Naba A., Ambalavanan N., Atabai K., Balestrini J., Bitterman P.B., Corley R.A., Ding B.S., Engler A.J. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018;73:77–104. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Cheng M., Lu T., Yang N., Ye S., Pan Y.H., Hong T., Dang S., Zhang W. A disintegrin and metalloproteinase with thrombospondin motifs 18 deficiency leads to visceral adiposity and associated metabolic syndrome in mice. Am. J. Pathol. 2018;188:461–473. doi: 10.1016/j.ajpath.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Zhu R., Pan Y.H., Sun L., Zhang T., Wang C., Ye S., Yang N., Lu T., Wisniewski T., Dang S. ADAMTS18 deficiency affects neuronal morphogenesis and reduces the levels of depression-like behaviors in mice. Neuroscience. 2019;399:53–64. doi: 10.1016/j.neuroscience.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of this article are available from the leading contact upon request.