Abstract
Objective
Klotho is an aging-suppressor gene which leads to accelerated aging when disrupted. This study was designed to investigate whether glutathione reductase (GR), a critical intracellular antioxidant enzyme, is involved in the pathogenesis of kidney damages associated with accelerated aging in Klotho-haplodeficient (KL+/–) mice.
Methods and results
Klotho-haplodeficient (KL+/–) mice and WT mice were used. We found that Klotho haplodeficiency impaired kidney function as evidenced by significant increases in plasma urea and creatinine and a decrease in urinary creatinine in KL+/– mice. The expression and activity of GR was decreased significantly in renal tubular epithelial cells of KL+/– mice, suggesting that Klotho deficiency downregulated GR. We constructed adeno-associated virus 2 (AAV2) carrying GR full-length cDNA (AAV-GR). Interestingly, in vivo AAV-GR delivery significantly improved Klotho deficiency-induced renal functional impairment and structural remodeling. Furthermore, in vivo expression of GR rescued the downregulation of the reduced glutathione/oxidized glutathione (GSH/GSSG) ratio, which subsequently diminished oxidative damages in kidneys, as evidenced by significant decreases in renal 4-HNE expression and urinary 8-isoprostane levels in KL mice.
Conclusion
This study provides the first evidence that Klotho deficiency-induced kidney damage may be partly attributed to downregulation of GR expression. In vivo delivery of AAV-GR may be a promising therapeutic approach for aging-related kidney damage.
Keywords: Klotho, Glutathione reductase, GSH/GSSG ratio, Kidney damage, Oxidative stress
1. Introduction
Aging is defined as the age-related decline in intrinsic physiological function essential for survival and fertility [1]. Progressive renal recession is a common phenomenon in the aging process, and aging-related declines in renal function are associated with a progressive loss of functioning nephrons [2]. Population-based studies show that about half of adults over age 70 had decreased renal function [[3], [4], [5], [6]], suggesting that aging is associated with declining renal function in the elderly. Kidney aging is an important process that contributes to declining lifespan [7]; however, its pathogenesis remains largely unclear.
Klotho, an anti-aging gene, extends the lifespan when overexpressed, and shortens the lifespan when disrupted [8,9]. The Klotho protein is expressed in the kidneys, predominately in distal convoluted tubules [8]. In humans, Klotho levels decline in the aged population, and it has been reported that Klotho levels in humans at age 70 are only about half of those seen in humans at age 40 [10]. In a previous study, we reported that aging-related kidney damage is associated with decreased Klotho expression [2]. More recently, we showed that Klotho gene deficiency causes kidney damage in an adult murine model [11,12], indicating that Klotho may be a potential pathological factor for kidney aging. It is not known, however, how Klotho deficiency causes kidney damage.
Glutathione reductase (GR) is a potent substrate-specific enzyme that plays an essential role in catalyzing an oxidized form of glutathione (glutathione disulfide, GSSG) into a reduced form (sulfhydryl form glutathione, GSH) [13]. Our preliminary study demonstrated that renal GR protein expression and activity were significantly decreased in Klotho-deficient mice. Thus, we hypothesized that in vivo overexpression of GR would attenuate Klotho deficiency-induced kidney damage through its anti-oxidative stress (anti-OS) effects. In our current study, we mimicked the accelerated aging process in KL+/– mice, to investigate the potential role GR in Klotho deficiency-induced kidney damage.
2. Methods
A detailed Methods section is available in the Online Supplementary Materials.
2.1. Construction of recombinant adeno-associated virus (AAV) with mouse GR gene
The procedure for constructing the recombinant adeno-associated virus (AAV)-2 carrying the mouse GR full-length cDNA (AAV-GR) was performed as we described previously [[14], [15], [16], [17]]. AAV carrying green fluorescent protein (AAV-GFP) was constructed and served as a reporter gene construct. For details, please refer to the Online Supplementary Materials.
2.2. Animal study protocols
This study was performed according to the guidelines of the National Institutes of Health (NIH) on the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Oklahoma Health Science Center.
Three groups of Klotho-deficient heterozygous (KL+/–) mice and one group of age-matched WT mice, of which all had 129Sv background, were used (4–6 mice per group, all males, all 18-months- old). Briefly, the three groups of KL+/– mice received AAV-GFP, AAV-GR, and PBS, respectively. Viral particles were delivered intravenously via the tail vein at 2 × 108 particles per mouse (0.2 mL). The WT group received PBS and served as control. Body weight was measured and urine was collected weekly, and the animals were euthanized at the end of 9th week after gene delivery. Plasma was collected for measuring creatinine and urea. After perfusion, kidneys were collected and separated into three parts. One part was saved in −80 °C for molecular assays and the others were embedded in paraffin or optimal cutting temperature compound for histological and immunohistochemical analysis.
2.3. Measurements of renal function parameters
Plasma urea level was detected with a urea assay kit (DIUR-500; BioAssay Systems, Hayward, CA, USA). Plasma and urinary creatinine levels were detected with a creatinine assay kit (DICT-500; BioAssay Systems). Urinary albumin concentration was measured with a mouse-specific microalbuminuria ELISA kit (Albuwell M; Exocell, Philadelphia, PA, USA). All renal function parameters were measured according to manufacturers’ instructions.
2.4. Measurement of urinary 8-isoprostane and renal GSH/GSSG ratio
The 8-isoprostane level in urine was measured using an ELISA kit (OxiSelect™ Oxidative DNA Damage ELISA Kit; Cell Biolabs, Inc., San Diego, CA, USA). The GSH/GSSG ratio in kidneys was detected with a fluorometric assay kit (GSH/GSSG Ratio Detection Assay Kit; Abcam Inc., Cambridge, MA, USA) according to manufacturer's instruction [18].
2.5. Immunohistochemical analysis and histological examination of kidneys
This procedure was described in our previous studies [9,11,19,20]. For details, please refer to the Online Supplementary Materials.
2.6. Western blots
Standardized protocols were used as in our previous studies [[21], [22], [23], [24], [25]], with details available in the Online Supplementary Materials.
2.7. Identification of GFP expression
This procedure was described in our previous studies [14,26]. For details, please refer to the Online Supplementary Materials.
2.8. Measurement of in situ superoxide production
In situ superoxide production was measured in aortas using the oxidation-sensitive dye dihydroethidium (DHE) [[27], [28], [29]]. For details, please see the Online Supplementary Materials.
2.9. Statistical analyses
All data were analyzed by one-way ANOVA. Unpaired t-test was used for comparisons between the two groups. Significance was set at 95% confidence limit.
3. Results
3.1. Klotho deficiency caused renal function impairment which was associated with a decreased expression and activity of GR in kidney
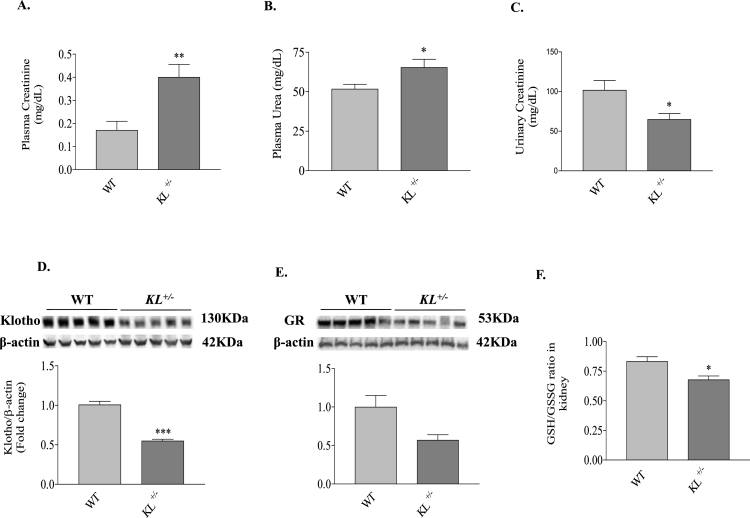
To study the role of endogenous Klotho in the development of kidney aging, we detected the renal functions of 18-month-old KL+/– and age-matched littermate WT mice. Renal functional impairment in Klotho-deficient mice was evidenced by the significant increases in plasma creatinine and plasma urea (Fig. 1A and B), as well as significantly decreased excretion of creatinine in urine (Fig. 1C). Western blot analysis confirmed that Klotho protein expression in kidneys of KL+/– mice was about 50% of that of wild-type (WT) mice (Fig. 1D). Notably, GR protein expression was decreased significantly in kidneys of KL+/– mice (Fig. 1E). The GSH/GSSG ratio regulated by GR, an indicator of oxidative stress, declined significantly in kidneys of KL+/– mice (Fig. 1F).These results revealed, for the first time, that GR expression protein and activity were down-regulated in kidneys due to Klotho deficiency during the development of renal function impairment.
Fig. 1.
Klotho deficiency impaired renal function which was associated with decreased expression of GR and GSH/GSSG ratio in kidneys. (A) Plasma creatinine concentration. (B) Urinary creatinine concentration. (C) Plasma urea concentration. (D) Representative western blot bands and quantitative analysis of Klotho expression in kidney. (E) Representative western blot bands and quantitative analysis of GR expression in kidney. (F) GSH/GSSG ratio in kidney. n = 4–5 mice/group. *P < 0.05 **P < 0.01 vs WT.
3.2. AAV-GR delivery attenuated klotho deficiency-induced renal functional and structural damage
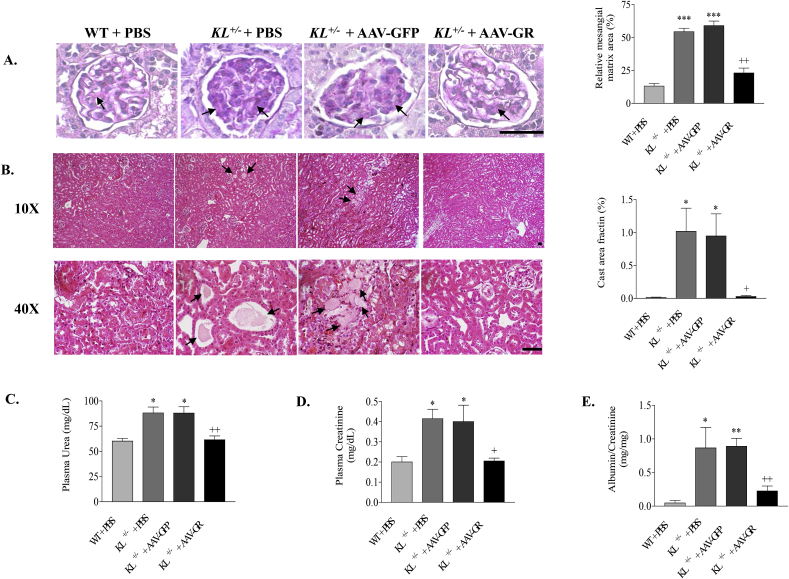
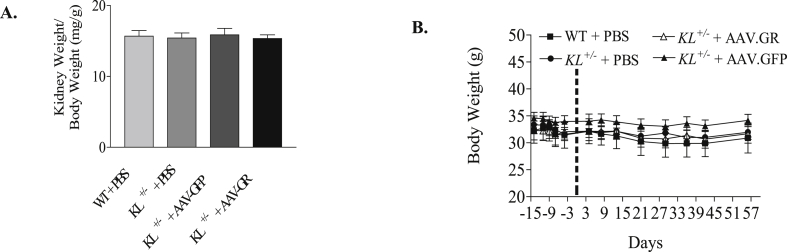
Since GR protein expression was notably downregulated by Klotho deficiency, we performed in vivo expression of GR in KL+/– mice to test whether GR supplement could attenuate kidney damage induced by Klotho deficiency. Consistent with the renal functional change, PAS staining showed extracellular matrix expansion in Klotho-deficient mice treated with either PBS or GFP. AAV delivery of the GR gene attenuated extracellular matrix expansion in glomeruli (Fig. 2A). Tubular cast formation, another sign of kidney aging [7], was found notably increased in medullary region of Klotho-deficient mice compared with that of WT mice by Hematoxylin and Eosin (HE) staining. In vivo expression of GR significantly attenuated tubular cast formation in kidneys of Klotho-deficient mice (Fig. 2B). Plasma urea, plasma creatinine and urinary albumin of Klotho-deficient mice were significantly decreased to WT level by GR gene delivery (Fig. 2C–E), suggesting improved renal function. On the other hand, kidney and body weights (Fig. 3) were not affected by AAV gene delivery, suggesting the safety of AAV gene transfer. These results indicated that the downregulation of GR may be involved in Klotho deficiency-induced renal structural and functional damage.
Fig. 2.
GR gene delivery abolished Klotho deficiency induced renal structural and functional damage. (A) Representative photomicrographs and quantitative analysis of PAS-stained kidney sections. Arrows indicate PAS-positive mesangial material (pink). Relative mesangial matrix area is expressed as PAS-positive mesangial matrix per total glomerular tuft cross-sectional area. An average value was obtained from analyses of 20 glomeruli per mouse. (B) Representative photomicrographs of H&E-stained kidney sections. Arrows indicate tubular cast (protein) formations. Semi-quantitative analysis of tubular cast formation in medulla is expressed as area fraction. Scale bars, 50 μm. (C) Plasma urea concentration. (D) Plasma creatinine concentration. (E) Urinary albumin (normalized to urinary creatinine). n = 4–6 mice/group. *P < 0.05 **P < 0.01 vs WT + PBS; +P < 0.05 ++ P < 0.01 vs KL+/- + AAV-GFP. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
GR gene delivery did not alter kidney weight or body weight in KL±mice. (A) Kidney weight (normalized by body weight). (B) Time course of body weight changes. n = 4–6 mice/group.
3.3. AAV-GR delivery ameliorated klotho-deficiency-induced renal fibrosis
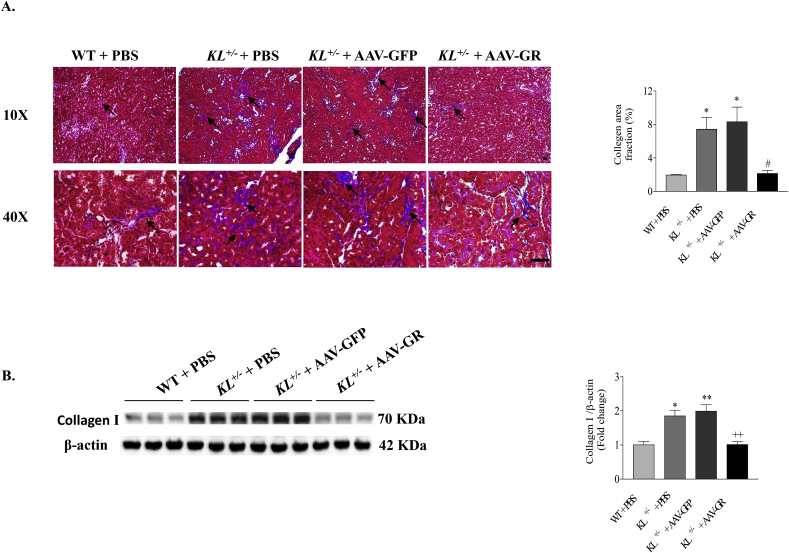
Masson's trichrome staining showed that collagen deposition (blue staining) was increased in renal tubular interstitium (Fig. 4A). Western blot confirmed the enhanced expression of collagenⅠin kidneys (Fig. 4B) of KL+/– mice versus WT mice. These results suggested that heterogeneous Klotho deficiency caused tubulointerstitial fibrosis. Notably, 9 weeks after AAV-GR gene delivery, tubulointerstitial collagen deposition and collagenⅠprotein expression were significantly reduced to control level (Fig. 4), indicating that in vivo overexpression of GR abolished renal fibrotic formation induced by Klotho deficiency.
Fig. 4.
GR gene delivery attenuated Klotho deficiency induced renal fibrosis. (A) Representative photomicrographs of Masson's trichrome-stained kidney sections. Arrows indicate trichrome-positive collagenous components in cortical interstitium (blue staining). Semi-quantification of the area fraction of collagen deposition in kidneys. Scale bars, 50 μm. (B) Representative Western blot bands and quantitative analysis of type I collagen. The relative protein expression was normalized to β-actin first and then calculated as fold changes of the controls (WT + PBS). n = 4–6 mice/group. *P < 0.05 **P < 0.01 vs WT + PBS; +P < 0.05 ++ P < 0.01 vs KL+/- + AAV-GFP. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. AAV-GR gene delivery attenuated klotho-deficiency-induced renal oxidative damage
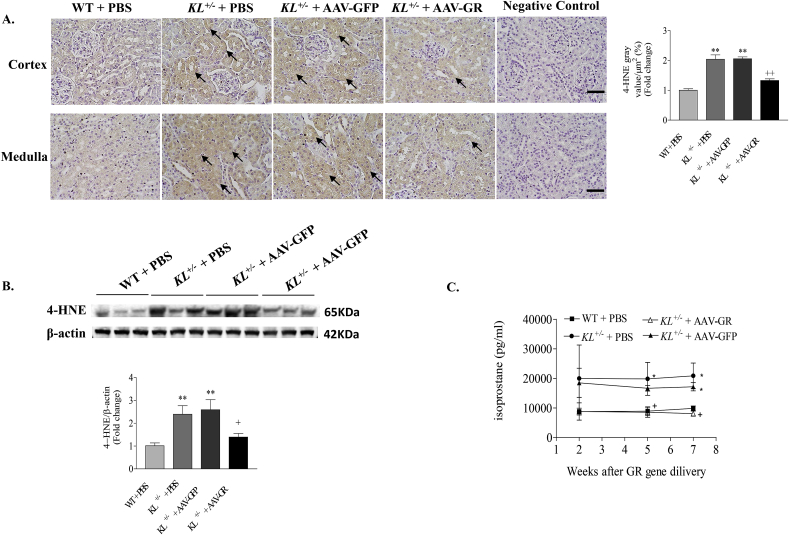
Immunohistochemical analysis showed that 4-HNE adducts were mainly found in renal tubules in KL+/– mice, which could be significantly attenuated by in vivo expression of GR (Fig. 5A). Western blot confirmed that the 4-HNE level was significantly increased in kidneys of KL+/– mice relative to WT, which was notably diminished after GR gene transfer (Fig. 5B), indicating that in vivo expression of GR reduced renal lipid peroxidation damages due to Klotho deficiency.
Fig. 5.
GR gene delivery attenuated Klotho deficiency induced renal oxidative damage. (A) Representative photomicrographs of 4-HNE immunostaining in kidney sections (brown color). Semi-quantification of 4-HNE staining in kidneys. Upper and lower photomicrographs represent cortex and medulla area, respectively. Scale bars, 50 μm. (B) Representative Western blot bands and quantitative analysis of 4-HNE. The relative protein expression was normalized to β-actin first and then calculated as fold changes of the controls (WT + PBS). (C) Time course of urinary 8-isoprostane concentration. n = 4–6 mice/group. *P < 0.05 **P < 0.01 vs WT + PBS; +P < 0.05 ++ P < 0.01 vs KL+/- + AAV-GFP. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To confirm the anti-oxidative effect of AAV-GR in kidney, we monitored urinary 8-isoprostane concentration, another marker of renal oxidative stress [[30], [31], [32], [33]], at different time points after AAV-GR delivery. A significantly higher urinary level of 8-isoprostane was found in KL+/– mice versus WT mice (Fig. 5C). Two weeks after GR gene transfer, urinary 8-isoprostane was reduced in Klotho-deficient mice although it did not reach statistically significant difference. The level of 8-isoprostane in urine in KL ±mice was significantly decreased to the control level at fifth week after AAV-GR delivery and everlasting (Fig. 5C). Thus, in vivo expression of GR gene effectively reduced renal oxidative stress caused by Klotho deficiency.
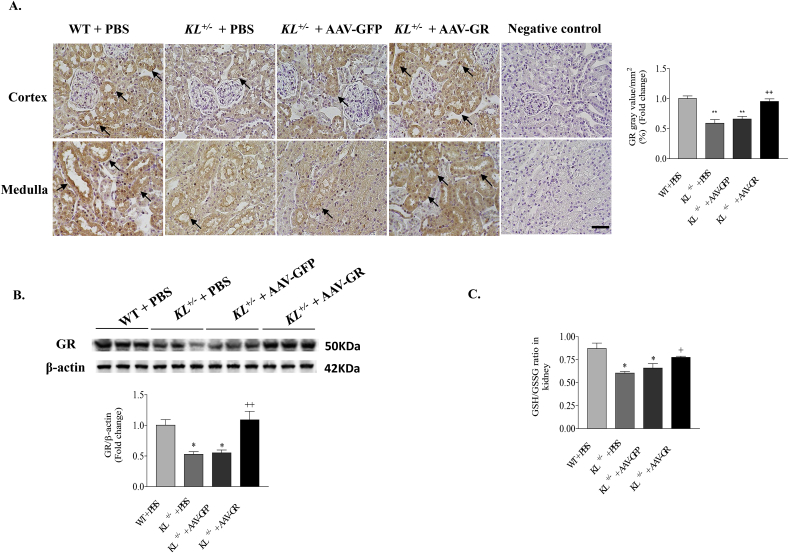
3.5. AAV-GR delivery rescued the downregulation of GR protein expression and activity in kidneys of KL+/– mice
Immunohistochemical analysis indicated that AAV-GR delivery significantly increased GR protein expression in the renal tubule epithelial cells of KL+/– mice (brown staining, Fig. 6A). This result was further confirmed by western blot data (Fig. 6B). Associated with the recovery of GR protein expression, the GSH/GSSG ratio in kidneys of KL+/– mice was significantly increased to the control level after GR overexpression (Fig. 6C), indicating that in vivo expression of GR also rescued the downregulated GR activity due to Klotho deficiency. These data suggested that AAV-GR delivery restored GR protein expression, which consequently rescued the renal GSH/GSSG ratio in Klotho-deficient mice.
Fig. 6.
GR gene delivery restored GR protein expression and GSH/GSSG ratio in kidneys of Klotho-deficient mice. (A) Representative photomicrographs of GR immunostaining in kidney sections (brown color). Semi-quantification of GR staining in mice kidneys. Upper and lower photomicrographs represent cortex and medulla area, respectively. Scale bars, 50 μm. (B) Representative Western blot bands and quantitative analysis of GR. The relative protein expression was normalized to β-actin first and then calculated as fold changes of the controls (WT + PBS). (C) GSH/GSSG ratio in kidneys. n = 4–6 mice/group. *P < 0.05 **P < 0.01 vs WT + PBS; +P < 0.05 ++ P < 0.01 vs KL+/- + AAV-GFP. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We further assessed the superoxide levels in kidneys using DHE staining. Superoxide levels were increased significantly in kidneys in KL ± mice (Supplemental Figure S1). AAV-GR delivery effectively rescued klotho deficiency-induced superoxide accumulation. This result suggest that upregulation of superoxide levels may be partly attributed to the downregulation of GR expression and GSH/GSSG levels due to klotho deficiency.
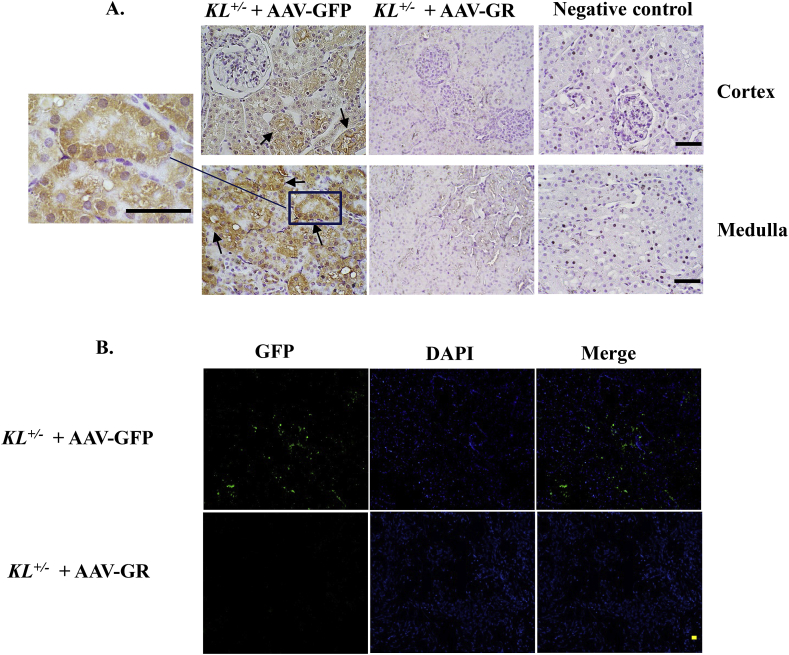
3.6. Expression of GFP in kidneys
To determine whether AAV-based transgenes still exist at end of the experiment, we detected the expression of GFP, the reporter gene in kidneys, using a GFP-specific antibody. IHC analysis revealed that the GFP expression was only found in kidneys of KL+/– mice treated with AAV. GFP (Fig. 7A). Positive staining (brown staining) was found in renal tubule epithelial cells. GFP staining was missing in kidneys of AAV-GR-treated group. Consistent with IHC result, immunofluorescent GFP signal (green fluorescence) was only found in AAV-GFP group (Fig. 7B), indicating that the GFP reporter gene was still expressed in mice 9 weeks after delivery of AAV. GFP. These results suggested that AAV achieved a long-term expression of transgenes.
Fig. 7.
Expression of GFP in kidneys. (A) Representative photomicrographs of GFP immunostaining in kidney sections (brown color) using a GFP-specific antibody. (B) Representative fluorescence photomicrographs of GFP expression in kidneys of KL± mice treated with AAV-GFP. Tissue sections showing GFP expression, DAPI staining, and a merge of GFP and DAPI, respectively. No GFP expression was found in mice treated with AAV-GR. Upper and lower photomicrographs represent cortex and medulla area, respectively. Scale bars, 50 μm n = 4–6 mice/group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Kidney aging, which also known as renal senescence is a complex, multifactorial process characterized by anatomical and functional changes accumulating during life span [34]. Population-based studies have documented that impaired renal function is common in the elderly [35]. Age-related kidney damage has been confirmed even after excluding other confounding factors [5,36,37]. However, how aging deteriorates renal function remains unclear.
Klotho was originally identified as an aging-suppressor gene [38], mutation of which results in multiple premature aging phenotypes [8]. The klotho levels decline in the aged population [10]. In this study, we found obvious kidney structure remodeling manifested by glomerular mesangial matrix expansion (Fig. 2A), renal tubular cast formation (Fig. 2B) and tubular interstitial fibrosis (Fig. 4) in Klotho-haplodeficient mice. Associated with these structural changes, kidney function was also markedly impaired as proved by increased plasma creatinine, plasma urea, and urinary creatinine (Fig. 1A–C) in this accelerated aging model, which had a half renal Klotho level compared to WT mice (Fig. 1D). It is interesting that Klotho deficiency downregulated GR protein expression and activity in kidneys (Fig. 1D and E) because the relationship of Klotho and GR has never been investigated. GR, which catalyzes the GSSG back to reduced GSH form, is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell [13]. It is reported that overexpression of GR increases the resistance of flies exposed to and extends flies’ life span under hyperoxic conditions [39], however, little is known about whether GR plays a role in kidney disease. In present study, we found that in vivo expression of GR abolished the Klotho deficiency-induced renal structural and functional damage (Fig. 2). This finding provided the first evidence that Klotho deficiency caused kidney aging partly through downregulation of GR.
Notably, GR activity which was measured by the ratio of GSH/GSSG, was also rescued coincident with the recovery of protein expression after GR gene transfer (Fig. 6), suggested that the recovery of GR activity may be due to the restoration of downregulation of its protein expression due to Klotho deficiency. The GSH metabolism may not be affected by in vivo expression of GR which did not affect the total GSH and GSSG levels in kidneys (Supplemental Figure S4). It is well demonstrated that GR is critical in maintaining redox balance in all types of cells. This may also partially explain why Klotho gene deficiency causes extensive aging phenotypes in nearly all types of cells. The limitation of this in vivo study is that it does not address how Klotho regulates GR protein expression and activity. On the other hand, the Klotho protein regulates transcription factors of antioxidant response elements (AREs) [40], the function of which is associated with GR expression [[41], [42], [43]]. Thus, Klotho may functionally interact with GR and regulate its activity through AREs. This hypothesis, however, needs to be validated.
Oxidative stress (OS) is a state in which oxidation exceeds the antioxidant capacity in the body secondary to a loss of the balance between them, which causes profound alterations of various biological structures, including cellular membranes, lipids, proteins and nucleic acids [44]. Among other organs, the kidney is an organ highly vulnerable to damage caused by OS [45]. Although Klotho is regarded as a regulator of OS [46], the underlying mechanism of the anti-oxidant effect of Klotho remains poorly understood. Indeed, in our study, renal oxidative stress was observed in the accelerated kidney aging model as evidenced by significant increases in 4-HNE expression in the kidney and 8-isoprostane in urine (Fig. 5), which was associated with kidney damage induced by klotho deficiency (Fig. 2). This finding is noteworthy because it at least partially explained the mechanism how Klotho involved in the pathological process of kidney aging, that is, through modulating OS in aged kidneys.
Although it is known that OS contributes to kidney damage and aging [45,47], little is known what causes OS in aging-related kidney damage. To our knowledge, this is the first study to show that downregulation of GR activity played an important role in OS induced by Klotho deficiency as evidenced by the fact that in vivo expression of GR restored GSH/GSSG ratio (Fig. 6) which subsequently abolished the elevation of 4-HNE and 8-isoprostane due to Klotho deficiency (Fig. 5). Superoxide generation is known as the major source of reactive oxygen species (ROS) in vascular system which contributed to vascular dysfunction [14,15,48]. However, it may not be the major mechanism of OS in Klotho deficiency induced kidney damage [45]. In vivo studies have found accumulated oxidative damage occurs from decreased levels of endogenous anti-oxidants rather than increased ROS production [45]. Thus, Klotho deficiency-induced elevation of superoxide levels may be secondary to the impaired antioxidant system due to downregulation of GSH levels which can be rescued by AAV-GR delivery (Supplemental Fig. S2). Glutathione (GSH) is a major endogenous antioxidant that participates directly in neutralization of H2O2 which in turn leads to a decrease in superoxide (O2), as well as maintains exogenous antioxidants (Vit C and E) in their reduced (active) forms [49]. In this manner, GSH protects the cell and delays the development of pathologies [45]. Upon interaction with ROS (e.g., H2O2), GSH was converted to its oxidized form, GSSG. GR is critical to cell survival because it recycles GSH, the active form of glutathione. Thus, this study suggests that the suppressed GR activity which resulted in decreased anti-oxidative defense was involved in the development of OS in kidney aging, which can be attenuated by GR gene delivery. This finding may potentially change our concept upon kidney aging.
In vivo GR gene transfer would lead to GR expression as AAV.GR carries GR full-length cDNA. Although we expect that GR gene transfer may not directly affect expression of other genes associated with the antioxidative defense system, improvement in oxidative stress by GR gene transfer may ameliorate cellular function which indirectly improves expression of other genes associated with the antioxidative defense system. In addition, an increase in the bioavailability of reduced GSH, a substrate of glutathione peroxidase (GPx), may enhance GPx activity and increase decomposition of H2O2 into water which decreases oxidative stress. A decrease in H2O2 levels would facilitate dismutation of superoxide (O2−) to H2O2 via superoxide dismutase (SOD). The activity of glutathione s-transferases (GSTs) is dependent upon a steady supply of GSH. An increase in the ratio of GSH/GSSG or bioavailability of GSH may increase GSTs activity and its detoxification effect on xenobiotics.
Upregulation of mTOR activity is involved in the aging process [[50], [51], [52]]. We found that mTOR activity was increased in kidneys of KL ±mice (Supplemental Fig. 2), which may be involved in Klotho deficiency-induced kidney damage. Interestingly, upregulation of mTOR activity was likely due to increased ROS and oxidative damage as a result of downregulation of GR expression/activity and GSH levels which can be rescued by in vivo AAV-GR delivery. Interestingly, in vivo expression of GR also increased Klotho protein expression in kidneys of KL ± mice (Supplemental Fig. S3). This may be attributed to the enhanced antioxidant effects by AAV-GR delivery which subsequently improved cell function although the detailed mechanism requires further investigation.
GSH is a major antioxidant in mammalian cells. A decrease in GSH/GSSG ratio would result in oxidative damage leading to cellular dysfunction. Thus, supplement with exogenous glutathione (GSH) may protect cells from oxidative damage and improve organ function. It has been reported that GHS decreases oxidative stress levels and improved kidney functions in kidney diseases models in which GSH levels are downregulated [53,54]. For example, treatment with GSH could inhibit oxidative stress and abnormal angiogenesis, and improve cellular immune responses in patients with chronic kidney disease (CKD) [55]. Thus, GSH is an attractive antioxidant agent. A future study is warranted for investigating whether treatment with GSH improves kidney function in Klotho-haplodeficient (KL+/-) mice.
Perspective
To our knowledge, this is the first study demonstrating that Klotho deficiency downregulates GR expression and activity in tubular epithelial cells, which may mediate Klotho deficiency-induced kidney damage. This finding is significant, as it points to a new direction for understanding the pathogenesis of kidney aging. AAV-GR delivery may be an effective therapeutic approach for aging-related kidney damage.
Translational statement
This is the first exciting report showing that Klotho deficiency downregulates GR expression and activity in kidney cells, which may mediate Klotho deficiency-induced kidney damage. This finding is significant, as it points to a new direction for understanding the pathogenesis of kidney aging. AAV-GR delivery may be an effective therapeutic approach for aging-related kidney damage.
Source of funding
This work was supported by National Institutes of Health (NIH) R01 AG049780, AG062375 and HL122166.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
None.
Acknowledgements
We would like to thank Dr. Nathan Tipton for his critical editing of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101692.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuo Z., Lei H., Wang X., Wang Y., Sonntag W., Sun Z. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Age (Dordr) 2011;33:261–274. doi: 10.1007/s11357-010-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaeffner E.S., Ebert N., Delanaye P., Frei U., Gaedeke J., Jakob O., Kuhlmann M.K., Schuchardt M., Tolle M., Ziebig R., van der Giet M., Martus P. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Combet S., Geffroy N., Berthonaud V., Dick B., Teillet L., Verbavatz J.M., Corman B., Trinh-Trang-Tan M.M. Correction of age-related polyuria by dDAVP: molecular analysis of aquaporins and urea transporters. Am. J. Physiol. Ren. Physiol. 2003;284:F199–F208. doi: 10.1152/ajprenal.00167.2002. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y.T., Chiu H.C., Su H.M., Yang J.F., Voon W.C., Lin T.H., Lai W.T., Sheu S.H. Lower hemoglobin concentrations and subsequent decline in kidney function in an apparently healthy population aged 60 year and older. Clinica Chimica Acta; Int. J. Clinic. Chem. 2008;389:25–30. doi: 10.1016/j.cca.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J., Astor B.C., Greene T., Eknoyan G., Levey A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. Off. J. Nat. Kidney. Found. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein J.R., Anderson S. The aging kidney: physiological changes. Adv. Chron. Kidney Dis. 2010;17:302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y.I. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Sun Z. Current understanding of klotho. Ageing Res. Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao N.M., Zhang Y.M., Zheng Q., Gu J. Klotho is a serum factor related to human aging. Chin. Med. J. 2004;117:742–747. [PubMed] [Google Scholar]
- 11.Zhou X., Chen K., Lei H., Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J. Am. Soc. Nephrol. 2015;26:121–132. doi: 10.1681/ASN.2013101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X., Chen K., Wang Y., Schuman M., Lei H., Sun Z. Antiaging gene klotho regulates adrenal CYP11B2 expression and aldosterone synthesis. J. Am. Soc. Nephrol.: JASN (J. Am. Soc. Nephrol.) 2016;27:1765–1776. doi: 10.1681/ASN.2015010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Kuro-o M., Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012;11:410–417. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Fan J., Wang S., Sun Z. Secreted klotho attenuates inflammation-associated aortic valve fibrosis in senescence-accelerated mice P1. Hypertension. 2018;71:877–885. doi: 10.1161/HYPERTENSIONAHA.117.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen P.G., Sun Z. AAV delivery of endothelin-1 shRNA attenuates cold-induced hypertension. Hum. Gene Ther. 2017;28:190–199. doi: 10.1089/hum.2016.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meulendyke K.A., Ubaida-Mohien C., Drewes J.L., Liao Z., Gama L., Witwer K.W., Graham D.R., Zink M.C. Elevated brain monoamine oxidase activity in SIV- and HIV-associated neurological disease. J. Infect. Dis. 2014;210:904–912. doi: 10.1093/infdis/jiu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y., Kuro-o M., Sun Z. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology. 2013;154:3855–3863. doi: 10.1210/en.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao D., Zuo Z., Tian J., Ali Q., Lin Y., Lei H., Sun Z. Activation of SIRT1 attenuates klotho deficiency-induced arterial stiffness and hypertension by enhancing AMP-activated protein kinase activity. Hypertension. 2016;68:1191–1199. doi: 10.1161/HYPERTENSIONAHA.116.07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K., Zhou X., Sun Z. Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension. 2015;66:1006–1013. doi: 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y., Sun Z. In vivo pancreatic beta-cell-specific expression of antiaging gene klotho: a novel approach for preserving beta-cells in type 2 diabetes. Diabetes. 2015;64:1444–1458. doi: 10.2337/db14-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y., Chen J., Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension. 2016;67:564–573. doi: 10.1161/HYPERTENSIONAHA.115.06825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K., Sun Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J. Mol. Med. (Berl.) 2019;97:1615–1625. doi: 10.1007/s00109-019-01841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K., Sun Z. Activation of DNA demethylases attenuates aging-associated arterial stiffening and hypertension. Aging Cell. 2018 doi: 10.1111/acel.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah M., Sun Z. Klotho deficiency accelerates stem cells aging by impairing telomerase activity. J Gerontol A Biol Sci Med Sci. 2018 doi: 10.1093/gerona/gly261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosswhite P., Sun Z. Inhibition of phosphodiesterase-1 attenuates cold-induced pulmonary hypertension. Hypertension. 2013;61:585–592. doi: 10.1161/HYPERTENSIONAHA.111.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Skelley L., Wang B., Mejia A., Sapozhnikov V., Sun Z. AAV-based RNAi silencing of NADPH oxidase gp91(phox) attenuates cold-induced cardiovascular dysfunction. Hum. Gene Ther. 2012;23:1016–1026. doi: 10.1089/hum.2012.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Sun Z. Thyroid hormone induces artery smooth muscle cell proliferation: discovery of a new TRalpha1-Nox1 pathway. J. Cell Mol. Med. 2010;14:368–380. doi: 10.1111/j.1582-4934.2008.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivara M.B., Yeung C.K., Robinson-Cohen C., Phillips B.R., Ruzinski J., Rock D., Linke L., Shen D.D., Ikizler T.A., Himmelfarb J. Effect of coenzyme Q(10) on biomarkers of oxidative stress and cardiac function in hemodialysis patients: the CoQ(10) biomarker trial. Am. J. Kidney Dis. 2017;69:389–399. doi: 10.1053/j.ajkd.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markova I., Hüttl M., Oliyarnyk O., Kacerova T., Haluzik M., Kacer P., Seda O., Malinska H. The effect of dicarbonyl stress on the development of kidney dysfunction in metabolic syndrome - a transcriptomic and proteomic approach. Nutr. Metab. 2019;16:51. doi: 10.1186/s12986-019-0376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi J., Contag S.A., Chen K., Su Y., Figueroa J.P., Chappell M.C., Rose J.C. Sex-specific effect of antenatal betamethasone exposure on renal oxidative stress induced by angiotensins in adult sheep. Am. J. Physiol. Ren. Physiol. 2014;307:F1013–F1022. doi: 10.1152/ajprenal.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenarczyk M., Cohen E.P., Fish B.L., Irving A.A., Sharma M., Driscoll C.D., Moulder J.E. Chronic oxidative stress as a mechanism for radiation nephropathy. Radiat. Res. 2009;171:164–172. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolignano D., Mattace-Raso F., Sijbrands E.J., Zoccali C. The aging kidney revisited: a systematic review. Ageing Res. Rev. 2014;14:65–80. doi: 10.1016/j.arr.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Coresh J., Selvin E., Stevens L.A., Manzi J., Kusek J.W., Eggers P., Van Lente F., Levey A.S. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 36.Manjunath G., Tighiouart H., Ibrahim H., MacLeod B., Salem D.N., Griffith J.L., Coresh J., Levey A.S., Sarnak M.J. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 37.Bowling C.B., Muntner P. J Gerontol a-Biol. 2012;67:1379–1386. doi: 10.1093/gerona/gls173. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y., Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr. Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mockett R.J., Sohal R.S., Orr W.C. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. Faseb. J: Off. Pub. Feder. Am. Soc. Exp. Biol. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- 40.Ravikumar P., Ye J., Zhang J., Pinch S.N., Hu M.C., Kuro-o M., Hsia C.C., Moe O.W. alpha-Klotho protects against oxidative damage in pulmonary epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L566–L575. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mojzikova R., Dolezel P., Pavlicek J., Mlejnek P., Pospisilova D., Divoky V. Partial glutathione reductase deficiency as a cause of diverse clinical manifestations in a family with unstable hemoglobin (Hemoglobin Hana, beta63(E7) His-Asn) Blood Cell Mol. Dis. 2010;45:219–222. doi: 10.1016/j.bcmd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Wu K.C., Cui J.Y., Klaassen C.D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci: Off. J. Soc. Toxicol. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iskusnykh I.Y., Popova T.N., Agarkov A.A., Pinheiro de Carvalho M.A., Rjevskiy S.G. Expression of glutathione peroxidase and glutathione reductase and level of free radical processes under toxic hepatitis in rats. J. Toxicol. 2013;2013:870628. doi: 10.1155/2013/870628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betteridge D.J. What is oxidative stress? Metab. Clin. Exp. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 45.Small D.M., Coombes J.S., Bennett N., Johnson D.W., Gobe G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 46.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 47.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 48.Bengtsson S.H., Gulluyan L.M., Dusting G.J., Drummond G.R. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin. Exp. Pharmacol. Physiol. 2003;30:849–854. doi: 10.1046/j.1440-1681.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 49.Pompella A., Visvikis A., Paolicchi A., De Tata V., Casini A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 50.Johnson S.C., Rabinovitch P.S., Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shavlakadze T., Zhu J., Wang S., Zhou W., Morin B., Egerman M.A., Fan L., Wang Y., Iartchouk O., Meyer A., Valdez R.A., Mannick J.B., Klickstein L.B., Glass D.J. Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J Gerontol A Biol Sci Med Sci. 2018;73:845–852. doi: 10.1093/gerona/glx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mannick J.B., Del Giudice G., Lattanzi M., Valiante N.M., Praestgaard J., Huang B., Lonetto M.A., Maecker H.T., Kovarik J., Carson S., Glass D.J., Klickstein L.B. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 53.Akpinar H., Akpinar O. The effects of dexmedetomidine on biomarkers of oxidative stress and antioxidants in kidney. Bratisl. Lek. Listy. 2018;119:476–480. doi: 10.4149/BLL_2018_087. [DOI] [PubMed] [Google Scholar]
- 54.Veljkovic A.R., Nikolic R.S., Kocic G.M., Pavlovic D.D., Cvetkovic T.P., Sokolovic D.T., Jevtovic T.M., Basic J.T., Laketic D.M., Marinkovic M.R., Stojanovic S.R., Djordjevic B.S., Krsmanovic M.M. Protective effects of glutathione and lipoic acid against cadmium-induced oxidative stress in rat's kidney. Ren. Fail. 2012;34:1281–1287. doi: 10.3109/0886022X.2012.723661. [DOI] [PubMed] [Google Scholar]
- 55.Zuo M.H., Tang J., Xiang M.M., Long Q., Dai J.P., Yu G.D., Zhang H.G., Hu H. Clinical observation of the reduced glutathione in the treatment of diabetic chronic kidney disease. J. Cell. Biochem. 2019;120:8483–8491. doi: 10.1002/jcb.28135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.