Abstract
Bacteria identified in the oral cavity are highly complicated. They include approximately 1000 species with a diverse variety of commensal microbes that play crucial roles in the health status of individuals. Epidemiological studies related to molecular pathology have revealed that there is a close relationship between oral microbiota and tumor occurrence. Oral microbiota has attracted considerable attention for its role in in‐situ or distant tumor progression. Anaerobic oral bacteria with potential pathogenic abilities, especially Fusobacterium nucleatum and Porphyromonas gingivalis, are well studied and have close relationships with various types of carcinomas. Some aerobic bacteria such as Parvimonas are also linked to tumorigenesis. Moreover, human papillomavirus, oral fungi, and parasites are closely associated with oropharyngeal carcinoma. Microbial dysbiosis, colonization, and translocation of oral microbiota are necessary for implementation of carcinogenic functions. Various underlying mechanisms of oral microbiota‐induced carcinogenesis have been reported including excessive inflammatory reaction, immunosuppression of host, promotion of malignant transformation, antiapoptotic activity, and secretion of carcinogens. In this review, we have systemically described the impact of oral microbial abnormalities on carcinogenesis and the future directions in this field for bringing in new ideas for effective prevention of tumors.
Keywords: cancer, carcinogenesis, infection, oral microbiota
Oral microbiota has been playing an important role in the development of cancer throughout human body. Thus we try to summarize the current researches about this topic.
1. INTRODUCTION
Currently, cancer is the most disturbing disease in humans, affecting almost all regions in the body. Many factors such as chemicals, radiation, and physical agents are associated with its occurrence. 1 Among all the causes of cancer, role of microbes had been disregarded for a long time until landmark studies in the early 1990s established Helicobacter pylori (H. Pylori) as a causative agent of gastric cancer, resulting in a paradigm shift in implicating infectious agents as meaningful contributors to the process of gastric cancer. 2 Subsequent studies placed the worldwide risk of cancers from microbial infections at 16.1%, further implicating the role of microbiota. 3 The number of human cancers associated with viruses has grown. Human papillomavirus (HPV) is linked to cervical carcinoma, Epstein‐Barr virus (EBV) is linked to nasopharyngeal carcinoma, and hepatitis B virus (HBV) is linked to hepatocellular carcinoma. 4 The most classical example of bacterial tumor induction is the induction of gastric cancer by H. Pylori through host‐microbial interaction. 5 Schistosoma haematobium is closely associated with bladder squamous cell carcinoma 6 and Opisthorchis viverrini is linked to cholangiocarcinoma. 7 Treatments aimed at decreasing the infectious damage seem to improve the prognosis of infection‐associated cancers. A recent study has proven that eradication of H. Pylori seems to counteract the development of gastric adenocarcinoma. 8 Actinomycin C and D and mitomycin C have been recognized for their antitumor effect. 9 These studies have proven the role of microbial dysbiosis in cancer progression. According to the updated researches, a large group of tumor‐associated microbiota is implicated in tumorigenesis, while a small number of microbes have an antitumor effect. The recent isolation of a group of 11 commensal bacterial strains from gut microbiota has been shown to manipulate the anticancer immunity of the host. 10 Even before this discovery, isolation of Streptococcus pyogenes from neck cancer had laid out the groundwork for potential use of microbiota as anticancer agents. 11 Many animal models have been established to confirm the anticancer role of these microbes. 12 , 13 , 14 This phenomenon indicates that the interaction between microbiota and cancer is a complex one with some of the microorganisms showing tumorigenic effect and others exhibiting anticancer function.
The oral cavity harbors a huge collection of microbes including bacteria, fungi, viruses, and bacteriophages and is considered one of the greatest microbiological reservoirs in human body. 15 Some well‐studied periodontal organisms have now emerged as focal points for the developing association between oral microbial dysbiosis and cancer. 16 Fusobacterium nucleatum (F. nucleatum) from the oral cavity is considered a pivotal promoting factor for colorectal cancer (CRC). Moreover, periodontal health status has been confirmed to be associated with esophageal cancer and pancreatic cancer. Microorganisms from other parts of the body have been identified to play a role in tumor progression and chemoresistance. 17 These correlations between oral microbiota and carcinogenesis have shed some light on the ongoing explorations in this field, confirming the opinion that oral microbiota might contribute significantly to tumorigenesis through multiple mechanisms and a deep understanding of these mechanisms might help in resisting tumor progression. Correlation of oral microbiota with throat cancer and pancreatic cancer has been elucidated by Wang et al and Karpinsky. 18 , 19 Impact of oral microbiota on distant tumors was reported by Mascitti et al. 20 Underlying roles of oral microbiota as effective clinical biomarkers for the diagnosis of gastrointestinal carcinomas were summarized by Zhang et al and Chen et al. 21 , 22 Preliminary oral microbial carcinogenic mechanisms were summarized by Karpinski. 23 Based on the existing reviews focusing on this topic, we performed a systematic review of the existing studies, updates in the knowledge about this field, and the current research progress.
2. A COHORT OF ORAL MICROBIOTA WITH A STRONG IMPACT ON CARCINOGENESIS
Currently, methods have been developed to evaluate and decide the status of infection. These advances have helped build the association between oral microbiota and tumorigenesis. A considerable number of oral bacteria have been linked to carcinogenesis. Among them, F. nucleatum and Porphyromonas gingivalis (P. gingivalis) have been implicated in the progression of various carcinomas. Some of the other anaerobic and aerobic bacteria have also been reported to show a correlation with carcinogenesis. The role of HPV in the progression of oropharyngeal carcinoma has been widely acknowledged. Abnormalities related to oral fungi and parasites might also be associated with carcinogenesis, though the evidence regarding their role is lacking (Figure 1; Tables 1 and 2).
FIGURE 1.
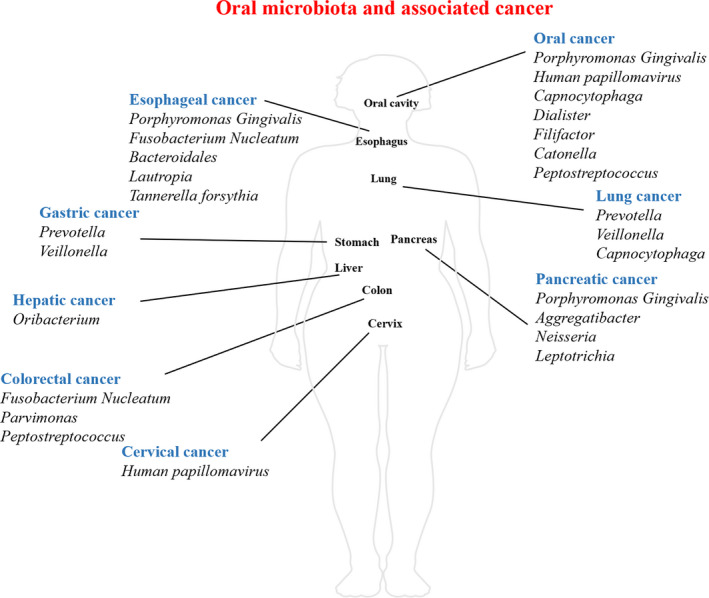
Distribution of oral microbiota and associated cancer. This figure describes the distribution of oral microbiota through human body, and their influence on certain types of cancers. Besides oral cavity, esophagus, pancreas, colon, lung, liver, stomach as well as cervix are also correlated with spread of oral microbiota
TABLE 1.
Detection of certain oral microbiota in cancerous sites
| Detecting method | Associated cancer type | Sample type | Microbiome | References |
|---|---|---|---|---|
| 16S rRNA sequencing | Oral squamous cell carcinoma | Tissues |
Actinomyces Parvimonas Fusobacterium nucleatum Pseudomonas aeruginosa Dialister |
24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 |
| 16S rDNA sequencing | Pancreatic cancer | Saliva |
Porphyromonas gingivalis Aggregatibacter Actinomycetemcomitans Fusobacterium Streptococcus Neisseria Capnocytophaga |
|
| Lung cancer | Saliva |
Capnocytophaga Veillonella |
||
| Throat cancer | Saliva |
Aggregatibacter Pseudomonas Bacteroides Ruminiclostridium |
||
| Gastric cancer | Saliva |
Streptococcus Prevotella Prevotella7 Veillonella |
||
| Metagenomic sequencing | Colorectal cancer | Fecal | Fusobacterium | 36, 37 |
| Esophageal cancer | Saliva | Streptococcus pneumoniae | ||
| Pan‐pathogen array | Oral squamous cell carcinoma | Tissues | Bacteria, viruses, fungi, etc | 38 |
| qPCR | Colorectal cancer | Tissues | F. nucleatum | 37, 39, 40 |
| Hepatic cancer | Saliva |
Oribacterium Clostridium sp. |
||
| Oral carcinoma | Saliva | Enterobacteriaceae | ||
| Immunological staining | Esophageal cancer | Tissues | P. gingivalis | 41, 42 |
| Oral squamous cell carcinoma | Tissues | P. gingivalis | ||
| Antibody detection | Esophageal cancer | Serum | P. gingivalis | 43, 44, 45 |
| Pancreatic cancer | Serum | P. gingivalis | ||
| Fluorescence in‐situ hybridization | Colorectal cancer | Tissues | F. nucleatum | 46, 47 |
TABLE 2.
Impact of oral microbiota on carcinogenesis
| Microbiome | Type | Associated cancer | Spreading passage | Effect | References |
|---|---|---|---|---|---|
| Fusobacterium nucleatum | Gram negative | Colorectal cancer | Digestive tract | Promoting | 24, 25, 26, 27, 36, 37, 39, 40, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 |
| Esophageal cancer | |||||
| Porphyromonas gingivalis | Gram negative | Oral squamous cell cancer | In‐situ oral cavity | Promoting | 41, 42, 43, 44, 45, 57, 58, 59, 60, 61, 62 |
| Pancreatic cancer | Blood stream | ||||
| Esophageal cancer | Digestive tract | ||||
| Human papillomavirus | DNA virus | Cervical cancer | Sexual behavior | Promoting | 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 |
| Oral cancer | |||||
| Head and neck cancer |
2.1. Fusobacterium nucleatum
Fusobacterium is a Gram‐negative anaerobic bacillus mainly located in the oral cavity and in the gastrointestinal tract. It is a natural inhabitant of the oral cavity. However, Fusobacterium has long been considered an opportunistic infectious agent in humans. 48 During in‐situ oral squamous cell carcinoma (OSCC), bioinformatic prediction analysis of sequencing data indicated that a high abundance of F. nucleatum subspecies Polymorphum was found in oral cancer tissues. 24 F. nucleatum has recently been implicated in CRC. 49 A higher relative abundance of Fusobacterium in CRC samples was identified by 16S rRNA sequencing. 25 Increased Fusobacterium levels in stools of CRC patients were detected using whole‐genome shotgun sequencing. 36 Fecal samples from other cohorts were also studied using metagenomic sequencing to confirm the presence of Fusobacterium and its species, suggesting that microbial signatures could be used as non‐invasive biomarkers. 37 The presence of F. nucleatum was validated after sequencing using quantitative polymerase chain reaction (qPCR) to detect its specific gene markers. 37 With F. nucleatum‐specific primer sets, it was confirmed that F. nucleatum detected in the CRC samples showed a high similarity with that detected in the saliva, 39 indicating the possible oral origin of F. nucleatum in CRC samples. With the help of fluorescence in‐situ hybridization technique, F. nucleatum‐specific fluorescent probe was used to reveal the significant abundance of F. nucleatum in colorectal tumors. 46 The invasion of F. nucleatum into CRC cells was also demonstrated with the help of fluorescent probe. 47 Patients with cancerous tissues having higher loads of F. nucleatum DNA had shorter survival durations, 50 making it a potential biomarker for prognosis. Studies have also revealed a close relationship between F. nucleatum‐positive CRC and diet, 51 , 75 demonstrating the complexity of the bacteria‐cancer interacting network. The presence of F. nucleatum was also identified in esophageal squamous cell carcinoma (ESCC) by measuring its DNA level using qPCR. 40 The cancer‐specific survival showed a negative correlation with the relative abundance of F. nucleatum, suggesting that F. nucleatum might be a prognostic biomarker for ESCC. 40 Recently, high F. nucleatum burden exhibited a poor chemotherapeutic response in two cohorts, 52 suggesting that F. nucleatum might impair the effect of chemotherapy and upregulate the drug tolerance in ESCC. Moreover, high levels of F. nucleatum were observed in gastric cancer tissues when compared with gastritis and intestinal metaplasia, 26 confirming its promoting role in digestive tract carcinoma. However, during the progression of pancreatic cancer, phylum Fusobacteria was associated with lower pancreatic cancer risk. 27 F. nucleatum harbors several membrane‐related and surface‐associated proteins, whose function is to interact with microorganisms and host cells. 53 , 54 This kind of interaction may lead to the pathogenic ability of F. nucleatum to bind and/or invade multiple cell types including oral and colonic epithelial cells. 55 , 56
2.2. Porphyromonas gingivalis
Porphyromonas gingivalis is a Gram‐negative anaerobic pathogenic bacterium involved in the destructive process of periodontitis. 57 Due to its property of perturbing epithelial tissues and host defense mechanisms, P. gingivalis has recently been considered to have potential influence on the development of tumors. P. gingivalis, a common oral pathogenic strain, was proven to be present at OSCC sites, detected by the strong positive immunohistochemical staining by rabbit P. gingivalis polyclonal antibodies. 41 As orodigestive tract is a continuous smooth passage, P. gingivalis, which has substantial abilities of mobility and invasion when compared with other oral bacteria, could possibly spread through the whole area and accelerate the process of in‐situ tumorigenesis. The chemotherapeutic resistance induced by colonization of P. gingivalis was also observed, 58 suggesting that periodontitis was an obstacle for the treatment of oral cancer. The shift of P. gingivalis from the oral cavity to other areas of orodigestive tract can easily occur with the passage of food and water. It was reported almost 15 years ago that poor oral health status showed a dose‐dependent association with esophageal squamous dysplasia, a precancerous lesion of ESCC. 59 Connection between oral bacteria and esophageal cancer has recently been confirmed through a clinical research suggesting that higher levels of P. gingivalis were associated with a higher incidence of ESCC. 60 Positive intensity of immunohistochemical staining for P. gingivalis was much more significant in ESCC tissues compared to adjacent or normal tissues, 42 indicating a possible relationship between P. gingivalis and ESCC. Median serum levels of P. gingivalis immunoglobulin (Ig) G and IgA were higher in ESCC patients than in controls, 43 demonstrating that a combination of IgG and IgA could be used in the diagnosis of ESCC patients. In addition to cancers in the upper digestive tract, pancreatic cancers also possess a strong association with oral bacteria. Tooth loss, one of the most common oral pathologies, was positively linked to pancreatic cancer. 61 Considering the fact that periodontitis is a major cause of tooth loss, further studies revealed that periodontal diseases also shared a positive relationship with risk of pancreatic cancer. 62 Association of P. gingivalis with higher risk of pancreatic cancer was demonstrated by 16S rRNA sequencing of oral wash samples. 27 Based on previous studies, serum antibody level of P. gingivalis could serve as a clinical biomarker for pancreatic cancer, as P. gingivalis serum antibody level was positively correlated with orodigestive cancer mortality. 44 This discovery paved the way to explore the role of serum IgG and subsequently, plasma antibodies against P. gingivalis were confirmed to increase in patients with pancreatic cancer, while antibodies against commensal oral bacteria showed a negative correlation with pancreatic cancer risk. 45 Infection and invasion of bacteria into the pancreas is observed in pancreatitis. 76 , 77 Moreover, in pancreatic abscess, a biofilm of microorganisms was also observed. 78 Thus, the existing pathologies of pancreas might induce the accumulation of oral microorganisms and their pathological effect might elicit carcinogenesis in the pancreas. Associations between pancreatic cancer and abundance of P. gingivalis in oral wash samples as well as between pancreatic cancer and increased levels of antibodies against P. gingivalis have been proven. 27 , 45 Pathogens can enter the pancreas through diverse routes including blood stream, bile duct, small bowel, and reflux into the pancreatic duct. 79 , 80 Thus, it might be speculated that P. gingivalis originating in the oral cavity may spread to the cancer sites or may disturb the immune system to accelerate the development of pancreatic cancer.
2.3. Other anaerobic bacteria
In addition to F. nucleatum and P. gingivalis, some of the other oral anaerobic bacteria were also listed as potential carcinogens, as they have some pathogenic properties similar to those of F. nucleatum and P. gingivalis. Their clinical relationship with tumor progression has been identified using multiple methods.
The genus Streptococcus contains more than 100 recognized species, most of which are anaerobic and are associated with human or animal hosts. 81 Some of them are normal inhabitants of the oral cavity, but some species exhibit high pathogenicity. Increased levels of Streptococcus in saliva were associated with a higher risk of gastric cancer. 28 Peptostreptococcus and Peptococcus were highly abundant in OSCC 29 and CRC samples. 30 However, it was also reported that Streptococcus pneumoniae was associated with a lower risk of esophageal adenocarcinoma. 60 Functions of different species of Streptococcus are found to be very different from one another. Many of these functions are linked to multiple oral and systemic diseases. As a predominant salivary genus, variation in its levels is proposed to be linked to abnormalities including cancers. Advances in sequencing techniques would be better equipped to explain the roles of different species of Streptococcus in carcinogenesis.
In addition, some other anaerobic oral bacteria with potential pathogenic properties are widely involved in tumorigenesis. High levels of Filifactor and Catonella were demonstrated in OSCC samples, 29 while Actinomyces and its parent taxa up to the phylum level were significantly abundant in paired normal tissues when compared with OSCC tissues. 31 Higher levels of Aggregatibacter in saliva were observed to denote a high risk of pancreatic cancer, 27 while Leptotrichia was related with lower pancreatic cancer risk. 27 A recent study that used 16S rRNA sequencing reported that Aggregatibacter was highly associated with throat cancer. 32 Prevotella and Prevotella 7 are considered to be associated with a lower risk of gastric cancer. 28 Bacteroides was related with a high risk of throat cancer in one study, 32 while higher abundance of order Bacteroidales in ESCC was also validated in a large population. 27 Ruminiclostridium was shown to be related to throat cancer. 32 Similarly, Clostridium sp. was also linked to OSCC. 33 Increased levels of Veillonella in saliva samples were considered to predict a high risk of gastric cancer and lung cancer. 28 , 82 Loss of Neisseria in saliva was validated as a marker for pancreatic cancer. 83 Elevated levels of Capnocytophaga were associated with a high risk for OSCC, while relatively lower levels of Capnocytophaga were observed in saliva from patient with pancreatic cancer. 83 Oribacterium level was higher in the tongue coat from patients with liver carcinoma. 34 Leptotrichia was more abundant in patients with pancreatic cancer in one study while its level was decreased in the saliva of patients with pancreatic cancer in another study. 27 , 83 Lautropia was also suggested to act as a marker for ESCC.
2.4. Aerobic bacteria
Aerobic bacteria, referred to as the bacteria whose survival is dependent on aerobic local environment, play crucial roles in the oral cavity. Unlike many anaerobic bacteria whose shared pathogenesis is linked to different types of oral diseases, most of the aerobic oral bacteria are located in the superficial areas and act as commensal bacteria to maintain oral microbial balance. Only a few aerobic bacteria have pathogenic abilities. 84 Apart from anaerobic oral bacteria who have close relationships with various types of carcinomas possibly due to their pathogenic abilities and special vital capacity deep inside human tissues, 85 some of the aerobic bacteria located in the oral cavity were also observed to be linked to carcinogenesis.
Parvimonas was highly decreased in normal oral sites, 31 while it was highly abundant in OSCC samples. 29 Salivary Parvimonas level was decreased in CRC. 29 , 30 Dialister showed high accumulation in OSCC samples. 29 Genus Pseudomonas was found to be highly associated with throat cancer using 16S rRNA sequencing 32 and its species Pseudomonas aeruginosa was predicted to accumulate in OSCC. 24 In oral biofilm from central lesions of oral carcinomas, level of Enterobacteriaceae, which is not a common oral inhabitant, was also increased to some extent. 33
2.5. Viruses
Apart from oral bacteria, viruses might also contribute to tumorigenesis. HPV is the most acknowledged virus associated with oral carcinogenesis.
Human papillomavirus infection is a major cause of cervical cancer, which is the third most prevalent cancer in women in the United States. It is also a causative factor for the development of oral cancer. 63 Among all types of HPV, some are not involved in the pathogenesis, while others exhibit a potential for cancer development. HPV 16 and HPV 18 are involved in approximately 70% of all cervical cancers. 64 These are indicated as high‐risk types of HPV. Recent researches have identified an increased incidence of HPV infection (approximately 50%) in head and neck squamous cell cancers. 65 , 66 HPV 16 was found to be the most frequent type in at least 90% of these cases. 67 Thus, high‐risk viruses such as HPV 16 are associated with cervical cancer as well as with OSCC, suggesting a common link among different types of HPV‐related cancers. This could be explained partly by changes in sexual behaviors. High‐risk sexual behaviors such as oral and genital sex have been observed to be linked with HPV transmission through oral cavity and genital sites. 68 , 69 , 70 Recent birth cohorts show an increased incidence of high‐risk sexual behaviors including practice of premarital sex, a greater average number of lifetime sexual partners, and oral sex. 71 , 72 Such sexual behaviors combined with some sexually transmitted diseases could partly account for the mutual communication of HPV from the genital sites to oral ones and vice versa. 73 A recent authoritative review summarized the clinical evidence from a large cohort of oropharyngeal cancer patients and found that 72.7% of the oropharyngeal carcinomas in the twenty‐first century were HPV positive largely due to oral sexual behaviors. 74 This conclusion from a large clinical sample strongly supports the correlation between HPV infection and oropharyngeal carcinoma.
2.6. Fungi and parasites
Though oral fungi and parasites are considered minorities in the oral microbial ecosystem, they are also suspected as carcinogenic candidates, as fungi and parasites located in other parts of human body have been discovered to promote tumor progression. However, little is known about the potential role of oral fungi and parasites in carcinogenesis.
Recently, pan‐pathogen array technology (PathoChip) was applied to describe the microbial biomarkers unique to human OSCC tissues. In addition to oncogenic bacteria and viruses, parasites and fungi were also detected in the array. 38 It could be speculated that variations in the levels of oral parasites and fungi might be associated with oral carcinogenesis, but lack of adequate evidence prevents us from arriving at a definite conclusion. Further studies are necessary for better elucidation of the role of parasites and fungi in carcinogenesis.
3. TUMORIGENESIS‐ASSOCIATED BIOLOGICAL BEHAVIORS OF ORAL MICROBIOTA
Though ample evidence about the link between oral bacteria and cancers has emerged, little is known about how oral microbiota can influence the process of carcinogenesis. Before the direct oncogenic impact on carcinogenesis, some important tumorigenesis‐associated microbial behaviors may be observed. For oral in‐situ carcinogenesis, colonization and survival of microbiota as well as subsequent microbial dysbiosis are prerequisites, after which microbial oncogenic effect could be exerted successfully. For systemic distal carcinogenesis, translocation ability is a crucial factor in the traffic of microbiota from the oral cavity to other parts of human body (Figure 2).
FIGURE 2.
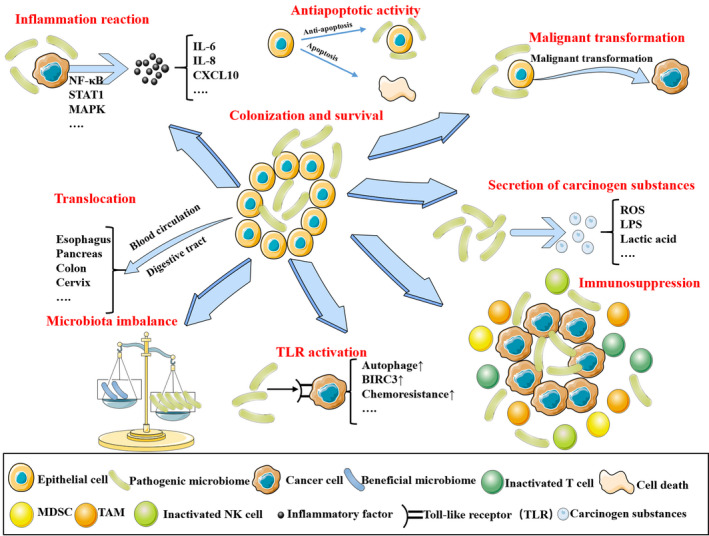
Mechanisms of the microbiota‐associated carcinogenesis. The figure depicts seven common and well‐acknowledged mechanisms for the microbiota‐associated carcinogenesis. After successful colonization and survival, pathogenic microbiota could promote the development of cancer via inflammation response, malignant transformation of epithelial cell, immunosuppression, induction of microbiota imbalance, promotion of antiapoptotic activity, and secretion of carcinogen substances. Besides in‐situ colonization, oral microbiota could translocate into other parts of human body through blood stream or digestive tract
3.1. Colonization and survival of oral microbiota inside tissues
The first step in the implementation of microbial carcinogenesis is colonization and survival of cancerous in‐situ microbiomes. Prolonged and persistent colonization by pathogenic microbiota enables further carcinogenic effect. Based on its ability to infect and colonize the basal epithelial cells of the skin or the mucosa and to use the differentiation pathway of the epithelial cells to complete its lifecycle, 86 HPV could persistently survive in the oral cavity and in the vagina as a potential source of carcinogenesis. Traditional opinions support that other parts of the body such as the genital tract can be ideal sites for the virus to be latent, whereas the oral cavity is thought to be able to defend against the invasion by pathogens. However, it was reported recently that HPV could also infect gingival tissues. 87 , 88 This could be explained by the fact that some high‐risk factors such as human immunodeficiency virus infection, smoking, tobacco use, and immunosuppression may damage the defense mechanism of the oral cavity. 89 Moreover, periodontitis might also favor the persistence of HPV in the oral cavity due to destruction of the integrity of mucosal barrier. 90 Differentiation pathway of the epithelial cells is necessary for HPV to complete its lifecycle, 86 as replication of viral DNA occurs only within the basal layers of the epithelial cells that are destined to undergo maturity and senescence. 91 Often, the movement of basal epithelial cells during wound healing is necessary for the lifecycle and the spread of viral particles, which takes approximately 2‐3 weeks. 92 F. nucleatum harbors several membrane occupation and recognition nexus repeat‐containing proteins, whose function is to interact with the microorganisms and the host cells. 53 , 54 Such an interaction acts as a groundwork for the pathogenic ability of F. nucleatum to bind and/or invade multiple cell types including oral and colonic epithelial cells. 55 , 56 The localization of F. nucleatum to cancerous tissues is mainly attributed to fusobacterial protein Fap2, which can recognize and bind to Gal/GalNAc, a membrane protein overexpressed in CRC cells. 93 The Fap2‐dependent‐specific binding mechanism instead of normal endocytosis suggests a unique interaction of F. nucleatum with CRC cells. Moreover, the unique FadA adhesin of F. nucleatum could help in the binding to E‐cadherin and activation of β‐catenin signaling pathway, thus regulating the inflammatory and oncogenic responses. 94 The variations in the survival rate of P. gingivalis in acidic conditions at different pH values in combination with the distribution of P. gingivalis in different regions of the upper digestive tract could partly play a role in the colonization of P. gingivalis in the upper digestive tract. 95 This finding supports the causative role of P. gingivalis in ESCC, as it clarifies the possibility for the colonization of P. gingivalis.
3.2. Microbial dysbiosis in the oral cavity
Various commensal oral bacteria have a negative correlation with the risk of pancreatic cancer. It is a well‐known fact that oral diseases commonly originate from changes in the balance of microbial ecology, 96 , 97 which means decrease in the number of beneficial bacteria and increase in the number of harmful ones. Microbial dysbiosis has been widely linked to inflammation‐associated CRC, 98 which is a new insight focusing not only on the effect of specific pathogens but also on the imbalance of the entire microbial ecosystem. Many types of oral bacteria with a change in the diversity of their species may lead to functional alteration of the oral ecosystem during carcinogenesis. Abnormality in the relative microbial levels is a common sign suggestive of oral microbial imbalance. Abnormal changes in the oral microbial richness have been observed to be linked to different types of cancers. A recent cross‐cohort meta‐analysis revealed a greater richness of oral microbial species in CRC tissues, indicating an influx of bacteria originating in the oral cavity. 30 In contrast, lower richness and diversity were observed in the salivary samples of patients with acute myelocytic leukemia when compared with healthy controls. 99 Microbial diversity of the tongue coat in liver cancer patients also showed an obvious increase when compared with healthy population. 34 Tongue coat, salivary samples, and subgingival samples collected from patients with gastric cancer revealed a reduced microbial diversity in the tongue coat of patients and surprisingly, an increased microbial diversity in the salivary and the subgingival samples. 48 , 75 Recently, microbial diversity of saliva was shown to decrease with throat cancer progression. 32 Inflammation and immunodeficiency are the two most noticeable outcomes of dysbiosis. 100 They are also among the crucial mechanisms of carcinogenesis. Oral cavity serves as an open environment, which is under the influence of many external factors such as smoking and drinking. Recent studies identified dysbiosis of oral cavity caused by excessive smoking and drinking. 101 , 102 Consistent with these observations, smoking and drinking are risk factors for the development of many types of cancers. Hence, it might be speculated that oral microbial imbalance associated with alterations caused by external factors is another mechanism for tumorigenesis. It could also be speculated that the imbalance of microbiota reflects a damaged immune function, imposing a pathogenic influence on carcinogenesis globally.
3.3. Microbial translocation from the oral cavity to other parts of the body
The translocation ability of oral microbiota is important for the development of systemic cancers associated with oral infection in addition to many oral bacterial diseases. It is speculated that blood circulation is a probable pathway, as periodontal bacteria always migrate to other regions such as atherosclerotic lesions 103 and brains of patients with Alzheimer's disease 104 through blood vessels. Orodigestive tract is also a plausible passage, as movement of bacteria could cooperate with the flow of food and fluid into the digestive system fluently. This finding was confirmed by the presence of oral bacteria in distal esophagus. 105 Bleeding is a common complication during periodontal therapy. Through this route, oral microbiota could enter the blood circulation and colonize the regions suitable for their survival. Moreover, some mobile oral bacteria could move toward distant regions through the airway and the digestive tract during drinking or eating. Thus, there is ample evidence to speculate that after translocation, oral microbiota could contribute to cancer development in regions other than the oral cavity. Indeed, the microbial translocation ability may be an initial mechanism for the development of distant carcinomas.
4. MECHANISMS UNDERLYING THE ROLE OF ORAL MICROBIOTA IN CARCINOGENESIS
Evidently, the role of oral microbiota is a double‐edged sword in carcinogenesis, as both antitumor and pro‐tumor microbial functions have been discovered. Some prevalent opinions about oral microbial oncogenic mechanisms have been summarized and discussed in the following subsections. However, further exploration is still needed in this field.
4.1. Excessive inflammatory reaction in response to microbiota
Though moderate inflammatory reaction is protective against tumorigenesis, excessive inflammatory response is reportedly a primary contributor to carcinogenesis in many types of cancers. 106 , 107 , 108 Invasion of pathogenic microbiota could also elicit various inflammatory diseases. 109 Hence, the carcinogenic effect of the microbiota is partly due to their induction of inflammation. Many oral microorganisms associated with carcinogenesis are pathogenic bacteria or conditional pathogenic bacteria such as Porphyromonas, Prevotella, and Fusobacterium, which could induce chronic inflammatory reaction. Production of some well‐known inflammatory mediators and effect molecules is highly elevated due to the imbalance of oral microbiota. Local concentrations of cytokines such as interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6) as well as matrix metalloproteinases (MMPs) are highly elevated, as observed in the pathogenesis of periodontitis. 110 IL‐1β acts as a motivator for the progress of inflammation due to its ability to release prostaglandins, tumor necrosis factor, and IL‐6. 111 Moreover, IL‐1β itself has been considered to have a great potential to promote tumor metastasis 112 and malignant transformation in OSCC. 113 As a principal downstream signaling molecule, IL‐6 is also involved in cancer initiation, promotion, progression, and metastasis. 114 The translational value of IL‐6 as a therapeutic target in cancer treatment also underlines its importance in the inflammation‐associated cancer pathogenesis. 115 Low levels of bacteria‐induced tumor necrosis factor are also reported to be associated with tumor promotion. 116 Microbiota‐induced or IL‐1β‐induced overexpression of MMPs is a crucial factor in tumor migration, as degradation of extracellular matrix and loss of cell‐cell or cell‐matrix adhesion caused by MMPs might contribute considerably to tumor invasion and tumor spread. 117 Some inflammatory signaling pathways commonly activated by these cytokines such as nuclear factor‐κB (NF‐κB), Wnt, and JAK‐STAT3 cascades are also associated with carcinogenesis from genetic perspective, which might explain the influence of microbiota on cancer development to some extent. Analysis of microarray data has revealed changes in several hub genes such as IL‐6, signal transducer and activator of transcription 1, and C‐X‐C motif ligand 10 after P. gingivalis infection. Most of these could act as upstream regulators of carcinogenesis. 32 Using molecular biological technology, some explanations for this phenomenon have emerged. The virulence of bacteria is mainly due to the inflammatory response of invaded tissues, which could also account for the progression of many types of cancers. Interleukin‐8 (IL‐8) is a key factor in inflammation that has been found to participate in P. gingivalis‐induced oral carcinogenesis. 93 Similarly, inflammation‐associated classical cascades, such as NF‐κB, mitogen‐activated protein kinases, programmed death‐ligand 1 (B7‐H1), and programmed death‐ligand 2 (B7‐DC), have been identified to intervene in the malignancy of oral cancer cells. 93 , 118 A recent study suggested that blockade of immune checkpoints could effectively diminish the carcinogenic influence of P. gingivalis in ESCC. 119 F. nucleatum can also induce high‐level expression of some pro‐inflammatory cytokines including IL‐6 and IL‐8, 31 which are key promoters in inflammation‐related diseases.
4.2. Pathogen‐induced immunosuppression of host
Immunosuppression is also known to play an important role in the development of many types of cancers. 120 Many pathogenic microbes are involved in the suppression of host immunological function. Microbiota‐associated immunosuppression is an important contributor to carcinogenesis. Complicated techniques of immune evasion allow HPV to remain undetected for a long time and to deploy its oncogenic effect persistently. 121 Proteins E6 and E7 play a significant roles in this process. E6 reduces surface expression of CDH1, 122 a key component for antigen presentation, and the expression of toll‐like receptor 9, 123 a recognition receptor for viral pathogens. E7 decreases the expression of transporter associated with antigen processing 1, preventing the activation of T lymphocytes. 124 Downregulation of proinflammatory cytokines such as tumor necrosis factor α (TNF‐α) and upregulation of anti‐inflammatory cytokines such interleukin‐10 also take place during infection. 125 P. gingivalis has been proven to suppress the immune system by invasion of host cells and disruption of immune‐related signaling pathways, 126 , 127 which might influence the malignant process of pancreatic cancer. The intra‐tumoral bacteria also play a key role in this process. By recruitment of tumor‐infiltrating immune cells, F. nucleatum creates a proinflammatory tumor‐associated environment consisting of many myeloid‐derived suppressor cells and tumor suppressing immune cells, which is conducive for neoplasia. 128 M2 polarization of macrophages always contributes substantially to tumor‐associated environment. This phenomenon is also observed in CRC associated with F. nucleatum infection. 129 Another pathway exploited by F. nucleatum is inhibition of natural killer cells and various T lymphocytes via Fap2 binding to T‐cell immunoreceptor with Ig and ITIM domains in these cells, affecting antitumor immune activities. 130
4.3. Induction of malignant transformation
After stable colonization and survival, microbial effect on the epithelial cells to promote their malignant transformation is crucial for the initial phase of carcinogenesis. After initial colonization, some key proteins derived from HPV launch the malignant transformation of epithelial cells. Among these, the most important proteins are E6 and E7, which have the ability to prolong cell cycle, activate cellular proliferation, and prevent apoptosis. 131 Due to the impact of E6 and E7, infected cells cease to undergo apoptosis and remain involved in persistent cell cycle. This ability is explained by the interaction between RB1, RBL1, and E7 132 , 133 as well as by degradation of TP53 induced by E6. 134 With accumulation of genetic alterations, transformation from normal cell to invasive cancer cell finally takes place. Prolonged and repetitive exposure to P. gingivalis may be implicated in the malignant transformation of normal oral epithelial cells, 135 suggesting that severe periodontitis might contribute substantially to oral carcinogenesis. Furthermore, acquisition of cancer stem cell properties via P. gingivalis infection is thought to play a role in enhancing the aggressiveness of oral cancer cells. 82 Based on the finding that F. nucleatum could interact with intestinal epithelium as well as with oral epithelium, studies have identified that F. nucleatum could initiate the host molecules of normal oral epithelium and promote the predisposition to malignant transformation through epithelial‐mesenchymal transition. 136 Many oral anaerobic bacteria share a similar ability to interact with epithelial cells and long‐term exposure to these bacteria might result in epithelial malignant transformation. Thus, interactions between oral microbiota and normal epithelial cells could result in a great number of changes in gene expression including changes in mRNAs, miRNAs, and LncRNAs. Many of these genetic alterations such as P53 downregulation are associated with malignant transformation. Hence, along with the positive role of oral microbiota in promoting cancer development, it is speculated that oral microbiota can also guide the malignant transformation of normal epithelium to initiate tumorigenesis. If this phenomenon is verified in the future, oral microbiota might be regarded as a cancer‐initiating factor rather than just a risk factor. Thus, greater attention ought to be paid to the association between oral microbiota and cancer.
4.4. Promotion of antiapoptotic activity
The antiapoptotic ability of cancer cells is a malignant feature that could lead to long‐term proliferation and chemoresistance of carcinoma. P. gingivalis‐induced promotion of antiapoptotic activity is the best example to explain the role of oral microbiota in this phenomenon. Reportedly, P. gingivalis induces changes in the intrinsic mitochondrial apoptotic activity via JAK1/AKT/STAT3 pathway. 137 , 138 Phosphorylation of BAD and enhancement of the ratio of BCL2 to BAX caused by P. gingivalis infection could significantly reduce the apoptotic activity of epithelial cells. 139 Moreover, secretion of antiapoptotic enzyme nucleoside diphosphate kinase by this bacteria cleaves adenosine triphosphate and prevents the activation of P2X7, decreasing the apoptotic activity modulated by these molecules. Activation of toll‐like receptor 4 by F. nucleatum results in upregulation of autophagy and downregulation of apoptosis, leading to a chemoresistance phenotype. 140 Since many of these cancer‐associated oral microbes share the same pathogenic properties and could activate or inactivate similar receptors or signaling pathways, it could be speculated that many microbiota‐associated cancer phenotypes such as chemoresistance might be explained partly by the promotion of antiapoptotic activity.
4.5. Production of carcinogenic substances
A recent study has reported a significant carcinogenic role of a metabolic genetic toxic substance named colibactin secreted by Escherichia coli. 141 This great discovery has ignited interest in the microbiota‐produced carcinogens. Similar to intestinal microbiota, substances produced by oral microbiota might also be associated with carcinogenesis. Reactive oxygen species are cell metabolic products generated during microbiota‐induced inflammation. 142 Their contribution to cancer pathogenesis has been verified in various processes such as cellular transformation, tumor survival, invasion, angiogenesis, and metastasis. 143 Similarly, reactive nitrogen species might also exhibit similar potential. Some known oral peroxigenic bacteria including Streptococcus oralis, S. mitis, S. sanguinis, S. gordonii, S. oligofermentans, Lactobacillus fermentum, L. jensenii, L. acidophilus, L. minutus, and Bifidobacterium adolescentis 144 might pose carcinogenic influence. Lipopolysaccharide (LPS) is a pathogenic substance commonly shared by many anaerobic oral bacteria. Its ability of activating inflammatory process is widely linked to inflammation‐associated cancer pathogenesis. Many cancer‐associated cytokines such as IL‐1β, IL‐6, and TNF‐α are elevated due to LPS stimulus during oral infection. 145 Similarly, volatile sulfur compounds produced by some Gram‐negative oral bacteria exhibit toxic and inflammation‐inducing effect and might play a role in carcinogenesis. 146 , 147 Gingipains produced by P. gingivalis are major pathogenic substances whose pathologic features have been reported in periodontitis and Alzheimer's disease. They might also be linked with tumorigenesis, as their ability to activate inflammatory signaling and MMP9 might promote tumor migration to some extent. 148 Many oral bacteria such as genera Lactobacillus, Lactococcus, Bifidobacterium, Streptococcus, Leuconostoc, and Pediococcus have the potential to produce lactic acid. 149 Studies have identified the role of lactic acid in the immunosuppression of cancer sites, making it a useful therapeutic target. 150 , 151 On the other hand, overproduction of acidic substances could result in low pH and hypoxic microenvironment, which is greatly suitable for tumor metastasis. 152 Moreover, many taxa isolated from oral cancer samples include aciduric species, 153 demonstrating the link between oral microbiota‐derived acidic products and carcinoma (Table 3).
TABLE 3.
Impact of oral microbial carcinogen on carcinogenesis
| Carcinogen | Source | Effect | References |
|---|---|---|---|
| ROS, RNS | Streptococcus oralis, S. mitis, S. sanguinis, S. gordonii, S. oligofermentans, Lactobacillus fermentum, L. jensenii, L. acidophilus, L. minutus, and Bifidobacterium adolescentis |
|
142, 143, 144 |
| VSCs | Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum |
|
146, 147 |
| LPS | Gram negative oral bacteria |
|
145 |
| Gingipains | P. gingivalis |
|
148 |
| Lactic acid | Lactobacillus, Lactococcus, Bifidobacterium, Streptococcus, Leuconostoc Pediococcus |
|
149, 150, 151, 152, 153 |
Although multiple pathways of microbiota‐induced carcinogenesis have been reported, the most prevalent and well‐studied mechanisms include direct influence on cancer cells and indirect effect via immune regulation. The existing observations could shed more light on other pathways that remain to be discovered.
As strong clinical correlation has been discovered between microbiota and carcinogenesis, studies about pathogenic mechanisms of microbiota‐induced cancer are making great progress currently. However, more details are needed to clarify the basic biology of cancer‐associated oral microbiota, the interaction between microbiota and host cells, and the mechanism used by oral microbiota to influence other microorganisms and to shape microenvironments.
5. CONCLUSIONS
The concept of microbiota‐associated cancer is currently a hot topic with great attention from microbiome researchers and tumor researchers. Oral cavity serves as one of the largest microbial storage in human body and the microbial variations in the oral cavity might be highly linked with malignancies. From clinical information and basic research, some evidence about oral microbiota and carcinogenesis has been discovered. However, further studies are still necessary for an accurate and complete understanding of this relationship.
The abundance of oral bacteria specifically associated with cancer appears to be a clinical biomarker to some extent, although further exploration is still necessary. Oral health detection indices could be used to predict multiple global health problems, as the oral cavity could act as a window toward deeper areas of human body. If this clinical phenomenon is confirmed, early prediction of some types of cancers using microbial detection might be a crucial breakthrough. The testing samples of oral microbiome vary among salivary samples, tissue samples, fecal samples, and blood samples. Microbial RNA expression level, protein level, or specific serum antibody level might be selected as detection index. However, selecting the index that could accurately predict cancer‐associated microbial variation needs further clinical experiments. Due to the multiformity and accuracy of microbial detection techniques, oral microbial status has a great potential to be a biomarker for related cancers and to help arrive at precise diagnosis and prediction.
Once the mechanisms of microbial carcinogenesis are fully elucidated, their application in therapy might be explored. Currently, combination therapy is a well‐accepted cancer treatment due to the complexity associated with cancer formation and development. As microbial factors could contribute to cancer development and chemoresistance, the use of antimicrobial treatment might be included in the combination strategy. Removal of pathogenic microbiota might overcome the negative influence of infectious factors and contribute to enhancing the effect of chemotherapy. Further clinical studies are necessary to prove whether anti‐pathogen therapy could be applied to clinical treatment. If anti‐pathogen treatment could be confirmed as a useful approach in clinical therapy, survival and prognosis of cancer patients might be improved significantly.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
Jiwei Sun and Qingming Tang contributed equally to this review and should be considered co‐first author. Conceptualizations, Jiwei Sun and Qingming Tang; literature collecting and preparation, Jiwei Sun and Qingming Tang; writing‐original draft preparation, Jiwei Sun and Qingming Tang; writing‐review and editing, Jiwei Sun, Qingming Tang, Shaoling Yu, Mengru Xie, Yanling Xie, and Guangjin Chen; Supervision, Lili Chen.
ETHICS APPROVAL
This study was approved by Tongji Medical College, Huazhong University of Science and Technology.
ACKNOWLEDGMENTS
This work was funded by Young Elite Scientist Sponsorship Program by CAST (2018QNRC001, to QT), and Key Supporting Program by Health Commission of Hubei Province (WJ2019C001, to LC).
Sun J, Tang Q, Yu S, et al. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020;9:6306–6321. 10.1002/cam4.3206
Jiwei Sun and Qingming Tang equally contributed to this review.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357(6348):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim SS, Ruiz VE, Carroll JD, Moss SF Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305(2):228‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607‐615. [DOI] [PubMed] [Google Scholar]
- 4. Rochford R, Korir A, Newton R. Viral‐associated malignancies in Africa: are viruses 'infectious traces' or 'dominant drivers'? Curr Opin Virol. 2016;20:28‐33. [DOI] [PubMed] [Google Scholar]
- 5. Peek RM Jr, Blaser MJ Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 6. Honeycutt J, Hammam O, Fu CL, Hsieh MH. Controversies and challenges in research on urogenital schistosomiasis‐associated bladder cancer. Trends Parasitol. 2014;30(7):324‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saengboonmee C, Seubwai W, Wongkham C, Wongkham S. Diabetes mellitus: possible risk and promoting factors of cholangiocarcinoma: association of diabetes mellitus and cholangiocarcinoma. Cancer Epidemiol. 2015;39(3):274‐278. [DOI] [PubMed] [Google Scholar]
- 8. Doorakkers E, Lagergren J, Engstrand L, Brusselaers N Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut. 2018;67(12):2092‐2096. [DOI] [PubMed] [Google Scholar]
- 9. Davis W, Larionov LF. Progress in chemotherapy of cancer. Bull World Health Organ. 1964;30:327‐341. [PMC free article] [PubMed] [Google Scholar]
- 10. Tanoue T, Morita S, Plichta DR, et al. A defined commensal consortium elicits CD8 T cells and anti‐cancer immunity. Nature. 2019;565(7741):600‐605. [DOI] [PubMed] [Google Scholar]
- 11. Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin Orthop Relat Res. 1893;1991(262):3‐11. [PubMed] [Google Scholar]
- 12. Zhang L, Gao L, Zhao L, et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid‐based small interfering RNAs. Can Res. 2007;67(12):5859‐5864. [DOI] [PubMed] [Google Scholar]
- 13. Tian Y, Guo B, Jia H, et al. Targeted therapy via oral administration of attenuated Salmonella expression plasmid‐vectored Stat3‐shRNA cures orthotopically transplanted mouse HCC. Cancer Gene Ther. 2012;19(6):393‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu B, Wang J, Cheng L, Liang J. Role of JNK and NF‐kappaB pathways in Porphyromonas gingivalis LPS‐induced vascular cell adhesion molecule‐1 expression in human aortic endothelial cells. Mol Med Rep. 2013;8(5):1594‐1600. [DOI] [PubMed] [Google Scholar]
- 15. Baker JL, Bor B, Agnello M, Shi W, He X. Ecology of the oral microbiome: beyond bacteria. Trends Microbiol. 2017;25(5):362‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10(3):e1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67(4):326‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang L, Yin G, Guo Y, et al. Variations in oral microbiota composition are associated with a risk of throat cancer. Front Cell Infect Microbiol. 2019;9:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karpinski TM. The microbiota and pancreatic cancer. Gastroenterol Clin North Am. 2019;48(3):447‐464. [DOI] [PubMed] [Google Scholar]
- 20. Mascitti M, Togni L, Troiano G, et al. Beyond head and neck cancer: the relationship between oral microbiota and tumour development in distant organs. Front Cell Infect Microbiol. 2019;9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Niu Q, Fan W, Huang F, He H. Oral microbiota and gastrointestinal cancer. Onco Targets Ther. 2019;12:4721‐4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Chen X, Yu H, Zhou H, Xu S. Oral microbiota as promising diagnostic biomarkers for gastrointestinal cancer: a systematic review. Onco Targets Ther. 2019;12:11131‐11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karpinski TM. Role of oral microbiota in cancer development. Microorganisms. 2019;7(1):20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Al‐Hebshi NN, Nasher AT, Maryoud MY, et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7(1):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drewes JL, White JR, Dejea CM, et al. High‐resolution bacterial 16S rRNA gene profile meta‐analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsieh YY, Tung SY, Pan HY, et al. Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep. 2018;8(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population‐based nested case‐control study. Gut. 2018;67(1):120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J, Xu S, Xiang C, et al. Tongue coating microbiota community and risk effect on gastric cancer. Journal of Cancer. 2018;9(21):4039‐4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao H, Chu M, Huang Z, et al. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7(1):11773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas AM, Manghi P, Asnicar F, et al. Metagenomic analysis of colorectal cancer datasets identifies cross‐cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25(4):667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahn SH, Chun S, Park C, Lee JH, Lee SW, Lee TH. Transcriptome profiling analysis of senescent gingival fibroblasts in response to Fusobacterium nucleatum infection. PLoS One. 2017;12(11):e0188755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geng F, Wang Q, Li C, et al. Identification of potential candidate genes of oral cancer in response to chronic infection with Porphyromonas gingivalis using bioinformatical analyses. Front Oncol. 2019;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34(4):304‐308. [PubMed] [Google Scholar]
- 34. Lu H, Ren Z, Li A, et al. Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci Rep. 2016;6:33142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan X, Yang M, Liu J, et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5(10):3111‐3122. [PMC free article] [PubMed] [Google Scholar]
- 36. Zeller G, Tap J, Voigt AY, et al. Potential of fecal microbiota for early‐stage detection of colorectal cancer. Mol Syst Biol. 2014;10:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu J, Feng Q, Wong SH, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non‐invasive biomarkers for colorectal cancer. Gut. 2017;66(1):70‐78. [DOI] [PubMed] [Google Scholar]
- 38. Banerjee S, Tian T, Wei Z, et al. Microbial signatures associated with oropharyngeal and oral squamous cell carcinomas. Sci Rep. 2017;7(1):4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Komiya Y, Shimomura Y, Higurashi T, et al. Patients with colorectal cancer have identical strains of in their colorectal cancer and oral cavity. Gut. 2019;68(7):1335‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamamura K, Baba Y, Nakagawa S, et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res. 2016;22(22):5574‐5581. [DOI] [PubMed] [Google Scholar]
- 41. Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3(4):209‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao S, Li S, Ma Z, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect Agents Cancer. 2016;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao SG, Yang JQ, Ma ZK, et al. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer. 2018;18(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33(5):1055‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michaud DS, Izard J, Wilhelm‐Benartzi CS, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62(12):1764‐1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han YW Fusobacterium nucleatum: a commensal‐turned pathogen. Curr Opin Microbiol. 2015;23:141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brennan CA, Garrett WS Fusobacterium nucleatum – symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17(3):156‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3(7):921‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamamura K, Izumi D, Kandimalla R, et al. Intratumoral levels predict therapeutic response to neoadjuvant chemotherapy in esophageal squamous cell carcinoma. Clin Cancer Res. 2019;25(20):6170‐6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Manson McGuire A, Cochrane K, Griggs AD, et al. Evolution of invasion in a diverse set of Fusobacterium species. MBio. 2014;5(6):e01864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zanzoni A, Spinelli L, Braham S, Brun C. Perturbed human sub‐networks by Fusobacterium nucleatum candidate virulence proteins. Microbiome. 2017;5(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Strauss J, Kaplan GG, Beck PL, et al. Invasive potential of gut mucosa‐derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17(9):1971‐1978. [DOI] [PubMed] [Google Scholar]
- 56. Han YW, Shi W, Huang GT, et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000;68(6):3140‐3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mysak J, Podzimek S, Sommerova P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014:476068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Woo BH, Kim DJ, Choi JI, et al. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget. 2017;8(29):46981‐46992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sepehr A, Kamangar F, Fahimi S, Saidi F, Abnet CC, Dawsey SM. Poor oral health as a risk factor for esophageal squamous dysplasia in northeastern Iran. Anticancer Res. 2005;25(1b):543‐546. [PubMed] [Google Scholar]
- 60. Peters BA, Wu J, Pei Z, et al. Oral microbiome composition reflects prospective risk for esophageal cancers. Can Res. 2017;77(23):6777‐6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stolzenberg‐Solomon RZ, Dodd KW, Blaser MJ, Virtamo J, Taylor PR, Albanes D. Tooth loss, pancreatic cancer, and Helicobacter pylori . Am J Clin Nutr. 2003;78(1):176‐181. [DOI] [PubMed] [Google Scholar]
- 62. Michaud DS, Joshipura K, Giovannucci E, Fuchs CS. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99(2):171‐175. [DOI] [PubMed] [Google Scholar]
- 63. He YF, Zhang MY, Feng LD, Yin YH, Zhang R, Di W. Risk of cervical cancer and precancerous diseases in the oral HPV carriers. Zhonghua fu chan ke za zhi. 2013;48(8):611‐616. [PubMed] [Google Scholar]
- 64. de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta‐analysis. Lancet Infect Dis. 2007;7(7):453‐459. [DOI] [PubMed] [Google Scholar]
- 65. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus‐related and ‐unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612‐619. [DOI] [PubMed] [Google Scholar]
- 66. Majchrzak E, Szybiak B, Wegner A, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol. 2014;48(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467‐475. [DOI] [PubMed] [Google Scholar]
- 68. Pickard RK, Xiao W, Broutian TR, He X, Gillison ML. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sex Transm Dis. 2012;39(7):559‐566. [DOI] [PubMed] [Google Scholar]
- 69. D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263‐1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chung CH, Bagheri A, D'Souza G. Epidemiology of oral human papillomavirus infection. Oral Oncol. 2014;50(5):364‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29(8):779‐792. [DOI] [PubMed] [Google Scholar]
- 72. Premarital sexual experience among adolescent women–United States, 1970–1988. MMWR Morb Mortal Wkly Rep. 1991;39(51‐52):929‐932. [PubMed] [Google Scholar]
- 73. Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686‐698. [DOI] [PubMed] [Google Scholar]
- 74. Chow LQM. Head and neck cancer. N Engl J Med. 2020;382(1):60‐72. [DOI] [PubMed] [Google Scholar]
- 75. Liu L, Tabung FK, Zhang X, et al. Diets that promote colon inflammation associate with risk of colorectal carcinomas that contain Fusobacterium nucleatum . Clin Gastroenterol Hepatol. 2018;16(10):1622‐31.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schmid SW, Uhl W, Friess H, Malfertheiner P, Buchler MW. The role of infection in acute pancreatitis. Gut. 1999;45(2):311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Widdison AL. Pathogenesis of pancreatic infection. Ann R Coll Surg Engl. 1996;78(4):350‐353. [PMC free article] [PubMed] [Google Scholar]
- 78. Brook I, Frazier EH. Microbiological analysis of pancreatic abscess. Clin Infect Dis. 1996;22(2):384‐385. [DOI] [PubMed] [Google Scholar]
- 79. Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut. 1994;35(9):1306‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fritz S, Hackert T, Hartwig W, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200(1):111‐117. [DOI] [PubMed] [Google Scholar]
- 81. Andam CP, Hanage WP. Mechanisms of genome evolution of Streptococcus. Infect Genet Evol. 2015;33:334‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ha NH, Woo BH, Kim DJ, et al. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015;36(12):9947‐9960. [DOI] [PubMed] [Google Scholar]
- 83. Torres PJ, Fletcher EM, Gibbons SM, Bouvet M, Doran KS, Kelley ST. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ. 2015;3:e1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schuster GS. Oral flora and pathogenic organisms. Infect Dis Clin North Am. 1999;13(4):757‐774. [DOI] [PubMed] [Google Scholar]
- 85. Evaldson G, Heimdahl A, Kager L, Nord CE. The normal human anaerobic microflora. Scand J Infect Dis Suppl. 1982;35:9‐15. [PubMed] [Google Scholar]
- 86. Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32(Suppl 1):S7‐15. [DOI] [PubMed] [Google Scholar]
- 87. Madinier I, Doglio A, Cagnon L, Lefebvre JC, Monteil RA. Southern blot detection of human papillomaviruses (HPVs) DNA sequences in gingival tissues. J Periodontol. 1992;63(8):667‐673. [DOI] [PubMed] [Google Scholar]
- 88. Hormia M, Willberg J, Ruokonen H, Syrjanen S. Marginal periodontium as a potential reservoir of human papillomavirus in oral mucosa. J Periodontol. 2005;76(3):358‐363. [DOI] [PubMed] [Google Scholar]
- 89. Beachler DC, D'Souza G. Oral human papillomavirus infection and head and neck cancers in HIV‐infected individuals. Curr Opin Oncol. 2013;25(5):503‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stubenrauch F, Laimins LA. Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol. 1999;9(6):379‐386. [DOI] [PubMed] [Google Scholar]
- 91. Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 92. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2 Suppl):S5‐S10. [DOI] [PubMed] [Google Scholar]
- 93. Abed J, Emgard JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor‐expressed Gal‐GalNAc. Cell Host Microbe. 2016;20(2):215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E‐cadherin/beta‐catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yuan X, Liu Y, Kong J, et al. Different frequencies of Porphyromonas gingivalis infection in cancers of the upper digestive tract. Cancer Lett. 2017;404:1‐7. [DOI] [PubMed] [Google Scholar]
- 96. Papapanou PN. Population studies of microbial ecology in periodontal health and disease. Annals of periodontology. 2002;7(1):54‐61. [DOI] [PubMed] [Google Scholar]
- 97. Papapanou PN, Baelum V, Luan WM, et al. Subgingival microbiota in adult Chinese: prevalence and relation to periodontal disease progression. J Periodontol. 1997;68(7):651‐666. [DOI] [PubMed] [Google Scholar]
- 98. Yu LC. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang Y, Xue J, Zhou X, et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS One. 2014;9(7):e102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Levy M, Thaiss CA, Elinav E. Metagenomic cross‐talk: the regulatory interplay between immunogenomics and the microbiome. Genome Med. 2015;7:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yu G, Phillips S, Gail MH, et al. The effect of cigarette smoking on the oral and nasal microbiota. Microbiome. 2017;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fan X, Peters BA, Jacobs EJ, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gaetti‐Jardim E Jr, Marcelino SL, Feitosa AC, Romito GA, Avila‐Campos MJ. Quantitative detection of periodontopathic bacteria in atherosclerotic plaques from coronary arteries. J Med Microbiol. 2009;58(Pt 12):1568‐1575. [DOI] [PubMed] [Google Scholar]
- 104. Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol Immunol. 2002;17(2):113‐118. [DOI] [PubMed] [Google Scholar]
- 105. Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc Natl Acad Sci USA. 2004;101(12):4250‐4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10(4):153‐169. [DOI] [PubMed] [Google Scholar]
- 107. Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. Eur J Cancer. 2006;42(6):735‐744. [DOI] [PubMed] [Google Scholar]
- 108. Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol. 2013;49(9):887‐892. [DOI] [PubMed] [Google Scholar]
- 109. Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358(6361):359‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cardoso EM, Reis C, Manzanares‐Cespedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130(1):98‐104. [DOI] [PubMed] [Google Scholar]
- 111. Hou LT, Liu CM, Liu BY, Lin SJ, Liao CS, Rossomando EF. Interleukin‐1beta, clinical parameters and matched cellular‐histopathologic changes of biopsied gingival tissue from periodontitis patients. J Periodontal Res. 2003;38(3):247‐254. [DOI] [PubMed] [Google Scholar]
- 112. Weichand B, Popp R, Dziumbla S, et al. S1PR1 on tumor‐associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL‐1beta. J Exp Med. 2017;214(9):2695‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lee CH, Chang JS, Syu SH, et al. IL‐1beta promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230(4):875‐884. [DOI] [PubMed] [Google Scholar]
- 114. Taniguchi K, Karin M. IL‐6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54‐74. [DOI] [PubMed] [Google Scholar]
- 115. Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL‐6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor‐alpha as a tumour promoter. Eur J Cancer. 2006;42(6):745‐750. [DOI] [PubMed] [Google Scholar]
- 117. Bourboulia D, Stetler‐Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20(3):161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Groeger S, Domann E, Gonzales JR, Chakraborty T, Meyle J. B7–H1 and B7‐DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis . Immunobiology. 2011;216(12):1302‐1310. [DOI] [PubMed] [Google Scholar]
- 119. Yuan X, Liu Y, Li G, et al. Blockade of immune‐checkpoint B7–H4 and lysine demethylase 5B in esophageal squamous cell carcinoma confers protective immunity against P gingivalis infection. Cancer Immunol Res. 2019;7(9):1440‐1456. [DOI] [PubMed] [Google Scholar]
- 120. Krisl JC, Doan VP. Chemotherapy and transplantation: the role of immunosuppression in malignancy and a review of antineoplastic agents in solid organ transplant recipients. Am J Transplant. 2017;17(8):1974‐1991. [DOI] [PubMed] [Google Scholar]
- 121. Piersma SJ. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011;4(3):361‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hubert P, Caberg JH, Gilles C, et al. E‐cadherin‐dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus‐associated (pre)neoplastic lesions. J Pathol. 2005;206(3):346‐355. [DOI] [PubMed] [Google Scholar]
- 123. Hasan UA, Bates E, Takeshita F, et al. TLR9 expression and function is abolished by the cervical cancer‐associated human papillomavirus type 16. J Immunol. 2007;178(5):3186‐3197. [DOI] [PubMed] [Google Scholar]
- 124. Einstein MH, Leanza S, Chiu LG, et al. Genetic variants in TAP are associated with high‐grade cervical neoplasia. Clin Cancer Res. 2009;15(3):1019‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mota F, Rayment N, Chong S, Singer A, Chain B. The antigen‐presenting environment in normal and human papillomavirus (HPV)‐related premalignant cervical epithelium. Clin Exp Immunol. 1999;116(1):33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. The host cytokine response to Porphyromonas gingivalis is modified by gingipains. Oral Microbiol Immunol. 2009;24(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Duncan L, Yoshioka M, Chandad F, Grenier D. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage‐like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb Pathog. 2004;36(6):319‐325. [DOI] [PubMed] [Google Scholar]
- 128. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor‐immune microenvironment. Cell Host Microbe. 2013;14(2):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen T, Li Q, Wu J, et al. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4‐dependent mechanism. Cancer Immunol Immunother. 2018;67(10):1635‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gur C, Ibrahim Y, Isaacson B, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 132. Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus‐16 induces degradation of retinoblastoma protein through the ubiquitin‐proteasome pathway. Can Res. 1996;56(20):4620‐4624. [PubMed] [Google Scholar]
- 133. Munger K, Basile JR, Duensing S, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20(54):7888‐7898. [DOI] [PubMed] [Google Scholar]
- 134. Tommasino M, Accardi R, Caldeira S, et al. The role of TP53 in cervical carcinogenesis. Hum Mutat. 2003;21(3):307‐312. [DOI] [PubMed] [Google Scholar]
- 135. Geng F, Liu J, Guo Y, et al. Persistent Exposure to Porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front Cell Infect Microbiol. 2017;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhang S, Li C, Liu J, et al. Fusobacterium nucleatum promotes epithelial‐mesenchymal transiton through regulation of the lncRNA MIR4435‐2HG/miR‐296‐5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3‐kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis . Infect Immun. 2004;72(7):3743‐3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis . Cell Microbiol. 2007;9(8):1997‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yao L, Jermanus C, Barbetta B, et al. Porphyromonas gingivalis infection sequesters pro‐apoptotic Bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25(2):89‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pleguezuelos‐Manzano C, Puschhof J, Huber AR, et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E coli . Nature. 2020;580(7802):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang J, Wang X, Vikash V, et al. ROS and ROS‐mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95‐105. [DOI] [PubMed] [Google Scholar]
- 144. Abranches J, Zeng L, Kajfasz JK, et al. Biology of oral streptococci. Microbiol Spectrum. 2018;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kang JY, Shin HS. Effects of 1,7‐substituted methylxanthine derivatives on LPS‐stimulated expression of cytokines and chemokines in Raw 264.7 and HK‐2 cells. J Microbiol Biotechnol. 2015;25(2):296‐301. [DOI] [PubMed] [Google Scholar]
- 146. Milella L. The negative effects of volatile sulphur compounds. J Vet Dent. 2015;32(2):99‐102. [DOI] [PubMed] [Google Scholar]
- 147. Ianaro A, Cirino G, Wallace JL. Hydrogen sulfide‐releasing anti‐inflammatory drugs for chemoprevention and treatment of cancer. Pharmacol Res. 2016;111:652‐658. [DOI] [PubMed] [Google Scholar]
- 148. Inaba H, Sugita H, Kuboniwa M, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16(1):131‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Karpinski TM, Szkaradkiewicz AK. Characteristic of bacteriocines and their application. Polish J Microbiol. 2013;62(3):223‐235. [PubMed] [Google Scholar]
- 150. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Investig. 2013;123(9):3685‐3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell‐derived lactic acid on human T cells. Blood. 2007;109(9):3812‐3819. [DOI] [PubMed] [Google Scholar]
- 152. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hooper SJ, Crean SJ, Fardy MJ, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56(Pt 12):1651‐1659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


