Highlights
-
•
Around 85% caregivers and 94% providers agreed vaccines were safe and effective.
-
•
An immediate decline in vaccine confidence were reported after vaccine incident.
-
•
Vaccine confidence regained following government and public health responses.
-
•
Providers overwhelmed by workload and psychological pressure after vaccine incident.
Keywords: Vaccine, Confidence, Acceptance, Vaccine incident, China
Abstract
Introduction
The Changchun Changsheng Vaccine Incident (CCVI) occurred mid-2018 and involved irregularities in the manufacture and quality control of diphtheria-tetanus-acellular-pertussis and rabies vaccines. This study investigates vaccine confidence amongst Chinese caregivers and vaccination-service providers (VSPs) six months after the CCVI.
Methods
Quantitative surveys were conducted in January 2019 with 2124 caregivers of children and 555 VSPs in three areas in China. The proportions of respondents who agreed to the four statements from the Vaccine Confidence Index™ were used to measure vaccine confidence. Descriptive and univariate analyses were performed to study the level of vaccine confidence. Semi-structured interviews were conducted with 48 caregivers, 43 VSPs and 9 immunization program managers. Interviews were analyzed thematically using a combination of deductive and inductive coding. Media surveillance was conducted to monitor public responses to the CCVI.
Results
Media surveillance indicated that public attention to vaccine-related issues increased sharply immediately post-CCVI but declined rapidly thereafter. Six months post-CCVI, 96.0% of caregivers and the same proportion of VSPs reported that vaccination was important and compatible with their religious beliefs. 82.7% and 88.2% of caregivers agreed that vaccines were safe and effective. 92.8% and 94.6% of VSPs agreed that vaccines were safe and effective. Both caregivers and VSPs reported an immediate decline in vaccine confidence post-CCVI. In most cases this trust was regained over time following government and public health responses, however some people remained hesitant about vaccinating their children. Many VSPs were overwhelmed by consultations, workload and psychological pressure after the CCVI.
Conclusion
After an initial decline, vaccine confidence recovered to pre-incident levels six months after the CCVI. However, some caregivers moved from the higher to the lower end of the vaccine confidence spectrum, pointing to the need to promote the acceptance of vaccination especially given the need for new vaccines to control the coronavirus epidemic.
1. Introduction
In 2019 vaccine hesitancy was identified as a top ten threats to global health by the World Health Organization (WHO) [1]. The Strategic Advisory Group of Experts on Immunization identified lack of confidence as a key factor underlying hesitancy [2]. Public trust in vaccines is dynamic, context specific and driven by multiple factors, including emotions, culture and politics [3]. These factors can be exacerbated when concerns have some basis in vaccine safety or quality issues. Maintaining public trust in vaccination is increasingly important when it comes to resilience in the face of new and emerging disease outbreaks, such as COVID-19, to optimize the acceptance of a new COVID vaccine when it becomes available.
In July 2018, Changchun Changsheng Biotechnology Co. Ltd was charged by China Food and Drug Administration (now renamed National Medical Products Administration) with two counts of malpractice: (i) manufacturing and selling 250,000 substandard diphtheria-tetanus-acellular-pertussis (DTaP) vaccines (Nov 2017) and (ii) illegal production of freeze dried rabies vaccines (July 2018) [4]. One of these contraventions resulted in substandard DTaP vaccines being administered to 215,184 Chinese children [5], [6]. Although no cases of long-term sequelae related to the substandard rabies and DTaP vaccines have been officially reported, claims that the substandard vaccines were poisonous were published on internet and social media sites [7], [8], [9]. The Changchun Changsheng vaccine incident (CCVI) and media reporting increased public anxiety about the safety of vaccines and the regulation of vaccine production in China [10]. This anxiety was evident in a survey conducted in August 2018 which found that self-scored confidence (scale ranging from 0 for no confidence to 9 for very confident) among the Chinese public declined from 6.7 to 3.2 [6].
In the months after the CCVI, substandard vaccines were recalled, and regulatory investigations conducted. Results from these investigations were published and the government adopted and implemented the first Vaccine Administration Act, which aimed to tighten vaccine regulation [4], [11]. Whether these institutional responses eased public concern is not clear. In this study we addressed this question and investigated vaccine confidence of Chinese caregivers (those with responsibility for children < 6 years) and vaccination service providers (VSPs, certificated health professionals who provide immunization related services in vaccination clinics or obstetrics departments) six months after the CCVI.
2. Methods
2.1. Study design and sites
We conducted a mixed-methods cross-sectional study in January 2019 in Anhui and Shaanxi provinces and Shenzhen megacity in Guangdong province. Anhui, located in Middle China, was one of the provinces where vaccines associated with the CCVI were administered. To avoid causing emotional distress to participants we only recruited participants from areas where the CCVI-associated vaccines were not administered. Shaanxi, located in less-developed West China, was not involved in the CCVI. Shenzhen megacity, located in the most developed eastern coast area and not involved in the CCVI, was selected for its proximity to Hong Kong, where immunization services and available vaccines differ from those in mainland China. Shenzhen residents can access immunization services in Shenzhen and Hong Kong. We selected five districts/counties in total, which included one urban district from Shenzhen city, one urban district and one rural county separately from Anhui and Shaanxi provinces.
2.2. Quantitative data collection and analysis
Questionnaires were administered to caregivers of children aged 0–6 and VSPs. Caregivers were enrolled through a two-stage clustered sampling method. At district/county level, 3–4 communities were selected to represent lower, median and higher social-economic tiers within each district. At community level, caregivers were recruited from vaccination clinics (for children <3 years) and kindergartens (for children aged 3–6 years). Caregivers who visited the sampled clinics on a set day were invited to scan QR code to fill in questionnaires on their mobile phones or to fill in paper-based questionnaires delivered by field investigators. Caregivers of children from a class in the sampled kindergarten were invited to complete the mobile-phone-based questionnaires. We disseminated 2178 questionnaires to caregivers (298 paper-based, 1880 mobile-phone-based) and received 2124 valid questionnaires from caregivers with a response rate of 97.5%. We invited all (≈600) VSPs working in the sampled areas to complete a mobile-phone-based survey and obtained 555 valid questionnaires from VSPs with a response rate of 95.0%. Respondents received an electronic currency worth 5 CNY (0.7 USD) for completing the mobile-phone survey, or a small gift of equal value for the paper-based questionnaire.
The questionnaires were developed by the research team and piloted in a non-study-site community with 30 caregivers and 10 VSPs. The contents of the questionnaires covered social demographic characteristics, vaccine confidence, experiences of vaccine hesitancy, vaccine related information seeking behaviors and experiences of the CCVI. Four statements from the Vaccine Confidence Index™ were used to measure vaccine confidence: “vaccines are important for children to have”, “overall I think vaccines are safe”, “overall I think vaccines are effective”, and “vaccines are compatible with my religious beliefs” [12]. Each respondent was asked to rate the extent to which they agreed with these statements on a Likert scale: strongly agree, tend to agree, neutral or do not know, tend to disagree, strongly disagree. The responses to the four statements were grouped into two categories: agree (including “strongly agree” and “tend to agree”) and disagree (including “strongly disagree”, “tend to disagree” and “neutral or don’t know”). The proportions of respondents who agreed to the four statements were used as the measure of vaccine confidence. Descriptive analyses were performed to depict the levels of vaccine confidence in the target population. Univariate analyses were performed to compare the levels of vaccine confidence by districts and vaccine types, using Chi-square or Fisher’s exact tests for categorical measures.
2.3. Qualitative data collection and analysis
Semi-structured interviews, which lasted between 30 and 60 mins, were conducted with caregivers and VSPs. Three caregivers and three VSPs were interviewed in each community. Caregivers were purposively sampled according to their children’s vaccination status: (i) received all central government-funded Expanded Program on Immunization (EPI) vaccines on time, (ii) received all EPI vaccines but some later than recommended, and (iii) refused at least one EPI vaccine. VSPs were sampled according to their job title, including directors of vaccination clinics, vaccination staff and pediatrician. In addition, one Centre for Disease Control and Prevention (CDC) immunization program manager from each area was interviewed. In total, we conducted 48 interviews with caregivers, 43 interviews with VSPs and nine interviews with immunization program managers. Interviews were audio-recorded, transcribed and downloaded to NVivo 11, where they were analyzed thematically using a combination of deductive and inductive coding [13]. The analysis presented here focuses on interviewees experiences with the CCVI and how it affected their vaccine confidence.
2.4. Media surveillance data collection and analysis
Media surveillance was conducted from June 2018 to January 2019 (one month prior to CCVI to six months after the CCVI) to assess media and public responses to the CCVI. The Chinese term “疫苗” (means ‘vaccine’) was used as the key search term to collect data from Baidu (the most popular search engine in China) and WeChat (China’s most popular social media app). Media reactions to the CCVI were measured by the daily number of online news reports and WeChat posts, whereas public reactions were measured by the Baidu search index and the daily number of WeChat messages.
The Fudan University School of Public Health, and the London School of Hygiene & Tropical Medicine Ethics Committees approved the study protocol [FDU IRB#2018-10-0703, LSHTM Ethics Ref 16016]. Electronically signed informed consent was obtained from respondents of mobile-phone-based survey. Written informed consent was obtained from respondents of paper-based questionnaire and interviews.
3. Results
3.1. Quantitative results
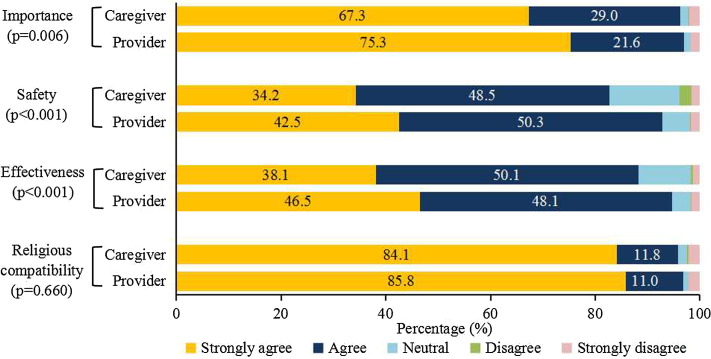
Participants’ social and demographic characteristics are summarized in Table 1 . Six months after the CCVI, over 95.0% of caregiver and VSPs respondents thought vaccination was both important and compatible with their religious beliefs (Fig. 1 ). Caregivers were less likely to agree that vaccines were safe and effective in comparison with VSPs; 82.7% versus 92.8% for safety (P < 0.001) and 88.2% versus 94.6% for effectiveness (P < 0.001).
Table 1.
Characteristics of participants in quantitative survey.
| Caregivers | Vaccination-service providers | |
|---|---|---|
| Total | 2124 | 555 |
| County/city | ||
| Rural county, Anhui province | 419(19.73) | 73(13.15) |
| Urban city, Anhui province | 436(20.53) | 100(18.02) |
| Rural county, Shaanxi province | 410(19.30) | 53(9.55) |
| Urban city, Shaanxi province | 448(21.09) | 197(35.50) |
| Shenzhen city | 411(19.35) | 132(23.78) |
| Gender | ||
| Male | 496(23.35) | 72(12.97) |
| Female | 1628(76.65) | 483(87.03) |
| Age (years, mean ± SD) | 33.71 (7.85) | 35.06(8.22) |
| Education | ||
| High school or below | 803 (37.81) | 46(8.29) |
| Junior college | 575 (27.07) | 237(42.70) |
| Bachelor degree or above | 746 (35.12) | 272(49.01) |
| Relationship with children | ||
| Mother | 1511 (71.14) | – |
| Father | 421 (19.82) | – |
| Grandparents and others | 192 (9.04) | – |
SD: Standard Deviation.
Fig. 1.
Vaccine confidence among caregivers and vaccination-service providers.
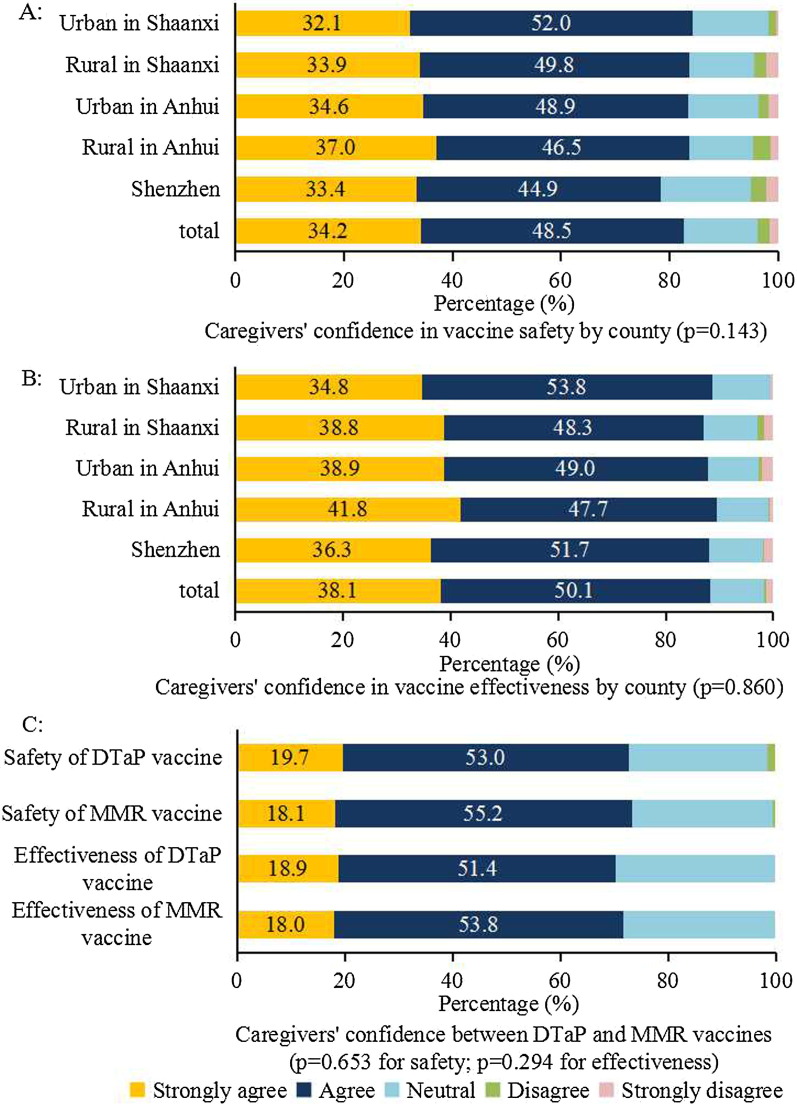
Caregivers’ confidence in vaccine safety and effectiveness did not differ significantly between county or vaccine type (Fig. 2 ). Despite DTaP vaccines involvement in the CCVI, respondents did not rate them as less safe than other vaccines and there were no significant differences in overall vaccine confidence between Anhui province (where some substandard DTaP vaccines were used) and other provinces.
Fig. 2.
Caregivers’ vaccine confidence by county and vaccine type. Notes: diphtheria-tetanus-acellular-pertussis (DTaP) vaccine, Measles-mumps-rubella (MMR) vaccine.
3.2. Qualitative results
3.2.1. Experiences of the CCVI
Caregivers reported feeling worried, angry or scared when they first heard about the CCVI. Their immediate responses were to examine their children’s vaccination records to check whether they received the substandard vaccines, consult health professionals, discuss the issue with friends, and follow the reporting of the investigations. The information they received reassured some, but others continued to have concerns about vaccine safety and effectiveness.
“Our first reaction was that ‘we don't want to get vaccinated. We can't trust it. There are so many fake vaccines that have such a huge impact on the society. The children suffered. That's our first reaction. Later, I slowly recognized that (the vaccination services here) had nothing to do with (the CCVI), not here. There is no fake vaccine (in this clinics). There is no problem. So, we will trust.” (Caregiver 12, female, age 57, Shenzhenwan Community, Shengzen city)
Six months after the CCVI more than half of the caregivers described how their confidence in vaccines had been restored because: (i) the involved vaccines were not widely used throughout China, (ii) their children were not directly involved, (iii) no serious adverse events following immunization were reported, (iv) the healthcare system and the government were trustworthy, and (v) local CDCs published investigation results promptly. Other caregivers however remained concerned and decided to (i) postpone vaccination (3/48), (ii) seek imported rather than domestic vaccines (6/48), (iii) vaccinate their children outside of mainland China, (iv) accept the EPI vaccines but refuse non-EPI vaccines (2/48) or (v) dubiously accept vaccines (10/48).
Many caregivers stated that they did not think that the quality of VSPs services changed after the CCVI. Caregivers reported that they received reassurance from VSPs that the vaccines provided at their clinics were not involved in the CCVI. However, they also reported lack of communication with VSPs about vaccine safety and effectiveness linked to the CCVI. Caregivers understood the pressure and challenges that the VSPs faced after the CCVI but expected them to remain accountable and professional.
VSPs reported feeling angry, powerless, upset and stressed after the CCVI occurred. According to them the CCVI happened because the manufacturers were driven by financial interest and lacked moral and ethical integrity.
“Money, profits, it’s all about that. It is the pecuniary interest, this their backstage problem, we ordinary people do not know, but it is still driven by interests, money” (VSP 37, female, age 47, Shahe Community, Shenzhen city).
After the incident their immediate action was to check the bar codes of the vaccines used at their clinics to see if they were part of the substandard batches. Health facility managers also hosted meetings with the health staff to provide detailed information about the CCVI. Despite this input many VSPs felt they were left to deal with the aftermath of the CCVI; reassuring anxious caregivers and bearing the brunt of their anger.
“Parents were furious and talking about their children non-stop. He [the parent] couldn’t find anyone to blame, there were us [health care providers], I was the only one he knew he could blame. It was so scary […] but I was furious about the event too. Who can I blame? The scandal made our work so passive. It was like a nightmare.” (VSP 24, female, age 28, Xiyuan Community, Anhui province).
Numerous caregivers presented at vaccination clinics to ask questions and in some instances, mothers had more up-to-date knowledge about the CCVI than VSPs. Regardless of which vaccine their child was due to receive parents also wanted strong reassurance and evidence about the safety of individual vaccines. A few VSPs reported feeling “threatened” by the level of interrogation targeted at them especially when caregivers presented in large groups.
3.2.2. What caregivers and VSPs expected of CCVI institutional response
Caregivers expected the government to (a) strengthen vaccine regulation, (b) punish involved individuals and institutes, (c) disseminate public information, and (d) invest more in vaccination clinics. Only three out of 48 caregivers commented directly on the governmental response to the CCVI; they thought the government response had been timely and systematic but questioned whether the penalties applied to offenders were sufficiently deterrent.
With regards to CDC’s response, four caregivers stated that CDC did a good job of providing official information and conducting internal investigations. These activities partially met other caregivers’ expectations of CDC’s role which they thought included: (a) recommending appropriate vaccines, (b) collaborating with vaccination service providers to strengthen the delivery system, and (c) providing professional information.
A significant number of VSPs and immunization managers recommended building a more comprehensive, stricter regulatory system for vaccines with harsher laws and punishments for offenders.
“Follow up (of the incident) is not strict enough…it is a national problem. These are things (vaccine) for children …this is very serious” (VSP 30, female, age 30, Yunyang County, Shaanxi province).
3.3. Media and the public reactions following the CCVI
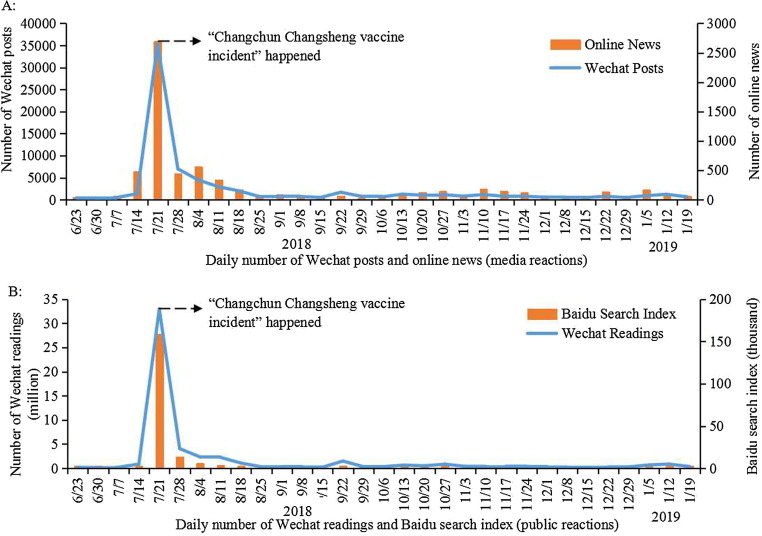
Media and public interest in vaccination increased sharply and declined rapidly post-CCVI (Fig. 3 ). Two weeks before the CCVI, there were on average only 62 online news articles and 483 WeChat posts on vaccines daily. This increased to 2698 and 35,310 per day the first week after the CCVI was reported publicly. Public responses changed even more rapidly than online news. The average number of vaccine-related WeChat posts per day increased from 0.86 million to 33 million in the first week of the CCVI. An even greater change in the Baidu search index was also observed. However, one month later, both media reports and public reactions declined to the level before the CCVI and remained stable until January 2019.
Fig. 3.
Media and public reactions following the ‘Changchun Changsheng’ vaccine incident in China, June 2018–January 2019.
4. Discussion
We investigated vaccine confidence of Chinese caregivers and VSPs six months after the CCVI. We found that over 95.0% of the respondents thought vaccination was both important and compatible with their religious beliefs. 82.7% of caregivers agreed that vaccines were safe while 92.8% of VSPs held the same perception. 88.2% of caregivers agreed that vaccines were effective while 94.6% of VSPs believed so. Caregivers and VSPs had intense negative emotional experiences during the CCVI. Their immediate reactions and the institutional responses of government and CDC eased some of them, but others continued to have concerns about vaccine safety and effectiveness. We also found a gap between the expectation of anxious caregivers and the capacity of VSPs, and an insufficient communication with the caregivers about the governmental responses to the CCVI.
Caregivers and VSPs surveyed in this study conveyed restored and continuing trust in vaccination six months after the CCVI. Reported vaccine confidence rates were close to the European average, and higher than the global average [14], [15]. Similar proportions of caregiver survey respondents and European members of the public agreed that vaccines were safe (82.7% vs 82.8%) and effective (88.2% vs 87.8%) with Chinese caregivers more likely to consider vaccines as important (96.2% vs 90.0%) [14]. Furthermore, our survey documented more positive views on vaccines than the 2018 Global Monitor survey, which found that the proportions of people perceived vaccines were safe and effective were 68.0% and 77.0% in East Asia (China, Japan, Mongolia, South Korea, Taiwan), 59.0% and 77.0% in West Europe (Austria, Belgium, France, Germany, Luxembourg, Netherlands, Switzerland) and 79.0% and 84.0% in global average, respectively [15].
We observed an immediate decline of vaccine confidence following the CCVI and a sequent recovery of vaccine confidence six months after the incident. One month after the CCVI, two studies reported significant decreases in confidence in domestic vaccines [6], [10]. In January 2019, six months after the CCVI, vaccine confidence in the provinces we surveyed was higher (safe 82.7%, effective 88.2%, important 96.2%) than levels reported in China in 2018 using the same instrument (safe 73.0%, effective 79.0%, important 91.0%) [15]. This recovery in confidence could be attributed to respondents’ understanding of the incident, their trust towards the government and healthcare system, and the prompt publication of information by public health institutions. Similar phenomena on vaccine confidence recovery were reported by studies in China [16], [17]. Vaccine incidents due to misinformation, quality or delivery problems have occurred repeatedly in China since 2000 [7]. After the hepatitis B virus (HBV) vaccine incident in 2013, during which coincidental infant deaths following HBV vaccination were subject to a lot of media attention, the proportion of caregivers who regarded domestic vaccines as safe decreased from 85.0% to 26.7%, and recovered to 70.0% six months later [16]. The HBV vaccination uptake also returned to the pre-incident level after two months. Similar change of vaccine confidence was reported after the Shandong vaccine incident which involved illegal vaccine sales [17].
Although public trust in vaccines was restored, we observed a clear move from strong to moderate agreement with vaccine confidence statements. The proportion of the public who strongly agreed that vaccines were safe and effective decreased from 47.0%-34.2% and 49.0%-38.1% respectively between July 2018 (Global Monitor) [18] and January 2019 (our survey). Our qualitative findings confirm these trends and provide some evidence that concerns ignited by the CCVI were leading to changes in vaccination behaviors. These findings are a warning sign that repeated vaccine quality incidents cumulatively impact vaccine confidence and could affect coverage, especially if the governmental response is not prompt. Hence, vaccine safety monitoring systems in China need to be strengthened in order to detect and respond timely to quality issues in order to avoid any potential crisis of confidence.
There was a gap existed between the expectations of anxious caregivers and the capacity of VSPs to address their questions. Our qualitative analysis indicated that consulting health professionals was the most frequent action taken by worried caregivers after the CCVI. VSPs play an essential role in building and sustaining public confidence in vaccines [19]. However, some of VSPs in our study were overwhelmed by increased questioning and demands for information. Many of the VSPs felt their own knowledge was more out-of-date than that of caregivers and they reported psychological pressure due to the CCVI investigations and media reporting. This indicates that VSPs need more technical and emotional support, particularly in times of crisis. Some general strategies and materials have been proposed by WHO, UNICEF and other organizations, but locally tailored and culturally sensitive materials and approaches are also needed [20], [21].
There was insufficient communication about and subsequent dissatisfaction with the governmental responses to the CCVI. This was illustrated by the observed lack of awareness of governmental responses in our interviews. Many caregivers were unaware about how the government and CDC responded following the CCVI. Apart from a few caregivers who had positive views on the government’s response, most caregivers and VSPs wanted the government and CDC to do more to strengthen the vaccine regulatory system. A month prior to this study, the Chinese legislature completed a public online consultation about a new Vaccine Administration Act [11]. None of the caregivers or VSPs mentioned the Act when they were invited to comment on government responses during our interviews.
This study has two limitations. The cross-sectional study design limited its capacity to illustrate the change of vaccine confidence over time. To monitor the change of vaccine confidence in China, we are planning to conduct a follow-up survey in late 2020. Facing the challenges caused by the coronavirus pandemic, we will also investigate the impact of the pandemic on the sentiments of caregivers towards general vaccine and the new COVID vaccine which is under development. Besides, our target populations were caregivers of children and VSPs. Their sentiments towards vaccine did not represent the public opinion in China.
5. Conclusions
Six months after the CCVI, the proportion of caregivers expressing confidence in vaccines in the provinces we surveyed recovered from the initial decline post CCVI [6] to the level before the CCVI [18]. However, a dip in level of confidence from strong to moderate agreement indicates that caregivers have residual and ongoing concerns about vaccine safety and effectiveness. Findings from our study suggest that while rapid actions from government and CDC [4], [11] have helped to alleviate immediate vaccine confidence concerns, there is a need for increased support for VSPs and better communication about government initiatives to strengthen vaccine monitoring and regulation.
Author contributions
ST led the design and execution of the study, analyzed qualitative data, and wrote the manuscript. FYS co-led the study design, analyzed qualitative data, and contributed to writing the manuscript. TC co-led the study design, participated in qualitative data analysis, and revised the manuscript. XZ, KH participated in the study design and qualitative data analysis. MJ, LR, HY participated in the study design and reviewed the manuscript. FD participated in quantitative data analysis. ZH co-led the design and execution of the study, analyzed quantitative data, and contributed to writing the manuscript. HL guided the design and execution of the study, and reviewed the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Ziru Deng and Mark Francis who supported part of the data analysis, Xinyu Zhou who analyzed media surveillance data, the data collection teams, China CDC, the provincial and county level CDCs, vaccination clinics who facilitated the research fieldwork and all the survey and interview participants.
Funding
This research was funded by the National Institute for Health Research (EPIDZL9012) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care.
This research was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation at London School of Hygiene and Tropical Medicine in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or PHE.
References
- 1.World Health Organization. Ten threats to global health in 2019 online2019 [cited 2019 December 09]. Available from: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019.
- 2.Noni E MacDonald, the SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161-4. [DOI] [PubMed]
- 3.Larson HJ, Mnookin S. Trust and Confidence in Vaccines: Tales of Three Vaccines, Lessons for Others. In: Bloom B, Lambert PH, editors. The Vaccine Book. Amsterdam ; Boston: Elsevier, AP; 2016. p. 529–40.
- 4.Baidu-pedia. Changchun Changsheng vaccine incident online: Baidu-pedia; 2018 [updated 2019 August 19; cited 2019 December 19]. Available from: https://baike.baidu.com/item/长春长生疫苗事件.
- 5.Guo Z., Bai L., Gong S. Some comments on the scandal of rabies vaccine in China. Vaccine. 2019;37(30):3936–3937. doi: 10.1016/j.vaccine.2019.05.085. [DOI] [PubMed] [Google Scholar]
- 6.Han B., Wang S., Wan Y., Liu J., Zhao T., Cui J. Has the public lost confidence in vaccines because of a vaccine scandal in China. Vaccine. 2019;37(36):5270–5275. doi: 10.1016/j.vaccine.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 7.Yan J, Ouyang Z, Vinnikova A, Chen M. Avoidance of the threats of defective vaccines: how a vaccine scandal influences parents' protective behavioral response. Health communication. 2020:1–10. [DOI] [PubMed]
- 8.Yao K-H, Jia J. Enhance research, prevention and control of pertussis for protecting public confidence in vaccination: focus on the adverse events of vaccine with insufficient potency and its long-term impacts. Chinese journal of contemporary pediatrics. 2018;20(1):1–4. doi: 10.7499/j.issn.1008-8830.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancet. Vaccine scandal and confidence crisis in China. The Lancet. 2018;392(10145):360. [DOI] [PubMed]
- 10.Zhou M., Qu S., Zhao L., Kong N., Campy K.S., Wang S. Trust collapse caused by the Changsheng vaccine crisis in China. Vaccine. 2019;37(26):3419–3425. doi: 10.1016/j.vaccine.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W. Public consultation on Vaccine Administration Act Xinhua Net: Xinhua Net; 2018 [updated Novermber 11, 2018; cited 2020 February 22]. Available from: http://www.xinhuanet.com/politics/2018-11/11/c_1123696553.htm.
- 12.Larson H., de Figueiredo A., Xiahong Z., Schulz W., Verger P., Johnston I. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyatzis RE. Transforming qualitative information: thematic analysis and code development. Thousand Oaks: Sage Publications; 1998.
- 14.Larson H, Figueiredo Ad, Karafillakis E, Rawal M. State of vaccine confidence in the EU 2018. Luxembourg: Publications Office of the European Union, 2018.
- 15.Gallup. Wellcome global monitor – first wave findings 2019. Available from: https://wellcome.ac.uk/sites/default/files/wellcome-global-monitor-2018.pdf.
- 16.Yu W., Liu D., Zheng J., Liu Y., An Z., Rodewald L. Loss of confidence in vaccines following media reports of infant deaths after hepatitis B vaccination in China. Int J Epidemiol. 2016;45(2):441–449. doi: 10.1093/ije/dyv349. [DOI] [PubMed] [Google Scholar]
- 17.Cao L., Zheng J., Cao L., Cui J., Xiao Q. Evaluation of the impact of Shandong illegal vaccine sales incident on immunizations in China. Human Vacc Immunotherap. 2018;14(7):1672–1678. doi: 10.1080/21645515.2018.1473697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.[dataset] Dataset and Crosstabs for All Countries from Wellcome Global Monitor [Internet]. Gallup. 2019 [cited 02 May 2020]. Available from: https://wellcome.ac.uk/sites/default/files/wgm2018-dataset-crosstabs-all-countries.xlsx.
- 19.Flicoteaux R., Pulcini C., Carrieri P., Schwarzinger M., Leport C., Verger P. Correlates of general practitioners’ recommendations to patients regarding vaccination for the 2009–2010 pandemic influenza (A/H1N1) in France: implications for future vaccination campaigns. Vaccine. 2014;32(20):2281–2287. doi: 10.1016/j.vaccine.2014.02.074. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Improving vaccination demand and addressing hesitancy online2019 [updated 16 August 2019; cited 2019 December 09]. Available from: https://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/.
- 21.European Centre for Disease Prevention and Control. Addressing misconceptions on measles vaccination online [cited 2019 Novemver 22]. Available from: https://www.ecdc.europa.eu/en/measles/prevention-and-control/addressing-misconceptions-measles.