Abstract
Objectives
Maternal pre-pregnancy weight is known to affect foetal development. However, it has not yet been clarified if gestational weight gain is associated with childhood behavioural development.
Methods
We performed a pooled analysis of two prospective birth cohorts to investigate the association between gestational weight gain and childhood problem behaviours, and the effect modification of maternal pre-pregnancy BMI. In total, 378 mother–child pairs from the Maastricht Essential Fatty Acids Birth cohort (MEFAB) and 414 pairs from the Rhea Mother–Child cohort were followed up from early pregnancy to 6–7 years post-partum. At follow up, parents assessed their children’s behaviour, measured as total problems, internalizing and externalizing behaviours, with the Child Behaviour Checklist. We computed cohort- and subject-specific gestational weight gain trajectories using mixed-effect linear regression models. Fractional polynomial regressions, stratified by maternal pre-pregnancy BMI status, were then used to examine the association between gestational weight gain and childhood problem behaviours.
Results
In the pre-pregnancy overweight/obese group, greater gestational weight gain was associated with higher problem behaviours. On average, children of women with overweight/obesity who gained 0.5 kg/week scored 25 points higher (on a 0–100 scale) in total problems and internalizing behaviours, and about 18 points higher in externalizing behaviours than children whose mothers gained 0.2 kg/week. Inconsistent results were found in the pre-pregnancy normal weight group.
Conclusions for Practice
Excessive gestational weight gain in women with pre-pregnancy overweight/obesity might increase problem behaviours in school-age children. Particular attention should be granted to avoid excessive weight gain in women with a pre-pregnancy overweight or obesity.
Electronic supplementary material
The online version of this article (10.1007/s10995-020-02962-y) contains supplementary material, which is available to authorized users.
Keywords: Gestational weight gain, Pre-pregnancy BMI, Internalizing, Externalizing, Problem behaviours
Significance
What is already known on this subject?: Strong evidence suggests that maternal weight before pregnancy may be associated with foetal development. However, little is known about the consequences of inadequate gestational weight gain; recent studies suggest that there might be an association with offspring’s neurocognitive and behavioural development.
What does this study add?: Children born to women with pre-pregnancy overweight/obesity who gained 0.5 kg/week during pregnancy showed higher scores in all three assessed behaviour scales, compared to children whose mothers gained 0.2 kg/week. Conversely, no clear association appeared in children born to women with a pre-pregnancy weight in the normal range.
Introduction
Excessive gestational weight gain (GWG) is a global burden that may have serious consequences on children’s physical and psychological development, especially when combined with a pre-existing overweight status (Institute of Medicine and National Research Council, 2009; Van Lieshout, 2013; World Health Organization, 2016).
An important aspect of childhood psychological development concerns problem behaviours, a group of psychopathological disorders that affects stress reactivity. Internalizing behaviours are typified by anxious and depressive traits, whereas aggressiveness and hyperactivity characterise externalizing behaviours (Achenbach & Resorta, 2001). Early-onset problem behaviours are deemed precursors of several adverse outcomes in later life, including psychiatric disorders and delinquency (e.g., Ferdinand et al., 2004; Reef, van Meurs, Verhulst, & van der Ende, 2010).
To date, only one study has investigated the influence of GWG on childhood problem behaviours, reporting no association (Pugh et al., 2016). Conversely, other studies found poor childhood behavioural outcomes were associated with excessive GWG when combined with pre-pregnancy overweight/obesity (Aubuchon-Endsley et al., 2017; Rodriguez et al., 2008). This study, therefore, aimed to examine the association between GWG and problem behaviours in school-age children. The possible effect modification of maternal pre-pregnancy body mass index (BMI) status was assessed, based on previous evidence of the correlation between GWG and pre-pregnancy weight (Institute of Medicine and National Research Council, 2009).
Methods
Study Participants
The Maastricht Essential Fatty Acid Birth (MEFAB) cohort is a prospective birth cohort established in the South of Netherlands (van der Wurff et al., 2015). Between 1989 and 1995, 1334 pregnant women were recruited during their first antenatal visit. After excluding all women with cardiovascular, neurological, renal or metabolic conditions and those who did not provide blood samples for fatty acid assessment, 1203 women were included in the cohort. Of these, 750 were eligible for the 7-year follow-up evaluation.
The Rhea Mother-Child Cohort recruited pregnant women during their first-trimester ultrasound examinations in Crete, Greece, between 2007 and 2008. Eligible women were resident of the Heraklion region and did not present any communication disorder. A total of 1363 singleton pregnancies were followed-up until delivery, as previously described (Chatzi et al., 2017).
To meet the inclusion criteria for this study, participants had to attend a minimum of two prenatal visits during which body weight was measured at least once and provide complete information on child behaviour. Consequently, this study included a total of 378 mother–child pairs from MEFAB (50.4% of participants eligible for the follow up) and 414 from Rhea (30.4% of participants followed-up until delivery).
In accordance with the Declaration of Helsinki, the MEFAB study was approved by the Medical Ethics Committee, University Hospital, Maastricht/ Maastricht University, while the Rhea study was approved by the Ethics Committee of the University Hospital in Heraklion. Written informed consent was obtained from all participants included in the study.
Results of the non-response analyses are presented in the Online Resource 1. The mothers of children with follow-up data were less likely to have overweight/obesity in MEFAB (27.25% vs. 34.20%), although only a small difference was observed in the median early pregnancy BMI (22.90 (21.49, 25.23) vs. 23.46 (21.22, 26.30)). Children included in the Rhea study were more likely to have highly educated parents (40.58% vs. 30.78%) and mothers who were less likely to smoke (17.52% vs. 22.78%). In both cohorts, children with follow-up data weighted about 100 g more at birth than children without follow-up data (MEFAB: 3304.09 g (520.98) vs. 3205.14 g (583.27); Rhea: 3213.18 g (453.36) vs. 3121.06 g (488.82)). Other differences were of minor entity and not expected to affect participation rate.
Weight in Pregnancy
In MEFAB, hospital staff measured women’s weight in four occasions during pregnancy: at study entry (median; interquartile range (IQR): week 10.14; 8.29, 12.29), during the second (week 21.86; 21.00, 22.86) and third study visits (week 32; 31.43, 32.57), and at delivery (39.43 weeks; 38.29, 40.43). In Rhea, trained midwives measured women’s weight during clinical visits in the first (week 12; 11, 13) and third (week 32; 30, 35) trimesters, while data on women’s weight at delivery was self-reported and collected during telephone interviews conducted 8–10 weeks after giving birth (final gestational age: week 38; 38, 39).
Pre-pregnancy BMI
In MEFAB, pre-pregnancy BMI (kg/m2) was calculated using the measured first trimester weight as a proxy of weight before conception, since no information was recorded regarding pre-pregnancy weight. In Rhea, given the relatively late recruitment (median: week 12), information on self-reported pre-pregnancy weight, collected at study entry, was used to compute pre-pregnancy BMI.
Due to a limited number of women falling into the underweight (BMI < 18.5; n = 25, 3.05%) and obese (BMI ≥ 30; n = 81, 9.88%) pre-pregnancy BMI categories, pre-pregnancy BMI status was computed as normal (BMI < 25 kg/m2) vs. overweight/obese (BMI ≥ 25 kg/m2).
Child Problem Behaviours
The Child Behaviour Checklist (CBCL) 4/18 and its revised version, the CBCL 6/18, were used in MEFAB and Rhea, respectively, to assess children’s problem behaviours as perceived by their parents (Achenbach & Edelbrock, 1983; Achenbach & Resorta, 2001). The CBCL has demonstrated good psychometric properties and reliability (Achenbach & Resorta, 2001). Both versions of the CBCL forms have been validated for use in both Dutch and Greek populations (De Groot, Koot, & Verhulst, 1994; Roussos et al., 1999). This study assessed the three CBCL broadband scales: total problems, internalizing and externalizing behaviours. To allow comparability between studies, age-standardized T-scores (with a mean of 50 and a standard deviation of 10) were used. T-scores can range from 0 to 100; high values (i.e., above 63) indicate clinical levels of symptomatology.
Statistical Analysis
Computation of GWG Trajectories (Analyses Stratified by Cohort)
To increase modelling precision, this step of the analysis included all women with available information on at least one measure of gestational weight and at least two measures of gestational age at which weight (or other data) was collected, independently of the availability of follow-up data (n = 1227 in MEFAB and n = 1353 in Rhea; median (IQR) number of measurements per woman: 4 (4, 4) in MEFAB, 3 (2, 3) in Rhea; percentage of women with one weight measurement: 0.24% in MEFAB, 6.95% in Rhea. Additional information is provided in the Online Resource 2). The linearity of the association was explored in each cohort separately; no evidence of deviation from linearity was found. Mixed-effect linear regression models with two levels (i.e., random intercepts for participants and random slopes for measurement occasion) were, then, used to model maternal weight during pregnancy against gestational age. The best linear unbiased predicted slope of gestational weight was obtained for each woman and used as the exposure in the subsequent step of the analyses (Chen et al., 2015). In this study, we refer to the predicted slope of gestational weight as weekly gestational weight gain (wGWG), as it represents the average weekly increment in weight during pregnancy.
Multivariable Regressions (Pooled Analyses)
The associations between wGWG and childhood problem behaviours were assessed with multivariable regression analyses with the best-fitting fractional polynomials of wGWG, since these associations did not follow a linear pattern. Interaction between maternal pre-pregnancy BMI status and wGWG were tested for all outcomes. Furthermore, given the role of sex on prenatal brain development (e.g., Reinius & Jazin, 2009)), the interaction between wGWG and children’s sex was evaluated. Subsequent analyses were stratified based on the effect modifier’s categories in case of statistically significant interactions.
We used a Directed Acyclic Graph (Online Resource 3; (Textor, van der Zander, Gilthorpe, Liśkiewicz, & Ellison, 2016) to identify the covariates to control for. The final covariate set included: maternal age, smoking and alcohol consumption, parental education and parity. In addition, maternal first trimester (MEFAB) or pre-pregnancy (Rhea) weight, children’s age at assessment and a cohort indicator variable were adjusted for. The children’s sex was controlled for in the non-stratified analyses (i.e., those for which a significant interaction with children’s sex was not found).
For ease of interpretation, we used the MIMRGNS command (Klein, 2014) in Stata to predict problem behaviour scores at the 5th, 25th, 50th, 75th and 95th percentiles of wGWG, while keeping constant all other variables included in the model.
To increase the sample size and reduce the bias due to missing values, multiple imputation of missing covariate data was performed using chained equations where 50 completed datasets were generated, separately for the two cohorts (White, Royston, & Wood, 2011). An imputation model including all exposures, outcomes, covariates and additional auxiliary variables was constructed. Auxiliary variables comprised maternal height, subject-specific mean weight in pregnancy, birth weight, gestational age, pregnancy outcomes, children’s BMI at follow-up, breastfeeding status and day-care attendance.
Several sensitivity analyses were performed to assess the robustness of our results. First, we excluded women who gave birth before week 37 of pregnancy, since a preterm birth might influence both wGWG and child development. Second, we included only women with complete information on weight during pregnancy, to rule out the possibility that the group of women with fewer weight measurements differed from the group with complete data. Third, we repeated the analyses in each cohort separately to evaluate potential heterogeneity. Fourth, we additionally controlled for breastfeeding and day-care attendance, since these might independently influence the outcomes. Fifth, we repeated the analyses excluding pre-pregnancy underweight and obese women. Sixth, complete-case data analyses were performed by including only participants without missing covariate data. Seventh, we additionally controlled for maternal Mediterranean diet score, calculated based on women’s early-pregnancy dietary intakes (Chatzi et al., 2008). For these analyses, data was restricted to the Rhea cohort, as no information on dietary intake during pregnancy was available for women included in MEFAB. Finally, we assessed the possible mediating effect of delivery mode, birth weight, gestational age, gestational diabetes and children’s BMI on the main associations. The possible mediating effects of child’s blood leptin and tumour necrosis factor (TNF ), measured at 4 years, and cord-blood leptin were assessed in the Rhea cohort as post-hoc analyses.
All statistical analyses were conducted with either Stata version 14.2 (StataCorp, 2015) or R version 3.5.1 (R Core Team, 2008), with α set at 0.05.
Results
Population characteristics subdivided by cohort are presented in Table 1 (by maternal pre-pregnancy BMI status) and in the Online Resource 4 (by problem behaviour category). In both cohorts, a higher percentage of women with a pre-pregnancy BMI in the overweight/obese range had a low level of education compared to normal-weight women. Furthermore, there was a tendency for children born to women with overweight/obesity to have higher problem behaviour scores compared to children born to women with a normal BMI.
Table 1.
Population’s characteristic
| MEFAB | Rhea | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Normal weighta | Overweight/obesea | p-value | n | Normal weighta | Overweight/obesea | p-value | ||
| Maternal characteristics | |||||||||
| Age at delivery (years) | 378 | 29.81 (3.90) | 28.90 (4.12) | 0.047 | 413 | 29.63 (4.50) | 30.70 (4.92) | 0.026 | |
| Ancestry/nationality (% Caucasian/Greek)b | 377 | 272 (98.91%) | 101 (99.02%) | 0.926 | 410 | 249 (95.04%) | 144 (94.74%) | 0.893 | |
| Pre-pregnancy BMI (kg/m2)a | 378 | 21.90 (1.77) | 27.28 (25.79, 29.34) | < 0.001 | 414 | 21.96 (1.87) | 28.40 (26.22, 32.70) | < 0.001 | |
| Smoking during pregnancy (% ever smokers) | 376 | 63 (22.99%) | 31 (30.39%) | 0.141 | 371 | 40 (16.74%) | 25 (18.94%) | 0.593 | |
| Alcohol during pregnancy (% ever drinkers) | 376 | 8 (2.92%) | 5 (4.90%) | 0.350 | 361 | 65 (29.02%) | 36 (26.28%) | 0.574 | |
| Parity | |||||||||
| No children | 378 | 200 (72.73%) | 81 (78.64%) | 0.314 | 408 | 127 (49.22%) | 58 (38.67%) | 0.041 | |
| One child | 62 (22.55%) | 16 (15.53%) | 97 (37.60%) | 60 (40.00%) | |||||
| Two or more children | 13 (4.73%) | 6 (5.83%) | 34 (13.18%) | 32 (21.33%) | |||||
| Level of education | |||||||||
| Low | 263 | 40 (20.94%) | 27 (37.50%) | 0.001 | 414 | 13 (4.96%) | 20 (13.16%) | 0.009 | |
| Middle | 72 (37.70%) | 32 (44.44%) | 143 (54.58%) | 70 (46.05%) | |||||
| High | 79 (41.36%) | 13 (18.06%) | 106 (40.46%) | 62 (40.79%) | |||||
| Weight in pregnancy (kg) | |||||||||
| First trimester | 378 | 61.04 (6.63) | 77.52 (11.15) | < 0.001 | 342 | 60.77 (6.68) | 80.59 (14.06) | < 0.001 | |
| Second trimester | 377 | 65.24 (6.63) | 80.85 (11.12) | < 0.001 | – | – | – | – | |
| Third trimester | 376 | 69.56 (7.16) | 84.71 (11.20) | < 0.001 | 362 | 70.91 (8.12) | 88.68 (13.91) | < 0.001 | |
| At delivery | 375 | 72.73 (7.69) | 87.88 (11.91) | < 0.001 | 377 | 73.24 (8.72) | 87.04 (14.48) | < 0.001 | |
| Caesarean section (% yes) | 376 | 20 (7.33%) | 10 (9.71%) | 0.447 | 413 | 114 (43.68%) | 91 (59.87%) | 0.002 | |
| Gestational diabetes mellitus (% yes) | 376 | 3 (1.10%) | 2 (1.94%) | 0.525 | 379 | 20 (8.40%) | 23 (16.31%) | 0.019 | |
| Children’s characteristics | |||||||||
| Gestational age (weeks) | 378 | 39.83 (1.53) | 39.90 (1.83) | 0.680 | 411 | 38.28 (1.50) | 37.94 (1.73) | 0.035 | |
| Birth weight (g) | 377 | 3303.64 (509.51) | 3305.29 (553.33) | 0.978 | 409 | 3221.00 (437.39) | 3199.67 (480.88) | 0.647 | |
| Sex (% male) | 378 | 150 (54.55%) | 55 (53.40%) | 0.842 | 414 | 139 (53.05%) | 93 (61.18%) | 0.108 | |
| Breastfeeding (% ever breastfed) | 268 | 98 (50.00%) | 27 (37.50%) | 0.069 | 401 | 223 (88.14%) | 121 (81.76%) | 0.077 | |
| Day-care attendance (% yes) | 270 | 166 (84.69%) | 55 (74.32%) | 0.049 | 413 | 57 (21.84% | 27 (17.76%) | 0.321 | |
| Age at survey (years) | 268 | 7.28 (0.26) | 7.27 (0.28) | 0.902 | 413 | 6.57 (0.28) | 6.58 (0.28) | 0.616 | |
| BMI at survey (kg/m2) | 264 | 15.12 (14.31, 16.14) | 15.62 (14.59, 17.35) | 0.001 | 412 | 16.03 (14.99, 17.69) | 16.94 (15.73, 19.30) | < 0.001 | |
| Total problems | 378 | 50.31 (11.30) | 52.82 (10.87) | 0.054 | 414 | 51.29 (9.75) | 52.32 (8.37) | 0.275 | |
| Internalizing behaviours | 378 | 51.65 (10.78) | 52.46 (9.38) | 0.506 | 414 | 51.26 (9.04) | 52.04 (8.66) | 0.391 | |
| Externalizing behaviours | 378 | 50.28 (10.60) | 53.32 (10.52) | 0.013 | 414 | 53.40 (9.72) | 54.61 (8.11) | 0.198 | |
aMaternal BMI was computed using first-trimester weight in MEFAB and pre-pregnancy BMI in Rhea
bAncestry/nationality was coded as Caucasian/other in MEFAB and Greek/other in Rhea; values are expressed as mean (SD), median (IQR) or number (%) as appropriate; p-values are calculated using Student’s t-test for continuous variables or chi square for categorical variables
Mean wGWG was 0.40 (SD = 0.11) kg/week in MEFAB, and 0.41 (SD = 0.05) kg/week in Rhea (p = 0.407). The mean intercept of the linear regression between gestational weight and gestational age was 60.99 kg (SD = 10.71) in MEFAB and 63.26 kg (SD = 12.99) in Rhea (p = 0.008). This value can be compared with the reported pre-pregnancy weight in Rhea (mean = 65.79 kg; SD = 14.21; p < 0.0001).
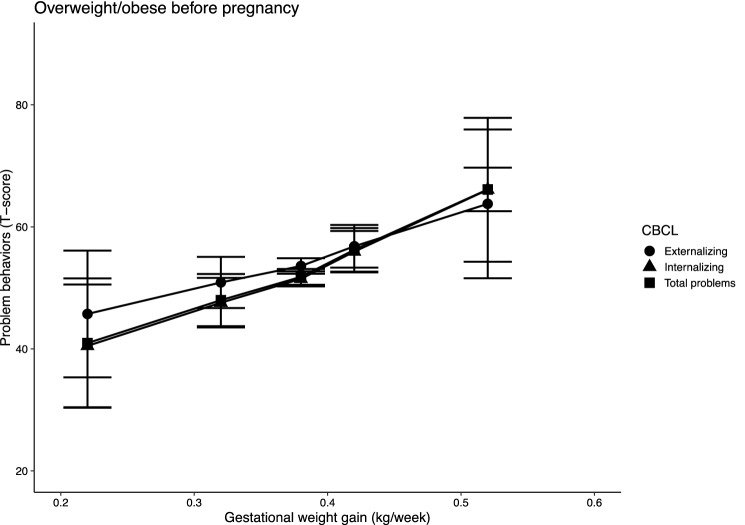
The interaction between wGWG and pre-pregnancy BMI was statistically significant on all three outcomes. Furthermore, in the normal pre-pregnancy BMI group statistically significant interactions between wGWG and children’s sex were found on total problems and internalizing behaviours. Besides, interactions between wGWG and children’s sex were not statistically significant on externalizing behaviours in the pre-pregnancy normal weight group and on any outcomes in the pre-pregnancy overweight/obese group. The analyses were, therefore, stratified to account for the two effect modifiers—i.e., pre-pregnancy BMI and children’s sex; results are presented in Figs. 1, 2, 3 and 4, and in the Online Resource 5. Percentiles of wGWG were calculated in each group separately; 5th and 95th percentiles of wGWG corresponded to approximately 0.25 kg/week and 0.55 kg/week, respectively, in all groups (exact estimates are reported in Tables 8 and 9, Online Resource 5). In the pre-pregnancy overweight-obesity group, scores of both total problems and internalizing behaviours were approximately 25 points higher in children born to women who gained the most amount of weight during their pregnancy, compared to children born to women who gained the least weight. Average scores at 5th and 95th percentiles of wGWG (95% confidence interval) were 40.95 (30.35, 51.55) and 66.13 (53.69, 78.57) for total problems, and 40.49 (30.43, 50.54) and 66.08 (54.28, 77.87) for internalizing behaviours. A smaller difference (i.e., 18 points) was found in externalizing behaviour scores (45.73 (35.34, 56.12) and 63.77 (51.58, 75.97), for the 5th and 95th percentiles of wGWG, respectively). It is worth noting that the average predicted problem-behaviour scores for children of women with overweight/obesity who gained about 0.5 kg/week fell within the clinical level of symptomatology (i.e., above 63).
Fig. 1.
Predicted problem behaviour scores in children of women with pre-pregnancy overweight or obesity. Note n = 255; models were adjusted for maternal first trimester (MEFAB) or pre-pregnancy (Rhea) weight, maternal age at delivery, smoking and alcohol consumption during pregnancy, parent’s level of education, parity, children’s sex and children’s age at assessment; 95% confidence intervals are shown
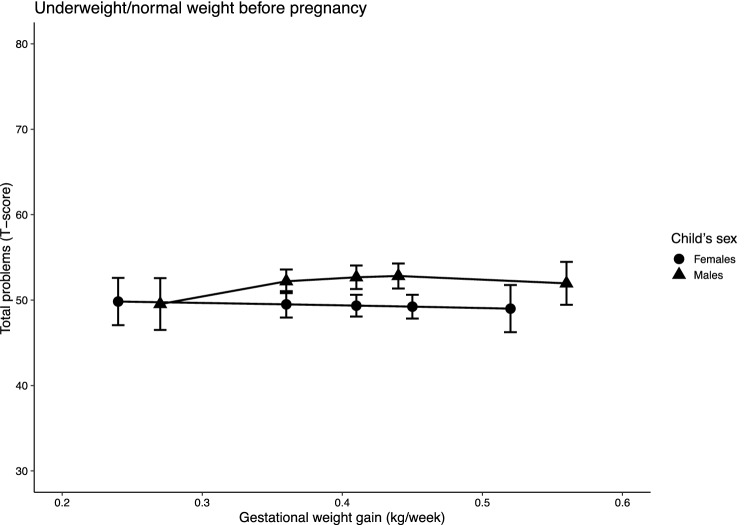
Fig. 2.
Predicted total problem scores by wGWG in children of women with pre-pregnancy BMI in the underweight or normal ranges, stratified by children’s sex. Note n = 289 (males) and 248 (females); models were adjusted for maternal first trimester (MEFAB) or pre-pregnancy (Rhea) weight, maternal age at delivery, smoking and alcohol consumption during pregnancy, parent’s level of education, parity and children’s age at assessment; 95% confidence intervals are shown
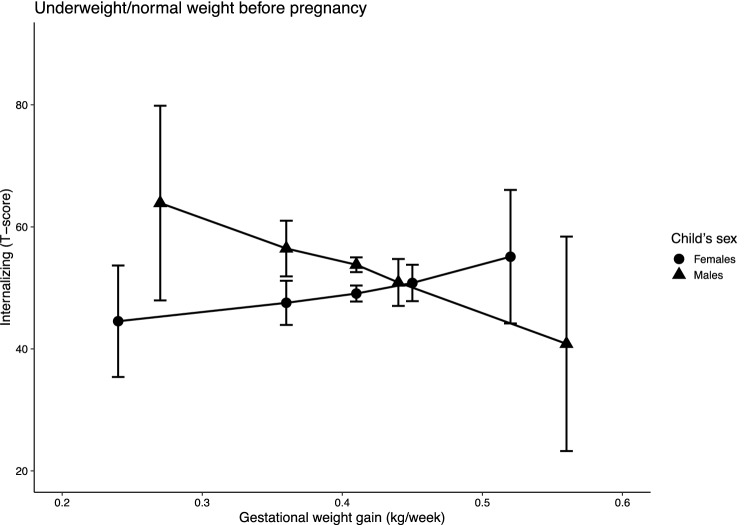
Fig. 3.
Predicted internalizing behaviour scores by wGWG in children of women with pre-pregnancy BMI in the underweight or normal ranges, stratified by children’s sex. Note n = 289 (males) and 248 (females); models were adjusted for maternal first trimester (MEFAB) or pre-pregnancy (Rhea) weight, maternal age at delivery, smoking and alcohol consumption during pregnancy, parent’s level of education, parity and children’s age at assessment; 95% confidence intervals are shown
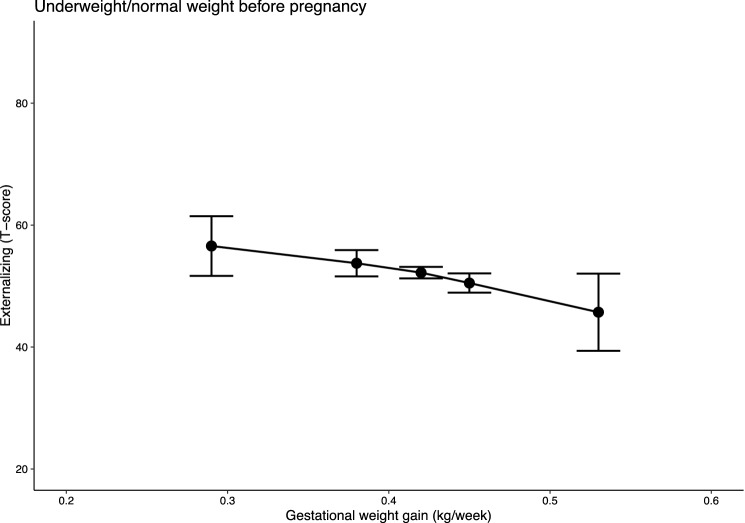
Fig. 4.
Predicted externalizing behaviour scores by wGWG in children of women with pre-pregnancy BMI in the underweight or normal ranges. Note n = 537; models were adjusted for maternal first trimester (MEFAB) or pre-pregnancy (Rhea) weight, maternal age at delivery, smoking and alcohol consumption during pregnancy, parent’s level of education, parity, children’s sex and children’s age at assessment; 95% confidence intervals are shown
However, in children born to women with a normal pre-pregnancy BMI, internalizing behaviour scores decreased by 23 points for increasing wGWG in males (63.90 (47.95, 79.84) and 40.83 (23.26, 58.41)), while increasing slightly (i.e., about 10 points) in females (44.53 (35.39, 53.67) and 55.11 (44.16, 66.05)). No association was observed in children of women with a normal weight for total problems (males: 49.53 (46.50, 52.56) and 51.96 (49.45, 54.47); females: 49.83 (47.05, 52.60) and 49.00 (46.24, 51.76)), and only a small reduction (i.e., about 10 points) was found in externalizing behaviour scores for increasing wGWG (56.56 (51.66, 61.46) and 45.71 (39.38, 52.03)).
Results of the sensitivity analysis are presented in the Online Resource 6. Overall, with the exception of the association between wGWG and internalizing behaviours in Rhea only, all regressions in the pre-pregnancy overweight/obese group showed similar estimates and a clear increase in the outcome’s predicted score. Besides, the associations between wGWG and problem behaviours in the normal-weight group were inconsistent. Finally, none of the performed mediation analyses highlighted a significant indirect effect, suggesting that the identified variables might not lie in the causal path between wGWG and problem behaviours.
Discussion
The aim of the present study was to evaluate the association between wGWG and problem behaviours in school-age children by pooling together individual data from two prospective European birth cohorts, MEFAB and Rhea. These results provide evidence for the association between maternal weight in pregnancy and behaviour problems in school-age children. In the overweight/obesity group, we observed a 25-point difference (on a 0–100 scale) in the average scores of the total problem and internalizing behaviour scales between children of women in the lower-end of the wGWG range and children of women in the higher-end of this range. Similarly, externalizing behaviour scores increased by about 18 points in children of women with the highest wGWG. Furthermore, our results showed that the offspring of women who gain excessive weight during pregnancy (i.e., about 0.5 kg/week) may attain mean problem behaviour scores in the clinical range of symptomatology (i.e., over 63). These results are likely to be of clinical relevance, considering that children with behaviour in the clinical range are at an increased risk of poor developmental outcomes, with higher odds for each unit increase in CBCL scores (Ferdinand et al., 2004; Reef et al., 2010).
In contrast, in children of women with a normal pre-pregnancy BMI, the associations were inconsistent. We observed a sex-specific trend of change for increasing wGWG in internalizing behaviours, with scores decreasing in males and slightly increasing in females. A reduction in externalizing behaviours for increasing wGWG was evident in males and females combined, while no association was observed with total problems.
To our knowledge, only one previous study has investigated the association between GWG by maternal pre-pregnancy BMI category and childhood problem behaviours, reporting no statistically significant association (Pugh et al., 2016). However, since the study population comprised only of low-income, high-risk women, these results cannot be directly compared with our findings, which were based on well educated, low-risk families. Other studies examined the association between GWG and infants’ neurobehavior (Aubuchon-Endsley, Bublitz, & Stroud, 2017) and attention deficit/hyperactivity disorder (ADHD) risk (Rodriguez et al., 2008), which are strongly related to problem behaviours in mid-childhood (Biederman, Monuteaux, Kendrick, Klein, & Faraone, 2005; Liu et al., 2010). Poor outcomes are reported in children of obese women who gained excessive weight during pregnancy (Aubuchon-Endsley et al., 2017; Rodriguez et al., 2008), supporting our findings.
If replicated, the results of the present study may have public health relevance, given the constantly rising number of overweight and obese women entering pregnancy (Institute of Medicine and National Research Council, 2009). In line with the American Institute of Medicine guidelines (Institute of Medicine and National Research Council, 2009), we showed that for overweight/obese women an adequate weight gain (i.e., approximately 0.22 kg/week) is associated with the lowest childhood problem behaviours. Given that 50–60% of overweight or obese women gain weight in excess during their pregnancy (Institute of Medicine and National Research Council, 2009), we recommend women with overweight/obesity should be closely monitored to prevent excessive GWG.
Maternal weight in pregnancy might influence the development of children’s behaviour via increased glucose levels and the consequent rise in insulin secretion by the foetus or through elevated levels of inflammatory cytokines. Additionally, obesity might result in leptin resistance, with consequent excessive leptin levels and disproportionate release of cortisol (Edlow, 2017). In fact, a previous study found that evening cortisol levels were elevated in women with pre-pregnancy obesity during the third trimester of pregnancy, with an even greater increase in cortisol levels observed in women with excessive GWG (Aubuchon-Endsley et al., 2014). Despite this evidence, in the present study, gestational diabetes mellitus did not mediate the association between wGWG and problem behaviours; however, only a small number of women in this study were diagnosed with gestational diabetes. Furthermore, we found no evidence of mediation by cord-blood leptin, serum pro-inflammatory cytokine TNF and leptin in children from the Rhea study. However, no more than 155 children were included in these analyses: the possible mediating effects by gestational diabetes, inflammatory cytokines and leptin cannot be completely ruled out.
Strengths of this study include the pooling of individual data from two European prospective birth cohorts, MEFAB and Rhea, which has led to greater generalisability of the results. Additional strengths include a centralised statistical-analysis approach with harmonised exposure, confounder and outcome variables and the assessment of children’s behaviour using similar versions of the CBCL.
Women’s weight in pregnancy was directly and repeatedly measured by hospital staff in both cohorts, with the exception of weight at delivery in Rhea, which was self-reported. Consequently, we were able to obtain precise estimates of wGWG, comparable between cohorts, by considering the trends of weight gain during pregnancy. Gestational weight trajectories were better described by linear patterns, in contrast with previous publications that showed non-linear GWG (e.g., Fraser et al., 2010). It should be noted, however, that, comparing the intercept of maternal weight’s trajectory with the self-reported pre-pregnancy weight in Rhea, a different pattern of weight gain could be hypothesized for the first weeks of pregnancy, with lower rates of weight gain in this period. Therefore, the frequency of weight measurements during pregnancy might not have been sufficient to capture the full complexity of GWG trajectory. Consequently, the GWG trajectory we described might resemble more the characteristic pattern of the second and third trimesters, overestimating slightly the weight gain in the first trimester.
A few limitations should also be considered. Pre-pregnancy BMI was based on first trimester measured weight in MEFAB and on self-reported pre-pregnancy weight in Rhea. Although not ideal, these methods represent common practice in epidemiological studies and clinical settings, generally being considered reliable and comparable (Headen, Cohen, Mujahid, & Abrams, 2017; Krukowski et al., 2016). In Rhea, delivery weight was self-reported 8–10 weeks postpartum. A systematic review has shown that the recall of delivery weight due to self-report is reproducible and valid (Headen et al., 2017), while underreporting of delivery weight, which tends to be more frequent than over-reporting, would most likely bias estimates toward the null (Schieve et al., 1999). Only few participants were classified as underweight or obese, precluding us from testing our hypotheses in these subgroups. Virtually all women included in this study were Caucasian, therefore these findings cannot be directly extended to other ethnic groups. Although the development of problem behaviours is influenced by several risk factors, we did not assess any child- or family-specific factors exclusively related to infancy or childhood. Nonetheless, these factors cannot be considered potential confounders of the association between GWG and problem behaviours, as they necessarily occur after the exposure (VanderWeele, 2019). Finally, despite their possible association with weight status before pregnancy or wGWG (Hartley, McPhie, Skouteris, Fuller-Tyszkiewicz, & Hill, 2015; Stuebe, Oken, & Gillman, 2009) and children’s development and behaviour (Borge et al., 2017; Madigan et al. 2018), we could not adequately control for maternal psychopathology, diet quality and physical activity before or during pregnancy.
Conclusions
Increasing wGWG, in combination with pre-pregnancy overweight/obesity, was associated with higher problem behaviours in school-age children. Less clear was the association between wGWG and problem behaviours in children of women with a normal pre-pregnancy BMI. Future studies should further examine the relationship between wGWG and childhood problem behaviours, assessing maternal psychopathology, diet quality and physical activity levels before and during pregnancy and including more women with a pre-pregnancy BMI in the obese or underweight ranges.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (PDF 127 kb)
Electronic supplementary material 2 (PDF 32 kb)
Electronic supplementary material 3 (PDF 183 kb)
Electronic supplementary material 4 (PDF 200 kb)
Electronic supplementary material 5 (PDF 164 kb)
Electronic supplementary material 6 (PDF 190 kb)
Acknowledgements
The authors would like to thank all participating families.
Abbreviations
- CBCL
Child Behaviour Checklist
- GWG
Gestational weight gain
- wGWG
Weekly gestational weight gain
- MEFAB
Maastricht Essential Fatty Acids Birth cohort
Funding
The MEFAB cohort was financially supported by the Dutch organization for Scientific Research (NWO, Grant Number 90462186) and by the University Hospital of Maastricht (Profilerings Fonds). The Rhea Mother–Child cohort was funded by the European Union H2020 (LIFECYCLE), FP7 (HELIX, CHICOS, EnviroGenomarkers, ENRIECO, ESCAPE) and FP6 (HiWate, NewGeneris) programs. Additional funding sources are: the National Strategic Reference Framework (ESPA) 2007-13, the General Secretariat for Research and Technology in Greece and the Research Committee of the University of Crete, Greece. Funders had no influence on any stage of the preparation of the present article.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Vaia L. Chatzi and Maurice P. Zeegers shared last authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achenbach TM, Edelbrock CS. Manual for the child behavior checklist and revised child behavior profile. Burlington, VT: T.M. Achenbach; 1983. [Google Scholar]
- Achenbach, T. M., & Resorta, L. A. (2001). Manual for ASEBA school-age forms and profiles. ASEBA.
- Aubuchon-Endsley NL, Bublitz MH, Stroud LR. Pre-pregnancy obesity and maternal circadian cortisol regulation: Moderation by gestational weight gain. Biological Psychology. 2014;102:38–43. doi: 10.1016/j.biopsycho.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubuchon-Endsley NL, Morales M, Giudice C, Bublitz MH, Lester BM, Salisbury AL, Stroud LR. Maternal pre-pregnancy obesity and gestational weight gain influence neonatal neurobehaviour. Maternal & Child Nutrition. 2017;13(2):e12317. doi: 10.1111/mcn.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Kendrick E, Klein KL, Faraone SV. The CBCL as a screen for psychiatric comorbidity in paediatric patients with ADHD. Archives of Disease in Childhood. 2005;90(10):1010–1015. doi: 10.1136/adc.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borge TC, Aase H, Brantsæter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: A systematic review and meta-analysis. British Medical Journal Open. 2017 doi: 10.1136/bmjopen-2017-016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi, L., Leventakou, V., Vafeiadi, M., Koutra, K., Roumeliotaki, T., Chalkiadaki, G., … Kogevinas, M. (2017). Cohort profile: The mother–child cohort in Crete, Greece (Rhea Study). International Journal of Epidemiology, 46(5), 1392–1393. 10.1093/ije/dyx084. [DOI] [PubMed]
- Chatzi, L., Torrent, M., Romieu, I., Garcia-Esteban, R., Ferrer, C., Vioque, J., … Sunyer, J. (2008). Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax, 63(6), 507–513. [DOI] [PubMed]
- Chen Y-H, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environmental Health. 2015;14:9. doi: 10.1186/1476-069X-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot A, Koot HM, Verhulst FC. Cross-Cultural Generalizability of the Child Behavior Checklist Cross-Informant Syndromes. Psychological Assessment. 1994 doi: 10.1037/1040-3590.6.3.225. [DOI] [Google Scholar]
- Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenatal Diagnosis. 2017 doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand RF, Heijmens Visser J, Hoogerheide KN, van der Ende J, Kasius MC, Koot HM, Verhulst FC. Improving estimation of the prognosis of childhood psychopathology; combination of DSM-III-R/DISC diagnoses and CBCL scores. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2004 doi: 10.1111/j.1469-7610.2004.00249.x. [DOI] [PubMed] [Google Scholar]
- Fraser, A., Tilling, K., Macdonald-Wallis, C., Sattar, N., Brion, M. J., Benfield, L., … Lawlor, D. A. (2010). Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation, 121(23), 2557–2564. [DOI] [PMC free article] [PubMed]
- Hartley E, McPhie S, Skouteris H, Fuller-Tyszkiewicz M, Hill B. Psychosocial risk factors for excessive gestational weight gain: A systematic review. Women and Birth. 2015;28(4):e99–e109. doi: 10.1016/j.wombi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obesity Reviews. 2017 doi: 10.1111/obr.12486. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine and National Research Council. (2009). In: K. M. Rasmussen, & A. L. Yaktine, (Eds.), Weight gain during pregnancy: Reexamining the guidelines. Washington, D.C.: National Academies Press. [PubMed]
- Klein, D. (2014). MIMRGNS: Stata module to run margins after mi estimate. In Statistical Software Components S457795. Boston: Boston College Department of Economics.
- Krukowski RA, West DA, DiCarlo M, Shankar K, Cleves MA, Saylors ME, Andres A. Are early first trimester weights valid proxies for preconception weight? BMC Pregnancy and Childbirth. 2016;16:357–362. doi: 10.1186/s12884-016-1159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Bann, C., Lester, B., Tronick, E., Das, A., Lagasse, L., … Bada, H. (2010). Neonatal Neurobehavior Predicts Medical and Behavioral Outcome. Pediatrics, 125(1), e90–e98. 10.1542/peds.2009-0204. [DOI] [PMC free article] [PubMed]
- Madigan, S., Oatley, H., Racine, N., Fearon, R. M. P., Schumacher, L., Akbari, E., … Tarabulsy, G. M. (2018). A Meta-Analysis of Maternal Prenatal Depression and Anxiety on Child Socioemotional Development. Journal of the American Academy of Child and Adolescent Psychiatry, 57(9), 645–657. 10.1016/j.jaac.2018.06.012. [DOI] [PubMed]
- Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, Bodnar LM. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG: An International Journal of Obstetrics and Gynaecology. 2016;123(13):2094–2103. doi: 10.1111/1471-0528.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Reef J, van Meurs I, Verhulst FC, van der Ende J. Children’s problems predict adults’ DSM-IV disorders across 24 years. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(11):1117–1124. doi: 10.1016/j.jaac.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Reinius B, Jazin E. Prenatal sex differences in the human brain. Molecular Psychiatry. 2009;14(11):988–989. doi: 10.1038/mp.2009.79. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A., Miettunen, J., Henriksen, T. B., Olsen, J., Obel, C., Taanila, A., … Järvelin, M. R. (2008). Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. International Journal of Obesity, 32, 550–557. [DOI] [PubMed]
- Roussos, A., Karantanos, G., Richardson, C., Hartman, C., Karajiannis, D., Kyprianos, S., … Zoubou, V. (1999). Achenbach’s Child Behavior Checklist and Teachers’ Report Form in a normative sample of Greek children 6-12 years old. European Child and Adolescent Psychiatry. 10.1007/s007870050125. [DOI] [PubMed]
- Schieve LA, Perry GS, Cogswell ME, Scanlon KS, Rosenberg D, Carmichael S, Ferre C. Validity of self-reported pregnancy delivery weight: An analysis of the 1988 National Maternal and Infant Health Survey. American Journal of Epidemiology. 1999 doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2015). Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
- Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. American Journal of Obstetrics and Gynecology. 2009 doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: The R package “dagitty”. International Journal of Epidemiology. 2016 doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- van der Wurff ISM, Groot RHM, Stratakis N, Gielen M, Hornstra G, Zeegers M. Maastricht essential fatty acid birth cohort. Lipid Technology. 2015;27(3):59–62. doi: 10.1002/lite.201500007. [DOI] [Google Scholar]
- Van Lieshout RJ. Role of maternal adiposity prior to and during pregnancy in cognitive and psychiatric problems in offspring. Nutrition Reviews. 2013;71(1):S95–101. doi: 10.1111/nure.12059. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. Principles of confounder selection. European Journal of Epidemiology. 2019;34(3):211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Good maternal nutrition: the best start in life. Copenhagen: WHO Regional Office for Europe; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (PDF 127 kb)
Electronic supplementary material 2 (PDF 32 kb)
Electronic supplementary material 3 (PDF 183 kb)
Electronic supplementary material 4 (PDF 200 kb)
Electronic supplementary material 5 (PDF 164 kb)
Electronic supplementary material 6 (PDF 190 kb)