Abstract
Although hyperglycemia, high blood pressure and aging increase the risk of developing kidney complications, some diabetes patients exposed to these risk factors do not develop kidney disease, suggesting the presence of endogenous protective factors. There is a growing need to understand these factors determining protection of the kidney in order to improve the design of preventive strategies and to enhance the processes responsible for renoprotection. The aim of this review was to present the existing molecular and epidemiological data on factors showing protective effects in diabetic kidney disease, and to summarize the evidence regarding their potential in the area of future clinical diagnostics, therapeutics and early preventive strategies. These include transcriptomic and proteomic studies regarding the anti‐inflammatory, anti‐fibrotic and regenerative factors that were associated with slower progression of renal function loss. Another focus is the new evidence regarding the evaluation of alterations in the regulatory epigenome and its involvement in the risk of diabetic kidney disease. Further effort is required to validate and extend these findings, and to define their potential for clinical implementation in the future.
Keywords: Biomarker, Diabetic kidney disease, Protective factor
Recent studies have characterized a number of novel risk and protective factors contributing to the variability in the interindividual risk of initiation and progression of diabetic kidney disease. Both these facets of diabetic nephropathy pathophysiology will inform the management of diabetes patients, and will be important to attenuate the incidence of end‐stage renal disease attributed to diabetic kidney disease. The aim of this review was to present the existing molecular and epidemiological data on factors showing protective effects in diabetic kidney disease, and to summarize the evidence regarding their potential in the area of future clinical diagnostics, therapeutics and early preventive strategies.
INTRODUCTION
Patients with diabetes mellitus are at increased risk of end‐stage renal disease (ESRD), and they contribute half of all new cases of ESRD in the USA population 1 , 2 . Studies of the natural history of diabetic nephropathy show that (during 15 years of diabetes) 20–30% patients with type 1 and type 2 diabetes will develop chronic kidney disease (CKD) and will likely progress to ESRD, whereas the remaining 60–70% are protected during their lifetime 3 , 4 . It could be hypothesized that this variation in the intra‐individual risk of ESRD is attributable to the varying intensity of specific endogenous protective factors in early disease stages. Knowing the nature of these mechanisms should facilitate the development of new preventive strategies to reduce the risk of ESRD and mortality.
Diabetic kidney disease (DKD) is considered a complex disease with a central role of chronic inflammation, progressive tubular damage and fibrosis of the tubulointerstitium 5 , 6 , 7 , 8 . This injury occurs as a result of an imbalance existing between the intensity of damaging (e.g., advanced glycated end‐products, free radicals, pro‐inflammatory and pro‐fibrotic molecules) and protective factors in the kidney (Figure 1). Some of the epidemiological studies from the past 10 years showed robust risk markers that predict kidney outcomes (ESRD, CKD stage 3 or progressive renal decline); however, the prognostic value of these markers in the early course of the disease is still limited 9 . These studies have been nicely reviewed elsewhere 9 , 10 . Recently, increasing studies were carried out giving more attention to a preclinical potential of various protective factors (e.g., survival, proliferative, anti‐inflammatory and anti‐oxidant factors) and their use as targets for prevention. The main focus of this review was to survey the existing human data on factors showing the protective effect in DKD, and to summarize the evidence regarding their role in the area of future preventive strategies. These, in addition to risk factors could be assessed as biomarkers, and then simultaneously incorporated into future prediction risk models. This approach would allow clinicians to more comprehensively ascertain the individual’s risk of ESRD. Alternatively, a better understanding of the protective factors and functional mechanisms underlying their regulation could aid the design and monitoring of the effect of future therapeutics, suited for strengthening natural physiological mechanisms.
Figure 1.
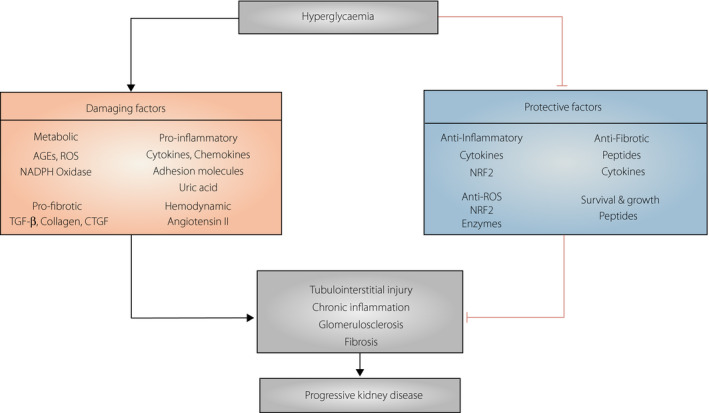
Different injury and protective mechanisms involved in the initiation and progression of Diabetic Kidney Disease. AGEs, advanced glycation end products; CTGF, connective tissue growth factor; NAPDH, nicotinamide adenine dinucleotide phosphate; NRF2, nuclear factor erythroid factor 2; ROS, reactive oxygen species; TGF‐β, transforming growth factor‐β; VEGF, vascular endothelial growth factor.
EPIDEMIOLOGICAL STUDIES IN DIABETIC NEPHROPATHY
Diabetic nephropathy is manifested by the presence of albuminuria, or progressive loss of kidney function 11 . Albuminuria is known to be a common risk factor of ESRD and CVD, and a reduction of urinary albumin excretion through any intervention results in a decrease in the future incidence of these outcomes 12 . However, many patients with type 1 diabetes or type 2 diabetes follow a pathway to progressive renal function loss before the onset of macroalbuminuria or even microalbuminuria, and despite improvements in their outcome resulting from multifactorial management. Progressive renal decline was characterized as a linear process that instantly precedes ESRD, and that commences when patients have normal renal function 11 . At this stage, only a subset of patients will develop clinically significant renal decline in the future. These high‐risk patients are currently indistinguishable from the patients with no renal decline; that is, in whom estimated glomerular filtration rate (eGFR) loss during the next decades will not exceed 1–2 mL/min/1.73 m2/year as an effect of aging, or the patients with slow decline, at low risk of ESRD in their expected lifetime 13 . Although the mechanisms underlying progressive renal decline are still poorly understood, for early prevention to be successful, better understanding of the factors associated with high variability of the rates of GFR loss is required. Unfortunately, studies in animal models of diabetic nephropathy (DN) have met with challenges, as a result of inefficacy to precisely reflect the human disease phenotype. Subsequently, epidemiologists have been working to identify risk factors for renal function loss in patients with type 1 and type 2 diabetes; some of these studies have led to a better understanding of the major molecular processes contributing to the disease risk, such as the role of chronic inflammation, progressive fibrosis and tubulointerstitial damage.
Today, inflammation is considered the major contributor to the pathology of the onset and progression of DKD, and is also involved in cardiovascular complications and all‐cause poor prognosis in diabetes 5 , 6 , 7 . From the early phase, many epidemiological studies of progressive renal decline have focused on the assessment of proteomic markers among pro‐inflammatory factors. For example, studies from the Joslin Diabetes Center have examined the contribution of inflammatory processes in the progression of renal function loss in people with type 1 and type 2 diabetes. Much attention has been paid to tumor necrosis factor‐alpha (TNF‐α) pathway markers in the prediction of early and late renal decline 14 , 15 , 16 . Circulating levels of TNF receptors (TNFR1 or TNFR2) were shown to be excellent predictors of progressive kidney disease in patients with a wide variety of CKD and albuminuria stages. Other robust markers include molecules implicated in fibrosis and matrix accumulation, and markers indicating tubulointerstitial damage. Less consideration has been given to the factors 17 , 18 that counteract these damage processes and whether these factors differ in their effect between CKD stages 18 , 19 , 20 . More recently, it has been shown that protective factors, including proliferative, survival, anti‐inflammatory and anti‐fibrotic factors, among others, are related to lower odds of progressive renal function loss. Taken together, all these studies provided promising candidate molecules associated with DKD; however, they often examined single markers. Sporadic studies tested a combined use of the interconnected pairs of biomarkers with opposing actions, such as epidermal growth factor (EGF) and monocyte chemoattractant protein‐1 (MCP‐1) or transforming growth factor (TGF)‐β1 and its endogenous counter‐regulator, bone morphogenetic protein‐7 (BMP‐7), which was shown to offer superior value compared with either of this markers alone. However, contemporary risk modeling, especially in multifactorial diseases, should not rely on one single biomarker, but rather require assessment of sets of biomarkers that would include pro‐inflammatory and other injury molecules, also incorporating protective factors, as well as their interactions, interconnections and interdependencies, that will in sum address the complexity of underlying processes. The interactions between proteins are often summarized in a form of an interaction network graph, where nodes represent biomarkers and edges represent interactions. For illustrative purposes, the robust biomarkers derived from previous human studies of progressive renal decline, including markers involved in inflammation, fibrosis, proximal tubulopathy and mitochondrial dysfunction processes, interconnected in a protein–protein interaction network model, are plotted in Figure 2. A putative model interconnects “protective” markers downregulated (highlighted in blue) and “risk” markers upregulated (highlighted in red) in diabetes patients with progressive renal loss through interaction terms extracted from the Search Tool for the Retrieval of Interacting Genes. In addition, biomarkers are grouped according to their predefined role in diabetic kidney pathophysiology.
Figure 2.
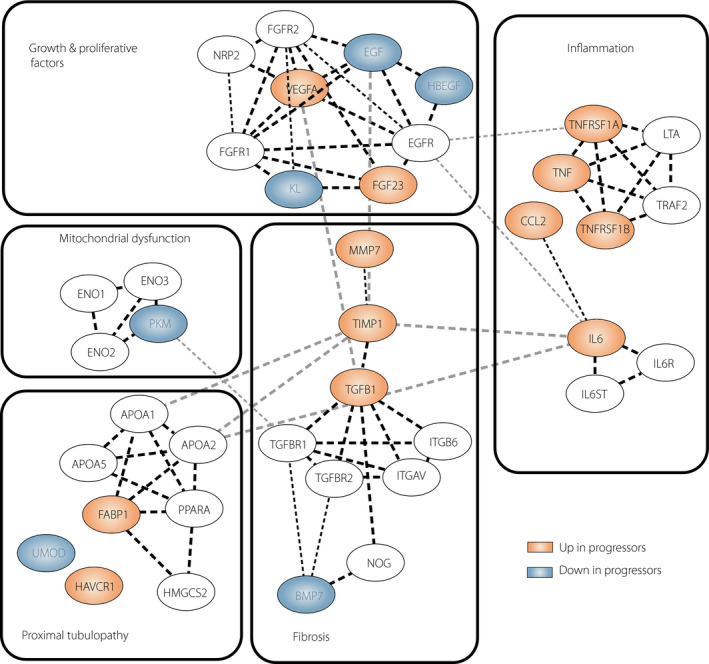
Protein–protein interaction network model for selected protective and risk biomarkers in progressive diabetic kidney disease. On the basis of available literature, biomarkers were grouped according to their involvement in the processes in diabetic kidney pathology (inflammation, fibrosis, proximal tubulopathy, growth and regeneration, and mitochondrial dysfunction). Interactions were derived from the Search Tool for the Retrieval of Interacting Genes/Proteins v10 database. Each node represents a candidate marker. Red nodes correspond to markers with high enrichment in progressors, and blue nodes correspond to markers downregulated in progressors. Selected markers involved are shown along with genes encoding molecules tightly connected (marked as white). The edges represent predicted protein–protein interactions, and the line thickness indicates the strength of data support. The interaction network was visualized using Cytoscape software (Institute for Systems Biology, Seattle, WA, USA). APOA, apolipoprotein A; BMP7, bone morphogenetic protein 7, CCL2; monocyte chemoattractant protein‐1; ENO, enolase; EGF; epidermal growth factor, EGFR, epidermal growth factor receptor; FABP1, liver fatty acid binding protein; FGF23, fibroblast growth factor 23; FGFR, fibroblast growth factor receptor; HAVCR1, kidney injury molecule‐1; HBEGF, heparin‐binding epidermal growth factor; HMGCS2, 3‐hydroxy‐3‐methylglutaryl‐CoA synthase 2; IL6, interleukin 6; IL6R; interleukin 6 receptor; IL6ST, glycoprotein 130; ITGAV, integrin alpha‐V; ITGB6, integrin beta‐6; KL, Klotho; LTA, lymphotoxin alpha; MMP7, matrix metalloproteinase‐7; NRP2, neuropilin‐2; NOG, noggin; PKM, pyruvate kinase PKM; PPARA, Peroxisome proliferator‐activated receptor alpha; TGFB1, transforming Growth Factor Beta 1; TGFBR, TGF beta receptor; TIMP1, tissue inhibitor of metalloproteinase 1; TNFRSF, tumor necrosis factor receptor; TRAF2, TNF receptor‐associated factor 2; VEGFA, vascular endothelial growth factor A; UMOD; uromodulin.
HUMAN STUDIES OF PROTECTIVE FACTORS FOR RENAL FUNCTION LOSS
Prospective studies assessing protective factors for progressive renal function loss are summarized in Table 1. Several protective tubular proteins, including EGF, Klotho, uromodulin, BMP‐7, have been associated with a slower decline in eGFR in diabetes patients. The data are most consistent for urinary EGF – a key survival factor expressed on the apical membrane of distal tubule cells 21 – with urinary concentrations decreasing during acute renal injury 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 . Tissue and urine levels of EGF were positively correlated with GFR across CKD stages, and were associated with eGFR trajectories and slower progression to ESRD during follow up in some studies 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , but it has not always been an independent predictor of progression 23 . Profiles of tissue expression and urinary excretion of EGF were characterized by the studies utilizing systems biology approaches; including proteomic, as well as transcriptomic, data combined with clinical follow‐up data 22 , 23 . It has been shown that tissue and urinary EGF represents a regulator of multiple pathways, and an important marker in the prediction of the future loss of kidney function. Furthermore, these observations were validated in other cohorts consisting of a various etiologies of kidney diseases 22 . The effect of EGF was the strongest, and was independent from clinical factors and other markers 22 . In a cohort of 642 normoalbuminuric type 2 diabetes patients with normal renal function at baseline, low urinary EGF was associated with the increased risk of kidney outcomes (CKD stage 3, rapid GFR decline or a composite of both outcomes), suggesting the role in the onset of DN. However, the incremental prognostic value of adding urine EGF to the established risk model was not significant 23 . In agreement with these publications, we have found increased odds of early progressive renal decline associated with decreased urinary levels of EGF in the longitudinal cohort involving 1032 type 2 diabetes patients enrolled into the Joslin Kidney Study 24 . Relevant to this observation was the finding that the effect of EGF became much stronger when urinary EGF excretion was standardized by urinary MCP‐1 levels, and expressed as the EGF‐to‐MCP‐1 ratio. The effect of this ratio was very strong in univariate as in multiple logistic analyses when controlled for clinical risk factors and other markers, including plasma TNF receptor 1 and plasma kidney injury molecule 1. This indicates that this ratio might be causally related to or it measures an intensity of a specific disease process that contributed to early renal decline, which was independent from other examined determinants. It was suggested that downregulation of EGF with simultaneous upregulation of MCP‐1 reflected increased apoptosis in the proximal tubules, and might reflect an index of tubulointerstitial damage in reflux nephropathy 25 . Recently, a strong association between levels of the EGF‐to‐MCP‐1 ratio and the risk of DKD onset and progression has been observed in type 2 diabetes patients from two smaller studies in early and late DKD 26 , 27 . It is uncertain whether decreased EGF expression only reflects the damaged tubules or decreased EGF affects the development of tubulointerstitial injury. It is a protective cytokine regulating tubular recovery after injury, but the specific protective mechanisms are not fully understood. Future studies should dissolve the questions as to whether treatment to increase EGF or supplementation of EGF will improve the prognosis of the DKD patients 28 , and to address EGF and the EGF‐to‐MCP‐1 ratio role in DN.
Table 1.
Epidemiological studies of the protective markers for progressive kidney disease in cohorts of patients with type 1 and type 2 diabetes
| Marker | Outcome | Role in DN | Author | Study design | Population |
|---|---|---|---|---|---|
| EGF | ESRD or 40% eGFR loss | Cell regeneration | Ju et al. 22 | Prospective | Four cohorts with different CKD etiologies |
| EGF | CKD3, eGFR loss 5% per year | Betz et al. 23 | Prospective | T2D (NA; CKD Stage 1‐2) | |
| EGF, EGF/MCP1 | 30% GFR loss | Nowak et al. 24 | Prospective | T2D (NA, low albuminuria; CKD Stage 1‐2) | |
| EGF, EGF/MCP1 | Prevalence of DN, GFR slope | Wu et al. 26 | Cross‐sectional/prospective | T2D (high albuminuria; early/advanced CKD) | |
| EGF, EGF/MCP1 | 25% GFR loss | Satrpoj et al. 27 | Prospective | T2D (varying albuminuria and CKD stages) | |
| Klotho | 30% GFR loss |
Anti‐fibrotic Anti‐inflammatory |
Drew et al. 33 | Prospective | Elderly, 40% with diabetes (CKD stage 1–3) |
| Klotho | eGFR slope | Kim et al. 34 | Prospective | T2D (NA; CKD stage 1–2) | |
| Klotho | 50% eGFR loss | Fountoulakis et al. 35 | Cross‐sectional/prospective | T2D (NA and low albuminuria; CKD <3b) | |
|
BMP7 BMP7/TGF1beta |
Doubling of serum creatinine, death due to kidney disease |
Anti‐fibrotic Anti‐inflammatory Anti‐apoptotic |
Wong et al. 38 | NCC within prospective | T2D (NA and low albuminuria; various CKD stages) |
| Uromodulin | 30% GFR loss, renal transplant | Inconclusive | Sejdu et al. 39 | Prospective | T1D, T2D (varying albuminuria and CKD stages) |
| Uromodulin | 30% GFR loss, MA or both | Schlatzer et al. 40 | Nested case‐control | T1D (NA; CKD stage 1) | |
| Uromodulin | GFR loss 3 mL/min per year | Bjornstad et al. 41 , 42 | Prospective | T1D (NA; CKD stage 1) | |
| Uromodulin | 20% GFR loss | Colombo et al. 43 | Prospective | T1D (varying degrees of albuminuria; CKD stage 2–3) | |
| Uromodulin | CKD stage 3 | Sjaarda et al. 44 | Prospective/mendelian randomization |
T2D, IFG (CKD 1–2) |
BMP7, bone morphogenetic protein 7; CVD, cardiovascular disease; EGF, epidermal growth factor; IFG, impaired fasting glucose; MCP‐1, monocyte chemoattractant protein‐1; NCC, nested case control; T1D, type 1 diabetes; T2D, type 2 diabetes; TGF‐β1, transforming growth factor‐β1.
Some markers with established function have more recently been identified as kidney protective. For example, soluble Klotho, a fibroblast growth factor 23 coreceptor, which is expressed mainly in the kidney tubules, was originally identified as an anti‐aging gene 29 . Klotho appears to be a negative upstream regulator of renal fibrosis, also controlling multiple fibrosis‐implicated processes. It has been shown that Klotho deficiency turns the kidney prone to damage from oxidative stress, ischemia and inflammation, whereas its supplementation (in acute as well as chronic settings) attenuates fibrosis‐related kidney diseases 30 , 31 . Circulating Klotho is decreased in mouse models of diabetes and in patients in early stages of CKD, even in the absence of GFR loss, and it could be further related to a higher risk of eGFR decline 32 , 33 . In the most recent meta‐analysis that considered eight prospective studies (although just two studies were carried out on diabetes 34 , 35 , it was found that decreased Klotho levels were associated with increased adverse kidney outcomes, which indicated that Klotho could be a prognostic biomarker for patients with CKD 36 . The meta‐analysis highlighted the variability in the results from the studies included, related to differences in study population, size and sample selection, which points to the need for future research.
Promising findings were recently presented for a TGF‐β1 inhibitor, BMP‐7, which is involved in renal morphogenesis. As a member of the TGF‐β family, BMP‐7 was first speculated to be a pro‐fibrotic factor, but recently an abundance of research presented a strong anti‐fibrotic, anti‐inflammatory and anti‐apoptotic effect. BMP‐7 was identified as one of potential genes for DN in the most recent genome‐wide association study in the Juvenile Diabetes Research Foundation Diabetic Nephropathy Collaborative Research Initiative, which has assembled 20,000 participants with type 1 diabetes, with and without DKD 37 . Earlier, a proteomic analysis carried in type 2 diabetes patients from the ADVANCE trial, showed a graded increase in the risk of CKD in individuals with lower circulating BMP‐7 levels or significantly higher TGF‐β1 levels 38 . When added to clinical predictors, the two markers were able to increase the area under the receiver operating characteristic curve from 0.73 to 0.94, which was highly significant. It is important to recall that the study design intended on matching for multiple risk factors at baseline, which makes the results of the study less‐reflective of its discriminative value in clinical settings.
Uromodulin (UMOD), a most abundant urine protein with a long history in DN, was examined as a predictor for progressive kidney disease in five studies of type 1 diabetes; however, the results of these studies are conflicting 39 , 40 , 41 , 42 , 43 . The authors of the studies in non‐diabetic CKD hypothesized that the observed differences in the magnitude and the direction of the observed association might depend on the timing of the injury and CKD stage, as well as the population being studied and its related comorbid conditions. Rare mutations in UMOD cause autosomal dominant tubulointerstitial kidney disease, which leads to CKD and ESRD. In epidemiological analysis of prediabetes and type 2 diabetes with established cardiovascular risk factors, increased blood UMOD levels were associated with a decreased risk of incident CKD 44 . However, when utilizing a Mendelian randomization approach, based on GWAS results for the UMOD gene, an opposite association emerged, suggesting the causality of higher UMOD levels as a risk factor for DKD 44 . These inconsistent findings might arise from the fact that patients with low UMOD present a reduced tubular mass. Therefore, it is possible that decreased UMOD reflects tubular functional reserve that is decreased in normoalbuminuria 41 , 42 , 44 .
Despite the advancement in proteomic technologies, only few large‐scale studies have been carried out in DN, and no studies have been specifically carried out regarding the identification of circulating protective markers. One exception is the recent assessment of the determinants of DKD in a cohort of Joslin Medalists. The study was built on the findings from the long‐lasting cohort set up in the Joslin Diabetes Center, specifically to study protective factors of diabetes complications. It involves patients who have had type 1 diabetes for ≥50 years with approximately half of the cohort never reaching DN 45 , 46 , irrespective of the glycemic control they experienced. The study used kidney biopsies and proteomic as well as metabolomic approaches to identify markers whose expression patterns might explain the profound protection in this cohort. To screen for markers underlying protection from DN, the authors utilized untargeted metabolomics, as well as an aptamer‐based array that measures 1123 proteins in plasma 47 , 48 . The circulating levels of 120 proteins were increased in diabetes patients who were protected from CKD compared with those with CKD stage 3b, which shows potential protective factors against DN. The results of integrated network analysis identified enhancement/upregulation of several novel (pyruvate kinase M2, amyloid precursor protein), as well as previously known pathways (EGFR) in CKD‐protected Medalists 48 . Subsequently, the presence of plasma pyruvate kinase M2 has been confirmed in a general population of patients with type 1 and type 2 diabetes. Higher plasma pyruvate kinase M2 at baseline has been independently associated with a slower GFR decline and slower progression to ESRD in type 1 diabetes patients from the Joslin Kidney Study 47 . The validation and extension of these findings in longitudinal cohorts of diabetes patients would be desirable. Also, as circulating proteins might originate from different sources, the functional interpretation of the exact role of the identified proteins remains to be explored.
Of other candidate proteins, various anti‐inflammatory and anti‐fibrotic molecules, growth factors, and anti‐oxidant factors were mechanistically characterized in the context of kidney pathology. However, not many have ever been studied in the context of human DKD in prospective studies. A recurring conclusion in published transcriptomic studies is the importance of oxidative stress, which is underlying all facets of kidney pathophysiology, including vascular, glomerular and tubular dysfunction for DN progression 49 , 50 . The anti‐oxidant factors were previously reported in the context of DKD, although mainly in functional or small cross‐sectional studies 49 , 50 , 51 , 52 , 53 . In addition, investigation of other protective factors in diabetes patients, such as growth factors, adenosine, immune proteins, fibroblast growth factor receptors and netrins, is in early stages 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 .
CLINICAL APPLICATION OF THE PROTECTIVE FACTORS: FUTURE POSSIBILITIES
Clinical management of patients with diabetes could be significantly improved if it would be possible to estimate if they develop DN and how fast their GFR decline or their likely response to targeted treatment would be. Protective factors could encompass several advantages in this area. As kidney‐specific markers, they are expected to provide superior specificity and are less confounded by non‐renal processes. Second, they might represent unique processes that are not captured by clinical characteristics, as well as other markers (as shown for EGF). Third, their expression is often downregulated according to the decreased GFR, so their levels are not affected by filtrated protein, which suggests they could add significant prognostic value to albuminuria. Bioinformatic analyses have shown a considerable fraction of the protective factors mapping to extracellular space (with highest enrichment in the kidney), which suggests their utility as biomarkers or drug targets 18 . The future will tell if adding the protective markers into established risk factors (ACR, GFR, TNFR1) will confirm these markers as useful targets for the prevention of DN in clinical settings.
One important clinical problem that emerged from epidemiological studies is the importance of accurate tests of renal decline in non‐albuminuric diabetes patients, for both clinical settings and drug trials 62 , 63 , 64 , 65 . Very recently, it was documented that although the presence of albuminuria influences the rate of eGFR decline 63 , 66 , with macroalbuminuric patients presenting the most pronounced loss in eGFR 63 , 66 , as many as 48% patients progress to CKD stage 3 remaining normoabuminuric 63 . Previously, studies of Joslin Clinic patients identified 9% of patients with type 1 diabetes and 20% of patients with type 2 diabetes (with normal eGFR at study entry) whose renal function had significantly and persistently declined without any increase in the albumin‐to‐creatinine ratio 13 , 64 , realizing the need for new tests to identify people at risk for eGFR loss earlier than albuminuria. Since the recognition of early renal decline in normoalbuminuria, later studies in type 1 and type 2 diabetes have extended their findings about previously known biomarkers of renal loss 65 . These biomarkers are implicated in the mechanisms that result in a decline in eGFR, but are not directly linked to the progression of albuminuria. Again, these extended studies have confirmed that the described biomarkers could be useful in risk stratification of early renal decline in low albuminuria (microalbuminuria), and might also be clinically important if only the patients with normoalbuminuria are considered. In regard to the latter, tubular markers (e.g., increased plasma kidney injury molecule 1, and decreased plasma uromodulin, urine EGF, and EGF‐to‐MCP‐1 ratio) were evaluated in well‐designed cohorts of diabetes patients, and showed promise as independent predictors of early renal decline 24 , 41 , 42 , 65 , 67 . In line with this, data provided by recent analysis of Japan’s nationwide renal biopsy registry have shown the extent of interstitial fibrosis and tubular atrophy as the strongest histological predictor of renal decline in non‐albuminuric DKD 66 . Additionally, markers implicated in kidney fibrosis (e.g., markers of collagen formation and degradation, N‐acetyl‐seryl‐aspartyl‐lysyl‐proline, and matrix metalloproteinase‐7‐to‐ WAP four‐disulfide core domain 2 ratio) were associated with the eGFR trajectory in several pilot studies in normoalbuminuria 68 , 69 , 70 , 71 , 72 . Future research is required to establish whether they improve the stratification of patients regarding progression risk. The direct application of the results of epidemiological studies lies in the inclusion of the preselected risk and protective markers into multimarker prognostic tests built on sets of molecules involved in independent biological processes. The generation of such multimarker tests is currently being investigated and is expected to deliver clinical benefit through enhanced capacity to predict the individual’s rate of disease progression. Consistently, an improved multitherapeutic approach in DN could cover blocking mechanisms of injury, as well as enhancing the nephroprotective mechanisms; for example, by stimulating endogenous programs of anti‐inflammatory and anti‐reactive oxygen species response, enhancing endogenous mechanisms to normalize aberrant (DNA) methylation or improving the sensitivity to cell survival factors. To make the second task feasible, it would be essential to better understand the factors determining protection of the kidney in order to design strategies to enhance the processes necessary for recovery. These goals require multiple experimental as well as epidemiological approaches. Whereas utility as a disease marker is determined by carrying out more studies in humans, their evaluation as a drug target necessitates further validation in a setting of animal studies or clinical trials. Although it is possible that some of the discussed candidate proteins simply indicate ongoing protection, early results from studies in animals are encouraging 30 , 47 , 73 , 74 , 75 . So far, only activators of anti‐inflammatory and anti‐oxidant transcription factor nuclear factor erythroid factor 2 have reached clinical trials (Table 2). Although with these data, bardoxolone methyl has improved eGFR in DN, further precise tailoring of this therapy on the background of its safety remains an important clinical issue 76 . An intriguing possibility is that the profiling of protective pathways will allow early identification of individuals whose predisposition is attributable to defects in single protective mechanisms, and then applying targeted treatment. Whereas further investigation is required, therapeutics are assembled making the future clinical trials feasible.
Table 2.
Current human interventional studies targeting pathways that involve transcription‐related factor 2 nuclear factor erythroid factor 2
| Target protein | MOA: DRUG (generic name) | Indications | Status | References for human interventional studies in DN |
|---|---|---|---|---|
|
NRF2 NF‐B and STATs |
NRF2 agonist (activates KEAP1‐Nrf2 pathway), NF‐B inhibitor, bardoxolone methyl (RTA 402) |
(BEACON), CKD stage 4, T2D |
Terminated RCT, phase III |
PMID: 24206459 PMID: 24169612 PMID: 24903467 |
|
NRF2 NF‐B and STATs |
Bardoxolone methyl (RTA 402) |
(BEACON), CKD stage 4, T2D |
Post‐hoc analysis |
PMID: 29402767 PMID: 30318163 PMID: 31377056 |
|
NFR2 NF‐B and the STATs |
Bardoxolone methyl (RTA 402) |
(PHOENIX); CKD stage 3–4, T1D, other rare CKD |
Completed RCT, phase II | NCT03366337 |
|
NFR2 NF‐B and the STATs |
Bardoxolone methyl (RTA 402) |
(TSUBAKI) CKD patients with T2D |
Completed RCT, phase II | NCT02316821 |
|
NRF2 NF‐B and STATs |
Bardoxolone methyl (RTA 402) |
(AYAME) CKD stage 3–4, T1D or T2D |
Active RCT, phase III | NCT03550443 |
|
NRF2 NF‐B and STATs |
Bardoxolone methyl (RTA 402) |
(EAGLE) CKD stage >4, CKD, Alport |
Phase III RCT, recruiting | NCT03749447 |
|
NRF2 SIRT1 |
SIRT1 agonist, upregulates NRF2 (resveratrol) | Non‐diabetic CKD stage 3–4 | Completed RCT, phase III | NCT02433925 |
Data for human interventional studies and respective national clinical trial (NCT) numbers were accessed from www.clinicaltrials.gov on 1 November 2019.
CKD, chronic kidney disease; DN, diabetic nephropathy; MOA, mechanism of action; NF‐κB, nuclear factor Kappa B; NRF2, nuclear factor erythroid factor 2; PMID, PubMed identifier; RCT, randomized clinical trial; SIRT1, sirtuin 1; STATs, signal transducers and activators of transcription; T1D, type 1 diabetes; T2D, type 2 diabetes.
Another focus of contemporary translational research activity involves exploring the changes in the epigenome and its involvement in DN. Epigenetic profiling could be valuable for diagnostic tests development and might facilitate precision medicine approaches. Epigenome is a main mechanism relating environmental exposures to altered gene function, and it is responsive to modifiable environmental factors. Pinpointing the modified sequences, which reflect plasticity of the human genome altered by the environmental exposures, could inform tailored strategies to prevent deregulated gene function. Although epigenetic studies in human DN are in very early stages, their preliminary findings are briefly outlined in the following section.
STUDIES REGARDING EPIGENETIC FACTORS IN HUMAN DIABETIC NEPHROPATHY
Gene regulation is maintained through activity of transcription factors, genetic mechanisms and epigenetic mechanisms, that include DNA methylation, histone modifications and non‐coding RNAs. DNA methylation, together with histone acetylation, mutually regulate chromatin structure, genomic stability, and maintain tissue homeostasis and gene expression, and disruption of these mechanisms leads to diabetes and its complications. Earlier, a study in DN was published that has utilized transcriptome profiling of fibroblasts from 100 type 1 diabetes patients from the longstanding Genetics of Kidneys in Diabetes cohort. It has suggested that patients who were protected from DN might undergo more robust epigenetic modifications resulting in enriched expression of genes involved in cell healing and repair pathways, and that epigenetic factors play a role in DN risk 77 .
Variations in the methylation of the loci in genes involved in fibrosis occur in animal models and patients with DN, and they coincide with expression of the downstream transcripts in the kidney tissue, suggesting a functional mechanism of gene regulation. In experimental studies, methylation of protective genes was altered, whereas its reversal has attenuated fibrosis 75 , 78 , 79 . One example is Klotho, which is associated with less renal decline. Its decreased expression is maintained through altered methylation of its promoter, whereas the correction of this defect increased Klotho expression, and subsequently attenuated pro‐fibrotic gene expression and renal fibrosis in experimental studies 79 .
Given the species‐specific differences in gene regulation, model organism studies of epigenetic factors cannot substitute studies carried out on humans. The introduction of new techniques has facilitated the implementation of epigenome‐wide association studies. The majority of these studies compared DNA methylation in patients with and without DN using microarray technology, and utilized whole blood or blood leukocytes. Potential sites with differential methylation in DKD have been found at loci that either encode genes previously implicated in the kidney pathology or contain regulatory elements controlling DN relevant genes 80 , 81 , 82 . Nevertheless, there is very little overlap in the cytosine‐phosphate‐guanine (CpG) sites identified between those studies. The largest epigenome‐wide association study carried out for non‐diabetic CKD included 4,859 participants, and found multiple differentially methylated loci; four of them predicted eGFR decline, corresponded to changes in downstream transcripts and coincided with fibrosis in kidney tissue 83 . Among these genes, a protective variant has been identified in renal PTPN6 that is related to interleukin‐4 signaling. Furthermore, there have been efforts to understand epigenetic regulation of gene expression in kidney tubules. Two cross‐sectional studies have assessed CpGs in microdissected kidney tissue from people without diabetes and patients with type 2 diabetes and various degrees of kidney disease. In the first study, Ko et al. 84 tested a small cohort with expression data from renal tissue; a set of CpGs related to the fibrotic pathway were found and were correlated with downstream gene expression, which was associated with DN. Interestingly, a set of genes known to be related to kidney development, including genes encoding transcription factors, also showed significant cytosine methylation changes. In a follow‐up analysis in a similar study setting, the authors used omics data (gene expression, clinical, histology, methylation) to build predictive models for kidney function decline 85 . Only methylation changes have improved the baseline models. CpG found at the EGF promoter was associated with eGFR decline, which was also suggested as a major mechanism for decreased expression level of kidney EGF in DN. Adding this CpG site has offered an improvement in model performance available for traditional factors, suggesting the potential as a biomarker. Larger epigenome‐wide scale changes studies, aided by novel analyses, could continue to enhance the understanding of the complex pathogenesis of DN, assisting the future design of molecularly targeted preventive interventions and, perhaps, delivery of novel biomarkers.
In addition to the classical epigenetic modifications that include chromatin remodeling through DNA methylation and histone modifications, non‐coding RNAs are other mechanisms of gene regulation and are involved in attenuating the progression of DN through direct gene silencing. Kato et al. 86 first expressed a hypothesis that TGF‐β1 might play an important role in the early stages of the development of DN, whereas some non‐coding RNAs regulate the key molecules in the TGF‐β1 pathway. This hypothesis was recently explored by Pezzolesi et al. 87 , who assessed several extracellular microRNAs (miRNAs) involved in the TGF‐β1 pathway; two miRNAs – let‐7c‐5p and miR‐29a‐3p – were associated with a 50% reduction in the risk of rapid progression to ESRD in type 1 diabetes patients with normal renal function at baseline. The authors concluded that miRNAs could be considered as preclinical indicators to evaluate changes and to predict the risk of DN. However, in contrast to tissue miRNA profiling, human studies of extracellular miRNAs are in the early phase. The majority of published data have utilized a cross‐sectional design and evaluated the levels of miRNAs in healthy diabetes patients versus patients with DKD. Studies using urine met with challenges, due to difficulties in the assessment of urinary miRNAs, and because of their low abundance and high intra‐individual variability of measured concentrations. However, it is anticipated that novel approaches, such as measurement of miRNAs encapsulated in urinary microvesicles originating from the kidney, will facilitate future research by providing more robust markers of kidney processes in DN 88 .
CONCLUSION
Over the past decade, there has been a significant advancement in the characterization of the risk and protective factors associated with progressive renal function loss in diabetes. As well as providing insights into pathophysiological mechanisms in DN progression, these factors, when combined into a multimarker prognostic test, could capture individuals’ risk of future renal function loss. The available data support significant differential methylation for a human kidney protective mechanism, and have highlighted changes in pathways encompassing known disease biomarkers, such as EGF. Significant questions remain regarding the role of the protective factors, and the underlying epigenetic mechanisms, as targets for future pharmacological and behavioral interventions.
Disclosure
The author declares no conflict of interest.
Acknowledgments
I thank Professor Andrzej Krolewski (Joslin Diabetes Center) whose leadership was a great inspiration to this work. Research support was provided by Medical University of Bialystok (grant number: SUB/1/DN/20/002/4407).
J Diabetes Investig 2020; 11: 1085–1096
References
- 1. Collins AJ, Foley RN, Herzog C, et al US renal data system 2010 annual data report. Am J Kidney Dis 2011; 57(Suppl 1): A8‐e1‐526. [DOI] [PubMed] [Google Scholar]
- 2. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 2016; 12: 73–81. [DOI] [PubMed] [Google Scholar]
- 3. Adler AI, Stevens RJ, Manley SE, et al UKPDS GROUP: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 4. Retnakaran R, Cull CA, Thorne KI, et al UKPDS Study Group: Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006; 55: 1832–1839. [DOI] [PubMed] [Google Scholar]
- 5. Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 2016; 12: 13–26. [DOI] [PubMed] [Google Scholar]
- 6. Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol 2005; 16: 1537–1538. [DOI] [PubMed] [Google Scholar]
- 7. Navarro‐Gonzalez JF, Mora‐Fernandez C, Muros de Fuentes M, et al Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 2011; 7: 327–340. [DOI] [PubMed] [Google Scholar]
- 8. Fernandez‐Fernandez B, Ortiz A, Gomez‐Guerrero C, et al Therapeutic approaches to diabetic nephropathy — beyond the RAS. Nat Rev Nephrol 2014; 10: 325–346. [DOI] [PubMed] [Google Scholar]
- 9. Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease. Diabetologia 2018; 61: 996–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Persson F, Rossing P. Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl 2018; 8: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 2015; 38: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neuen BL, Young T, Heerspink HJL, et al SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2019; 7: 845–854. [DOI] [PubMed] [Google Scholar]
- 13. Krolewski AS, Skupien J, Rossing P, et al Fast renal decline to end‐stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int 2017; 91: 1300–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolkow PP, Niewczas MA, Perkins B, et al Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 2008; 19: 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gohda T, Niewczas MA, Ficociello LH, et al Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012; 23: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niewczas MA, Gohda T, Skupien J, et al Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 2012; 23: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niewczas MA, Pavkov ME, Skupien J, et al A signature of circulating inflammatory proteins and development of end‐stage renal disease in diabetes. Nat Med 2019; 25: 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perco P, Mayer G. Endogenous factors and mechanisms of renoprotection and renal repair. Eur J Clin Invest 2018; 48: e12914. [DOI] [PubMed] [Google Scholar]
- 19. Chen T, Cao Q, Wang Y, et al M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int 2019; 95: 760–773. [DOI] [PubMed] [Google Scholar]
- 20. Sharma R, Kinsey GR. Regulatory T cells in acute and chronic kidney diseases. Am J Physiol Renal Physiol 2018; 314: F679–F698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 2013; 83: 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ju W, Nair V, Smith S, et al and PKU‐IgAN Consortium. Tissue transcriptome‐driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015; 7: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Betz BB, Jenks SJ, Cronshaw AD, et al Urinary peptidomics in a rodent model of diabetic nephropathy highlights epidermal growth factor as a biomarker for renal deterioration in patients with type 2 diabetes. Kidney Int 2016; 89: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 24. Nowak N, Skupien J, Smiles AM, et al Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int 2018; 93: 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chertin B, Farkas A, Puri P. Epidermal growth factor and monocyte chemotactic peptide‐1 expression in reflux nephropathy. Eur Urol 2003; 44: 144–149. [DOI] [PubMed] [Google Scholar]
- 26. Wu L, Li XQ, Chang DY, et al Associations of urinary epidermal growth factor and monocyte chemotactic protein‐1 with kidney involvement in patients with diabetic kidney disease. Nephrol Dial Transplant 2020; 35: 291–297. [DOI] [PubMed] [Google Scholar]
- 27. Satirapoj B, Dispan R, Radinahamed P, et al Urinary epidermal growth factor, monocyte chemoattractant protein‐1 or their ratio as predictors for rapid loss of renal function in type 2 diabetic patients with diabetic kidney disease. BMC Nephrol 2018; 19: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Isaka Y. Epidermal growth factor as a prognostic biomarker in chronic kidney diseases. Ann Transl Med 2016; 4(Suppl 1): S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurosu H, Yamamoto M, Clark JD, et al Suppression of aging in mice by the hormone Klotho. Science 2005; 309: 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haruna Y, Kashihara N, Satoh M, et al Amelioration of progressive renal injury by genetic manipulation of Klotho gene. PNAS 2007; 104: 2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuro‐o M. The Klotho proteins in health and disease. Nature Rev Nephrol 2019; 15: 27–44. [DOI] [PubMed] [Google Scholar]
- 32. Shimamura Y, Hamada K, Inoue K. Serum levels of soluble secreted α‐Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clinical and Experimental Nephrol 2012; 6: 722–729. [DOI] [PubMed] [Google Scholar]
- 33. Drew DA, Katz R, Kritchevsky S, et al Association between soluble Klotho and change in kidney function: the health aging and body composition study. J Am Soc Nephrol 2017; 28: 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SS, Song SH, Kim IJ. Decreased plasma α‐Klotho predict progression of nephropathy with type 2 diabetic patients. J Diabetes Complications 2016; 30: 887–892. [DOI] [PubMed] [Google Scholar]
- 35. Fountoulakis N, Maltese G, Gnudi L, et al Reduced levels of anti‐ageing hormone Klotho predict renal function decline in type 2 diabetes. J Clin Endocrinol Metab 2018; 103: 2026–2032. [DOI] [PubMed] [Google Scholar]
- 36. Liu Q, Yu L, Feng J‐H, et al The prognostic role of klotho in patients with chronic kidney disease: a systematic review and meta‐analysis. Dis Markers 2019; 2019: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salem RM, Todd JN, Sandholm N, et al Genome‐wide association study of diabetic kidney disease highlights biology involved in glomerular basement membrane collagen. J Am Soc Nephrol 2019; 30: 2000–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong MG, Perkovic V, Woodward M, et al Circulating bone morphogenetic protein‐7 and transforming growth factor‐b1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int 2013; 83: 189–91. [DOI] [PubMed] [Google Scholar]
- 39. Sejdiu I, Torffvit O. Decreased urinary concentration of Tamm‐Horsfall protein is associated with development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetic patients. Scand J Urol Nephrol 2008; 42: 168–174. [DOI] [PubMed] [Google Scholar]
- 40. Schlatzer D, Maahs DM, Chance MR, et al Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Diabetes Care 2012; 35: 549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bjornstad P, Pyle L, Cherney DZI, et al Plasma biomarkers improve prediction of diabetic kidney disease in adults with type 1 diabetes over a 12‐year follow‐up: CACTI study. Nephrol Dial Transplant 2018; 33: 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bjornstad P, Wiromrat P, Johnson RJ, et al Serum uromodulin predicts less coronary artery calcification and diabetic kidney disease over 12 years in adults with type 1 diabetes: the CACTI study. Diabetes Care 2019; 42: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colombo M, Valo E, McGurnaghan SJ, et al Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia 2019; 62: 1616–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sjaarda J, Gerstein HC, Yusuf S, et al Blood HER2 and Uromodulin as Causal Mediators of CKD. J Am Soc Nephrol 2018; 29: 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun JK, Keenan HA, Cavallerano JD, et al Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50‐year Medalist Study. Diabetes Care 2011; 34: 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keenan HA, Costacou T, Sun JK, et al Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50‐year Medalist Study. Diabetes Care 2007; 30: 1995–1997. [DOI] [PubMed] [Google Scholar]
- 47. Gordin D, Shinjo T, St‐Louis R, et al Activation of PKM2 Protects against Diabetic Kidney Disease from Mice to Men. Diabetes 2018; 67(Supp 1): 91. [Google Scholar]
- 48. Gordin D, Shah H, Shinjo T, et al Characterization of glycolytic enzymes and pyruvate kinase m2 in type 1 and 2 diabetic nephropathy. Diabetes Care 2019; 42: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brezniceanu ML, Liu F, Wei CC, et al Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 2008; 57: 451–459. [DOI] [PubMed] [Google Scholar]
- 50. Mootha VK, Lindgren CM, Eriksson KF, et al PGC‐1alpha‐responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267–273. [DOI] [PubMed] [Google Scholar]
- 51. Ishizaka Y, Yamakado M, Toda A, et al Relationship between estimated glomerular filtration rate, albuminuria, and oxidant status in the Japanese population. BMC Nephrol 2013; 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karamouzis I, Sarafidis PA, Karamouzis M, et al Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol 2008; 28: 397–404. [DOI] [PubMed] [Google Scholar]
- 53. Jiang T, Huang Z, Lin Y, et al The protective role of NRF2 in streptozocin‐induced diabetic nephropahy. Diabetes 2010; 59: 850–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dai C, Yang J, Bastacky S, et al Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol 2004; 15: 2637–2647. [DOI] [PubMed] [Google Scholar]
- 55. Kim YC, An JN, Kim JH, et al Soluble cMet levels in urine are a significant prognostic biomarker for diabetic nephropathy. Sci Rep 2018; 8: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liang G, Song L, Chen Z, et al Fibroblast growth factor 1 ameliorates diabetic nephropathy by an anti‐inflammatory mechanism. Kidney Int 2018; 93: 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang S, Li Y, Fan J, et al Interleukin‐22 ameliorated renal injury and fibrosis indiabetic nephropathy through inhibition of NLRP3 inflammasome activation. Cell Death Dis 2017; 8: e2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu C, Ke X, Wang Y, et al The level of netrin‐1 is decreased in newly diagnosed type 2 diabetes mellitus patients. BMC Endocrine Disorders 2016; 16: 33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yim J, Kim G, Lee BW, et al Relationship between circulating netrin‐1 concentration, impaired fasting glucose, and newly diagnosed type 2 diabetes. Front Endocrinol (Lausanne) 2018; 9: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mohamed R, Jayakumar C, Ranganathan PV, et al Kidney proximal tubular epithelial‐specific overexpression of netrin‐1 suppresses inflammation and albuminuria through suppression of COX‐2‐mediated PGE2 production in streptozotocin‐induced diabetic mice. Am J Pathol 2013; 181: 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muraoka H, Hasegawa K, Sakamaki Y, et al Role of Nampt‐Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephropathy. Cell Rep 2019; 27: 199–212. [DOI] [PubMed] [Google Scholar]
- 62. Tsalamandris C, Allen TJ, Gilbert RE, et al Progressive decline in renal function in diabetic patients with and without albuminuria. Diabetes 1994; 43: 649–655. [DOI] [PubMed] [Google Scholar]
- 63. Vistisen D, Andersen GS, Hulman A, et al Progressive decline in estimated glomerular filtration rate in patients with diabetes after moderate loss in kidney function even without albuminuria. Diabetes Care 2019; 42: 1886–1894. [DOI] [PubMed] [Google Scholar]
- 64. Perkins BA, Ficociello LH, Ostrander BE, et al Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007; 18: 1353–61. [DOI] [PubMed] [Google Scholar]
- 65. Krolewski AS, Niewczas MA, Skupien J, et al Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 2014; 37: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yamanouchi M, Furuichi K, Hoshino J, et al Nonproteinuric versus proteinuric phenotypes in diabetic kidney disease: a propensity score‐matched analysis of a nationwide, biopsy‐based cohort study. Diabetes Care 2019; 42: 891–902. [DOI] [PubMed] [Google Scholar]
- 67. Nowak N, Skupien J, Niewczas MA, et al Increased plasma kidney injury molecule‐1 suggests early progressive renal decline in non‐proteinuric patients with type 1 diabetes. Kidney Int. 2016; 89: 459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morita M, Uchigata Y, Hanai K, et al Association of urinary type IV collagen with GFR decline in young patients with type 1 diabetes. Am J Kidney Dis. 2011; 58: 915–20. [DOI] [PubMed] [Google Scholar]
- 69. Araki S, Haneda M, Koya D, et al Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Diabetes Care 2010; 33: 1805–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pilemann‐Lyberg S, Rasmussen DGK, Hansen W, et al Markers of collagen formation and degradation reflect renal function and predict adverse outcomes in patients with type 1 diabetes. Diabetes Care 2019; 42: 1760–1768. [DOI] [PubMed] [Google Scholar]
- 71. Ihara K, Skupien J, O’Neil KV. Association Between Renal Fibrosis and Early Renal Decline in Type 2 Diabetes [Abstract]. J Am Soc Nephrol 2019; 30(Suppl): 900A–901A. [Google Scholar]
- 72. Nitta K, Nagai T, Mizunuma Y, et al N‐Acetyl‐seryl‐aspartyl‐lysyl‐proline is a potential biomarker of renal function in normoalbuminuric diabetic patients with eGFR ≥ 30 ml/min/1.73 m(2). Clin Exp Nephrol. 2019; 23: 1004–1012. [DOI] [PubMed] [Google Scholar]
- 73. Neyra JA, Hu MC. Potential application of Klotho in human chronic kidney disease. Bone 2017; 100: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang S, Chen Q, Simon TC, et al Bone morphogenic protein‐7, a novel therapy for diabetic nephropathy. Kidney Int 2003; 63: 2037–2049. [DOI] [PubMed] [Google Scholar]
- 75. Tampe B, Tampe D, Muller C, et al Tet3‐mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7‐induced reversal of kidney fibrosis. J Am Soc Nephrol 2014; 25: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cuadrado A, Rojo AI, Wells G, et al Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 2019; 18: 295–317. [DOI] [PubMed] [Google Scholar]
- 77. Caramori ML, Kim Y, Goldfine AB, et al Differential gene expression in diabetic nephropathy in individuals with type 1 diabetes. J Clin Endocrinol Metab 2015; 100: E876–82. [DOI] [PubMed] [Google Scholar]
- 78. Liu L, Zou J, Guan Y, et al Blocking the histone lysine 79 methyltransferase DOT1L alleviates renal fibrosis through inhibition of renal fibroblast activation and epithelial‐mesenchymal transition. FASEB J 2019; 33: 11941–11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Young G‐H, Wu V‐C. Klotho methylation is linked to uremic toxins and chronic kidney disease. Kidney Int 2012; 81: 611–612. [DOI] [PubMed] [Google Scholar]
- 80. Sapienza C, Lee J, Powell J, et al DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics 2011; 6: 20–28. [DOI] [PubMed] [Google Scholar]
- 81. Chen Z, Miao F, Patterson AD, et al Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Natl Acad Sci USA 2016; 113: 3002–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Qiu C, Hanson RL, Fufaa G, et al Cytosine methylation predicts renal function decline in American Indians. Kidney Int 2018; 93: 1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chu AY, Tin A, Schlosser P, et al Epigenome‐wide association studies identify DNA methylation associated with kidney function. Nat Commun 2017; 8: 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ko YA, Mohtat D, Suzuki M, et al Cytosine methylation changes in enhancer regions of core pro‐fibrotic genes characterize kidney fibrosis development. Genome Biol 2013; 14: R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gluck C, Qiu C, Han SY, et al Kidney cytosine methylation changes improve renal function decline estimation in patients with diabetic kidney disease. Nature Commun 2019; 10: 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kato M, Zhang J, Wang M, et al MicroRNA‐192 in diabetic kidney glomeruli and its function in TGF‐beta‐induced collagen expression via inhibition of E‐box repressors. Proc Natl Acad Sci 2007; 104: 3432–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pezzolesi MG, Satake E, McDonnell KP, et al Circulating TGF‐β1‐regulated miRNAs and the risk of rapid progression to ESRD in Type 1 Diabetes. Diabetes 2015; 64: 3285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 2016; 27: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


