Abstract
Introduction
Resuscitation using blood products is critical during the acute postinjury period. However, the optimal target haemoglobin (Hb) levels have not been adequately investigated. With the restrictive transfusion strategy for critically injured patients (RESTRIC) trial, we aim to compare the restrictive and liberal red blood cell (RBC) transfusion strategies.
Methods and analysis
This is a cluster-randomised, crossover, non-inferiority trial of patients with severe trauma at 22 hospitals that have been randomised in a 1:1 ratio based on the use of a restrictive or liberal transfusion strategy with target Hb levels of 70–90 or 100–120 g/L, respectively, during the first year. Subsequently, after 1-month washout period, another transfusion strategy will be applied for an additional year. RBC transfusion requirements are usually unclear on arrival at the emergency department. Therefore, patients with severe bleeding, which could lead to haemorrhagic shock, will be included in the trial based on the attending physician’s judgement. Each RBC transfusion strategy will be applied until 7 days postadmission to the hospital or discharge from the intensive care unit. The outcomes measured will include the 28-day survival rate after arrival at the emergency department (primary), the cumulative amount of blood transfused, event-free days and frequency of transfusion-associated lung injury and organ failure (secondary). Demonstration of the non-inferiority of restrictive transfusion will emphasise its clinical advantages.
Ethics and dissemination
The trial will be performed according to the Japanese and International Ethical guidelines. It has been approved by the Ethics Committee of each participating hospital and The Japanese Association for the Surgery of Trauma (JAST). Written informed consent will be obtained from all patients or their representatives. The results of the trial will be disseminated to the participating hospitals and board-certified educational institutions of JAST, submitted to peer-reviewed journals for publication, and presented at congresses.
Trial registration number
UMIN Clinical Trials Registry; UMIN000034405. Registered 8 October 2018.
Keywords: accident & emergency medicine, haematology, blood bank & transfusion medicine, trauma management
Strengths and limitations of this study.
During the acute postinjury period, the appropriate strategy for red blood cell transfusion has not been investigated.
This trial will be the first to determine the optimal haemoglobin level during the acute postinjury period in patients with severe trauma.
This multicentre trial will have a cluster-randomised, crossover non-inferiority design.
The two study interventions will be restricted or liberal red blood cell transfusion initiated immediately after the patient’s arrival at the emergency department.
Each red blood cell transfusion strategy will be defined by a target haemoglobin level rather than by the actual patient’s haemoglobin level.
Introduction
Bleeding is a major cause of death after severe trauma. Although early haemostatic procedures are most important, resuscitation using crystalloid and blood products also plays a crucial role in the early phase of management of patients with severe trauma. While the transfusion of fresh frozen plasma has been widely evaluated in the management of trauma-associated coagulopathy during the acute postinjury period,1–7 the transfusion of red blood cells (RBCs) has not been investigated adequately, and the optimal target levels of haemoglobin (Hb) in the early phase of treatment remain unclear.
The European guidelines for the management of major bleeding and coagulopathy recommend target Hb levels of 70–90 g/L.8 This is based mainly on the results of a posthoc analysis of the Transfusion Requirements in Critical Care (TRICC) trial,9 which compared a restrictive transfusion strategy (target Hb level: 70–90 g/L) with a liberal transfusion strategy (target Hb level: 100–120 g/L).10 However, as the TRICC trial included critically ill patients after admission to intensive care units (ICUs), information regarding the patient characteristics, haemostatic procedures and transfusion before admission to the ICUs remains unclear.10 Furthermore, the trial excluded patients with active blood loss.10 Therefore, it is not appropriate to apply the results of this trial and its posthoc analysis to patients in the early phase of severe trauma.9 10 The European guidelines also state that ‘it should be emphasised that this study was neither designed nor powered to answer these questions with precision’ in the rationale section.8
A low Hb level is a possible cause of hypoxic damage to various organs. In patients with traumatic brain injury, a low Hb level is associated with particularly concerning neurological outcomes.11 Recently, a randomised controlled trial that compared two Hb transfusion thresholds (70 g/L or 100 g/L) in patients with traumatic brain injury indicated no differences in the neurological outcomes and mortality rates between the use of low and high Hb transfusion thresholds.12 However, 38% of the patients included in that study were not transfused with any packed RBCs.12
To address the above-mentioned lack of clarity regarding the clinical impacts of a restrictive RBC transfusion strategy in trauma patients during the acute postinjury period, we are conducting a cluster-randomised, crossover non-inferiority trial to compare restrictive and liberal RBC transfusion strategies.
Methods and analysis
Trial design
The restrictive transfusion strategy for critically injured patients (RESTRIC) trial is a cluster-randomised, crossover non-inferiority multicentre trial of patients with severe trauma. This pragmatic trial aims to reproduce real-world settings as closely as possible. The RESTRIC trial applies a cluster-randomised design that enables the initiation of study interventions immediately after arrival at the emergency department (ED) and a crossover design to reduce the confounding effects between different hospitals.
Twenty-two hospitals in Japan are participating in the RESTRIC trial (table 1). These hospitals are tertiary emergency medical facilities that provide emergency and intensive care treatments to patients with severe trauma. The participating hospitals have been randomised into two study schedules at a 1:1 ratio based on a precreated random assignment table to either a restrictive transfusion strategy (target Hb level: 70–90 g/L) or a liberal transfusion strategy (target Hb level: 100–120 g/L). After the randomisation, the hospitals will apply the first transfusion strategy for 1 year (first study period). After a washout period of 1 month, after the end of the first study period, the second transfusion strategy will be applied for another 1 year (second study period; figure 1).
Table 1.
List of participating hospitals and ethics committee
| Participating hospitals | Ethics committees |
| Principal institution | |
| Department of Emergency and Critical Care Medicine, Tohoku University Hospital | Ethics Committee Tohoku University Graduate School of Medicine |
| Project management | |
| Department of Emergency Medicine, Hokkaido University Hospital | The Institutional Review Board of Hokkaido University Hospital |
| Other participating institutions | |
| Advanced Critical Care and Emergency Centre, Okayama University Hospital | Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital, Ethics Committee |
| Advanced Critical Care Centre, Gifu University Hospital | Medical Review Board of Gifu University Graduate School of Medicine |
| Advanced Emergency and Critical Care Centre, Saitama Red Cross Hospital | Hospital ethical committee of Saitama Red Cross |
| Advanced Trauma, Emergency and Critical Care Centre, Oita University Hospital | The Institutional Review Board of Interventional Clinical Research of Oita University Hospital |
| Department of Emergency Medicine, Gunma University Graduate School of Medicine | Institutional Review Board of Gunma University Hospital |
| Department of Acute Care Surgery, Shimane University Faculty of Medicine | The Shimane University Institutional Committee on Ethics |
| Department of Emergency and Critical Care Medicine, Chiba University Graduate School of Medicine | Chiba University Certified Clinical Research Review Board |
| Department of Emergency and Critical Care Medicine, Fukuoka University Hospital | Institutional Review Board of Fukuoka University Hospital |
| Department of Emergency and Critical Care Medicine, Japan Red Cross Maebashi Hospital | Research Review Board of Japan Red Cross Maebashi Hospital |
| Department of Emergency and Critical Care Medicine, Juntendo University Urayasu Hospital | The Ethics Committee of the Juntendo University Urayasu Hospital |
| Department of Emergency and Critical Care Medicine, Nippon Medical School | Ethics Committee of Nippon Medical School Hospital |
| Department of Emergency and Critical Care Medicine, Nippon Medical School Tama Nagayama Hospital | Ethics Committee of Nippon Medical School Tamanagayama Hospital |
| Department of Emergency and Critical Care Medicine, Tokyo Saiseikai Central Hospital | Research Ethics Committee, Tokyo Saiseikai Central Hospital |
| Department of Emergency and Critical Care Medicine, Wakayama Medical University | The Ethical Review Board of Wakayama Medical University |
| Department of Emergency Medicine, Division of Acute Care Surgery, Teikyo University School of Medicine | Teikyo University Institutional Review Board |
| Emergency and Critical Care Centre, Kochi Health Sciences Centre | Institutional Review Board, Kochi Health Sciences Center |
| Senri Critical Care Medical Centre, Saiseikai Senri Hospital | Ethical committee Saiseikai Senri Hospital |
| Senshu Trauma and Critical Care Centre, Rinku General Medical Centre | Ethics Committee for Clinical Research, Rinku General Medical Centre |
| Shock and Trauma Centre, Nippon Medical School Chiba Hokusoh Hospital | The Ethical Review Board of Nippon Medical School Chiba Hokusoh Hospital |
| Trauma and Acute Critical Care Centre, Tokyo Medical and Dental University Hospital of Medicine | Medical Research Institute Tokyo Medical and Dental University |
Figure 1.
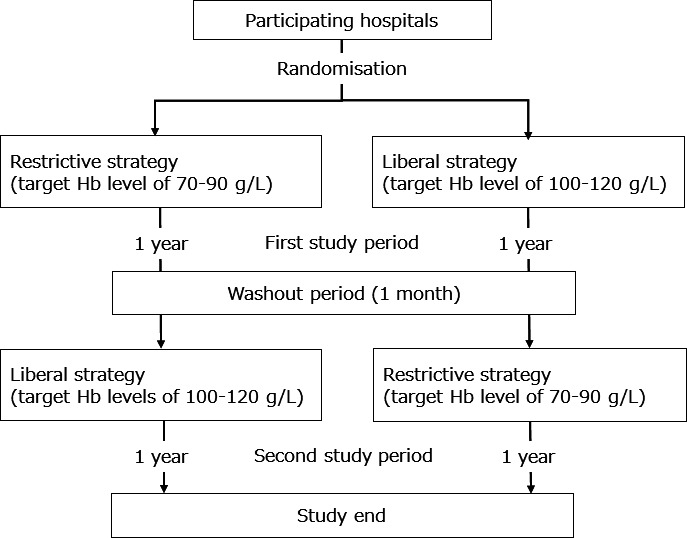
Flowchart of the randomisation and crossover of the participating hospitals. Hb, haemoglobin.
The allocated transfusion strategy is posted in each hospital in order to provide opt-out opportunities to patients and their next of kin. The allocated transfusion strategy will be applied for all trauma patients during the initial phase after arrival at the ED. After obtaining the consent for registration from the patients or their representatives, the patients will be registered in the trial and the transfusion strategy will be applied until a defined period. If the registration to the trial is declined, the transfusion strategy will be continued based on the physician’s decision.
Patients
On arrival at the ED, the requirement for RBC transfusion is usually unclear. Therefore, the inclusion criteria include trauma patients aged ≥20 years with one of the following complications based on the judgement of the attending physician (figure 2):
Figure 2.
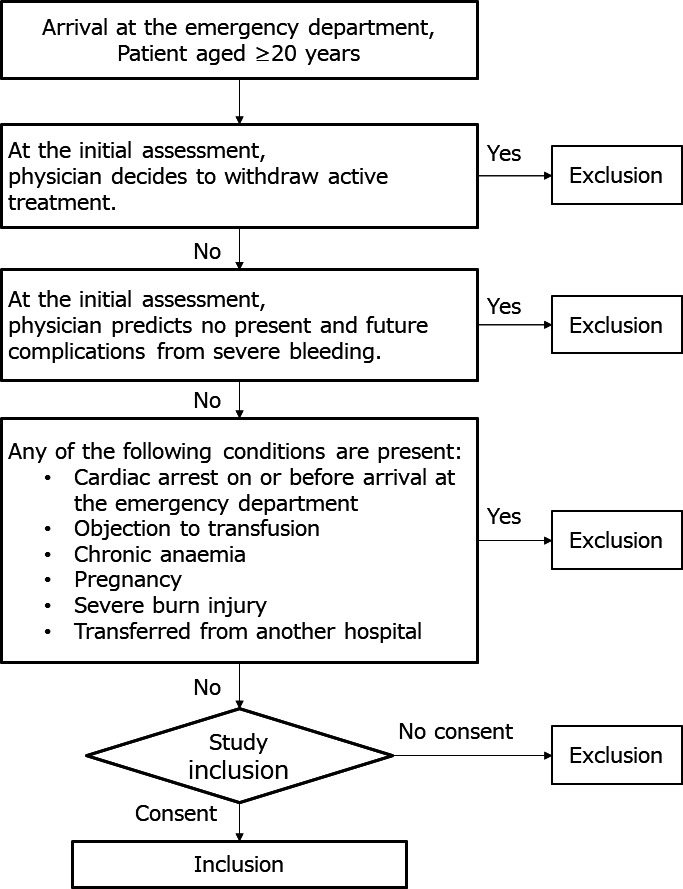
Flowchart of the patient enrolment process.
Severe bleeding that can result in circulatory shock.
Suspicion of such bleeding after arrival at the ED.
Possibility of inducing such bleeding by surgical procedures during the acute phase of trauma.
The following exclusion criteria have been set:
Cardiac arrest before or on arrival at the hospital.
Transfer from another hospital.
Physician’s decision to withdraw active treatment at the initial assessment.
Severe burn injuries (≥15% of the body surface).
Pregnancy.
Chronic anaemia (Hb level ≤70 g/L).
Known objection to blood transfusions.
Intervention
In severe trauma patients with active bleeding, RBC transfusion is frequently initiated before confirming a decrease in Hb levels. Therefore, each RBC transfusion strategy is defined by the target Hb level rather than the current Hb level. The timing of RBC transfusion initiation in a patient with active bleeding is determined by the attending physician based not only on the Hb levels but also on haemodynamic instability. Either of the RBC transfusion strategies will be applied to patients until (1) 7 days after admission to the hospital, (2) discharge from the ICU, (3) decision to withdraw active treatment or (4) death.
Assessments and follow-up
Clinical assessments and treatments will be performed as necessary based on the attending physician’s judgement. The schedule of trial assessments is presented in table 2. The assessment data will be recorded in the electronic trial data capture system (NorthNet, https://www.crmic-huhp.jp/northnet/edc/). Patients will be followed for 28 days. If a patient is discharged from the hospital prior to 28 days after arrival at the ED, the investigators will contact the patient (or their representative, as appropriate) by telephone to collect information regarding the patient’s status.
Table 2.
Schedule of assessments
| Arrival at ED | 6 hour | 12 hours | 24 hours | 48 hours | Day 7 | Discharge from ICU | Discharge from hospital | Day 28 | |
| Informed consent | ○ | ||||||||
| Check inclusion/exclusion criteria | ○ | ||||||||
| Patient assessment | ○ | ||||||||
| Physiologic severity | ○ | ||||||||
| Abbreviated Injury Scale | ○ | ||||||||
| Surgical intervention and IVR | ├────○────┤ | ||||||||
| Laboratory data | ○ | ||||||||
| Haemoglobin level | ├─────────────────────○──────────────────────┤ | ||||||||
| Cumulative amount of transfusion | ○ | ○ | ○ | ○ | ○ | ○ | |||
| Organ failure (renal/respiratory/hepatic) | ├─────────────────────○──────────────────────┤ | ||||||||
| TRALI | ├───────────────────────────────────────────○───────────────────────────────────────┤ | ||||||||
| Complications | ├───────────────────────────────────────────○───────────────────────────────────────┤ | ||||||||
| Mortality | ○ | ○ | ○ | ||||||
| Discharge destination | ○ | ||||||||
| Glasgow outcome scale | ○ | ||||||||
| Event-free days (free of ventilator/catecholamine/ICU) | ○ | ||||||||
Complications include deep venous thrombosis, pulmonary embolism, acute myocardial infarction, ischaemic bowel necrosis and sepsis.
IVR, interventional radiology; TRALI, transfusion-related acute lung injury; ICU, intensive care unit.
Safety monitoring
A safety monitoring board comprising two independent experts who are not involved in the conduct of the trial will oversee the safety of the trial. Significant adverse events (SAEs) will be recorded immediately in the patient’s medical record and in the electronic data capture system (NorthNet, https://www.crmic-huhp.jp/northnet/edc/) which are same as the system that recorded the assessment data of patients. The treating physician will immediately report any SAEs to the site investigator, who will in turn report them to the chief of each site and the principal investigator. The principal investigator will then consult with the safety monitoring board about the SAEs. The board will review and examine the report and send written recommendations made in response to the principal investigator.
Primary outcome
To evaluate the non-inferiority of the restrictive transfusion strategy to the liberal transfusion strategy, we will assess the 28-day survival rate after arrival at the ED (tables 2 and 3) as the primary outcome measure. Patients with incomplete information regarding survival/death on the 28th day after arrival at the ED will be defined as dropout and will be excluded from the primary outcome analysis.
Table 3.
Primary and secondary outcomes
| Outcome | Definition/annotation |
| Primary outcome | |
| 28 day survival rate after arrival at the ED | Patients whose survival/death information on 28th day after arrival at the ED is unclear are defined as drop-outs and will be excluded from the primary outcome analysis |
| Secondary outcome | |
| Time to death during the first 28 days after arrival at the ED | |
| In-hospital survival rate | |
| Cumulative transfusion amounts | |
| Red blood cell concentrate | Cumulative amounts during the first 1, 7 and 28 days after arrival at the ED |
| Fresh-frozen plasma | Cumulative amounts during the first 1, 7 and 28 days after arrival at the ED |
| Platelet concentrate | Cumulative amounts during the first 1, 7 and 28 days after arrival at the ED |
| Event-free days during the first 28 days after arrival at the ED | |
| Ventilator-free days | When the patient dies during the first 28 days after the arrival at ED, the free days are defined as zero |
| Catecholamine-free days | When the patient dies during the first 28 days after the arrival at ED, the free days are defined as zero |
| ICU-free days | When the patient dies during the first 28 days after the arrival at ED, the free days are defined as zero |
| Organ failure during the first 7 days after arrival at the ED | |
| Renal failure | Stage III defined by the Kidney Disease Improving Global Guidelines |
| Hepatic failure | Total bilirubin level ≥6 mg/dL as per the Sequential Organ Failure Assessment score |
| Respiratory failure | Moderate acute respiratory distress syndrome according to the Berlin definition |
| Complications during in-hospital stay or the first 28 days after arrival at the ED | |
| Deep venous thrombosis | Presence or absence should be diagnosed using clinical imaging |
| Pulmonary embolism | Presence or absence should be diagnosed using clinical imaging |
| Cerebral infarction | Presence or absence should be diagnosed using clinical imaging |
| Acute myocardial infarction | Presence or absence should not be diagnosed using only an elevation of cardiac biomarkers |
| Bowel ischaemia | Presence or absence should not be diagnosed using laboratory data |
| Transfusion-associated lung injury | Presence, possibility or absence are defined using the Toronto definition |
| Sepsis | Presence or absence should be diagnosed using the Sepsis-3 definition |
| Glasgow outcome scale score at discharge from the hospital | Good recovery, moderate disability, severe disability, persistent vegetative state or death |
ED, emergency department; ICU, intensive care unit.
Secondary outcomes
The secondary outcome measures will be: (1) the time to death during the first 28 days, (2) in-hospital survival rate, (3) cumulative amounts of RBC concentrate, fresh-frozen plasma and platelet cell concentrate transfused during days 1, 7 and 28, (4) ventilator-free, catecholamine-free and ICU-free days during the first 28 days, (5) frequency of organ failure (renal, hepatic and respiratory) during the first 7 days, (6) rates of each complication (deep venous thrombosis, pulmonary embolism, cerebral infarction, myocardial infarction, bowel ischaemia, transfusion-associated lung injury (TRALI) and sepsis) during the first 28 days and (7) the Glasgow Outcome Scale at discharge from the hospital (tables 2 and 3).13 If the patient dies during the first 28 days after admission to the hospital, each event-free day will be defined as zero. Renal failure is defined as stage III as per the Kidney Disease Improving Global Guidelines.14 Hepatic failure is defined as a total bilirubin level ≥6 mg/dL, as per the Sequential Organ Failure Assessment score.15 Respiratory failure is defined as moderate acute respiratory distress syndrome as per the Berlin definition.16 Deep venous thrombosis, pulmonary embolism and cerebral infarction will be diagnosed via clinical imaging, whereas myocardial infarction and bowel ischaemia will not be diagnosed solely from the elevation of cardiac biomarkers and laboratory data, respectively. TRALI is defined according to the Toronto definition,17 and sepsis is defined according to sepsis-3.18
Sample size
In our previous retrospective multicentre observational study, wherein data were collected from 796 patients with severe trauma from 15 hospitals during a 1-year period,19–26 241 patients received RBC concentrates during the first 24 hours after arrival at the ED and 25% of the patients transfused with RBC concentrates died within 28 days after arrival at the ED. Based on these results, we assumed a mortality rate of 25% at 28 days after arrival in the ED among patients receiving a liberal RBC transfusion strategy. To evaluate the non-inferiority of the restrictive versus liberal transfusion strategy at 28 days postarrival at the ED, we set both the interclass and interperiod correlation coefficients at 0.05 and the non-inferiority margin at 3%. The non-inferiority margin was defined based on statistically acceptable tolerance and clinically acceptable margin referenced previous large clinical trials in the same field10 27–30
Assuming that 17 hospitals participate and are randomised as a cluster, the present study would require the inclusion of 170 patients for each of the transfusion strategies to reach a power of 80% and a one-sided alpha level of 2.5%, based on a previous study.31 Therefore, we set the total target sample size for this study at 400 patients, considering a possible variation in the cluster size, the inclusion of non-appropriate patients and dropouts during follow-up. According to previous studies, this number of patients will allow us to study the outcomes for 2 years.19–26
Statistical plan
All analyses of the primary outcome will be adjusted for clustering within sites. The analysis will use a mixed model with adjustment for intervention, the period as a fixed effect and the sites and the interaction of site with period as a random effect.32 The non-inferiority margin will be set at P0−P1 <0.03 (P0, 28-day survival rate for liberal transfusion; P1, 28-day survival rate for restrictive transfusion). Therefore, we will evaluate whether the lower limit of the 95% CI of P0−P1 exceeds the non-inferiority margin (3%) or not. We will use the full analysis set for our primary outcome analysis after excluding cases with missing primary outcome values. We will follow the principle of intention-to-treat for the primary analysis and a per-protocol for sensitivity analysis to ensure that no cases deviate intentionally from the target Hb levels.
The secondary outcomes will be analysed as follows. (1) Kaplan-Meier curves with log rank statistics will be used to assess the survival rate during the first 28 days after arrival at the ED, (2) the number of in-hospital survival patients will be tabulated, (3) summary statistics of the cumulative amounts of transfused RBC concentrate, fresh-frozen plasma and platelet cell concentrate during days 1, 7 and 28 after arrival at the ED will be created using graphs plotted over time, (4) summary statistics of the event-free days (ie, ventilator-free, catecholamine-free and ICU-free days) will be calculated, (5) the proportions of organ failure and complications will be calculated, (6) the Glasgow Outcome Scale will be measured at discharge from hospital.
Subgroup analyses will be performed to investigate the effects of the interventions on patients according to sex, age (<60 or ≥60 years), Injury Severity Score (<16 or ≥16 years), head trauma and performance of definitive surgical procedures within 6 hours of arrival at the ED. The results of both unadjusted and covariate data-adjusted analyses will be assessed. Furthermore, we will perform a posthoc power analysis if the numbers of the participating institutions and included patients differ from the planned numbers.
Patient and public involvement
No patient is involved.
Ethics and dissemination
Ethical approval and consent to participate
The clinical trial will be conducted according to the principles of the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects published by the Ministry of Health, Labour and Welfare of Japan and the Japanese Ministry of Education, Culture, Sports, Science and Technology. Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our trial. The present trial is registered with the UMIN Clinical Trials Registry and has been approved by the Ethics Committee of each participating hospital (table 1) and the Japanese Association for the Surgery of Trauma (Ethics Committee of the Japanese Association for the Surgery of Trauma). Written informed consent will be obtained from all patients or their representatives. The trial information form in Japanese and patient consent forms in Japanese and English were provided as supplement files (online supplemental files 1-3).
bmjopen-2020-037238supp001.pdf (434.6KB, pdf)
bmjopen-2020-037238supp002.pdf (126.6KB, pdf)
bmjopen-2020-037238supp003.pdf (22.7KB, pdf)
Dissemination
The results of trial will be disseminated to the participating hospitals and board-certified educational institutions of The Japanese Association for The Surgery of Trauma, submitted to peer-reviewed journals for publication, and presented at congresses.
Expected outcomes
The RESTRIC trial will compare the outcomes of the restrictive versus liberal RBC transfusion strategy in trauma patients during the acute postinjury period. To the best of our knowledge, the RESTRIC trial will be the first to clarify the optimal target Hb levels in patients with severe trauma during this period.
Although previous studies, such as the TRICC trial and its posthoc analysis,9 10 initiated the study interventions after admission to the ICU, the RESTRIC trial has been designed to initiate the study interventions immediately after arrival at ED and to continue these interventions through the early phase of severe trauma. In patients with severe trauma, management before admission to an ICU is as important as that after admission to an ICU. If the restrictive RBC transfusion strategy is found to be non-inferior to the liberal RBC transfusion strategy, the former will be considered advantageous in clinical settings during the acute postinjury period because it will help reduce the total amount of RBC transfusion. This reduction in RBC transfusion will reduce (a) the risk of transfusion-related complications such as TRALI, (b) RBC transfusion-related immunomodulation and (c) the costs associated with RBC transfusion.17 33
Trial status
At first, the trial protocol V.1.3 was approved at 11 October 2018. The latest protocol is V.1.7 that has been approved at 19 December 2019 after minor changes (online supplemental file 4). In May 2019, 12 participating institutions were randomised as a cluster, and the trial was started. The first patient was included on 11 May 2019. Subsequently, 10 more institutions have joined the trial and have been randomised. The last participating institution began the trial in October 2019. Patients will be recruited until October 2021 and followed up thereafter.
bmjopen-2020-037238supp004.pdf (1.7MB, pdf)
Supplementary Material
Acknowledgments
We would like to thank Akiyoshi Hagiwara (Niizashiki Chuo General Hospital), Atsushi Shiraishi (Emergency and Trauma Centre, Kameda Medical Centre), Daizoh Saitoh (Division of Traumatology Research Institute, National Defence Medical College), Kazuhisa Yoshiya (Department of Traumatology and Acute Critical Medicine, Osaka University Graduate School of Medicine) and Shinji Nakahara (Department of Emergency Medicine, Teikyo University School of Medicine) for supporting the RESTRIC trial. We would also like to thank Editage (https://online.editage.jp/) for English language editing.
Footnotes
Contributors: MH conceived and designed the RESTRIC trial and drafted the manuscript. TT drafted the statistical plan. HI calculated the sample size and developed the statistical plan. SK is the principal investigator and supervised the planning of the RESTRIC trial. MH, TT, HI, DK, KS, TO, TY, YK, AE, KI, YM and SK discussed the plan of the RESTRIC trial, revised the manuscript for important intellectual content and read and approved the final manuscript.
Funding: This study was supported in part by research grants from The General Insurance Association of Japan and The Marumo Emergency Medical Research Promotion Fund.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Borgman MA, Spinella PC, Perkins JG, et al. . The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support Hospital. J Trauma 2007;63:805–13. 10.1097/TA.0b013e3181271ba3 [DOI] [PubMed] [Google Scholar]
- 2.Shaz BH, Dente CJ, Nicholas J, et al. . Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion 2010;50:493–500. 10.1111/j.1537-2995.2009.02414.x [DOI] [PubMed] [Google Scholar]
- 3.Cotton BA, Reddy N, Hatch QM, et al. . Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 2011;254:598–605. 10.1097/SLA.0b013e318230089e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, et al. . The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013;148:127–36. 10.1001/2013.jamasurg.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson PI, Sørensen AM, Larsen CF, et al. . Low hemorrhage-related mortality in trauma patients in a level I trauma center employing transfusion packages and early thromboelastography-directed hemostatic resuscitation with plasma and platelets. Transfusion 2013;53:3088–99. 10.1111/trf.12214 [DOI] [PubMed] [Google Scholar]
- 6.Langan NR, Eckert M, Martin MJ. Changing patterns of in-hospital deaths following implementation of damage control resuscitation practices in US forward military treatment facilities. JAMA Surg 2014;149:904–12. 10.1001/jamasurg.2014.940 [DOI] [PubMed] [Google Scholar]
- 7.Holcomb JB, Tilley BC, Baraniuk S, et al. . Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015;313:471–82. 10.1001/jama.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossaint R, Bouillon B, Cerny V, et al. . The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care 2016;20:100. 10.1186/s13054-016-1265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntyre L, Hebert PC, Wells G, et al. . Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? J Trauma 2004;57:563–8. discussion 68. 10.1097/01.ta.0000136158.93864.54 [DOI] [PubMed] [Google Scholar]
- 10.Hebert PC, Wells G, Blajchman MA, et al. . A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. transfusion requirements in critical care Investigators, Canadian critical care Trials Group. N Engl J Med 1999;340:409–17. [DOI] [PubMed] [Google Scholar]
- 11.Sena MJ, Rivers RM, Muizelaar JP, et al. . Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med 2009;35:480–8. 10.1007/s00134-008-1289-z [DOI] [PubMed] [Google Scholar]
- 12.Robertson CS, Hannay HJ, Yamal J-M, et al. . Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 2014;312:36–47. 10.1001/jama.2014.6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480–4. 10.1016/s0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 14.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, de Mendonça A, Cantraine F, et al. . Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793–800. 10.1097/00003246-199811000-00016 [DOI] [PubMed] [Google Scholar]
- 16.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. . Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 17.Kleinman S, Caulfield T, Chan P, et al. . Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion 2004;44:1774–89. 10.1111/j.0041-1132.2004.04347.x [DOI] [PubMed] [Google Scholar]
- 18.Shankar-Hari M, Phillips GS, Levy ML, et al. . Developing a new definition and assessing new clinical criteria for septic shock: for the third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:775–87. 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiraishi A, Kushimoto S, Otomo Y, et al. . Effectiveness of early administration of tranexamic acid in patients with severe trauma. Br J Surg 2017;104:710–7. 10.1002/bjs.10497 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura Y, Ishikura H, Kushimoto S, et al. . Fibrinogen level on admission is a predictor for massive transfusion in patients with severe blunt trauma: analyses of a retrospective multicentre observational study. Injury 2017;48:674–9. 10.1016/j.injury.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 21.Kudo D, Kushimoto S, Shiraishi A, et al. . The impact of preinjury antithrombotic medication on hemostatic interventions in trauma patients. Am J Emerg Med 2017;35:62–5. 10.1016/j.ajem.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa M, Maekawa K, Kushimoto S, et al. . Hyperfibrinolysis in severe isolated traumatic brain injury may occur without tissue hypoperfusion: a retrospective observational multicentre study. Crit Care 2017;21:222. 10.1186/s13054-017-1811-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayakawa M, Kushimoto S, Watanabe E, et al. . Pharmacokinetics of recombinant human soluble thrombomodulin in disseminated intravascular coagulation patients with acute renal dysfunction. Thromb Haemost 2017;117:851–9. 10.1160/TH16-07-0547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa M, Maekawa K, Kushimoto S, et al. . High D-dimer levels predict a poor outcome in patients with severe trauma, even with high fibrinogen levels on arrival: a multicenter retrospective study. Shock 2016;45:308–14. 10.1097/SHK.0000000000000542 [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara A, Kushimoto S, Kato H, et al. . Can Early Aggressive Administration of Fresh Frozen Plasma Improve Outcomes in Patients with Severe Blunt Trauma?--A Report by the Japanese Association for the Surgery of Trauma. Shock 2016;45:495–501. 10.1097/SHK.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo A, Shiraishi A, Otomo Y, et al. . Development of Novel Criteria of the "Lethal Triad" as an Indicator of Decision Making in Current Trauma Care: A Retrospective Multicenter Observational Study in Japan. Crit Care Med 2016;44:e797–803. 10.1097/CCM.0000000000001731 [DOI] [PubMed] [Google Scholar]
- 27.Hébert PC, Wells G, Blajchman MA, et al. . A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. transfusion requirements in critical care Investigators, Canadian critical care Trials Group. N Engl J Med 1999;340:409–17. 10.1056/NEJM199902113400601 [DOI] [PubMed] [Google Scholar]
- 28.Villanueva C, Colomo A, Bosch A, et al. . Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. 10.1056/NEJMoa1211801 [DOI] [PubMed] [Google Scholar]
- 29.Hajjar LA, Vincent J-L, Galas FRBG, et al. . Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559–67. 10.1001/jama.2010.1446 [DOI] [PubMed] [Google Scholar]
- 30.Mazer CD, Whitlock RP, Fergusson DA, et al. . Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med 2017;377:2133–44. 10.1056/NEJMoa1711818 [DOI] [PubMed] [Google Scholar]
- 31.Giraudeau B, Ravaud P, Donner A. Sample size calculation for cluster randomized cross-over trials. Stat Med 2008;27:5578–85. 10.1002/sim.3383 [DOI] [PubMed] [Google Scholar]
- 32.Morgan KE, Forbes AB, Keogh RH, et al. . Choosing appropriate analysis methods for cluster randomised cross-over trials with a binary outcome. Stat Med 2017;36:318–33. 10.1002/sim.7137 [DOI] [PubMed] [Google Scholar]
- 33.Remy KE, Hall MW, Cholette J, et al. . Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion 2018;58:804–15. 10.1111/trf.14488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037238supp001.pdf (434.6KB, pdf)
bmjopen-2020-037238supp002.pdf (126.6KB, pdf)
bmjopen-2020-037238supp003.pdf (22.7KB, pdf)
bmjopen-2020-037238supp004.pdf (1.7MB, pdf)


