Abstract
Monitoring anti-TNF agents in inflammatory bowel disease (IBD) patients may be helpful in optimizing outcomes. We aimed to evaluate potential correlations among demographic, clinical, laboratory, or imaging parameters, as well as serum levels of infliximab (IFX) and adalimumab (ADA) and their respective antibodies, in the clinical management of IBD patients.
A cross-sectional study of 95 patients with Crohn's disease (CD) or ulcerative colitis (UC) in maintenance therapy with infliximab or adalimumab was performed. Drug trough levels and anti-drug levels were determined using ELISA-based assays.
Regarding the serum IFX dosage, patients with higher relative C-reactive protein (CRP) levels had significantly lower relative serum IFX levels (<3 μg/mL) (P = .028). In contrast, higher concentrations of anti-IFX antibodies were found in patients who were not on concomitant immunomodulators (P = .022) and who had more biological-related adverse events (P = .001) and higher levels of CRP (P = .042). Serum CRP levels were also negatively correlated with IFX (CC = −0.315; P = .033) but positively correlated with the presence of IFX antibodies (CC = 0.327; P = .027). Serum albumin dosage showed a positive correlation with levels of both IFX (CC = 0.379; P = .004) and ADA (CC = 0.699; P = .003).
Although anti-TNF-α trough levels and immunogenicity do not show a significant correlation with disease outcome, our results reinforce the use of combination therapy for patients treated with infliximab. Moreover, we confirmed the presence of significant associations between anti-TNF-α trough levels and immunogenicity with body mass index (BMI), the concomitant use of immunomodulators, the rates of side effects, and laboratory markers, including serum albumin and CRP.
Keywords: adalimumab, Crohn's disease, immunogenicity, inflammatory bowel disease, infliximab, therapeutic drug monitoring, ulcerative colitis
1. Introduction
Inflammatory bowel diseases (IBDs) are chronic and progressive diseases including ulcerative colitis (UC) and Crohn's disease (CD) that affect the gastrointestinal tract. The fact that the incidence of IBD has been increasing globally in recent years is a matter of great concern due to high morbidity and cost.[1–3]
The major aim when treating patients with IBD is to control the inflammatory response and maintain clinical and endoscopic remission. Some of the most widely used anti-TNF-α biological therapies (infliximab and adalimumab) have limitations, including infusion reactions and a high rate of primary and secondary nonresponders, despite their undisputed success. Not all patients respond to this type of therapy, with approximately 30% of patients failing the induction response (nonprimary responders), and a significant proportion of initial responders tend to lose the response over time (approximately 40–60%). In patients initially regarded as responders, the annual risk of response loss has been reported as 13% per patient/year.[4] In fact, various studies in the literature show controversial and often contradictory results in this regard.[5,6]
The monitoring of serum levels of biological drugs and the formation of anti-drug antibodies have emerged as useful tools in the follow-up of patients, and they enable physicians to optimize treatment and maintain drugs at effective concentrations for longer periods of time. In addition, serum levels and anti-drug antibodies may have significant impacts on the cost of treatment in these patients, avoiding overdoses or the use of ineffective drugs.[7,8] The rationale for monitoring drug levels and their respective anti-drug antibodies relies on the fact that they can help a physician to objectively understand the reason for a potential treatment failure and to define the next steps in patient management. Moreover, a proactive action provides a great opportunity to maximally optimize and increase the chances of success.[7] In practice, the best time to check anti-drug antibodies is on the day of the application of the next dose, just before the application of the drug, particularly in situations of treatment failure.[9] Because the cost of routinely measuring the serum level of anti-TNF-α and its antibodies is still high, particularly in developing countries, attempts to identify other laboratory, clinical, or endoscopic markers, which may correlate with those tests, appear to be logical and justifiable.
The goal of the present study was to measure serum levels of anti-TNF-α biological drugs and their respective antibodies to identify correlations with sustained clinical response, nonresponse, and loss of drug response in IBD patients.
2. Materials and methods
2.1. Study design, selection of patients, and ethical considerations
Patients were consecutively recruited at the outpatient unit of the Policlínica Piquet Carneiro of the State University of Rio de Janeiro, Brazil, from July 2015 to November 2016. The study protocol was approved by the institutional research board and the ethical committee of the University Hospital of the State University of Rio de Janeiro (Approval number: CAAE 30711014.6.0000.5259) and was performed in accordance with the Helsinki declaration. All the participants signed an informed consent form prior to entry into the study. The study was of cross-sectional design with prospective patient inclusion. A total of 95 patients with IBD were selected, and the diagnosis of CD or UC was confirmed by routine clinical, endoscopic and/or radiological, and histological parameters. All patients were using either infliximab in the maintenance phase, with doses of 5 mg/kg or 10 mg/kg every 6 or 8 weeks after being given the induction phase with infliximab 5 mg/kg at weeks 0, 2 and 6 at the beginning of treatment, or adalimumab at doses of 20 or 40 mg SC weekly or every 2 weeks as postinduction therapy with 160/80/40 mg every 2 weeks.
Sociodemographic data, diagnosis, duration of disease, and specific phenotypes were recorded. Clinical activity evaluation was performed using the Harvey-Bradshaw score for CD and the Truelove or Mayo index for UC. To evaluate the laboratory activity, we used the complete blood count, concentrations of serum hemoglobin and albumin, erythrocyte sedimentation rate (ESR), titrated C-reactive protein (CRP), and the dosage of fecal calprotectin. A simplified endoscopic score was used for colonoscopic evaluation and for the assessment of mucosal healing. A modified score was used for enterography analysis by magnetic resonance. The following criteria were used to define the response to treatment in 3 distinct groups, according to clinical, laboratory, and imaging parameters: nonresponse or primary failure, that is, patients not responding to anti-TNF-α induction therapy; loss of response or secondary failure, that is, patients who responded to induction therapy and then relapsed from disease activity; and a good or sustained response to treatment, that is, patients who responded to anti-TNF-α induction therapy and maintained the response with maintenance therapy.
The exclusion criteria were patients who declined to participate in the study, HIV-positive patients, and patients who had not used a biological therapy for more than 9 weeks.
2.2. Determination of infliximab, adalimumab, anti-infliximab, and anti-adalimumab blood antibody concentrations
Venous blood samples were harvested in serum tubes immediately before infliximab infusion and adalimumab application to ensure that the drugs were at the lowest possible blood concentration in all patients. The collection tubes were centrifuged at 3000 rpm for 10 min at room temperature. The serum was then transferred into cryotubes and stored at −20 °C until analysis. Serum levels of infliximab and anti-infliximab antibodies as well as adalimumab and anti-adalimumab antibodies were assessed simultaneously by Lisa Tracker Duo Infliximab and Lisa Tracker Duo Adalimumab enzyme-linked immunosorbent assay (ELISA)-based techniques (Theradiag, France), respectively. All assays were performed according to protocols provided by the kit manufacturers.
2.3. Statistical analysis
Individual characteristics were analyzed using simple descriptive statistics. Differences between the distributions of the selected variables were evaluated with either the Chi-square test or Fisher's exact test for categorical data. The correlation between numeric variables was assessed using Spearman's rank correlation coefficient. All tests were two-tailed, and statistical significance was established at P values of less than .05. Statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL).
2.4. Data sharing and data availability accessibility
Study materials are available upon request to interested researchers. The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
3. Results
3.1. Study population and laboratory results
Blood samples from 95 patients were evaluated. Among the selected patients, 85 (89.47%) had CD, and 10 (10.53%) had UC. Sixty-three patients (66.32%) were on infliximab therapy, while 32 (33.68%) were on adalimumab therapy.
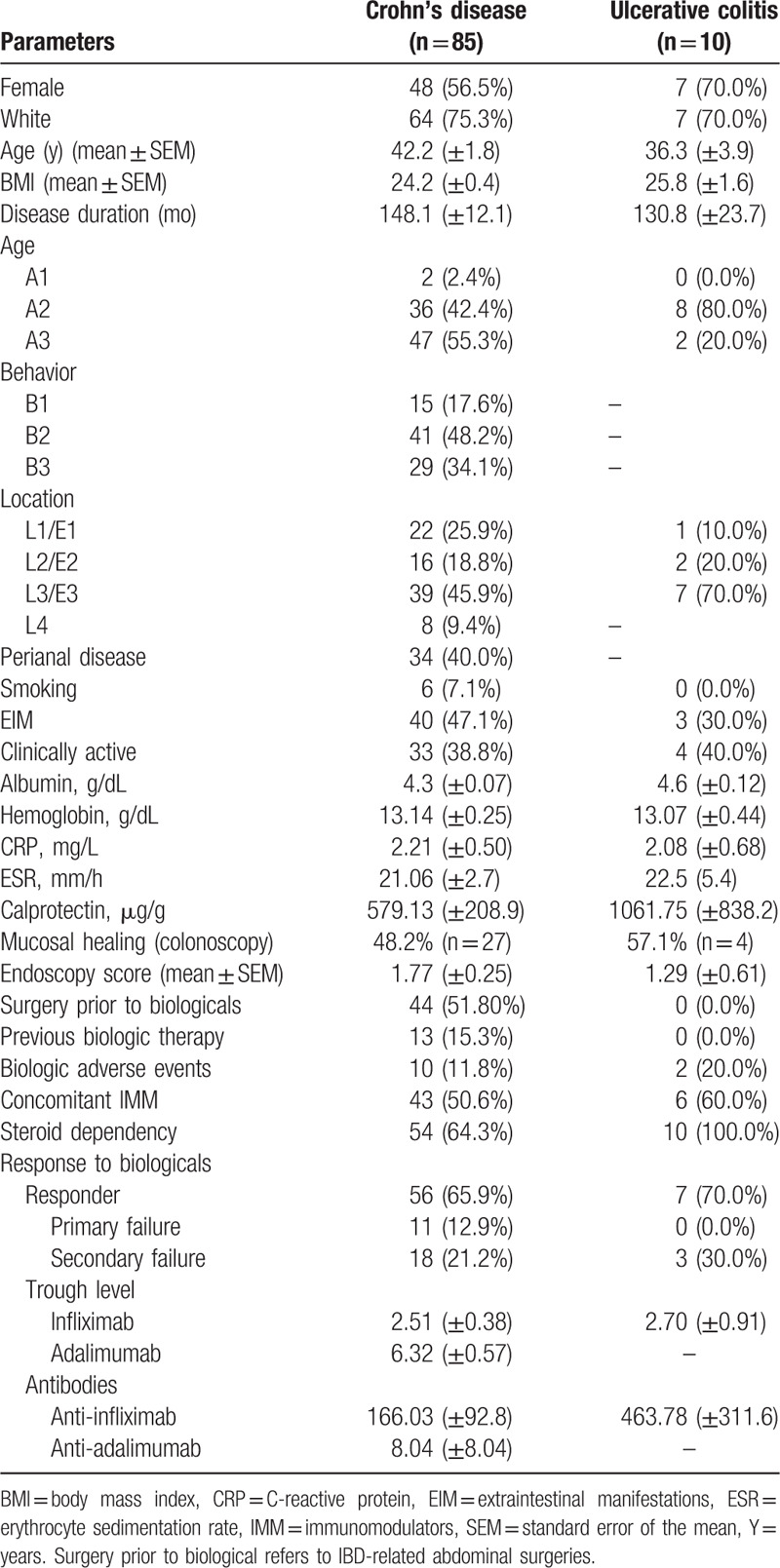
Among the patients with CD, 56 (65.9%) were responders (sustained response), 11 (12.9%) were primary nonresponders (primary failure), and 18 (21.2%) were secondary nonresponders (secondary failure). Among the patients with UC, 7 (70%) were responders, and 3 (30.0%) were secondary nonresponders; there were no reports of patients with UC who were primary nonresponders in this study. Details of the demographic and clinical characteristics of the patients and the respective laboratory results are described in Table 1.
Table 1.
Patient demographics and medical characteristics.
3.2. Comparison of infliximab and adalimumab trough concentrations and anti-drug antibody concentrations with several medical parameters
In accordance with previous studies in the literature regarding serum infliximab,[5,6,10–15] we considered a ≥3 μg/mL cut-off as the therapeutic level related to the satisfactory clinical response to treatment. We considered levels of anti-infliximab antibodies greater than 0 (zero) to be positive.
Table 2 shows a comparative analysis of serum infliximab levels and anti-infliximab antibodies in relation to several clinical, endoscopic, and laboratory parameters. We found that patients with higher CRP levels had significantly lower levels of serum infliximab (<3 μg/mL) (P = .028). In contrast, high levels of anti-IFX antibodies were detected among the patients who were not using immunomodulators concomitantly (P = .022), who had more side effects related to biologicals (P = .001), and who had high levels of CRP (P = .042).
Table 2.
Agreement of infliximab and anti-infliximab serum concentrations with selected clinical, endoscopic, and laboratory variables.
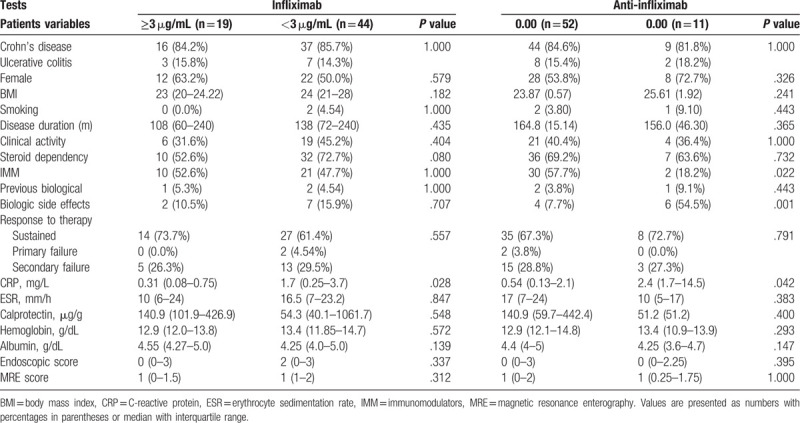
Table 3 shows a comparative analysis of serum adalimumab levels and anti-adalimumab antibodies in relation to several clinical, endoscopic, and laboratory parameters. In accordance with previous studies in the literature regarding serum adalimumab,[16–18] we adopted a ≥3 μg/mL cut-off as the therapeutic level related to good clinical response to treatment. We considered levels of anti-adalimumab antibodies greater than 0 (zero) to be positive.
Table 3.
Agreement of adalimumab and anti-adalimumab serum concentrations with selected clinical, endoscopic, and laboratory variables.
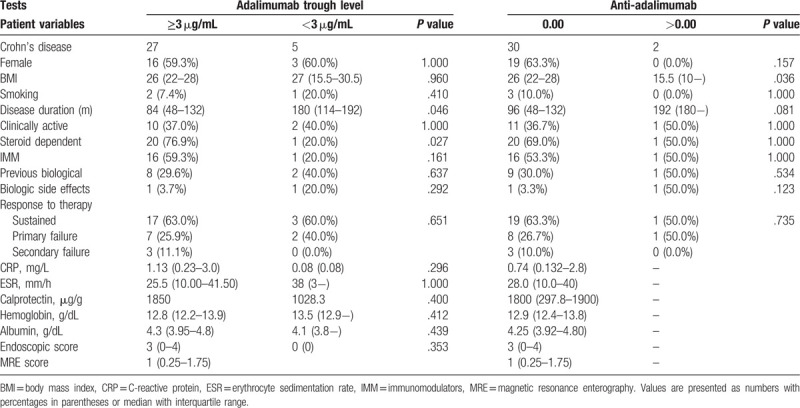
Table 3 shows that patients with lower serum adalimumab levels had a longer disease duration since diagnosis (P = .046). Lower body mass index (BMI) was significantly associated with higher levels of anti-ADA antibodies, despite the relatively small number of individuals in the study (P = .036). However, no significant difference was found between drug levels and their respective antibodies and the clinical responses presented by the patients.
Table 4 shows a series of correlations between numeric variables assessed by Spearman's rank correlation coefficient. A relatively weak negative correlation was found between BMI and serum infliximab level (CC = −0.292; P = .02). Serum CRP levels were also negatively correlated with infliximab (CC = −0.315; P = .033) but were positively correlated with anti-infliximab antibodies (CC = 0.327; P = .027). This means that patients with adequate serum levels of infliximab (high) present a satisfactory therapeutic response with reduced levels of inflammatory markers including serum CRP. In contrast, patients with low serum levels of infliximab had high CRP, and anti-infliximab antibodies were present. Serum albumin dosage was positively correlated with serum infliximab levels (CC = 0.379; P = .004) and adalimumab (CC = 0.699; P = .003).
Table 4.
Correlations between trough levels and antibodies with clinical, laboratory and imaging variables.

4. Discussion
In the last 2 decades, biological agents, particularly monoclonal anti-TNF-α antibodies, have become the mainstay of IBD therapy. However, in Brazil, this change has progressively occurred only in the last decade. Some side effects and a relevant rate of primary and secondary nonresponders have been reported and represent a critical limitation for the treatment of IBD patients. To address this issue, we proposed a pilot study to measure anti-TNF-α trough levels and immunogenicity, for the first time, in an area regarded as having low IBD prevalence, to investigate potential associations with specific disease outcomes. In addition, we evaluated whether anti-TNF-α trough levels and anti-drug antibodies are associated with parameters routinely used in the follow-up of patients.
Findings from several previous studies have shown that anti-TNF-α trough levels are consistently associated with a sustained clinical response[10] and mucosal healing.[11,13] The results from a recent study appear to corroborate this information, as the best clinical outcomes were associated with elevated infliximab trough levels, which were even higher than 10 μg/mL.[11] In agreement with a previous report,[19] we identified a correlation between serum infliximab and CRP concentrations, which indicates that patients with higher trough levels, especially within the therapeutic range, have concomitant low levels of inflammatory markers. However, fecal calprotectin, another noninvasive marker routinely used to assess the activity of intestinal inflammation in IBD, did not show a significant association with anti-TNF-α or anti-drug antibodies in this study. It is likely that the relatively small number of samples analyzed for fecal calprotectin in this study might have influenced the results. To identify other potentially useful laboratory biomarkers related to the response to therapy and/or to anti-TNF-α trough levels and anti-drug antibodies, we analyzed ESR, hemoglobin, and albumin. While results involving ESR and hemoglobin did not show any significant association with anti-TNF-α trough levels or anti-drug antibodies, patients who had higher serum albumin concentrations also had higher serum levels of infliximab and adalimumab. In accordance with our findings, a previous study identified a relationship between serum albumin and the pharmacokinetics of infliximab. In particular, patients with higher relative serum albumin concentrations maintained higher serum infliximab levels, reduced clearance, and longer half-lives compared to patients with low serum albumin.[20,21] Albumin is the most abundant protein in human plasma and has a well-established role in the transport and metabolism of drugs.[22] Albumin binds with high affinity to various drugs, whereas non-albumin-bound (“free”) drugs are more likely to be excreted because binding to the protein may decrease the rate of drug elimination.[22] In addition to its several critical physiological roles, albumin metabolism can also be affected by both chronic and acute disorders. In fact, low serum albumin concentration has been associated with poor outcomes in several health conditions and has been used as a marker of underlying pathologic processes, including malnutrition and inflammation.[23] Here, we reinforce the importance of albumin concentration as an adjuvant biomarker in the follow-up of chronic inflammatory disorders such as IBD and present for the first time its association with adalimumab, in addition to the already described relationship with infliximab levels.
Regarding the concomitant use of immunomodulators, it has been previously demonstrated that drugs such as azathioprine are capable of reducing immunogenicity, preventing the formation of anti-drug antibodies during biological therapy.[24,25] In fact, previous data have shown that combination therapy is more effective than azathioprine or infliximab monotherapy in achieving parameters of deep remission.[26] The results of this study appear to confirm the beneficial effect of this association in relation to infliximab but not adalimumab. Although the number of patients in this study on combination therapy with adalimumab is smaller than that with infliximab, our results appear to corroborate the findings from a recent investigation, which demonstrated that the clinical efficacy of a combination of adalimumab and azathioprine did not differ from that of adalimumab monotherapy in the long term.[27] Of note, in addition to potential effects on the clinical efficacy and development of anti-drug antibodies, in the present study, we also detected more side effects among patients with antibodies against infliximab. This finding appears to reinforce the notion that the beneficial effect of concomitant azathioprine during biologic therapy may not be directly synergistic but rather may indirectly increase serum levels of infliximab and/or prevent the formation of anti-drug antibodies.
In terms of individual characteristics, it is interesting to note that patients with a higher BMI had lower concentrations of serum infliximab, in agreement with previous studies in the literature.[21,28] In fact, previous pharmacokinetic studies analyzing anti-TNF-α therapies have demonstrated that higher body weight is consistently associated with increased drug clearance and lower trough serum levels.[29,30] In a study in which the influence of BMI on the response to biologic therapy was assessed in patients with UC, investigators identified a higher risk of surgery and hospitalization for each kg/mm2 increase in BMI. Such an association was observed in weight-based dose and fixed-dose treatments. In addition, in contrast to our results, a significant negative correlation was observed between BMI and adalimumab serum levels but not infliximab serum levels.[21] Nevertheless, in another study addressing BMI and biological therapy, obese patients with either CD or UC (BMI > 30) on infliximab had a higher risk of flares and loss of response to treatment compared to nonobese patients.[28] Our BMI findings appear to have important clinical implications because the prevalence of obesity is increasing rapidly in developing countries,[31] and rates appear to increase in parallel with IBD.[32,33] In this sense, physicians taking care of overweight and obese patients with IBD should be prepared to consider more aggressive treatments and the concomitant use of immunomodulators, in addition to the close monitoring of patients using biologic therapy and potentially directly targeting obesity via a multidisciplinary approach.
We acknowledge some limitations in our analysis, mostly due to the study design, reflecting the exploratory nature of the investigation. Although this work constitutes a pilot study, it highlights important aspects involving a comparative analysis of anti-TNF-α trough levels and immunogenicity related to therapy among patients from a low IBD prevalence area. Among the patients consecutively enrolled in this study, the majority had a sustained primary response. Interestingly, we present higher response rates (clinical remission) (65.9% CD and 70% UC) than most reports in the literature (overall average of 40%).[34] A possible explanation for this may rely on the fact that this study is a simple transversal analysis, considering that a single sample per patient may not be sufficiently consistent to predict the outcome. For example, patients with current low trough levels may not have manifested a loss of response yet. In contrast, patients whose samples already reveal the presence of anti-drug antibodies may still have a satisfactory response to the biologic agent in question. Moreover, some technical aspects also need to be addressed. For example, while sustained high levels of anti-drug antibodies usually lead to permanent loss of response, it has also been suggested that anti-drug antibodies may be transient and may not always result in permanent or progressive immunogenicity and worse clinical outcome. Therefore, it has been suggested that infliximab trough levels should be measured at week 14 and at the time of loss of response; whenever trough levels are undetectable or low, anti-infliximab antibodies should be assessed and followed-up to rule out sustained formation of anti-drug antibodies.[35] This represents a limitation of this pilot study, in which we present only a transversal analysis of the patients. Another controversial issue emerges from the question of whether the measurement of anti-infliximab antibodies by ELISA is reliable in the presence of serum infliximab. Thus, it has been proposed that samples with measurable serum infliximab should probably be considered inconclusive for anti-drug antibodies.[36] Although different study designs may render the data difficult to interpret and compare, it is likely that the specific characteristics of our cohort might have influenced the results.
Here, we speculate that the specificities of this study population may involve demographic features, including genetic background and epigenetic modifications as well as the possibility of a more naïve nature of the patients, with a relatively more recent use of biologic agents in general. It is also interesting to note that during the last decade, while the use of anti-TNF-α agents has become progressively more common in Brazil, mainly due to availability in the public health system, which covers approximately the entire population, availability of the respective monitoring tests has not followed in parallel. Therefore, further investigations analyzing local epidemiological aspects of IBD and the clinical response, including follow-up studies to address long-term remission, the occurrence of failures and adverse events, will be critically important. Moreover, it will also be crucial to investigate the cost-effectiveness of promoting monitoring trough levels and immunogenicity through the national health system. In this regard, not only direct costs but also indirect costs, including the need for surgery, additional medication, hospitalization, and temporary and permanent disability, would be of paramount importance for planning novel health policies for IBD in the country.
In conclusion, although the results obtained in this IBD cohort study do not show a clear correlation between anti-TNF-α trough levels and immunogenicity with disease outcomes, we confirmed significant associations with BMI, the concomitant use of immunomodulators, the rate of side effects, and laboratory markers, including serum albumin, and CRP. In particular, the results of this study reinforce the use of combination therapy for patients treated with infliximab, for initial reduction of side effects and to minimize the loss of response in the long term. Prospective controlled trials will be necessary to further investigate the most appropriate approaches to monitor patients under biologic therapy, particularly individuals who lose the response. Moreover, studies addressing the cost-effectiveness of monitoring trough levels and immunogenicity for patients with IBD under biological therapy will be critically important both at the individual level to implement more effective personalized medicine as well as at the population level to possibly influence public health strategies.
Acknowledgments
The authors thank the Brazilian research foundations CNPq and FAPERJ for their financial support.
Author contributions
Grinman AB and Souza MG participated in the conception and design of the study, the acquisition, analysis, and interpretation of data, and the drafting of the manuscript. Bouskela E, Carvalho ATP, and de Souza HSP participated in the conception and design of the study, obtained funding, analyzed, and interpreted data, and critically revised the manuscript for important intellectual content. All authors gave final approval of the submitted version of the manuscript.
Conceptualization: Eliete Bouskela, Ana Teresa P. Carvalho.
Data curation: Maria das Graças C. de Souza, Heitor S. de Souza.
Formal analysis: Eliete Bouskela, Heitor S. de Souza.
Funding acquisition: Heitor S. de Souza.
Investigation: Ana B. Grinman, Maria das Graças C. de Souza.
Methodology: Ana B. Grinman, Maria das Graças C. de Souza.
Project administration: Eliete Bouskela, Ana Teresa P. Carvalho.
Resources: Ana Teresa P. Carvalho.
Supervision: Eliete Bouskela, Ana Teresa P. Carvalho.
Validation: Maria das Graças C. de Souza, Eliete Bouskela, Ana Teresa P. Carvalho.
Visualization: Ana B. Grinman, Maria das Graças C. de Souza, Eliete Bouskela, Ana Teresa P. Carvalho.
Writing – original draft: Ana B. Grinman, Ana Teresa P. Carvalho.
Writing – review & editing: Heitor S. de Souza.
Heitor S. de Souza: 0000-0002-3647-7324.
Footnotes
Abbreviations: ADA = adalimumab, BMI = body mass index, CD = Crohn's disease, CRP = C-reactive protein, EIM = extraintestinal manifestations, ESR = erythrocyte sedimentation rate, IBD = inflammatory bowel disease, IFX = infliximab, IMM = immunomodulator, UC = ulcerative colitis.
How to cite this article: Grinman AB, Souza Md, Bouskela E, Carvalho AT, Souza HS. Clinical and laboratory markers associated with anti-TNF-alpha trough levels and anti-drug antibodies in patients with inflammatory bowel diseases. Medicine. 2020;99:10(e19359).
Supported by grants from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro-FAPERJ and Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq.
This work was supported by grants from the Brazilian Research Council (CNPq) (302401/2016-4) and the FAPERJ (Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro) (E26/202.781/2017).
This manuscript, including related data and tables, has not been previously published and is not under consideration elsewhere.
The authors have no conflicts of interest to disclose.
References
- [1].Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–17. [DOI] [PubMed] [Google Scholar]
- [2].Chachu KA, Osterman MT. How to diagnose and treat IBD mimics in the refractory IBD patient who does not have IBD. Inflamm Bowel Dis 2016;22:1262–74. [DOI] [PubMed] [Google Scholar]
- [3].Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature reviews. Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- [4].Pariente B, Pineton de Chambrun G, Krzysiek R, et al. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:1199–206. [DOI] [PubMed] [Google Scholar]
- [5].Steenholdt C. Use of infliximab and anti-infliximab antibody measurements to evaluate and optimize efficacy and safety of infliximab maintenance therapy in Crohn's disease. Dan Med J 2013;60:B4616. [PubMed] [Google Scholar]
- [6].Jiang W, Li X. Molecular analysis of inflammatory bowel disease: clinically useful tools for diagnosis, response prediction, and monitoring of targeted therapy. Mol Diagn Ther 2015;19:141–58. [DOI] [PubMed] [Google Scholar]
- [7].Vande Casteele N, Herfarth H, Katz J, et al. American gastroenterological association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57. e836. [DOI] [PubMed] [Google Scholar]
- [8].Doherty G, Katsanos KH, Burisch J, et al. European Crohn's and Colitis Organisation topical review on treatment withdrawal [‘Exit Strategies’] in inflammatory bowel disease. J Crohn's Colitis 2018;12:17–31. [DOI] [PubMed] [Google Scholar]
- [9].Mitrev N, Vande Casteele N, Seow CH, et al. Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;46:1037–53. [DOI] [PubMed] [Google Scholar]
- [10].Schmitz EM, van de Kerkhof D, Hamann D, et al. Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits. Clin Chem Lab Med 2016;54:1211–9. [DOI] [PubMed] [Google Scholar]
- [11].Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc 2016;14:550–7. e552. [DOI] [PubMed] [Google Scholar]
- [12].Kang B, Choi SY, Kim HS, et al. Mucosal healing in paediatric patients with moderate-to-severe luminal Crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohn's Colitis 2016;10:1279–86. [DOI] [PubMed] [Google Scholar]
- [13].Papamichael K, Rakowsky S, Rivera C, et al. Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment Pharmacol Ther 2018;47:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koga A, Matsui T, Takatsu N, et al. Trough level of infliximab is useful for assessing mucosal healing in Crohn's disease: a prospective cohort study. Intest Res 2018;16:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Greener T, Kabakchiev B, Steinhart AH, et al. Higher infliximab levels are not associated with an increase in adverse events in inflammatory bowel disease. Inflamm Bowel Dis 2018;24:1808–14. [DOI] [PubMed] [Google Scholar]
- [16].Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies-to-adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal 2013;78-79:39–44. [DOI] [PubMed] [Google Scholar]
- [17].Zittan E, Kabakchiev B, Milgrom R, et al. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn's disease. J Crohn's Colitis 2016;10:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut 2016;65:249–55. [DOI] [PubMed] [Google Scholar]
- [19].Roblin X, Marotte H, Leclerc M, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohn's Colitis 2015;9:525–31. [DOI] [PubMed] [Google Scholar]
- [20].Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- [21].Kurnool S, Nguyen NH, Proudfoot J, et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther 2018;47:1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang F, Bian C, Zhu L, et al. Effect of human serum albumin on drug metabolism: structural evidence of esterase activity of human serum albumin. J Struct Biol 2007;157:348–55. [DOI] [PubMed] [Google Scholar]
- [23].Vincent JL, Dubois MJ, Navickis RJ, et al. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Anna Surg 2003;237:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut 2007;56:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis 2017;23:650–60. [DOI] [PubMed] [Google Scholar]
- [26].Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naive patients with Crohn's disease – a SONIC post hoc analysis. Aliment Pharmacol Ther 2015;41:734–46. [DOI] [PubMed] [Google Scholar]
- [27].Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn's disease: a prospective, randomized trial. J Crohn's Colitis 2016;10:1259–66. [DOI] [PubMed] [Google Scholar]
- [28].Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2118–24. [DOI] [PubMed] [Google Scholar]
- [29].Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 2014;20:2247–59. [DOI] [PubMed] [Google Scholar]
- [30].Sharma S, Eckert D, Hyams JS, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn's disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis 2015;21:783–92. [DOI] [PubMed] [Google Scholar]
- [31].Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pringle PL, Stewart KO, Peloquin JM, et al. Body mass index, genetic susceptibility, and risk of complications among individuals with Crohn's disease. Inflamm Bowel Dis 2015;21:2304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nature reviews. Gastroenterol Hepatol 2017;14:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol 2009;104:760–7. [DOI] [PubMed] [Google Scholar]
- [35].Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. [DOI] [PubMed] [Google Scholar]
- [36].Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014;63:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


