Abstract
Aims/hypothesis
This study aimed to examine if beta-aminoisobutyric acid (BAIBA) is (i) secreted by skeletal muscle in humans during exercise, (ii) associated with insulin secretory function in vivo, and (iii) directly linked with acute glucose-mediated insulin release by pancreatic beta cells in vitro.
Methods
Following 2-weeks of single-leg immobilization, plasma BAIBA concentrations were measured in the brachial artery and the femoral veins of each leg in healthy male subjects, at rest and during two-legged dynamic knee-extensor exercise. During a 2-h hyperglycamic clamp, insulin secretory function and levels of plasma BAIBA were assessed in non-diabetic individuals, non-diabetic individuals following 24-h hyperglycemia and patients with type 2 diabetes. Direct effects of BAIBA on acute glucose-mediated insulin release were probed in INS-1832/3 cells under normal and ‘diabetes-like’ conditions. Finally, the effect of BAIBA on mitochondrial function was assessed in INS-1832/3 cells using extracellular flux analysis.
Results
(i) BAIBA is released from skeletal muscle at rest and during exercise under healthy conditions but is suppressed during exercise following leg immobilization, (ii) plasma BAIBA concentrations inversely associate with insulin secretory function in humans, (iii) BAIBA lowers mitochondrial energy metabolism in INS-1 832/3 cells in parallel with decreased insulin secretion
Conclusion/interpretation: BAIBA is a myokine released by skeletal muscle during exercise and indepedantly alters the triggering pathway of insulin secretion in cultured INS-1832/3 cells.
Keywords: Exercise, Muscle contraction, Myokine, Type 2 diabetes, Glucolipotoxicity, Pancreatic beta-cell, BAIBA
Abbreviations: BAIBA, beta-aminoisobutyric acid; T2D, type 2 diabetes; RPMI, Roswell Parks Memorial Institute; HEPES, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); β-cell, pancreatic beta-cell; BSA, bovine serum albumin; GSIS, glucose-stimulated insulin secretion; DAPI, 4′,6-disamidino-2-phenylindole
1. Introduction
Circulating biomarkers can identify exploitable targets to prevent and/or treat disease. Chronic hyperglycemia is associated with the failure of insulin secretion in type 2 diabetes [1]. Understanding the involvement of specific circulating factors that regulate changes in insulin secretory function will help to advance our understanding of diabetes pathophysiology. β-Aminoisobutyric acid (BAIBA), also known as 3-amino-2-methylpropanoic acid, is a nonproteinogenic amino acid. It is a known catabolite of thymine and valine metabolism in mammals. Recent work has identified BAIBA as a potential myokine - a cytokine or other peptide, produced, expressed and released by muscle fibers which exerts paracrine or endocrine effects. Specifcially in 2014 Roberts and colleagues showed that BAIBA is secreted from primary muscle cells and increased by 20% in mice with access to a running wheel and elevated by 17% following 20 weeks of aerobic exercise in sedentary and healthy subjects [2]. Morover supplementation of BAIBA increases the expression of brown adipocyte-specific genes in white adipose tissue, increases hepatic β-oxidation and leads to a decrease in body fat in mice [2]. These findings suggest that enhanced plasma BAIBA could have important systemic functions.
The role of BAIBA as a systemic mediator has been supported by several other studies, recently reviewed by Tanianskii et al. (2019) [3]. Briefly, in 2018, Kitase and colleagues confirmed BAIBA secretion ex vivo in perfused mouse extensor digitorum longus and isolated soleus muscles [4]. Shi et al. showed that BAIBA prevents hepatic ER-stress in vitro and in mouse models [5]. In humans, BAIBA is a marker of fatty acid β-oxidation which inversely associates with cardiometabolic risk factors [2,6]. In relation to diabetes, treatment with BAIBA in mice has been shown to avert diet-induced obesity and improve glucose metabolism in streptozotocin- and diet-induced diabetes [5,7,8]. Furthermore, intracellular BAIBA concentrations are negatively associated with first-phase insulin secretion in the clonal 832/13 beta-cell line [9]. Based on many of these studies it has been hypothesized that muscle-derived BAIBA could be a mechanistic component of the established beneficial effects of physical exercise in chronic metabolic disease [10,11]. Importantly, however, this has yet to be confirmed in humans and evidence for an acute exercise effect on BAIBA secretion, which is more consistent with what we understand about myokine release, is lacking. In fact, Morales et al. (2017) failed to demonstrate acute changes in plasma BAIBA concentration following a 350 kcal exercise at 70% of VO2 max [12]. In contrast and more recently, Stautemas et al. (2019) were the first to demonstrate elevations in plasma BAIBA levels by acute aerobic exercise in humans [13]. Interestingly, plasma and urine BAIBA levels primarily consist of R-BAIBA, which is derived from thymine metabolism in the cytosol of liver and kidney cells [14]. On the other hand, S-BAIBA, which is derived from valine in the mitochondria of primarily skeletal muscle, only constitutes 2% of total plasma BAIBA [13]. That being said, in relation to the suggested role of BAIBA as a myokine, both R- and S-BAIBA are elevated by acute exercise [13].
BAIBA is detectable in plasma and urine and its concentration is influenced by single nucleotide polymorphisms in the alanine-glyoxylate aminotransferase 2 (AGXT2) gene [15]. In vitro and in vivo murine studies have shown that downregulation of AGXT2 subsequently alters BAIBA concentration [16]. Given the strong association between an increased risk of type 2 diabetes in humans and polymorphisms in the hepatic nuclear factor 4α gene (HNF4A) [17], a major regulator of AGXT2 expression [16], such observations provide possible further evidence for a role of BAIBA in diabetes [2,9]. Since BAIBA is secreted by primary muscle cells and intracellular BAIBA concentrations are inversely related to first phase insulin secretion in vitro, in this paper we conducted a series of experiments to examine (i) the effect of acute exercise on BAIBA release from healthy and immobilized skeletal muscle in healthy humans, (ii) the relationship between plasma BAIBA levels and insulin secretion in non-diabetic individuals with and without acute hyperglyceamia and patients with type 2 diabetes, and (iii) the effect of exogenous BAIBA exposure on acute insulin release by pancreatic beta-cells in response to glucose under healthy and pathophysiological conditions.
2. Materials and methods
2.1. BAIBA release by healthy and immobilized human skeletal muscle during acute exercise
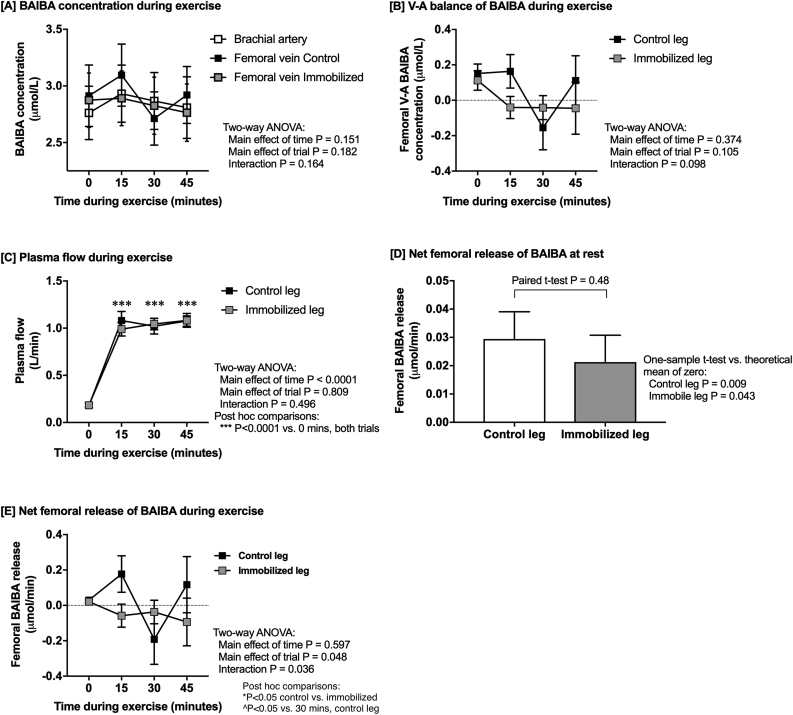
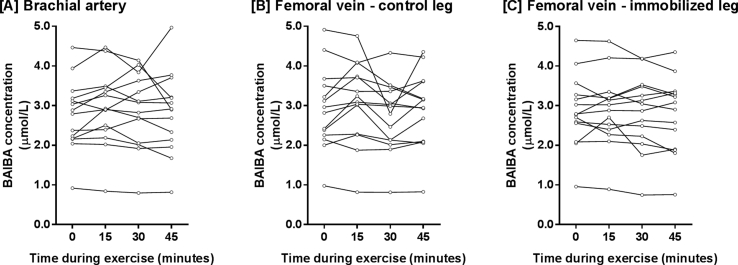
Plasma samples collected by arterio-venous (A-V) balance methodology in a study described in detail in Ref. [18] were assessed for BAIBA using a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Scientific) coupled to an Ultimate 3000 UHPLC (Thermo Scientific). A-V balance methods were used to assess specific metabolite changes directly from skeletal muscle, since the A-V balance method measures metabolite changes across a single tissue. Thus if a change in a metabolite is observed then the flux of that metabolite into or out of the tissue in question has changed. The study was approved by the Ethical Committee of the Capital Region of Denmark (H-4-2010-85). In brief, N = 14 young healthy males (age 23 ± 1 y, BMI 24.0 ± 0.7 kg/m2, VO2max 47.6 ± 1.7 mL/kg/min) completed 14-days of single-leg immobilization (an experimental model for local acute insulin-insensitivity [[19], [20], [21], [22], [23]]) before coming to the lab following an overnight fast. Lines were placed in a brachial artery and the femoral veins in both legs. Blood samples were collected at baseline and every 15-min during a 45-min two-legged dynamic knee-extensor exercise at 50% of pre-determined maximal power output to mimic a moderate-intensity bout, known to induce a change in metabolism and up-regulate key exercise-related signalling events in skeletal muscle. Plasma samples were separated and stored at −80 °C until measurement of BAIBA concentrations by liquid chromatography/mass spectrometry (see Supplemental Methods for full methodological details and Supplemental Fig. S1 for individual BAIBA levels). Leg blood flow was measured by Doppler ultrasound at the same time points and femoral net release of BAIBA in both legs was calculated using the Fick principle, as femoral venous BAIBA concentration minus arterial concentration multiplied by plasma flow (calculated as blood flow multiplied by 1 – hematocrit fraction).
2.2. Circulatory plasma BAIBA levels and insulin secretory function of healthy individuals with or without acute hyperglycemia and patients with type 2 diabetes
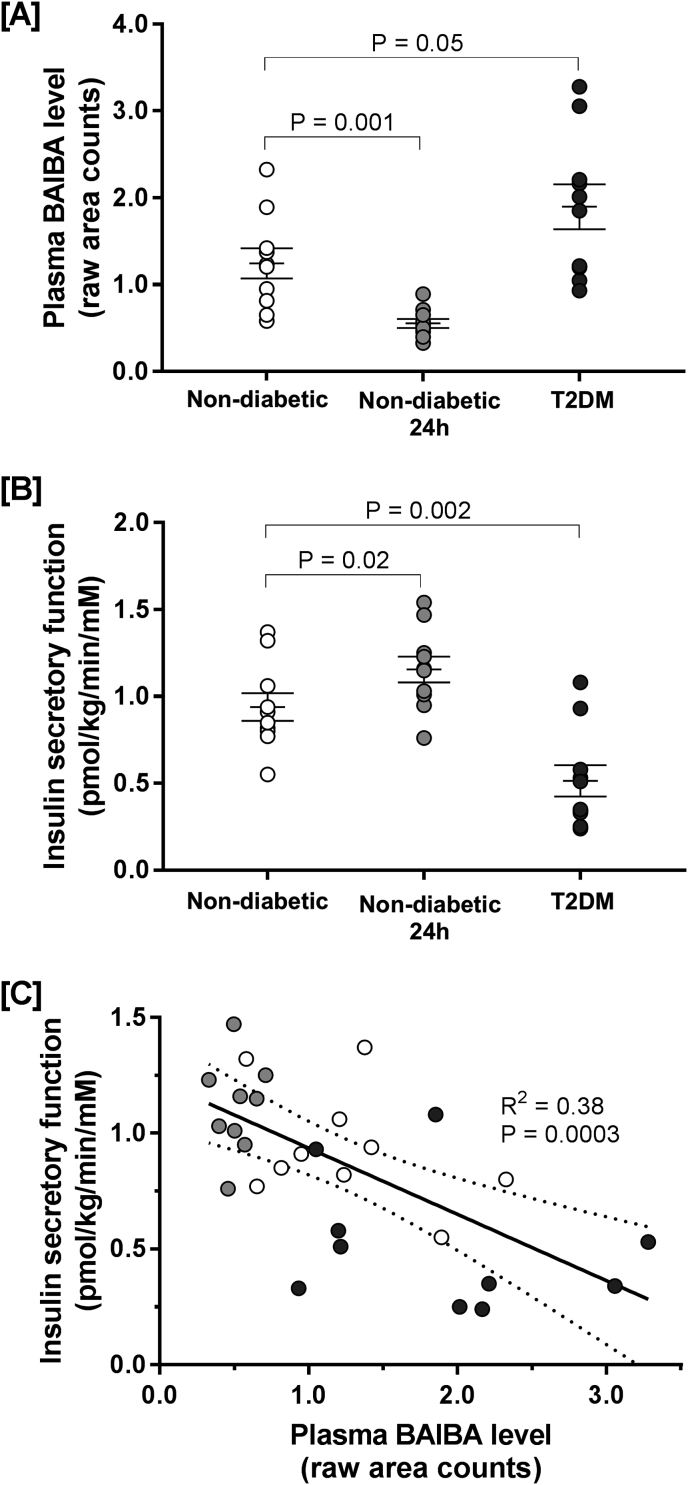
Plasma samples previously collected in a study described in detail in Ref. [24] were used for metabolomic analyses to search for circulating metabolites associated with pancreatic beta-cell insulin secretion. The study was approved by the Ethical Committee of the Capital Region of Denmark (H32010127) and registered on clinicaltrials.gov (NCT01375270). In brief, we recruited N = 10 non-diabetic individuals (2 female, 8 male, age 56 ± 3 y, BMI 31.3 ± 1.2 kg/m2, HbA1c 37.7 ± 1.1 mmol/mol) and N = 10 age- and BMI-matched individuals with type 2 diabetes (T2D; 2 female, 8 male, age 56 ± 3 y, BMI 30.0 ± 1.2 kg/m2, HbA1c 51.2 ± 2.3 mmol/mol, time since diagnosis 5 ± 1 y). Following an overnight fast, insulin secretory function (insulin secretion rate derived from C-peptide deconvolution, divided by plasma glucose) was measured during a 2-h hyperglycemic clamp at 5.4 mM glucose above basal glucose levels (4.5–5.5 mM), venous samples were collected and plasma stored at −80 °C until metabolomic profiles were analyzed liquid chromatography/mass spectrometry and gas chromatography/mass spectrometry (full methodological details are available in the Supplemental Methods). In non-diabetic subjects, the hyperglycemic clamp was extended to 24-h to replicate an acute model of hyperglycemia. Insulin secretory function was re-measured after 24-h and further plasma samples were collected for metabolomic analysis.
2.3. Insulin secretion by INS-1832/13 cells
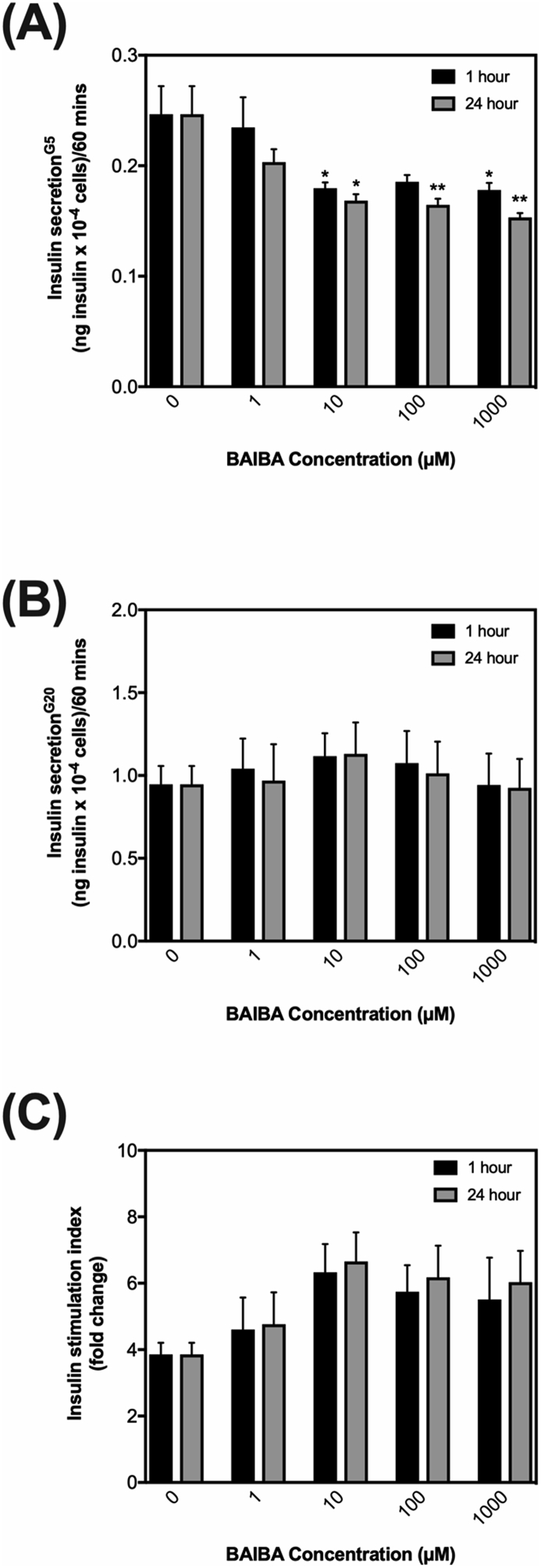
INS-1832/3 cells grown in RPMI media containing 5 mM glucose, 5% (v/v) FBS, 10 mM HEPES, 1 mM sodium pyruvate, 50 units/ml penicillin, 50 μg/mL streptomycin, 50 μM 2-mercaptoethanol and 1x GlutaMAX™ (ThermoFisher, Massachusetts, USA) were seeded at 8 × 104 cells/well in 96-well microplates and incubated in the presence or absence of sodium palmitate (20 nM) at different glucose concentrations (5, 11, or 20 mM) for 24 h (Note: Palmitate was conjugated to bovine serum albumin (BSA) at a molar ratio of 7:1, yielding an unbound free fatty acid concentration of 20 nM as estimated assuming binding parameters reported by Huber et al. [25]). Cells were then treated with or without BAIBA (DL-3-Aminoisobutyric Acid, A0281, >98% purity, Tokyo Chemical Industry, Oxford, UK) at 0, 1, 10, 100 or 1000 μMole/L for 1 or 24 h. After treatment with BAIBA, insulin secretion was measured under 5 mM or 20 mM glucose as described previously [26]. Insulin was assessed by homogenous time-resolved fluorescence (#62IN1PEG, Cisbio Bioassays, France). Absolute insulin secretion concentrations were normalized to cell density using 4′,6-diamidino-2-phenyl-indole (DAPI) fluorescence as previously described [26].
2.4. Mitochondrial respirometry of INS-1832/3 cells
INS-1832/3 cells seeded at 8 × 104 cells/well in XFe96 V3-PS well plates (Agilent Technologies, Santa Clara, California, USA) were treated with or without BAIBA (10 μMole/L) for 1 h and assessed for mitochondrial respiration using a Seahorse XFe96 Extracellular Flux Analyser (Agilent Technologies, Santa Clara, California, USA) as previously described [27]. Mitochondrial oxygen uptake rates were normalized to cell density using DAPI fluorescence as previously described [26]. For clarity, the methodological design of the respirometry experiment is described in the Supplemental Methods and Supplemental Fig. S2.
2.5. Statistics
In human study 1, two-way ANOVA determined the effect of time (baseline, 15, 30, 45 min) and trial (control vs. leg immobilization) on plasma BAIBA, plasma flow, and net balance, with Tukey’s post hoc comparisons where appropriate. D’Agostino & Pearson tests were used to test for normality and F-tests were used to test for homogeneity of variance. Data diverging from parametric assumptions were log-transformed before analysis (in cases where negative raw values were present, a constant was added to all values so they were all positive before log-transformation). In human study 2, paired t-tests were used to compare means between baseline and 24 h in healthy subjects, unpaired t-tests were used to compare means between healthy and T2D groups, and linear regression analysis was used to explore the relationship between plasma BAIBA and insulin secretory function. The effect of BAIBA vs. vehicle on in vitro insulin secretion and mitochondrial respiration was examined using paired t-tests or one-way ANOVA with Bonferroni post hoc comparisons, where appropriate. The authors who measured plasma BAIBA concentrations (DO & WD) and the author who completed the statistical analysis (TS) were blinded to the trial identity of the samples in human study 2. Trial IDs were decoded (by FD) following statistical analysis. Data indicate mean ± SEM and statistical significance was accepted when P ≤ 0.05.
3. Results
3.1. Femoral BAIBA release differs between healthy and immobilized skeletal muscle during acute exercise
Using femoral arterial-venous balance methodology, we examined to what extent BAIBA is released from exercising human skeletal muscle and whether single leg immobilization (a model of local acute insulin-insensitivity) influences BAIBA release. Following 2-weeks of single-leg immobilization, BAIBA levels were measured in blood plasma taken from the brachial artery and femoral vein of each leg in healthy male subjects, at rest and during two-legged dynamic knee-extensor exercise. The time course of change in plasma BAIBA concentration, the venous minus arterial net balance for BAIBA, and plasma flow are shown in Fig. 1A–C. The pattern of femoral A-V BAIBA release and uptake during exercise was significantly different between control and immobilized legs (Fig. 1B), however this does not control for plasma blood flow, which increases significantly in the imobilised and control legs during exercise. Of note, no signficiant differences in plasma blood flow at rest nor during exercise were observed between the imobilised and control legs - P = 0.809 (Fig. 1C). To calculate the femoral net release of BAIBA (Fig. 1E) the venous minus arterial net balance was multiplied by plasma flow (Fig. 1C). At rest, there was a statistically significant net release of BAIBA in both legs when compared with a theoretical mean of zero (Fig. 1D). Of importance, at rest, leg immobilization had no significant effect on the net release of BAIBA (Fig. 1D). However, during exercise, a different pattern of BAIBA release occurred between the control and immobilized leg, with a net uptake of BAIBA in the immobilized leg (Fig. 1E - grey symbols) and a pulsatile pattern of release and uptake of BAIBA in the control leg (Fig. 1E - black symbols). The pulsatile pattern of BAIBA release and uptake during exercise in the control leg (Fig. 1E – black symbols) occurred due to a net release of BAIBA from 0.02 μMole/L to 0.2 μMole/L BAIBA after 15 min of exercise and then a net uptake of BAIBA from 0.2 μMole/L BAIBA to −0.2 μMole/L BAIBA during the next 15 min of exercise and finally an increase in BAIBA release during the final 15 min of exercise from −0.2 μMole/L BAIBA to 0.1 μMole/L BAIBA. The mean net uptake and release across all time points in the control leg were significantly different from the immobilized leg (Fig. 1E). Importantly the drop in the net femoral release of BAIBA after 30-min of exercise in the control leg (Fig. 1E) was not induced by sample dilution since no decreases were observed at this time point for hematocrit, IL-6, or FFAs (data not shown). The decrease in the net femoral release of BAIBA at 30-min in the control leg was also not explained by a single outlier because this pattern is evident in several subjects (Supplemental Fig. S1).
Fig. 1.
The effect of leg immobilization on femoral arterial-venous BAIBA net balance. The femoral arterial-venous net balance was determined for BAIBA in N = 14 healthy individuals at rest and during 45-min of two-legged dynamic knee-extensor exercise (at 50% Wmax) following 2-weeks of single-leg immobilization. Plasma BAIBA concentrations (panel A), venous minus arterial BAIBA concentrations (panel B), plasma flow (panel C), and net femoral release of BAIBA at rest (panel D) and during exercise are displayed as mean averages ± SEM.
3.2. Circulatory plasma BAIBA inversely correlates with the rate of insulin secretion in humans in vivo
Fasting circulatory plasma BAIBA was significantly higher in individuals with type 2 diabetes compared with non-diabetic controls (1.90 ± 0.26 vs. 1.25 ± 0.17 raw area counts; Fig. 2A). When non-diabetic controls were exposed to acute (24hr) hyperglycemia, circulating plasma BAIBA levels were significantly lowered from 1.25 ± 0.17 to 0.55 ± 0.05 raw area counts (Fig. 2A). Unsurprisingly, the rate of insulin secretion was significantly lower in individuals with type 2 diabetes compared to non-diabetic controls (Fig. 2B), while exposure to acute (24hr) hyperglycemia significantly elevated insulin secretion in non-diabetic individuals (Fig. 2B). By plotting the rates of insulin secretion as a function of plasma BAIBA levels, it becomes apparent that the level of circulatory plasma BAIBA inversely correlates with the rate of insulin secretion (Fig. 2C).
Fig. 2.
The relatonship between plasma BAIBA concentration and insulin secretory function in humans. Panel A: BAIBA (identified and measured by metabolomic profiling) was measured in plasma samples collected from N = 10 non-diabetic individuals following an overnight fast (non-diabetic; white circles) and after 24-h of exposure to acute experimental hyperglycemia (non-diabetic 24 h; grey circles), and following an overnight fast in N = 10 age- and BMI-matched individuals with type 2 diabetes (T2DM; black circles). Panel B: Insulin secretory function (insulin secretion rate divided by plasma glucose) was also measured in the same subjects. Panel C: the relationship between plasma BAIBA concentrations and insulin secretory function. Individual subject data points are plotted on each panel. Lines on panels A and B represent mean averages ± SEM.
3.3. BAIBA directly mediates insulin secretion by INS-1832/13 cells
Given the link between plasma BAIBA levels and insulin secretion in vivo, we next explored if exogenous BAIBA alters glucose-mediated insulin secretion directly from pancreatic beta-cells (β-cell) in vitro using the β-cell line, INS-1832/3. We exposed INS-1832/3 cells to a range of different BAIBA concentrations (0, 1 μM, 10 μM, 100 μM and 1000 μM) for 1 or 24 h and investigated its effect on acute glucose-mediated insulin release at a submaximal insulin stimulating glucose concentration (5 mM) and maximal insulin stimulating glucose concentration (20 mM). Exogenous BAIBA significantly decreased insulin release mediated by 5 mM glucose at concentrations between 10-1000 μM at both 1 and 24 h of exposure (Fig. 3A). Exogenous BAIBA had no significant effect on acute insulin release mediated by 20 mM glucose (Fig. 3B). Exogenous BAIBA appears to increase the fold change between insulin secreted by 5 vs 20 mM glucose, referred to as the insulin stimulation index (Fig. 3C). However, it is important to note that this apparent increase is not statistically significant and is related to the lowering effect of BAIBA on insulin release mediated by 5 mM glucose (Fig. 3A).
Fig. 3.
The effect of BAIBA treatment on insulin release by insulin-secreting INS-1832/3 cells. Cells were exposed to culture medium containing either 0, 1, 10, 100 or 1000 μM BAIBA for 1 h (black bars) or 24 h (grey bars) and assessed for insulin release by 5 mM (Panel A) or 20 mM glucose (Panel B). The insulin stimulation index was calculated by assessing the fold change in insulin secreted from 5 mM glucose. (GSIS) above 5 mM glucose (panel A) was calculated as the fold change in absolute insulin release from 5 mM to 20 mM glucose (Panel C). Data represent means ± SEM from 3 to 5 independent experiments with each condition replicated 3–5 times in each experiment. ∗,∗∗,∗∗∗ indicates P ≤ 0.05, P < 0.01 or P < 0.001 vs. control cells without BAIBA treatment unless otherwise shown.
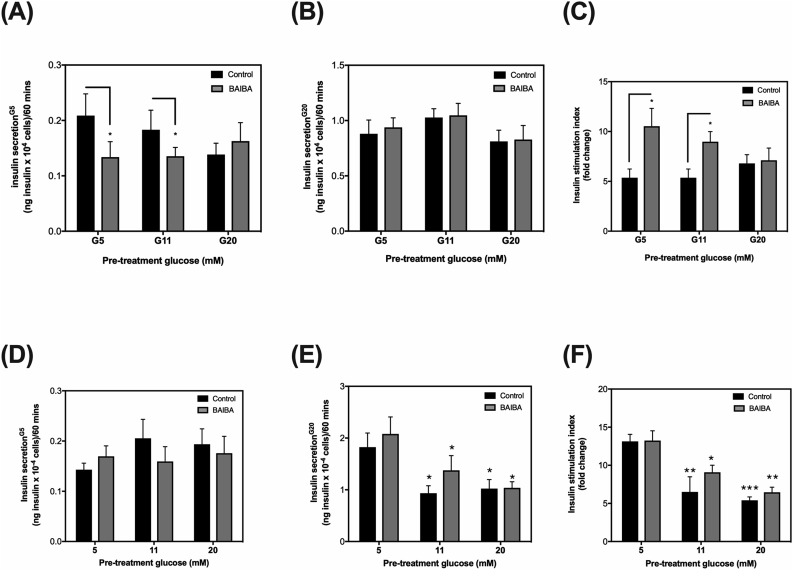
Given that BAIBA altered acute glucose-mediated insulin release by INS-1832/3 cells grown under 5 mM glucose, we next compared the effect of 1 h BAIBA exposure on acute glucose-mediated insulin release of INS-1832/3 cells under “acute hyperglycemic-like” conditions (24-h pre-incubation in culture medium with 5, 11- or 20-mM glucose) to mimic our model of acute hyperglycemia in humans (Fig. 2). Under these conditions, we used a single dose of 10 μMole/L BAIBA for 1 h as this was the lowest concentration and time frame that exerted an effect on insulin secretion in ‘healthy INS-1832/3 cells’ (Fig. 3A). Consistent with our previous observation (Figs. 3A), 10 μMole/L BAIBA significantly lowered acute glucose-mediated insulin release of INS-1832/3 cells challenged with 5 mM glucose (Fig. 4A), but not 20 mM glucose (Fig. 4B). In INS-1832/3 cells grown in culture media containing 11 or 20 mM glucose for 24 h – an in vitro model of acute hyperglycemia, 1 h exposure to 10 μMole/L BAIBA lowered acute 5 mM glucose-mediated insulin release by cells grown with 11 but not 20 mM glucose (Fig. 4A) and had no significant effect on acute 20 mM glucose-mediated insulin release (Fig. 4B). Interestingly, in cells grown in media with 5 or 11 mM glucose, BAIBA almost doubled the insulin stimulation index from around 5 to around 10 which was completely blunted in cells grown in culture media with 20 mM glucose (Fig. 4C). Importantly, this apparent increase in the insulin stimulation index in cells exposed to BAIBA (Fig. 4C) is owing to a decrease in insulin release under basal conditions (Fig. 4A). It should also be noted that our experimental hyperglycemic model of incubating INS-1832/3 cells to either 11 mM or 20 mM glucose for 24 h induced no significant change in the insulin stimulation index nor acute insulin release mediated by glucose when compared to cells grown under 5 mM glucose (Fig. 4A–C).
Fig. 4.
The effect of BAIBA treatment on insulin release by insulin-secreting INS-1832/3 cells exposed to experimental ‘diabetes-like conditions’. Cells were pre-exposed to diabetes-like conditions for 24-h: 5 mM, 11 mM or 20 mM glucose in the absence (Panels: A-C) or presence of palmitate (Panels: D-F), and then treated with (grey bars) or without (black bars) 10 μM BAIBA for 1 h. The insulin stimulation index (C and F) was assessed as the fold change in insulin secreted from 5 mM glucose to 20 mM glucose. Data represent means ± SEM from 3 to 5 independent experiments with each condition replicated 3–5 times in each experiment. ∗,∗∗,∗∗∗ indicates P ≤ 0.05, P < 0.01 or P < 0.001 vs. G5 control cells without BAIBA treatment unless otherwise shown.
To further understand if BAIBA alters insulin secretion by β-cells under type 2 diabetes-like conditions, we next compared the effect of 1 h BAIBA exposure on acute glucose-mediated insulin release by INS-1832/3 cells exposed to glucolipotoxic conditions - 24 h palmitate plus 5, 11 or 20 mM glucose. In cells pre-exposed to 5 mM glucose plus palmitate, the decreasing effect of BAIBA on acute 5 mM glucose-mediated insulin release (Fig. 4A) is abolished, in fact, in palmitate treated cells, BAIBA modestly increases insulin release from 0.14 (ng insulinx10−4 cells/60 min) to 0.17 (ng insulin × 10−4 cells/60 min) (Fig. 4D). In INS-1832/3 cells pre-exposed to 11 mM or 20 mM glucose plus palmitate, acute 5 mM glucose-mediated insulin release was not altered by BAIBA (Fig. 4D). Consistent with our findings in Fig. 4B, BAIBA has no significant effects on acute 20 mM glucose-mediated insulin release by cells pre-exposed to 5 mM, 11 mM or 20 mM glucose plus palmitate (Fig. 4E). In line with these observations, BAIBA had no significant effect on the insulin stimulation index of cells pre-exposed to 5 mM, 11 mM or 20 mM glucose plus palmitate (Fig. 4F).
3.4. BAIBA lowers mitochondrial energy metabolism of INS-1832/3 cells
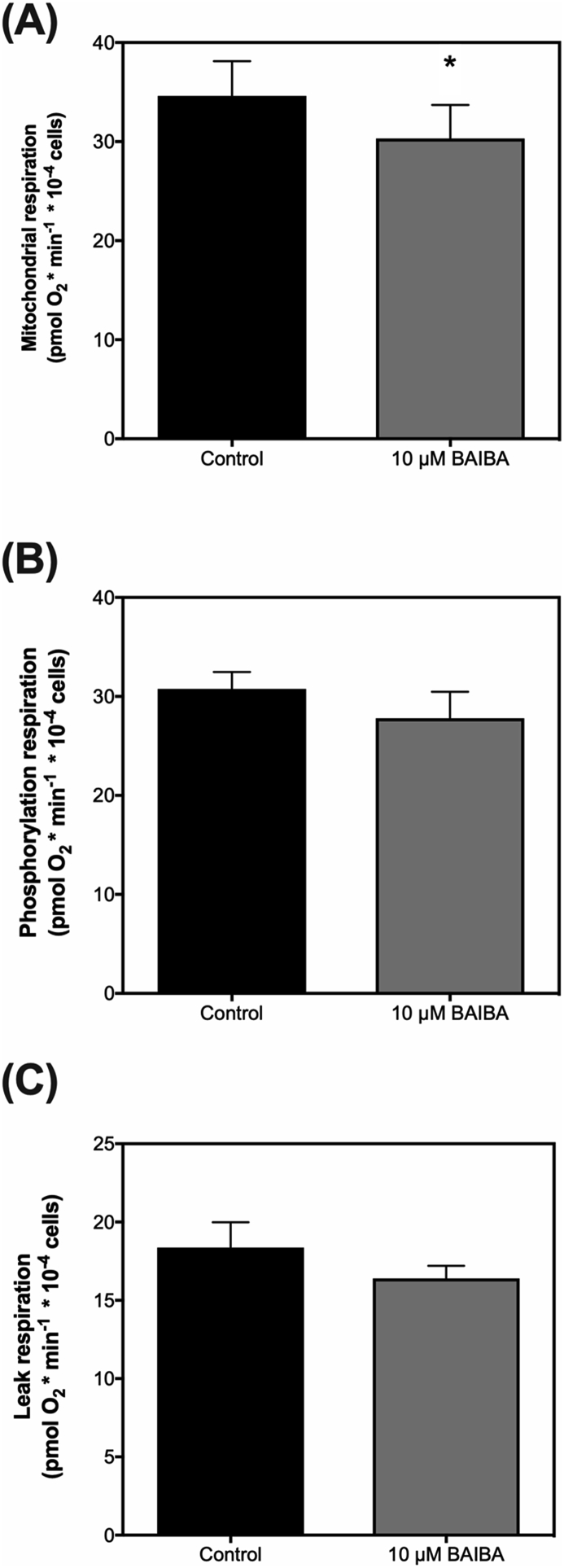
Mitochondrial energy metabolism tightly regulates the triggering of insulin secretion in β-cells through direct control over the phosphorylation potential. Thus any change in the triggering of insulin secretion by β-cells would associate with changes in mitochondrial respiration. Therefore to examine if the effect of BAIBA on acute insulin release by INS-1832/3 cells (Fig. 4A), is linked with changes in the triggering pathway of insulin secretion, we next probed mitochondrial energy metabolism of INS-1832/3 cells using Seahorse Extracellular Flux Analysis. In corroboration with the effect of BAIBA on acute insulin release (Fig. 4A), extracellular flux analysis revealed that BAIBA significantly lowers mitochondrial oxygen consumption rate from 49 (pmol O2∙min−1∙10−4 cells) to 44 (pmol O2∙min−1∙10−4 cells) in INS-1832/3 cells (Fig. 5A). In line with the effect on absolute mitochondrial oxygen consumption rate, ATP coupled (phosphorylation) respiration (Fig. 5B) and oligomycin-resistant (leak) respiration (Fig. 5C) were also modestly lowered by BAIBA treatment.
Fig. 5.
Mitochondrial respiratory activity of insulin-secreting INS-1832/3 cells exposed to BAIBA. Cells incubated in serum-free RPMI culture medium containing ±10 μM BAIBA for 1 h were assessed for mitochondrial respiratory activity using seahorse extracellular flux analysis. Mitochondrial oxygen uptake rates were examined in assay media containing 5 mM glucose, G5 (Panel A). Mitochondrial respiration linked to ADP-phosphorylation (measured from the amount of mitochondrial oxygen uptake inhibited by the ATP synthase inhibitor oligomycin; Panel B), and mitochondrial respiration linked with proton leak (measured from the amount of mitochondrial oxygen uptake resistant to the ATP synthase inhibitor oligomycin; Panel C) were also calculated from the raw trace. Data represent means ± SEM from 3 separate experiments spread across 3 experimental days with each condition repeated 4–5 times in each individual experiment. ∗ indicates P ≤ 0.05 vs. control cells without BAIBA treatment.
4. Discussion
This is the first study to demonstrate a net release of BAIBA from skeletal muscle at rest and during leg-kicking exercise (Fig. 1). We show that following leg immobilization (an in-vivo model of inactivity-induced insulin resistance), exercise-induced release of BAIBA is prevented and net uptake occurs (Fig. 1). Together these data may indicate that the release of BAIBA by skeletal muscle during exercise is linked with skeletal muscle insulin sensitivity. Of particular interest was the pulsatile pattern of BAIBA release and uptake that occurred in the control leg during exercise (Fig. 1E). The pulsatile pattern of release and uptake in the control leg appeared to occur mainly due to a significant uptake of BAIBA after 30 min of exercise which was apparent in 10 of the 14 subjects (Supplemental Fig. S1). These data may suggest that exercise causes pulsatile metabolism of intermediates that release BAIBA for example branch-chain amino acids or thymine, however no evidence currently exists to prove this. To our knowledge this is the first study to show evidence for such metabolic pulsatility in skeletal muscle. However, it is important to note that the pulsatile pattern of release and uptake of BAIBA was only explored during 45 min of leg extension exercise in this study. Therefore further investigation is warranted to understand the kinetics of BAIBA release during different kinds of exercise over more varied durations and to exclude the possibility that a metabolic phenomenom occurred during our acute leg extension exercise study.
Given that skeletal muscle, as shown here, and the liver, as shown previously [14], are key organs for BAIBA release, it was interesting to find that insulin secretory function in humans with and without acute hyperglyceamia and type 2 diabetes inversley correlated with whole body plasma levels of BAIBA (Fig. 2). Consistently, in vitro, we found that BAIBA lowered insulin release by INS-1832/3 cells at a submaximal insulin stimulating glucose concentration concomitantly with a decrease in mitochondrial energy metabolism (Fig. 4, Fig. 5). Further analysis revealed that the lowering effect of BAIBA on acute glucose (5 mM) mediated insulin release is abolished by pre-exposure of cells to 20 mM glucose and/or palmitate (Fig. 4). Collectively, our data provide evidence that (i) BAIBA is released directly by human skeletal muscle, (ii) BAIBA release by human skeletal muscle during exercise is blunted in a model of insulin-resistance, (iii) whole body plasma levels of BAIBA inversely correlate with plasma insulin in non diabetic individuals with and without acute hyperglyceamia and in patients with type-2 diabetes, and (iv) BAIBA mediates acute glucose-induced insulin release by pancreatic beta-cells in vitro.
To date, the therapeutic benefits of BAIBA have been attributed to suppress inflammation, improve insulin sensitivity of skeletal muscle, improve insulin signalling in mice with type 2 diabetes and browning of white fat [2,5]. Except for some human plasma measurements showing that BAIBA inversely correlates with cardiometabolic risk factors and increases during prolonged and acute exercise [2,13], previous data is largely limited to rodents. In our first set of experiments we demonstrate that human skeletal muscle is a source for the net release of BAIBA, both at rest and during leg-kicking exercise (Fig. 1). Moreover, following leg immobilization in healthy individuals (Fig. 1D), the net release of BAIBA during exercise is abolished (Fig. 1E). Given that our model of leg immobilization has been shown previously to lower insulin sensitivity and glucose tolerance [28,29], these data indicate that exercise may only mediate BAIBA release from insulin-sensitive muscle in healthy individuals. However, femoral A-V balance does not directly probe metabolite release from skeletal muscle but represents changes in muscle, adipose, and endothelial cells, thus we assume that the change is related to muscle because it is the largest organ in the femoral bed. Moreover, we cannot exclude the possibility that BAIBA release and/or uptake occurs from other tissues during exercise or from skeletal muscle following chronic physical inactivity or metabolic disease. In fact, measuring plasma BAIBA concentrations in non-diabetic and type-2 diabetic patients showed that fasting plasma BAIBA is elevated in patients with type 2 diabetes and suppressed by acute experimental hyperglycaemia (Fig. 2A). Given that whole body plasma BAIBA levels are elevated at rest in type-2 diabetes patients (Fig. 2), it is probable that either BAIBA release or reduced BAIBA uptake occurs in individuals with lower insulin sensitivity. That said, the exact organs responsible for the changes in BAIBA release and/or uptake during acute and prolonged insulin-resistance was not explored in this study and requires further investigation. Morover, the extent by which exercise alters this, if at all, still remains to be understood.
More interestingly, and possibly more relevant to diabetes research, data from human study 2 highlights an inverse relationship between plasma BAIBA concentration and plasma insulin concentration (Fig. 2C). For example, during acute hyperglycaemia, when insulin secretion is exacerbated (Fig. 2B), plasma BAIBA levels were lower than healthy controls (Fig. 2A). On the other hand, in patients with type 2 diabetes that have impaired insulin secretion (Fig. 2B), plasma BAIBA levels were notably higher than controls (Fig. 2A). Although we cannot conclude from these data if the change in baseline plasma BAIBA level is a result of changes in muscle net balance. That said, our leg immobilization data (Fig. 1) raise the possibility that the decrease in plasma BAIBA level following 24-h hyperglycaemia (Fig. 2A) may be related to changes in muscle net balance.
To understand if BAIBA influences insulin secretion directly, we showed that BAIBA lowers acute insulin release by normal INS-1832/3 cells when challenged with 5 mM glucose (Fig. 3, Fig. 4B). These data corroborate our findings in vivo that show an inverse relationship between BAIBA level and insulin secretory function (Fig. 2). Interestingly, the effect of BAIBA on acute insulin release was only apparent in cells challenged with a submaximal insulin-stimulating glucose concentration (Fig. 3, Fig. 4B), suggesting that the effect of BAIBA on insulin release is overcome by a high glucose challenge in normal cells. Moreover, the effect of BAIBA on acute insulin release was abolished by (gluco)lipotoxic conditions, since BAIBA did not affect insulin release in INS-1832/3 cells pre-exposed to 5, 11 or 20 mM glucose plus palmitate (Fig. 4D). Given that pre-exposure of INS-1 cells to higher glucose (11 mM and 20 mM) plus palmitate as shown here (Fig. 4E and F) and previously [30,31] leads to decreased insulin secretion, this correlates with the finding that elevated plasma BAIBA levels associate with lower insulin secretory function in patients with type 2 diabetes (Fig. 2). However, since BAIBA has no direct effect on insulin secretion by INS-1832/3 cells under diabetic-like conditions (Fig. 4D, E and 4F), it is unlikely that the elevated level of plasma BAIBA shown in patients with type 2 diabetes contributes to the loss of insulin secretion in these individuals (Fig. 2). That said, to support this finding, the direct effect of BAIBA on insulin release should be explored with isolated human iselts.
By probing mitochondrial oxygen consumption rates of INS-1832/3 cells, we identify that BAIBA lowers mitochondrial energy metabolism of INS-1832/3 cells, linking the lowering effect of BAIBA on acute insulin release (Fig. 3A) to changes in mitochondrial energy metabolism (Fig. 5). Since mitochondrial energy metabolism tightly regulates KATP dependant insulin secretion through direct control over the phosphorylation potential [32,33]. Lower rates of mitochondrial respiration and consequent ADP phosphorylation will result in less insulin release at submaximal glucose concentrations. Therefore, BAIBA likely mediates acute insulin release by INS-1832/3 cells through changes in the pathway of KATP dependant insulin secretion. Collectively, our in vitro data suggest that BAIBA influences insulin release by healthy β-cells through changes in mitochondrial energy metabolism but has no direct effect on insulin secretory function of β-cells exposed to ‘diabetic-like’ conditions. Overall, our findings suggest a link between physical inactivity, plasma BAIBA level and modifications of β-cell insulin secretion.
Skeletal muscle is an insulin-sensitive tissue that plays a major role in glucose metabolism in the basal and postprandial state, as well as during and following exercise. Following a decrease in insulin sensitivity, β-cells compensate by increasing insulin release to meet demand. This is seen following, for example, high fat feeding or intralipid infusion [34] or physical inactivity/bed rest [28,29,35]. On the contrary, when insulin sensitivity is increased (by exercise, for example), β-cells adapt, whereby insulin release is lowered since less insulin is required to fulfil its function [36]. The inverse relationship between insulin secretion and insulin sensitivity in humans was first described by Kahn in 1993 [37]. Strikingly, to date, it is still not fully understood how β-cells make such careful functional adjustments in accordance with the function of a peripheral tissue. Since β-cells secrete insulin, which in turn signals to muscle cells to translocate GLUT4 to facilitate an increase in glucose disposition from the blood, it is prudent to assume that a skeletal muscle-derived factor provides feedback information to β-cells [38,39]. This study suggests that BAIBA may function in this way, although further investigation is warranted to unravel the underlying feedback mechanisms that likely exist between the endocrine pancreas and insulin-sensitive tissues. That being said, it is important to note, that this study is not without limitations, for example the effect of exercise, plasma BAIBA level and insulin secretory function was not explored in individuals with type 2 diabetes or obesity-induced insulin resistance. Due to the methods used, we were unable to calculate the μMole/L concentration of plasma BAIBA in Fig. 2 so a direct comparison of plasma BAIBA level between older and younger individuals cannot be drawn from this study. Importantly, the extent by which BAIBA release by skeletal muscle leads to inter-organ crosstalk also needs to be confirmed in vivo. Moreover the direct effect of BAIBA was only assessed in INS-1832/3 cells and therefore requires confirmation in human β-cells and/or isolated pancreatic islets. The concentration of BAIBA used in vitro was also on the higher level from what has been typically measured in blood plasma in healthy individuals (Fig. 1.). Future work is necessary to understand the exact mechanism(s) and significance by which BAIBA regulates insulin secretion in human β-cells and how post-exercise BAIBA regulation and training mediates adaptations, as well as in vivo effects of BAIBA supplementation in humans with and without type 2 diabetes. Furthermore, the basaline concentration of BAIBA within tissues such as the pancreas and its change, if any at all, during exercise also warrants exploration in order to better replicate physiological/pathological BAIBA concentrations for future in vitro investigation.
To conclude, it is evident that BAIBA is released into circulation from skeletal muscle, at a rate that is increased by muscle contraction (an insulin-sensitizing stimulus) and in vitro modifies insulin release by healthy β-cells. Indeed further work is required to confirm possible gender differences of BAIBA release as well as specific enantiomers of BAIBA that are released by contracting human skeletal muscle and to what extent skeletal muscle-derived enantiomers of BAIBA influence human β-cell insulin release. Nonetheless, further investigation of BAIBA and the molecular signalling processes by which it propagates its functions are warranted, particularly since this metabolite is a possible mediator of insulin secretion.
Grant funding
This work was supported by a European diabetes research program grant from the European Foundation for the Study of Diabetes (EFSD)/Astra Zeneca (Solomon). The A-V balance studies were supported by the UNIK (Investment Capital for University Research) research programme ‘Food, Fitness & Pharma for Health and Disease’ (Danish Ministry of Science, Technology and Innovation), The Nordea Foundation, A.P. Møller and Hustru Chastine Mc-Kinney Møllers Foundation. The Phenome Centre Birmingham was funded through a Medical Research Council grant (MR/M009157/1).
CRediT authorship contribution statement
Jonathan P. Barlow: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Visualization, Project administration. Kristian Karstoft: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - review & editing. Andreas Vigelsø: Methodology, Validation, Formal analysis, Writing - review & editing. Martin Gram: Methodology, Validation, Formal analysis, Writing - review & editing, Funding acquisition. Jørn W. Helge: Investigation, Validation, Formal analysis, Writing - review & editing. Flemming Dela: Methodology, Validation, Formal analysis, Writing - review & editing, Supervision, Funding acquisition. Kirk Pappan: Investigation, Validation, Formal analysis, Writing - review & editing. Donna O’Neil: Investigation, Validation, Formal analysis. Warwick Dunn: Investigation, Validation, Formal analysis, Supervision, Funding acquisition. Thomas P.J. Solomon: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Visualization, Project administration, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare no competing interest.
Acknowledgements
The authors thank the Mitochondrial Profiling Centre at the University of Birmingham for providing support for mitochondrial respiratory experiments. The authors also wish to thank Miss Camilla S. Christensen and Dr Dan P. Christensen from the University of Copenhagen who helped initiate pilot work examining the effect of BAIBA on INS-1 cells. Finally, the authors would like to thank the research participants for their time. Since completion of the work, Thomas Solomon is now affiliated with Blazon Scientific, London, UK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100053.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Defronzo R.A. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts L.D., Boström P., O’Sullivan J.F., Schinzel R.T., Lewis G.D., Dejam A. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metabol. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanianskii D., Jarzebska N., Birkenfeld A., O’Sullivan J., Rodionov R. Beta-aminoisobutyric acid as a novel regulator of carbohydrate and lipid metabolism. Nutrients. 2019;524:11. doi: 10.3390/nu11030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitase Y., Vallejo J.A., Gutheil W., Vemula H., Jaehn K., Yi J. Beta-aminoisobutyric acid, L-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22:1531–1544. doi: 10.1016/j.celrep.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi C.-X., Zhao M.-X., Shu X.-D., Xiong X.-Q., Wang J.-J., Gao X.-Y. β-aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep. 2016;21924:6. doi: 10.1038/srep21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krug S., Kastenmüller G., Stückler F., Rist M.J., Skurk T., Sailer M. The dynamic range of the human metabolome revealed by challenges. Faseb J. 2012;26:2607–2619. doi: 10.1096/fj.11-198093. [DOI] [PubMed] [Google Scholar]
- 7.Begriche K., Massart J., Toby A.A., Igoudjil A., Lettéron P., Fromenty B. β-Aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity. 2008;16:2053–2067. doi: 10.1038/oby.2008.337. [DOI] [PubMed] [Google Scholar]
- 8.Begriche K., Massart J., Fromenty B. Effects of β-aminoisobutyric acid on leptin production and lipid homeostasis: mechanisms and possible relevance for the prevention of obesity. Fund Clin Pharmacol. 2010;24:269–282. doi: 10.1111/j.1472-8206.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang M., Joseph J.W. Assessment of the metabolic pathways associated with glucose-stimulated biphasic insulin secretion. Endocrinology. 2014;155:1653–1666. doi: 10.1210/en.2013-1805. [DOI] [PubMed] [Google Scholar]
- 10.Ginter E., Simko V. Recent data on obesity research: β-aminoisobutyric acid. Bratisl Lek Listy. 2014;115:492–493. doi: 10.4149/bll_2014_095. [DOI] [PubMed] [Google Scholar]
- 11.Kammoun H.L., Febbraio M.A. Come on BAIBA light my fire. Cell Metabol. 2014;19:1–2. doi: 10.1016/j.cmet.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Morales F.E., Forsse J.S., Andre T.L., McKinley-Barnard S.K., Hwang P.S., Anthony I.G. BAIBA does not regulate UCP-3 expression in human skeletal muscle as a response to aerobic exercise. J Am Coll Nutr. 2017;36:1–10. doi: 10.1080/07315724.2016.1256240. [DOI] [PubMed] [Google Scholar]
- 13.Stautemas J., Kuilenburg A.B.P.V., Stroomer L., Vaz F., Blancquaert L., Lefevere F.B.D. Acute aerobic exercise leads to increased plasma levels of R- and S-β-Aminoisobutyric acid in humans. Front Physiol. 2019;1240:10. doi: 10.3389/fphys.2019.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuilenburg ABP van, Stroomer A.E.M., Lenthe H van, Abeling N.G.G.M., Gennip AH van. New insights in dihydropyrimidine dehydrogenase deficiency: a pivotal role for beta-aminoisobutyric acid? Biochem J. 2004;379:119–124. doi: 10.1042/bj20031463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittel A., Müller F., König J., Mieth M., Sticht H., Zolk O. Alanine-glyoxylate aminotransferase 2 (AGXT2) polymorphisms have considerable impact on methylarginine and β-aminoisobutyrate metabolism in healthy volunteers. PloS One. 2014;9:e88544. doi: 10.1371/journal.pone.0088544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdin D.V., Kolobov A.A., Brocker C., Soshnev A.A., Samusik N., Demyanov A.V. Diabetes-linked transcription factor HNF4α regulates metabolism of endogenous methylarginines and β-aminoisobutyric acid by controlling expression of alanine-glyoxylate aminotransferase 2. Sci Rep. 2016;6:35503. doi: 10.1038/srep35503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson S., Raeder H., Eide S.A., Midthjell K., Hveem K., Søvik O. Studies in 3,523 Norwegians and meta-analysis in 11,571 subjects indicate that variants in the hepatocyte nuclear factor 4 alpha (HNF4A) P2 region are associated with type 2 diabetes in Scandinavians. Diabetes. 2007;56:3112–3117. doi: 10.2337/db07-0513. [DOI] [PubMed] [Google Scholar]
- 18.Vigelsø A., Gram M., Dybboe R., Kuhlman A.B., Prats C., Greenhaff P.L. The effect of age and unilateral leg immobilization for 2 weeks on substrate utilization during moderate-intensity exercise in human skeletal muscle. J Physiol. 2016;594:2339–2358. doi: 10.1113/jp271712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crossland H., Skirrow S., Puthucheary Z.A., Constantin-Teodosiu D., Greenhaff P.L. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: different routes to similar end-points. J Physiol. 2019;597:1259–1270. doi: 10.1113/jp275444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter E.A., Kiens B., Mizuno M., Strange S. Insulin action in human thighs after one-legged immobilization. J Appl Physiol. 1989;67:19–23. doi: 10.1152/jappl.1989.67.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Dirks M.L., Wall B.T., van de Valk B., Holloway T.M., Holloway G.P., Chabowski A. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65:2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 22.Kawamoto E., Koshinaka K., Yoshimura T., Masuda H., Kawanaka K. Immobilization rapidly induces muscle insulin resistance together with the activation of MAPKs (JNK and p38) and impairment of AS160 phosphorylation. Physiological Reports. 2016;4:e12876. doi: 10.14814/phy2.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gram M., Vigelsø A., Yokota T., Hansen C.N., Helge J.W., Hey-Mogensen M. Two weeks of one-leg immobilization decreases skeletal muscle respiratory capacity equally in young and elderly men. Exp Gerontol. 2014;58:269–278. doi: 10.1016/j.exger.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Solomon T.P.J., Knudsen S.H., Karstoft K., Winding K., Holst J.J., Pedersen B.K. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab. 2012;97:4682–4691. doi: 10.1210/jc.2012-2097. [DOI] [PubMed] [Google Scholar]
- 25.Huber A.H., Kampf J.P., Kwan T., Zhu B., Kleinfeld A.M. Fatty acid-specific fluorescent probes and their use in resolving mixtures of unbound free fatty acids in equilibrium with albumin. Biochemistry. 2006;45:14263–14274. doi: 10.1021/bi060703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlow J., Jensen V.H., Affourtit C. Uncoupling protein-2 attenuates palmitoleate protection against the cytotoxic production of mitochondrial reactive oxygen species in INS-1E insulinoma cells. Redox Biology. 2015;4:14–22. doi: 10.1016/j.redox.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Affourtit C., Brand M.D. Vol. 457. Elsevier; 2009. pp. 405–424. (Chapter 23 measuring mitochondrial bioenergetics in INS-1E insulinoma cells). [DOI] [PubMed] [Google Scholar]
- 28.Mikines K.J., Dela F., Tronier B., Galbo H. Effect of 7 days of bed rest on dose-response relation between plasma glucose and insulin secretion. Am J Physiol. 1989;257:E43–E48. doi: 10.1152/ajpendo.1989.257.1.e43. [DOI] [PubMed] [Google Scholar]
- 29.Mikines K.J., Richter E.A., Dela F., Galbo H. Seven days of bed rest decrease insulin action on glucose uptake in leg and whole body. J Appl Physiol. 1991;70:1245–1254. doi: 10.1152/jappl.1991.70.3.1245. [DOI] [PubMed] [Google Scholar]
- 30.Barlow J., Affourtit C. Novel insights into pancreatic β-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells. Biochem J. 2013;456:417–426. doi: 10.1042/bj20131002. [DOI] [PubMed] [Google Scholar]
- 31.Barlow J., Jensen V.H., Jastroch M., Affourtit C. Palmitate-induced impairment of glucose-stimulated insulin secretion precedes mitochondrial dysfunction in mouse pancreatic islets. Biochem J. 2016;473:487–496. doi: 10.1042/bj20151080. [DOI] [PubMed] [Google Scholar]
- 32.Henquin J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 33.Patterson J.N., Cousteils K., Lou J.W., Fox J.E.M., MacDonald P.E., Joseph J.W. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. J Biol Chem. 2014;289:13335–13346. doi: 10.1074/jbc.m113.521666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann O.P., Dahl D.B., Brechtel K., Machann J., Haap M., Maier T. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 35.Knudsen S.H., Hansen L.S., Pedersen M., Dejgaard T., Hansen J., van Hall G. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J Appl Physiol. 2012;113:7–15. doi: 10.1152/japplphysiol.00189.2011. [DOI] [PubMed] [Google Scholar]
- 36.Mikines K.J., Sonne B., Tronier B., Galbo H. Effects of training and detraining on dose-response relationship between glucose and insulin secretion. American Journal of Physiology - Endocrinology and Metabolism. 1989;256:E588–E596. doi: 10.1152/ajpendo.1989.256.5.E588. [DOI] [PubMed] [Google Scholar]
- 37.Kahn S.E., Prigeon R.L., McCulloch D.K., Boyko E.J., Bergman R.N., Schwartz M.W. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 38.Barlow J.P., Solomon T.P. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am J Physiol-Endoc M. 2018;314:E297–E307. doi: 10.1152/ajpendo.00353.2017. [DOI] [PubMed] [Google Scholar]
- 39.Barlow J., Solomon T.P.J. Conditioned media from contracting skeletal muscle potentiates insulin secretion and enhances mitochondrial energy metabolism of pancreatic beta-cells. Metab, Clin Exp. 2019;91:1–9. doi: 10.1016/j.metabol.2018.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.