Abstract
Metastatic disease is the major cause of death among cancer patients. A class of genes, named metastasis suppressors, has been described to specifically regulate the metastatic process. The metastasis suppressor genes are downregulated in the metastatic lesion compared to the primary tumor. In this review, we describe the body of research surrounding the first metastasis suppressor identified, Nm23. Nm23 overexpression in aggressive cancer cell lines reduced their metastatic potential in vivo with no significant reduction in primary tumor size. A complex mechanism of anti-metastatic action is unfolding involving several known Nm23 enzymatic activities (nucleotide diphosphate kinase, histidine kinase, and 3′–5′ exonuclease), protein–protein interactions, and downstream gene regulation properties. Translational approaches involving Nm23 have progressed to the clinic. The upregulation of Nm23 expression by medroxyprogesterone acetate has been tested in a phase II trial. Other approaches with significant preclinical success include gene therapy using traditional or nanoparticle delivery, and cell permeable Nm23 protein. Recently, based on the inverse correlation of Nm23 and LPA1 expression, a LPA1 inhibitor has been shown to both inhibit metastasis and induce metastatic dormancy.
Keywords: Nm23, Metastasis suppressor, Therapeutics, Histidine protein kinase, Metastasis, NDPK, NME
1. Background
Metastasis is a critical factor in the prognosis of cancer patients and is a major contributor to mortality. Although surgery, radiotherapy, and chemotherapy have improved, the goal of dramatically increasing patient survival remains to be achieved. As such, the metastasis research field has focused on identifying new therapeutic strategies. The discovery of a new class of genes, the metastasis suppressor genes, has therefore received much attention. Nm23 (NME) was the first metastasis suppressor identified [1] on the basis of its reduced expression in a comparative hybridization screen of poorly versus highly metastatic sublines of the murine melanoma cell line K-1735. Transfection and knockout mouse experiments, discussed below, confirmed a metastasis suppressive function. This gene was independently identified as a regulator of imaginal disc differentiation late in Drosophila development as Awd [2, 3]. Thus far, ten human homologues (Nm23-H1–Nm23-H10) have been described with Nm23-H1 and Nm23-H2 being the most studied. They have been separated into two groups based on sequence homology. Group I (H1–H4) includes proteins with nucleotide diphosphate kinase (NDPK) activity and 58–88 % similarity; however, group II (H6–H10) consists of more divergent proteins with only 25–45 % similarity [4].
Since the establishment of Nm23 as the first metastasis suppressor gene, many experiments have been performed attempting to elucidate the exact biochemical mechanism of its anti-metastatic function. Herein, we review the role of Nm23 in the control of metastasis across several cancer cell lines and tumor types. In addition, we examine new therapeutic strategies proposed and tested in in vivo models in order to restore the anti-metastatic function of Nm23-H1.
2. Biochemical functions of Nm23
Multiple biochemical functions have been identified for Nm23 proteins, and several may contribute to the anti-metastatic properties (for a critical review, see [5]). In the 1950s, Drs. Paul Berg and Hans Krebs independently identified a biochemical activity that removed the terminal phosphate from a nucleotide triphosphate (NTP) and added it to a nucleotide diphosphate (NDP). This NDPK activity [6, 7] was subsequently reported to be encoded by Nm23 [8]. Reported functions for the NDPK activity of Nm23 may include maintenance of nucleotide pools, activation of the Gβγ subunit of G proteins by Nm23-H2 [9], and mutator phenotypes [10]. Nm23-H1 is known to be a multifunctional enzyme with at least two other biochemical activities described to date: a histidine protein kinase (HPK) and a 3′–5′ exonuclease. The histidine protein kinases are well known in prokaryotic and lower eukaryotic systems, where they control cellular responses to the environment, but are poorly understood in mammalian cells. The NDPK activity forms the first part of an HPK reaction, in which a high-energy phosphate is transferred from an NTP to Nm23 on a histidine residue. Nm23-P then transfers this phosphate to a protein, either to a high-energy histidine or aspartic acid residue, or possibly an irreversible lower-energy serine or other amino acid phosphorylation. Reported substrates for Nm23 as an HPK include aldolase C, ATP citrate lyase, and the kinase suppressor of Ras [11], the last of which has been linked to Erk-Map kinase activation (review in [12, 13]).
The laboratory of David Kaetzel reported a 3′–5′ DNA exonuclease activity for NDPK-A/Nm23-H1 [14]. Using a series of Nm23-H1 mutations transfected into 1205LU melanoma cells, both the histidine involved in its NDPK and HPK activities and a lysine involved in its exonuclease activity were shown to be partially responsible for metastasis suppression [15]. From these observations, the authors demonstrated, in the melanoma cell line WM793 and in MEFs, a role for the Nm23-H1 NDPK and 3′–5′ exonuclease activities in the repair of UV irradiation-induced DNA lesions [16]. The authors speculate that Nm23-H1 expression reduces the acquisition of mutations that promote transition to the metastatic phenotype in melanoma cells.
The anti-metastatic property of Nm23 has also been associated with its ability to form specific protein–protein interactions (Table 1). In general, ruling out nonspecific interactions due to Nm23 “stickiness” has been critical; bonafide interactions are typically confirmed by two way co-immunoprecipitations and alter the function of one of the proteins involved. A number of pathways potentially involved in metastasis are affected by Nm23 interaction. For several proteins, Nm23-H1 interaction essentially “titers out” the amount of free, prometastatic protein. Examples of such titering include Prune [17, 18] and some viral proteins [ 19, 20]. In other cases, more complex interactions occur modulating cytoskeletal architecture or scaffolds for the Erk-Map kinase pathway.
Table 1.
Nm23-H1 binding proteins and the pathway(s) regulated by the interaction
| Protein | Regulated pathway | Phenotype | Isoform | Reference |
|---|---|---|---|---|
| Tiam1 | Rac pathway | Rac1 inactivation | Nm23-H1 | [68] |
| Ksr | Erk pathway | Ksr degradation | Nm23-H1 | [11] |
| Rps3 | Erk pathway | Ark pathway inactivation | Nm23-H1 | [69] |
| Lbc | Rho GTPase pathway | Rho GTPase pathway inhibition | Nm23-H2 | [70] |
| Rad | Ras pathway | Ras pathway inactivation | Nm23-H1 | [71] |
| Dbl-1 | cdc42 | Cdc42 GTPase inhibition | Nm23-H1 | [72] |
| ARF6 | Endocytosis | E-cadherin-mediated edocytosis | Nm23-H1 | [73] |
| STRAP | TGFβ pathway | p53-induced apoaptosis and cell cycle arrest | Nm23-H1 | [74] |
| EBNA-1-3C | Transcriptional regulation | Increased MMP9 and αV integrin expression | Nm23-H1 | [20] |
| ERα | Transcriptional regulation | Reduced cathepsin and Bcl-2 expression | Nm23-H1 | [75] |
| ERβ | Transcriptional regulation | Increased estrogen-mediated transcription | Nm23-H2 | [76] |
| RORaa | Transcriptional regulation | N/A | Nm23-H1, Nm23-H2 | [77] |
| Oct-1 | Transcriptional regulation | N/A | Nm23-H1 | [78] |
| SET | Transcriptional regulation | Granzyme A-mediated cell death | Nm23-H1 | [79] |
| Vimentin | Cytoskeleton modulation | Increased intermediate filaments density | Nm23-H1 | [80] |
| Tubulin | Cytoskeleton modulation | N/A | Nm23-H1 | [81] |
| Icap-1a | Cell adhesion | N/A | Nm23-H2 | [82] |
| Plakoglobin | Cell adhesion | Increased protein stability | Nm23-H1, Nm23-H2 | [83] |
| APE1 | DNA repair | Increased APE1 endonuclease activity | Nm23-H1 | [15] |
| Prune | Pro-metastasis | Increase cAMP-PDE prune activity | Nm23-H1 | [84] |
| MDM2 | p53 degradation | Inhibition Nm23 anti-motility ability | Nm23-H2 | [85] |
| HPV 16E7 | Cervical cancer | Ubiquitin-proteosome-dependent Nm23 degradation | Nm23-H1, Nm23-H2 | [86] |
| GAPDH | Glycolytic pathway | Phosphoglycerate mutase activation | Nm23-H1 | [87] |
| Aurora-A | Mitosis | N/A | Nm23-H1 | [88] |
The interaction has been shown only by two-hybrid and GST pull down experiments
A final activity of Nm23 is the regulation of gene expression, which may be influenced by Nm23 participation in transcriptional complexes. The role of Nm23 protein binding as part of a transcriptional complex is only partially understood but may be critical to downstream changes in gene expression. A gene expression profile of MDA-MB-435 cells over-expressing Nm23-H1 or its mutant forms lacking the metastasis suppressive property revealed an inverse correlation between the expression of Nm23-H1 and EDG2 (LPAR1), the first in the family of lysophosphatidic acid receptors [21]. EDG2 over-expression in MDA-MB-435 cells was able to enhance their motility and metastasis [22], suggesting it may serve as a target of Nm23-H1-mediated suppression. Interestingly, recent results showed that the LPAR1 inhibitor Debio 0719 dramatically decreased metastasis in mice [23].
3. Nm23 anti-metastatic properties
3.1. Transfection studies
According to the definition, metastasis suppressors only block the spread of cancer to distant sites without reducing primary tumor formation or size. As such, in vitro experiments (such as soft agar cloning, wound scratch assays, and Boyden chamber chemotaxis assays) may reveal information about component parts of the metastatic process but are not sufficient for studying metastasis. The metastatic cascade is a complex, multi-step process that requires the interaction between cancer cells and the tissue microenvironment. Therefore, in vivo studies are necessary to evaluate the ability of a gene, or chemotherapeutic, to suppress metastasis. Both syngeneic and xenograft animal models have been utilized to assess the in vivo effect of Nm23-H1 in spontaneous (primary tumor formation induced metastasis) and experimental (after injection into the bloodstream) metastasis assays. From these experiments, results showed that cancer cells exogenously expressing Nm23-H1 were significantly inhibited in their capacity to form metastatic colonies, though primary tumor formation was largely unaffected (Table 2). Cell lines from multiple histological types of cancer were metastasis suppressed by Nm23. However, in no experiment did overexpression of Nm23 completely abrogate metastasis. This may indicate the importance of obtaining a critical level of Nm23 over-expression within the cancer cells in order to achieve successful metastatic suppression. More likely, the difference in suppression among cancer types, and within subtypes, can be attributed to the varying pathways active among the differing cancers. Complete inhibition may require cooperation from multiple metastasis suppressor genes acting on several pathways.
Table 2.
Ectopic expression of Nm23 in higH1y metastatic cancer cells reduced metastasis formation
| Tumor type | Origin | Cell line | Reduction in metastases (%) | Gene | Reference |
|---|---|---|---|---|---|
| Breast carcinoma | Human | MDA-MB-435 | 50–90 | Nm23-H1 | [89] |
| Breast carcinoma | Human | MDA-MB-435 | 40–100 | Nm23-H1, Nm23-H2 | [90] |
| Breast carcinoma | Human | MDA-MB-231- BAG | 40–50 | Nm23-H1, Nm23-H2 | [91] |
| Breast carcinoma | Rat | MTC/MTLn3 | 30–70 | Nm23-H1, Nm23-H2 | [92] |
| Breast carcinoma | Murine | 4T1 | 60–70 | Nm23-H1, Nm23-M1 | [23] |
| Melanoma | Murine | K-1735 TK | 58–96 | Nm23-H1 | [93] |
| Melanoma | Murine | B16-sublines | 54–90 | Nm23-M1, Nm23-M2 | [94] |
| Melanoma | Murine | B16F10 | 93 | Nm23-H1 | [95] |
| Melanoma | Human | 1205LU | 62 | Nm23-H1 | [15] |
| Melanoma | Murine | MelJuSo | 40–80 | Nm23-H1 | [96] |
| Colon | Murine | colon26 | 55 | Nm23-H2 | [97] |
| Colon | Human | HT-29 | 90 | Nm23-H1 | [98] |
| Oral squamous cell carcinoma | Human | LMF4 | 80–90 | Nm23-H2 | [25] |
Several exceptions to these observations are noteworthy. First, an exception to the consensus of primary tumor data has been reported, where Nm23-H1/Nm23-H2−/+ knockout mice developed fewer melanomas after exposure to UV radiation [16]. Second, it remains unclear that Nm23-H2 is as potent of a metastasis suppressor as Nm23-H1. Some reports clearly show a metastasis suppressive activity while others do not, and the data are limited by reporting only certain endpoints without full metastasis assays. Lee et al. showed that when stably overexpressing Nm23-H2 in NIH3T3 fibroblasts, HLK3 hepatocytes and oral carcinoma cells (LMF4), a pro-oncogenic effect was observed, namely, tumor formation in nude mice and increased c-myc, cyclin D1, and NF-kB expression [24, 25]. Nm23-H2 silencing did not show the same effect on invasiveness of liver and colon cancer cells indicating a specific role of the H1 isoform as a negative regulator of cancer cell invasion [26]. Finally, in neuroblastoma and hematopoietic tumors, Nm23 overexpression may have the opposite effect.
It is interesting to note that Nm23-H1 has been shown to suppress multiple steps of the metastatic cascade, including invasion, tumor cell survival, and colonization. Multiple transfection studies have described less invasive primary tumors when Nm23 is overexpressed. Horak et al. showed that Nm23 was able to suppress metastasis at the initial steps in metastatic colonization [21]. In experimental metastasis assays, fluorescently labeled MDA-MB-435 cells over-expressing Nm23-H1 showed equivalent arrival in the lungs as control transfectants, but a 10-fold reduction in the retention of tumor cells within murine lungs over time.
Pathway analyses of Nm23 overexpressing tumor cells have suggested multiple downstream effects. Using in vitro studies, Boissan et al. described the role of Nm23 in the maintenance of E-cadherin-mediated intercellular adhesion and limitation of the invasive potential, and identified MAPK, Rac1-GTPase, and Akt signaling as pathways involved in this process [26].
3.2. Nm23-M1 null/SV40 transgenic mouse
The Nm23-H1 knockout mouse model confirmed a role for Nm23-H1 in metastasis suppression. The Nm23-H1 knockout shows a normal phenotype except for defects in mammary gland development [27]. However, a phenotype appeared under two experimental situations promoting hepatocellular carcinoma, either when bred with mice expressing the SV40 virus large T antigen in the liver or when mice are injected with the toxin diethylnitrosamine. In both cases, the development of liver tumors was unaffected, but a two-fold increase in lung metastases was observed [28].
Distinct results were obtained for Nm23-H2. The NME1−/−, NME2−/− double KO showed a perinatal death, while heterozygotes displayed an erythrocyte development phenotype [29]. Analyzing the MEF cells from the double knockout, a reduction in receptor coupled G proteins (beta adrenergic receptor coupled Gs protein) in the plasma membrane was observed and this phenotype was rescued by Nm23-H2 but not Nm23-H1 re-expression [30, 31]. Moreover, the Nm23-H2−/− mouse showed no evident phenotype except for a defect in cytokine production in T cells due to a dysfunction in the KCa3.1 channels [32], although metastasis has not been analyzed.
In conclusion, both transfection and Nm23-H1 knockout mice confirm a metastasis suppressive role for Nm23-H1. Additional supportive data emanate from cell permeable Nm23 proteins, discussed below. The role of Nm23-H2 overlaps but may also be more complex.
3.3. Nm23 expression in tumor and metastatic tissues
Nm23 protein levels have been estimated in numerous tumor cohorts (Table 3). Reductions in Nm23-H1 expression have been significantly associated with aggressive behavior in gastric carcinoma, ovarian cancer, melanoma, breast cancer, and some other cancers [33–35]. Nm23 expression was inversely correlated to tumor aggressiveness using several tumor and patient parameters such as overall survival, disease-free survival, lymph node status, tumor stage, and expression of specific markers (i.e., hormone receptors). Discordant conclusions have been published in cohort studies. The size of cohort used to evaluate the correlation Nm23-metastatic disease can affect the results of the analyses. Antibody specificity, grading schemes, and other technical factors also contribute to varying results. Other studies have examined Nm23 expression in metastatic lesions. Reduced expression of Nm23 in metastases, as compared to histologically similar primary tumors, was observed in gastric, head and neck, non-small cell lung, uterine, and breast carcinomas, and melanoma [36–42].
Table 3.
Nm23 expression in human carcinoma cohort studies
| Tumor type | Cohort size | Reference |
|---|---|---|
| Inverse correlation between Nm23 expression and metastasis | ||
| Breast cancer | 27 | [99] |
| Breast cancer | 71 | [100] |
| Breast cancer | 168 | [101] |
| Breast cancer | 390 | [102] |
| Breast cancer | 163 | [103] |
| Breast cancer | 130 | [104] |
| Breast cancer | 128 | [105] |
| Breast cancer | 73 | [106] |
| Melanoma | 30 | [107] |
| Melanoma | 157 | [108] |
| Melanoma | 21 | [109] |
| Melanoma | 32 | [110] |
| Melanoma | 120 | [111] |
| Prostate cancer | 346 | [112] |
| Ovarian carcinoma | 50 | [113] |
| Gastric cancer | 24 | [114] |
| Gastric cancer | 59 | [36] |
| Gastric cancer | 413 | [34] |
| Hepatocellular carcinoma | 21 | [115] |
| Hepatocellular carcinoma | 47 | [116] |
| Hepatocellular carcinoma | 43 | [117] |
| Non-small-cell lung cancer | 381 | [118] |
| Non-small-cell lung cancer | 104 | [119] |
| Non-small-cell lung cancer | 285 | [120] |
| Oral squamous cell carcinoma | 86 | [121] |
| Positive correlation between Nm23 expression and metastasis | ||
| Neuroblastoma | 76 | [43] |
| Osteosarcoma | 25 | [44] |
| Non-small-cell lung carcinoma | 36 | [122] |
| Myelogenous leukemia | 110 | [123] |
| Peripheral T-cell lymphoma | 137 | [124] |
| Peripheral T-cell lymphoma | 102 | [125] |
| Lymphomas | 606 | [126] |
| Diffuse large B-cell lymphoma | 172 | [126] |
| Hodgkin lymphoma | 128 | [127] |
| Acute myelogenous leukemia | 102 | [49] |
| Thyroid carcinomas | 86 | [128] |
In stark contrast to the above results, high Nm23-H1 levels were associated with a significant reduction in survival for patients with neuroblastoma, osteosarcoma, and hematological malignancies [43–45]. Obviously, Nm23 may play a tissue-specific role, and different regulatory mechanisms may act in different tumors.
Although the amino acid sequence of Nm23 does not contain a conserved secretion signal peptide, it has been detected in conditioned medium of some tumor cell lines and patients’ sera [46–48]. It is unclear how Nm23 protein is secreted into the extracellular space. However, several studies using lymphomas and leukemias evaluated the serum level of Nm23-H1 protein using an ELISA detection system and correlated Nm23 levels with the clinical outcome of patients. One hypothesis is that the major portion of serum Nm23 levels results from erythrocyte death. In vitro experiments using these cancer types suggested that extracellular Nm23 protein may promote lymphoma and leukemia growth and survival by autocrine and/or paracrine mechanisms [49–51]. It has been suggested that extracellular Nm23-H1 indirectly increases the survival of the immature CD34+/CD11b− cells or primary cultured mononuclear cells (PBMNCs), by inducing the production of inflammatory cytokines (IL-1b, IL-6, IL-8, GM-SCF, and MCP-1) from the more mature CD34−/CD11b+ cells.
4. Therapeutic strategies using Nm23-H1
The development of new therapeutic strategies to prevent, halt, or shrink metastases is greatly needed. For the last two decades, several therapeutic strategies using Nm23-H1 have been reported in solid tumor model systems.
4.1. Re-expression of Nm23-H1
The re-expression of Nm23-H1 in breast cancer cells by medroxyprogesterone acetate (MPA) has been reported [52] through the glucocorticoid receptor in triple-negative (estrogen and progesterone receptor negative, HER2 normal) breast cancer. MPA bound to the glucocorticoid receptor and the glucocorticoid response element, altering nucleosomal structure and permitting transcription factor binding to the transcription factor binding sites. Treatment with MPA elevated Nm23-H1 expression in progesterone receptor negative, glucocorticoid receptor positive MDA-MB-231 and MDA-MB-435 human breast carcinoma cell lines in vitro. No effect on proliferation was observed in vitro, but soft agar colonization of metastatic breast cancer cell lines was reduced by approximately 50 % in vitro. MPA had no effect on colony formation of MDA-MB-231T cells expressing antisense Nm23-H1 [53], suggesting some degree of specificity.
Whether MPA-mediated re-expression of Nm-23-H1 would inhibit metastatic progression in vivo was examined through xenograft experiments with MDA-MB-231T cells, in which these cells were intravenously injected into the mice. MPA treatment induced Nm23-H1 expression in MDA-MB-231T cells in vivo, decreasing the metastatic colonization in the lungs of MPA-treated mice by 27–36 % in two independent experiments. Moreover, high levels of Nm23-H1 were detected in 43 % of pulmonary lesions of MPA-treated mice compared with 13 % of metastases in untreated mice. Side effects of MPA treatment included weight gain and decreased bone density. Based on these data, a phase II clinical trial of MPA with metronomic chemotherapy was conducted (personal communication, Dr. Kathy Miller, Indiana University).
Multiple other compounds and drugs have been reported to elevate Nm23 expression. These include thujone, a monoterpene natural product. Injection of thujone into mice bearing B16F-10 melanoma cells improved survival and upregulated Nm23 among other molecular changes [54]. Another natural product, the polysaccharide LMPAB, inhibited B16 melanoma metastasis, elevated Nm23 expression, and inhibited protease expression [55]. Again, using the B16 model system, amentoflavone significantly lowered pulmonary metastases and increased Nm23 expression, among other markers [56]. In vitro data also identified the anti-inflammatory compounds indomethacin and acetylsalicylic acid, a vitamin D analog, all-trans retinoic acid, the anti-oxidant l-carnosine, and gamma linolenic acid as up-regulating Nm23 proteins. All of these compounds are nonspecific, in that multiple additional mechanisms of action are anticipated, and the sum of risks and benefits will determine efficacy rather than effects on a single pathway.
4.2. Gene therapy
Two Nm23 delivery methods have been reported. One is an adeno-associated virus (AAV)-mediated transduction and the other is a non-viral nanoparticle-mediated transduction. The effectiveness of the former method was examined through an orthotopic transplantation model with SW626-M4 human ovarian adenocarcinoma cell lines expressing low Nm23, in which the tumor fragments formed by these cells in nude mice were implanted in the ovarian serosa. In more than 95 % of ovarian tumor cells in mice intraperitoneally injected with AAV-Nm23-H1, increased Nm23 expression was observed. The experiments demonstrated that the number of animals developing liver metastasis in the AAV-Nm23-H1-treated group reduced to 60 % of that in the PBS- or AAV-LacZ-treated group, and that the AAV-Nm23-H1 treatment increased the time and percentage of survival (46 %, at 140 days) as compared with the PBS- or AAV-LacZ-treated animals (0 %, all died 35 days earlier) [57].
The effectiveness of the nanoparticle method was examined through experimental metastasis experiments with the B16F10 mouse melanoma cell line. The nanoparticle was a poly-l-lysine-modified iron oxide nanoparticle (IONP-ALL), which was formed by modifying poly-l-lysine to the surface of iron oxide nanoparticles. Positively charged IONP-PLL bound to negatively charged DNA phosphate groups and showed greater resistance to DNase-1-mediated DNA degradation compared with naked DNA. IONP-PLL/DNA complexes that were intravenously injected into mice were distributed into multiple organs such as the brain, lungs, spleen, and kidneys [58]. In metastasized B16F10 cells in the lung derived from the mice intravenously injected with IONP-PLL carrying Nm23-H1 plasmid, strong expression of Nm23 was observed. In the IONP-PLL/NM23-H1-treated group, metastasis was clearly suppressed compared with the group treated with free Nm23-H1 plasmid. The groups of mice treated with empty IONP-PLL, naked Nm23 plasmid DNA, or saline all developed metastases similarly. The intravenous injection of IONP-PLL carrying Nm23-H1 plasmid significantly extended the survival time of the mice inoculated with B16F10 [59]. Although the delivery system also leads to Nm23 expression in normal tissues, no side effects have been observed. Both approaches are limited by general problems with gene therapy strategies.
4.3. Protein therapy using cell permeable Nm23
The effectiveness of protein-based therapy to deliver cell permeable Nm23 protein (CP-Nm23) as an anti-metastatic agent has been reported [60]. CP-Nm23 is the fused protein composed of hydrophobic macromolecule transduction domains (MTD) and Nm23. MTD is an improved amino acid sequence derived from a membrane-translocating motif composed of 12 amino acids from a hydrophobic signal sequence from fibroblast growth factor 4. The MTD fused with eGFP was quickly incorporated into several human cancer cell lines such as MDA-MB-435 (breast cancer cells), HCT116 (colon cancer cells), and A549 (lung cancer cells) in vitro, and murine organs such as the liver, spleen, and lung in vivo [60].
Biological effects of CP-Nm23 were examined through in vitro experiments using MDA-MB-231 and MDA-MB-435 human breast cancer cell lines and xenograft experiments with MDA-MB-231 cells. Both cell lines incorporating CP-Nm23 proteins reduced MEK and ERK phosphorylation, consistent with Ksr data, and EDG2 and gene expression alterations previously described. CP-Nm23 inhibited metastasis-associated phenotypes including cell migration, adhesion, matrigel invasion, and anchorage-independent growth in vitro. Moreover, CP-Nm23 not only suppressed the establishment of lung metastases in the in vivo models but also cleared already established metastases, thereby greatly prolonging the survival of animals harboring disseminated tumor cells. Indeed, the mice injected with CP-Nm23 showed enhanced survival (90 %) after 40 weeks as compared with control mice (20 %). Bouin’s solution and vimentin staining confirmed the reduction or lack of lung metastases in the treated mice. [60]
4.4. LPA1, inversely expressed with Nm23, as a therapeutic target
An inverse correlation between Nm23-H1 levels and the expression of a therapeutic target in inhibiting metastasis progression was reported [22]. The target is known as LPA1 (also known as EGD2 or LPAR1), one of the G protein-coupled receptors for serum lysophosphatidic acid (LPA). LPA is produced from lysophosphatidylcholine in the cell membrane and in plasma by autotaxin. It is now known that the effects of LPA at physiological concentrations are mediated by five bona fide, high-affinity cognate receptors (LPA1–LPA5), low-affinity cognate receptors (P2Y5, P2Y10, and GPR87) and perhaps by additional recently proposed or as yet unidentified receptors [61, 62]. These signaling pathways have been shown to prompt aspects of tumorigenesis and metastatic progression [63].
Gene expression profiling of MDA-MB-435 cells expressing wild-type Nm23-H1, compared with those expressing mutant forms of Nm23-H1 (P96S and S120G) lacking the anti-motility property, identified LPA1 as downregulated by wild-type but not by mutant Nm23-H1 expression. In vitro experiments demonstrated that LPA1 overexpression significantly restored motility to MDA-MB-435 cells expressing Nm23-H1. Further, LPA1 knockdown via RNA interference reduced the motility of MDA-MB-435 and MDA-MB-231 cells. Moreover, in serial sections of human breast cancer specimens, an inverse pattern of Nm23-H1 and LPA1 staining was observed. In hepatocellular carcinomas developed from Nm23 knockout mice, elevated expression of LPA1 was also observed. Given the above data, the hypothesis that an LPA1 inhibitor can prevent the metastatic progression of breast cancer cells was examined through spontaneous animal experiments with 4T1 murine mammary carcinoma and xenograft experiments with MDA-MB-231T cells [23].
Debio 0719 (Debiopharm S.A., Lausanne, Switzerland) is an antagonist for LPA receptors and selectively inhibits LPA receptor-mediated actions, especially through LPA1 and LPA3. The IC50 of 0719 for LPA1 is 60 nM, with less potent inhibition of LPA3 at 660 nM and LPA2 at 2 μM. In vitro experiments demonstrated that 0719 had no effect on proliferation of 4T1 and MDA-MB-231T cells but inhibited their motility. In a spontaneous metastasis assay using 4T1 murine mammary cells, 0719 had no effect on primary tumor formation but inhibited lung metastasis by 89 % and liver metastasis by 73 %. The metastasis inhibitory activity of 0719 was confirmed in MDA-MB-231T experimental metastasis assays using both lung metastasis counts and animal survival as endpoints.
Analysis of the histologic section of the primary and metastatic tumors derived from the mice inoculated with 4T1 cells revealed that the Ki67 labeling of primary tumors from mice treated with vehicle or 0719 was comparable (P=0.86). In contrast, in vehicle-treated liver metastases, 15.3 % of tumor cells were Ki67 positive and declined 65 to 5.4 % in the 0719-treated lesions (P=0.005). Similar trends were observed in 4T1 lung metastases. No detectable cleaved caspase 3 was observed at any site. Thus, LPA1 pathway interruption halted tumor proliferation in a site-specific manner, suggestive of an induction of metastatic dormancy. Dormancy has been proposed to result from the reciprocal down-regulation of the proliferative Erk-Map kinase pathway and up-regulation of the p38 stress Map kinase pathway [64–67]. This signaling pattern was observed in 4T1 cells in the liver and lung of 0719-treated mice but not primary tumors. The dormancy status induced by 0719 treatment was shown to be reversible, and discontinuation of 0719 administration in experimental metastasis assays with MDA-MB-231T cells led to a decrease in survival with pulmonary metastasis evident at necropsy [23].
5. Conclusion
The metastasis suppressor field has identified a series of proterns with non-obvious mechanisms of action that are teaching us new lessons about the regulation of metastatic colonization and providing new therapeutic leads. For solid tumors, Nm23-H1 is unarguably a metastasis suppressor with a complex mechanism of action. New translational approaches that have been developed around this gene, while still in their infancy, have shed light on new approaches to prevent or halt metastatic progression (Fig. 1).
Fig. 1.
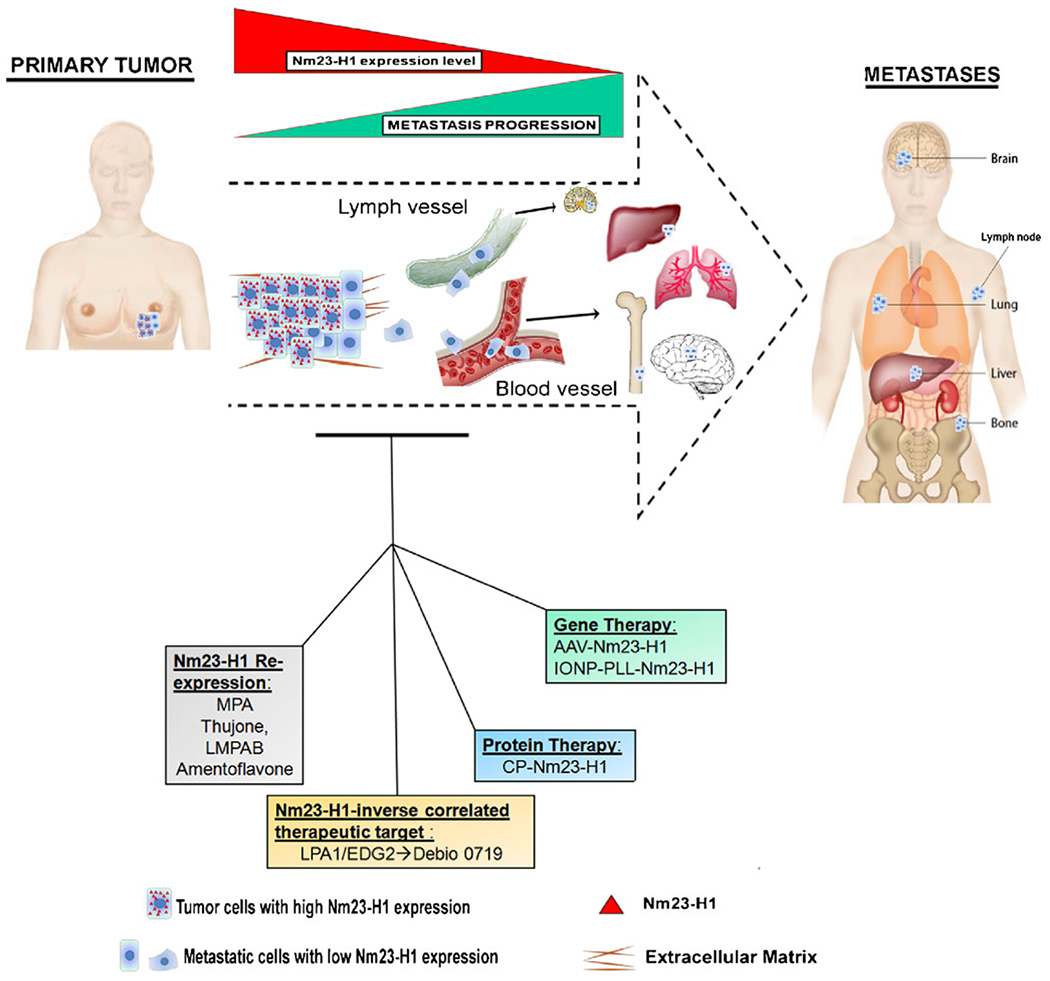
Several therapeutic strategies using Nm23-H1 have been reported and tested in in vivo models in order to restore the metastatic suppression property of Nm23. Nm23-H1 expression increased after the treatment with Medroxyprogesterone acetate (MPA) or other natural compounds ( thujone, LMPAB, amentoflavone). Three Nm23-H1 delivery methods, the adeno-associated virus (AAV)- Nm23-H1, the poly-L-lysine-modified iron oxide nanoparticle (IONP-PLL)-Nm23-H1 and the cell permeable Nm23-H1 protein (CP-Nm23), have been reported. Lysophosphatidylcholine receptor 1 (LPA1), a new therapeutic target mediator of metastatic dormancy was identified , based on its inverse correlation to Nm23 expression
New tools are needed in the metastasis field for continued progress. These include tools for the analysis of phosphohistidine and the HPK function of Nm23. More generally, newer metastatic models that recapitulate important aspects and the heterogeneity of human disease will help prove the generality of translational findings.
Acknowledgments
This research was supported by the Intramural Program of National Cancer Institute.
References
- 1.Steeg PS, et al. (1988). Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Research, 48 (22), 6550–6554. [PubMed] [Google Scholar]
- 2.Dearolf CR, Hersperger E, & Shearn A (1988). Developmental consequences of awdb3, a cell-autonomous lethal mutation of Drosophila induced by hybrid dysgenesis. Developmental Biology, 129(1), 159–168. [DOI] [PubMed] [Google Scholar]
- 3.Dearolf CR, et al. (1988). Molecular consequences of awdb3, a cell-autonomous lethal mutation of Drosophila induced by hybrid dysgenesis. Developmental Biology, 129(1), 169–178. [DOI] [PubMed] [Google Scholar]
- 4.Lacombe ML, et al. (2000). The human Nm23/nucleoside diphosphate kinases. Journal of Bioenergetics and Biomembranes, 32 (3), 247–258. [DOI] [PubMed] [Google Scholar]
- 5.Steeg PS, Zollo M, &Wieland T. (2011). A critical evaluation of biochemical activities reported for the nucleoside diphosphate kinase/Nm23/Awd family proteins: opportunities and missteps in understanding their biological functions. Naunyn-Schmiedeberg’s Archives of Pharmacology, 384(4–5), 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg P, & Joklik WK (1953). Transphosphorylation between nucleoside polyphosphates. Nature, 172(4387), 1008–1009. [DOI] [PubMed] [Google Scholar]
- 7.Krebs HA, & Hems R (1953). Some reactions of adenosine and inosine phosphates in animal tissues. Biochimica et Biophysica Acta, 12(1–2), 172–180. [DOI] [PubMed] [Google Scholar]
- 8.Wallet V, et al. (1990). Dictyostelium nucleoside diphosphate kinase higH1y homologous to Nm23 and Awd proteins involved in mammalian tumor metastasis and Drosophila development. Journal of the National Cancer Institute, 82(14), 1199–1202. [DOI] [PubMed] [Google Scholar]
- 9.Hippe HJ, et al. (2003). Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gbeta subunits. Specific activation of Gsalpha by an NDPK B.Gbetagamma complex in H10 cells. Journal of Biological Chemistry, 278(9), 7227–7233. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, et al. (1995). The gene for nucleoside diphosphate kinase functions as a mutator gene in Escherichia coli. Journal of Molecular Biology, 254(3), 337–341. [DOI] [PubMed] [Google Scholar]
- 11.Hartsough MT, et al. (2002). Nm23-H1 metastasis suppressor phosphorylation of kinase suppressor of Ras via a histidine protein kinase pathway. Journal of Biological Chemistry, 277(35), 32389–32399. [DOI] [PubMed] [Google Scholar]
- 12.Steeg PS, Horak CE, & Miller KD (2008). Clinicaltranslational approaches to the Nm23-H1 metastasis suppressor. Clinical Cancer Research, 14(16), 5006–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steeg PS, et al. (2003). Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Cancer Letters, 190(1), 1–12. [DOI] [PubMed] [Google Scholar]
- 14.Ma D, McCorkle JR, & Kaetzel DM (2004). The metastasis suppressor NM23-H1 possesses 3′-;5′ exonuclease activity. Journal of Biological Chemistry, 279(17), 18073–18084. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, et al. (2011). Metastasis suppressor function of NM23-H1 requires its 3––5′ exonuclease activity. International Journal of Cancer, 128(1), 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarrett SG, et al. (2012). Metastasis suppressor NM23-H1 promotes repair of UV-induced DNA damage and suppresses UV-induced melanomagenesis. Cancer Research, 72(1), 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garzia L, et al. (2008). Phosphorylation of nm23-H1 by CKI induces its complex formation with h-prune and promotes cell motility. Oncogene, 27(13), 1853–1864. [DOI] [PubMed] [Google Scholar]
- 18.Reymond A, et al. (1999). Evidence for interaction between human PRUNE and nm23-H1 NDPKinase. Oncogene, 18(51), 7244–7252. [DOI] [PubMed] [Google Scholar]
- 19.Kaul R, et al. (2007). Epstein-Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. Journal of Virology, 81(19), 10352–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian C, Cotter MA 2nd, & Robertson ES (2001). Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23-H1: a molecular link to cancer metastasis. Nature Medicine, 7(3), 350–355. [DOI] [PubMed] [Google Scholar]
- 21.Horak CE, et al. (2007). Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Research, 67(15), 7238–7246. [DOI] [PubMed] [Google Scholar]
- 22.Horak CE, et al. (2007). Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Research, 67(24), 11751–11759. [DOI] [PubMed] [Google Scholar]
- 23.Jean-Claude A Marshall JCA., J Collins JW, Nakayama J, Horak HE, Liewehr DJ, et al. (2012). Inhibition of LPA1/EDG2 Induces Metastatic Dormancy in Breast Cancer. JNCI, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MJ, et al. (2011). Pro-oncogenic potential of NM23-H2 in hepatocellular carcinoma. Experimental & Molecular Medicine, 44(3): 214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki H, et al. (1999). Overexpression of nm23-H2/NDP kinase B in a human oral squamous cell carcinoma cell line results in reduced metastasis, differentiated phenotype in the metastatic site, and growth factor-independent proliferative activity in culture. Clinical Cancer Research, 5(12), 4301–4307. [PubMed] [Google Scholar]
- 26.Boissan M, et al. (2010). Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Research, 70 (19), 7710–7722. [DOI] [PubMed] [Google Scholar]
- 27.Arnaud-Dabernat S, et al. (2003). Knockout mice as model systems for studying nm23/NDP kinase gene functions. Application to the nm23-M1 gene. Journal of Bioenergetics and Biomembranes, 35(1), 19–30. [DOI] [PubMed] [Google Scholar]
- 28.Boissan M, et al. (2005). Increased lung metastasis in transgenic NM23-Null/SV40 mice with hepatocellular carcinoma. Journal of the National Cancer Institute, 97(11), 836–845. [DOI] [PubMed] [Google Scholar]
- 29.Postel EH, et al. (2009). Targeted deletion of Nm23/nucleoside diphosphate kinase A and B reveals their requirement for definitive erythropoiesis in the mouse embryo. Developmental Dynamics, 238(3), 775–787. [DOI] [PubMed] [Google Scholar]
- 30.Hippe HJ, et al. (2009). The interaction of nucleoside diphosphate kinase B with Gbetagamma dimers controls heterotrimeric G protein function. Proceedings of the National Academy of Sciences of the United States of America, 106 (38), 16269–16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hippe HJ, et al. (2011). Nucleoside diphosphate kinase B is required for the formation of heterotrimeric G protein containing caveolae. Naunyn-Schmiedeberg’s Archives of Pharmacology, 384(4–5), 461–472. [DOI] [PubMed] [Google Scholar]
- 32.Di L, et al. (2010). Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel KCa3.1, resulting in defective T cell activation. Journal of Biological Chemistry, 285 (50), 38765–38771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirotkovic-Skerlev M, et al. (2005). Expression of c-myc, erbB-2, p53 and nm23-H1 gene product in benign and malignant breast lesions: coexpression and correlation with clinicopathologic parameters. Experimental and Molecular Pathology, 79(1), 42–50. [DOI] [PubMed] [Google Scholar]
- 34.Muller W, et al. (1998). Expression of nm23 in gastric carcinoma: association with tumor progression and poor prognosis. Cancer, 83(12), 2481–2487. [DOI] [PubMed] [Google Scholar]
- 35.Kapitanovic S, et al. (2004). nm23-H1 expression and loss of heterozygosity in colon adenocarcinoma. Journal of Clinical Pathology, 57(12), 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar Dhar D, et al. (1999). nm23 in the primary and metastatic sites of gastric carcinoma. Relation to AFP-producing carcinoma. Oncology, 56(2), 122–128. [DOI] [PubMed] [Google Scholar]
- 37.Betke H, et al. (1998). The role of nm23 in melanoma progression and its prognostic significance. Polish Journal of Pathology, 49(2), 93–96. [PubMed] [Google Scholar]
- 38.Guan-Zhen Y, et al. (2007). Reduced protein expression of metastasis-related genes (nm23, KISS1, KAI1 and p53) in lymph node and liver metastases of gastric cancer. International Journal of Experimental Pathology, 88(3), 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takes RP, et al. (2001). Expression of genetic markers in lymph node metastases compared with their primary tumours in head and neck cancer. The Journal of Pathology, 194(3), 298–302. [DOI] [PubMed] [Google Scholar]
- 40.Ohta Y, et al. (2000). Increased vascular endothelial growth factor and vascular endothelial growth factor-c and decreased nm23 expression associated with microdissemination in the lymph nodes in stage I non-small cell lung cancer. The Journal of Thoracic and Cardiovascular Surgery, 119(4 Pt 1), 804–813. [DOI] [PubMed] [Google Scholar]
- 41.Sarac E, et al. (1998). nm23 expression in carcinoma of the uterine cervix. European Journal of Gynaecological Oncology, 19(3), 312–315. [PubMed] [Google Scholar]
- 42.Terasaki-Fukuzawa Y, et al. (2002). Decreased nm23 expression, but not Ki-67 labeling index, is significantly correlated with lymph node metastasis of breast invasive ductal carcinoma. International Journal of Molecular Medicine, 9(1), 25–29. [PubMed] [Google Scholar]
- 43.Leone A, et al. (1993). Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastomas. Oncogene, 8(4), 855–865. [PubMed] [Google Scholar]
- 44.Oda Y, et al. (2000). Comparison of histological changes and changes in nm23 and c-MET expression between primary and metastatic sites in osteosarcoma: a clinicopathologic and immunohistochemical study. Human Pathology, 31(6), 709–716. [DOI] [PubMed] [Google Scholar]
- 45.Niitsu N, et al. (2001). Serum nm23-H1 protein as a prognostic factor in aggressive non-Hodgkin lymphoma. Blood, 97(5), 1202–1210. [DOI] [PubMed] [Google Scholar]
- 46.Okabe-Kado J, et al. (1995). Inhibitory action of nm23 proteins on induction of erythroid differentiation of human leukemia cells. Biochimica et Biophysica Acta, 1267(2-3), 101–106. [DOI] [PubMed] [Google Scholar]
- 47.Okabe-Kado J (2002). Serum nm23-H1 protein as a prognostic factor in hematological malignancies. Leukemia & Lymphoma, 43 (4), 859–867. [DOI] [PubMed] [Google Scholar]
- 48.Anzinger J, et al. (2001). Secretion of a nucleoside diphosphate kinase (Nm23-H2) by cells from human breast, colon, pancreas and lung tumors. Proceedings of the Western Pharmacology Society, 44, 61–63. [PubMed] [Google Scholar]
- 49.Niitsu N, et al. (2000). Plasma levels of the differentiation inhibitory factor nm23-H1 protein and their clinical implications in acute myelogenous leukemia. Blood, 96(3), 1080–1086. [PubMed] [Google Scholar]
- 50.Okabe-Kado J, et al. (2009). Extracellular NM23-H1 protein inhibits the survival of primary cultured normal human peripheral blood mononuclear cells and activates the cytokine production. International Journal of Hematology, 90(2), 143–152. [DOI] [PubMed] [Google Scholar]
- 51.Lilly AJ, et al. (2011). Nm23-h1 indirectly promotes the survival of acute myeloid leukemia blast cells by binding to more mature components of the leukemic clone. Cancer Research, 71 (3), 1177–1186. [DOI] [PubMed] [Google Scholar]
- 52.Ouatas T, Halverson D, & Steeg PS (2003). Dexamethasone and medroxyprogesterone acetate elevate Nm23-H1 metastasis suppressor gene expression in metastatic human breast carcinoma cells: new uses for old compounds. Clinical Cancer Research, 9 (10 Pt 1), 3763–3772. [PubMed] [Google Scholar]
- 53.Palmieri D, et al. (2005). Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. Journal of the National Cancer Institute, 97(9), 632–642. [DOI] [PubMed] [Google Scholar]
- 54.Siveen KS,& Kuttan G (2011). Thujone inhibits lung metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Canadian Journal of Physiology and Pharmacology, 89(10), 691–703. [DOI] [PubMed] [Google Scholar]
- 55.Niu YC, et al. (2009). A low molecular weight polysaccharide isolated from Agaricus blazei Murill (LMPAB) exhibits its anti-metastatic effect by down-regulating metalloproteinase-9 and up-regulating Nm23-H1. The American Journal of Chinese Medicine, 37(5), 909–921. [DOI] [PubMed] [Google Scholar]
- 56.Guruvayoorappan C, & Kuttan G (2008). Amentoflavone inhibits experimental tumor metastasis through a regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase, lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in lung tissues of C57BL/6 mice. Immunopharmacology and Immunotoxicology, 30 (4), 711–727. [DOI] [PubMed] [Google Scholar]
- 57.Li J, et al. (2006). Inhibition of ovarian cancer metastasis by adeno-associated virus-mediated gene transfer of nm23H1 in an orthotopic implantation model. Cancer Gene Therapy, 13(3), 266–272. [DOI] [PubMed] [Google Scholar]
- 58.Xiang JJ, et al. (2003). IONP-PLL: a novel non-viral vector for efficient gene delivery. The Journal of Gene Medicine, 5(9), 803–817. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, et al. (2009). Nanoparticle delivery of anti-metastatic NM23-H1 gene improves chemotherapy in a mouse tumor model. Cancer Gene Therapy, 16(5), 423–429. [DOI] [PubMed] [Google Scholar]
- 60.Lim J, et al. (2011). Cell-permeable NM23 blocks the maintenance and progression of established pulmonary metastasis. Cancer Research, 71(23), 7216–7225. [DOI] [PubMed] [Google Scholar]
- 61.Choi JW, et al. (2010). LPA receptors: subtypes and biological actions. Annual Review of Pharmacology and Toxicology, 50, 157–186. [DOI] [PubMed] [Google Scholar]
- 62.Ishii I, et al. (2004). Lysophospholipid receptors: signaling and biology. Annual Review of Biochemistry, 73, 321–354. [DOI] [PubMed] [Google Scholar]
- 63.Mills GB, & Moolenaar WH (2003). The emerging role of lysophosphatidic acid in cancer. Nature Reviews. Cancer, 3(8), 582–591. [DOI] [PubMed] [Google Scholar]
- 64.Aguirre-Ghiso JA, et al. (2003). ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38 (SAPK). Cancer Research, 63(7), 1684–1695. [PubMed] [Google Scholar]
- 65.Aguirre-Ghiso JA (2007). Models, mechanisms and clinical evidence for cancer dormancy. Nature Reviews. Cancer, 7(11), 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aguirre-Ghiso JA, Ossowski L, & Rosenbaum SK (2004). Green fluorescent protein tagging of extracellular signalregulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Research, 64(20), 7336–7345. [DOI] [PubMed] [Google Scholar]
- 67.Sosa MS, et al. (2011). ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clinical Cancer Research, 17(18), 5850–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Otsuki Y, et al. (2001). Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proceedings of the National Academy of Sciences of the United States of America, 98(8), 4385–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SH, & Kim J (2006). Reduction of invasion in human fibrosarcoma cells by ribosomal protein S3 in conjunction with Nm23-H1 and ERK. Biochimica et Biophysica Acta, 1763(8), 823–832. [DOI] [PubMed] [Google Scholar]
- 70.Iwashita S, et al. (2004). Lbc proto-oncogene product binds to and could be negatively regulated by metastasis suppressor nm23-H2. Biochemical and Biophysical Research Communications, 320(4), 1063–1068. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J, et al. (1999). Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proceedings of the National Academy of Sciences of the United States of America, 96(26), 14911–14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murakami M, et al. (2008). The suppressor of metastasis Nm23-H1 interacts with the Cdc42 Rho family member and the pleckstrin homology domain of oncoprotein Dbl-1 to suppress cell migration. Cancer Biology & Therapy, 7(5), 677–688. [DOI] [PubMed] [Google Scholar]
- 73.Palacios F, et al. (2002).ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nature Cell Biology, 4(12), 929–936. [DOI] [PubMed] [Google Scholar]
- 74.Seong HA, Jung H, & Ha H (2007). NM23-H1 tumor suppressor physically interacts with serine-threonine kinase receptor-associated protein, a transforming growth factor-beta (TGF-beta) receptor-interacting protein, and negatively regulates TGF-beta signaling. Journal of Biological Chemistry, 282(16), 12075–12096. [DOI] [PubMed] [Google Scholar]
- 75.Curtis CD, et al. (2007). Interaction of the tumor metastasis suppressor nonmetastatic protein 23 homologue H1 and estrogen receptor alpha alters estrogen-responsive gene expression. Cancer Research, 67(21), 10600–10607. [DOI] [PubMed] [Google Scholar]
- 76.Rayner K, et al. (2008). Discovery of NM23-H2 as an estrogen receptor beta-associated protein: role in estrogen-induced gene transcription and cell migration. The Journal of Steroid Biochemistry and Molecular Biology, 108(1-2), 72–81. [DOI] [PubMed] [Google Scholar]
- 77.Paravicini G, et al. (1996). The metastasis suppressor candidate nucleotide diphosphate kinase NM23 specifically interacts with members of the ROR/RZR nuclear orphan receptor subfamily. Biochemical and Biophysical Research Communications, 227(1), 82–87. [DOI] [PubMed] [Google Scholar]
- 78.Zheng L, Roeder RG, & Luo Y (2003). S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell, 114(2), 255–266. [DOI] [PubMed] [Google Scholar]
- 79.Fan Z, et al. (2003). Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell, 112(5), 659–672. [DOI] [PubMed] [Google Scholar]
- 80.Otero AS (2000). NM23/nucleoside diphosphate kinase and signal transduction. Journal of Bioenergetics and Biomembranes, 32(3), 269–275. [DOI] [PubMed] [Google Scholar]
- 81.Pinon VP, et al. (1999). Cytoskeletal association of the A and B nucleoside diphosphate kinases of interphasic but not mitotic human carcinoma cell lines: specific nuclear localization of the B subunit. Experimental Cell Research, 246(2), 355–367. [DOI] [PubMed] [Google Scholar]
- 82.Fournier HN, et al. (2002). Integrin cytoplasmic domainassociated protein 1alpha (ICAP-1alpha ) interacts directly with the metastasis suppressor nm23-H2, and both proteins are targeted to newly formed cell adhesion sites upon integrin engagement. Journal of Biological Chemistry, 277(23), 20895–20902. [DOI] [PubMed] [Google Scholar]
- 83.Aktary Z, et al. (2010). Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene, 29(14), 2118–2129. [DOI] [PubMed] [Google Scholar]
- 84.D’Angelo A, et al. (2004). Prune cAMP phosphodiesterase binds nm23-H1 and promotes cancer metastasis. Cancer Cell, 5(2), 137–149. [DOI] [PubMed] [Google Scholar]
- 85.Polanski R, et al. (2011). MDM2 interacts with NME2 (non-metastatic cells 2, protein) and suppresses the ability of NME2 to negatively regulate cell motility. Carcinogenesis, 32(8), 1133–1142. [DOI] [PubMed] [Google Scholar]
- 86.Mileo AM, et al. (2006). Multiple interference of the human papillomavirus-16 E7 oncoprotein with the functional role of the metastasis suppressor Nm23-H1 protein. Journal of Bioenergetics and Biomembranes, 38(3-4), 215–225. [DOI] [PubMed] [Google Scholar]
- 87.Engel M, et al. (1998). Glyceraldehyde-3-phosphate dehydrogenase and Nm23-H1/nucleoside diphosphate kinase A. Two old enzymes combine for the novel Nm23 protein phosphotransferase function. Journal of Biological Chemistry, 273(32), 20058–20065. [DOI] [PubMed] [Google Scholar]
- 88.Du J, & Hannon GJ (2002). The centrosomal kinase Aurora-A/STK15 interacts with a putative tumor suppressor NM23-H1. Nucleic Acids Research, 30(24), 5465–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leone A, et al. (1993). Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene, 8(9), 2325–2333. [PubMed] [Google Scholar]
- 90.Bhujwalla ZM, et al. (1999). Nm23-transfected MDA-MB-435 human breast carcinoma cells form tumors with altered phospholipid metabolism and pH: a 31P nuclear magnetic resonance study in vivo and in vitro. Magnetic Resonance in Medicine, 41 (5), 897–903. [DOI] [PubMed] [Google Scholar]
- 91.Russell RL, et al. (1998). Relationship of nm23 to proteolytic factors, proliferation and motility in breast cancer tissues and cell lines. British Journal of Cancer, 78(6), 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuda M, et al. (1996). Decreased expression of nucleoside diphosphate kinase alpha isoform, an nm23-H2 gene homolog, is associated with metastatic potential of rat mammary-adenocarcinoma cells. International Journal of Cancer, 65(4), 531–537. [DOI] [PubMed] [Google Scholar]
- 93.Leone A, et al. (1991). Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell, 65(1), 25–35. [DOI] [PubMed] [Google Scholar]
- 94.Baba H, et al. (1995). Two isotypes of murine nm23/nucleoside diphosphate kinase, nm23-M1 and nm23-M2, are involved in metastatic suppression of a murine melanoma line. Cancer Research, 55(9), 1977–1981. [PubMed] [Google Scholar]
- 95.Parhar RS, et al. (1995). Effects of cytokine-mediated modulation of nm23 expression on the invasion and metastatic behavior of B16F10 melanoma cells. International Journal of Cancer, 60(2), 204–210. [DOI] [PubMed] [Google Scholar]
- 96.Miele ME, et al. (1997). Suppression of human melanoma metastasis following introduction of chromosome 6 is independent of NME1 (Nm23). Clinical & Experimental Metastasis, 15 (3), 259–265. [DOI] [PubMed] [Google Scholar]
- 97.Tagashira H, et al. (1998). Reduced metastatic potential and cmyc overexpression of colon adenocarcinoma cells (Colon 26 line) transfected with nm23-R2/rat nucleoside diphosphate kinase alpha isoform. International Journal of Molecular Medicine, 2 (1), 65–68. [DOI] [PubMed] [Google Scholar]
- 98.Suzuki E, et al. (2004). nm23-H1 reduces in vitro cell migration and the liver metastatic potential of colon cancer cells by regulating myosin light chain phosphorylation. International Journal of Cancer, 108(2), 207–211. [DOI] [PubMed] [Google Scholar]
- 99.Bevilacqua G, et al. (1989). Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Research, 49(18), 5185–5190. [PubMed] [Google Scholar]
- 100.Hennessy C, et al. (1991). Expression of the antimetastatic gene nm23 in human breast cancer: an association with good prognosis. Journal of the National Cancer Institute, 83(4), 281–285. [DOI] [PubMed] [Google Scholar]
- 101.Charpin C, et al. (1998). Prognostic significance of Nm23/NDPK expression in breast carcinoma, assessed on 10-year follow-up by automated and quantitative immunocytochemical assays. The Journal of Pathology, 184(4), 401–407. [DOI] [PubMed] [Google Scholar]
- 102.Dong SW, et al. (2011). Expression patterns of ER, HER2, and NM23-H1 in breast cancer patients with different menopausal status: correlations with metastasis. Molecular Diagnosis & Therapy, 15(4), 211–219. [DOI] [PubMed] [Google Scholar]
- 103.Heimann R, Ferguson DJ, & Hellman S (1998). The relationship between nm23, angiogenesis, and the metastatic proclivity of node-negative breast cancer. Cancer Research, 58(13), 2766–2771. [PubMed] [Google Scholar]
- 104.Tokunaga Y, et al. (1993). Reduced expression of nm23-H1, but not of nm23-H2, is concordant with the frequency of lymph-node metastasis of human breast cancer. International Journal of Cancer, 55(1), 66–71. [DOI] [PubMed] [Google Scholar]
- 105.Royds JA, et al. (1993). Nm23 protein expression in ductal in situ and invasive human breast carcinoma. Journal of the National Cancer Institute, 85(9), 727–731. [DOI] [PubMed] [Google Scholar]
- 106.Bal A, et al. (2008). Expression of nm23 in the spectrum of preinvasive, invasive and metastatic breast lesions. Diagnostic Pathology, 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrari D, et al. (2007). Dermatopathological indicators of poor melanoma prognosis are significantly inversely correlated with the expression of NM23 protein in primary cutaneous melanoma. Journal of Cutaneous Pathology, 34(9), 705–712. [DOI] [PubMed] [Google Scholar]
- 108.McDermott NC, et al. (2000). Immunohistochemical expression of nm23 in primary invasive malignant melanoma is predictive of survival outcome. The Journal of Pathology, 190(2), 157–162. [DOI] [PubMed] [Google Scholar]
- 109.Sarris M, et al. (2004). Cytoplasmic expression of nm23 predicts the potential for cerebral metastasis in patients with primary cutaneous melanoma. Melanoma Research, 14(1), 23–27. [DOI] [PubMed] [Google Scholar]
- 110.Dome B, Somlai B, & Timar J (2000). The loss of NM23 protein in malignant melanoma predicts lymphatic spread without affecting survival. Anticancer Research, 20(5C), 3971–3974. [PubMed] [Google Scholar]
- 111.Pacifico MD, et al. (2005). nm23 as a prognostic marker in primary cutaneous melanoma: evaluation using tissue microarray in a patient group with long-term follow-up. Melanoma Research, 15(5), 435–440. [DOI] [PubMed] [Google Scholar]
- 112.Andolfo I, et al. (2011). Correlation of NM23-H1 cytoplasmic expression with metastatic stage in human prostate cancer tissue. Naunyn-Schmiedeberg’s Archives of Pharmacology, 384(4–5), 489–498. [DOI] [PubMed] [Google Scholar]
- 113.Mandai M, et al. (1994). Expression of metastasis-related nm23-H1 and nm23-H2 genes in ovarian carcinomas: correlation with clinicopathology, EGFR, c-erbB-2, and c-erbB-3 genes, and sex steroid receptor expression. Cancer Research, 54(7), 1825–1830. [PubMed] [Google Scholar]
- 114.Hsu NY, et al. (1999). Expression of nm23 in the primary tumor and the metastatic regional lymph nodes of patients with gastric cardiac cancer. Clinical Cancer Research, 5(7), 1752–1757. [PubMed] [Google Scholar]
- 115.Yamaguchi A, et al. (1994). Expression of human nm23-H1 and nm23-H2 proteins in hepatocellular carcinoma. Cancer, 73(9), 2280–2284. [DOI] [PubMed] [Google Scholar]
- 116.Guo H, et al. (2010). Prognostic significance of co-expression of nm23 and p57 protein in hepatocellular carcinoma. Hepatology Research, 40(11), 1107–1116. [DOI] [PubMed] [Google Scholar]
- 117.An R, et al. (2010). Expressions of nucleoside diphosphate kinase (nm23) in tumor tissues are related with metastasis and length of survival of patients with hepatocellular carcinoma. Biomedical and Environmental Sciences, 23(4), 267–272. [DOI] [PubMed] [Google Scholar]
- 118.Hsu NY, et al. (2007). Prognostic significance of expression of nm23-H1 and focal adhesion kinase in non-small cell lung cancer. Oncology Reports, 18(1), 81–85. [PubMed] [Google Scholar]
- 119.Goncharuk VN, et al. (2004). Co-downregulation of PTEN, KAI-1, and nm23-H1 tumor/metastasis suppressor proteins in non-small cell lung cancer. Annals of Diagnostic Pathology, 8(1), 6–16. [DOI] [PubMed] [Google Scholar]
- 120.Ohta Y, et al. (2001). The predictive value of vascular endothelial growth factor and nm23 for the diagnosis of occult metastasis in non-small cell lung cancer. Japanese Journal of Cancer Research, 92(3), 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang YF, et al. (2004). Prognostic significance of nm23-H1 expression in oral squamous cell carcinoma. British Journal of Cancer, 90(11), 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Engel M, et al. (1993). High levels of nm23-H1 and nm23-H2 messenger RNA in human squamous-cell lung carcinoma are associated with poor differentiation and advanced tumor stages. International Journal of Cancer, 55(3), 375–379. [DOI] [PubMed] [Google Scholar]
- 123.Yokoyama A, et al. (1996). Differentiation inhibitory factor nm23 as a new prognostic factor in acute monocytic leukemia. Blood, 88(9), 3555–3561. [PubMed] [Google Scholar]
- 124.Niitsu N, et al. (2003). Expression of nm23-H1 is associated with poor prognosis in peripheral T-cell lymphoma. British Journal of Haematology, 123(4), 621–630. [DOI] [PubMed] [Google Scholar]
- 125.Niitsu N, Nakamine H, & Okamoto M (2011). Expression of nm23-H1 is associated with poor prognosis in peripheral T-cell lymphoma, not otherwise specified. Clinical Cancer Research, 17 (9), 2893–2899. [DOI] [PubMed] [Google Scholar]
- 126.Niitsu N, et al. (2004). Clinical significance of intracytoplasmic nm23-H1 expression in diffuse large B-cell lymphoma. Clinical Cancer Research, 10(7), 2482–2490. [DOI] [PubMed] [Google Scholar]
- 127.Niitsu N, et al. (2008). A clinicopathological study of nm23-H1 expression in classical Hodgkin’s lymphoma. Annals of Oncology, 19(11), 1941–1946. [DOI] [PubMed] [Google Scholar]
- 128.Ferenc T, et al. (2004). Analysis of nm23-H1 protein immunoreactivity in follicular thyroid tumors. Polish Journal of Pathology, 55 (4), 149–153. [PubMed] [Google Scholar]


