Summary
Insulin regulates glucose metabolism through thousands of regulatory mechanisms; however, which regulatory mechanisms are keys to control glucose metabolism remains unknown. Here, we performed kinetic trans-omic analysis by integrating isotope-tracing glucose flux and phosphoproteomic data from insulin-stimulated adipocytes and built a kinetic mathematical model to identify key allosteric regulatory and phosphorylation events for enzymes. We identified nine reactions regulated by allosteric effectors and one by enzyme phosphorylation and determined the regulatory mechanisms for three of these reactions. Insulin stimulated glycolysis by promoting Glut4 activity by enhancing phosphorylation of AS160 at S595, stimulated fatty acid synthesis by promoting Acly activity through allosteric activation by glucose 6-phosphate or fructose 6-phosphate, and stimulated glutamate synthesis by alleviating allosteric inhibition of Gls by glutamate. Most of glycolytic reactions were regulated by amounts of substrates and products. Thus, phosphorylation or allosteric modulator-based regulation of only a few key enzymes was sufficient to change insulin-induced metabolism.
Subject Areas: Biological Sciences, Mathematical Biosciences, Systems Biology, Proteomics, Metabolomics, Metabolic Flux Analyisis, Omics
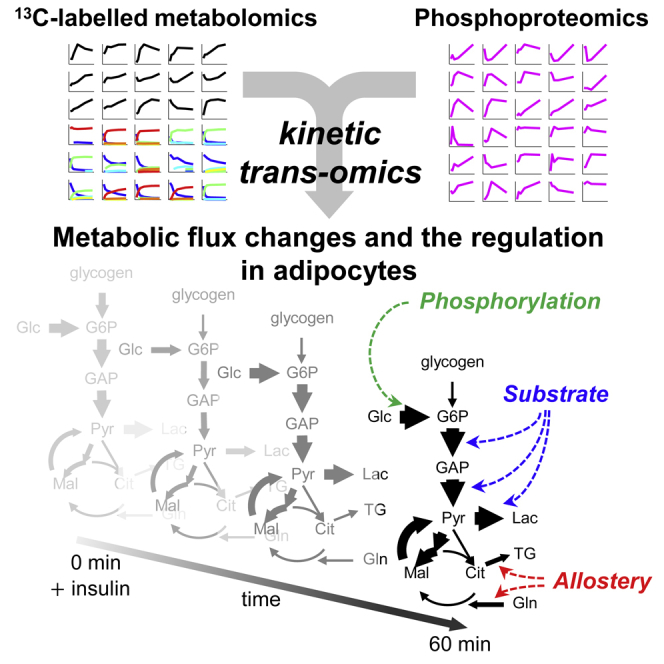
Graphical Abstract
Highlights
-
•
We developed a kinetic trans-omic network of insulin-stimulated adipocyte metabolism
-
•
Flux changes over time through the glucose metabolism were quantitatively estimated
-
•
Nine fluxes were controlled by allosteric regulation and one by phosphorylation
-
•
Key regulators were phosphorylated AS160 and the allosteric regulators of Gls and Acly
Biological Sciences; Mathematical Biosciences; Systems Biology; Proteomics; Metabolomics; Metabolic Flux Analyisis; Omics
Introduction
Cellular metabolism controls the storage of nutrients, production of energy, and synthesis of precursors of cellular components. In addition to maintaining metabolic homeostasis, cells change their metabolism in response to internal and external conditions by dynamic changes in fluxes of metabolites (Plum et al., 2006; Ray et al., 2012; Salih and Brunet, 2008). Cellular metabolism is regulated not only by the concentrations of substrates, products, and metabolic enzymes but also by acute changes in the activities of the metabolic enzymes, which are regulated by post-translational modifications of the enzymes, such as phosphorylation, as well as by allosteric effectors (Cairns et al., 2011; Kochanowski et al., 2015; Saltiel and Kahn, 2001; Yugi and Kuroda, 2017). To investigate the regulation of cellular metabolism, comprehensive studies of intracellular molecules have been conducted, using methods such as mass spectrometry(MS)-based phosphoproteomics (Humphrey et al., 2015; Jünger and Aebersold, 2014; Macek et al., 2009) and metabolomics (Hirayama et al., 2014; Peng et al., 2015; Zamboni et al., 2015). However, key regulatory mechanisms controlling cellular metabolism remain unknown, because the phosphorylation state of many proteins and the concentrations of many metabolites are dynamically changing. Together, changes in protein phosphorylation and the abundances of substrates, products, and allosteric regulators alter the activity of numerous enzymes. One approach to explore how cellular metabolism is controlled is through integration of multi-omic data with biochemical reaction data (Gerosa et al., 2015; Hackett et al., 2016; Kawata et al., 2018; Krycer et al., 2017; Yugi et al., 2014). This approach is called trans-omic analyses (Yugi et al., 2016, 2019). In previous trans-omic studies of mammalian cells responding to external hormone insulin, multi-omic data were integrated to construct qualitative trans-omic networks of hormone-induced metabolism (Kawata et al., 2018; Krycer et al., 2017; Yugi et al., 2014). However, qualitative trans-omic networks do not directly indicate how much each regulatory mechanism affects cellular metabolism. Quantitative, time-resolved, multi-omic data are required to identify the contributions of specific regulatory mechanisms to hormone-induced changes in metabolism. The temporally changing data need to be incorporated into a kinetic mathematical model that includes metabolic enzymes, their substrates, products, and allosteric effectors, along with relevant phosphorylated proteins. In this study, we described trans-omic networks by kinetic equations together with time-resolved multi-omic data. We refer to this approach as kinetic trans-omic analysis. The kinetic trans-omic analysis enables identification of key regulatory mechanisms that likely influence downstream molecules from many candidate regulatory mechanisms through model selection. Moreover, the kinetic trans-omic analysis provides quantitative understanding of the relative contributions of specific regulatory mechanisms to cellular metabolism in response to key perturbations.
Insulin is a key hormone that regulates cellular glucose metabolism. Adipocytes respond to insulin by changing the flux of glucose through various metabolic pathways (Dimitriadis et al., 2011; Giorgino et al., 2005; Krycer et al., 2017, 2020b; 2020a; Quek et al., 2020). In 14C-glucose labeling experiments with adipocytes (Cahill et al., 1959; Katz et al., 1966), insulin increases fluxes through the metabolic pathways of glucose uptake, glycolysis, oxidative pentose phosphate (PP) pathway, glycogen synthesis, glycerol synthesis, and fatty acid synthesis. In these studies, fluxes are determined using the assumption that metabolism achieves a new steady-state condition with constant concentrations of substrates and products after insulin stimulation. However, this assumption is physiologically inaccurate particularly in the context of mammalian physiology. For example, in vivo insulin-stimulated changes in the fluxes through metabolic pathways are highly dynamic and are probably never at a steady state (Krycer et al., 2017, 2020a).
Various regulatory mechanisms have been reported for glucose metabolism in adipocytes or for enzymes that participate in pathways that use carbon from glucose. For example, phosphorylation controls the abundance of glucose transporter type 4 (Glut4) at the plasma membrane. Insulin stimulates glucose uptake by promoting the translocation of Glut4 into the plasma membrane (Dimitriadis et al., 2011; Giorgino et al., 2005). Akt-dependent phosphorylation of AS160, a GTPase-activating protein (GAP) for Rab GTPases, promotes Glut4 translocation into the plasma membrane. Rabs are required for the movement of Glut4 vesicles to the plasma membrane, thus phosphorylation-mediated de-inhibition of Rabs triggers Glut4 exocytosis (Huang and Czech, 2007). Once glucose is transported into the cell by Glut4, glucose flows into glycolysis. In many cell lines and organs, phosphofructokinase 1 (Pfk1) is allosterically activated by fructose 2,6-bisphosphate (F2,6BP) and inhibited by ATP and citrate (Mor et al., 2011; Passonneau and Lowry, 1963; Schöneberg et al., 2013), whereas pyruvate kinase is allosterically activated by fructose 1,6-bisphosphate (F1,6BP) and inhibited by ATP (Israelsen and Vander Heiden, 2015; Munday et al., 1980). Regulation of fatty acid synthesis is also complex. When insulin stimulates glycolysis in adipose tissue, ATP-citrate lyase (Acly) is allosterically activated by intermediates of glucose metabolism, including glucose 6-phosphate (G6P), fructose 6-phosphate (F6P), fructose 1,6-bisphosphate (F1,6BP), F2,6BP, and phosphoenolpyruvate (Potapova et al., 2000). Phosphorylation of Acly also increases the activity of the enzyme, whereas phosphorylation of fatty acid synthase (Fasn) and acetyl-CoA carboxylases 1 (Acc1) decrease their activities (Song et al., 2018).
Although many different regulatory mechanisms for enzymatic processes have been reported, many are based on experiments using different cells in different conditions or on kinetic measurements of isolated enzymes. Consequently, the relative contribution of different regulatory mechanisms to metabolic homeostasis in intact adipocytes upon insulin stimulation remains unclear. Moreover, previous reports are based on data obtained from cells or using enzymes under steady-state conditions. Little is known about the regulatory mechanisms in glucose metabolism during a dynamically changing condition such as in response to insulin (Krycer et al., 2017).
In this study, we identify the key regulatory mechanisms and the relative contributions of these events to glucose metabolism in insulin-stimulated adipocytes. We performed kinetic trans-omic analysis by developing a trans-omic network model based on kinetic equations. We selected the appropriate model with time-resolved metabolomic and phosphoproteomic data acquired under identical experimental conditions (Humphrey et al., 2013; Krycer et al., 2017; Quek et al., 2020). We performed 13C-metabolic flux analysis under non-steady-state conditions to estimate the dynamic changes in glucose metabolic flux using metabolomic data from 13C-labeling MS experiments. Using reaction kinetics, we described the estimated 13C-metabolic flux through each biochemical reaction. Our model includes regulation by enzyme phosphorylation, allosteric effectors, as well as the concentrations of substrates and products. With the kinetic trans-omic analysis, we identified the key regulatory mechanisms, including regulatory events and molecules, of glycolysis and fatty acid biosynthesis in insulin-stimulated adipocytes: insulin stimulated flux through glycolysis by promoting glucose transport/Glut4 translocation via phosphorylation of AS160 at S595. Insulin stimulated flux through Gpat_Acly by allosteric activation by G6P or F6P. Insulin stimulated flux through glutaminase by alleviating allosteric inhibition by glutamate. Our analysis indicated that most glycolytic reactions were regulated by amounts of substrates and products rather than by changes in phosphorylation or by allosteric effectors. Thus, with experimental and modeling approaches, our kinetic trans-omic analysis demonstrates the importance of taking a holistic approach to understand how protein modifications and metabolite regulators work in a dynamically changing complex biochemical network.
Results
Overview of Kinetic Trans-omic Analysis
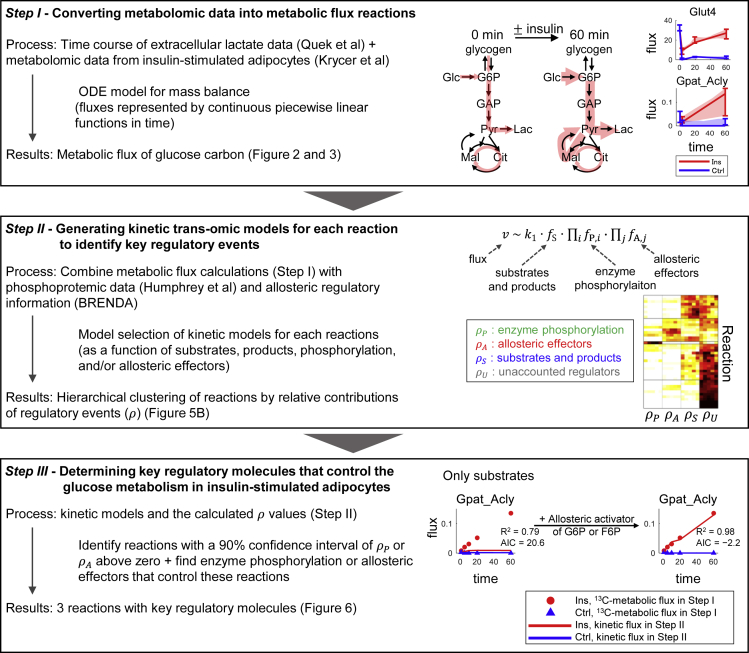
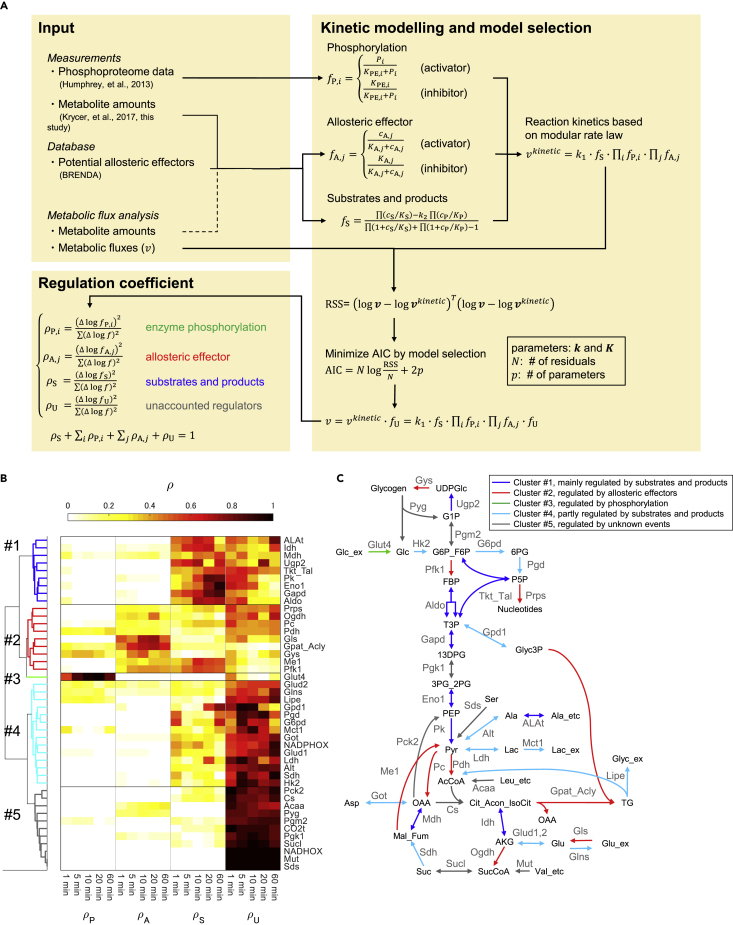
Here, we developed a kinetic trans-omic analysis and identified the key regulatory mechanisms, such as changes in enzyme phosphorylation, allosteric effectors, substrates, and products, for glucose metabolic flux in insulin-stimulated adipocytes (Figure 1). The cells used for the analysis were mouse 3T3-L1 cells differentiated into adipocytes (Green and Kehinde, 1975). The kinetic trans-omic analysis consisted of the following three steps. Step I: we used mass balances in the metabolic network to model flux changes from studies of adipocytes treated with or without insulin (Krycer et al., 2017; Quek et al., 2020). The data were from time-resolved 13C-labeled metabolomic data and extracellular lactate studies; Step II: we developed a kinetic trans-omic model using phosphoproteomic data (Humphrey et al., 2013) and information from a database of allosteric regulators (Placzek et al., 2017) and quantified the contribution of regulatory events mediated by enzyme phosphorylation, allosteric effectors, substrates, and products to the observed changes in metabolic flux; Step III: we identified key regulatory molecules involving phosphorylation of specific proteins or specific allosteric effectors that control flux through insulin-regulated reactions.
Figure 1.
Summary of Kinetic Trans-omic Analysis to Identify Key Regulatory Mechanisms in Insulin-Stimulated Adipocytes
The procedures consist of three steps by integrating 13C-labeled metabolic data and phosphoproteomic data together with kinetic mathematical modeling.
Step I: Converting Metabolomic Data into Metabolic Flux Reactions
We calculated glucose metabolic flux changes in insulin-stimulated adipocytes using 13C-metabolic flux analysis under non-steady state (Figure S1). We used 13C-labeled metabolomic data (Krycer et al., 2017) and extracellular lactate data (Quek et al., 2020) from adipocytes with (Ins) or without insulin (Ctrl) stimulation (Figures 2 and S2 and Data S1A). Adipocytes were exposed to medium containing [U-13C]glucose-containing media in the presence or absence of 100 nM insulin. Intracellular metabolites labeled with 13C were quantified by MS at six time points over a total of 60 min.
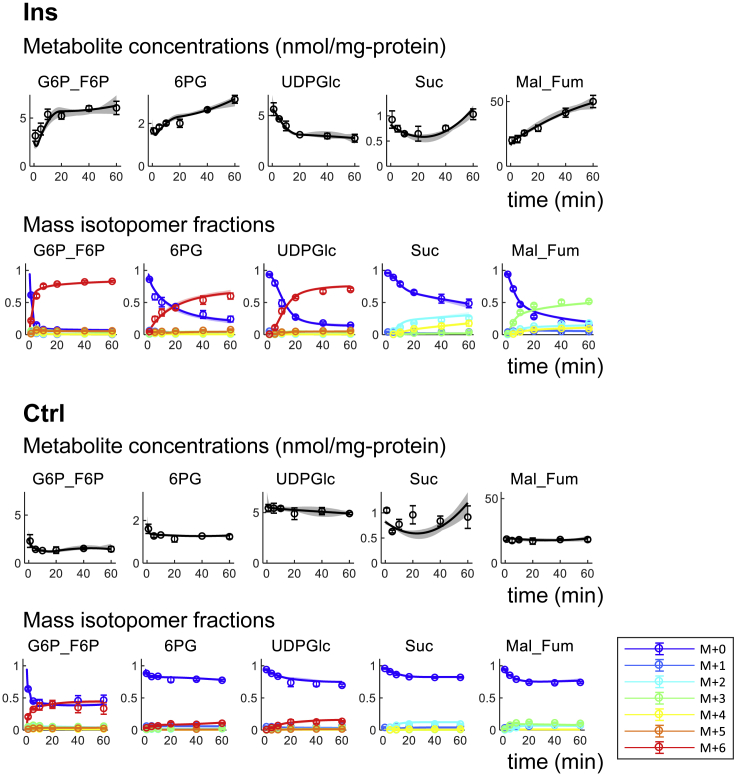
Figure 2.
Measured and Estimated Metabolic Concentrations and Mass Isotopomer Fractions in the Ins and Control Ctrl Conditions
The circles and the error bars indicate the mean and the standard deviations of the measurements from three separate experiments (Krycer et al., 2017). The lines and the shaded areas indicate optimal estimates with the 90% confidence intervals for the values calculated with the model. M + i indicates mass isotopomer with i carbons labeled with 13C. Note that media were exchanged at 0 min in both conditions. All measured and estimated metabolites concentrations and their mass isotopomer fractions are shown in Figure S2, metabolites are defined in Table S1, and estimated concentrations are shown in Table S2 and Data S1.
For the Ins and Ctrl samples, we calculated flux changes from the metabolite concentrations and mass isotopomer fractions using an ordinary differential equation (ODE) model for mass balance with fluxes represented by continuous piecewise linear functions in time (Figure S1, see Transparent Methods). Briefly, we defined reaction stoichiometry and carbon atom transitions in glucose metabolism (Table S1) and developed an ODE model for mass balance of the intracellular metabolites and their mass isotopomers. Some of the adjacent metabolites and their isotopic ratios, such as G6P and F6P, and malate and fumarate, were similar (Figure S2). Therefore, we considered these metabolites to be in rapid equilibrium and summed them as one metabolite, G6P_F6P and Mal_Fum, in the model (Figure 3A, Table S1). For those metabolites that were not measured, such as pyruvate (Pyr) and succinyl-CoA (SucCoA), we used the model to predict their concentrations and mass isotopomer fractions (Figure S2). To represent the non-steady-state nature of the system, we described fluxes as continuous piecewise linear functions in the time domain (Abate et al., 2012; Leighty and Antoniewicz, 2011) (Figure S1). This approach enabled representation of flux changes without using reaction kinetics. Because adipocytes were stimulated with insulin at 0 min, the metabolite concentrations and metabolic fluxes at 0 min in both the Ins and Ctrl conditions were set at the same values. We then calculated optimal flux changes in the Ins and Ctrl conditions (Figure S3, Table S2). To obtain optimal flux changes, we minimized variance-weighted residual sum of squared errors between measured concentrations and mass isotopomer fractions of metabolites and those predicted by the model. Metabolite concentrations and mass isotopomer fractions were highly correlated between measurements and predictions (Figures 2 and S2), indicating that metabolite concentrations and mass isotopomer fractions are reasonably explained by the model.
Figure 3.
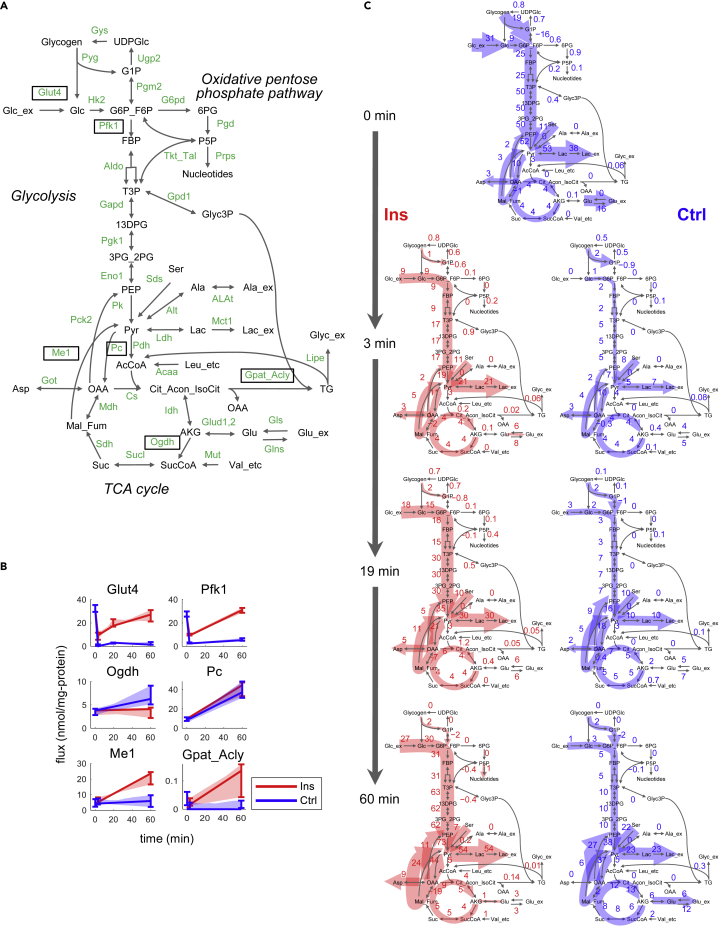
Metabolic Flux Changes in Glucose Metabolism in Adipocytes With or Without Insulin Stimulation
(A) Metabolic network used for metabolic flux analysis. Green characters besides arrows are abbreviations of enzymes or proteins that mediate the reactions (see Table S1 for definitions). Reactions shown in (B) are boxed. Reactions for NADH oxidation, NADPH oxidation, and CO2 transport are not shown but are included in the model for metabolic flux analysis
(B) Estimated flux changes over time through representative reactions of Glut4, Pfk1, Ogdh, Pc, Me1, and Gpat_Acly. The lines and the shaded areas indicate optimal estimates with the 90% confidence intervals. The error bars indicate the 90% confidence intervals at switch times (times when the slope of flux changes), as well as at 0 and 60 min. Different number of time intervals (and switch times) were set among reactions (see Transparent Methods, Figure S1). The confidence intervals were calculated from 200 sets of sampled parameters under constraints based on the covariance matrix of the estimated parameters (see Transparent Methods), and the optimal estimates can be outside the confidence intervals. Fluxes at 0 min between the Ins and Ctrl conditions are set to be the same. All estimated flux changes are shown in Figure S3 and Table S2.
(C) Changes in fluxes in glucose metabolism in adipocytes in the Ins and Ctrl conditions. Numbers besides reactions are the optimal estimates of fluxes with the unit of nmol/mg-protein/min. The thickness of the red or blue shading indicates the relative flux. Fluxes between 2-oxoglutarate (AKG) and glutamate (Glu) are the summation of fluxes through NADH-dependent glutamate dehydrogenase, NADPH-dependent glutamate dehydrogenase, alanine aminotransferase, and aspartate aminotransferase. The negative value of the flux represents backward direction of the reaction, and the direction is defined in Table S1.
See also Figures S3, S6, and S7, Tables S1 and S2, and Data S1.
Insulin increased fluxes through Glut4 and glycolysis (Figures 3A–3C). Flux through Pfk1 (Figures 3A–3C) in glycolysis increased from 9 nmol/mg-protein/min at 3 min to 31 nmol/mg-protein/min at 60 min in the Ins condition, whereas it remained relatively steady (2–5 nmol/mg-protein/min) between these times in the Ctrl condition. Thus our metabolic flux model indicates that the flux through Pfk1 in glycolysis was markedly increased by insulin stimulation, which is consistent with previous findings (Cahill et al., 1959; Katz et al., 1966). Decreased flux through Glut4 and Pfk1 from 0 to 3 min in both the Ins and Ctrl conditions is likely due to the effect of media change, because fluxes through Glut4 and Pfk1 decreased similarly in both Ins and Ctrl conditions (Quek et al., 2020).
In the Ins condition, fluxes through pyruvate carboxylase (Pc) and malic enzyme 1 (Me1) (Figures 3A–3C) increased over time, resulting in a flux cycle between malate and fumarate on one side and pyruvate on the other. At 60 min in the Ins condition, flux through this cycle became larger than the flux through the tricarboxylic acid (TCA) cycle (Figure 3C). In contrast to the flux cycle through Pc and Me1, fluxes through oxidative TCA cycle in the TCA cycle in the Ins condition did not change (Figure 3C). This result indicates that insulin does not have a large influence on fluxes through oxidative TCA cycle.
Fluxes through oxidative PP pathways were less than 2 nmol/mg-protein/min (Figure 3C) in both the Ins and Ctrl conditions. The oxidative PP pathway produces NADPH for reducing power in cells. In the Ins condition, the flux through oxidative PP pathway was much smaller than flux through Me1, which also produces NADPH. Thus, our results are consistent with a previous study showing that insulin-stimulated adipocytes generate most of their cellular reducing power from the Me1 reaction, rather than the oxidative PP pathway (Liu et al., 2016).
Insulin stimulated fluxes through triacylglycerol (TG) biosynthesis (Figures 3A–3C). We represented this process as a merged reaction, Gpat_Acly, consisting of glycerol-3-phosphate acyltransferase (Gpat) and Acly (see Transparent Methods). Stimulation of TG biosynthesis is a well-known action of insulin (Cahill et al., 1959; Dimitriadis et al., 2011; Giorgino et al., 2005; Katz et al., 1966). Note that reactions of Gpat and Acly were merged as one reaction in our metabolic network to maintain the stoichiometric flux balance from glycerol 3-phosphate and citrate to TG (see Transparent Methods). The synthesis of fatty acids in TG requires considerable NADPH molecules. Our model predicted that these were derived primally from increased flux through the Me1 reaction (Figures 3B and 3C). Notably, the intracellular level of NADPH remained stable in both the Ins and Ctrl cells (Figure S2). Thus, our model indicated that insulin stimulated an increase in glucose flux through Me1 to meet the NADPH demand for insulin-stimulated TG synthesis.
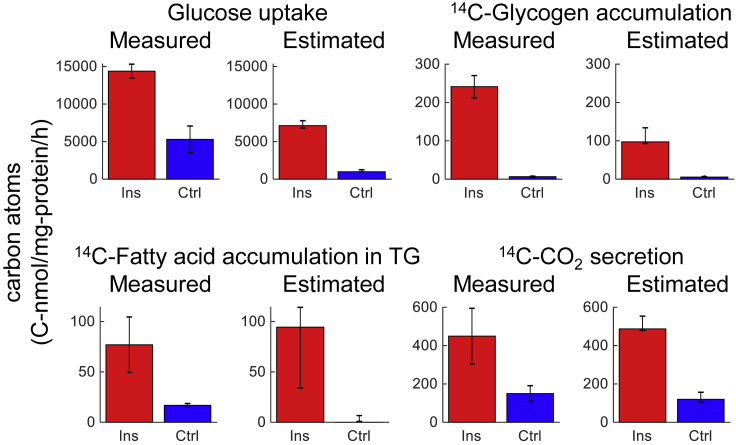
We validated the flux predictions from the model with alternate experiments using natural glucose or 14C-glucose as a tracer (Quek et al., 2020). These data were not used to generate the model to calculate flux. We used the model to predict fluxes involved in glucose uptake, 14C-glycogen accumulation, 14C-fatty acid accumulation in TG, and 14C-CO2 secretion and compared those predictions with the experimental data (Figure 4). In both the model and the experiments, insulin stimulated glucose uptake, 14C-glycogen accumulation, 14C-fatty acid accumulation in TG, and 14C-CO2 secretion. The measured and calculated fluxes for each of these reactions were of a similar order of magnitude, indicating that the model is sufficiently accurate to use to quantitatively identify key regulatory events.
Figure 4.
Validation of the Estimated Flux with Experimental Data Using 14C-Glucose
Error bars indicate 90% confidence intervals for the measurements and the estimates. The 90% confidence intervals for the measurements were calculated by the means ± z × the standard errors, where z is the Z score for 90% confidence intervals (1.6449). Estimated amounts were obtained by integrating sum of products of corresponding fluxes and mass isotopomer fractions of precursor metabolites (see Transparent Methods).
Step II: Generating Kinetic Trans-omic Models for Each Reaction to Identify Key Regulatory Events
Time-resolved metabolomic and phosphoproteomic data implicitly represent quantitative information of regulation of fluxes by changes in enzyme phosphorylation, allosteric effectors, substrates, and products. To quantify the contributions of these regulatory events to the differences in the predicted fluxes between the Ins and Ctrl conditions, we used time-resolved metabolomic and phosphoproteomic data acquired under the same conditions (Figure 5). We developed kinetic models for each flux using modular rate law (Liebermeister et al., 2010) (Figure 5A).
Figure 5.
Key Regulatory Events by Enzyme Phosphorylation, Allosteric Effectors, Substrates, and Products
(A) Overview of the method for quantifying the contribution of regulatory events. Delta (Δ) indicates difference between the Ins and Ctrl conditions.
(B) Clustering of reactions by key regulatory events. Hierarchical clustering was performed on regulation coefficients of ρP, ρA, ρS, and ρU at 1, 5, 10, 20, and 60 min using Euclidean distance as a metric with the Ward method. The data matrix used for the clustering contains 44 rows for reactions and 4,020 columns (5 time points × 4 ρ × 201 optimal and sampled parameters) (Table S4), and the mean of ρ among optimal and sampled parameters is shown as color in the heatmap. See Figures S4A and S8, Tables S3 and S4, and Data S1.
(C) The metabolic network shown with each reaction colored according to its cluster classification.
In the modular rate law, flux is defined as a product of the kinetic constant k1 and a function for the amounts of substrates and products, and other functions for enzyme phosphorylation and allosteric effectors. We assumed that enzyme amounts remained constant during the 60 min after insulin stimulation. We believe that this is a valid assumption because the duration of the experiments was much shorter than the 31-h median half-life of proteins (Sandoval et al., 2013). The enzyme amounts were implicitly included in k1. We used the amounts of substrates and products from metabolomic data in adipocytes with or without insulin stimulation (Krycer et al., 2017). If the data were not available, we used the calculated amounts from our metabolic flux analysis (Figure S2). We also used the enzyme phosphorylation sites and the amounts of the phosphorylation from phosphoproteomic data in insulin-stimulated adipocytes (Humphrey et al., 2013). Metabolites, as potential allosteric effectors for the corresponding metabolic enzymes, were identified from the BRENDA database (Placzek et al., 2017), and their amounts were obtained from metabolomic data with or without insulin stimulation (Krycer et al., 2017), or from the amounts calculated in our metabolic flux analysis (Figure S2). All phosphorylation sites and allosteric effectors are listed in Table S3. Kinetic parameters in the modular rate law were calculated by minimizing residual sum of squared errors between the fluxes defined by the modular rate law and the 13C-metabolic fluxes calculated from metabolic flux analysis (see Transparent Methods).
In our trans-kinetic model with 44 reactions, we identified 82 candidate phosphorylation sites (80 unique sites on 25 enzymes) and 170 candidate allosteric effectors for 35 reactions (Table S3). To determine the contributions of each phosphorylation, allosteric effector, and substrate and product to the fluxes, we modeled the 44 reactions with and without phosphorylation and allosteric effectors, and performed model selection using a stepwise selection method based on Akaike's Information Criterion (AIC) (Burnham et al., 2011; Yamashita et al., 2007). With this model selection, we identified enzyme phosphorylation and allosteric effectors, which gives a better representation of 13C-metabolic flux by reaction kinetics (Figure 5A, see Transparent Methods). Briefly, we developed models of each reaction in which the modular rate law contained k1, and a function for substrate and product, along with a function for phosphorylation, a function for allosteric effectors, or a function for both phosphorylation and allosteric effectors. We performed stepwise model selection for each reaction independently using optimal flux predictions from the metabolic flux analysis and selected the model with the smallest AIC for each reaction. The model finally selected represents regulatory events by substrates, products, and the selected enzyme phosphorylation and/or allosteric effectors.
We next quantified contributions of the regulatory events to the differences in the predicted fluxes between the Ins and Ctrl conditions by defining a regulation coefficient ρ for each regulatory event: enzyme phosphorylation (ρP), allosteric effectors (ρA), substrates and products (ρS), and unaccounted regulators (ρU). The sum of all ρ equals 1, and a larger ρ indicates a larger contribution to flux at each time point. We calculated ρ values and the confidence intervals for all 44 reactions at each time point (Figure S4A, Table S4).
To reveal patterns of regulation and to identify key regulatory events in glucose metabolism in insulin-stimulated adipocytes, we performed hierarchical clustering analysis based on the calculated ρ at each time point. Such analysis resulted in five clusters for the 44 reactions (Figures 5B and 5C). Two clusters contain reactions primarily regulated by either phosphorylation or allosteric effectors. Cluster #3 contains only one reaction, Glut4, a rate-limiting reaction for glucose uptake. This is the only reaction with a large ρP, indicating that flux through this reaction is regulated by phosphorylation. Cluster #2 is characterized by reactions with larger ρA, indicating that fluxes through these reactions are regulated by allosteric effectors. Cluster #2 contains nine reactions, including Gpat_Acly, glutaminase (Gls), and glycogen synthase (Gys), which are associated with synthesis or degradation of cellular macromolecules (lipid, protein, and glycogen, respectively). This cluster also includes Pc and Me1, which are reactions that replenish intermediates in the TCA cycle.
Two clusters contain reactions primarily or partially regulated by changes in substrates and products (Figures 5B and 5C). Cluster #1 is characterized by reactions with large ρS values, indicating that fluxes through these reactions are mainly regulated by substrates and products. Cluster #1 contains many reactions in glycolysis, including aldolase (Aldo), glyceraldehyde-3-phosphate dehydrogenase (Gapd), enolase 1 (Eno1), and pyruvate kinase (Pk). This result indicates that most glycolytic fluxes were regulated by changes in the amounts of substrates and products rather than phosphorylation or allosteric regulation. Cluster #4 is characterized by a high ρU, indicating unknown regulation, and a moderate ρS, indicating that fluxes through these reactions are partly regulated by substrates and products. Cluster #5 is characterized by large ρU, and the mechanism for regulation of flux through these reactions are mostly unknown.
This analysis revealed key regulatory events in glucose metabolism in insulin-stimulated adipocytes. Among the 44 reactions in glucose metabolism, insulin regulated only nine reactions (20%, Cluster #2) primarily through allosteric effectors and only one reaction (2%, Cluster #3) primarily through phosphorylation. Changes in the amounts of substrates and products had a large effect on nine reactions (20%, Cluster #1) and a partial effect on 14 reactions (32%, Cluster #4). Eleven reactions (25%, Cluster #5) had unknown mechanisms of regulation by insulin.
To assess the accuracy of our modeling, we compared the calculated Michaelis-Menten constants in this study with the experimental values from BRENDA (Placzek et al., 2017). We used reactions with time-averaged ρU smaller than 0.5 because the fluxes of these reactions were well explained by known regulation by substrates, products, and, where appropriate, enzyme phosphorylation or allosteric effectors. We compared 20 estimated KS and KP values with KM values in BRENDA and found that these values showed positive correlation (Pearson's correlation coefficient = 0.47, p = 0.036) (Figure S4B), supporting reasonable accuracy of parameter estimation.
Step III: Determining Key Regulatory Molecules that Control the Glucose Metabolism in Insulin-Stimulated Adipocytes
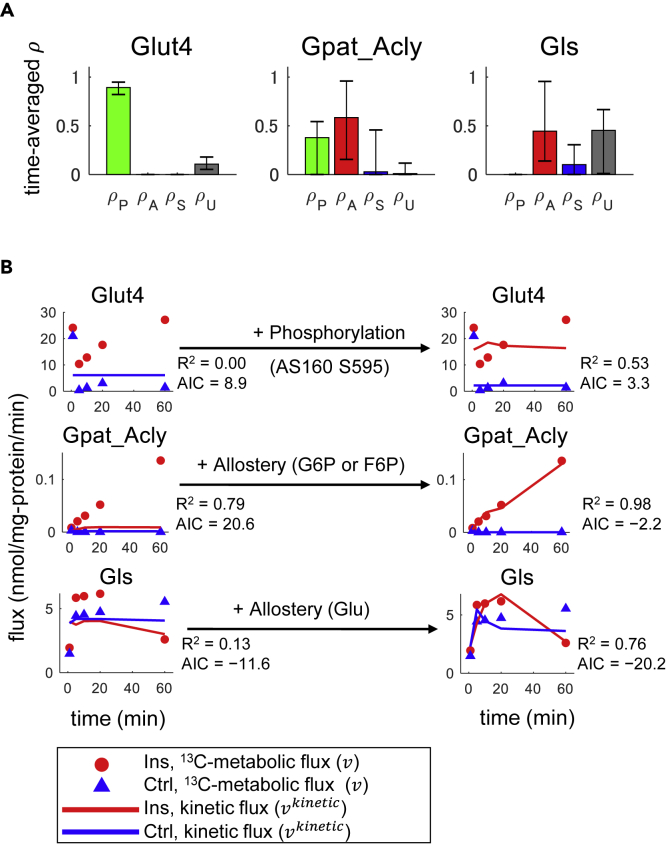
To select the reactions to investigate in detail for regulation by phosphorylation or allosteric effectors, we calculated the average ρ values for each regulatory event over the entire 60 min time course (Figure S5A). We selected reactions for which ρP or ρA had a 90% confidence interval above zero. Only three reactions met these criteria: Glut4 with a time-averaged ρP of 0.89 (90% confidence interval of 0.82–0.95), Gpat_Acly with a ρA of 0.58 (90% confidence interval of 0.16–0.96), and Gls with ρA of 0.46 (90% confidence interval of 0.14–0.96) (Figure 6A). Thus, we investigated phosphorylation that could regulate Glut4 (glucose uptake) and allosteric effectors that could regulate Gpat_Acly (TG synthesis) or Gls (glutaminase). For these reactions, we determined key regulatory molecules as the most robustly selected regulatory molecules for the estimation errors of metabolic fluxes (Transparent Methods).
Figure 6.
Determination of Key Regulatory Molecules
(A) Time-averaged regulation coefficients for reactions with 90% confidence intervals of ρP or ρA > zero. Error bars indicate the 90% confidence intervals. The confidence intervals were calculated from 200 sets of sampled parameters (see Transparent Methods).
(B) Effect of key regulatory molecules on kinetic flux models. The dots indicate estimated flux changes over time by metabolic flux analysis under non-steady-state conditions (), and the lines indicate kinetic fluxes () for the indicated metabolic enzymes. Kinetic fluxes with (right) or without (left) the indicated key regulatory molecules. Shown are the regulatory molecules that produced the smallest AIC when added to the model calculated with substrates and products. R2 and AIC values are indicated. A lower or more negative AIC value represents better fit. Note that kinetic fluxes through Glut4 without regulatory molecules in the Ins and Ctrl conditions are the same and the red and blue lines are overlapped. See also Figure S5A, Tables S3 and S4, and Data S1.
The reaction mediated by Glut4 had 17 candidate regulatory molecules (17 phosphorylation sites of proteins of Glut4 and AS160) (Table S3). Among the candidate regulatory molecules of phosphorylation, phosphorylation of AS160 at S595 was determined as the key regulatory molecule of flux through Glut4 (Figures 6B and S5B). This result is consistent with earlier observations that the phosphorylation site S595 is located within a consensus phopho-Akt substrate motif (RXRXXS∗/T∗) (Treebak et al., 2010) and phosphorylation of AS160 in response to insulin results in Glut4 translocation via Rabs (Huang and Czech, 2007), indicating the validity of our kinetic trans-omic analysis.
The reaction mediated by Gpat_Acly had 19 candidate regulatory molecules, including seven phosphorylation sites of proteins and 12 allosteric effectors, such as G6P, F6P, and F1,6BP, F2,6BP, and phosphoenolpyruvate. Among the candidate regulatory molecules, G6P or F6P was determined as the key regulatory molecule of flux through Gpat_Acly (Figures 6B and S5B). This result is consistent with the earlier observations that both G6P and F6P are allosteric activators for Acly (Potapova et al., 2000), an important step in fatty acid synthesis (Song et al., 2018). Because G6P and F6P are highly correlated, it is difficult to distinguish the separate influence of these two allosteric effectors on Gpat_Acly flux. Although phosphorylation of Acly by Akt has been shown to activate this reaction (Song et al., 2018), phosphorylation of Acly was not determined as the key regulatory molecule for Gpat_Acly. The effect of phosphorylation of Acly did not meet the criteria of ρP above zero with a 90% confidence interval. This is possibly because of lack of phosphoproteomic data in the control condition, resulting in inaccurate prediction of the contribution of phosphorylation to Acly regulation. Addition of phosphoproteomic data in the Ctrl condition is needed to conclude whether phosphorylation of Acly is a key regulatory molecule in the glucose metabolism in insulin-stimulated adipocytes.
The reaction mediated by Gls had 17 candidate regulatory molecules, including 17 allosteric effectors, such as glutamate, glutamine, ATP, and G6P. Among the candidate regulatory molecules, glutamate, the allosteric inhibitor of the reaction, was determined as the key regulatory molecule of flux through Gls (Figures 6B and S5B).
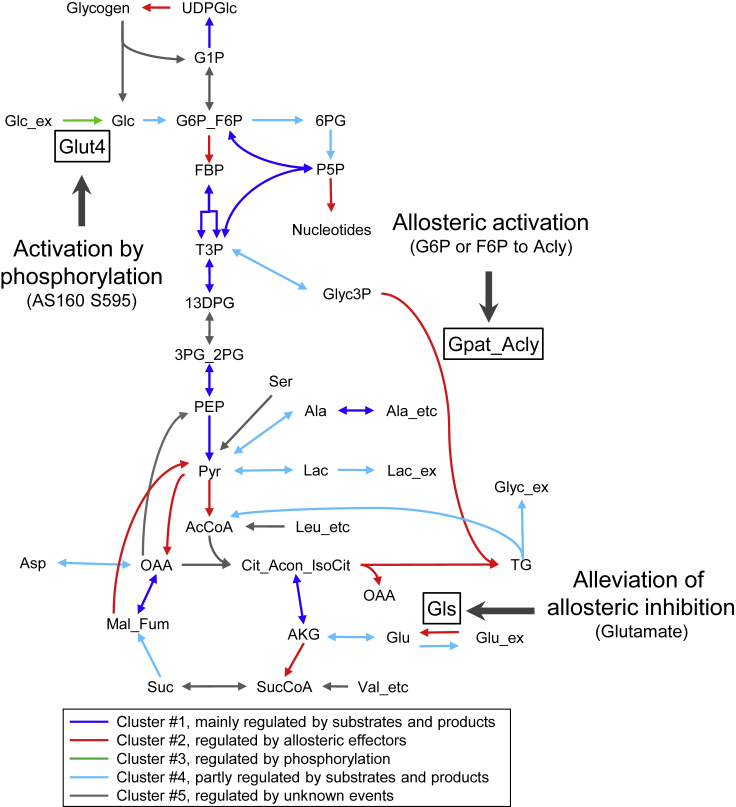
Thus, our kinetic trans-omic model predicted that insulin regulates flux changes through three key reactions. Phosphorylation of AS160 at S595 promoted flux through the Glut4 reaction to stimulate glucose uptake, allosteric activation of Acly in Gpat_Acly by G6P or F6P stimulated TG synthesis, and alleviation of allosteric inhibition of Gls by glutamate stimulated glutamate synthesis (Figure 7).
Figure 7.
Key Regulatory Mechanisms in the Glucose Metabolism in Insulin-Stimulated Adipocytes
The colors of reactions correspond to the clusters in Figure 5B. Reactions in Figure 6 are emphasized by boxes and green and red bold arrows because the key regulatory molecules were selected for the reactions. In insulin-stimulated adipocytes, the reaction mediated by Glut4 is stimulated by phosphorylation of the protein AS160 at S595, the reaction mediated by Gpat_Acly is stimulated by the allosteric effectors G6P or F6P, and the reaction mediated by Gls is stimulated by alleviating the allosteric effector glutamate.
Discussion
We developed a kinetic trans-omic analysis and identified key regulatory mechanisms, such as key regulatory events and molecules, controlling the activity of the proteins and enzymes mediating the reactions (Figure 7). The analysis relied on previously acquired metabolomic (Krycer et al., 2017; Quek et al., 2020) and phosphoproteomic data (Humphrey et al., 2013), as well as data acquired as part of this study. This study describes the methodologies and provides insights into how glucose metabolism is regulated by insulin. An advantage that this study has over previous trans-omic studies (Gerosa et al., 2015; Hackett et al., 2016; Kawata et al., 2018; Krycer et al., 2017; Yugi et al., 2014) is that we assessed non-steady-state flux to identify key regulatory mechanisms in metabolisms. Different flux values among time in both Ins and Ctrl conditions facilitate an accurate kinetic modeling in our kinetic trans-omic analysis. Moreover, by evaluating the regulation of metabolism by insulin in a dynamic state, this study represents a more physiologically relevant condition than studies of steady-state conditions.
Our kinetic trans-omic analysis revealed that three reactions—two controlled by allosteric effectors (reactions of Gpat_Acly controlled by G6P or F6P and Gls controlled by glutamate) and one controlled by phosphorylation (the Glut4 reaction controlled by AS160)—were primary regulatory events in insulin-induced changes in metabolic flux of adipocytes. Except for the Glut4 reaction, which our model indicated was almost exclusively regulated by phosphorylation, our model indicates that most reactions were at least partially or predominantly controlled by amounts of substrates and products. Allosteric regulation strongly contributed to the flux through nine reactions, including those of Gpat_Acly and Gls. Taken together, these findings indicated that insulin modulation of glycolytic flux in adipocytes is principally regulated by increased glucose uptake through Glut4, rather than by regulating the activity of glycolytic enzymes.
Previously, we described flux changes as B-spline functions (Quek et al., 2020); here, we used piecewise linear functions where the number of time intervals were different among reactions. The B-spline method is potentially able to represent more complex flux changes over time than the present method, whereas the present method can represent flux changes with smaller number of parameters than Quek et al.'s method. The present method would facilitate reduction in estimation errors by preventing overfitting. We compared the predicted fluxes using two methods (Figures S6 and S7) and identified high correlations (Pearson's correlation coefficient >0.6) for fluxes through glycolysis, including Glut4, glyceraldehyde -3-phosphate dehydrogenase (Gapd), phosphoglycerate kinase 1 (Pgk1), and enolase (Eno); through the oxidative PP pathway including G6P dehydrogenase (G6pd) and 6-phosphogluconate dehydrogenase (Pgd); and through lactate dehydrogenase (Ldh) and the lactate transporter (Mct1), indicating that our model predicted the fluxes through these reactions with reasonable accuracy. These results indicate that fluxes calculated by each method are, in principle, consistent in glucose metabolism proximal to glucose uptake. A consistent difference between the two approaches was in the fluxes in the early time points in the Ctrl condition for which the B-spline method calculated fluxes larger than those calculated by the method using the piecewise linear function (Figures S6 and S7B). This would be because fluxes in the Ins and Ctrl conditions are independently calculated in the Quek et al.'s study, whereas fluxes at 0 min in both the Ins and Ctrl conditions were set at the same parameter values in the present study. As adipocytes were stimulated with insulin at 0 min, fluxes at 0 min in the Ins and Ctrl conditions should be the same values and the present study may provide physiologically more relevant fluxes in the early time points than the Quek et al.'s study.
Kinetic trans-omic analyses of metabolic flux under steady-state conditions have been reported. These studies included changes in gene expression (transcriptomic data) (Gerosa et al., 2015) or changes in protein abundance (proteomic data) (Hackett et al., 2016). These studies were of either Escherichia coli growing exponentially on eight different carbon sources (Gerosa et al., 2015) or yeast grown under 25 different chemostat conditions (Hackett et al., 2016). We did not consider changes in enzyme abundance (through altered gene expression or protein degradation) as a regulatory event of flux because we focused on an acute metabolic response to insulin in a short (60 min) timescale. Measuring protein abundance and incorporating such data into reaction kinetics would require analysis of a longer timescale.
According to another recent study (Tanner et al., 2018), reactions mediated by the glucose transporter (Glut1, Glut3, and Glut5), hexokinase (Hk2), phosphofructokinase (Pfk1). and the lactate transporter (Mct4) are rate limiting in a pathway from glucose uptake to lactate secretion in mammalian immortalized baby mouse kidney (iBMK) cells. In addition, overexpression of phosphofructo-2-kinase/fructose-2,6-bisphosphatases 1 and 3 (Pfkfb1 and Pfkfb3), which produces F2,6BP, activates flux in the pathway. In this study, we obtained similar results for the glucose transporter and hexokinase. The contribution of phosphorylation of AS160 to the flux differences between the Ins and Ctrl conditions (time-averaged ρP) for Glut4 was 0.89 (Figure 6A), indicating that glucose uptake is the key reaction of glucose metabolism in insulin-stimulated adipocytes. In our model, Hk2 had a small contribution of substrates to the flux differences between the Ins and Ctrl conditions (time-averaged ρS of 0.12) (Figure S5A), indicating that the contribution of changes in substrate concentrations are small. Hk2 had a larger contribution from unknown regulators (time-averaged ρU of 0.88) (Figure S5A), indicating that the mechanism of insulin-dependent regulation remains to be determined. In contrast to Tanner et al. (2018), phosphofructokinase was not rate limiting in our model. We measured F1,6BP and F2,6BP separately by ion chromatography-MS (Hirayama et al., 2020) and found that the regulatory molecules for Pfk1 in insulin-stimulated adipocytes were the substrate F6P and allosteric effectors, including F6P and ADP (Figure S5A, Table S3). Measured F2,6BP amounts were similar between the Ins and Ctrl conditions (Figure S8), indicating that this potential allosteric effector did not regulate Pfk1 fluxes. Thus, comparison of our study and that of Tanner et al. (2018) indicated that key regulatory mechanisms of glycolytic flux depend on cell type and experimental conditions, and that multi-omic measurements from samples under the same experimental condition is necessary to identify key regulatory mechanisms.
Here, we classified reactions in glucose metabolism in adipocytes into five clusters using hierarchical clustering based on regulation coefficients, which represents contribution of regulatory events by enzyme phosphorylation, allosteric effectors, substrates, and products, to flux differences between the Ins and Ctrl conditions (Figure 5B). In Quek et al., 2020, we classified reactions into eight clusters using k-means clustering based on flux differences between the Ins and Ctrl conditions. In Krycer et al., 2017, we classified metabolites into five clusters using hierarchical clustering based on differences in metabolite concentrations between the Ins and Ctrl conditions. In all three studies, we found that reactions in the TCA cycle were classified into multiple clusters: four clusters in this study (Figures 5C and 7) and in Krycer et al. (2017) and three clusters in Quek et al. (2020). Thus, collectively these three studies indicated that the TCA cycle is regulated as functionally separate blocks, rather than as a single cycle, in the insulin-stimulated adipocytes.
In this study, flux estimation and identification of regulatory mechanisms of metabolism were sequentially performed. Flux estimation and identification of regulatory mechanisms can be performed in parallel by developing a kinetic model of metabolism directly from multi-omic data, which requires explicit knowledge of regulatory mechanisms and simultaneous flux estimation and identification of regulatory mechanisms for all reactions, resulting in combinatorial explosion and large computational cost. By contrast, our method does not require explicit knowledge of regulatory molecules for flux and identification of the regulatory molecules can be performed for each reaction independently, because flux is already estimated before kinetic modeling. Thus, our sequential method has a lower computational cost. Furthermore, our method can be applied to any metabolic pathway and can predict the contribution of unknown regulators to flux, leading to new hypotheses about metabolic regulation.
In conclusion, we developed a kinetic trans-omic analysis using time-resolved 13C-glucose-labeled metabolomic and phosphoproteomic data from insulin-stimulated adipocytes and identified the enzyme phosphorylation and allosteric effectors with the greatest impact on glucose flux in response to insulin. Our approach can be widely applied to analyze the regulation in metabolism both in vitro and in vivo, and can provide new insights into metabolic diseases, such as type 2 diabetes.
Limitation of the Study
Although we successfully quantitatively identified key regulatory events in insulin-stimulated adipocytes, there remain reactions with large ρU values (Cluster #5 in Figure 5B), wherein the mechanism for regulation of flux is mostly unknown. A reason for this cluster could be the relatively large estimation errors for the metabolic fluxes of these reactions. For example, to simplify the analysis we did not compartmentalize reactions into different subcellular regions, such as between the cytoplasm and mitochondria. This might cause inaccurate fluxes calculations, especially in the TCA cycle. We used [U-13C]glucose as the source of the tracer, and alternate source of tracers, such as 13C-glutamine (Walther et al., 2012) and partially 13C-labeled glucose, in which only a part of the carbon atoms is 13C-labeled, could improve estimation precision of overall fluxes in glucose metabolism in mammal cells (Jang et al., 2018; Munger et al., 2008; Walther et al., 2012). Large estimation errors for the concentrations of metabolites lacking experimental measurements, such as pyruvate and oxaloacetate (Figure S2), could also cause large ρU values for reactions for which substrates, products, and allosteric effectors are such metabolites. Another possibility for the large ρU could be that main regulatory events of these reactions are not included in our candidate regulatory molecules (substrates, products, enzyme phosphorylation, and allosteric effectors).
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shinya Kuroda (skuroda@bs.s.u-tokyo.ac.jp).
Materials Availability
This study did not generate new unique reagents. Metabolomic and phosphoproteomic data from insulin-stimulated 3T3-L1 adipocytes in our previous studies (Humphrey et al., 2013; Krycer et al., 2017; Quek et al., 2020) are used in this study.
Data and Code Availability
The datasets generated during this study are in the published article. The MATLAB code for 13C-metabolic flux analysis under non-steady-state conditions and kinetic modeling to identify key regulatory mechanisms are available at GitHub (https://github.com/Satoshi-Ohno/Ohno_et_al_2020).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to Dr. Shinsuke Uda, Dr. Hiroyuki Kubota, Dr. Fumio Matsuda, Dr. Hiroshi Shimizu, Dr. Yoshihiro Toya, and our laboratory members for fruitful discussion. We thank Nancy R. Gough, BioSerendipity, LLC, for writing and editorial assistance. The computational analysis of this work was performed in part with support of the super computer system of National Institute of Genetics (NIG), Research Organization of Information and Systems (ROIS). This research was facilitated by access to Sydney Mass Spectrometry, a core research facility at the University of Sydney.
This work was supported by the Creation of Fundamental Technologies for Understanding and Control of Biosystem Dynamics, CREST (JPMJCR12W3) from the Japan Science and Technology Agency (JST), and by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number (17H06300, 17H06299, 18H03979). S.O. was funded by JSPS KAKENHI Grant Number JP17K14864. L.-E.Q. was funded by the Judith and David Coffey Fund, and Cancer Institute NSW. J.R.K. was funded by an NHMRC Early Career Fellowship (APP1072440), Australian Diabetes Society Skip Martin Early Career Fellowship, and Diabetes Australia Research grant. D.E.J. is an NHMRC Senior Principal Research Fellow (APP1019680). K.Y. was funded by JSPS KAKENHI Grant Number JP15H05582 and JP18H05431, and ‘‘Creation of Innovative Technology for Medical Applications Based on the Global Analyses and Regulation of Disease-Related Metabolites,” PRESTO (JPMJPR1538) from JST. A.H. was funded by the Research on Development of New Drugs (GAPFREE) from the Japan Agency for Medical Research and Development, AMED. T.S. was funded by the AMED-CREST from AMED. A.H. and T.S. were funded from Yamagata prefectural government and the City of Tsuruoka.
Author Contributions
S.O., D.E.J., and S.K. conceived the project. S.O. performed the modeling. L.-E.Q., J.R.K., A.H., S.I., F.S., K.S., and T.S. performed experiments. S.O., L.-E.Q., K.Y., and J.R.K. analyzed the data. S.O. and S.K. wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101479.
Contributor Information
David E. James, Email: david.james@sydney.edu.au.
Shinya Kuroda, Email: skuroda@bs.s.u-tokyo.ac.jp.
Supplemental Information
Data S1 include sheets A–F: A, experimental data used for 13C-metabolic flux analysis; B, parameter bounds for 13C-metabolic flux analysis; C, estimated parameter values in 13C-metabolic flux analysis; D, estimated parameter values in kinetic models; E, Michaels-Menten constants obtained from the BRENDA database; F, correspondence of reactions between this study and the Quek et al.’s study (Quek et al., 2020).
References
- Abate A., Hillen R.C., Aljoscha Wahl S., Wahl S.A. Piecewise affine approximations of fluxes and enzyme kinetics from in vivo13C labeling experiments. Int. J. Robust Nonlin. Control. 2012;22:1120–1139. [Google Scholar]
- Burnham K.P., Anderson D.R., Huyvaert K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011;65:23–35. [Google Scholar]
- Cahill G.F., Jeanrenaud B., Leboeuf B., Renold A.E. Effects of insulin on adipose tissue. Ann. N. Y. Acad. Sci. 1959;82:4303–4311. doi: 10.1111/j.1749-6632.1959.tb44921.x. [DOI] [PubMed] [Google Scholar]
- Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G., Mitrou P., Lambadiari V., Maratou E., Raptis S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011;93:S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- Gerosa L., Haverkorn van Rijsewijk B.R.B., Christodoulou D., Kochanowski K., Schmidt T.S.B., Noor E., Sauer U. Pseudo-transition analysis identifies the key regulators of dynamic metabolic Adaptations from steady-state data. Cell Syst. 2015;1:270–282. doi: 10.1016/j.cels.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Giorgino F., Laviola L., Eriksson J.W. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol. Scand. 2005;183:13–30. doi: 10.1111/j.1365-201X.2004.01385.x. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hackett S.R., Zanotelli V.R.T., Xu W., Goya J., Park J.O., Perlman D.H., Gibney P.A., Botstein D., Storey J.D., Rabinowitz J.D. Systems-level analysis of mechanisms regulating yeast metabolic flux. Science. 2016;354:aaf2786. doi: 10.1126/science.aaf2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama A., Wakayama M., Soga T. Metabolome analysis based on capillary electrophoresis-mass spectrometry. Trends Anal. Chem. 2014;61:215–222. [Google Scholar]
- Hirayama A., Tabata S., Kudo R., Hasebe M., Suzuki K., Tomita M., Soga T. The use of a double coaxial electrospray ionization sprayer improves the peak resolutions of anionic metabolites in capillary ion chromatography-mass spectrometry. J. Chromatogr. A. 2020;1619:460914. doi: 10.1016/j.chroma.2020.460914. [DOI] [PubMed] [Google Scholar]
- Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Humphrey S.J., Yang G., Yang P., Fazakerley D.J., Stöckli J., Yang J.Y., James D.E. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 2013;17:1009–1020. doi: 10.1016/j.cmet.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S.J., James D.E., Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends Endocrinol. Metab. 2015;26:676–687. doi: 10.1016/j.tem.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Israelsen W.J., Vander Heiden M.G. Pyruvate kinase: function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015;43:43–51. doi: 10.1016/j.semcdb.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C., Chen L., Rabinowitz J.D. Metabolomics and isotope tracing. Cell. 2018;173:822–837. doi: 10.1016/j.cell.2018.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jünger M.A., Aebersold R. Mass spectrometry-driven phosphoproteomics: patterning the systems biology mosaic. Wiley Interdiscip. Rev. Dev. Biol. 2014;3:83–112. doi: 10.1002/wdev.121. [DOI] [PubMed] [Google Scholar]
- Katz J., Landau B.R., Bartsch G.E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J. Biol. Chem. 1966;241:727–740. [PubMed] [Google Scholar]
- Kawata K., Hatano A., Yugi K., Kubota H., Sano T., Fujii M., Tomizawa Y., Kokaji T., Tanaka K.Y., Uda S. Trans-omic analysis reveals selective responses to induced and basal insulin across signaling, transcriptional, and metabolic networks. iScience. 2018;7:212–229. doi: 10.1016/j.isci.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanowski K., Sauer U., Noor E. Posttranslational regulation of microbial metabolism. Curr. Opin. Microbiol. 2015;27:10–17. doi: 10.1016/j.mib.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Krycer J.R., Yugi K., Hirayama A., Fazakerley D.J., Quek L.-E., Scalzo R., Ohno S., Hodson M.P., Ikeda S., Shoji F. Dynamic metabolomics reveals that insulin primes the adipocyte for glucose metabolism. Cell Rep. 2017;21:3536–3547. doi: 10.1016/j.celrep.2017.11.085. [DOI] [PubMed] [Google Scholar]
- Krycer J.R., Elkington S.D., Diaz-Vegas A., Cooke K.C., Burchfield J.G., Fisher-Wellman K.H., Cooney G.J., Fazakerley D.J., James D.E. Mitochondrial oxidants, but not respiration, are sensitive to glucose in adipocytes. J. Biol. Chem. 2020;295:99–110. doi: 10.1074/jbc.RA119.011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krycer J.R., Quek L.E., Francis D., Fazakerley D.J., Elkington S.D., Diaz-Vegas A., Cooke K.C., Weiss F.C., Duan X., Kurdyukov S. Lactate production is a prioritized feature of adipocyte metabolism. J. Biol. Chem. 2020;295:83–98. doi: 10.1074/jbc.RA119.011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighty R.W., Antoniewicz M.R. Dynamic metabolic flux analysis (DMFA): a framework for determining fluxes at metabolic non-steady state. Metab. Eng. 2011;13:745–755. doi: 10.1016/j.ymben.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Liebermeister W., Uhlendorf J., Klipp E. Modular rate laws for enzymatic reactions: thermodynamics, elasticities and implementation. Bioinformatics. 2010;26:1528–1534. doi: 10.1093/bioinformatics/btq141. [DOI] [PubMed] [Google Scholar]
- Liu L., Shah S., Fan J., Park J.O., Wellen K.E., Rabinowitz J.D. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat. Chem. Biol. 2016;12:345–352. doi: 10.1038/nchembio.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macek B., Mann M., Olsen J.V. Global and site-specific quantitative phosphoproteomics: principles and Applications. Annu. Rev. Pharmacol. Toxicol. 2009;49:199–221. doi: 10.1146/annurev.pharmtox.011008.145606. [DOI] [PubMed] [Google Scholar]
- Mor I., Cheung E.C., Vousden K.H. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb. Symp. Quant. Biol. 2011;76:211–216. doi: 10.1101/sqb.2011.76.010868. [DOI] [PubMed] [Google Scholar]
- Munday K.A., Giles I.G., Poat P.C. Review of the comparative biochemistry of pyruvate kinase. Comp. Biochem. Physiol. 1980;67:403–411. doi: 10.1016/0305-0491(76)90315-1. [DOI] [PubMed] [Google Scholar]
- Munger J., Bennett B.D., Parikh A., Feng X.-J.J., McArdle J., Rabitz H.A., Shenk T., Rabinowitz J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passonneau J.V., Lowry O.H. P-Fructokinase and the control of the citric acid cycle. Biochem. Biophys. Res. Commun. 1963;13:372–379. [Google Scholar]
- Peng B., Li H., Peng X.-X. Functional metabolomics: from biomarker discovery to metabolome reprogramming. Protein Cell. 2015;6:628–637. doi: 10.1007/s13238-015-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek S., Schomburg I., Chang A., Jeske L., Ulbrich M., Tillack J., Schomburg D. BRENDA in 2017: new perspectives and new tools in BRENDA. Nucleic Acids Res. 2017;45:D380–D388. doi: 10.1093/nar/gkw952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L., Belgardt B.F., Brüning J.C. Central insulin action in energy and glucose homeostasis. J. Clin. Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova I.A., El-Maghrabi M.R., Doronin S.V., Benjamin W.B. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of atp:citrate lyase by phosphorylated sug. Biochemistry. 2000;39:1169–1179. doi: 10.1021/bi992159y. [DOI] [PubMed] [Google Scholar]
- Quek L.-E., Krycer J.R., Ohno S., Yugi K., Fazakerley D.J., Scalzo R., Elkington S.D., Dai Z., Hirayama A., Ikeda S. Dynamic 13C flux analysis captures the reorganization of adipocyte glucose metabolism in response to insulin. iScience. 2020;23:100855. doi: 10.1016/j.isci.2020.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P.D., Huang B.-W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih D.A., Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A.R., Kahn C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sandoval P.C., Slentz D.H., Pisitkun T., Saeed F., Hoffert J.D., Knepper M.A. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J. Am. Soc. Nephrol. 2013;24:1793–1805. doi: 10.1681/ASN.2013030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg T., Kloos M., Brüser A., Kirchberger J., Sträter N. Structure and allosteric regulation of eukaryotic 6-phosphofructokinases. Biol. Chem. 2013;394:977–993. doi: 10.1515/hsz-2013-0130. [DOI] [PubMed] [Google Scholar]
- Song Z., Xiaoli A.M., Yang F. Regulation and metabolic significance of De Novo lipogenesis in adipose tissues. Nutrients. 2018;10:1–22. doi: 10.3390/nu10101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner L.B., Goglia A.G., Wei M.H., Sehgal T., Parsons L.R., Park J.O., White E., Toettcher J.E., Rabinowitz J.D. Four key steps control glycolytic flux in mammalian cells. Cell Syst. 2018;7:49–62. doi: 10.1016/j.cels.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak J.T., Taylor E.B., Witczak C.A., An D., Toyoda T., Koh H.-J., Xie J., Feener E.P., Wojtaszewski J.F.P., Hirshman M.F. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am. J. Physiol. 2010;298:C377–C385. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther J.L., Metallo C.M., Zhang J., Stephanopoulos G. Optimization of 13C isotopic tracers for metabolic flux analysis in mammalian cells. Metab. Eng. 2012;14:162–171. doi: 10.1016/j.ymben.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Yamashita K., Kamimura R. A stepwise AIC method for variable selection in linear regression. Commun. Stat. Theory Methods. 2007;36:2395–2403. [Google Scholar]
- Yugi K., Kuroda S. Metabolism as a signal generator across trans-omic networks at distinct time scales. Curr. Opin. Syst. Biol. 2017;8:59–66. [Google Scholar]
- Yugi K., Kubota H., Toyoshima Y., Noguchi R., Kawata K., Komori Y., Uda S., Kunida K., Tomizawa Y., Funato Y. Reconstruction of insulin signal flow from phosphoproteome and metabolome data. Cell Rep. 2014;8:1171–1183. doi: 10.1016/j.celrep.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Yugi K., Kubota H., Hatano A., Kuroda S. Trans-Omics: how to reconstruct biochemical networks across multiple “omic” layers. Trends Biotechnol. 2016;34:276–290. doi: 10.1016/j.tibtech.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Yugi K., Ohno S., Krycer J.R., James D.E., Kuroda S. Rate-oriented trans-omics: integration of multiple omic data on the basis of reaction kinetics. Curr. Opin. Syst. Biol. 2019;15:109–120. [Google Scholar]
- Zamboni N., Saghatelian A., Patti G.J. Defining the metabolome: size, flux, and regulation. Mol. Cell. 2015;58:699–706. doi: 10.1016/j.molcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 include sheets A–F: A, experimental data used for 13C-metabolic flux analysis; B, parameter bounds for 13C-metabolic flux analysis; C, estimated parameter values in 13C-metabolic flux analysis; D, estimated parameter values in kinetic models; E, Michaels-Menten constants obtained from the BRENDA database; F, correspondence of reactions between this study and the Quek et al.’s study (Quek et al., 2020).
Data Availability Statement
The datasets generated during this study are in the published article. The MATLAB code for 13C-metabolic flux analysis under non-steady-state conditions and kinetic modeling to identify key regulatory mechanisms are available at GitHub (https://github.com/Satoshi-Ohno/Ohno_et_al_2020).