Abstract
Objectives:
Humans exhibit significant ecogeographic variation in bone size and shape. However, it is unclear how significantly environmental temperature influences cortical and trabecular bone, making it difficult to recognize adaptation vs. acclimatization in past populations. There is some evidence that cold-induced bone loss results from sympathetic nervous system activation and can be reduced by nonshivering thermogenesis (NST) via uncoupling protein (UCP1) in brown adipose tissue (BAT). Here we test two hypotheses: 1) low temperature induces impaired cortical and trabecular bone acquisition, and 2) UCP1, a marker of NST in BAT, increases in proportion to degree of low temperature exposure.
Methods:
We housed wildtype C57Bl/6J male mice in pairs at 26°C (thermoneutrality), 22°C (standard), and 20°C (cool) from 3 weeks to 6 or 12 weeks of age with access to food and water ad libitum (N=8/group).
Results:
Cool housed mice ate more but had lower body fat at 20°C vs. 26°C. Mice at 20°C had markedly lower distal femur trabecular bone volume fraction, thickness, and connectivity density and lower midshaft femur cortical bone area fraction vs. mice at 26°C (p<0.05 for all). UCP1 expression in BAT was inversely related to temperature.
Discussion:
These results support the hypothesis that low temperature was detrimental to bone mass acquisition. Nonshivering thermogenesis in brown adipose tissue increased in proportion to low temperature exposure but was insufficient to prevent bone loss. These data show that chronic exposure to low temperature impairs bone architecture, suggesting climate may contribute to phenotypic variation in humans and other hominins.
Keywords: temperature, sympathetic tone, nonshivering thermogenesis, trabecular bone
Hominin skeletal phenotype varies geographically, such that populations at high latitudes tend to have broader, more massive bodies with shorter distal limb segments compared to populations at lower latitudes (Churchill 1998; Foster and Collard 2013; Holliday 1997; Holliday and Hilton 2009; Katzmarzyk and Leonard 1998; Pearson 2000; Ruff 2002; Ruff 1994). According to Bergmann’s Law and Allen’s Rule, these shape differences increase fitness in cold climates by lowering surface area:volume ratio and decreasing heat loss (Allen 1877; Bergmann 1847; Ruff 1993). However, the extent to which these patterns reflect genetic adaptation vs. developmental plasticity during growth is largely unknown. It is also unclear whether temperature directly influences human variation in aspects of skeletal phenotype such as cortical bone cross-sectional geometry or trabecular bone microarchitecture.
Several recent observations suggest the skeleton may have developmentally plastic responses to temperature. First, the well-known historical positive correlations of latitude with body mass and bi-iliac breadth and negative correlations with surface area/mass ratio and distal limb segment length (Roberts 1953; Ruff 1994) have weakened in recent years (Katzmarzyk and Leonard 1998). This trend is clearly due in part to secular trends in body mass, but potentially also reflects reduced cold exposure. Second, in retrospective data, cold-adapted populations tend to have more robust bones than heat-adapted populations do, perhaps reflecting their higher body mass (Pearson 2000). However, other studies of cold-dwelling humans found low cortical thickness and bone mineral density as well as accelerated bone loss with aging, although it is unclear whether these patterns are due to temperature, high fat and protein intake, or both (Harper et al. 1984; Lazenby 1997; Leslie et al. 2006; Leslie et al. 2008; Mazess and Mather 1974; Mazess and Mather 1975; Thompson and Gunness-Hey 1981; Wallace et al. 2014). Finally, in animal models, changes in limb bone length can be produced experimentally within a single generation simply by raising mice in cold or warm temperatures (Serrat et al. 2008), and increasing housing temperature to mouse thermoneutrality reduces trabecular bone loss (Iwaniec et al. 2016). These provocative studies demonstrate that at least in rodents, bone responds dynamically to temperature, such that the cold-dwelling skeletal phenotype may not be entirely genetically mediated.
If bone exhibits developmentally plastic responses to temperature, then it is essential to understand how interactions between cold stress and thermogenic mechanisms alter skeletal acquisition. In mammals, temperature is primarily maintained by basal metabolic rate, augmented during cold challenge by mechanisms such as shivering and nonshivering thermogenesis (NST). NST occurs in mitochondria-rich adipocytes called brown adipose tissue (BAT) and has recently been the subject of renewed interest. Although the constitutive BAT depots present in human newborns regress after infancy (Heaton 1972), a type of BAT known as beige fat can be induced in adults in response to cold exposure (Cypess et al. 2009; Saito et al. 2009; Sharp et al. 2012; van Marken Lichtenbelt et al. 2009; Virtanen et al. 2009; Wu et al. 2012). Such active BAT is found after cold challenge in 50–90% of adults under age 35, but only 8% of adults over 35 (Saito et al. 2009; van Marken Lichtenbelt et al. 2009). BAT is more abundant in winter than in summer, and in outdoor vs. indoor workers (Au-Yong et al. 2009; Huttunen et al. 1981). This environmental responsiveness suggests that beige fat may have aided hominin expansion to colder regions. High-latitude populations have higher basal metabolic rates than predicted for their lean mass, potentially due in part to nonshivering thermogenesis in BAT (Leonard and Levy 2015), and it is reasonable to hypothesize that earlier cold-adapted hominins also relied in part on BAT thermogenesis (Steegmann 2007).
BAT is also a potential mediator of skeletal responses to cold stress (reviewed in Devlin 2015; Lowell and Spiegelman 2000). Cold exposure increases sympathetic tone, leading to bone loss via hypothalamic signaling to beta2-adrenergic receptors on osteoblasts. Cold exposure activates uncoupling protein-1 (UCP1) in the inner mitochondrial membrane to divert fatty acid and glucose oxidation to instead generate heat (Nicholls and Rial 1999), which would increase body temperature and reduce sympathetic tone. In animal models, in the absence of BAT, chronic SNS activation induces bone loss (Kajimura et al. 2011). The Misty mouse model of dysfunctional BAT has high sympathetic tone and low bone mass that is mitigated by the beta-adrenergic antagonist propranolol, suggesting that BAT might protect bone mass by buffering the skeleton against SNS-induced bone loss (Motyl et al. 2013). In support of positive skeletal effects of BAT, recent studies in humans report that BAT is positively associated with bone mineral density (BMD) and bone cross-sectional area in subadults and adults of both sexes (Bredella et al. 2012; Bredella et al. 2014; Lee et al. 2013; Ponrartana et al. 2012).
To delineate the mechanistic relationships between low temperature, nonshivering thermogenesis in brown adipose tissue, and skeletal acquisition, here we used controlled experiments in mice housed at sub-thermoneutral temperatures at or near thermoneutrality (Gaskill et al. 2012) as a model for exposure to low temperature in humans. Human thermoneutrality ranges from ~26–32 °C when unclothed (Erikson et al. 1956; Hardy and Dubois 1937; Kingma et al. 2014) and from ~20–25 °C in clothing (Kingma et al. 2012; Kingma et al. 2014; Lodhi and Semenkovich 2009), although individual tolerance depends on factors including body shape, fat mass, and acclimatization. The equivalent thermoneutral temperature in the mouse is a subject of ongoing debate. Laboratory mice are typically housed at 20–22 °C, but recent studies have argued that thermoneutrality for mice is closer to 30 °C and that mice housed at standard temperature are chronically cold stressed (Cannon and Nedergaard 2011; Gaskill et al. 2012; Gordon 2012; Karp 2012; Lodhi and Semenkovich 2009; Overton 2010). In contrast, Speakman and Keijer (2012) noted that both humans and mice have energy expenditure of ~1.6–1.8 times basal metabolic rate at 22 °C, such that on a metabolic basis, this temperature is appropriate for mice being used to model humans. However, a more recent study using indirect calorimetry found that energy expenditure in singly housed mice is 1.8 times basal metabolic rate at 30 °C and increases to 3.1 times basal metabolic rate at 22 °C (Fischer et al. 2018). Mice at 22 °C also exhibit significantly higher respiratory quotient, glucose uptake and UCP1 activity in BAT (David et al. 2013). These studies show that mice expend increased energy on nonshivering thermogenesis when housed at or below standard vivarium temperature.
Here we compare the effects of housing at temperatures from 20–26 °C to test the following hypotheses: 1) that low temperature reduces skeletal acquisition in young, rapidly growing animals, 2) and that UCP1 protein expression as a marker of increased NST in BAT is proportional to the decrease in housing temperature. For hypothesis 1), we predicted that exposure to low temperature during rapid skeletal growth would increase sympathetic tone, leading to weaker trabecular microarchitecture, cortical bone cross-sectional geometry, and bone strength. Given evidence from early studies that cold exposure increases bone marrow adiposity, we predicted that cool housed mice would have more and larger adipocytes in distal femur bone marrow (Huggins and Blocksom 1936). For hypothesis 2), we predicted that increased UCP1 expression in BAT may partially or completely prevent low temperature-induced bone loss by increasing body temperature and blunting the rise in sympathetic tone. Overall, we predicted that BMD, cortical and trabecular bone parameters, and histological indices of bone formation would be negatively correlated with low temperature and positively correlated with UCP1 levels as a marker of NST.
MATERIALS AND METHODS
Mice and diet intervention
We obtained 3-week-old male C57BL/6J (B6) mice (The Jackson Laboratory, Bar Harbor, ME) and assigned them to housing at 20 °C (low temperature, “cool”), 22 °C (standard housing temperature, “control”), or 26 °C (near thermoneutrality, “warm”) and a normal diet ad libitum (PicoLab 5L0D, LabDiets, St. Louis, MO, 13% kcal/fat, 30% kcal/protein, 57% kcal/carbohydrate) until 6 or 12 weeks of age (N=8/group).
Baseline body mass and bone mineral density did not differ among groups. Mice were housed in pairs to provide socialization but minimize elevated nest temperatures. Toth et al. (2015) tested the effect of housing density on cage temperature at 22 °C, 26 °C, and 30 °C and found no significant difference in ambient temperature when density increased from one mouse to two mice per cage. Therefore, to avoid introducing an additional variable by singly housing (Nagy et al. 2002), we maintained mice at two per cage. Mouse body mass and food intake were measured two to three times weekly on a digital scale. All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee.
Peripheral dual-energy x-ray absorptiometry
In vivo assessment of whole body bone mineral density (BMD, g/cm2), bone mineral content (BMC, g), and body composition was performed at 3, 6, 9, and 12 weeks of age using peripheral dual-energy x-ray absorptiometry under inhaled isoflurane (pDXA, PIXImus I, GE Lunar Corp.), as previously described (Bouxsein et al. 2008; Bouxsein et al. 2005; Devlin et al. 2016).
Euthanasia and specimen harvesting/preparation
At the conclusion of the experiment, mice were sacrificed by CO2 inhalation. Blood was collected by cardiac puncture to assess indices of bone turnover (Devlin et al. 2014). Epididymal white adipose and interscapular brown adipose depots were dissected, snap frozen in liquid nitrogen, and stored at −80°C (Murray et al. 2000). Femurs were harvested and cleaned of soft tissue. The right femur was prepared for imaging and biomechanical testing by wrapping in saline soaked gauze, and then stored airtight at −20°C. The left femur was prepared for histology in 70% ethanol at 4°C.
Histology
Left femora were fixed in 70% ethanol and undecalcified bones were embedded in polymethylmethacrylate (N=4–5/group). Sagittal sections (4–8 μm thick) were cut with Jung 2065 and 2165 microtomes (Leica Biosystems, Buffalo Grove, IL) and stained with Von Kossa/tetrachrome stain for assessment of marrow adiposity in the distal femoral metaphysis using Bioquant OSTEO software (Bioquant Image Analysis, Nashville, TN). Although marrow lipids are removed during tissue processing, adipocytes remain identifiable as round, unstained spaces (Aguirre et al. 2007). The region of interest included the secondary spongiosa of the distal femur beginning 150 microns proximal to the growth plate and extending proximally 1200 microns, and mediolaterally across the entire bone section at a margin of 150 microns from the cortical edges. Measurements included the number of adipocytes and the percentage of adipocyte volume per tissue volume. Terminology followed the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (Dempster et al. 2013).
mRNA Isolation and Quantification
To quantify expression of NST marker UCP1, total RNA was prepared from liquid nitrogen snap-frozen BAT via standard TRIzol extraction (Sigma, St. Louis, MO). RNA was recovered in 30 μl RNase-free water, quantitated on a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and treated with Promega RQ1 RNase-free DNase to remove any residual genomic DNA (Promega M6101, Madison, WI). Preparation and generation of cDNA was made with the Promega GoScript Reverse Transcriptase System (Promega A5000, Madison, WI) according to manufacturer’s instructions.
Gene expression was measured on a CFX384 Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA) at the University of Michigan Molecular Biology Core. 30 ng of cDNA was used per 15 μl PCR reaction with SYBR Green PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific 4309155, Waltham, MA) according to manufacturer’s recommendations. All reactions were performed in triplicate. Specific primers (Invitrogen, Carlsbad, CA) were used at 300 nmol per reaction for either target gene UCP1 (F 5’ GGA TGG TGA ACC CGA CAA CT 3’, R 5’ CTT GGA TCT GAA GGC GGA CT 3’) or housekeeping gene cyclophilin (F 5’ CAA ATG CTG GAC CAA ACA CAA 3’). Primer reaction specificity was verified via melting curve analysis and efficiency varied between 101% and 98% for UCP1 and cyclophilin, respectively, when spanning 5 orders of magnitude. UCP1 relative gene expression was determined by the ΔΔ-Ct method (Livak and Schmittgen 2001).
Protein Isolation and Quantification
To quantify UCP1 expression, total protein was extracted via standard methods in RIPA buffer (Thermo Scientific, Waltham, MA) with protease (Sigma, St. Louis, MO) and phosphatase (Roche, Basel, Switzerland) inhibitors added. Protein concentration was determined via BCA assay (Pierce, Rockford, IL). Western blot followed standard SDS-PAGE procedures using Mini-PROTEAN Tetra Cell (Bio-Rad, Hercules, CA) equipment. After transfer, gel was stained with 0.1% Coomassie G-250 (Thermo Scientific, Waltham, MA) and imaged to visualize total protein loading. Primary antibodies for rabbit anti-UCP1 (Thermo Fisher Scientific #PA1–24894) and mouse anti-GAPDH antibody (Ambion #AM4300, Carlsbad, CA) were used 1:1k and 1:2k, respectively, in TBST+5% nonfat dry milk. HRP-conjugated secondary antibodies for anti-rabbit and anti-mouse (Thermo Fisher Scientific #65–6120 and #31430, Rockford, IL) were used 1:30,000 in TBST+5% nonfat dry milk, bands visualized by ECL (Advantsa, Menlo Park, CA) and signals captured on a c600 imaging system (Azure Biosystems, Dublin, CA). Bands were quantitated using ImageJ density values, and UCP1 expression determined via total protein normalization (Aldridge et al. 2008; Gilda and Gomes 2013).
Serum leptin measurement
Terminal blood samples were collected in MiniCollect serum separation tubes (Grenier Bio-One, Kremsumunster Austria) at time of sacrifice by cardiac puncture, stored on wet ice, and serum isolated by centrifuge. Serum was stored at −80°C until analysis. Serum leptin was measured in duplicate by ELISA (Crystal Chem, Downers Grove, IL) according to manufacturer’s instructions and quantitated on microplate reader (Molecular Devices, Sunnyvale, CA).
Mouse trabecular and cortical bone morphology by microCT
Assessment of bone morphology and microarchitecture was performed with high-resolution microcomputed tomography (eXplore Locus SP, GE Healthcare, Chalfont, UK) using scan parameters of 80 kVP, 0.45 mA. In brief, the metaphysis of the distal femur was scanned using an 18 μm isotropic voxel size. Images were reconstructed, filtered, and thresholded using a specimen-specific threshold (Meinel et al. 2005; Ridler and Calvard 1978). Morphometric parameters were computed using a direct 3D approach that does not rely on any assumptions about the underlying structure (Hildebrand et al. 1999; Hildebrand and Rüegsegger 1997). Cortical and trabecular regions of interest were evaluated using Microview (v. 2.2, GE Healthcare, London, ON, Canada). The trabecular region of interest began 270 microns proximal to the distal growth plate and extended proximally for 10% of the total length of the bone (Bouxsein et al. 2010). For the trabecular bone region we assessed bone volume fraction (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular separation (TbSp, μm), trabecular number (Tb.N, 1/mm), and connectivity density (Conn.D 1/mm3). The cortical region of interest consisted of 50 slices centered at the femoral midshaft. Cortical bone parameters included the total cross-sectional area, cortical bone area (TA and BA, mm2), bone area fraction (BA/TA, %), cortical thickness (mm), maximum and minimum area moments of inertia (Imax and Imin, mm4), and polar moment of inertia (J). All methods followed American Society for Bone and Mineral Research guidelines (Bouxsein et al. 2010).
Bone strength testing
After completion of μCT, the strength of the femoral midshaft was assessed by 4-point bending using previously described methods (Sinder et al. 2015; Smith et al. 2013). Briefly, frozen specimens were thawed in calcium-buffered saline. A low force mechanical testing system (858 Minibionix II; MTS Systems Corporation, Eden Prairie, MN, USA) was used to apply a constant displacement rate of 0.05 mm/sec in the anterior-posterior direction, with the anterior side in compression. Upper and lower supports were 2.075 mm and 6.35 mm apart, respectively. Force-displacement data were used to determine structural properties (maximum load, stiffness and postyield displacement). Mechanical stiffness from four-point bending was adjusted for μCT-derived femoral midshaft area moment of inertia to calculate estimated material properties (Jepsen et al. 2015).
Statistical Analysis
Statistical analyses were run in SPSS 24 (IBM, Armonk, NY) using general linear models to test for effects of temperature and/or a temperature*age interaction. If there was a significant effect of temperature or temperature*age for a particular variable, the GLM was stratified by age to obtain p values for temperature (20 °C vs. 22 °C vs. 26 °C) within mice at 6 or 12 weeks of age. Given the influence of body mass on long bone cortical cross-sectional geometry, femoral cortical variables (Ct.Th, Ct.Ba, Ct.TA, Imax, Imin, J) were adjusted by regressing against body mass and testing for significance using the residuals (Lang et al. 2005). The significance level for major effects was set at alpha=0.05. All graphs were made using GraphPad Prism 7.0c for Mac OSX (GraphPad Software, La Jolla, CA).
RESULTS
Body size and composition, whole-body BMD, and hormone levels
Body mass and femur length did not differ across housing temperatures (Fig. 1A, Table 1), although at 6, 9, and 12 weeks of age, cool mice ate 12%, 36%, and 27% more than control mice and 22%, 70%, and 53% more than warm mice, respectively (p<0.005 for all, Fig. 1B). Cool mice had shorter bodies at 9 and 12 weeks of age (p<0.05 for both, Fig. 1C) and shorter tails at 6, 9, and 12 weeks of age (p<0.05 for all, Table 1).
Figure 1.
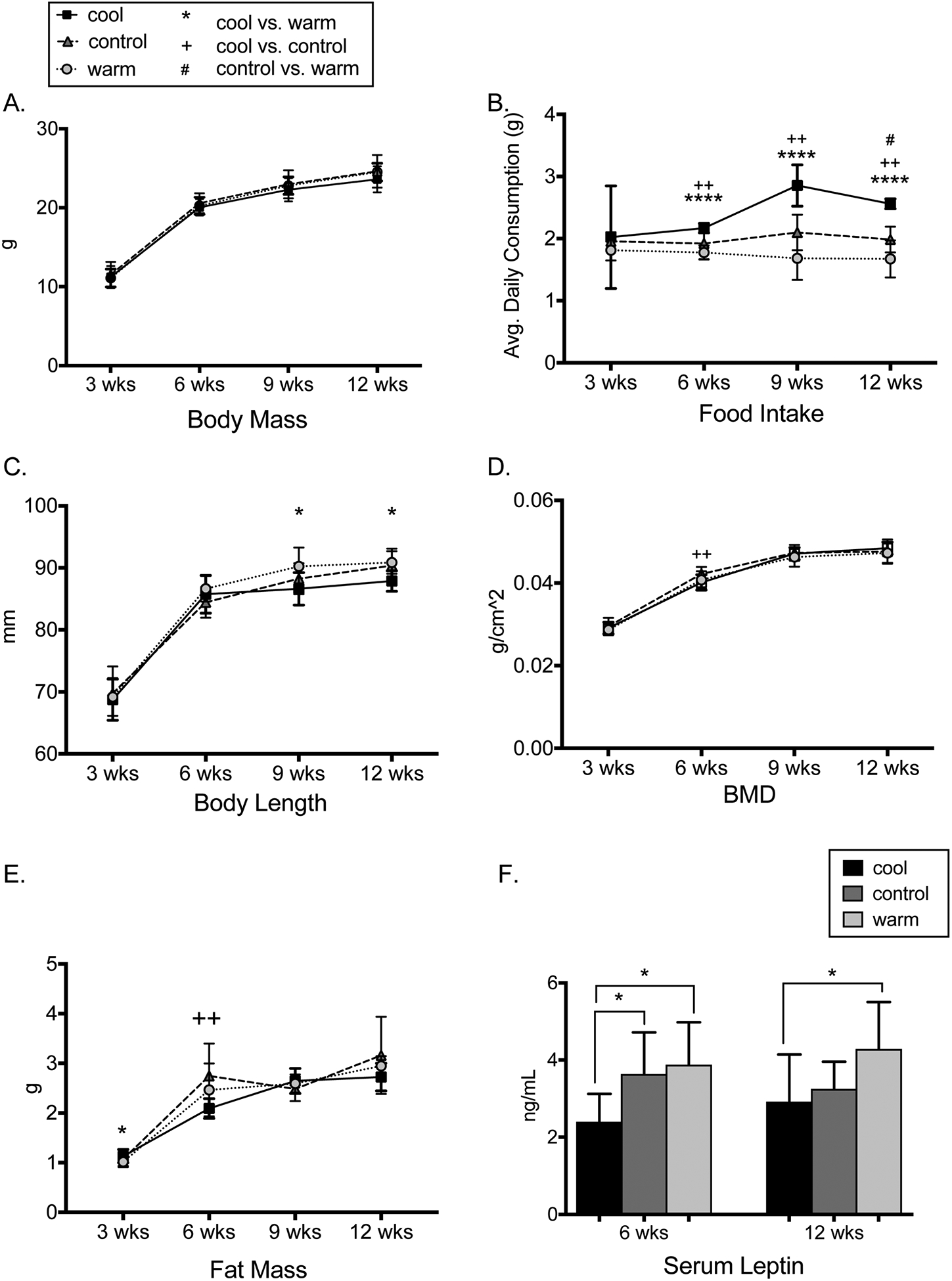
A. Body mass did not differ by temperature (N=8/group). B. Cool vs warm mice ate less at 6, 9, and 12 weeks (p<0.0001 for all, N=4 cages/group). C. Cool vs warm mice had shorter bodies at 9 and 12 weeks (p<0.05, N=8/group). D. Bone mineral density was lower in cool vs control mice at 6 weeks (p=0.009) but did not otherwise differ (N=8/group). E. Cool vs. control mice had lower fat mass at 6 weeks (p=0.002, N=8/group). F. Cool vs warm mice had lower serum leptin at 6 and 12 weeks (p<0.05, N=8/group). Error bars represent + 1 SD.
Table 1.
Body length, bone mineral content, and body composition in male C57BL/6J mice housed at 20, 22, or 26°C from 3 wks to 6 or 12 wks of age (N=8/group)
| 6 wks of age | 9 wks of age | 12 wks of age | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20°C (cool) | 22°C (control) | 26°C (warm) | 20°C (cool) | 22°C (control) | 26°C (warm) | 20°C (cool) | 22°C (control) | 26°C (warm) | ||||||||||||
| SD | SD | SD | SD | SD | SD | SD | SD | SD | ptemp 6 wks | ptemp 9 wks | ptemp 12 wks | |||||||||
| Femur lengtd (mm) | 0.10 | 0.40 | 0.40 | 0.8 | 0.2 | 0.1 | NS | NS | NS | |||||||||||
| Tail length (mm) | 3.4 | 2.8 | 2.3 | 2.1 | 1.6 | 2.6 | 2.9 | 4.2 | 2.9 | <0.0001 | 0.041 | 0.004 | ||||||||
| BMC (g) | 0.022 | 0.023 | 0.016 | 0.034 | 0.022 | 0.018 | 0.036 | 0.065 | 0.014 | NS | NS | NS | ||||||||
| Lean mass (g) | 0.99 | 1.31 | 1.22 | 1.41 | 1.43 | 1.05 | 1.38 | 1.33 | 0.95 | NS | NS | NS | ||||||||
Longitudinal DXA showed differences in body composition across temperatures. BMD was 5.1% lower in cool vs. control mice at 6 weeks of age (p<0.01) but not 9 or 12 weeks of age (Fig 1D). BMC (g) did not differ at any age (Table 1). Compared to control and warm mice, cool mice had lower %body fat at 6 weeks of age but not at 9 or 12 weeks of age (Fig. 1E), while lean mass did not differ between groups (Table 1). Serum leptin was lower in cool and control vs. warm mice at 6 weeks and in cool vs. warm mice at 12 weeks of age (p<0.05 for all, Fig. 1F).
Trabecular bone at distal femur and cortical bone at midshaft femur
In the distal femur, there were no effects of temperature on trabecular bone microarchitecture at 6 weeks of age. At 12 weeks of age, trabecular bone volume fraction was 29–31% lower (p<0.05, Fig. 2A, B) and connectivity density was 14–23% lower in control and cool vs. warm housed mice, respectively (p<0.05 for both, Fig. 2C). Cool mice also had 9% lower trabecular number and 9% higher trabecular spacing (p<0.05 for both, Table 2) vs. warm mice, but there were no significant effects of temperature on trabecular thickness (Table 2).
Figure 2.
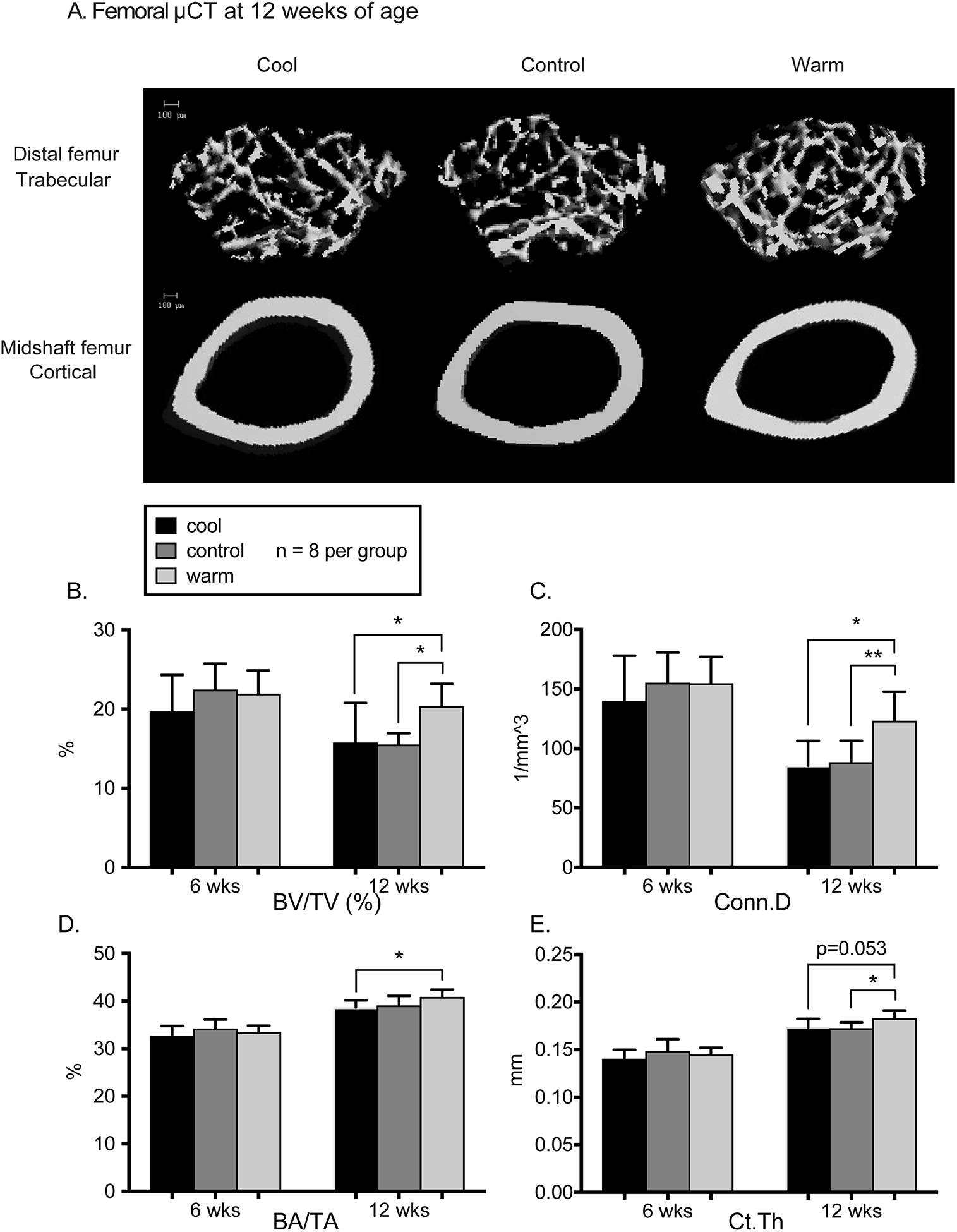
A. MicroCT reconstructions of trabecular bone in the distal femur and cortical bone in the midshaft femur. B. In the distal femur, trabecular bone volume fraction (BV/TV) was lower for cool and control vs warm mice at 12 weeks (p<0.05). C. Connectivity density was lower for cool and control mice vs warm at 12 weeks (p=0.03 and p=0.008). D. In the cortical midshaft femur, bone area fraction (BA/TA) of cool mice was lower than warm mice (p=0.041) at 12 weeks. E. Cortical thickness at 12 weeks was significantly lower in control vs. warm (p=0.03) but not cool vs. warm mice (p=0.053). There were no significant differences at 6 weeks of age. Error bars represent 1 SD. N=8/group for all data shown.
Table 2.
Distal femoral trabecular bone microarchitecture and midshaft femoral cortical bone cross-sectional geometry in male C57BL/6J mice housed at 20, 22, or 26°C from 3 wks to 6 or 12 wks of age (N=8/group)
| 6 wks of age | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20°C (cool) | 22°C (control) | 26°C (warm) | 20°C (cool) | 22°C (control) | 26°C (warm) | |||||||
| SD | SD | SD | SD | SD | SD | ptemperature | ||||||
| BV/TV (%) | 4.6 | 3.3 | 2.9 | 5.0 | 1.4 | 2.8 | 0.031 | |||||
| Tb.N (/mm) | 0.62 | 0.24 | 0.34 | 0.30 | 0.24 | 0.34 | 0.035 | |||||
| Tb.Th (mm) | 0.004 | 0.007 | 0.005 | 0.008 | 0.002 | 0.005 | NS | |||||
| Tb.Sp (mm) | 0.022 | 0.010 | 0.010 | 0.015 | 0.011 | 0.011 | 0.018 | |||||
| Conn.D (/mm) | 0.021 | 0.019 | 0.014 | 0.015 | 0.020 | 0.015 | 0.019 | |||||
| BA/TA (%) | 2.1 | 1.9 | 1.4 | 1.5 | 2.0 | 1.5 | 0.037 | |||||
| BA (mm2) | 0.04 | 0.06 | 0.04 | 0.07 | 0.03 | 0.03 | NS | |||||
| TA (mm2) | 0.05 | 0.10 | 0.10 | 0.17 | 0.10 | 0.07 | NS | |||||
| Ct.Th (mm) | 0.009 | 0.013 | 0.007 | 0.009 | 0.006 | 0.008 | 0.022 | |||||
| Imax (mm4) | 0.016 | 0.032 | 0.026 | 0.044 | 0.030 | 0.017 | NS | |||||
| Imin (mm4) | 0.005 | 0.014 | 0.009 | 0.023 | 0.009 | 0.011 | NS | |||||
| J (mm4) | 0.021 | 0.044 | 0.034 | 0.066 | 0.036 | 0.026 | NS | |||||
Significant difference vs. warm (p<0.05, GLM and Tukey test);
p=0.053
In midshaft femur cortical bone cross-sectional geometry, there were no significant differences across temperatures at 6 weeks of age. At 12 weeks of age, cortical bone area fraction was 6% lower in cool mice (p<0.05, Fib. 2A, D), and cortical bone thickness was lower in control (p<0.05) but not cool (p=0.053) vs. warm mice (Fig. 2E). Other cortical bone properties, including cortical bone area, total area, Imax, Imin, and J, did not differ across temperatures (Table 2).
Bone marrow adiposity and bone strength
Histology of the distal femur showed that the number (Ad.N/BS) and volume (Ad.V/TV) of adipocytes in the marrow cavity were highly variable across individuals and were not affected by temperature (Table 3). Mechanical testing of the femoral diaphysis indicated no temperature-induced differences in stiffness, maximum load, or postyield displacement (Table 3).
Table 3.
Distal femoral marrow adiposity at 12 wks of age (N=4–5/group) and midshaft femoral bone strength testing at 6 and 12 wks of age (N=8/group) in male C57BL/6J mice housed at 20, 22, or 26°C beginning at 3 wks of age
| 6 wks of age | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20°C (cool) | 22°C (control) | 26°C (warm) | 20°C (cool) | 22°C (control) | 26°C (warm) | ||||||||
| SD | SD | SD | SD | SD | SD | ptemperature | |||||||
| Ad.N/BS (%) | 43.0 | 16.2 | 26.2 | NS | |||||||||
| Ad.V/TV (%) | 0.19 | 0.16 | 0.16 | NS | |||||||||
| Maximum load (N) | 1.6 | 1.5 | 1.3 | 3.5 | 4.2 | 2.7 | NS | ||||||
| Stiffness (N/mm) | 12.1 | 13.8 | 9.5 | 30.3 | 29.2 | 17.0 | NS | ||||||
| Postyield displacement (mm) | 0.35 | 0.31 | 0.29 | 0.21 | 0.44 | 0.35 | NS | ||||||
| Estimated elastic modulus (GPa) | 0.49 | 0.56 | 0.49 | 0.74 | 1.31 | 0.85 | NS | ||||||
UCP1 mRNA and protein in brown adipose tissue
As measured by RT-PCR, the relative mRNA abundance of the nonshivering thermogenesis marker UCP1 in BAT was 58% higher for cool vs. warm mice at 6 weeks of age (p<0.05) and 66–8% higher in cool and control vs. warm mice 12 weeks of age p<0.005 for both, N=8 individuals/group, Fig. 3). Following RT-PCR, a subset of mice was used for UCP1 protein detection via Western blotting (N=3–4 from each temperature, Fig. 3). UCP1 protein expression in BAT total protein lysate was 50–55% higher in cool and control vs. warm mice at 6 weeks and 66% higher in cool vs. warm mice at 12 weeks of age (p<0.01 for all, Fig. 3).
Figure 3.
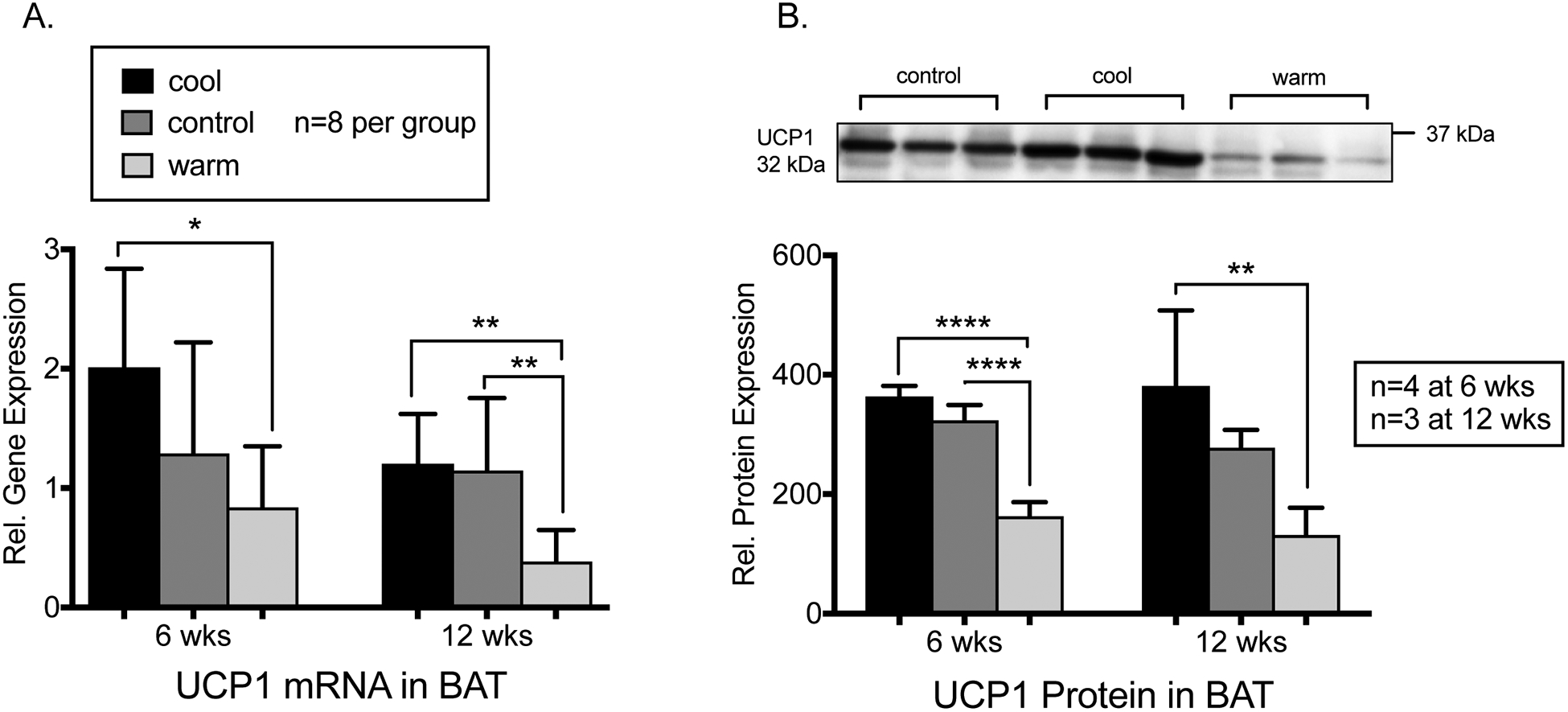
A. Relative mRNA of NST marker UCP1 in BAT by qRT-PCR was significantly higher for cool vs. warm mice at 6 (p=0.017) and 12 weeks (p=0.004). UCP1 mRNA was also increased in control vs. warm mice at 12 weeks (p=0.005) with N=8 individuals per group. B. Western blot of UCP1 from BAT total protein lysate shows increased expression for cool vs. warm mice at 6 (n=4 per group, p<0.001) and 12 weeks (N=3 per group, p=0.009) and for control vs. warm mice at 6 weeks (N=4 per group, p<0.001). Representative blot with anti-UCP1 signal shown for 12 week BAT with one individual per lane. Error bars represent 1 SD.
DISCUSSION
In this study, we tested two hypotheses: 1) that exposure to low temperature impairs bone acquisition during rapid skeletal growth, and 2) that upregulation of UCP1 is proportional to the decrease in temperature. We predicted that low temperature would increase sympathetic tone, leading to decreased trabecular microarchitecture, cortical bone cross-sectional geometry, and bone strength, but also higher UCP1 expression as a marker of NST in BAT. To test these hypotheses, we raised 3-week-old C57J/B6 male mice to 6 or 12 weeks of age at 20°C (low temperature, “cool”), 22°C (standard housing, “control”), and 26°C (near thermoneutrality, “warm”).
In partial support of our first hypothesis, the results indicate that low temperature is deleterious to overall skeletal size and to bone mass. Compared to mice housed near thermoneutrality, cool-housed mice had shorter bodies and tails, lower trabecular bone volume fraction, sparser, less connected trabeculae in the distal femur, lower midshaft femur cortical bone volume fraction, and a trend towards lower cortical thickness (p=0.053), at 12 weeks of age. Despite higher food intake, cool mice had lower fat mass at 6 weeks of age and lower serum leptin levels at 6 and 12 weeks, although their body mass did not differ from warm-housed mice.
Contrary to our first hypothesis, cool-housed mice did not have lower BMD, higher marrow adiposity, or lower midshaft diaphyseal bone strength. The most likely explanation is that the range of temperatures we used was too small. In terms of bone strength, cool housed mice had thinner cortices but not lower polar moments of inertia, indicating that the distribution of bone mass allowed maintenance of bending strength. More generally, the finding that a modest temperature change had greater effects in trabecular than cortical bone is consistent with the observation that trabecular bone is more responsive than cortical bone to external stimuli, perhaps due to its relatively higher surface area and turnover rate (Bilezikian et al. 2002). Similarly, although Huggins and Blocksom (1936) found that transplanting vertebrae from the tail to the abdominal cavity of rats decreased marrow adiposity, the temperature difference from outside to inside the body was greater than the range of temperatures used here.
In support of our second hypothesis, low temperature upregulated nonshivering thermogenesis. At 6 weeks of age, cool mice had significantly more UCP1 mRNA and cool and control mice had significantly more UCP1 protein compared to warm mice. At 12 weeks of age, cool and control mice had significantly more UCP1 mRNA, and cool mice had significantly more UCP1 protein compared to warm mice. The mRNA values reflect transcription of the gene, while protein levels reflect its existing expression. This pattern shows that mice at both 20 °C and 22 °C had chronically increased UCP1 activity in response to these temperatures. Although this relatively modest 6°C difference between cool and warm housing temperatures was sufficient to increase UCP1 expression but insufficient to restore normal bone architecture, it is possible that bone loss would have been even greater in its absence. Alternatively, it is possible that UCP1 upregulation helps to maintain temperature homeostasis, but that being warmer does not help to preserve bone mass.
Comparison to previous studies of temperature and bone in animal models
These data are consistent with older studies demonstrating reduced longitudinal growth, bone length, and tail length in experimental animals housed at sub-thermoneutral temperatures (Al-Hilli and Wright 1983; Ashoub 1958; Sumner 1909; Weaver and Ingram 1969). More recently, Serrat et al. (2013; 2008) found that mice housed at 7 °C had shorter bodies and shorter limb bones than mice raised at 27 °C, despite higher food intake and no difference in body mass. Importantly, cold exposure reduced blood flow and solute transport to limb bone growth plates in vivo (Serrat et al. 2008; 2009), whereas cold-induced reductions in growth were mitigated by exercise (Serrat et al. 2010) and hindlimb heating (Serrat 2014; 2015). These studies strongly suggest circulation to the growth plate underlies temperature-induced differences in limb length, and reduced circulation may contribute to the decreased trabecular bone architecture we observed. In the current study, mice housed at 20–22 °C had shorter bodies and tails but not shorter femurs than mice at 26 °C. As noted above, the low temperature we used was sufficient to change growth of the most distal extremities (e.g. the tail), but may not have been enough to affect limb bone growth. The shorter overall body length could reflect a combination of decreased skull and/or vertebral length, which we will measure in future studies. It should also be noted that although femur length did not change, trabecular bone volume in the distal femur was significantly lower, demonstrating that temperature can affect internal bone architecture without altering the external appearance of the bone. Consistent with our findings, Iwaniec et al. (2016) showed that housing mice at thermoneutrality (32°C) rather than the standard housing temperature of 22°C slowed the rate of age-related trabecular bone loss, demonstrating that even a mild temperature decrease is deleterious to the skeleton.
There is growing evidence that the mechanism underlying such cold-induced bone loss involves upregulation of sympathetic tone. Cold exposure increases sympathetic nervous system (SNS) activation in mammals, leading to warming mechanisms such as shivering, piloerection, vasoconstriction, and nonshivering thermogenesis in BAT. However, multiple studies in animal models have shown that chronic SNS activation also induces bone loss via β-adrenergic receptors expressed on osteoblasts, such that mice lacking the beta2-adrenergic receptor are protected from bone loss (Bonnet et al. 2008; Kajimura et al. 2011). Most compellingly, the Misty mouse strain has dysfunctional BAT, high sympathetic tone, and low bone mass, the latter of which is reduced when mice are treated with the beta-adrenergic antagonist propranolol (Motyl et al. 2013). These data suggest that nonshivering thermogenesis in BAT might protect the skeleton by warming the organism and therefore reducing SNS-induced bone loss (Motyl and Rosen 2011).
Fewer studies have addressed the effects of sympathetic tone on bone mass in humans, although it has been noted that patients taking “beta blocker” drugs, which inhibit sympathetic signaling to osteoblast beta-adrenergic2 receptors, have higher BMD and lower risk of fracture compared to the background population (Bonnet et al. 2007; Yang et al. 2012). More generally, multiple studies have shown that BAT is associated with higher bone mass in humans, including higher femoral total area and bone cross-sectional area in both men and women of varying BMI and age, and BMD in lean, young women (Bredella et al. 2012; Bredella et al. 2014; Lee et al. 2013). BAT mass is highest in humans during adolescent peak bone mass acquisition, and as in adults, is positively correlated with femoral cortical bone area and total cross-sectional area in children and adolescents (Gilsanz et al. 2012; Ponrartana et al. 2012). However, more work is needed to understand how interactions between BAT and the skeleton affect bone mass, and to test the role of other factors such as muscle mass.
Relevance for understanding how temperature affects bone in humans
In humans, high latitude populations under chronic cold exposure generally exhibit a wider pelvis, shorter stature, and shorter distal limb segments, as well as lower bone density and cortical thickness and accelerated rates of bone loss, compared to lower latitude populations (Harper et al. 1984; Katzmarzyk and Leonard 1998; Lazenby 1997; Leslie et al. 2006; Leslie et al. 2008; Mazess and Mather 1974; Mazess and Mather 1975; Pearson 2000; Roberts 1953; Ruff 1994; Thompson and Gunness-Hey 1981; Wallace et al. 2014). For example, skeletal analyses and bone mineral density values suggested earlier onset of age-related bone loss in indigenous cold-dwelling populations vs. populations of European ancestry (Harper et al. 1984; Mazess and Mather 1974; Mazess and Mather 1975; Thompson and Gunness-Hey 1981; Wallace et al. 2014). However, the extent of bone loss attributable to cold exposure is difficult to disentangle from other factors, including ancestry, consumption of a high protein, low calcium diet (Mazess and Mather 1974), and metabolic changes such as cold-induced hyperthyroidism (Lazenby 1997). Hyperthyroidism is particularly interesting in this context because elevated thyroid hormone is a stimulus of NST in BAT, and previous studies have shown that hyperthyroidism in response to chronic cold exposure, known as the polar T3 syndrome, both increases sympathetic tone and accelerates bone turnover (Allain and McGregor 1993; Schneider et al. 1994; Schneider et al. 1995). It is also interesting to note that accelerated bone loss in cold-dwelling humans appears as early as the 4th decade of life, at around the same age when studies of cold-responsive BAT suggest its abundance begins to decline (Harper et al. 1984; Mazess and Mather 1974; Mazess and Mather 1975; Wallace et al. 2014).
Although more work is needed to determine the timing and extent of bone loss with age in cold-dwelling humans, the reported patterns are generally consistent with our findings in the mouse model. In cool housed mice, the decrements in bone volume fraction, trabecular number, and connectivity density vs. warm housed mice are greater in 12 week old vs. 6 week old animals, similar to the accelerated age-related bone loss seen in humans. However, in young, rapidly growing mice up to 12 weeks of age, low temperature is more deleterious to trabecular bone microarchitecture than it is to bone mineral density or cortical bone properties, whereas in aging humans, cold decreases cortical bone thickness and BMD. Studies of old cool-exposed mice are needed to determine how low temperature affects the aging skeleton. More generally, impaired bone acquisition occurred in spite of higher uncoupling protein expression in brown adipose tissue, suggesting that although higher brown adipose tissue mass is linked to higher bone mass both in humans and in animal models, it is insufficient to normalize bone acquisition during chronic exposure to low temperature. Our results also demonstrate that developmental plasticity of the skeleton during subadult growth can occur in response to temperature as well as to mechanical loading, and thus climate may underlie some of the phenotypic variation observed among human populations.
Limitations and future directions
There are several limitations to this study. First, we assessed only males and used a single mouse strain, and it is possible that there are strain-specific and/or sex-specific responses to temperature. Second, as discussed above, the narrow range of temperature variation we used likely diminished the differences among our experimental groups. In addition, it is not clear whether the relationship between temperature and bone is linear or whether there are thresholds for the entire body or for particular regions. These questions will be addressed in future work focused on comparisons between temperature and bone growth in specific skeletal regions. Third, we raised mice only to 12 weeks of age, and therefore were not able to assess how low temperature and UCP1 upregulation affect the skeleton during aging. Finally, we did not directly measure sympathetic tone or body temperature in the mice. Future studies will use mouse enclosures that generate greater temperature differences and will include quantification of additional outcomes such as body temperature, activity level, energy expenditure, and metabolism.
CONCLUSION
In this study, we sought to test the developmental plasticity of cortical and trabecular bone in response to low temperature. We hypothesized that low temperature would induce proportional decreases in cortical and trabecular bone acquisition, as well as proportional increases in nonshivering thermogenesis in BAT. In support of our hypotheses, cool-housed mice had lower trabecular bone mass in the femur compared to warm housed mice, despite greater food intake and increased UCP1 expression in BAT. These results support the hypothesis that the growing skeleton has phenotypic plasticity in response to temperature. Upregulation of UCP1 in brown adipose tissue leading to increased nonshivering thermogenesis may have mitigated bone loss but was not sufficient to prevent it.
ACKNOWLEDGEMENTS
AR and MD planned experiments and drafted the manuscript. AR, CT, MD, MC, CM, LS, TS, and TB performed experiments, collected data and analyzed data. MC, CM, LS, TS, and TB reviewed and edited the manuscript. Funding for this project was provided by NSF BCS-1638553 to MD. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30 AR069620. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Paul Thurmond, Elizabeth Vernasco-Price, Joseph Sanders, and Kathie Hutyra for assistance with mouse experiments. We are grateful to Drs. Alon Kahana, Phillip E. Kish, and Alfonso Saera-Vila for assistance with protein and mRNA experiments. We thank two anonymous reviewers for helpful comments that improved the manuscript.
Funding for this project was provided by NSF BCS-1638553 to MD. Research reported in this publication was supported by NIAMS P30AR069620.
Literature Cited
- Aguirre JI, Leal ME, Rivera MF, Vanegas SM, Jorgensen M, and Wronski TJ. 2007. Effects of basic fibroblast growth factor and a prostaglandin E2 receptor subtype 4 agonist on osteoblastogenesis and adipogenesis in aged ovariectomized rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 22(6):877–888. [DOI] [PubMed] [Google Scholar]
- Al-Hilli F, and Wright EA. 1983. The effects of changes in the environmental temperature on the growth of bone in the mouse. Radiological and morphological study. Br J Exp Pathol 64(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- Aldridge GM, Podrebarac DM, Greenough WT, and Weiler IJ. 2008. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods 172(2):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain TJ, and McGregor AM. 1993. Thyroid hormones and bone. The Journal of endocrinology 139(1):9–18. [DOI] [PubMed] [Google Scholar]
- Allen JA. 1877. The influence of physical conditions in the genesis of species. Radical Review 1:108–140. [Google Scholar]
- Ashoub MA. 1958. Effect of two extreme temperatures on growth and tail-length of mice. Nature 181(4604):284. [DOI] [PubMed] [Google Scholar]
- Au-Yong IT, Thorn N, Ganatra R, Perkins AC, and Symonds ME. 2009. Brown adipose tissue and seasonal variation in humans. Diabetes 58(11):2583–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C 1847. Ueber die verhaltnisse der warmeokonomie der thiere zu ihrer grosse. Gottinger Studien 3:595–708. [Google Scholar]
- Bilezikian JP, Raisz LG, and Rodan GA. 2002. Principles of bone biology. San Diego: Academic Press. [Google Scholar]
- Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, and Benhamou CL. 2007. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40(5):1209–1216. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Pierroz DD, and Ferrari SL. 2008. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. Journal of musculoskeletal & neuronal interactions 8(2):94–104. [PubMed] [Google Scholar]
- Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, and Müller R. 2010. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. Journal of bone and mineral research 25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, and Ferrari SL. 2008. Mice Lacking Beta-Adrenergic Receptors Have Increased Bone Mass, But Are Not Protected from Deleterious Skeletal Effects of Ovariectomy. Endocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, and Ferrari SL. 2005. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, and Klibanski A. 2012. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. The Journal of clinical endocrinology and metabolism 97(4):E584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Gill CM, Rosen CJ, Klibanski A, and Torriani M. 2014. Positive effects of brown adipose tissue on femoral bone structure. Bone 58:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J. 2011. Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of experimental biology 214(Pt 2):242–253. [DOI] [PubMed] [Google Scholar]
- Churchill SE. 1998. Cold adaptation, heterochrony, and Neandertals. Evol Anthropol 7(2):46–61. [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A et al. 2009. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine 360(15):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JM, Chatziioannou AF, Taschereau R, Wang H, and Stout DB. 2013. The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comp Med 63(5):386–391. [PMC free article] [PubMed] [Google Scholar]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, and Parfitt AM. 2013. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 28(1):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ. 2015. The “Skinny” on brown fat, obesity, and bone. American journal of physical anthropology 156 Suppl 59:98–115. [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Brooks DJ, Conlon C, Vliet M, Louis L, Rosen CJ, and Bouxsein ML. 2016. Daily leptin blunts marrow fat but does not impact bone mass in calorie-restricted mice. The Journal of endocrinology 229(3):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Van Vliet M, Motyl K, Karim L, Brooks DJ, Louis L, Conlon C, Rosen CJ, and Bouxsein ML. 2014. Early-Onset Type 2 Diabetes Impairs Skeletal Acquisition in the Male TALLYHO/JngJ Mouse. Endocrinology 155(10):3806–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson H, Krog J, Andersen KL, and Scholander PF. 1956. The critical temperature in naked man. Acta physiologica Scandinavica 37(1):35–39. [DOI] [PubMed] [Google Scholar]
- Fischer AW, Cannon B, and Nedergaard J. 2018. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Molecular metabolism 7:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster F, and Collard M. 2013. A reassessment of Bergmann’s rule in modern humans. PloS one 8(8):e72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, and Garner JP. 2012. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PloS one 7(3):e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilda JE, and Gomes AV. 2013. Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Anal Biochem 440(2):186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, and Hu HH. 2012. Changes in brown adipose tissue in boys and girls during childhood and puberty. The Journal of pediatrics 160(4):604–609 e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ. 2012. Thermal physiology of laboratory mice: Defining thermoneutrality. Journal of Thermal Biology 37(8):654–685. [Google Scholar]
- Hardy JD, and Dubois EF. 1937. Regulation of Heat Loss from the Human Body. Proceedings of the National Academy of Sciences of the United States of America 23(12):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AB, Laughlin WS, and Mazess RB. 1984. Bone mineral content in St. Lawrence Island Eskimos. Human biology 56(1):63–78. [PubMed] [Google Scholar]
- Heaton JM. 1972. The distribution of brown adipose tissue in the human. Journal of anatomy 112(Pt 1):35–39. [PMC free article] [PubMed] [Google Scholar]
- Hildebrand T, Laib A, Muller R, Dequeker J, and Ruegsegger P. 1999. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 14(7):1167–1174. [DOI] [PubMed] [Google Scholar]
- Hildebrand T, and Rüegsegger P. 1997. A new method for the model independent assessment of thickness in three-dimensional images. J Micros 185:67–75. [Google Scholar]
- Holliday TW. 1997. Body proportions in Late Pleistocene Europe and modern human origins. Journal of human evolution 32(5):423–448. [DOI] [PubMed] [Google Scholar]
- Holliday TW, and Hilton CE. 2009. Body proportions of circumpolar peoples as evidenced from skeletal data: Ipiutak and Tigara (Point Hope) versus Kodiak Island Inuit. American journal of physical anthropology. [DOI] [PubMed] [Google Scholar]
- Huggins C, and Blocksom BH. 1936. Changes in Outlying Bone Marrow Accompanying a Local Increase of Temperature within Physiological Limits. J Exp Med 64(2):253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Hirvonen J, and Kinnula V. 1981. The occurrence of brown adipose tissue in outdoor workers. European journal of applied physiology and occupational physiology 46(4):339–345. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Philbrick KA, Wong CP, Gordon JL, Kahler-Quesada AM, Olson DA, Branscum AJ, Sargent JL, DeMambro VE, Rosen CJ et al. 2016. Room temperature housing results in premature cancellous bone loss in growing female mice: implications for the mouse as a preclinical model for age-related bone loss. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27(10):3091–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen KJ, Silva MJ, Vashishth D, Guo XE, and van der Meulen MC. 2015. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 30(6):951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, and Karsenty G. 2011. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med 208(4):841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL. 2012. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209(6):1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, and Leonard WR. 1998. Climatic influences on human body size and proportions: ecological adaptations and secular trends. American journal of physical anthropology 106(4):483–503. [DOI] [PubMed] [Google Scholar]
- Kingma B, Frijns A, and van Marken Lichtenbelt W. 2012. The thermoneutral zone: implications for metabolic studies. Frontiers in bioscience 4:1975–1985. [DOI] [PubMed] [Google Scholar]
- Kingma BR, Frijns AJ, Schellen L, and van Marken Lichtenbelt WD. 2014. Beyond the classic thermoneutral zone: Including thermal comfort. Temperature (Austin) 1(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DH, Sharkey NA, Lionikas A, Mack HA, Larsson L, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, Stitt JP et al. 2005. Adjusting data to body size: a comparison of methods as applied to quantitative trait loci analysis of musculoskeletal phenotypes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 20(5):748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby RA. 1997. Bone loss, traditional diet, and cold adaptation in Arctic populations. American Journal of Human Biology 9(3):329–341. [DOI] [PubMed] [Google Scholar]
- Lee P, Brychta RJ, Collins MT, Linderman J, Smith S, Herscovitch P, Millo C, Chen KY, and Celi FS. 2013. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 24(4):1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard WR, and Levy SB. 2015. Contributions of brown adipose tissue to human metabolic adaptation: Comparative and evolutionary perspectives. American journal of physical anthropology 156(S60). [Google Scholar]
- Leslie WD, Metge CJ, Weiler HA, Doupe M, Wood Steiman P, and O’Neil JD. 2006. Bone density and bone area in Canadian Aboriginal women: the First Nations Bone Health Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 17(12):1755–1762. [DOI] [PubMed] [Google Scholar]
- Leslie WD, Weiler HA, Lix LM, and Nyomba BL. 2008. Body composition and bone density in Canadian White and Aboriginal women: the First Nations Bone Health Study. Bone 42(5):990–995. [DOI] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, and Semenkovich CF. 2009. Why we should put clothes on mice. Cell metabolism 9(2):111–112. [DOI] [PubMed] [Google Scholar]
- Lowell BB, and Spiegelman BM. 2000. Towards a molecular understanding of adaptive thermogenesis. Nature 404(6778):652–660. [DOI] [PubMed] [Google Scholar]
- Mazess RB, and Mather W. 1974. Bone mineral content of North Alaskan Eskimos. The American journal of clinical nutrition 27(9):916–925. [DOI] [PubMed] [Google Scholar]
- Mazess RB, and Mather WE. 1975. Bone mineral content in Canadian Eskimos. Human biology 47(1):44–63. [PubMed] [Google Scholar]
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, Vunjak-Novakovic G, and Kaplan D. 2005. Silk implants for the healing of critical size bone defects. Bone 37(5):688–698. [DOI] [PubMed] [Google Scholar]
- Motyl KJ, Bishop KA, DeMambro VE, Bornstein SA, Le P, Kawai M, Lotinun S, Horowitz MC, Baron R, Bouxsein ML et al. 2013. Altered thermogenesis and impaired bone remodeling in Misty mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 28(9):1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl KJ, and Rosen CJ. 2011. Temperatures rising: brown fat and bone. Discov Med 11(58):179–185. [PMC free article] [PubMed] [Google Scholar]
- Murray I, Havel PJ, Sniderman AD, and Cianflone K. 2000. Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology 141(3):1041–1049. [DOI] [PubMed] [Google Scholar]
- Nagy TR, Krzywanski D, Li J, Meleth S, and Desmond R. 2002. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obesity research 10(5):412–415. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, and Rial E. 1999. A history of the first uncoupling protein, UCP1. Journal of bioenergetics and biomembranes 31(5):399–406. [DOI] [PubMed] [Google Scholar]
- Overton JM. 2010. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 34 Suppl 2:S53–58. [DOI] [PubMed] [Google Scholar]
- Pearson OM. 2000. Activity, climate, and postcranial robusticity: implications for modern human origins and scenarios of adaptive change. Curr Anthropol 41(4):569–607. [PubMed] [Google Scholar]
- Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, and Gilsanz V. 2012. Brown adipose tissue and its relationship to bone structure in pediatric patients. The Journal of clinical endocrinology and metabolism 97(8):2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler T, and Calvard S. 1978. Picture thresholding using an iterative selection method. IEEE Trans on Systems, Man and Cybernetics SMC-8(8):630–632. [Google Scholar]
- Roberts DF. 1953. Body weight, race, and climate. American journal of physical anthropology 11:533–558. [DOI] [PubMed] [Google Scholar]
- Ruff C 2002. Variation in Human Body Size and Shape. Annual Review of Anthropology 31(1):211–232. [Google Scholar]
- Ruff CB. 1993. Climatic adaptation and hominid evolution: The thermoregulatory imperative. Evol Anthropol 2:53–60. [Google Scholar]
- Ruff CB. 1994. Morphological adaptation to climate in modern and fossil hominids. American journal of physical anthropology 37(S19):65–107. [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K et al. 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58(7):1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DL, Barrett-Connor EL, and Morton DJ. 1994. Thyroid hormone use and bone mineral density in elderly women. Effects of estrogen. JAMA : the journal of the American Medical Association 271(16):1245–1249. [PubMed] [Google Scholar]
- Schneider DL, Barrett-Connor EL, and Morton DJ. 1995. Thyroid hormone use and bone mineral density in elderly men. Archives of internal medicine 155(18):2005–2007. [PubMed] [Google Scholar]
- Serrat MA. 2013. Allen’s rule revisited: temperature influences bone elongation during a critical period of postnatal development. Anat Rec (Hoboken) 296(10):1534–1545. [DOI] [PubMed] [Google Scholar]
- Serrat MA. 2014. Environmental temperature impact on bone and cartilage growth. Compr Physiol 4(2):621–655. [DOI] [PubMed] [Google Scholar]
- Serrat MA, King D, and Lovejoy CO. 2008. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proceedings of the National Academy of Sciences of the United States of America 105(49):19348–19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrat MA, Schlierf TJ, Efaw ML, Shuler FD, Godby J, Stanko LM, and Tamski HL. 2015. Unilateral heat accelerates bone elongation and lengthens extremities of growing mice. J Orthop Res 33(5):692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrat MA, Williams RM, and Farnum CE. 2009. Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy. J Appl Physiol (1985) 106(6):2016–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrat MA, Williams RM, and Farnum CE. 2010. Exercise mitigates the stunting effect of cold temperature on limb elongation in mice by increasing solute delivery to the growth plate. J Appl Physiol (1985) 109(6):1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V et al. 2012. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS one 7(11):e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, and Kozloff KM. 2015. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone 71:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Bigelow EM, and Jepsen KJ. 2013. Systematic evaluation of skeletal mechanical function. Curr Protoc Mouse Biol 3:39–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, and Keijer J. 2012. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Molecular metabolism 2(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmann AT Jr. 2007. Human cold adaptation: an unfinished agenda. American journal of human biology : the official journal of the Human Biology Council 19(2):218–227. [DOI] [PubMed] [Google Scholar]
- Sumner FB. 1909. Some effects of external conditions upon the white mouse. Journal of Experimental Zoology 7(1):97–155. [Google Scholar]
- Thompson DD, and Gunness-Hey M. 1981. Bone mineral-osteon analysis of Yupik-Inupiaq skeletons. American journal of physical anthropology 55(1):1–7. [DOI] [PubMed] [Google Scholar]
- Toth LA, Trammell RA, and Ilsley-Woods M. 2015. Interactions Between Housing Density and Ambient Temperature in the Cage Environment: Effects on Mouse Physiology and Behavior. J Am Assoc Lab Anim Sci 54(6):708–717. [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, and Teule GJ. 2009. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine 360(15):1500–1508. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S et al. 2009. Functional brown adipose tissue in healthy adults. The New England journal of medicine 360(15):1518–1525. [DOI] [PubMed] [Google Scholar]
- Wallace IJ, Nesbitt A, Mongle C, Gould ES, and Grine FE. 2014. Age-related variation in limb bone diaphyseal structure among Inuit foragers from Point Hope, northern Alaska. Archives of osteoporosis 9:202. [DOI] [PubMed] [Google Scholar]
- Weaver ME, and Ingram DL. 1969. Morphological Changes in Swine Associated with Environmental Temperature. Ecology 50(4):710–713. [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G et al. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Nguyen ND, Eisman JA, and Nguyen TV. 2012. Association between beta-blockers and fracture risk: a Bayesian meta-analysis. Bone 51(5):969–974. [DOI] [PubMed] [Google Scholar]


