Abstract
Background/Aims
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder which has a close relationship with the central nervous system (CNS). Vagus nerve (VN) is the major connector between CNS and the enteric nervous system (ENS). This study aimed to investigate the possible changes of the dimension of VN with vagus ultrasonography (VU) in IBS.
Materials and Methods
A total of 119 patients consisting of 66 IBS patients appropriated with Rome IV criteria and 53 patients of the control group were enrolled in the study. All patients underwent colonoscopy to rule out organic pathologies, such as polyposis, inflammatory bowel syndromes, diverticulosis, and colon cancer. Right vagus (RV) and left vagus(LV) nerve areas and diameters were calculated with VU.
Results
There was no statistical difference in the RV (p=0.445) and LV (p=0.944) diameters between the two groups. In addition, there was no statistical difference in RV (p=0.549) and LV (p=0.874) areas between the IBS and control groups.
Conclusion
VU clearly depicted that there are no changes of dimension in VN areas and diameters in IBS. This might show that VN is only a transporter of abnormal neuronal stimulations according to IBS pathophysiology.
Keywords: Irritable bowel syndrome, ultrasonography, vagus nerve
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that presents with altered defecation habits or chronic and/or recurrent abdominal pain that exacerbates or cools down during defecation (1). Recent studies have shown that IBS is not a basic gastroenterological disorder, but a complex disorder with contributions from brain structures, such as the cortex, amygdala, hippocampus, anterior cingulate cortex, and insula, as well as the hypothalamic–hypophysis axis and endocrine systems (2, 3).
IBS is closely related to stress and psychiatric disorders. Stress leads to IBS symptoms via autonomic and neuroendocrine responses. Studies showed that individuals who were exposed to stress in the early period of their life have IBS symptoms in the later periods of their life (4). This shows that there is a strong relationship between the brain and the gastrointestinal system.
The vagus nerve (VN) is one of the major transit ways between brain–gut axis. It is well known that VN efferent fibers do not directly affect motor or secretory function of the gut, but affect the myenteric plexus. It is believed that parasympathetic efferents of VN might be synapsing directly with enteric motor neurons (5).
VN constitutes both the afferent and efferent pathways of the brain–gut interactions; in fact, the VN is the most important nerve of the brain–gut axis (3, 6). Although they exhibit similar symptoms, based on the pathophysiology, IBS can be classified into a top-down model or a bottom-up model (7). It is clear that the VN lies at the center of the pathophysiology in both the top-down or bottom-up models of IBS; however, VN dysfunction is disputable in IBS cases.
In the literature, the dysfunction of VN in IBS has been already shown (8), but possible morphological and/or dimensional changes of VN due to IBS have not been discussed yet. In this study, we aimed to investigate the possible changes of dimension in theVN in IBS patients using vagus ultrasonography (VU).
MATERIALS AND METHODS
This study was approved by Tekirdağ Namık Kemal University School of Medicine ethics committee with study approval number 2018.01.01.01. A total of 127 patients who referred to The Tekirdağ Namık Kemal University School of Medicine Research and Education Hospital Gastroenterology clinic between January 2018 and December 2018 were enrolled in this study. Informed consent was obtained from all patients. Patients aged under 18 years; patients who could not undergo colonoscopies; and patients with diabetes mellitus (DM), inflammatory bowel disease, chronic kidney disease, peripheral neuropathy, and tumoral lesions of VN constituted the exclusion criteria of the study. When the blood profiles were evaluated, seven patients had DM and one patient had chronic kidney failure; they were also excluded from the study.
A total of 66 patients were diagnosed with IBS based on the Rome IV criteria, and 53 of the patients had no IBS symptoms. Colonoscopy was performed on all patients for the exclusion of organic diseases, such as colon adenocarcinoma, inflammatory bowel syndromes, colonic polyposis, and diverticulosis. Colonoscopy examinations were performed by an experienced gastroenterologist.
Vagus ultrasound
The VU examinations were conducted by an experienced radiologist who was blinded to the patient status using an Aplio XG (Toshiba, Tokyo, Japan). The right and left VNs were examined separately at the level of the thyroid gland in transverse sections using a 6–14 MHz linear probe. The common carotid artery (CCA) and internal jugular vein (IJV) were detected, and the VN was found at the level of the thyroid cartilage in the vagina caroticum between the CCA and the IJV (Figure 1). The axial area of the VN was measured at this level, and the VN diameter was measured in the long axis (Figures 2 and 3) (9).
Figure 1.
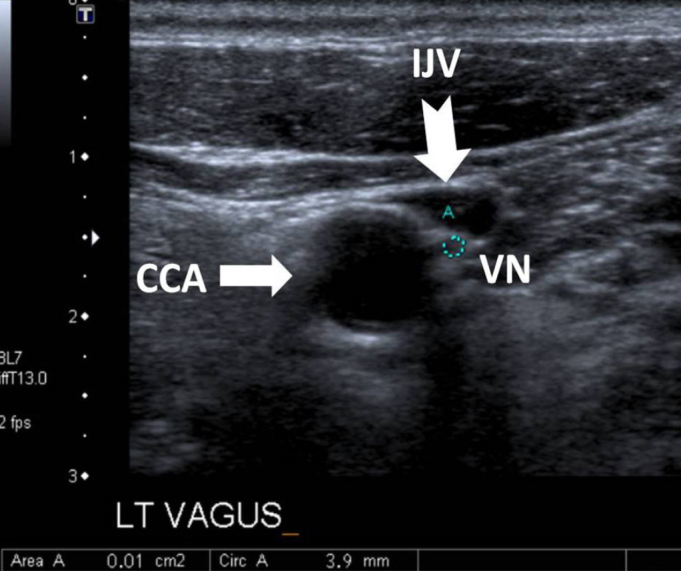
At the level of the thyroid gland, axial neck ultrasound with a linear probe shows the common carotid artery (CCA) (white arrow) and internal jugular vein (IJV) (white-tailed arrow). Between these structures, in the vagina caroticum, vagus nerve (VN) can be seen as a hypoechoic round image.
Figure 2.
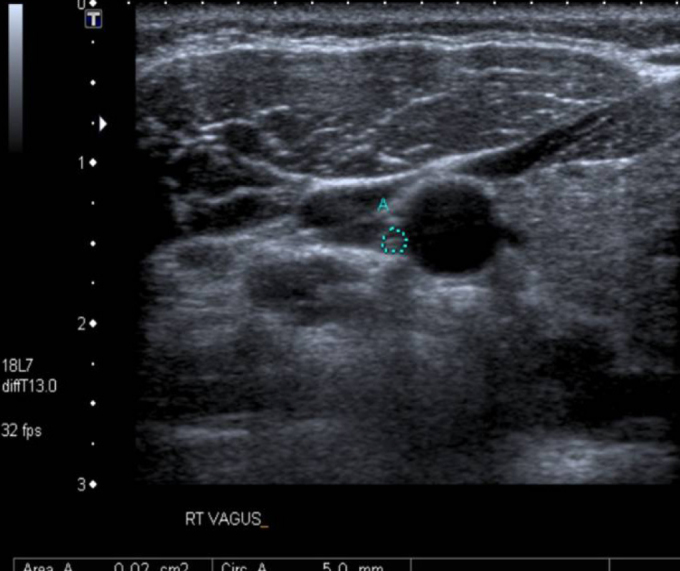
In the axial section, the right VN area was calculated as 2 mm2 (dotted area).
Figure 3.
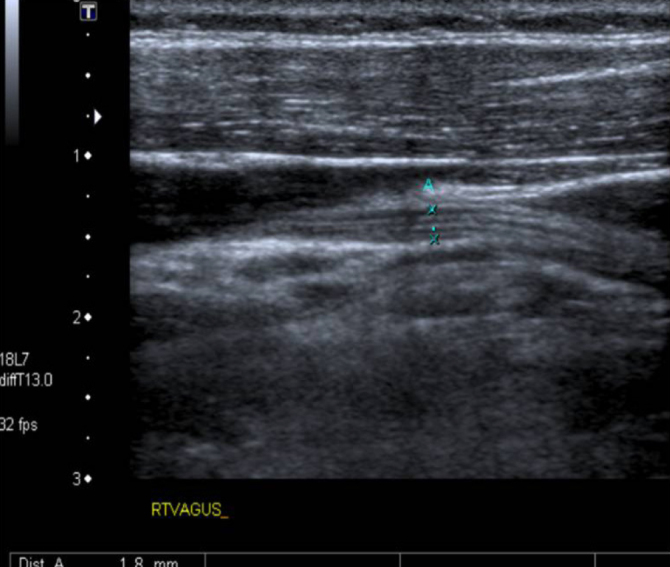
VN can be clearly seen in the longitudinal axis. In this axis, VN diameter was calculated as 1.8 mm. Fibers of VN can also be seen in this axis.
Statistical Analysis
The data were analyzed using the Statistical Packages for the Social Sciences (SPSS) IBM SPSS Statistics for Windows version 17.0 (IBM Corp.; Armonk, NY, USA). The Kolmogorov–Smirnov test was used to determine whether the distribution of the continuous numerical variables was close to normal. The descriptive statistics were expressed as the mean±standard deviation or the median (quarterly distribution width) for the numerical variables. For the categorical variables, they were expressed as the number of cases and percentage.
The significance of the difference between the groups in terms of the age averages was examined using the Student’s t test, and the significance of the difference in terms of the gender distribution was examined using the continuity corrected chi-squared test. The significance of the differences between the groups in terms of VN diameter and area were evaluated using the Mann–Whitney U test. A p<0.05 was considered to be statistically significant.
RESULTS
The distributions of the males and females in the IBS and non-IBS groups have no statistical significance (p=0.489).The mean age of the non-IBS group was 53.5±8.9 years (range 18–76 years), and the mean age of the IBS group was 48.5±12.3 years old (range 34–71 years). Therefore, the IBS group was younger (p=0.014). The patients’ descriptive statistics are shown in Tables 1 and 2.
Table 1.
Demographic features of IBS and non-IBS groups.
| non-IBS (n=53) | IBS (n=66) | p | |
|---|---|---|---|
| Age (years) | 53.5±8.9 | 48.5±12.3 | 0.014† |
| 95% CI | 51.0–56.0 | 45.5–51.5 | |
| Gender | 0.489‡ | ||
| Female | 32 (60.4%) | 45 (68.2%) | |
| Male | 21 (39.6%) | 21 (31.8%) |
Student’s t test,
Continuity corrected chi-squared test.
95% CI: 95% confidence interval; IBS: irritable bowel syndrome.
Table 2.
The baseline characteristics of the study’s population (n=119).
| Variables | non-IBS (n=53) | IBS (n=66) |
|---|---|---|
| Glucose | 103.45±22.13 | 96±9.14 |
| Hct % | 39.79±4.63 | 40.01±4.39 |
| Hg g/dL | 13.32±1.71 | 13.20±1.67 |
| WBC uL | 6788±1870.93 | 6979.54±1704.27 |
| Platelet × 103/uL | 241.56±68.73 | 259.34±50.93 |
| Creatinine mg/dL | 0.74±0.14 | 0.88±0.20 |
| Urine mg/dL | 29.54±6.53 | 28.20±11.45 |
Values are presented as n (%) or mean±standard deviation.
Hct: hematocrit; Hg: hemoglobin; IBS: irritable bowel syndrome; WBC: white blood cell.
Between the IBS and the non-IBS groups, there were no statistically significant differences in the medians of the right vagus (RV) diameter (p=0.445), the medians of the left vagus (LV) diameter (p=0.944), and the means of the vagus diameter (p=0.651; Table 3).
Table 3.
VN diameter measurements of cases according to study groups.
| non-IBS (n=53) | IBS (n=66) | p† | |
|---|---|---|---|
| VN diameter (mm) | |||
| Right | 1.70 (1.50–1.95) | 1.70 (1.50–1.80) | |
| 95% CI | 1.60–1.90 | 1.60–1.70 | 0.445 |
| Left | 1.50 (1.20–1.70) | 1.50 (1.30–1.60) | |
| 95% CI | 1.30–1.60 | 1.40–1.60 | 0.944 |
| Mean | 1.60 (1.45–1.80) | 1.57 (1.44–1.71) | |
| 95% CI | 1.50–1.70 | 1.50–1.65 | 0.651 |
Data were shown as median (25th–75th) percentiles.
Mann–Whitney U test.
95% CI: 95% confidence interval; IBS: irritable bowel syndrome; VN: vagus nerve.
There were no statistically significant differences between the IBS and non-IBS groups in terms of the medians of the RV area (p=0.549), the medians of the LV area (p=0.874), and the means of the vagus area (p=0.670; Table 4).
Table 4.
VN area measurements of cases according to study groups.
| non-IBS (n=53) | IBS (n=66) | p† | |
|---|---|---|---|
| VN area (mm2) | |||
| Right | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | |
| 95% CI | 2.0–2.0 | 2.0–2.0 | 0.549 |
| Left | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | |
| 95% CI | 2.0–2.0 | 2.0–2.0 | 0.874 |
| Mean | 2.0 (1.5–2.5) | 2.0 (2.0–2.5) | |
| 95% CI | 2.0–2.0 | 2.0–2.0 | 0.670 |
Data were shown as median (25th–75th) percentiles.
Mann–Whitney U test.
95% CI: 95% confidence interval; IBS: irritable bowel syndrome; VN: vagus nerve.
DISCUSSION
The VN is the main nerve of the brain–gut axis, and it is the only pathway that allows integration between the brain and the gastrointestinal tract. The fact that VN dysfunction is more functional than morphological is consistent with IBS pathophysiology. Our study is the only one in the literature to show that there were no changes of dimension in the VN in the IBS cases. For that reason, according to our study, the pathological electrical activity in the brain and/or gut may not be because of dimensional changes in the VN in IBS patients.
The IBS prevalence is approximately 10–15% in industrialized countries (10), and it reaches up to 28% on gastroenterologist examinations (11). In IBS cases, the symptoms are predominantly classified by hypomotility and constipation due to vagal hypofunction or hypermotility and diarrhea due to vagal hyperfunction (12). The association between IBS and psychiatric disorders is an undeniable fact, and the prevalence of this association is between 50% and 90% (13). Moreover, other studies have emphasized that stress causes VN dysfunction (14, 15). In recent years, researchers have come to believe that the gut microbiome affects the brain–gut axis, and that it plays an important role in the etiology of functional gastrointestinal system diseases, such as IBS (16).
The enteric nervous system (ENS) is a complex structure that contains the submucosal plexus and myenteric plexus. It is controlled by hormones and cytokines that are integrated into the central nervous system (CNS) and that control the CNS itself. In the interactions between the ENS and the CNS, afferent knowledge of the VN plays an important role (5, 17).
It is known that 80% of VN fibers are afferent and 20% are efferent (18). In rats, the VN has been shown to perform gastrointestinal system innervation, except for the rectum (19). However, the innervation of the VN in humans remains controversial. Some authors (20) have suggested that the VN innervates up to the splenic flexure, whereas the remaining colonic loops are innervated by pelvic nerves. Another group of authors (3, 21) believes that the entire digestive tract maybe innervated by the VN. In any case, the VN is the most important pathway allowing information to travel through the brain–gut axis (18).
In the literature, there are many studies showing VN dysfunction in IBS. In their study, Smart and Atkinson (22) showed that the distal esophageal sphincter response, gastric secretion response, and cardiac pulsation response were decreased in IBS patients. Spaziani et al. (23) who evaluated the effects of rectal distention on the blood pressure, and, accordingly, determined the changes in the rectal baroreceptor sensitivity, showed that the baroreceptor stability was increased in IBS patients. Botha et al. (24) reported that deep inspiration and anticholinergic drugs, which increase vagal tone, reduce esophageal pressure and pain. Pellisier et al. (25) have shown that there is a high sympathetic and low parasympathetic tone in IBS which shows the dysfunction of VN. These studies have emphasized the relationships between vagal dysfunction and functional bowel diseases; however, none of these studies have discussed the morphological and/or dimensional changes of VN. Our study clearly depicted that there is not a dimensional differentiation of VN between IBS patients and the normal population.
In the literature, evaluation of nerves via ultrasonography was discussed (26). With the exception of our study, there are no studies in the literature that investigated the possible changes of dimension in the VN in IBS, a disease in which VN dysfunction is certain. In their study of diabetic patients, Tawfik et al. (27) reported, via VU guidance, that the VN was affected in diabetic neuropathy. In that study, the results showed that there were decreases in the VN diameter and area in the diabetic neuropathy cases when compared with the control group. Conversely, with VU guidance, our study clearly showed that the VN exhibits no changes of dimension in IBS cases. This situation might be due to the nature of the disease and it would provide a better understanding of the IBS. Evaluation of motor and sensorial nerve functions with electromyography accordingly with VN morphology/dimension via radiological modalities might be the next step of our study.
In our study, there was a statistically significant difference between the IBS and non-IBS groups in terms of age distribution; the IBS group was younger. This condition was evaluated in accordance with the pathophysiology and nature of IBS. Moreover, the fact that this was a single-center study and that there was an insufficient number of patients were among the limitations of this study. Studies with more patients will provide more accurate results.
In conclusion, the results of our study showed, with VU guidance, that there were no changes of dimension in the VN in IBS cases. The findings shown in this study might also depict the status of VN as a neurological pathway between CNS and ENS in the IBS pathophysiology which could provide a better understanding of the nature of the disease.
MAIN POINTS.
Vagus Nerve (VN) is the main way that connects the central nervous system and enteric nervous system.
VN has a critical role for transporting pathological nerve stimulations in Irritable Bowel Syndrome (IBS).
VN dimensions and echo patern can be evaluated by ultrasonography.
Ultrasonography showed that there are no dimensional changes of VN in IBS patients compared to normal population.
Footnotes
This study was presented at the 28th Year Annual Meeting of Turkish Society of Neuroradiology with International Participation, February 15–17, 2019, Istanbul, Turkey
Ethics Committee Approval: Ethics committee approval was received for this study from Ethics committee of Tekirdağ Namık Kemal University School of Medicine in 01.25.2018 with approval number 2018.01.01.01.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – O.O., R.M.; Design – O.O., T.İ.K.O.; Supervision – R.M., M.D.; Resource – O.O., R.M.; Materials - T.İ.K.O., M.D.; Data Collection and/orProcessing – O.O., T.İ.K.O.; Analysis and/orInterpretation – O.O., R.M.; Literature Search – O.O., R.M.; Writing – O.O., R.M.; Critical Reviews – T.İ.K.O., M.D.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gas-troenterology. 2006;130:1377–90. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Coss-Adame E, Rao SS. Brain and gut interactions in irritable bowel syndrome: new paradigms and new understandings. Curr Gastroenterol Rep. 2014;16:379. doi: 10.1007/s11894-014-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaz B, Sinniger V, Pellissier S. The vagus nerve in the neuro-immune axis: implications in the pathology of the gastrointestinal tract. Front Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer EA, Naliboff BD, Chang L, Coutinho SVV. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–24. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 5.Mulak A, Bonaz B. Irritable bowel syndrome: a model of the brain-gut interactions. Med Sci Monit. 2004;10:RA55–62. [PubMed] [Google Scholar]
- 6.Holtmann G, Shah A, Morrison M. Pathophysiology of functional gastrointestinal disorders: a holistic overview. DigDis. 2017;35:5–13. doi: 10.1159/000485409. [DOI] [PubMed] [Google Scholar]
- 7.Mayer EA, Naliboff BD, Chang L. Basic pathophysiologic mechanisms in irritable bowel syndrome. Dig Dis. 2001;19:212–8. doi: 10.1159/000050682. [DOI] [PubMed] [Google Scholar]
- 8.Pellissier S, Dantzer C, Mondillon L, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn’s disease and irritable bowel syndrome. PLoS One. 2014;9:e105328. doi: 10.1371/journal.pone.0105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knappertz VA, Tegeler CH, Hardin SJ, McKinney WM. Vagus nerve imaging with ultrasound: anatomic and in vivo validation. Otolaryngol Head Neck Surg. 1998;118:82–5. doi: 10.1016/S0194-5998(98)70379-1. [DOI] [PubMed] [Google Scholar]
- 10.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M. Pathophysiology in irritable bowel syndrome. Drug News Perspect. 2001;14:268–78. doi: 10.1358/dnp.2001.14.5.704648. [DOI] [PubMed] [Google Scholar]
- 12.Buckley MM, O’Mahony SM, O’Malley D. Convergence of neuro-endocrine-immune pathways in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2014;20:8846–58. doi: 10.3748/wjg.v20.i27.8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry. 2001;62(Suppl 8):38–45. discussion 46–7. [PubMed] [Google Scholar]
- 14.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CT, Chuang TY, Lu CL, et al. Abnormal vagal cholinergic function and psychological behaviors in irritable bowel syndrome patients: a hospital-based Oriental study. Dig Dis Sci. 1998;43:1794–9. doi: 10.1023/A:1018848122993. [DOI] [PubMed] [Google Scholar]
- 16.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–9. doi: 10.1016/0016-5085(93)90419-D. [DOI] [PubMed] [Google Scholar]
- 20.Netter FH. Atlas of Human Anatomy. Ardsley, USA: Ciba-Geigy Corporation; 1989. [Google Scholar]
- 21.Delmas J, Laux G. Anatomie Médico-Chirurgicale du Système Nerveux Végétatif: (Sympathique&Parasympathique) Paris: Masson; 1933. [DOI] [Google Scholar]
- 22.Smart HL, Atkinson M. Abnormal vagal function in irritable bowel syndrome. Lancet. 1987;2:475–8. doi: 10.1016/S0140-6736(87)91792-2. [DOI] [PubMed] [Google Scholar]
- 23.Spaziani R, Bayati A, Redmond K, et al. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol Motil. 2008;20:336–42. doi: 10.1111/j.1365-2982.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 24.Botha C, Farmer AD, Nilsson M, et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut. 2015;64:611–7. doi: 10.1136/gutjnl-2013-306698. [DOI] [PubMed] [Google Scholar]
- 25.Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. 2010;35:653–62. doi: 10.1016/j.psyneuen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Grimm A, Axer H, Heiling B, Winter N. Nerve ultrasound normal values-Readjustment of the ultrasound pattern sum score UPSS. Clin Neurophysiol. 2018;129:1403–9. doi: 10.1016/j.clinph.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Tawfik EA, Walker FO, Cartwright MS, El-Hilaly RA. Diagnostic ultrasound of the vagus nerve in patients with diabetes. J Neuroimaging. 2017;27:589–93. doi: 10.1111/jon.12452. [DOI] [PubMed] [Google Scholar]


