Abstract
Langerhans cells (LCs) are specialized dendritic cells (DCs) that play a defense role in recognizing foreign antigens, in tissue where antigenic exposures occur, as in the skin and mucous membranes. LCs are able to continuously move within the tissues thanks to dendritic contraction and distension performing their surveillance and/or phagocytosis role. These cells are characterized by the presence of Birbeck granules in their cytoplasm, involved in endocytosis. LCs have been characterized in several classes of vertebrates, from fish to mammals using different histological and molecular techniques. The aim of the present review is to define the state of art and the need of information about immunohistochemical markers of LCs in different classes of vertebrates. The most used immunohistochemical (IHC) markers are Langerin/CD207, CD1a, S-100 and TLR. These IHC markers are described in relation to their finding in different vertebrate classes with phylogenetical considerations. Among the four markers, Langerin/CD207 and TLR have the widest spectrum of cross reactivity in LCs.
Keywords: Dendritic cells, Markers, Immunohistochemistry, Mammals, Fish
1. Introduction
Dendritic cells (DCs) have been described in all lymphoid organs as in the liver, intestine and lungs (Vermaelen and Pauwels, 2005). Due to their ability to recognize and bind foreign antigens, DCs localize wherever there is an antigenic exposure, such as in the skin and mucous membranes. At this stage, the DCs are still immature, showing a high affinity in intercepting and binding the antigen, but a lower ability to stimulate T lymphocytes (Gallucci and Matzinger, 2001). If the detected antigen shows molecular traces of pathogens or tissue destruction, DCs change becoming mature. Once activated, DCs migrate to the lymph nodes, triggering the immune response and presenting antigenic peptides to specific T cells. There is a division within the family of DCs into myeloid DC (mDC) and plasmacytoid DC (pDC) (Spits et al., 2000). The pDCs, when exposed to viral antigens, release high doses of Interferon 1, and upon maturing, trigger an adaptive immune response. Langerhans cells belong to the myeloid line and are found in basal and suprabasal layers of the epidermis and in oral, nasal, pulmonary, corneal, vaginal, rectal mucosal epithelia (Romani et al., 2012). They can be characterized for the presence of Birbeck granules in their cytoplasm, which can play a role in endocytosis. Langerhans cells are in continuous movement, elongating the dendrites between the keratinocytes in the skin, playing their role as overseers (Nishibu et al., 2006). By means of these movements of contraction and dendritic distension, they can control even the most external layers of the skin, managing to infiltrate through the cellular junctions, up to the stratum corneum and also migrate to the underlying connective tissue (Lauriano et al., 2019). In a state of quiescence, LCs help to regulate the cell populations of the skin, while, in alert conditions and together with keratinocytes, trigger an immune response which aims to activate T lymphocytes (Klechevsky et al., 2008; Polak et al., 2012). Keratinocytes, through the release of cytokines, can modulate the functionality of LCs, modifying the type of induced response such as TNF alpha which is a powerful activator (Groves et al., 1995). Several studies report the presence of cells morphologically and functionally similar to the LCs of mammals, also in the other vertebrate classes (Kordon et al., 2016; Lauriano et al., 2014, 2018, 2019, 2020; Lovy et al., 2006, 2009; Zaghloul et al., 2017). These cells do not present only long dendrites but are characterized from cytoplasmic granules similar to the Birbeck ones and have been highlighted with LCs typical markers, such as CD207 and S-100, and for this reason they are called Langerhans–like cells.
Inflammation is an animal defense mechanism, which aims to sanitize organs and tissues, eliminating any pathogens (Loynes et al., 2018) and starting the repair process (Ferrero-Miliani et al., 2007; Medzhitov, 2010). It is an innate nonspecific mechanism because cellular or tissue damage begins by inducing a vascular reaction, releasing the flogosis chemical mediators. Vasodilatation occurs, increase in capillary permeability with the appearance of edema, and leukocyte inflation (Chertov et al., 2000). This characterizes the clinical picture of inflammation with calor, rubor, tumor, dolor e function laesa (Takeuchi and Akira, 2010). The first signal for this defensive mechanism is the release of cytokines (Mahla et al., 2013). Being implicated in inflammatory processes, LCs represent an excellent indicator of diagnosis of various pathologies. Many scientific studies have shown that these cells are easily detected by immunohistochemistry using antibodies to CD1a, CD207 (Langerin), S-100 and TLR. Frequently, these antibodies are associated with each other to confirm any pathological diagnosis. The aim of this review is to make know the state of art about most common immunohistochemical markers of Langerhans cells in Vertebrate. Characterization of these cells, using the same antibodies in the different vertebrate classes, can clarify the immune system phylogenesis and confirm their homology with mammalian Langerhans cells. Moreover, immunohistochemical characterization of these cells in vertebrate models, could be applied in the diagnosis of skin and mucous membrane human diseases.
1.1. Laboratory validation of primary antibodies
Immunohistochemistry is an experimental technique whose results are based on analyte interpretation, specific reagents and the proper use of controls. The concept of specificity is based on the property of antibodies to recognize specific epitopes. The binding of antibody to the target molecule in a mixture can be tested by means of absorption control prior to application to the tissue. This, however, does not demonstrate the specific positivity of the antibody in the tissue. The most stringent positive controls are carried out by the presence of the antigen that is known in another tissue of the same species examined (internal positive control) or using a different sample from another species (external positive control) which is known to contain the target molecule. A negative control demonstrates the reaction between the epitope of the target antigen and the paratope of the antibody. Although a manufacturer demonstrates specificity through Western Blot, this does not mean that this specificity also occurs in tissue samples. Commonly negative control is done by omitting the primary antibody, but this procedure does not demonstrate its bond specificity. For a valid negative control, serum or specific isotypical immunoglobulins shall be replaced at the same concentration as the primary antibody (Hewitt et al., 2014).
Over than the above cited immunohistochemistry laboratory procedures to highlight the specificity of an antibody, the literature review highlighted several strategies to validate the use of antibodies for research applications (Baker, 2015a, b; Bradbury and Plückthun, 2015; Couchman, 2009; Edfors et al., 2018; Uhlen et al., 2016;).
In one of the most complete study in defining how validate antibodies use for research applications Edfors and collaborators (2018) reported the results of the following five optimal methods: orthogonal methods, genetic knockdown, recombinant expression, independent antibodies, and capture mass spectrometry analysis. They showed that all these methods can be used for antibodies validation in a systematic and standardized way for Western Blot applications. The same methods can be also used for other antibodies research application as immunohistochemistry.
It is of fundamental importance the use of these methods in order to avoid false positive and doubtful results. Immunohistochemistry supported by the above described methods is then a powerful tool for tissue research applications in both mammalian and non-mammalian vertebrates.
The following paragraphs review the main antibodies using in the detection of Langerhans cells in mammalian and non-mammalian vertebrates.
2. Langerin/CD207
Langerin is a C-type lectin detectable in many cell types such as Langerhans cells (LCs) and dendritic cells (DCs), in most epithelial and connective tissues, which plays a role in the recognition of foreign antigens such as pathogens and bacteria (Mayer et al., 2007). Langerin acts as an inducer of Birbeck granule formation in human (Valladeau et al., 2000). In the study of Valladeau et al. (2002), Langerin/CD207 was used to mark the LCs, in mouse. The results showed that CD207, like the human one, also leads to the formation of pentalamellar membranes typical of Birbeck granules (Birbeck et al., 1961; Wolff, 1967), emphasizing the conservation of its function.
Several studies have shown the presence of similar LCs in zebrafish (He et al., 2017; Lin et al., 2019). It is still unclear whether these cells are actually LCs, as the ontogenesis of Langerin/CD207 in zebrafish is not known. Identifier markers such as Birbeck granules have been found in zebrafish. Lugo-Villarino et al. (2010) identified cells which were morphologically similar to DC of mammals in Danio rerio, showing that the cellular constituents of the antigen presentation process seem to be well preserved from teleosts to higher vertebrates. The presence of DCs-like cells in teleosts has been demonstrated in salmonids (Fuglem et al., 2010; Haugarvoll et al., 2006; Ohta et al., 2004); and in the turbot (Psetta maxima) (Hu et al., 2010). Further studies have shown the expression of markers such as MHC II (Koppang et al., 2004; Morrison et al., 2006; Olsen et al., 2011) and Langerin/CD207, highly conserved among vertebrates (Lovy et al., 2009). CD/207 antibody is considered one of the most efficient markers of Langerhans and dendritic cells.
2.1. Human
Pagliari et al. (2011) conducted research on LCs in paracoccidiomycosis (PCM). This fungal infection is caused by Paracoccidioides brasiliensis and occurs with evident skin and mucous membrane lesions. LCs were found in the skin of the control group specimens by immunoreaction with CD207 (Fig. 1 ). Furthermore, in the group with PCM lesions, the LCs were localized in the inflammatory infiltrates, in the dermis and in the corium of the lesions. Powell et al. (2017) showed that Langerin/CD207 is a marker to confirm LCs histiocytosis (LCH). By immunohistochemistry, the proliferation of positive CD1a and CD207 cells was noted, which, and, together with S-100, confirms that these are the most effective markers for the diagnosis of histiocytosis. Hattori et al. (2011) characterized the expression of CD207 in the cornea by confocal immunohistochemistry. LCs CD207 positive were localized in the epithelium and corneal stroma. Morphologically the LCs in the stroma showed a more rounded soma while those present in the epithelium were characterized by long dendrites and a smaller soma. These data allow to distinguish two different LCs resident populations in the stroma and epithelium.
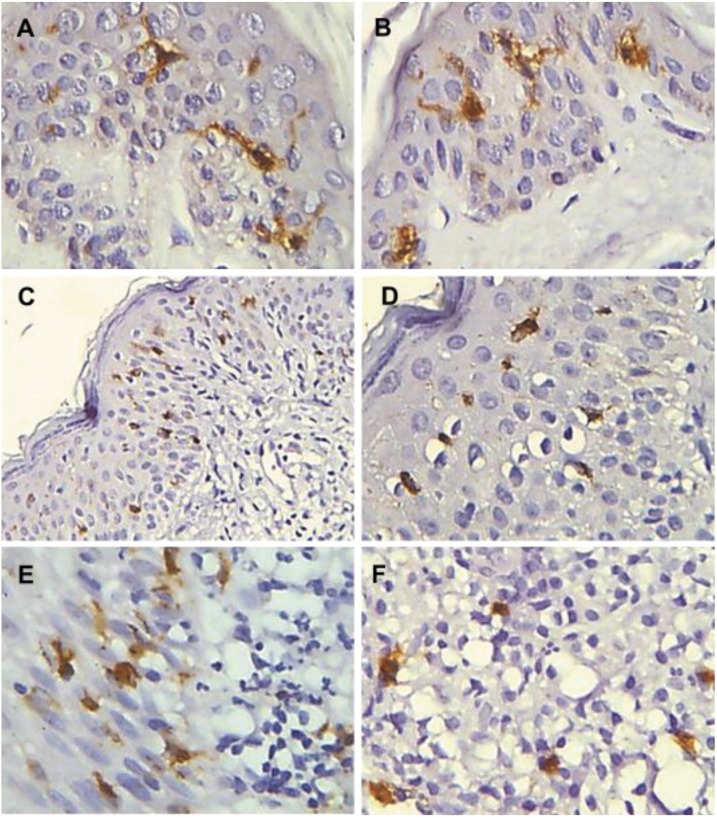
Fig. 1.
Immunohistochemical detection of langerin positive in Human normal skin and in cutaneous lesions. Positive langerin cells with long and short dendrites in the epidermis of normal skin (A, B); langerin positive cells with short dendrites slightly distributed in PCM skin lesions (C, D); positive Langerin cells distributed in the inflammatory infiltrate in the epidermis / dermis interface (E) and dermis (F). Streptavidin-biotin peroxidase method - x200 (C) and x400 (A, B, D, E, F). Reproduction from Pagliari et al., 2011. ELSEVIER LICENSE N° 4881280962191.
2.2. Mammals
Dauch et al. (2013) studied the role of LCs in diabetic mice with mechanical allodynia. By immunoreaction with CD207, an increase in the number of LCs in the skin of the paw was found in this model. This increase could be caused by a proliferation of LCs in response to the diabetic pathological condition, or an increased recall of immature LCs towards the epidermis. The data collected demonstrate an increase of CD207 positive cells in the subepidermal plexus under conditions of mechanical allodynia (Dauch et al., 2013). Pergolizzi et al. (2020a) testing the biological effects of green coffee beans in rat paw edema, demonstrated positive LCs in inflamed skin after carrageenan administration through immunohistochemical analysis with CD207 and S-100. In an ex vivo rabbit corneal keratitis model, Pergolizzi et al. (2020b) demonstrated the presence of Langerin/CD207 positive dendritic cells in basal epithelial layer and in the stroma. Lauriano et al. (2020), carried out a study on the expression of Langerin/CD207 in the respiratory system of Stenella coeruleoalba. Immunohistochemistry with CD207 has shown dendritic cells like LCs in the lung and associated lymph nodes. Dendritic cells strongly positive for CD207 have been found in the epithelium and in the connective tissue of the airways (Fig. 2 ). These results were confirmed by the presence of langerin positive cells in dolphin skin.
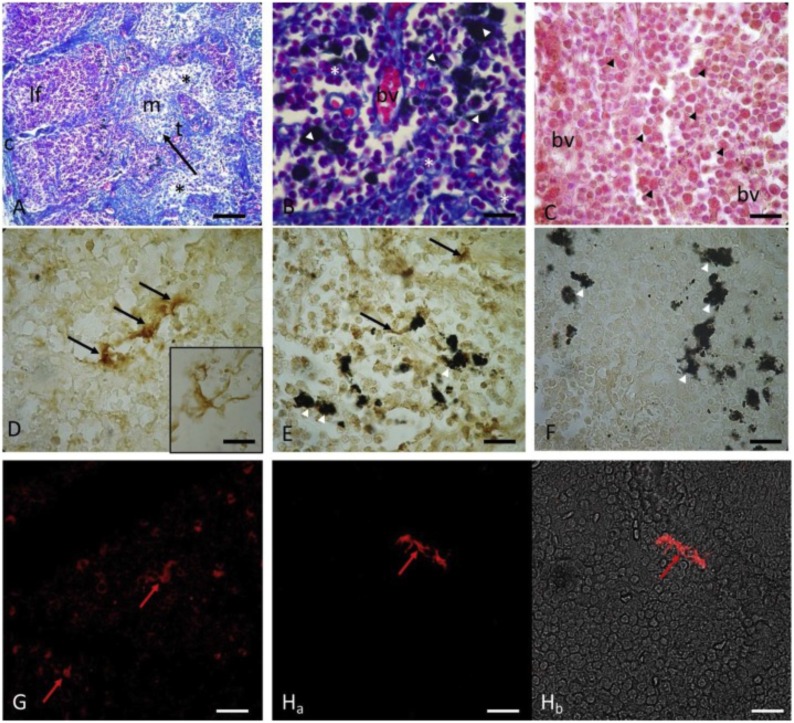
Fig. 2.
Immunoperoxidase and confocal immunofluorescence reactions with Langerin/DC 207 in dolphin (Stenella coeruleoalba) lymph nodes. The lymph node parenchyma capsule (c); the projections of the collagen septa that infiltrate the central portion of the lymph nodes dividing the parenchyma into the lymphoid follicles (If) in the cortex; the medulla (m) shows few immune cells (*) separated by sinusoids (black arrow) and trabeculae (t). Mallory's trichome stain (A). Alveolar macrophages (white arrowheads) and lymphocytic cords (white asterisks) are evident in the lymphoid follicles. Mallory's trichome stain (B). Blood vessels (bv) and granular eosinophils (black arrowheads). Hematoxylin/Eosin staining (C). Langerin/CD207 positive dendritic cells (black arrows and insert). Immunoperoxidase method (D). Langerin/CD207 positive dendritic cells (black arrow) in close contact with alveolar macrophages (white arrowhead). Immunoperoxidase method (E). Langerin/CD207 positive dendritic cells (red arrows) in the cortical region of the lymph nodes. Immunofluorescence method (G-H). Control experiments were conducted excluding the primary antibody (F). Magnifications: A 20x; B-C-d-E-F 100x, G–H 40 × . Scale bars: 3A (50 μm), 3B-C-d-E-F-G-H (20 μm). Reproduction from Lauriano et al., 2020. ELSEVIER LICENSE N° 4881300568867 (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.3. Fishes
Lovy et al. (2009) characterized LCs in the hematopoietic organs of salmonids. Specimens of Atlantic salmon (Salmo salar) and rainbow trout (Oncorhyncus mykiss) were used for the research. Spleen incubation with CD207 revealed the presence of positive langerin cells. The Atlantic salmon cephalic kidney also showed positivity to the reaction with CD207. These data suggest the possibility to characterize with Langerin/CD207, Langerhans-like cells in primitive vertebrates. Kordon et al. (2016) carried out research on LCs in Ictalurus punctatus, channel catfish. Using antibodies to CD207, LCs-like cells have been labeled in the spleen and cephalic kidney of the catfish (Fig. 3 ). Since these cells have been identified in the organs proper to the catfish's immune system, they could also have functional competences, as well as morphological similarities, with those of mammals. Electron microscopy also describes granules in the cytoplasm of these cells, like to Birbeck granules in mammalian LCs. These results clarify the similarity between teleost and mammalian Langerhans cells. CD207 has been proposed as a potential marker of DCs in the dogfish Scyliorhinus canicular (Lauriano et al., 2019). In the study, the authors highlighted the presence of DC-like cells in the gut associated lymphoid tissue (GALT) using a panel of antibodies composed by Langerin/CD207, TLR2 and S-100.
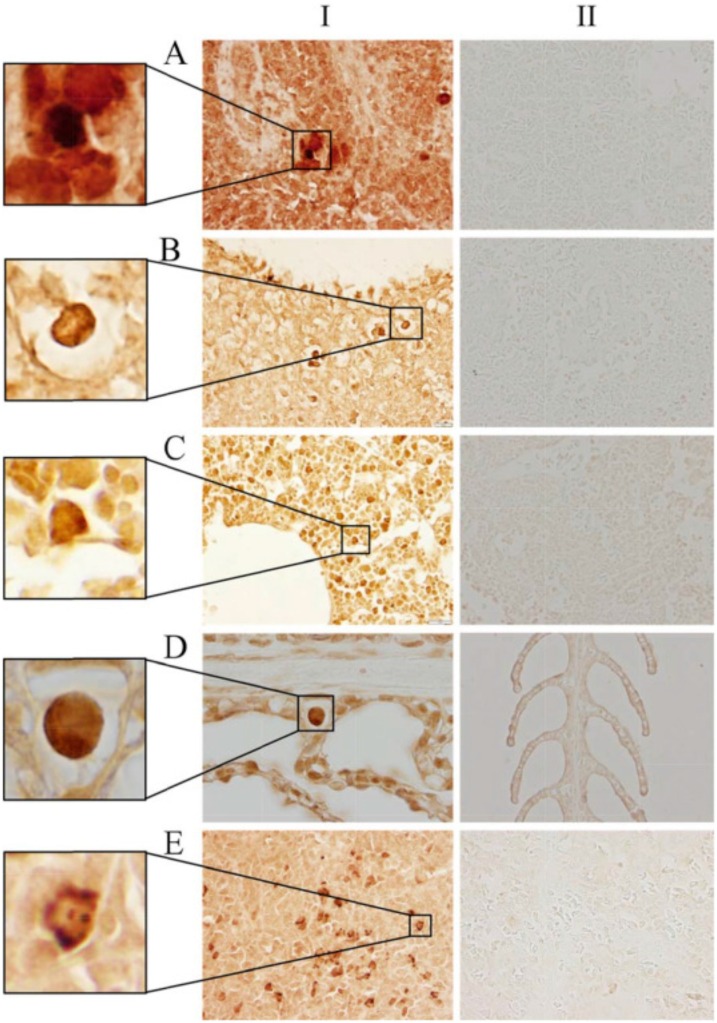
Fig. 3.
Langerin/CD207 positive cells in the spleen, anterior kidney, and gill of catfish and in Rainbow trout spleen with pAbs in different concentrations; the inserts on the left are magnified images of selected areas. Panel I: Catfish spleen, Abs dilution 1:50 (A); Catfish spleen, Abs dilution 1: 500 (B); Catfish anterior kidney, Abs dilution 1: 500 (C); Catfish gill, Abs dilution 1: 500 (D); Rainbow trout spleen (positive control), Abs dilution 1:50 (E). Panel II shows the respective negative controls (normal goat IgG). Immunoperoxidase method. Photomicrographs (600x). Reproduction from Kordon et al., 2016. ELSEVIER LICENSE N° 4881300043294.
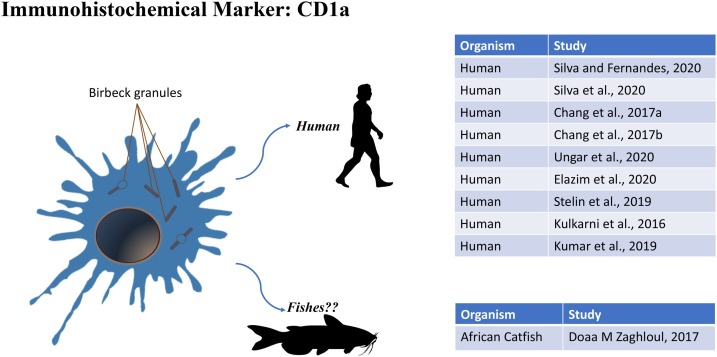
3. CD1a
CD1a (Cluster of Differentiation 1a) is a human protein related to the Major Histocompatibility Complex (MHC), which play the role of mediators in antigen presentation to T lymphocytes. Phylogenetically, CD1 genes have not been found in basal vertebrates as fish (Reinink and Van Rhijn, 2016), despite an immunohistochemical study reported Langerhans-like cells in the spleen of African catfish (Clarias gariepinus) (Zaghloul et al., 2017). Other studies revealed CD1 genes in reptiles (Yang et al., 2015), birds (Miller et al., 2005; Salomonsen et al., 2005), and marsupials (Baker and Miller, 2007; Cheng and Belov, 2014). It is probable that CD1 proteins arose in a common ancestor of placental mammals from a primordial form of CD1.
3.1. Human
CD1a is often used in diagnostics, associated with CD207, to confirm the presence of full-blown pathology, as shown by the data obtained by da Silva (2020) and Fernandes (2020). da Silva et al. (2020), studied how immature DCs, plasmacytoid DCs and LCs, were distributed in oral submucosal fibrosis (OSMF), associated squamous cell carcinoma (OSMF-OSCC), oral leukoplakia (OL) and OSCC. The study was conducted by immunohistochemistry with CD207, CD1a and CD303. The data obtained show a reduction of CD207+ and CD1a + cells in all groups, except in oral leukoplakia for CD1a in OL, claiming that such reductions could be associated with the development of these pathologies, as an indicator of malignant neoplastic transformation. Furthermore, this study showed that CD1a and CD207 are effective in identifying immature DC and Langerhans cells. CD1a is also frequently associated with S-100, as highlighted by Chang et al. (2017b)in a study carried out on LCs in odontogenic keratocysts (OKS). Anti-CD1a and anti-S100 antibodies have been used to mark LCs. The presence of LCs is closely related to the inflammatory stage with an increasing trend from mild to severe inflammatory state (Chang et al., 2017a). Ungari et al. (2020) carried out a research on LCs histiocytosis (LCH) of a mammary lymph node in an 18-year-old woman. The biopsy showed CD1a-, CD207- and S-100-positive LCs. Xu et al. (2018) carried out a study on the effect of a topical ointment with 0.03 % of tacrolimus in the treatment of UVB irradiation. The results showed a strong reversal of irradiation damage with an increase in CD1positive LCs (Xu et al., 2018). Abd Elazim et al. (2020) carried out a research on cryopeeling in the treatment of solar freckles, in comparison with peeling with trichloroacetic acid (TCA). CD1a positive LCs were found only in cryopeeling treatment. An immunohistochemical study on CD1a positive LCs and CD57 positive Natural Killer (NK) was conducted by Talwar et al.(2009) on gingivitis. The results showed an increase in Natural Killer cells against a decrease in LCs. They concluded that the reduction of LCs is probably related to the regulation of NK cells. Kulkarni et al. (2016) used CD1a to characterize immature LCs in Oral Lichen Planus. The results obtained showed a significant increase in the expression of CD1a in LCs under pathological conditions both in the epithelium and in the connective tissue. In a recent study, Kumar et al. (2019) on OLP confirm the presence of positive CD1a LCs in the suprabasal and spinous layers of OLP lesions.
3.2. Fishes
A study by Zaghloul et al. (2017), conducted on the African catfish, allowed to characterize Langerhans-Like cells in the spleen of this fish. The spleen consisted of a white pulp and a red pulp, rich in blood vessels. The immunohistochemical reaction with S-100 and CD1a showed the presence of LCs in the red pulp around the vessels. Three types of granules, similar to Birbeck granules were found in the cytoplasm of these cells. Nevertheless, as already said in the section 3., CD1a genes have never been found in fishes; this gives rise a question about the immunoreactivity of CD1a in LCs-like in the study by Zaghloul et al. (2017). Is it possible that African catfish possess CD1a in LCs-like? Future molecular and immunohistochemical combined studies are needed to answer the question.
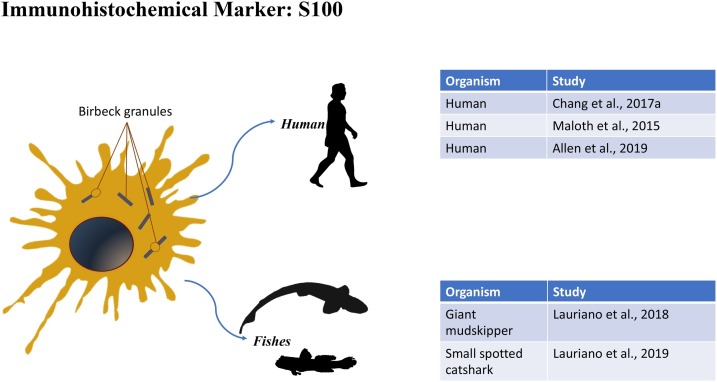
4. S-100
S-100 s are a family of heterodimeric proteins that bind calcium. To these belongs calprotectin, which binds calcium and zinc and is present in the cytoplasm of monocytes, neutrophils, and macrophages. Calprotectin also shows a bacteriostatic activity, suggesting an important role in the body's defense systems (Alesci et al., 2014). S-100 s have been characterized in many vertebrates as mammals, birds, reptiles, amphibians, and fishes and it is well conserved in the different groups. These proteins are absent in non-vertebrate organisms. It seems that S-100 s arose 460 million years ago before vertebrates move to land (Morgan et al., 2006; Ravasi et al., 2004). S-100 s differentiated then in two different lineages in fishes and tetrapod (Bobe and Goetz, 2000; Fonseca et al., 2011; Hsiao et al., 2003; Kraemer et al., 2008). Then S-100 genes remained intact during the last 165 million years. S-100 is used marker for melanocytes, Schwann cells, neuromast hair cells, nerve, and myoepithelial cells (Kahn et al., 1983; Montalbano et al., 2018), but it is often used to study Langerhans cells (Chang et al., 2017a). S-100 antibody has been used as marker for DCs in fish as described in the above section (2.3)
4.1. Humans
A study by Chang et al. (2017a), evaluated the LC count in dentigerous cysts, using S-100 as a marker. Through immunohistochemical investigation, LCs positivity was found in epithelial and in subepithelial connective tissue. This study has shown how LCs increase significantly from a mild or moderate to a severe inflammatory state. A study by Maloth et al. (2015) compared LCs data in oral Lichen planus (OLP) and oral squamous cell carcinoma (OSCC), by anti-S-100 antibodies. The study aimed to demonstrate the function of LCs in local immune response. Histomorphometric quantification was made only on S-100 positive cells, excluding those of the basal layer, identified as melanocytes. The positivity of the reaction has shown that LCs play crucial roles in various oral pathological conditions (Maloth et al., 2015). Also, in a study on the histiocytosis of LCs in an adult with diabetes insipidus conducted by Allen et al. (2019), the anti-S-100 antibody was used to highlight LCs in the tissue samples studied.
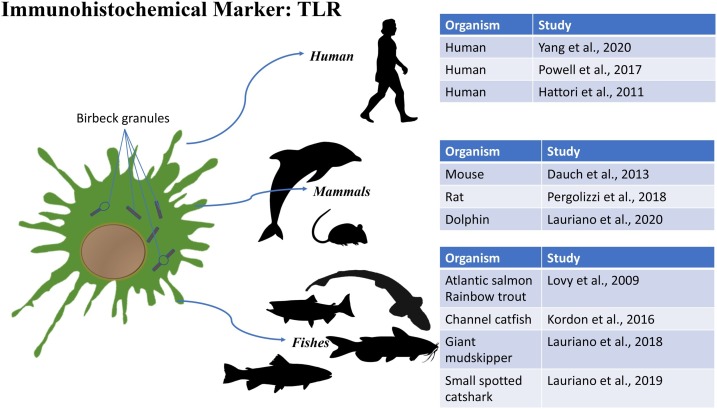
5. TLR
Inflammation triggers a cascade of signals that regulate chemical mediators and blood cells (Lawrence, 2009). During an inflammatory stage, the Pattern Recognition Receptor (PRRs) are activated (Mahla et al., 2013). They are proteins expressed by cells of the innate immune system such as Langerhans cells, macrophages, monocytes, and neutrophils (Alberts et al., 2002; Schroder and Tschopp, 2010). PRRs recognize Molecular Patterns associated with pathogens (PAMPs) (10) and associated with damage (DAMPs) (Seong and Matzinger, 2004). PRRs can be associated with the membrane (Toll-like receptor TLRs) (Janeway and Medzhitov, 2002; Takeda and Yamamoto, 2010) or immersed in the cytoplasm (Nod-like receptor NLRs and RIG-I-like receptor RLRs) (Takeuchi and Akira, 2010). TLRs are membrane receptors located on sentinel cells such as Langerhans cells and macrophages (Lauriano et al., 2016). For instance, toll-like receptor 2 (TLR2) recognize virus envelope glycoprotein B (gB1) inducing the secretion of interleukin-8 (IL8), involved in inflammatory states (Marino et al., 2019). Toll-like receptors (TLRs) are recognition molecules with key functions in the body's defense system. Since their structure is highly preserved over time, it has been hypothesized that they perform the same function in all vertebrate classes. In fact, 20 types of TLR have been isolated in many teleosts, such as rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Six types were found in all vertebrate taxa: TLR1, TLR3, TLR4, TLR5, TLR7 and TLR11 (Lauriano et al., 2016). Despite numerous types of TLRs have been characterized in the different classes of vertebrates, these receptors are structurally and genotypically high conserved (Marino et al., 2019) (Palti, 2011). For example, comparison between profiles of TLRs from two fish species (Danio rerio and Takifugu rubripes) revealed a core set of orthologous genes with high sequence conservation to human TLRs (Jault et al., 2004; Meijer et al., 2004; Oshiumi et al., 2003). In addition to these considerations, Purcell et al. (2006) demonstrated that the TLR-signaling molecules is conserved among vertebrates. The authors of this study, in order to validate their hypothesis, stimulated Oncorhynchus mykiss leukocytes with well-known mammalian TLR agonists. Stimulated rainbow trout’s leukocytes reacted showing different patterns of cytokine expression correspondent to mammalian responses. This allowed the authors to conclude that TLR-signaling genes are conserved among different vertebrate classes and they are able to recognize TLR ligands inducing a cascade of events. The above cited features of TLRs could be at the basis of the usefulness of TLR antibody to characterize LCs.
5.1. Humans
Tang et al. (2020), in a study on the verruca vulgaris lesion, highlighted LCs and plasmacytoid dendritic cells (pDCs) and their role in the cutaneous response to the virus. Verruca vulgaris is a chronic skin infection caused by the human papilloma virus (HPV). In this study CD1a, CD2AP, CD123, TLR7/9 were used. By immunohistochemistry, positive TLR9 cells, such as mononuclear cells with positive reaction cytoplasm, were detected in the dermis. In cases of skin lesion, they were present for 60 % compared to 7.7 % of cases without lesion. Furthermore, TLR9 was also present in the spinous layer keratinocytes, with a less marked reaction. The TLR7 positive cells were localized only in the dermis, without positivity in the epidermis. In contrast, pDCs showed high levels of TLR7/9 triggering the regulation of cytokines and IFN 1, determining the antiviral response.
5.2. Mammals
In a study carried out striped dolphin, Lauriano et al. (2014) marked whit TLR2 and S100 numerous Langerhans like cells in the skin. By means of anti-S100, several dendritic cells in the dolphin epidermis were labeled, which showed correspondence with LCs. These cells were multifaceted with long dendritic processes that infiltrated the keratinocytes, even reaching the stratum corneum. Dendritic cells have also been found in the dermis, but with a smaller body and less extensive processes.
5.3. Fishes
In the study on the mudskipper Periophtalmus schlosseri skin, Lauriano et al. (2018) marked dendritic cells similar to LCs, using TLR2, in addition to S-100, by immunohistochemistry and counterstain with H/E. TLR2 positive dendritic cells were similar to LCs in mammalian skin. It was noted that TLR2 was more present in cell bodies than in dendrites. TLR2 has also been reported to be an efficient marker for DCs in the GALT of the lesser spotted catshark Scyliorhinus canicula (Lauriano et al., 2019).
6. Conclusion
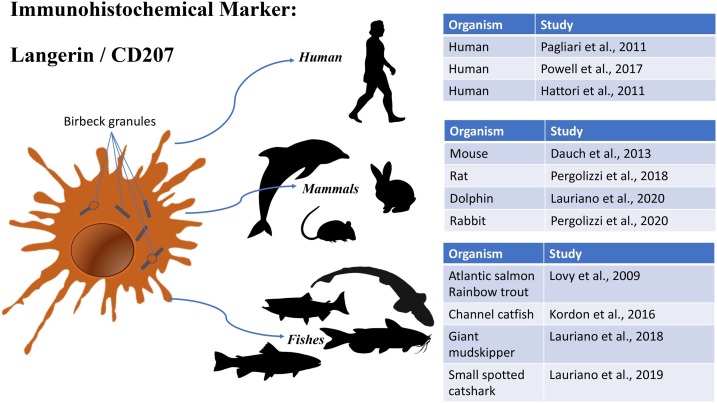
The use of specific markers for LCs is certainly useful not only to improve knowledge on phylogenesis and function of these cells in the different vertebrate classes, but also shows a diagnostic tool, allowing the identification of LCs in their different stages (immature and mature). In addition, the combined use of two or more of these markers allows a more effective characterization of the cell or an efficient diagnosis of a clinical picture. In order to summarize the markers for LCs, schematic representations are given in Fig. 4, Fig. 5, Fig. 6, Fig. 7 . Among the others Langerin/CD207 and TLR antibodies resulted to have the widest spectrum of cross reactivity in LCs of: mammals (human, mouse, rat, dolphin, rabbit) and fish (Atlantic salmon, rainbow trout, channel catfish, giant mudskipper, small spotted catshark). S-100 is an immunohistochemical marker useful for the detection of several cell types including LCs in both mammals and other vertebrate classes; despite this, S-100 marker is too generic and needs other immunoreaction experiments, in order to confirm the characterization of LCs. CD1a, on the contrary, is a good immunohistochemical marker of LCs, but is limited to mammalian species. Future studies on both immunoreactivity and gene conservation/evolution are necessary to state how LCs can be localized in all vertebrate classes using a single biological marker/target. For sure, Langerin/CD207 and TLR remain the main candidates to play this role. Furthermore, future studies on other Langerhans cells markers could be useful in the diagnosis of some pathologies. For example, IL-6 is a cytokine that serve as an important costimulatory factor of T lymphocyte activation; production of this cytokine has been suggested as a potential prognostic marker of COVID-19 disease severity (Russell et al., 2020). Langerhans Cells and lymph node dendritic cells express interleukin-6 (Cumberbatch, 1996). More studies on different molecules related with the IL-6 pathway in vertebrate models, could lead to perfection in future, diagnostic techniques applied human research.
Fig. 4.
Schematic representation of Langerin CD/207 cross reactivity on different vertebrate classes DCs.
Fig. 5.
Schematic representation of CD1a cross reactivity on vertebrate classes DCs.
Fig. 6.
Schematic representation of S100 cross reactivity on different vertebrate classes DCs.
Fig. 7.
Schematic representation of TLR cross reactivity on different vertebrate classes DCs.
CRediT authorship contribution statement
Alessio Alesci: Writing - original draft. Eugenia Rita Lauriano: Conceptualization, Writing - review & editing, Supervision. Marialuisa Aragona: Writing - review & editing, Data curation, Software. Gioele Capillo: Writing - review & editing, Software. Simona Pergolizzi: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
References
- Abd Elazim N.E., Makboul R., Botros S.N., Awad S.M. Cryopeeling versus trichloroacetic acid peeling in the treatment of solar lentigines: effect on epidermal Langerhans cells. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13288. [DOI] [PubMed] [Google Scholar]
- Alberts B., Johnson A., Lewis J., et al. Garland Science;; New York: 2002. Molecular Biology of the Cell. 4th edition. Innate Immunity. [Google Scholar]
- Alesci A., Cicero N., Salvo A., Palombieri D., Zaccone D., Dugo G., Bruno M., Vadalà R., Lauriano E.R., Pergolizzi S. Extracts deriving from olive mill waste water and their effects on the liver of the goldfish Carassius auratus fed with hypercholesterolemic diet. Nat. Prod. Res. 2014;28:1343–1349. doi: 10.1080/14786419.2014.903479. [DOI] [PubMed] [Google Scholar]
- Allen A., Matrova E., Ozgen B., Redleaf M., Emmadi R., Saran N. Langerhans’ cell histiocytosis of the temporal bone in an adult with central diabetes insipidus. Radiol. Case Reports. 2019;14:847–850. doi: 10.1016/j.radcr.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. Antibody anarchy: a call to order. Nature. 2015;527:545–551. doi: 10.1038/527545a. [DOI] [PubMed] [Google Scholar]
- Baker M. Reproducibility crisis: blame it on the antibodies. Nature. 2015;521:274–276. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- Baker M.L., Miller R.D. Evolution of mammalian CD1: marsupial CD1 is not orthologous to the eutherian isoforms and is a pseudogene in the opossum Monodelphis domestica. Immunology. 2007;121:113–121. doi: 10.1111/j.1365-2567.2007.02545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbeck M.S., Breathnach A.S., Everall J.D. An Electron microscope study of basal melanocytes and high-level clear cells (Langerhans cells) in vitiligo**From the chester beatty research institute, royal Cancer hospital, London, S.W. 3, and the departments of anatomy, and dermatology, St. mary’s hos. J. Invest. Dermatol. 1961;37:51–64. doi: 10.1038/jid.1961.80. [DOI] [Google Scholar]
- Bobe J., Goetz F.W. A S100 homologue mRNA isolated by differential display PCR is down-regulated in the brook trout (Salvelinus fontinalis) post-ovulatory ovary. Gene. 2000;257:187–194. doi: 10.1016/S0378-1119(00)00406-6. [DOI] [PubMed] [Google Scholar]
- Bradbury A., Plückthun A. Reproducibility: standardize antibodies used in research. Nature. 2015;518:27–29. doi: 10.1038/518027a. [DOI] [PubMed] [Google Scholar]
- Chang C.H., Wu Y.C., Wu Y.H., Sun A., Chen H.M., Lin H.P. Langerhans cells in 60 odontogenic keratocysts. J. Dent. Sci. 2017;12:283–290. doi: 10.1016/j.jds.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.H., Wu Y.C., Wu Y.H., Sun A., Kuo Y.S., Chiang C.P. S100 protein-positive Langerhans cells in 80 dentigerous cysts. J. Dent. Sci. 2017;12:405–412. doi: 10.1016/j.jds.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Belov K. Characterisation of non-classical MHC class I genes in the Tasmanian devil (Sarcophilus harrisii) Immunogenetics. 2014;66:727–735. doi: 10.1007/s00251-014-0804-3. [DOI] [PubMed] [Google Scholar]
- Chertov O., Yang D., Zack Howard O.M., Oppenheim J.J. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol. Rev. 2000;177:68–78. doi: 10.1034/j.1600-065X.2000.17702.x. [DOI] [PubMed] [Google Scholar]
- Couchman J.R. commercial antibodies: the good, bad, and really ugly. J. Histochem. Cytochem. 2009;57(1):7–8. doi: 10.1369/jhc.2008.952820. https://doi.org/10.1369%2Fjhc.2008.952820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M. Constitutive and inducible expression of interleukin-6 by Langerhans cells and lymph node dendritic cells. Immunology. 1996;87:513–518. doi: 10.1046/j.1365-2567.1996.504577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva L.C., Fonseca F.P., de Almeida O.P., de Almeida Mariz B.A.L., Lopes M.A., Radhakrishnan R., Sharma M., Kowalski L.P., Vargas P.A. CD1A+ and CD207+ cells are reduced in oral submucous fibrosis and orasquamous cell carcinoma. Med. Oral Patol. Oral y Cir. Bucal. 2020;25:e49–e55. doi: 10.4317/medoral.23177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauch J.R., Bender D.E., Luna-Wong L.A., Hsieh W., Yanik B.M., Kelly Z.A., Cheng H.T. Neurogenic factor-induced Langerhans cell activation in diabetic mice with mechanical allodynia. J. Neuroinflammation. 2013;10:64. doi: 10.1186/1742-2094-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edfors F., Hober A., Linderbäck K., Maddalo G., Azimi A., Sivertsson Å., Tegel H., Hober S., Szigyarto C.A.K., Fagerberg L., von Feilitzen K., Oksvold P., Lindskog C., Forsström B., Uhlen M. Enhanced validation of antibodies for research applications. Nat. Commun. 2018;9:4130. doi: 10.1038/s41467-018-06642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes D.T., van Heerden W.F.P., Ribeiro A.C.P., Brandão T.B., De Mello E.S., Rivera C., van Heerden M.B., Gondak R., Santos-Silva A.R., Vargas P.A., Lopes M.A. Different methods of cell quantification can lead to different results: a comparison of digital methods using a pilot study of dendritic cells in HIV-positive patients. Med. Oral Patol. Oral y Cir. Bucal. 2020;25:e431–e438. doi: 10.4317/medoral.23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca V.G., Rosa J., Laizé V., Gavaia P.J., Cancela M.L. Identification of a new cartilage-specific S100-like protein up-regulated during endo/perichondral mineralization in gilthead seabream. Gene Expr. Patterns. 2011;11:448–455. doi: 10.1016/j.gep.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Fuglem B., Jirillo E., Bjerkås I., Kiyono H., Nochi T., Yuki Y., Raida M., Fischer U., Koppang E.O. Antigen-sampling cells in the salmonid intestinal epithelium. Dev. Comp. Immunol. 2010;34:768–774. doi: 10.1016/j.dci.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Gallucci S., Matzinger P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Groves R.W., Allen M.H., Ross E.L., Barker J.N.W.N., MacDonald D.M. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br. J. Dermatol. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Hattori T., Chauhan S.K., Lee H., Ueno H., Dana R., Kaplan D.H., Saban D.R. Characterization of langerin-expressing dendritic cell subsets in the normal cornea. Investig. Ophthalmol. Vis. Sci. 2011;52:4598–4604. doi: 10.1167/iovs.10-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugarvoll E., Thorsen J., Laane M., Huang Q., Koppang E.O. Melanogenesis and evidence for melanosome transport to the plasma membrane in a CD83+ teleost leukocyte cell line. Pigment Cell Res. 2006 doi: 10.1111/j.1600-0749.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- He Y., Bao B., Li H. Using zebrafish as a model to study the role of epigenetics in hearing loss. Expert Opin. Drug Discov. 2017;12:967–975. doi: 10.1080/17460441.2017.1340270. [DOI] [PubMed] [Google Scholar]
- Hewitt S.M., Baskin D.G., Frevert C.W., Stahl W.L., Rosa-Molinar E. Controls for Immunohistochemistry: The Histochemical Society’s Standards of Practice for Validation of Immunohistochemical Assays. J. Histochem. Cytochem. 2014;62(10):693–697. doi: 10.1369/0022155414545224. https://doi.org/10.1369%2F0022155414545224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C., Der Ekker M., Tsai H.J. Skin-specific expression of ictacalcin, a homolog of the S100 genes, during zebrafish embryogenesis. Dev. Dyn. 2003;228:745–750. doi: 10.1002/dvdy.10411. [DOI] [PubMed] [Google Scholar]
- Hu Yhua, Zhang M., Sun L. Expression of Scophthalmus maximus CD83 correlates with bacterial infection and antigen stimulation. Fish Shellfish Immunol. 2010;29:608–614. doi: 10.1016/j.fsi.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jault C., Pichon L., Chluba J. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol. Immunol. 2004;40:759–771. doi: 10.1016/j.molimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kahn H.J., Marks A., Thom H., Baumal R. Role of antibody to S100 protein in diagnostic pathology. Am. J. Clin. Pathol. 1983;79:341–347. doi: 10.1093/ajcp/79.3.341. [DOI] [PubMed] [Google Scholar]
- Klechevsky E., Morita R., Liu M., Cao Y., Coquery S., Thompson-Snipes L.A., Briere F., Chaussabel D., Zurawski G., Palucka A.K., Reiter Y., Banchereau J., Ueno H. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppang E.O., Haugarvoll E., Hordvik I., Poppe T.T., Bjerkås I. Granulomatous uveitis associated with vaccination in the Atlantic salmon. Vet. Pathol. 2004;44:122–130. doi: 10.1354/vp.41-2-122. [DOI] [PubMed] [Google Scholar]
- Kordon A.O., Scott M.A., Ibrahim I., Abdelhamed H., Ahmed H., Baumgartner W., Karsi A., Pinchuk L.M. Identification of Langerhans-like cells in the immunocompetent tissues of channel catfish, Ictalurus punctatus. Fish Shellfish Immunol. 2016;58:253–258. doi: 10.1016/j.fsi.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Kraemer A.M., Saraiva L.R., Korsching S.I. Structural and functional diversification in the teleost S100 family of calcium-binding proteins. BMC Evol. Biol. 2008;8:48. doi: 10.1186/1471-2148-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni G., Sakki E.P., Kumar Y.V., Kolimi S., Perika R., Karthik K.V., Kumar K.M., Kalyan V.S. Expression of CD1a by Langerhan’s cells in oral lichen planus - A retrospective analysis. J. Clin. Diagn. Res. 2016;10 doi: 10.7860/JCDR/2016/19189.7966. ZC28–ZC3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar T.A., Veeravarmal V., Nirmal R.M., Amsaveni R., Nassar M.H.M., Kesavan G. Expression of cluster of differentiation 1a-positive langerhans cells in oral lichen planus. Indian J. Dermatol. 2019;64:41–46. doi: 10.4103/ijd.IJD_350_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriano E.R., Silvestri G., Kuciel M., Zuwała K., Zaccone D., Palombieri D., Alesci A., Pergolizzi S. Immunohistochemical localization of Toll-like receptor 2 in skin Langerhans’ cells of striped dolphin (Stenella coeruleoalba) Tissue Cell. 2014;46:113–121. doi: 10.1016/j.tice.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Lauriano E.R., Pergolizzi S., Capillo G., Kuciel M., Alesci A., Faggio C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016;59:250–255. doi: 10.1016/j.fsi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Lauriano E.R., Faggio C., Capillo G., Spanò N., Kuciel M., Aragona M., Pergolizzi S. Immunohistochemical characterization of epidermal dendritic-like cells in giant mudskipper, Periophthalmodon schlosseri. Fish Shellfish Immunol. 2018;74:380–385. doi: 10.1016/j.fsi.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Lauriano E.R., Pergolizzi S., Aragona M., Montalbano G., Guerrera M.C., Crupi R., Faggio C., Capillo G. Intestinal immunity of dogfish Scyliorhinus canicula spiral valve: a histochemical, immunohistochemical and confocal study. Fish Shellfish Immunol. 2019;87:490–498. doi: 10.1016/j.fsi.2019.01.049. [DOI] [PubMed] [Google Scholar]
- Lauriano E.R., Pergolizzi S., Lo Cascio P., Kuciel M., Zizzo N., Guerrera M.C., Aragona M., Capillo G. Expression of Langerin/CD207 in airways, lung and associated lymph nodes of a stranded striped dolphin (Stenella coeruleoalba) Acta Histochem. 2020;122 doi: 10.1016/j.acthis.2019.151471. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhou Q., Zhao C., Lin G., Xu J., Wen Z. An ectoderm-derived myeloid-like cell population functions as antigen transporters for langerhans cells in zebrafish epidermis. Dev. Cell. 2019;49 doi: 10.1016/j.devcel.2019.03.028. 605.e5-617.e5. [DOI] [PubMed] [Google Scholar]
- Lovy J., Wright G.M., Speare D.J. Morphological presentation of a dendritic-like cell within the gills of chinook salmon infected with Loma salmonae. Dev. Comp. Immunol. 2006;30:259–263. doi: 10.1016/j.dci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Lovy J., Savidant G.P., Speare D.J., Wright G.M. Langerin/CD207 positive dendritic-like cells in the haemopoietic tissues of salmonids. Fish Shellfish Immunol. 2009;27:365–368. doi: 10.1016/j.fsi.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Loynes C.A., Lee J.A., Robertson A.L., Steel M.J.G., Ellett F., Feng Y., Levy B.D., Whyte M.K.B., Renshaw S.A. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018;4:eaar8320. doi: 10.1126/sciadv.aar8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Villarino G., Balla K.M., Stachura D.L., Bañuelos K., Werneck M.B.F., Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahla R.S., Reddy M.C., Vijaya Raghava Prasad D., Kumar H. Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front. Immunol. 2013;4:248. doi: 10.3389/fimmu.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloth U.K., Dorankula Y.P.R., Pasupula A.P., Thokala M.R., Muddana K., Ramavath R. A comparative immunohistochemical analysis of langerhans cells in oral mucosa, oral lichen planus and oral squamous cell carcinoma. J. Clin. Diagn. Res. 2015;9 doi: 10.7860/JCDR/2015/14170.6235. ZC76–ZC7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino A., Pergolizzi S., Cimino F., Lauriano E.R., Speciale A., D’Angelo V., Sicurella M., Argnani R., Manservigi R., Marconi P. Role of herpes simplex envelope glycoprotein B and toll-like receptor 2 in ocular inflammation: an ex vivo organotypic rabbit corneal model. Viruses. 2019;11:819. doi: 10.3390/v11090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer W.J., Irschick U.M., Moser P., Wurm M., Huemer H.P., Romani N., Irschick E.U. Characterization of antigen-presenting cells in fresh and cultured human corneas using novel dendritic cell markers. Investig. Ophthalmol. Vis. Sci. 2007;48:4459–4467. doi: 10.1167/iovs.06-1184. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Meijer A.H., Gabby Krens S.F., Medina Rodriguez I.A., He S., Bitter W., Snaar-Jagalska B.E., Spaink H.P. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol. Immunol. 2004;40:773–783. doi: 10.1016/j.molimm.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Miller M.M., Wang C., Parisini E., Coletta R.D., Goto R.M., Lee S.Y., Barral D.C., Townes M., Roura-Mir C., Ford H.L., Brenner M.B., Dascher C.C. Characterization of two avian MHC-like genes reveals an ancient origin of the CD1 family. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8674–8679. doi: 10.1073/pnas.0500105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbano G., Capillo G., Laurà R., Abbate F., Levanti M., Guerrera M.C., Ciriaco E., Germanà A. Neuromast hair cells retain the capacity of regeneration during heavy metal exposure. Ann. Anat. 2018;218:183–189. doi: 10.1016/j.aanat.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Morgan R.O., Martin-Almedina S., Garcia M., Jhoncon-Kooyip J., Fernandez M.P. Deciphering function and mechanism of calcium-binding proteins from their evolutionary imprints. Biochim. Biophys. Acta - Mol. Cell Res. 2006 doi: 10.1016/j.bbamcr.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Morrison R.N., Koppang E.O., Hordvik I., Nowak B.F. MHC class II+ cells in the gills of Atlantic salmon (Salmo salar L.) affected by amoebic gill disease. Vet. Immunol. Immunopathol. 2006;109:297–303. doi: 10.1016/j.vetimm.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Nishibu A., Ward B.R., Jester J.V., Ploegh H.L., Boes M., Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Landis E., Boulay T., Phillips R.B., Collet B., Secombes C.J., Flajnik M.F., Hansen J.D. Homologs of CD83 from Elasmobranch and teleost fish. J. Immunol. 2004;173:4553–4560. doi: 10.4049/jimmunol.173.7.4553. [DOI] [PubMed] [Google Scholar]
- Olsen M.M., Kania P.W., Heinecke R.D., Skjoedt K., Rasmussen K.J., Buchmann K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011;30:859–869. doi: 10.1016/j.fsi.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Oshiumi H., Tsujita T., Shida K., Matsumoto M., Ikeo K., Seya T. Prediction of the prototype of the human Toll-like receptor gene family from the pufferfish, Fugu rubripes, genome. Immunogenetics. 2003;54:791–800. doi: 10.1007/s00251-002-0519-8. [DOI] [PubMed] [Google Scholar]
- Pagliari C., Fernandes E.R., Ferreira da Silva W.L., Alves de Lima Silva A., Stegun F.W., Seixas Duarte M.I. Revisiting Langerhans cells in paracoccidioidomycosis: expression of CD207/langerin in human cutaneous and mucosal lesions. Microbes Infect. 2011;13:1012–1017. doi: 10.1016/j.micinf.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Palti Y. Toll-like receptors in bony fish: from genomics to function. Dev. Comp. Immunol. 2011;35:1263–1272. doi: 10.1016/j.dci.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Pergolizzi S., D’Angelo V., Aragona M., Dugo P., Cacciola F., Capillo G., Dugo G., Lauriano E.R. Evaluation of antioxidant and anti-inflammatory activity of green coffee beans methanolic extract in rat skin. Nat. Prod. Res. 2020;34:1535–1541. doi: 10.1080/14786419.2018.1523161. [DOI] [PubMed] [Google Scholar]
- Pergolizzi S., Marino A., Capillo G., Aragona M., Marconi P., Lauriano E.R. Expression of Langerin/CD 207 and α-smooth muscle actin in ex vivo rabbit corneal keratitis model. Tissue Cell. 2020;66 doi: 10.1016/j.tice.2020.101384. [DOI] [PubMed] [Google Scholar]
- Polak M.E., Newell L., Taraban V.Y., Pickard C., Healy E., Friedmann P.S., Al-Shamkhani A., Ardern-Jones M.R. CD70-CD27 interaction augments CD8 T-cell activation by human epidermal langerhans cells. J. Invest. Dermatol. 2012;132:1636–1644. doi: 10.1038/jid.2012.26. [DOI] [PubMed] [Google Scholar]
- Powell P., Vitug G., Castro-Silva F., Ray A. A rare case of CD1a-negative Langerhans cell histiocytosis of the central nervous system in a child. Clin. Case Reports. 2017;5:1664–1667. doi: 10.1002/ccr3.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell M.K., Smith K.D., Aderem A., Hood L., Winton J.R., Roach J.C. Conservation of Toll-like receptor signaling pathways in teleost fish. Comp. Biochem. Physiol. - Part D Genomics Proteomics. 2006;1:77–88. doi: 10.1016/j.cbd.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasi T., Hsu K., Goyette J., Schroder K., Yang Z., Rahimi F., Miranda L.P., Alewood P.F., Hume D.A., Geczy C. Probing the S100 protein family through genomic and functional analysis. Genomics. 2004;84:10–22. doi: 10.1016/j.ygeno.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Reinink P., Van Rhijn I. Mammalian CD1 and MR1 genes. Immunogenetics. 2016;68:515–523. doi: 10.1007/s00251-016-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Brunner P.M., Stingl G. Changing views of the role of langerhans cells. J. Invest. Dermatol. 2012;132:872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19 - A systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsen J., Sørensen M.R., Marston D.A., Rogers S.L., Collen T., Van Hateren A., Smith A.L., Beal R.K., Skjødt K., Kaufman J. Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8668–8673. doi: 10.1073/pnas.0409213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Tschopp J. The inflammsomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Seong S.Y., Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Spits H., Couwenberg F., Bakker A.Q., Weijer K., Uittenbogaart C.H. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC)2 but not into pre-DC1: evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Yamamoto M. Current views of toll-like receptor signaling pathways. Gastroenterol. Res. Pract. 2010 doi: 10.1155/2010/240365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Talwar A., Arun K., Kumar T., Stelin S., Ramakrishan H. Immunohistological analysis of CD1a + langerhans cells and CD57 + natural killer cells in healthy and diseased human gingival tissue: a comparative study. J. Indian Soc. Periodontol. 2009;13:150–154. doi: 10.4103/0972-124x.60228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Zhu X., Han R., Zhou Q., Cheng H., Garcovich S. Expressionof langerhans cell and plasmacytoid dendritic cell markers, and toll-like receptor 7/9 signaling pathway proteins in verruca vulgaris lesions. Med. (United States) 2020;99 doi: 10.1097/MD.0000000000019214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M., Bandrowski A., Carr S., Edwards A., Ellenberg J., Lundberg E., Rimm D.L., Rodriguez H., Hiltke T., Snyder M., Yamamoto T. A proposal for validation of antibodies. Nat. Methods. 2016;13:823–827. doi: 10.1038/nmeth.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungari M., Ferrero G., Varotti E., Gusolfino M.D., Manotti L., Tanzi G., Trombatore M., Bertoni R. Langerhans cell histiocytosis of an intra-mammary lymph node in an 18-year-old woman. Pathologica. 2020;112:50–55. doi: 10.32074/1591-951X-27-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J., Ravel O., Dezutter-Dambuyant C., Moore K., Kleijmeer M., Liu Y., Duvert-Frances V., Vincent C., Schmitt D., Davoust J., Caux C., Lebecque S., Saeland S. Langerin, a novel C-type lectin specific to langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/S1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Valladeau J., Clair-Moninot V., Dezutter-Dambuyant C., Pin J.-J., Kissenpfennig A., Mattéi M.-G., Ait-Yahia S., Bates E.E.M., Malissen B., Koch F., Fossiez F., Romani N., Lebecque S., Saeland S. Identification of mouse Langerin/CD207 in langerhans cells and some dendritic cells of lymphoid tissues. J. Immunol. 2002;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- Vermaelen K., Pauwels R. Pulmonary dendritic cells. Am. J. Respir. Crit. Care Med. 2005;61A:170–177. doi: 10.1164/rccm.200410-1384SO. [DOI] [PubMed] [Google Scholar]
- Wolff K. The fine structure of the Langerhans cell granule. J. Cell Biol. 1967;35:468–473. doi: 10.1083/jcb.35.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.L., Feng Y.D., Song G.X., Gong Q.X., Yin L., Hu Y.Y., Luo D., Yin Z.Q. Tacrolimus reverses UVB irradiation-induced epidermal langerhans cell reduction by inhibiting TNF-α secretion in keratinocytes via regulation of NF-κB/p65. Front. Pharmacol. 2018;9:67. doi: 10.3389/fphar.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang C., Wang T., Bai J., Zhao Yu, Liu X., Ma Q., Wu X., Guo Y., Zhao Yaofeng, Ren L. Analysis of the reptile CD1 genes: evolutionary implications. Immunogenetics. 2015;67:337–346. doi: 10.1007/s00251-015-0837-2. [DOI] [PubMed] [Google Scholar]
- Zaghloul D., Derbalah A., Rutland C. Unique characterization of Langerhans cells in the spleen of the African catfish (Clarias gariepinus) Matters Sel. 2017 doi: 10.19185/matters.201703000005. [DOI] [Google Scholar]