Abstract
This study aims to assess the concentrations of size-fractioned particle mass (PM1.0, PM2.5, PM4.0, PM10) and number (PNC0.3, PNC0.5, PNC1.0, PNC2.5), bacteria, and fungi in a Materials Recycling Facility (MRF) in Brazil. The measurements were performed inside the waste processing shed (P1) and in the outdoor environment (P2) during working days in winter and spring of 2017, and summer of 2019. A total of 2,400 min of PM, 1,440 min of PNC, and 216 samples of bioaerosols were collected in the morning and afternoon. P1 has the strongest air contamination with mean values of 475.5 ± 563.7 µg m−3 for PM10, 58.6 ± 36.0 cm−3 for PNC0.3, 1,088.8 ± 825.2 colony-forming units per cubic meter (CFU m−3) for bacteria, and 2,738.3 ± 1,381.3 CFU m−3 for fungi. The indoor/outdoor ratios indicated the large influence of indoor sources due to the activities performed inside P1 that promote the generation and resuspension of pollutants. Gram-positive bacteria dominated with 58.6% of indoor samples. Overall, our results show a critical indoor air quality situation in a Brazilian MRF, which may cause several health risks for waste pickers. Finally, we call attention to the lack of occupational exposure limits for bioaerosols in industrial workplaces and mainly in MRFs.
Subject terms: Air microbiology, Atmospheric science
Introduction
Urbanization, population growth, the development of large conurbations, and the modification in society's lifestyle have led to an increase in the generation rates of solid waste, which has been causing economic, environmental, and social problems worldwide1–4. Hence, its management turned into a very important activity for municipalities5,6 mainly due to environmental and public health reasons, as solid waste can pose risks to human health and contaminate the atmosphere, soil, and water resources1,3,7.
Developing countries have the worst municipal solid waste (MSW) management and are more severely impacted by improperly managed waste8. In Brazil, for example, around 79 million tonnes of solid waste are generated per year or 1.04 kg/capita/day, while the global average is 0.74 kg/capita/day8,9. Of this amount, 30–40% could be recycled and reused (excluding composting), however, only 13% is sent to recycling10.
The recycling segment is an important stage of solid waste management and it has advantages due to aggregation of economic value, contribution to the reduction of disposed of or incinerated waste, and the reuse of materials in industries5,11. Recycling generally occurs through an informal sector with several people in low- and middle-income countries working as waste pickers because of economic reasons3,5,12–14. In Brazil, around 800,000 people work as waste pickers in at least 802 recycling cooperatives according to data from the National Movement of Collectors of Recyclable Materials (MNCR)15 and the Business Commitment to Recycling (CEMPRE)16. In a Materials Recycling Facility (MRF) in Londrina, located in the southern region of Brazil, the monthly income of each waste picker is approximately US$ 23517, but this value generally varies according to the amount of waste processed and from one place to another.
Waste management includes activities in which exposure to hazardous chemical agents and airborne pollutants, such as particulate matter (PM) and bioaerosols, may be abundant and may trigger adverse effects on human health18,19. Particles of the respirable-size fraction (< 10 µm) are of major concern as they are small enough to penetrate the respiratory system20–22. PM exposure has been related to adverse health effects, such as respiratory symptoms, aggravated asthma, decreased lung function, increased hospital admissions, shortened life expectancy, and mortality22–25. It also can be potentially involved in cases of Alzheimer’s and Parkinson’s diseases26. Furthermore, exposure to bioaerosols may also cause cardiovascular and respiratory diseases, including asthma, pneumonia, bronchitis, and tuberculosis, in addition to allergic reactions (such as conjunctivitis and sinusitis) and organic dust toxic syndrome27–30.
The quality of the recyclable materials is reduced when the population do not properly separate the MSW in their households and, consequently, the air at MRFs may be contaminated during the processes performed (mainly during sorting and baling)19,31,32. As MSW can be easily colonized by bacteria and fungi, these microorganisms might aerosolize upon handling, i.e., turn into bioaerosols, and workers may be exposed to numerous biological agents, which pose health hazards29,33,34. It is reported that people who work handling recyclable waste may present an elevated risk of irritation of the skin, eyes, and throat, and gastrointestinal, respiratory, and musculoskeletal problems compared to those of other jobs28,32,35.
Although it is already known that the exposure to several biological agents is high and that there is a great risk of occupational health problems for workers of this sector29,36, the assessment of bioaerosols, particle mass (PM), and particle number concentrations (PNC) suspended in the air of materials recycling facilities is scarce. Most studies have been focusing on the people who collect the waste33,37–40 and on composting facilities and landfill sites27,34,35,41–44.
The PM and bioaerosols emissions at MRFs may vary due to design, operational conditions, types of materials processed, and meteorological variables45–47, singular for each facility studied. Thus, it is important to evaluate the presence of these airborne pollutants in this occupational setting to provide possibilities of controlling its air pollution through specific intervention plans based on demand. Furthermore, we used evaluation methodologies that can be easily applied to identify insalubrious environments and to provide the implementation of corrective actions for reducing the exposure and harmful effects on workers’ health in the MSW management sector, especially in low- and middle-income countries. In this direction, this study aims to assess the PM, PNC, bacteria, and fungi concentrations in a Brazilian recycling cooperative, which is a typical environment of the MSW management in the country.
Results and discussion
Particle concentrations analysis
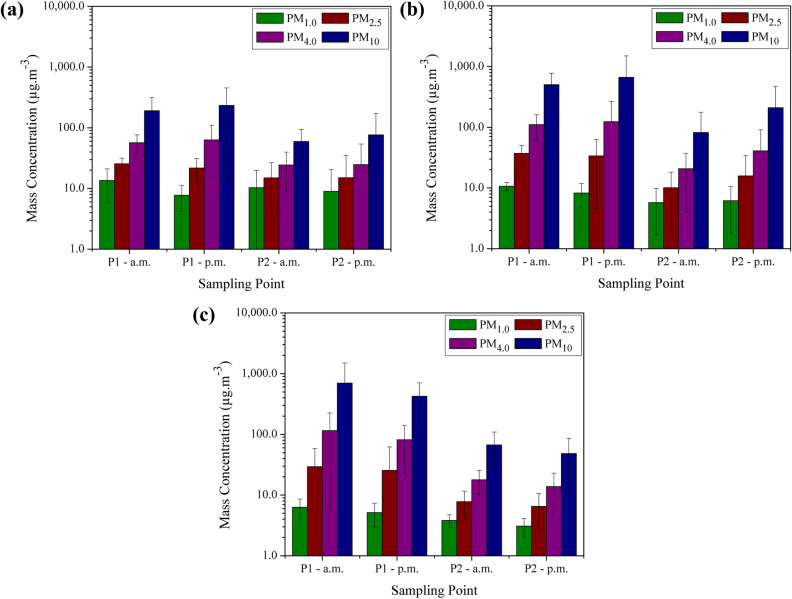
Mean PM results in winter, spring, and summer during the morning and afternoon at each sampling point are presented in Fig. 1. The highest values of mass concentration, considering all four particle sizes sampled, were observed in P1 in the afternoon for winter and spring, and in P1 in the morning for summer (Fig. 1a–c). P1 represents the waste processing shed, a site without windows and mechanical ventilation, in which occurs the storage, sorting, and processing of the recyclable materials. The waste is separated into big polypropylene bags that are then moved through the floor (promoting the resuspension of PM) and sent to processing, which consists of baling the waste that is later stored until commercialization. Depending on the amount of sorted waste, the transfer of big bags with a forklift to the outdoor environment also occurred and they were stored until processing.
Figure 1.
Mean mass concentrations (µg m−3) for all particle sizes measured during the morning and afternoon in winter (a), spring (b), and summer (c) in the waste processing shed (P1) and in the outdoor environment (P2).
Moreover, it was observed the presence of trucks during the samplings (emission sources of PM) to unload the materials collected in the city. It is important to point out that variations in PM concentrations may be expected throughout the day (morning vs. afternoon), depending mainly on the waste characteristics and the tasks being performed. As expected, the presence of indoor sources of PM, such as resuspension, fuel combustion, storage, and sorting of the recyclable waste, in addition to scarce particle dispersion resulted in indoor concentrations generally higher than the outdoor concentrations. More detailed information regarding the activities observed during the sampling periods is presented in the Supplementary Table S1.
Higher concentrations of the largest particle diameters were observed (Fig. 1a–c), as expected and also reported by other authors48,49. This characteristic is particularly predominant for PM4.0 and PM10, with the latter ranging (in mean values) from 59.5 to 233.9 µg m−3 in winter, from 82.2 to 663.9 µg m−3 in spring, and from 48.4 to 699.3 µg m−3 in summer. Mean PM2.5 concentrations ranged from 15.0 to 25.5 µg m−3, from 10.1 to 37.2 µg m−3, and from 6.5 to 29.3 µg m−3 in winter, spring, and summer, respectively. Beal et al.50 found mean PM2.5 values of 4.4 µg m−3 during summer and 10.3 µg m−3 in winter for the city of Londrina.
Overall, comparing the seasons studied, the mean concentrations were mostly higher in winter for PM1.0 and in spring for PM2.5, PM4.0, and PM10, which was the season with the lowest contribution to the removal of PM through wet deposition. It had 39.2 mm of rain during the sampling week, compared to 58.4 mm in winter, and 68.2 mm in summer, according to the Agronomic Institute of Parana (IAPAR)51. Light breezes were observed in all seasons analysed and did not considerably influence the concentrations outdoors (P2). These meteorological data are presented in the Supplementary Table S2. Also, it was noted a larger amount of waste to be processed during the winter samplings, followed by summer and spring. The main operational conditions and activities were observed during the three seasons studied regarding unloading, sorting, and processing (Supplementary Table S1), but the shed was organized differently with more workstations for the separation of waste into the big bags in winter and spring, which may have contributed to the obtained concentrations of pollutants.
The results for PM presented non-normal distribution and we verified that the mean concentrations in winter, spring, and summer are statistically different (p = 0.05) applying the Kruskal–Wallis test. This behaviour reflects the large variability in indoor sources and activities, as well as the influence of outdoor atmospheric conditions on the indoor environment.
In Brazil, there are regulatory standards for occupational safety and health according to the Ministry of Labour’s Consolidation of Labour Laws (CLT), as amended by the Law no. 6,514/1977, which establishes insalubrious activities or operations based on tolerance limits for exposure to heat, cold, noise, humidity, non-ionizing radiation, chemical and biological agents, among others52. However, there are no quantitative guidelines for particulate matter (only for mineral dust, such as asbestos, manganese and its compounds, and crystalline silica). Therefore, we used the maximum recommended value (MRV) of 80 µg m−3 for total aerosols in indoor air established by the Brazilian Health Regulatory Agency’s Resolution no. 09/200353. Concentrations obtained in this study were up to four, ten, and eleven times higher than the MRV in winter, spring, and summer, respectively, considering all four particle sizes sampled indoors. According to the World Health Organization (WHO)54, particulate matter from both indoor and outdoor sources is harmful to human health. Hence, the air quality guidelines recommended for PM (24-h mean: 50 µg m−3 for PM10 and 25 µg m−3 for PM2.5) can also be applied to indoor environments, which presented values several times higher than the standard for PM10 even taking into account that these guidelines are mean values for 24 h and the concentrations measured are for working hours.
The Brazilian regulation of 80 µg m−3 is not intended for indoor air at industrial environments or MSW management sites and occupational exposure limits for inhalable and total dust in the USA and Norway, for example, have been established as particulates not otherwise regulated, with guidelines of 10 mg m−355. Hazard identification, risk assessment and control are used to define and describe hazards in the workplace and to implement control measures, such as the use of personal protective equipment (PPE) at different levels of protection considering, for example, the concentrations of indoor air contaminants in industrial occupational locations56,57. The values measured in the MRF indicate that workers must use masks to attenuate their exposure to airborne particles but the use of inappropriate PPE, as well as its incorrect use, was a common reality observed on-site during our measurements, besides administrative workers (office) who do not use PPE.
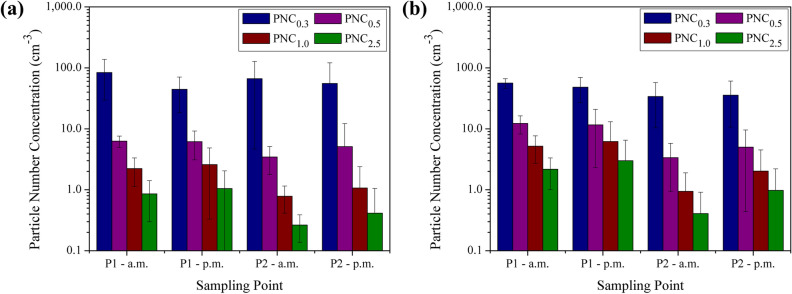
For the particle number concentrations (Fig. 2a,b), it was observed higher concentrations of the smallest sizes of particles measured, which is consistent and reported by other authors34,58–60. This tendency is prevalent for PNC0.3 that ranged from 44.5 to 83.7 cm−3 in winter and from 33.9 to 56.3 cm−3 in spring, in mean values.
Figure 2.
Mean number concentrations (cm−3) for all particle sizes measured during the morning and afternoon in winter (a) and spring (b) in the waste processing shed (P1) and in the outdoor environment (P2).
Considering the four different particle sizes measured, the highest mean concentrations were recorded in P1 in the morning for winter and spring probably due to the accumulation of pollutants during the non-working hours when the shed remains closed and there is no air exchange. Also due to the frequency of activities being carried out, including the higher number of trucks arriving at the MRF to unload the waste in the morning compared to the afternoon. As well as for PM, the values for PNC presented non-normal distribution and the mean concentrations are statistically different at the 5% level of significance between winter and spring.
Overall, the values were mostly higher in winter for PNC0.3 and in spring for PNC0.5, PNC1.0, and PNC2.5. The smallest size fraction may present a greater potential in impairing human health than larger particles due to lung deposition and systemic spread in the human body, reaching the heart, brain, liver, and kidneys, among other organs61–65.
Beal et al.50 reported a decrease in the mass concentration of PM as higher values of relative humidity were registered due to the wet deposition of the pollutants, whereas higher temperature values caused an increase in the mass concentration of PM2.5–10. Total and respirable PM were negatively correlated with humidity and positively correlated with temperature66. Pateraki et al.67 also found a positive correlation between PM10, PM2.5–10, and temperature and a negative correlation for the pollutants and relative humidity. The same characteristics were observed in the present study for the mass concentration of PM (Fig. 1a,b) and the meteorological variables, except in summer, as this was the only season that rain was noted during one of the sampling days in the afternoon, which probably influenced the concentrations of PM between both periods studied. Tables with mean, median, and standard deviation values for the meteorological variables sampled outdoors at the Londrina weather station of the IAPAR and inside the waste processing shed are presented in the Supplementary Tables S2 and S3, respectively.
The mass concentration values of PM2.5, PM10, and dust measured in different MSW management facilities in the world, including this study, are shown in Table 1. The results are variable depending on the activity executed by the workers (driver or waste handler) and the characteristics of each location, such as the type of solid waste received, the season, and the process performed (collection or sorting). In most studies in solid waste facilities, PM exposure is estimated by dust mass concentration28,29,36,46. This research presents results in mass and number concentrations for PM by size fractions, assessing more detailed information related to air pollution in a MRF.
Table 1.
Mean concentrations of PM2.5, PM10, and dust in municipal solid waste facilities around the world.
| Location | Sampling site | Independent variables | PM2.5 (µg m−3) | PM10 (µg m−3) | Dust (µg m−3) | Reference |
|---|---|---|---|---|---|---|
| Canada | MSW recycling plant | Sorting | 28 | |||
| Summer | – | – | 1,100.0 | |||
| Winter | 400.0 | |||||
| South Korea | MSW management plant | Collection and sorting | 29 | |||
| Driver | – | – | 600.0 | |||
| Waste handler | 900.0 | |||||
| Finland | MSW management plant | Optic sorting process hall | – | – | 1,200.0 | 36 |
| Optic sorting control room | 300.0 | |||||
| Waste receiving plant | 1,800.0 | |||||
| United Kingdom | MRFs | Sorting | – | – | 2,090.0 | 46 |
| 3,600.0 | ||||||
| South Korea | MSW processing plant | Collection | 73.3 | 300.2 | – | 68 |
| Sorting | 61.6 | 458.1 | ||||
| Summer | 93.1 | 247.3 | ||||
| Fall | 145.1 | 384.0 | ||||
| Portugal | Waste-sorting plants | WSP with mechanical ventilation | 27.5 | 149.0 | – | 69 |
| WSP without mechanical ventilation | 108.0 | 1,390.0 | ||||
| Brazil | MRF | Sorting and processing | Present study | |||
| Winter | 23.7 | 211.8 | – | |||
| Spring | 35.5 | 584.5 | ||||
| Summer | 27.4 | 562.4 | ||||
MSW municipal solid waste, MRFs materials recycling facilities, WSP waste-sorting plant, PM particulate matter, – not measured.
The PM1.0, PM2.5, PM4.0, and PM10 concentrations in this study are in the same range as the results found in other articles (Table 1). Despite the differences existing among the MRFs the highest values were observed predominantly during the sorting process28,29,36,46,68, and for people who directly handle the waste when evaluated the activity performed29, as well as noted in this research. Also, the absence of mechanical ventilation at the waste-sorting plant (WSP) in the study of Viegas et al.69 contributed to the mass concentration of PM2.5 and PM10. Thus, the particles may accumulate even more inside the waste shed analysed in our study due to the lack of a ventilation system and proper air exchange.
Regarding the season examined, our results diverge from those obtained by Lavoie and Guertin28, who found higher values in summer compared to winter, while Park et al.68 presented higher concentrations in fall when comparing to summer. In general, considering PM2.5 and PM10 in P1, the highest mean concentrations were registered in spring for the present study (Table 1), probably due to the more intense generation of PM from indoor sources and the infiltration of outdoor particles, as it was the season with the highest mean PM values in P2 and the lowest mean values of relative humidity (Supplementary Tables S2, S3), which probably caused a decrease in the pollutants removal via wet deposition. The amount of waste itself did not influence the concentrations obtained in spring, as it presented the lowest volume of waste to be processed compared to winter and summer. Therefore, the activities performed were substantial to the higher concentrations observed in spring.
Throughout the sampling week in August and February, respectively, 58.4 and 68.2 mm rainfall occurred compared to 39.2 mm in October51, which may have influenced the results since precipitation also contributes to the removal of atmospheric PM due to wet deposition50,70,71 (see Supplementary Tables S2, S3 that provide the data for all meteorological variables).
The indoor/outdoor (I/O) relationship was calculated for PM and PNC considering the samplings in the morning and afternoon in the three seasons studied (Table 2). The Brazilian Health Regulatory Agency’s Resolution no. 09/2003 recommended an I/O ratio lower than or equal to 1.5 for fungi, however, there is no specific ratio determined for PM. When the I/O ratio is higher than 1.5, a diagnosis of pollutant sources is necessary for a corrective intervention53.
Table 2.
Indoor/outdoor ratios for PM and PNC in winter, spring, and summer.
| Season | I/O | PM1.0 | PM2.5 | PM4.0 | PM10 | PNC0.3 | PNC0.5 | PNC1.0 | PNC2.5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Winter | P1/P2 | a.m | 1.3 | 1.7 | 2.3 | 3.2 | 1.3 | 1.8 | 2.8 | 3.2 |
| p.m | 0.9 | 1.4 | 2.5 | 3.1 | 0.8 | 1.2 | 2.4 | 2.5 | ||
| Spring | P1/P2 | a.m | 1.9 | 3.7 | 5.3 | 6.1 | 1.7 | 3.6 | 5.5 | 5.3 |
| p.m | 1.3 | 2.1 | 3.0 | 3.2 | 1.3 | 2.3 | 3.1 | 3.1 | ||
| Summer | P1/P2 | a.m | 1.6 | 3.8 | 6.5 | 10.5 | – | – | – | – |
| p.m | 1.7 | 3.9 | 5.9 | 8.8 | – | – | – | – | ||
– not measured.
The I/O ratios varied from 0.9 to 10.5 for PM depending on the season and period of the day analysed. Around 79% of the ratios were higher than 1.5, mainly for PM4.0 and PM10. During the morning samplings in spring and summer, the concentrations in P1 were much higher than in P2, resulting in values that were, respectively, four and seven times higher than 1.5 for PM10. Expressive values were also observed in the afternoon samples in summer as I/O ratios were four and six times higher than 1.5 for PM4.0 and PM10, respectively. This occurred probably due to the more intense generation of PM by indoor sources in spring and summer, although the infiltration of outdoor particles may also happen, whilst winter concentration values in P1 and P2 were considerably closer than in the other seasons. The mean concentration in P2 was higher in spring than in summer, probably due to the occurrence of 68.2 mm of rain during the sampling week in the latter season. Therefore, the ratios are generally higher in summer even though the mean concentrations are mostly higher in spring in P1.
For PNC, the values ranged from 0.8 to 5.5 and 75% of the ratios were higher than 1.5, mostly for PNC0.5, PNC1.0, and PNC2.5. As observed for mass concentration, the highest values were obtained in spring during the morning samplings, with ratios almost four times higher than 1.5 for PNC1.0 and PNC2.5.
In reviews regarding the relationship between indoor and outdoor PM, I/O ratios presented a wide range of values (tending to zero to over 10) due to the influence of distance to the sources, resuspension, type of ambient ventilation, air exchange rate, meteorological conditions, and the deposition velocity of particles72,73. Most studies presenting I/O data are performed in residential homes, commercial buildings, offices, schools, and universities and focus on measuring particle mass73,74. Thus, it is difficult to compare these environments to the occupational setting studied here, in addition to scarce information regarding PNC in indoor and outdoor air. Nevertheless, it is important to measure pollutants suspended in the indoor air to develop effective strategies to decrease human exposure and health risks associated with air pollution74–76.
Bioaerosols analysis
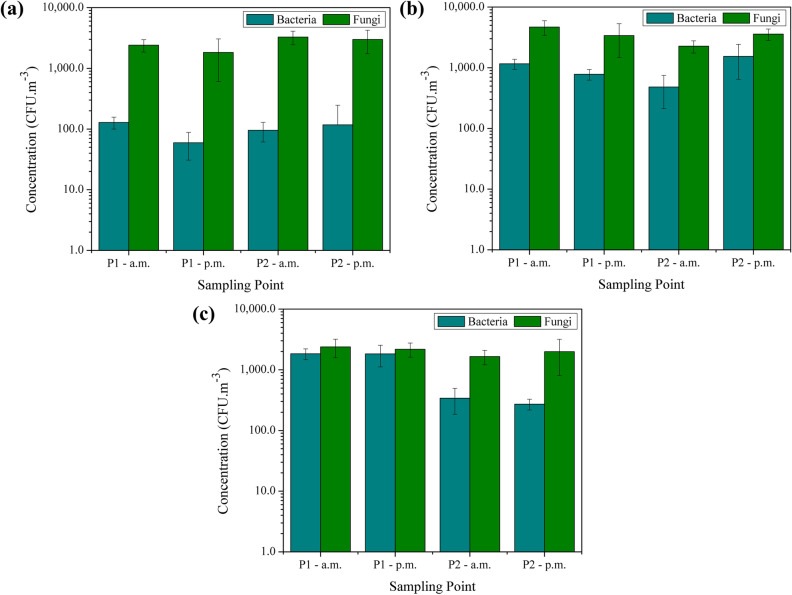
The mean concentrations of bacteria and fungi in the three seasons studied are shown in Fig. 3. The highest concentrations of bacteria were observed in P1 in the morning for winter and summer, and in P2 in the afternoon for spring. For fungi, this occurred in the morning in P2 for winter and in P1 for spring and summer. As aforementioned, P1 is the sampling point inside the waste processing shed, in which occurs activities related to the storage, sorting, and processing of the recyclable materials. On the other hand, P2 is the point located outdoors where there is an intense movement of trucks bringing the collected materials to the MRF. It was observed eventually the movement of other vehicles, such as cars and motorcycles, mainly from visitors. It also occurs the transfer of the big bags with sorted waste and the baled recyclable waste from the shed to the external environment using a forklift (promoting the resuspension of particles), which eventually becomes an open storage place and an emission source of airborne pollutants. Also, activities such as the storage and handling of the recyclable waste are considered as fundamental sources of bacteria and fungi at MRFs28.
Figure 3.
Mean concentrations of bacteria and fungi (CFU m−3) during the morning and afternoon in winter (a), spring (b), and summer (c) in the waste processing shed (P1) and in the outdoor environment (P2).
The mean values ranged from 59.3 to 128.4 colony-forming units per cubic meter (CFU m−3) for bacteria and from 1,826.1 to 2,407.1 CFU m−3 for fungi in winter, while in spring the concentrations ranged from 480.4 to 1,537.6 CFU m−3 and from 2,261.8 to 4,689.6 CFU m−3 for bacteria and fungi, respectively. In summer, the mean concentration values were between 271.7 and 1,838.5 CFU m−3 for bacteria, and between 1,649.7 and 2,386.3 CFU m−3 for fungi. Given the environments analysed and the activities performed during the samplings, we expected higher concentrations for bioaerosols, but our results are probably underestimated because of the method used. Hence, the results obtained in this study for bioaerosols using the settle plate method may be lower than the actual concentrations that workers are inhaling.
Overall, the highest values were obtained in summer for bacteria and in spring for fungi (Fig. 3a–c) as warm seasons are more favourable to the growth and reproduction of these microorganisms. The data presented non-normal distribution and statistical differences were observed at the 5% level of significance comparing the mean concentrations in winter, spring, and summer when applying the Kruskal–Wallis test.
Threshold values for bioaerosols are not yet established by the WHO, the United States Environmental Protection Agency (U.S. EPA), and the American Conference of Governmental Industrial Hygienists (ACGIH), for example, due to limited data and variability in the possible health effects caused by the air microflora30,77. On the other hand, different governmental and private organizations have established quantitative guidelines without considering the effects on human health77.
The Brazilian Health Regulatory Agency’s Resolution no. 09/2003 determined an MRV of 750 CFU m−3 for fungi in indoor air53. In Portugal, the MRV was reported in the Technical Note NT-SCE-02 of the National System for Energy and Indoor Air Quality Certification of Buildings as 500 CFU m−3 for bacteria and fungi78. In Denmark and some other Scandinavian countries, guidelines were established for total bacteria and Gram-negative bacteria as 10,000 and 1,000 CFU m−3, respectively, for an 8-h period28. Except for the Scandinavian countries, it is noteworthy that these guidelines were not established specifically for indoor air at MSW management sites, but we used these as reference values in the absence of more suitable ones.
Analysing the indoor setting in our study and the Brazilian Resolution, the concentration values of fungi in P1 were around three times higher than 750 CFU m−3 in winter and summer. In spring, the values were up to six times higher than the established MRV. Comparing with the Portuguese reference value, concentrations of fungi were almost five times higher than 500 CFU m−3 in P1 in winter and summer, and nine times higher than the MRV in spring. For bacteria, this was observed in spring and summer, when they were two and almost four times higher than the mentioned value, respectively. On the other hand, none of the concentrations exceeded the limit established in Denmark for total bacteria. As the Brazilian and Portuguese MRVs are aimed for indoor settings for public use with air-conditioning and/or buildings, we can assume that the values sampled in the MRF do not correspond to the activities carried out that generate airborne microorganisms due to the use of the settle plate technique. Thereby, although these results are not as high, the Occupational Safety and Health Administration (OSHA) of the United States related that 1,000 CFU m−3 indicates air contamination but not necessarily unsafe or hazardous conditions, which depend on the type of airborne microorganisms79.
Unfortunately, we only have this vague guideline in Brazil that urgently should be improved and specific regulations for waste pickers should be implemented due to the possible hazards of occupational exposure to bioaerosols. As stated in the regulatory standards of the Brazilian Ministry of Labour52, the biological agents are characterized qualitatively according to the activity performed, such as people who work in permanent contact with patients in isolation due to infectious diseases, wastewater, and urban waste (collection and industrialization). Thus, working with MSW is considered insalubrious, but no quantitative guidelines were established for waste pickers' health and safety in Brazil.
Meteorological variables, such as temperature and relative humidity, can affect the presence of bioaerosols in the air80. Dungan80 also related that airborne microorganism’s viability declines when temperature rises and relative humidity decreases. Frankel et al.81 found significant correlations between temperature, relative humidity, and the concentrations of bacteria and fungi, and so did Ren et al.82 for fungi in indoor air and Gamero et al.83 for fungi at a landfill site. Temperature and relative humidity, respectively, were negatively and positively correlated with bacterial and fungal concentrations84. On the other hand, Goh et al.85 related that indoor temperature and relative humidity were constant during their samplings, so these variables did not present any substantial impact on the concentrations of indoor fungi and bacteria. While the results in the literature are different, we found higher indoors (P1) concentrations of bacteria and fungi in the morning, when the temperature was lower (negative correlation), and humidity was higher (positive correlation), similar to Dungan80 and Green et al.84 (see Supplementary Table S3 for the meteorological data measured inside the waste processing shed). In P2, wind speed did not influence the bioaerosols concentrations (Supplementary Table S2).
The concentration values of bioaerosols, as total bacteria, Gram-negative bacteria, and fungi, measured in different MSW management facilities in the world, including this study, are shown in Table 3. Great variability in the parameters analysed and the results obtained in other studies is observed. The sampling methods were also different, and each sampling site has its characteristics regarding the type of solid waste received and the meteorological variables of the location.
Table 3.
Mean concentrations of bioaerosols in municipal solid waste facilities in the world.
| Location | Sampling site | Independent variables | Total Bacteria (103 CFU m−3) | GNB (103 CFU m−3) | Fungi (103 CFU m−3) | Reference |
|---|---|---|---|---|---|---|
| Canada | MSW recycling plant | Sorting | 28 | |||
| Summer | 21.9 | 0.5 | 19.2 | |||
| Winter | 6.1 | N.D. | 4.9 | |||
| South Korea | MSW management plant | Collection and sorting | 29 | |||
| Driver | 18.0 | 7.2 | 8.7 | |||
| Waste handler | 220.0 | 81.0 | 24.0 | |||
| Finland | MSW management plant | Optic sorting process hall | 68.9 | – | 220.0 | 36 |
| Optic sorting control room | 3.0 | 6.1 | ||||
| Waste receiving plant | 1.2 | 3.5 | ||||
| South Korea | MSW management plant | Collection | 11.0 | 12.0 | 3.7 | 86 |
| Sorting | 31.0 | 32.0 | 43.0 | |||
| Summer | 18.0 | 22.0 | 21.0 | |||
| Fall | 8.2 | 4.2 | 3.3 | |||
| Poland | MSW management plant | Sorting | 87 | |||
| Sorter | 65.0 | – | 102.0 | |||
| Machine operator | 29.0 | 126.0 | ||||
| Brazil | MRF | Sorting and processing | Present study | |||
| Winter | 0.09 | – | 2.1 | |||
| Spring | 1.0 | 4.0 | ||||
| Summer | 1.8 | 2.3 | ||||
MSW municipal solid waste, MRF materials recycling facility, CFU colony-forming unit, GNB gram-negative bacteria, N.D. not detected, – not measured.
The highest concentrations of bioaerosols were registered mainly during the sorting process28,29,36,86,87 and for the samplings performed in summer28,86. Regarding the season studied, Lavoie and Guertin28 claim that the concentration values of microorganisms measured during winter are always lower than during summer. In the present research, a similar result was observed as bacteria and fungi concentrations were lower in the winter compared to the summer. However, the highest average concentration for bacteria was registered in summer, while for fungi this occurred in spring (Table 3). Concentrations of fungi generally increase in spring and peak in summer88,89, although results are diverse in the literature. Černá et al.90 had higher fungi concentrations in spring in comparison to winter, while Nadal et al.91 presented higher values of bioaerosols in summer. When analysing different environments than the ones studied in this research, Gonçalves et al.92 found higher values at the beginning of spring in São Paulo, while Emygdio et al.93 presented a higher number of total spores in spring, but different genera had a large variability regarding seasonal characterization.
In general, the values measured in other studies presented in Table 3 are from 102 to 105 CFU m−3, while we obtained values ranging from 101 to 103 CFU m−3. The lack of a sampling protocol makes it difficult to compare the results since authors used different sampling methods, such as multi-stage impactors for the collection of airborne microorganisms on agar plates and air sampling pumps to measure the bioaerosols on polycarbonate or fibreglass filters. We used the settle plate method, which is a gravitational and passive (non-volumetric) sampling procedure used to enable the gathering of particles by the gravitational force on agar plates77 and the results are reproducible, reliable, and portray real conditions of the site studied94,95. Also, this technique is economically feasible, easily applicable, and allows samplings from different places at the same time without the disturbance of airflow94,95. However, it has limitations regarding the collection of smaller particles because the dry deposition of particles is a function of their mass, as well as the conversion of the number of CFUs counted on the agar plates in CFUs per cubic meter of air, presenting underestimated results compared to active sampling methods.
In Table 4 we present the I/O ratios that were calculated for bacteria and fungi concentrations in the morning and afternoon for the three seasons studied. The recommended I/O ratio for fungi is equal to or lower than 1.5 and there is no specific value for bacteria53. The values ranged from 0.5 to 6.7 for bacteria and from 0.6 to 2.1 for fungi. Four values, which represent 33.3%, were higher than 1.5 in spring and summer. This is an indication of the influence of activities performed inside the shed on levels of both bacteria and fungi observed indoors. Thus, higher indoor concentrations may be triggered by indoor bioaerosol sources96, but the infiltration of outdoor particles may also happen.
Table 4.
Indoor/outdoor ratios for bacteria and fungi in winter, spring, and summer.
| Season | I/O | Bacteria | Fungi | |
|---|---|---|---|---|
| Winter | P1/P2 | a.m | 1.4 | 0.7 |
| p.m | 0.5 | 0.6 | ||
| Spring | P1/P2 | a.m | 2.4 | 2.1 |
| p.m | 0.5 | 1.0 | ||
| Summer | P1/P2 | a.m | 5.4 | 1.4 |
| p.m | 6.7 | 1.1 | ||
In the present research, only four values were higher than 1.5, so the outdoor contribution to the concentration of airborne microorganisms is expressive probably due to the storage of recyclable items outside the waste shed at the MRF studied. Pictures of the outside environment (P2) are presented in the Supplementary Material. We did not find I/O ratios in similar environments (management of MSW), therefore no comparisons were made with other studies.
Although the results for bioaerosols are not as high as in other studies given the underestimation caused by the settle plate method, we observed a great diversity of bacterial and fungal colonies with different colours, shapes, margins, and textures through the macroscopic morphological characterization, which suggests genus and species richness in the air of the MRF. The results from the Gram staining technique showed that Gram-positive bacteria dominated the indoor samples of isolated colonies with 58.6%. Observing their microscopic morphology, the colonies of bacteria were then grouped into Gram-positive bacilli, Gram-positive cocci, Gram-negative bacilli, and Gram-negative cocci, which represented 46.6, 12.1, 24.1, and 17.2%, respectively.
Gram-positive bacteria also have been found as the most abundant airborne bacteria when analysing indoor samples in previous studies96–99. Airborne Gram-negative bacteria present brief periods of survival96, hence their comparatively small presence in the air of the MRF studied. Nevertheless, both Gram-positive and Gram-negative bacteria present cell wall components with pro-inflammatory agents, which may cause health problems such as respiratory symptoms18.
Conclusions
Bioaerosols, PM, and PNC concentrations were assessed in the environment of a Brazilian MRF, which is how the recyclable waste is managed in the country. The strongest air contamination by these pollutants was observed inside the waste processing shed (P1) due to the variety of activities that promote the generation and resuspension of pollutants. Mean values in P1 were 475.5 ± 563.7 µg m−3 for PM10, 58.6 ± 36.0 cm−3 for PNC0.3, 1,088.8 ± 825.2 CFU m−3 for bacteria, and 2,738.3 ± 1,381.3 CFU m−3 for fungi, considering all the seasons and periods of the day analysed.
The concentrations were predominantly higher in summer for bacteria and spring for fungi, PM, and PNC. The I/O ratios demonstrated the large influence of indoor sources on indoor concentrations, mainly for PM and PNC, which presented 79 and 75% of values higher than 1.5, respectively, during the three seasons studied.
Temperature and relative humidity may have influenced the behaviour found for these pollutants as notable correlations were observed among these parameters and the concentrations of PM and bioaerosols. Also, the operational conditions carried out at the MRF that result in airborne particles and microorganisms are responsible for this great variability of results. The studied MRF certainly has several genera and species of bacteria and fungi suspended in the air, which should be elucidated in future studies.
Our results show a critical indoor air quality situation with considerable concentration values mainly for particulate matter, which may cause several health risks for the waste pickers. Although we used an evaluation technique with easy application and economic feasibility, the bioaerosols results are most likely underestimated because of the passive sampling procedure. In Brazil, there is a lack of studies regarding the working environments and conditions at MRFs and the occupational health risks that waste pickers face daily. Hence, we worked toward a start to fill the gap of this knowledge. The temporal characteristics of particulate matter and bioaerosols emissions should be further explored given the high variability of activities occurring at the MRF during the samplings. The association between the exposure to these pollutants and the occurrence of occupational health problems also could be investigated in future studies.
It is suggested that the waste pickers use PPE, mainly gloves and masks, to mitigate the exposure to PM, PNC, and bioaerosols. We also recommend a detailed hazard identification, risk assessment and control to implement specific intervention plans based on the requirements of the MRF, such as in which areas and/or activities the use of PPE should be intensified, the real need of a ventilation system for the dispersion of pollutants, among other issues. For example, having enough doors and windows at MSW management sites is an economic and potential measure to improve air exchange naturally. Nevertheless, regulations for waste workers’ health and safety, in addition to design and operational conditions at MRFs should be implemented in Brazil considering the possible risks of occupational exposure to airborne pollutants and the opportunity of increasing natural ventilation. Moreover, it is imperative to have a better inspection and bigger investments in the infrastructure of MRFs to enhance the working settings for this important economic class and to minimize the occurrence of health problems, which generate high costs for the public health system.
Materials and methods
Study area characterization
A Materials Recycling Facility (MRF) located in Londrina, in the southern region of Brazil (23° 18′ 54″ S; 51° 12′ 52″ W), was used for the study. Londrina has an estimated population of 569,733 inhabitants according to the Brazilian Institute of Geography and Statistics (IBGE)100 and is a typical medium-sized city in Brazil. Furthermore, there is a daily production of 465 tonnes of municipal solid waste101, representing approximately 0.80 kg/capita/day.
The MRF is in the western region of Londrina, an urbanized area that has many residences, small industrial activities, and intense flow of light- and heavy-duty vehicles due to the proximity of a state highway. Furthermore, there is the movement of the trucks at the MRF itself that are used in the waste collection. The constructed area of the MRF is around 1,330 m2 represented by a shed and an office for its management. Moreover, there is an outdoor space where the processed waste is stored. The shed has a total area of 1,200 m2 even though the waste is stored, sorted, and processed by the waste pickers in an area of 920 m2, while the remaining 280 m2 are reserved for the processing of expanded polystyrene (Styrofoam) (Fig. 4). It has two large doors where the trucks enter to unload the recyclable waste, but it has no windows. Hence, two sampling points were selected for this study: the first inside the waste processing shed (P1) and the second in the outdoor environment (P2) (Fig. 4). We included pictures of the MRF as a Supplementary Material to provide a better view of the sampling sites (Supplementary Figs. S1, S2).
Figure 4.
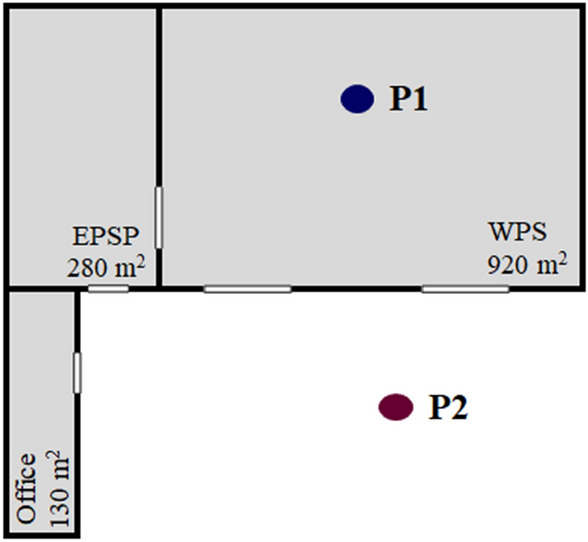
Sketch of the sampling points in the waste processing shed (P1) and in the outdoor environment (P2). EPSP expanded polystyrene processing, WPS waste processing shed.
The MRF studied is responsible for the collection of recyclable waste from approximately 90,000 households, which represents 39% of its total number in Londrina according to the Municipal Transit and Urbanization Company (CMTU)102. The collection is carried out with trucks from Monday to Friday in different areas determined by the CMTU, and then the recyclable waste is taken to the MRF for its sorting. The trucks enter the waste processing shed for the unloading of the materials to the floor. In 2017, workers used to have static screening tables to sort the materials in multiple workstations. In 2019, the MRF installed a conveyor to move the materials with two workstations along it. However, not all MRFs in Brazil have conveyors to separate the recyclable waste. The waste sorting process occurs manually by 26 workers, who put the materials in big polypropylene bags (90 × 90 × 110 cm with a loading weight of 1.5 tonnes) considering the following categories: paper, cardboard, metal, aluminium cans, plastic bags, PET bottles, Tetra Pak packages, Styrofoam, and residual waste. An average of 86.7 tonnes of recyclable waste is commercialized per month in the waste processing shed studied.
Sampling design and instrumentation
A total of 2,400 min of PM, 1,440 min of PNC, and 216 samples of bioaerosols were collected during working days in August and October of 2017, and in February of 2019, in the morning and afternoon. These months represent the respective seasons in Brazil: winter, spring, and summer. In every sampling campaign, 12 measurements were performed for PM and PNC of 60 min each, except for summer in which we carried out 16 measurements of PM (60 min each) and none for PNC due to operational obstacles. For bioaerosols, 72 samples were collected in every one of the seasons for 10 min. The data were clustered into morning and afternoon of each season studied.
Air temperature and relative humidity were measured using the Onset HOBO sensor (Model UX100-023, Onset Computer Corporation, USA), simultaneously with PM, PNC, and bioaerosols in the waste processing shed (P1). All measurements were performed around 1.2 m from the ground and the sampling apparatuses were placed in each environment as indicated in Fig. 4. Meteorological variables from the Agronomic Institute of Parana (Londrina weather station: 23° 21′ 16″ S; 51° 09′ 51″ W) were compiled to represent the conditions of the atmosphere outdoors during the sampling periods (see Supplementary Material).
PM and PNC measurements were carried out during 60 min each at the two sampling points simultaneously, with a flow rate of 2.83 L min−1 and 1 min of temporal resolution, using the Mass Particle Monitor (Model Aerocet 831, Met One Instruments, USA) and the Particle Counter Monitor (Model 804, Met One Instruments, USA). The first one was used for the measurement of the PM mass concentrations considering the sizes of 1.0 (PM1.0), 2.5 (PM2.5), 4.0 (PM4.0), and 10 μm (PM10) in diameter. The second one was used to measure the PNC for 0.3 (PNC0.3), 0.5 (PNC0.5), 1.0 (PNC1.0), and 2.5 μm (PNC2.5) in diameter.
Bioaerosols assessment
The settle plate method was used to collect bioaerosols samples (in triplicate) in Petri dishes containing Plate Count Agar (PCA), for the cultivation of bacteria, and Sabouraud Dextrose Agar (SDA), for the cultivation of fungi. Sampling was performed concomitantly with PM and PNC measurements in each of the three seasons studied and the exposure time of the agar plates to the air was 10 min53,103 at both collection points. In each experiment, we included contamination control plates that were not exposed to the air and all of them resulted in culture negative.
A new bioaerosols collection was performed in September of 2019 using the passive and active sampling procedures simultaneously to calculate their concentrations (in CFU m−3) since the equations found in the literature for the settle plate method using the colony-forming units (CFUs) presented different results94,104,105. Therefore, we established a relationship based on the results obtained from both sampling techniques to calculate the bioaerosols concentrations for the previous passive samplings.
The settle plate method sampling was done as aforementioned. For the active sampling, we used the MAS-100 NT equipment (MBV AG, Switzerland) in which bioaerosol collection occurs through the impaction process, i.e., the microorganisms can impact directly on the agar plate. The flow rate was 100 L min−1 resulting in 250 L of air collected in 2.5 min. The culture media utilized were Tryptic Soy Broth (TSB) for bacterial cultivation and Dichloran Rose Bengal Chloramphenicol (DRBC) for fungal cultivation. DRBC medium plates were also used for the settle plate sampling to evaluate a possible interference of the culture medium in the CFU count, which was not observed.
After sampling, agar plates were incubated at 35 °C ± 1 °C for 24 h for bacteria and at room temperature (± 25 °C) for 96 h for fungi106. Subsequently, the CFUs were counted in each Petri dish and we established ratios (7.4 for bacteria and 31.1 for fungi) between the concentrations obtained by the active sampling in CFU m−3 and the CFU values of the settle plate method. Then, the concentrations in CFU m−3 of the previous passive samplings were calculated, representing a correction since the growth of microorganisms on the agar plates is lower when using the settle plate method.
Bacterial colony morphology was observed through its form, elevation, and margin according to Rodina107 and then the colonies were isolated according to their macroscopic characteristics. Gram staining technique was applied for 58 isolated colonies of bacteria following the methodology described by Benson106 and microscopic morphological analysis was carried out observing the shape of cells: spheres (cocci), rods (bacilli), and spirals. Colony diversity was also analysed for fungi observing its main macroscopic aspects108.
Supplementary information
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001. The authors also acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Grant numbers 404104/2013-4 and 306862/2018-2-and the Araucaria Foundation. The authors would like to thank the managers of the Materials Recycling Facility who allowed us to perform this study and the Agronomic Institute of Parana for the meteorological data of the Londrina weather station. We also thank the reviewers for their valuable comments, which helped us to improve the article.
Author contributions
C.F.H.W. and E.H.D. worked on data collection and analysis. C.F.H.W., L.L.L.J., B.O.G., A.C.M. performed the experiments. C.F.H.W., K.V.M.C.P. and L.D.M. wrote the manuscript, then all authors reviewed it.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71787-0.
References
- 1.Giusti L. A review of waste management practices and their impact on human health. Waste Manag. 2009;29:2227–2239. doi: 10.1016/j.wasman.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Guerrero LA, Maas G, Hogland W. Solid waste management challenges for cities in developing countries. Waste Manag. 2013;33:220–232. doi: 10.1016/j.wasman.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Jacobi PR, Besen GR. Solid waste management in São Paulo: The challenges of sustainability. Estud. Avançados. 2011;25:135–158. [Google Scholar]
- 4.Lino FAM, Ismail KAR. Evaluation of the treatment of municipal solid waste as renewable energy resource in Campinas, Brazil. Sustain. Energy Technol. Assess. 2018;29:19–25. [Google Scholar]
- 5.Hoornweg D, Bhada-Tata P. What a Waste: A Global Review of Solid Waste Management. Urban Development Series Knowledge Papers. Geneva: World Bank; 2012. [Google Scholar]
- 6.Marino AL, Chaves GLD, Santos Junior JL. Do Brazilian municipalities have the technical capacity to implement solid waste management at the local level? J. Clean. Prod. 2018;188:378–386. [Google Scholar]
- 7.Porta D, Milani S, Lazzarino AI, Perucci CA, Forastiere F. Systematic review of epidemiological studies on health effects associated with management of solid waste. Environ. Heal. 2009;8:60. doi: 10.1186/1476-069X-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaza S, Yao LC, Bhada-Tata P, Van Woerden F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Geneva: World Bank; 2018. [Google Scholar]
- 9.ABRELPE (Brazilian Association of Public Cleaning and Special Waste Companies). Panorama dos resíduos sólidos no Brasil 2018/2019. (Brazilian Association of Public Cleaning and Special Waste Companies, 2019).
- 10.Silva P. A Organização Coletiva de Catadores de Material Reciclável no Brasil: Dilemas e Potencialidades sob a Ótica da Economia Solidária. Brazil: Springer; 2017. [Google Scholar]
- 11.Poole CJM, Basu S. Systematic review: Occupational illness in the waste and recycling sector. Occup. Med. (Chic. Ill.) 2017;67:626–636. doi: 10.1093/occmed/kqx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina M. The informal recycling sector in developing countries: Organizing waste pickers to enhance their impact. Gridlines. 2008;44:10. [Google Scholar]
- 13.del Moreno-Sánchez RP, Maldonado JH. Surviving from garbage: The role of informal waste-pickers in a dynamic model of solid-waste management in developing countries. Environ. Dev. Econ. 2006;11:371–391. [Google Scholar]
- 14.Wilson DC, Velis C, Cheeseman C. Role of informal sector recycling in waste management in developing countries. Habitat Int. 2006;30:797–808. [Google Scholar]
- 15.MNCR (National Movement of Collectors of Recyclable Materials). Mulheres São Maioria Entre Catadores de Materiais Recicláveis. https://www.mncr.org.br/noticias/noticias-regionais/mulheres-sao-maioria-entre-catadores-organizados-em-cooperativas (2014).
- 16.CEMPRE (Business Commitment to Recycling). CEMPRE Review 2019. (2019).
- 17.CEMPRE (Business Commitment to Recycling). Renda de Catadores Dispensa Complementação do Bolsa Família. https://cempre.org.br/cempre-informa/id/53/renda-de-catadores-dispensa-complementacao-do-bolsa-familia (2015).
- 18.Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 19.Vimercati L, et al. Respiratory health in waste collection and disposal workers. Int. J. Environ. Res. Public Health. 2016;13:1–8. doi: 10.3390/ijerph13070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S, Mandal P, Srivastava A. Quantification and characterization of size-segregated bioaerosols at municipal solid waste dumping site in Delhi. Proced. Environ. Sci. 2016;35:400–407. [Google Scholar]
- 21.Douglas P, et al. Respiratory hospital admission risk near large composting facilities. Int. J. Hyg. Environ. Health. 2016;219:372–379. doi: 10.1016/j.ijheh.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 22.WHO (World Health Organization) Health Effects of Particulate Matter: Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia. Geneva: World Health Organization; 2013. [Google Scholar]
- 23.Kim K-H, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Landrigan PJ, et al. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- 25.EPA. Health and Environmental Effects of Particulate Matter (PM). https://www.epa.gov/pm-pollution/health-and-environmental-effects-particulate-matter-pm (2018).
- 26.Roux J, et al. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ. Res. 2017;156:404–410. doi: 10.1016/j.envres.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 27.Breza-Boruta B. The assessment of airborne bacterial and fungal contamination emitted by a municipal landfill site in Northern Poland. Atmos. Pollut. Res. 2016;7:1043–1052. [Google Scholar]
- 28.Lavoie J, Guertin S. Evaluation of health and safety risks in municipal solid waste recycling plants. J. Air Waste Manag. Assoc. 2001;51:352–360. doi: 10.1080/10473289.2001.10464278. [DOI] [PubMed] [Google Scholar]
- 29.Park DU, Ryu SH, Kim SB, Yoon CS. An assessment of dust, endotoxin, and microorganism exposure during waste collection and sorting. J. Air Waste Manag. Assoc. 2011;61:461–468. doi: 10.3155/1047-3289.61.4.461. [DOI] [PubMed] [Google Scholar]
- 30.WHO (World Health Organization) WHO Guidelines for Indoor Air Quality: Dampness and Mold. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 31.Breum NO, Würtz H, Midtgaard U, Ebbehøj N. Dustiness and bio-aerosol exposure in sorting recyclable paper. Waste Manag. Res. 1999;17:100–108. [Google Scholar]
- 32.Schlosser O, Déportes IZ, Facon B, Fromont E. Extension of the sorting instructions for household plastic packaging and changes in exposure to bioaerosols at materials recovery facilities. Waste Manag. 2015;46:47–55. doi: 10.1016/j.wasman.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Lavoie J, Dunkerley CJ, Kosatsky T, Dufresne A. Exposure to aerosolized bacteria and fungi among collectors of commercial, mixed residential, recyclable and compostable waste. Sci. Total Environ. 2006;370:23–28. doi: 10.1016/j.scitotenv.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Byeon JH, Park CW, Yoon KY, Park JH, Hwang J. Size distributions of total airborne particles and bioaerosols in a municipal composting facility. Bioresour. Technol. 2008;99:5150–5154. doi: 10.1016/j.biortech.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Pahari AK, Dasgupta D, Patil RS, Mukherji S. Emission of bacterial bioaerosols from a composting facility in Maharashtra, India. Waste Manag. 2016;53:22–31. doi: 10.1016/j.wasman.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Lehtinen J, Tolvanen O, Nivukoski U, Veijanen A, Hänninen K. Occupational hygiene in terms of volatile organic compounds (VOCs) and bioaerosols at two solid waste management plants in Finland. Waste Manag. 2013;33:964–973. doi: 10.1016/j.wasman.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen EM, et al. Bioaerosol exposure in waste collection: A comparative study on the significance of collection equipment, type of waste, and seasonal variation. Ann. Occup. Hyg. 1997;41:325–344. [Google Scholar]
- 38.Lavoie J, Dunkerley CJ. Assessing waste collectors’ exposure to bioaerosols. Aerobiologia (Bologna). 2002;18:277–285. [Google Scholar]
- 39.Lee KH, et al. Occupational exposure to diesel particulate matter in municipal household waste workers. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0135229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ncube F, Ncube EJ, Voyi K. Bioaerosols, noise, and ultraviolet radiation exposures for municipal solid waste handlers. J. Environ. Public Health. 2017;2017:1–8. doi: 10.1155/2017/3081638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalvatzaki E, et al. Measurements of particulate matter concentrations at a landfill site (Crete, Greece) Waste Manag. 2010;30:2058–2064. doi: 10.1016/j.wasman.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 42.Chang MW, et al. Bioaerosols from a food waste composting plant affect human airway epithelial cell remodeling genes. Int. J. Environ. Res. Public Health. 2014;11:337–354. doi: 10.3390/ijerph110100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor DJ, Daly SM, Sodeau JR. On-line monitoring of airborne bioaerosols released from a composting/green waste site. Waste Manag. 2015;42:23–30. doi: 10.1016/j.wasman.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Kermani M, Farzadkia M, Kalantari RR, Bahmani Z. Fine particulate matter (PM2.5) in a compost facility: Heavy metal contaminations and health risk assessment, Tehran, Iran. Environ. Sci. Pollut. Res. 2018;25:15715–15725. doi: 10.1007/s11356-018-1625-y. [DOI] [PubMed] [Google Scholar]
- 45.Cioca LI, et al. Risk assessment in a materials recycling facility: Perspectives for reducing operational issues. Resources. 2018;7:85. [Google Scholar]
- 46.Gladding T, Thorn J, Stott D. Organic dust exposure and work-related effects among recycling workers. Am. J. Ind. Med. 2003;43:584–591. doi: 10.1002/ajim.10220. [DOI] [PubMed] [Google Scholar]
- 47.Pressley PN, Levis JW, Damgaard A, Barlaz MA, DeCarolis JF. Analysis of material recovery facilities for use in life-cycle assessment. Waste Manag. 2015;35:307–317. doi: 10.1016/j.wasman.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Maître A, et al. Municipal waste incinerators: Air and biological monitoring of workers for exposure to particles, metals, and organic compounds. Occup. Environ. Med. 2003;60:563–569. doi: 10.1136/oem.60.8.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viegas C, et al. Assessment of fungal contamination in waste sorting and incineration—case study in Portugal. J. Toxicol. Environ. Health Part A Curr. Issues. 2014;77:57–68. doi: 10.1080/15287394.2014.865583. [DOI] [PubMed] [Google Scholar]
- 50.Beal A, et al. Inorganic chemical composition of fine particulates in medium-sized urban areas: A case study of Brazilian cities. Aerosol Air Qual. Res. 2017;17:920–932. [Google Scholar]
- 51.IAPAR (Agronomic Institute of Parana). Dados diários de Londrina. https://www.iapar.br/modules/conteudo/conteudo.php?conteudo=1828 (2019).
- 52.Brazil. Normas Regulamentadoras (NR). https://sit.trabalho.gov.br/portal/index.php/seguranca-e-saude-no-trabalho/legislacao-sst/normas-regulamentadoras?view=default (2020).
- 53.Brazil. Resolução n° 09, de 16 de janeiro de 2003. Dispõe sobre padrões referenciais de qualidade do ar interior em ambientes climatizados artificialmente de uso público e coletivo. 1–35 (Official Gazette of the Federal Government, 2003).
- 54.WHO (World Health Organization) Air Quality Guidelines: Global Update 2005. Geneva: World Health Organization; 2006. [Google Scholar]
- 55.Eduard W, Heederik D, Duchaine C, Green BJ. Bioaerosol exposure assessment in the workplace: The past, present and recent advances. J. Environ. Monit. 2012;14:334–339. doi: 10.1039/c2em10717a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.OSHA (Occupational Safety and Health Administration) Personal Protective Equipment. New York: Occupational Safety and Health Administration; 2004. [Google Scholar]
- 57.Rout BK, Sikdar BK. Hazard identification, risk assessment, and control measures as an effective tool of occupational health assessment of hazardous process in an iron ore pelletizing industry. Indian J. Occup. Environ. Med. 2017;21:56–76. doi: 10.4103/ijoem.IJOEM_19_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costabile F, et al. Spatio-temporal variability and principal components of the particle number size distribution in an urban atmosphere. Atmos. Chem. Phys. 2009;9:3163–3195. [Google Scholar]
- 59.Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012;8:166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birmili W, et al. Variability of aerosol particles in the urban atmosphere of Dresden (Germany): Effects of spatial scale and particle size. Meteorol. Z. 2013;22:195–211. [Google Scholar]
- 61.Cassee FR, Héroux ME, Gerlofs-Nijland ME, Kelly FJ. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013;25:802–812. doi: 10.3109/08958378.2013.850127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heal MR, Kumar P, Harrison RM. Particles, air quality, policy and health. Chem. Soc. Rev. 2012;41:6606–6630. doi: 10.1039/c2cs35076a. [DOI] [PubMed] [Google Scholar]
- 63.Kumar P, et al. Ultrafine particles in cities. Environ. Int. 2014;66:1–10. doi: 10.1016/j.envint.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 64.Mueller MD, Hasenfratz D, Saukh O, Fierz M, Hueglin C. Statistical modelling of particle number concentration in Zurich at high spatio-temporal resolution utilizing data from a mobile sensor network. Atmos. Environ. 2016;126:171–181. [Google Scholar]
- 65.WHO (World Health Organization) Review of Evidence on Health Aspects of Air Pollution—REVIHAAP Project: First Results. Geneva: World Health Organization; 2013. [Google Scholar]
- 66.Jayamurugan R, Kumaravel B, Palanivelraja S, Chockalingam MP. Influence of temperature, relative humidity and seasonal variability on ambient air quality in a coastal urban area. Int. J. Atmos. Sci. 2013;2013:1–7. [Google Scholar]
- 67.Pateraki S, Asimakopoulos DN, Maggos T, Flocas HA, Vasilakos C. The role of wind, temperature and relative humidity on PM fractions in a suburban Mediterranean region. Fresenius Environ. Bull. 2010;19:2013–2018. [Google Scholar]
- 68.Park D, et al. Characteristics of particulate matter generated while handling municipal household waste. J. Occup. Health. 2013;55:503–510. doi: 10.1539/joh.13-0166-fs. [DOI] [PubMed] [Google Scholar]
- 69.Viegas S, Almeida-Silva M, Viegas C. Occupational exposure to particulate matter in 2 Portuguese waste-sorting units. Int. J. Occup. Med. Environ. Health. 2014;27:854–862. doi: 10.2478/s13382-014-0310-8. [DOI] [PubMed] [Google Scholar]
- 70.Dawson JP, Adams PJ, Pandis SN. Sensitivity of PM2.5 to climate in the Eastern US: A modeling case study. Atmos. Chem. Phys. 2007;7:4295–4309. [Google Scholar]
- 71.Santos TC, Carvalho VSB, Reboita MS. Avaliação da influência das condições meteorológicas em dias com altas concentrações de material particulado na Região Metropolitana do Rio de Janeiro. Eng. Sanitária Ambient. 2016;21:307–313. [Google Scholar]
- 72.Chen C, Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos. Environ. 2011;45:275–288. [Google Scholar]
- 73.Bo M, Salizzoni P, Clerico M, Buccolieri R. Assessment of indoor–outdoor particulate matter air pollution: A review. Atmosphere (Basel) 2017;8:20. [Google Scholar]
- 74.Cyrys J, Pitz M, Bischof W, Wichmann HE, Heinrich J. Relationship between indoor and outdoor levels of fine particle mass, particle number concentrations and black smoke under different ventilation conditions. J. Expo. Anal. Environ. Epidemiol. 2004;14:275–283. doi: 10.1038/sj.jea.7500317. [DOI] [PubMed] [Google Scholar]
- 75.Laumbach R, Meng Q, Kipen H. What can individuals do to reduce personal health risks from air pollution? J. Thorac. Dis. 2015;7:96–107. doi: 10.3978/j.issn.2072-1439.2014.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung DYC. Outdoor–indoor air pollution in urban environment: Challenges and opportunity. Front. Environ. Sci. 2015;2:1–7. [Google Scholar]
- 77.Kim KH, Kabir E, Jahan SA. Airborne bioaerosols and their impact on human health. J. Environ. Sci. (China) 2018;67:23–35. doi: 10.1016/j.jes.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Energy Agency. Nota Técnica-NT-SCE-02-Metodologia Para Auditorias Periódicas de QAI em Edifícios Existentes no Âmbito do RSECE. 1–50 (Energy Agency, 2009).
- 79.OSHA (Occupational Safety and Health Administration). Indoor Air Quality Investigation. https://www.osha.gov/dts/osta/otm/otm_iii/otm_iii_2.html (2020).
- 80.Dungan RS. Board-invited review: Fate and transport of bioaerosols associated with livestock operations and manures. J. Anim. Sci. 2010;88:3693–3706. doi: 10.2527/jas.2010-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frankel M, et al. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl. Environ. Microbiol. 2012;78:8289–8297. doi: 10.1128/AEM.02069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren P, Jankun TM, Belanger K, Bracken MB, Leaderer BP. The relation between fungal propagules in indoor air and home characteristics. Allergy Eur. J. Allergy Clin. Immunol. 2001;56:419–424. doi: 10.1034/j.1398-9995.2001.056005419.x. [DOI] [PubMed] [Google Scholar]
- 83.Gamero WBM, Ramírez MC, Parody A, Viloria A. Concentrations and size distributions of fungal bioaerosols in a municipal landfill. In: Tan Y, Shi Y, Tang Q, editors. Data Mining and Big Data Vol 10943. Berlin: Springer; 2018. pp. 244–253. [Google Scholar]
- 84.Green CF, Scarpino PV, Gibbs SG. Assessment and modeling of indoor fungal and bacterial bioaerosol concentrations. Aerobiologia (Bologna). 2003;19:159–169. [Google Scholar]
- 85.Goh I, Obbard JP, Viswanathan S, Huang Y. Airborne bacteria and fungal spores in the indoor environment: A case study in Singapore. Acta Biotechnol. 2000;20:67–73. [Google Scholar]
- 86.Park D, et al. Airborne bacteria and fungi associated with waste-handling work. Int. J. Occup. Environ. Health. 2013;19:311–318. doi: 10.1179/2049396713Y.0000000036. [DOI] [PubMed] [Google Scholar]
- 87.Krajewski JA, Tarkowski S, Cyprowski M, Szarapińska-Kwaszewska J, Dudkiewicz B. Occupational exposure to organic dust associated with municipal waste collection and management. Int. J. Occup. Med. Environ. Health. 2002;15:289–301. [PubMed] [Google Scholar]
- 88.Haas D, et al. Comparison of background levels of culturable fungal spore concentrations in indoor and outdoor air in southeastern Austria. Atmos. Environ. 2014;98:640–647. [Google Scholar]
- 89.Patel TY, et al. Variation in airborne fungal spore concentrations among five monitoring locations in a desert urban environment. Environ. Monit. Assess. 2018;190:634. doi: 10.1007/s10661-018-7008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Černá K, Wittlingerová Z, Zimová M, Janovský Z. Methods of sampling airborne fungi in working environments of waste treatment facilities. Int. J. Occup. Med. Environ. Health. 2016;29:493–502. doi: 10.13075/ijomeh.1896.00568. [DOI] [PubMed] [Google Scholar]
- 91.Nadal M, Inza I, Schuhmacher M, Figueras MJ, Domingo JL. Health risks of the occupational exposure to microbiological and chemical pollutants in a municipal waste organic fraction treatment plant. Int. J. Hyg. Environ. Health. 2009;212:661–669. doi: 10.1016/j.ijheh.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 92.Gonçalves FLT, et al. Indoor and outdoor atmospheric fungal spores in the São Paulo metropolitan area (Brazil): Species and numeric concentrations. Int. J. Biometeorol. 2010;54:347–355. doi: 10.1007/s00484-009-0284-6. [DOI] [PubMed] [Google Scholar]
- 93.Emygdio APM, Degobbi C, Gonçalves FLT, de Andrade MF. One year of temporal characterization of fungal spore concentration in São Paulo metropolitan area, Brazil. J. Aerosol Sci. 2018;115:121–132. [Google Scholar]
- 94.Pasquarella C, Pitzurra O, Savino A. The index of microbial air contamination. J. Hosp. Infect. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 95.Ghosh B, Lal H, Srivastava A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ. Int. 2015;85:254–272. doi: 10.1016/j.envint.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mirhoseini SH, Nikaeen M, Satoh K, Makimura K. Assessment of airborne particles in indoor environments: Applicability of particle counting for prediction of bioaerosol concentrations. Aerosol Air Qual. Res. 2016;16:1903–1910. [Google Scholar]
- 97.Zhu H, et al. Experimental study of indoor and outdoor airborne bacterial concentrations in Tempe, Arizona, USA. Aerobiologia (Bologna) 2003;19:201–211. [Google Scholar]
- 98.Aydogdu H, Asan A, Tatman Otkun M. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environ. Monit. Assess. 2010;164:53–66. doi: 10.1007/s10661-009-0874-0. [DOI] [PubMed] [Google Scholar]
- 99.CaboVerde S, et al. Microbiological assessment of indoor air quality at different hospital sites. Res. Microbiol. 2015;166:557–563. doi: 10.1016/j.resmic.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 100.IBGE (Brazilian Institute of Geography and Statistics). Brasil em Síntese. https://cidades.ibge.gov.br/brasil/pr/londrina/panorama (2019).
- 101.Silveira, F. Prefeitura de Londrina diz que Usina de Reciclagem Pode Aumentar a Capacidade de Tratamento do Lixo. https://g1.globo.com/pr/norte-noroeste/noticia/prefeitura-de-londrina-diz-que-usina-de-reciclagem-pode-aumentar-a-capacidade-de-tratamento-do-lixo.ghtml (2017).
- 102.CMTU (Municipal Transit and Urbanization Company). Coleta de Resíduos Recicláveis. https://cmtu.londrina.pr.gov.br/index.php/coleta-reciclavel.html (2019).
- 103.Tshokey T, Somaratne P, Agampodi S. Comparison of two air sampling methods to monitor operating room air quality and assessment of air quality in two operating rooms with different ventilation systems in the national hospital of Sri Lanka. Int. J. Infect. Control. 2016;12:1–8. [Google Scholar]
- 104.Hayleeyesus SF, Ejeso A, Derseh FA. Quantitative assessment of bio-aerosols contamination in indoor air of university dormitory rooms. Int. J. Health Sci. (Qassim) 2016;9:247–254. [PMC free article] [PubMed] [Google Scholar]
- 105.Omelyansky VL. Manual in Microbiology. Moscow: USSR Academy of Sciences; 1940. [Google Scholar]
- 106.Benson HJ. Microbiological Applications: Laboratory Manual in General Microbiology. New York: McGraw-Hill; 2002. [Google Scholar]
- 107.Rodina AG. Methods in Aquatic Microbiology. Baltimore: University Park Press; 1972. [Google Scholar]
- 108.Ellis D, Davis S, Alexiou H, Handke R, Bartley R. Descriptions of Medical Fungi. Adelaide: University of Adelaide; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.