ABSTRACT
Objective:
Previous studies have suggested that the use of an ankle–foot orthosis may cause disuse atrophy of the tibialis anterior muscle. The objective of this study was to explore gait and muscle activity changes in patients in the recovery phase of stroke with 2-month use of an ankle–foot orthosis that provided plantarflexion resistance.
Methods:
The participants were 19 patients in the recovery phase of stroke who were prescribed an ankle–foot orthosis that provided plantarflexion resistance. We measured ankle and shank tilt angles as well as electromyography activity of the tibialis anterior and the soleus during 10-m walk tests. Measurements were taken on three occasions. The first was 2 weeks after delivery of the orthosis, 1 and 2 months after the initial measurement, and the third 2 months later. Changes in gait parameters were analyzed between the first and second measurements and between the second and third measurements.
Results:
Between the second and third measurements, significant increases were observed in plantarflexion and shank forward tilt angles and the activity ratio of the tibialis anterior during loading response compared with other phases.
Conclusions:
Plantarflexion movement induced by an ankle–foot orthosis with plantarflexion resistance could increase the activity ratio of the tibialis anterior during loading response.
Keywords: electromyography, stroke patients, tibialis anterior
INTRODUCTION
Ankle–foot orthoses (AFO) are often used in the rehabilitation of stroke patients. However, some treatment strategies do not include the use of AFOs because of concerns about disuse atrophy of the tibialis anterior (TA) muscle.1,2) There is currently no consensus on this issue because no studies have demonstrated the effect of AFOs on TA activity. In normal gait, the TA is active during swing and loading response. During swing, TA muscle activity lifts the toe to secure foot clearance. During loading response, a force vector is applied to the heel at initial contact, resulting in plantarflexion of the ankle joint. At the same time, eccentric contraction of the TA muscle ensures gradual progression of plantarflexion and shank forward tilt.3) The eccentric contraction of the TA is important for this heel rocker function.
In general, AFOs can immediately improve foot drop during the stance and swing phases.4) Conventionally, AFOs that stop plantarflexion are used to improve gait in patients with stroke by preventing foot drop. However, stopping plantarflexion may inhibit the eccentric contraction of the TA muscle during loading response. In fact, the use of AFOs that stop flexion has been reported to decrease TA muscle activity. Hesse et al. reported that at an average of 34 days after stroke onset, TA muscle activity decreased more from swing to loading response when using an AFO with a plantarflexion stop (AFO-PS) than when not using an AFO. This suggests the possibility of long-term dependence on the AFO induced by disuse atrophy.1) A similar study by Lairamore et al. showed that at an average of 83 days after stroke onset, TA muscle activity during the swing phase decreased more when using a posterior leaf spring AFO (PAFO) than when not using an AFO.2) This supports the findings of Hesse et al. above. In contrast, Nikamp et al. reported that at an average of 30 days after stroke onset, TA muscle activity decreased more during swing when using a PAFO than when not using a PAFO, but there was no significant difference in muscle activity with or without a PAFO after 26 weeks.5)
The AFO-PS and PAFO used in these previous studies may have affected TA muscle activity by stopping plantarflexion during loading response. Recently, AFOs with plantarflexion resistance (AFO-PR) have been developed to aid the eccentric contraction of the TA muscle in the heel rocker.6,7) Because AFO-PRs allow for plantarflexion of the ankle joint in the heel rocker, they may alter TA muscle activity during gait in a different way to that of conventional AFOs.
Lee et al. reported that stroke recovery was relatively rapid during the first 4 weeks after onset, and then slowed between 3 and 6 months.8) Also, Branco et al. demonstrated functional recovery up to at least 24 weeks after acute stroke.9) Six months is considered the recovery phase and improvement of muscle activity is thought to be possible during this phase. It is necessary to investigate whether the use of an AFO that allows for plantarflexion during this period changes the muscle activity of the TA. Furthermore, the change in gait does not have an immediate effect but continuous walking practice is required over a period of time.6,7)
Against this background, in the current study we examined gait and muscle activity changes in patients in the recovery phase of stroke with 2-month use of an AFO-PR. The hypothesis was that AFO-PR use in the recovery phase of stroke would increase TA muscle activity as a result of plantarflexion movement of the ankle joint during loading response, with an accompanying increase in shank forward tilt.
MATERIALS AND METHODS
Participants
Patients with stroke have a diverse range of gaits, and differences in gait pattern can be assumed to significantly influence measurements. In this study, participants were selected by considering items that are expected to affect gait pattern. Also considered were the number of days from stroke onset and length of hospital stay.
The inclusion criteria of this study were patients with the first occurrence of stroke, patients in the recovery phase (within 90 days of onset of stroke), prescription of an AFO-PR between fiscal year 2017 and fiscal year 2019, lower limb Brunnstrom stage III or IV after completion of AFO use, the ability for the paralyzed foot and cane to contact the ground at the same time and for the nonparalyzed foot to make contact in front of the paralyzed foot (two-point gait with a cane on one side), and the extension thrust pattern was determined at the initial assessment for AFO use.10)
The extension thrust pattern was targeted based on a report by Yamamoto et al. demonstrating that AFO-PR use is delayed at the start of the plantarflexion moment during loading response. This function allows the gradual inclination of the shank in early midstance and results in reduced hyperextension of the knee joint in late midstance.11) Therefore, AFO-PRs are prescribed for patients with the extension thrust pattern, both generally and at rehabilitation facilities. The exclusion criteria were a planned hospital stay of less than 3 months and excessive equinovarus
This study was conducted in accordance with the Helsinki Declaration with approval from both the Ethics Review Committee of the International University of Health and Welfare Graduate School (Approval number 17-Ig-52) and the Ethics Committee of the Funabashi Municipal Rehabilitation Hospital (Approval number H29-17), where the participants were admitted. Participants received oral and written explanations of the aim and method of the study, and informed consent was obtained from all individual participants included in the study.
Ankle–Foot Orthosis Used in This Study
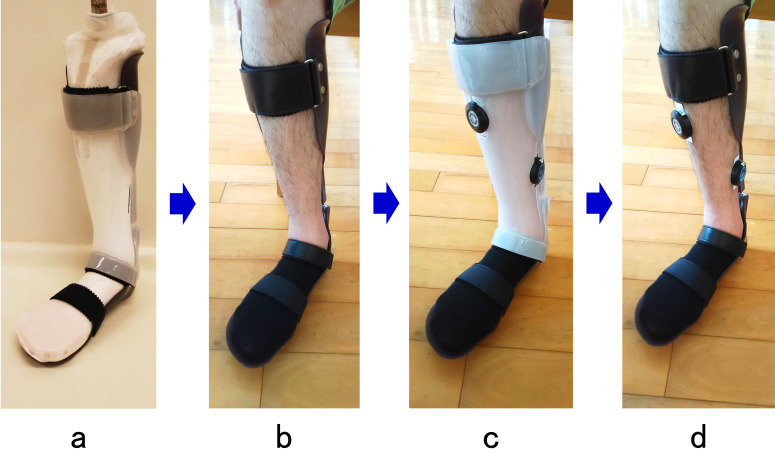
The AFO-PR used by the participants in this study (Ankle Joint Orthosis; Obara Kogyo, Funabashi, Japan: Fig. 1) is frequently prescribed at the participating facilities. Individual AFO-PRs were made for each participant using a bespoke 4-mm polypropylene sheet. The orthosis allows free dorsiflexion, and the initial dorsiflexion angle and plantarflexion resistance force can set between 0° to 9° and 0.5 to 2 Nm/degree, respectively. The settings for the initial dorsiflexion angle and plantarflexion resistance force of the AFO-PR were determined as deemed appropriate by physical therapists assigned to the participants and an orthotist who fitted the AFO, both immediately after completion of the AFO and also during the subsequent evaluation period. The initial dorsiflexion angle and the plantarflexion resistance force were adjusted based on observational gait analysis, considering the foot-to-floor contact angle and knee stability in early stance, which are visual indicators of ankle and knee kinematics.12)
Fig. 1.
Appearance of the AFO-PR and the parts used for adjusting the initial dorsiflexion angle and plantarflexion resistance. The initial dorsiflexion angle of the orthosis can be set to 0°, 3°, 6°, or 9° by changing the duralumin spacers, which come in four different thicknesses. The plantarflexion resistance force can be set to 0.5, 1.0, 1.5, or 2.0 Nm/degree by changing the urethane rubber parts, which come in four different levels of hardness.
Study Protocol
We took measurements at three different times. The first set of measurements was taken 2 weeks after the AFO was prescribed, which allowed the participants adequate time to become accustomed to using the AFO and to exclude any changes in gait pattern during this initial AFO testing phase. Subsequent measurements were taken 1 and 2 months later. A measurement period of 2 months was chosen because patients with stroke who are transferred from an acute hospital to the rehabilitation facility will stay there for 3 months on average, and their AFO will be prescribed a little less than a month after admission to the rehabilitation hospital. The total period of hospitalization is generally about 90 days, so we determined that taking three measurements during a 2-month stay in a recovery-phase ward would be appropriate.
The clinical parameters analyzed were the Functional Independence Measure (FIM),13) Berg Balance Scale (BBS),14) and Stroke Impairment Assessment Set (SIAS)15) scores obtained during regular assessments taken closest to the time of gait measurements. The SIAS foot pad test was used to focus on the dorsiflexion capacity of the ankle joint.
For the gait measurements, participants completed a 10-m walk test (10MWT)16) at a comfortable pace using a cane while wearing their own shoes and their own AFO-PR under the supervision of physiotherapists. A single measurement was taken after two or three practice walks to reduce physical and time burdens on participants. Measurements were repeated if an error occurred during data transmission from the measurement sensors or if the participant tripped or otherwise failed to walk continuously during the 10MWT. Walking without an AFO was not analyzed in this study because most participants could not walk safely without an AFO.
Outcome Measures and Measurement System
The following gait analysis parameters were measured: walking speed and stride (calculated from the walking time and number of steps during the 10MWT), cadence, shank forward and backward tilt angles (shank tilt angles), ankle joint angle, and electromyographic (EMG) activity of the TA and soleus (SOL) muscles.
To determine the shank tilt angles, the ankle joint angle, and EMG activity, we used a gait analyzer (Gait Judge System, Pacific Supply, Osaka, Japan) with a sampling frequency of 1000 Hz. A USB accelerometer was used for measuring the shank tilt angles, and a potentiometer was used for measuring the ankle joint angle. These devices were affixed to the main body of the AFO using a mounting jig that we manufactured specially (Fig. 2). To ensure consistent positioning of the EMG sensors, we made an EMG sensor positioning jig based on a positive model of the shank cast. The sensors were made of soft plastic and their positions were fixed for all three measurements (Fig. 3). An EMG sensor positioning jig was made for each participant, and the sensor positions were determined according to the SENIAM guidelines.17) EMG signals were recorded at a sampling rate of 1000 Hz and then high-pass filtered at 20 Hz and low-pass filtered at 2500 Hz to remove artefacts. Root-mean-square values for 50-ms intervals were calculated. Skin exfoliation and impedance checks were not performed to minimize the time burden. Foot switches were attached to the heels of the left and right soles as well as the midpoint of the 1st and 5th toe MP joints (Pressure sensor Flexi Force; Tekscan, South Boston, MA, USA). Foot switches were used to determine the start of the gait cycles during measurement. The position of the foot switch meant that the measured timing of toe-off could be earlier than the actual timing. However, heel rise was not observed during stance, so the time difference was not considered to be large.
Fig. 2.
USB accelerometer and potentiometer affixed to the main body of the AFO-PR using a mounting jig.
Fig. 3.
EMG sensor positioning jig and procedure. (a) Positive model and a jig. (b) AFO-PR worn by a patient, (c) ankle–foot orthosis with jig and attached sensors, (d) AFO-PR without the jig worn by the patient.
Data Processing
The data obtained during the 10MWT (between 8 and 17 gait cycles) were split into gait cycles based on the initial contact on the paretic side, as determined by the foot switches. The measured data were then averaged over the gait cycles. For the shank tilt angle, the angular velocity of the shank was automatically calculated using the gait analyzer software based on the accelerometer data from the USB accelerometer affixed to the shank. We then calculated the amount of displacement by taking the initial contact as 0°. For the ankle joint angle, we measured the initial contact as 0° and then calculated the subsequent angular displacement from the potentiometer data.
For the TA and SOL EMG activity and the ankle joint angle and shank tilt angle, a single gait cycle was separated into phases as follows: loading response on the paretic side, single support, pre-swing, and swing. For the ankle joint angle and shank tilt angle, we calculated the amount of displacement during each gait cycle phase. The EMG level is the average of the root-mean-square value obtained every 50 ms in each phase. Furthermore, the EMG activity was calculated by taking the average value during each phase based on %EMG activity normalized using the average values for a single gait cycle. Note that the average of each phase of a single gait cycle for EMG data is usually normalized to 100% of the EMG at maximum voluntary contraction. However, for patients with stroke, maximum voluntary contraction cannot be obtained because of problems such as spasticity and involuntary contractions, so we normalized the EMG data by taking the average values for a single gait cycle as 100%, as was done in a previous study.18) Because actual EMG levels are easily affected by the positioning of the EMG sensors, we used %EMG in this study, which can detect relative increases and decreases in muscle activity regardless of the actual level of muscle activity. Because the mean value of a single gait cycle was set to 100% and the EMG data were normalized, absolute comparisons cannot be made, but comparisons were done as a percentage of each phase of the gait cycle.
Statistical Analysis
The Shapiro–Wilk test was used to test the normality of the data (significance level P<0.05). Note that three measurement datasets were obtained for each of the 22 parameters examined (i.e., at each measurement time point). Friedman’s test was used for analysis if one or more of the datasets was not normally distributed. Otherwise, one-way analysis of variance was used. Bonferroni’s multiple comparison test was performed for parameters found to be significantly different by one-way analysis of variance or Friedman’s test at each time point (significance level P<0.05).19) All statistical analyses were performed using SPSS Statistics software version 25 (IBM, Armonk, NY, USA).
RESULTS
Table 1 shows the basic characteristics of participants and the results of the evaluation items. The participants were 19 patients with stroke and ranged in age from 36 to 84 years. The lesion sites were the putamen (7 participants), thalamus (5), putamen and thalamus (1), corona radiata (3), middle cerebral artery area (2), and medulla oblongata (1). For some patients, the plantarflexion resistance force setting was reduced during the study period. There were no participants whose resistance setting was increased or who could walk without an AFO. Therefore, we adjudged that all participants met the inclusion criteria throughout the study period.
Table 1. Participant characteristics and results for evaluated parameters at the first measurement, n=19.
| Item | Average (SD) or number of participants |
| Age | 59.3 (±14.0) years |
| Sex | Men 12 ∙ Women 7 |
| Paralyzed side | Right 12 ∙ Left 7 |
| Stroke type | Hemorrhagic 13 ∙ Infarction 6 |
| Brunnstrom stage | III 12 ∙ IV 7 |
| Raven’s Colored Progressive Matrices | 30.6 (±3.6) (Perfect score 36) |
| FIM | 93.9 (±18.3) (Perfect score 126) |
| BBS | 42.5 (±7.8) (Perfect score 76) |
| SIAS | 40.7 (±14.9) (Perfect score 56) |
| Stroke onset to delivery of AFO | 55.8 (±17.4) days |
FIM, Functional Independence Measure; BBS, Berg Balance Scale; SIAS, Stroke Impairment Assessment Set.
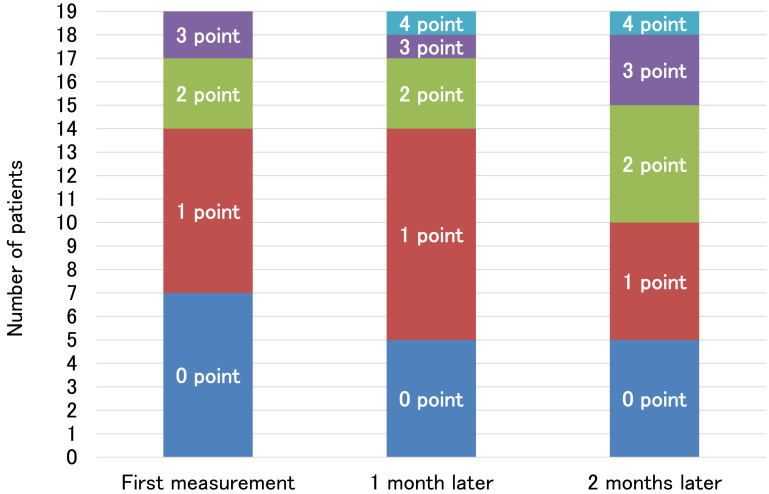
Figure 4 shows the SIAS foot pad test results for all 19 participants at first measurement, 1 month later, and 2 months later. These results show that the number of participants able to perform voluntary dorsiflexion, albeit inadequately, increased between the first and second months after the first measurement.
Fig. 4.
Results of the SIAS foot pad test.
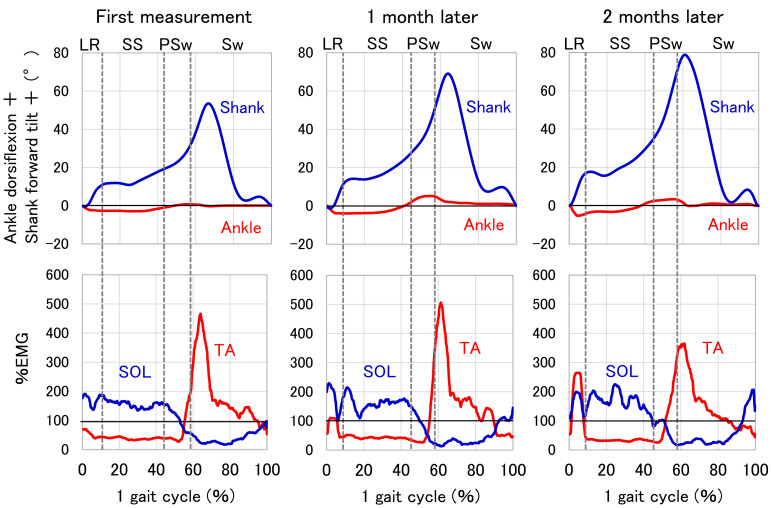
Figure 5 shows typical findings for angles and %EMGs throughout one gait cycle at first measurement, 1 month later, and 2 months later. During loading response, the shank forward tilt angle and %EMG of the TA show the greatest increase between the first and second months after the first measurement.
Fig. 5.
Typical examples of angles and EMG measurements. The angles were calculated relative to the value at initial contact. The %EMG is relative to the average of each trial. LR, loading response; SS, single support; PSw, pre-swing; Sw, swing; SOL, soleus muscle; TA, tibialis anterior.
Table 2 shows the measurement results for all 19 participants and Table 3 shows the percentage change between the first measurement and the measurement taken 1 month later (first to 1 month) and between the measurements taken 1 month later and 2 months later (1 month to 2 months).
Table 2. Participants' measurement results at first measurement, 1 month later, and 2 months later.
| Item | Unit | Time | Average | SD | Median | IR | P-value | |
| FIM total score | Points | First† | 105 | 24 | ||||
| 1 month† | 111 | 23 | 0.001 | |||||
| 2 months† | 114 | 14.5 | ||||||
| SIAS total score | Points | First | 42.5 | 7.9 | ||||
| 1 month | 43.9 | 7.9 | 0.001 | |||||
| 2 months | 45.1 | 7.8 | ||||||
| BBS total score | Points | First | 40.7 | 7.8 | ||||
| 1 month | 46.6 | 6.7 | 0.001 | |||||
| 2 months | 48.7 | 6.2 | ||||||
| Walking speed | m/s | First | 0.5 | 0.2 | ||||
| 1 month | 0.6 | 0.2 | 0.001 | |||||
| 2 months | 0.8 | 0.2 | ||||||
| Stride (normalized by height) |
% | First | 0.5 | 0.1 | ||||
| 1 month | 0.6 | 0.1 | 0.001 | |||||
| 2 months | 0.6 | 0.1 | ||||||
| Cadence | Steps/min | First | 72.9 | 17.8 | ||||
| 1 month | 81.5 | 15 | 0.001 | |||||
| 2 months | 90 | 15.8 | ||||||
| Ankle joint angle (amount of displacement +dorsiflexion) |
LR | Degree | First | –1.9 | 1.7 | |||
| 1 month | –2.3 | 1.7 | 0.001 | |||||
| 2 months | –2.8 | 1.6 | ||||||
| SS | First | 2 | 1.4 | |||||
| 1 month | 3.4 | 2 | 0.001 | |||||
| 2 months | 4.4 | 2.3 | ||||||
| PSw | First | 1.1 | 2.1 | |||||
| 1 month | 1.5 | 1.9 | 0.627 | |||||
| 2 months | 1.1 | 1.8 | ||||||
| Sw | First† | –1 | 1.5 | |||||
| 1 month† | –1.7 | 3.3 | 0.004 | |||||
| 2 months† | –2.9 | 2.8 | ||||||
| Shank tilt angle (amount of displacement +forward tilt) |
LR | Degree | First | 10.7 | 3.5 | |||
| 1 month | 11.3 | 4 | 0.001 | |||||
| 2 months | 13 | 3.8 | ||||||
| SS | First | 5.4 | 4.7 | |||||
| 1 month | 8.4 | 5.2 | 0.001 | |||||
| 2 months | 12.8 | 6.2 | ||||||
| PSw | First | 17.2 | 6.3 | |||||
| 1 month | 18 | 4.6 | 0.796 | |||||
| 2 months | 17.9 | 4.8 | ||||||
| Sw | First† | –37.8 | 16 | |||||
| 1 month† | –45.1 | 16.4 | 0.001 | |||||
| 2 months† | –54.2 | 22 | ||||||
| TA %EMG (average of each phase) |
LR | % | First | 98.7 | 29.4 | |||
| 1 month | 102.7 | 30.9 | 0.001 | |||||
| 2 months | 142.4 | 41.8 | ||||||
| SS | First | 75.3 | 32.6 | |||||
| 1 month | 68.2 | 34.4 | 0.489 | |||||
| 2 months | 75.1 | 31 | ||||||
| PSw | First† | 97.8 | 37.5 | |||||
| 1 month† | 99.7 | 28.1 | 0.810 | |||||
| 2 months† | 95.5 | 39 | ||||||
| Sw | First† | 140.5 | 48.2 | |||||
| 1 month† | 134.2 | 47.3 | 0.001 | |||||
| 2 months† | 124.1 | 48.5 | ||||||
| SOL %EMG (average of each phase) |
LR | % | First | 164.4 | 28.2 | |||
| 1 month | 163.8 | 29.2 | 0.586 | |||||
| 2 months | 159.1 | 22.9 | ||||||
| SS | First | 146.1 | 21 | |||||
| 1 month | 149.4 | 20 | 0.687 | |||||
| 2 months | 147.3 | 26.9 | ||||||
| PSw | First† | 95.5 | 30.5 | |||||
| 1 month† | 88.2 | 45 | 0.532 | |||||
| 2 months† | 86.9 | 33.6 | ||||||
| Sw | First | 57.8 | 13.9 | |||||
| 1 month | 55.7 | 15.2 | 0.575 | |||||
| 2 months | 58.8 | 15.3 | ||||||
†Nonparametric data; P-value, result of one way-analysis of variance or Friedman analysis. SD, standard deviation; IR, interquartile range.
Table 3. Percentage change between the first measurement and the measurement taken 1 month later and between the measurements taken 1 month later and 2 months later.
| Item | First to 1 month change rate (%) | 1 month to 2 months change rate (%) | |
| FIM total score | 5.7* | 2.7* | |
| SIAS total score | 3.3* | 2.7* | |
| BBS total score | 14.5* | 4.5* | |
| Walking speed | 23.5* | 20.6* | |
| Stride normalized by height | 9.8* | 8.9* | |
| Cadence | 11.8* | 10.4* | |
| Ankle joint angle (amount of displacement +dorsiflexion) | LR | 21.1* | 21.7* |
| SS | 70.0* | 29.4* | |
| PSw | 36.4 | –26.7 | |
| Sw | 70.0* | 70.6 | |
| Shank tilt angle (amount of displacement +forward tilt) | LR | 5.6 | 15.0* |
| SS | 55.6* | 52.4* | |
| PSw | 4.7 | –0.6 | |
| Sw | 19.3* | 20.2* | |
| TA %EMG (average of each phase) | LR | 4.1 | 38.7* |
| SS | –9.4 | 10.1 | |
| PSw | 1.9 | –4.2 | |
| Sw | –4.5 | –7.5* | |
| SOL %EMG (average of each phase) | LR | –0.4 | –2.9 |
| SS | 2.3 | –1.4 | |
| PSw | –7.6 | –1.5 | |
| Sw | –3.6 | 5.6 | |
First to 1 month change rate (%)=(1 month − first)/ first × 100; 1 month to 2 months change rate (%)=(2 months − 1 month)/1 month × 100.
*Significant difference according to Bonferroni's multiple comparison test (P <0.05).
FIM, BBS, SIAS, walking speed, stride, and cadence all showed significant increases over time for all combinations of time points (i.e., first to 1 month, first to 2 months, and 1 month to 2 months). Moreover, the results showed a nonlinear recovery trend, whereby the 1 month to 2 months increases were less than the first to 1 month increases.
For the displacement in the ankle joint angle and shank tilt angles in each phase of the gait cycle, the trends for the first measurement values and those at 1 month and 2 months later were as follows (Table 3). For the ankle joint angle, the plantarflexion angle during loading response, the dorsiflexion angle during single support, and the plantarflexion angle during swing increased significantly from first to 1 month, whereas, from 1 month to 2 months, only the plantarflexion angle during loading response and the dorsiflexion angle during single support increased. For the shank tilt angle, the forward tilt angle during single support and the backward tilt angle during swing increased significantly from first to 1 month, whereas, from 1 month to 2 months, the forward tilt angle during loading response, the forward tilt angle during single support, and the backward tilt angle during swing increased significantly. TA %EMG activity increased significantly during loading response compared with the other phases and decreased significantly during swing compared with the other phases from 1 month to 2 months. SOL %EMG activity showed no significant changes over the course of the study period. Further long-term effects could not be demonstrated because the AFO-PR was used for only 2 months.
DISCUSSION
The most important result of this study is that, contrary to previous studies, the use of an AFO-PR for 2 months significantly increased the TA %EMG activity during loading response compared with the other phases. The participants in this study showed less improvement in all time/distance factors and clinical evaluation parameters from 1 month to 2 months compared with that from first to 1 month. This is consistent with the nonlinear recovery process reported by Lee et al. and Branco et al., suggesting that this may be a general recovery trend.8,9)
Patients using an AFO-PR for 2 months during the recovery phase of stroke showed significant increases in ankle joint plantarflexion angle, shank forward tilt angle, and TA %EMG activity during loading response. Increases were observed in ankle joint dorsiflexion angle and in shank forward tilt angle during single support and in shank backward tilt angle during swing; however, a significant decrease in TA %EMG activity was noted during swing.
Hesse et al. reported that TA muscle activity decreased from the swing phase to the loading response, whereas Lairamore et al. reported a decrease during the swing phase only.1,2) The results of our study showed a significant decrease in TA %EMG activity during swing from 1 month to 2 months, consistent with the trend reported by Lairamore et al. During swing, even if TA muscle activity is inadequate because of the plantarflexion resistance of the AFO, TA muscle activity may decrease as a result of continued use of the AFO-PR because plantarflexion may be constrained. However, during loading response, TA %EMG activity increased, in contrast to the report by Hesse et al. However, neither of these two reports made before-and-after comparisons of muscle activity changes in the same participant.
Nikamp et al. reported made before-and-after comparisons of muscle activity changes in the same participant. Nikamp et al. reported that at an average of 30 days after stroke onset, TA muscle activity decreased more during swing when using a PAFO than when not using a PAFO, but there was no significant difference in muscle activity with or without a PAFO after 26 weeks.5) However, we found that in patients with stroke, 2-month use of an AFO-PR that allows plantarflexion of the ankle joint during loading response resulted in significant increases in TA %EMG activity during loading response compared with the other phases.
According to Swayne et al., intracortical excitability increases 3 months after stroke onset, and the cortical network is reorganized to maximize the efficiency of the remaining corticospinal tract as part of the recovery process for motor paralysis.20) Kamibayashi et al. reported that the excitability of the corticospinal tract to the TA increases compared with that of the rectus femoris, biceps femoris, and SOL as a result of robot-assisted passive stepping because of load-related afferent sensory inputs.21) With each gait cycle, the ankle joint plantarflexes during loading response, thereby passively extending the TA so that the excitability of the corticospinal tract and intracortical network increases as a result of the repeated afferent sensory inputs, and this increased excitability can be expected to facilitate muscle activity in the TA. However, continued AFO-PR use did not alter the pattern of SOL muscle activity, suggesting that the TA is more susceptible to continued use of an AFO-PR than the SOL is.
The ankle joint plantarflexion angle and the shank forward tilt angle increased during loading response from 1 month to 2 months. The increase in shank forward tilt angle may be a consequence of the increased TA muscle activity ratio (due to ankle joint plantarflexion during loading response with each repeated step) drawing the shank forward. The patients in this study were in the recovery phase of stroke and used an AFO-PR for 2 months; as a result, increases were observed in ankle joint plantarflexion angle and shank forward tilt angle during loading response from 1 month to 2 months, as well as an increase in TA %EMG activity. These results support our hypothesis that the use of an AFO-PR in patients in the recovery phase of stroke increases TA muscle activity because of plantarflexion movement of the ankle joint during loading response, with an accompanying increase in shank forward tilt. Therefore, plantarflexion movement induced by the AFO-PR increases the TA muscle activity ratio during loading response compared with the other phases.
In contrast to the other phases, TA %EMG activity during swing decreased from 1 month to 2 months. However, because the foot pad test showed an increase in the number of participants capable of voluntary dorsiflexion, it is unlikely that the TA was suffering from disuse. We speculate that the observed decrease in the TA activity ratio during the swing phase occurred because, in the current study, the EMG activity of each phase was normalized by the averaged EMG value of the whole gait cycle. Data on the absolute activity were not available, but the obtained data showed the relative activity in each phase. The results showed that the TA activity ratio during loading response significantly increased between 1 and 2 months after the first measurement. This increase induced the decrease in the TA activity ratio during swing phase.
There are some limitations to this study. The items analyzed consisted of TA and SOL muscle activity, as well as kinematic items limited to the ankle joint angle and the shank tilt angle; these items are less objective than three-dimensional (3D) motion data. The gait analyzer used in this study could not measure angles in the gait cycle as time-series data, so the angular displacement over a certain time period was analyzed instead. In gait analysis, measurement using a 3D motion analyzer is the gold standard, but it was thought that there was little need to consider the joint motion in the frontal and horizontal planes because the participants had either no equinovarus or only mild equinovarus. Therefore, we measured the shank tilt angle and the ankle joint angle in the sagittal plane, which can be measured using a highly versatile and portable measuring instrument that can be easily used even in facilities without a 3D motion analyzer. In addition, this study had only 19 participants, which is not sufficient to ensure the universality of the results. We did not perform a power calculation to determine the sample size. Because of the limited number of patients included in the study, we focused on the actual number of participants rather than the sample size. Finally, the study involved only patients using an AFO-PR, so measurement comparisons were not made against controls.
In conclusion, patients using an AFO-PR for 2 months during the recovery phase of stroke showed significant improvements in FIM, BBS, SIAS, walking speed, stride, and cadence as well as in ankle joint plantarflexion angle, shank forward tilt angle, and TA %EMG activity during loading response. The ankle joint dorsiflexion angle and the shank forward tilt angle during single support and the shank backward tilt angle during swing all increased; however, a significant decrease in TA %EMG activity was noted during swing. Therefore, we found that the plantarflexion movement allowed by an AFO with plantarflexion resistance could increase the TA muscle activity ratio during loading response. Further study including long-term AFO-PR use is required to verify the effect of the AFO-PR on the gait of patients in the recovery phase of stroke.
ACKNOWLEDGMENTS
We would like to thank all the participants in this study and the physical therapists at the target facilities. This article has the same content as a doctoral dissertation submitted to the International University of Health and Welfare Graduate School in 2019. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Both authors contributed equally in the preparation of this manuscript.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Hesse S,Werner C,Matthias K,Stephen K,Berteanu M: Non-velocity-related effects of a rigid double-stopped ankle–foot orthosis on gait and lower limb muscle activity of hemiparetic subjects with an equinovarus deformity. Stroke 1999;30:1855–1861. 10.1161/01.STR.30.9.1855 [DOI] [PubMed] [Google Scholar]
- 2.Lairamore C,Garrison MK,Bandy W,Zabel R: Comparison of tibialis anterior muscle electromyography, ankle angle, and velocity when individuals post stroke walk with different orthoses. Prosthet Orthot Int 2011;35:402–410. 10.1177/0309364611417040 [DOI] [PubMed] [Google Scholar]
- 3.Perry J,Burnfield JM: Gait Analysis: Normal and Pathological Function. SLACK Incorporated, Thorofare, New Jersey, 1992. pp. 19–47. [Google Scholar]
- 4.Daryabor A,Arazpour M,Aminian G: Effect of different designs of ankle–foot orthoses on gait in patients with stroke: A systematic review. Gait Posture 2018;62:268–279. 10.1016/j.gaitpost.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 5.Nikamp C,Buurke J,Schaake L,Palen J,Rietman J,Hermens H: Effect of long-term use of ankle–foot orthoses on tibialis anterior muscle electromyography in patients with sub-acute stroke: a randomized controlled trial. J Rehabil Med 2019;51:11–17. 10.2340/16501977-2498 [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto S,Fuchi M,Yasui T: Change of rocker function in the gait of stroke patients using an ankle foot orthosis with an oil damper: immediate changes and the short-term effects. Prosthet Orthot Int 2011;35:350–359. 10.1177/0309364611420200 [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S,Tanaka S,Motojima N: Comparison of ankle–foot orthoses with plantar flexion stop and plantar flexion resistance in the gait of stroke patients: a randomized controlled trial. Prosthet Orthot Int 2018;42:544–553. 10.1177/0309364618774055 [DOI] [PubMed] [Google Scholar]
- 8.Lee KB,Lim SH,Kim KH,Kim KJ,Kim YR,Chang WN,Yeom JW,Kim YD,Hwang BY: Six-month functional recovery of stroke patients. Int J Rehabil Res 2015;38:173–180. 10.1097/MRR.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branco JP,Oliveira S,Sargento-Freitas J,Laíns J,Pinheiro J: Assessing functional recovery in the first six months after acute ischemic stroke: a prospective, observational study. Eur J Phys Rehabil Med 2019;55:1–7. 10.23736/S1973-9087.18.05161-4 [DOI] [PubMed] [Google Scholar]
- 10.Kramers De Quervain IA,Simon SR,Leurgans S,Pease WS,McAllister DA: Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am 1996;78:1506–1514. 10.2106/00004623-199610000-00008 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S,Katsuhira J,Miura N: Gait change using an ankle–foot orthosis with an oil damper in a stroke patient with hyperextension of the knee joint in late stance. Int J Phys Med Rehabil 2015;3:262. [Google Scholar]
- 12.Kobayashi T,Orendurff MS,Hunt G,Lincoln LS,Gao F,LeCursi N,Foreman KB: An articulated ankle–foot orthosis with adjustable plantarflexion resistance, dorsiflexion resistance and alignment: a pilot study on mechanical properties and effects on stroke hemiparetic gait. Med Eng Phys 2017;44:94–101. 10.1016/j.medengphy.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodds TA,Martin DP,Stolov WC,Deyo RA: A validation of the Functional Independence Measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil 1993;74:531–536. 10.1016/0003-9993(93)90119-U [DOI] [PubMed] [Google Scholar]
- 14.Berg KO,Maki BE,Williams JI,Holliday PJ,Wood-Dauphinee SL: Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil 1992;73:1073–1080. [PubMed] [Google Scholar]
- 15.Liu M,Chino N,Tuji T,Masakado Y,Hase K,Kimura A: Psychometric properties of the Stroke Impairment Assessment Set (SIAS). Neurorehabil Neural Repair 2002;16:339–351. 10.1177/0888439002239279 [DOI] [PubMed] [Google Scholar]
- 16.Vos-Vromans DC,de Bie RA,Erdmann PG,van Meeteren NL: The responsiveness of the ten-meter walking test and other measures in patients with hemiparesis in the acute phase. Physiother Theory Pract 2005;21:173–180. 10.1080/09593980500212920 [DOI] [PubMed] [Google Scholar]
- 17.Hermens HJ,Freriks B,Disselhorst-Klug C,Rau G: Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10:361–374. 10.1016/S1050-6411(00)00027-4 [DOI] [PubMed] [Google Scholar]
- 18.Yang JF,Winter DA: Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil 1984;65:517–521. [PubMed] [Google Scholar]
- 19.Bavdek R,Zdolšek A,Strojnik V,Dolenec A: Peroneal muscle activity during different types of walking. J Foot Ankle Res 2018;11:50. 10.1186/s13047-018-0291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swayne OB,Rothwell JC,Ward NS,Greenwood RJ: Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex 2008;18:1909–1922. 10.1093/cercor/bhm218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamibayashi K,Nakajima T,Takahashi M,Akai M,Nakazawa K: Facilitation of corticospinal excitability in the tibialis anterior muscle during robot-assisted passive stepping in humans. Eur J Neurosci 2009;30:100–109. 10.1111/j.1460-9568.2009.06795.x [DOI] [PubMed] [Google Scholar]