Abstract
Gut microbiota have been emerging as important contributors to the regulation of host homeostasis. Accordingly, several substances converted by gut microbiota can have beneficial or adverse effects on human health. Among them, S-equol, which is produced from the isoflavone daidzein in the human and animal gut by certain microbiota, exerts estrogenic and antioxidant activities. Indoxyl sulfate, which is metabolized in the liver from indole converted from dietary tryptophan by bacterial tryptophanases in the colon, is known as a protein-bound uremic toxin. Trimethylamine N-oxide, which is generated via the oxidization of gut microbiota-derived trimethylamine by hepatic flavin monooxygenases, is known as an accelerator of atherosclerosis. The aforementioned gut-derived substances could be potential regulators of systematic tissue/organ function, including the vascular system. Macro- and microvascular complications of cardiovascular and metabolic diseases, including atherosclerosis, hypertension, and diabetes, occur systemically and represent the principal cause of morbidity and mortality. Vascular endothelial and smooth muscle dysfunction play pivotal roles in the development and progression of vasculopathies. We herein review the link between the aforementioned gut-derived substances and endothelial and vascular smooth muscle cell function. This information will provide a conceptual framework that would allow the development of novel preventive and/or therapeutic approaches against vasculopathies.
Keywords: blood pressure, endothelium, hypertension, indoxyl sulfate, S-equol, TMAO, vascular smooth muscle
Vascular dysfunction is undoubtedly associated with the onset and maintenance of hypertension, as well as with the initiation and development of vascular complications related to several chronic diseases, including diabetes, hypertension, and atherosclerosis.1–6 Blood vessels contain two primary cell types, endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), both of which exert an essential functions in sustaining vascular homeostasis.7
ECs constantly generate a number of vasoactive and trophic substances that regulate inflammation, VSMC growth, platelet function, plasmatic coagulation, and vasomotion under normal conditions.8,9 ECs play a pivotal role in vascular tone regulation by generating and releasing several factors, including endothelium-derived relaxing factors (EDRFs) and contracting factors.9–12 Among them, three EDRFs, including nitric oxide (NO), endothelium-derived hyperpolarizing factor (EDHF), and prostacyclin, and several endothelium-derived contracting factors, including angiotensin II, endothelin-1, vasoconstrictor prostanoids, and uridine adenosine tetraphosphate, have been well known.9–13 During aging and/or in several disease states, including hypertension, diabetes, hypercholesterolemia, and atherosclerosis, an imbalance between EDRFs and endothelium-derived contracting factors levels has been observed in different vasculatures.8–10 Therefore, manipulating the balance between endothelium-derived factors is an important strategy for preventing the initiation and development of vascular dysfunction and complications.
VSMCs are another major cell type that forms the blood vessels. Distinct from other mature cell types throughout the body, VSMCs do not terminally differentiate but maintain a remarkable plasticity.4–6,14 Fully differentiated medial VSMCs of mature blood vessels retain quiescence and express various genes and proteins related to important components that regulate contraction and relaxation, allowing them to regulate systemic and local blood pressure via vascular tone control.4,6,14 In response to vascular injury or changes in local environmental cues, differentiated/contractile VSMCs are capable of switching to a dedifferentiated phenotype characterized by increased proliferation, migration, and extracellular matrix synthesis consistent with the reduced expression of contractile markers.4–6,14 Given the key role of VSMC dysfunction in the remodeling process during the development of vascular diseases,4–6,14,15 determining causative factors and molecular mechanisms underlying abnormal proliferation, migration, apoptosis, senescence, and calcification in VSMCs is critical for a comprehensive understanding on the initiation and development of vascular dysfunction and for the establishment of therapies and preventive strategies against vascular diseases.
A growing body of evidence has suggested a relationship between gut microbiota and several cardiovascular and metabolic diseases.16–24 A number of substances derived from the gut microbiome, microbial metabolites, and bacterial structural components have been found to affect host homeostasis. Given the adverse or beneficial effects of such substances on many physiological functions, controlling gut dysbiosis, defined as deleterious changes to the composition or number of gut bacteria, has been an important strategy against the development and/or progression of numerous diet-related diseases, including cardiovascular diseases.16–24
The present review summarizes some of the experimental and clinical evidence indicating that gut-derived substances can affect vascular function, especially focusing on the relationship between cellular function and three substances, S-equol, indoxyl sulfate, and trimethylamine N-oxide (TMAO), in ECs and VSMCs.
S-EQUOL
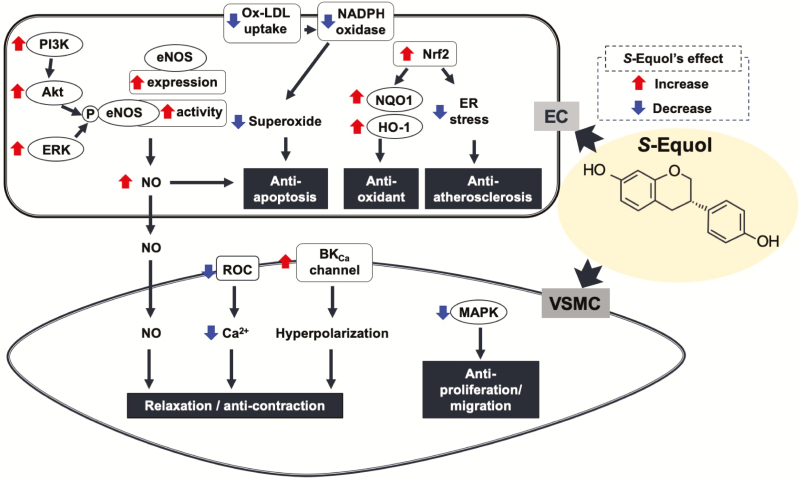
Equol [7-hydroxyl-3-(4-hydroxyphenyl)chroman] is produced from soy isoflavone daidzein in human and animal gut by certain bacterial biotypes that can across individuals.25,26 A number of studies have suggested that S-equol is responsible for the metabolic and cardiovascular benefits of soy.27–29 Considerable evidence has suggested that S-equol can affect several phenomena in not only ECs but also VSMCs (Figure 1).
Figure 1.
Effects of S-equol on vascular endothelial and smooth muscle cells. S-Equol has several beneficial effects, including anti-apoptosis, anti-oxidation, and anti-atherosclerosis; production of nitric oxide in endothelial cells; anti-proliferation and/or migration; and promotion of vascular smooth muscle cell relaxation. Details are provided in the text. Abbreviations: BKCa, large-conductance calcium-activated potassium channel; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; HO-1, heme oxygenase-1; MAPK, mitogen-activated protein kinase; NO, nitric oxide; Nrf2, NF-E2-related factor 2; NQO1, NAD(P)H:quinone oxidoreductase 1; Ox-LDL, oxidized low-density lipoprotein; PI3K, phosphoinositide 3-kinase; ROC, receptor-operated calcium channel; VSMC, vascular smooth muscle cell.
In VSMCs, S-equol inhibited the proliferation, collagen, and total protein syntheses, migration, and mitogen-activated protein kinase activity of human aortic smooth muscle cells (HASMCs) in a concentration-dependent manner,30 suggesting that S-equol may confer protective effects on the vascular system by inhibiting vascular remodeling and neointima formation.
In ECs, S-equol suppresses oxidized low-density lipoprotein-induced apoptosis via decreased superoxide production by nicotinamide adenine dinucleotide phosphate oxidase and increased NO production in human umbilical vein ECs (HUVECs)31 and inhibits H2O2-induced apoptosis by reducing intracellular reactive oxygen species (ROS) generation and increasing the expression of phosphorylated-p38 mitogen-activated protein kinase and Bcl-2 in bovine aortic ECs.32 These findings suggest that S-equol exerts antiapoptotic effects in ECs. Another study showed that S-equol acutely activates endothelial NO release at basal cytosolic Ca2+ levels by activating extracellular signal-regulated kinase (ERK) 1/2 and Akt independent of classic estrogen receptor (ER) signaling.33 Inhibiting mitochondrial ROS abolished S-equol-mediated activation of Akt, ERK1/2, endothelial NO synthase (eNOS) phosphorylation, and NO production, as well as the relationship between S-equol-stimulated mitochondrial ROS generation and epidermal growth factor receptor kinase transactivation and F-actin cytoskeleton reorganization.34 Hence, identifying novel actions of S-equol may provide valuable insights into therapeutic strategies that improve endothelial function in cardiovascular disease.
With regard to vascular tone regulation, S-equol can promote relaxation in a variety of arteries, including the carotid arteries, cerebral arteries,35 aorta,33,36,37 and basilar arteries.38 In rat carotid arteries, S-equol-induced relaxation via endothelium-, nitric oxide synthase (NOS)-, and K+ channel-independent pathways, which was preserved during angiotensin II-induced hypertension.35 Another study showed that S-equol-induced relaxation of rat thoracic aorta was NO dependent.33 In human uterine arteries, S-equol-induced relaxation was mediated by its calcium antagonistic action through its antagonism of receptor-dependent but not voltage-dependent Ca2+ channels.39S-Equol increased regional cerebral blood flow in rats and promoted concentration-dependent but endothelium-independent relaxation of rat cerebral basilar arteries, which was mediated by the large-conductance Ca2+-activated K+ (BKCa) channel.38 In fact, in stably expressed multiple K+ channels in HEK293 cells, S-equol inhibited several cardiac K+ currents at relatively high concentrations but increased BKCa current at very low concentrations, suggesting that S-equol was safe for cerebral vascular disorders.40 These evidences suggest that S-equol could cause endothelium-dependent and/or -independent relaxation depending on the artery.
Elevated insulin level, an important pathophysiological condition for type 2 diabetes, results from insulin-resistant states. Prolonged elevation of circulating insulin levels can promote several types of systematic dysfunctions, including vascular diseases.3 We very recently demonstrated that S-equol can prevent the augmentation of serotonin-induced contraction in carotid arteries receiving prolonged treatment with increased insulin levels, which may have been due to increased BKCa channel activity in carotid artery smooth muscle cells.41 Therefore, S-equol may possibly prevent the development of vascular dysfunction in high insulin conditions and type 2 diabetes.
In in vivo studies, a dietary soy protein-rich diet for 12–16 months can better modulate blood pressure in vivo, antioxidant and eNOS gene expression, and intracellular glutathione levels compared with a soy protein-deficient diet in male rats.42S-Equol treatment (low-dose, 10 mg/kg and high-dose, 20 mg/kg orally for 4 weeks) could dose-dependently decrease systolic blood pressure in deoxycorticosterone acetate-salt hypertensive rats by inhibiting angiotensin-converting enzyme activity and increasing the NO production.43 Removal of dietary soy isoflavones reduced endothelium-derived NO levels in ovariectomized rats, while S-equol supplementation (200 µg/day subcutaneously for 4 weeks starting at the 16th week after receiving an isoflavone-deficient diet) partially improved NO-mediated endothelial function.36 In addition, S-equol treatment (0.05% and 0.1% for 12–14 weeks) displayed antiatherosclerotic properties in apolipoprotein E knockout mice fed a high-fat diet by inhibiting endoplasmic reticulum stress through the activation of nuclear factor-erythroid 2-related factor 2 in ECs.44 The aforementioned in vivo studies imply that S-equol has potential benefits against vascular dysfunction among not only postmenopausal women but also those with hypertension and metabolic disorder-associated atherosclerosis.
Taking these in vitro and in vivo evidences together, S-equol appears to be a clinically safer alternative to feminizing estrogens for the prevention of cardiovascular diseases among both men and women.
Nonetheless, further investigations are required to elucidate the underlying mechanisms. For instance, S-equol exerts several biological effects by binding to ERs, with its binding affinity being stronger to ERβ than ERα.45,46 Given that ERs are not equally distributed among different tissues/cells, S-equol might have different effects depending on the ratio of ERα and ERβ isoforms present. Furthermore, S-equol may exert its effects by independently binding to ERs.33 Considering that the primary target(s) of S-equol in ECs and VSMCs related to the aforementioned effects remain unclear, future investigations will be required.
The production of S-equol has attracted considerable attention, with several excellent reviews having been written on S-equol-producing phenotype, S-equol-producing microorganisms, and S-equol-producing populations in the human gut.25,26S-Equol is produced by the action of gut bacteria in some individuals called S-equol-producers. The prevalence of S-equol-producers in Asian countries has been reported to be 50%–60%, with Western having a much lower prevalence (25%–30%) than Asian countries.47 Observational studies have suggested that S-equol production was associated with decreased risk of certain diseases or conditions, including obesity48, hypertension, and vascular dysfunction.48–51 Clinical trials have reported that the beneficial effect of soy on cardiovascular health, particularly on the normalization of lipids profiles,52,53 inflammatory markers,54 vascular function,54,55 and blood pressure,53,55 was only present or more pronounced in equol-producers than nonproducers, although others did not.56,57 However, most of these data were from nonprespecified subgroup analysis of equol-producers who were not randomized accordingly. Recently, Ahuja et al.58 investigated the cross-sectional association between dietary isoflavones and equol-producer status and coronary artery calcification, a biomarker of coronary atherosclerosis, among Japanese men. Their results subsequently showed that equol-producers had lower coronary artery calcification than equol nonproducers independent of cardiovascular risk factors. The absence of an inverse association between coronary arterial calcification and dietary isoflavones therefore indicated that equol may be an important factor for the atheroprotective properties of dietary isoflavones.58 Further prospective studies and clinical trials are warranted to expand on such observations.
Despite the growing body of evidence suggesting a relationship between microbiota profile and cardiovascular diseases, some questions about S-equol currently remain unresolved. For instance, the extent of differences in S-equol production under physiological and pathophysiological states have yet to be determined. Considering that the actual microorganisms involved in S-equol production currently remain unknown and that the production phenotype and S-equol production itself may be modified by dietary habits, drug consumption, and disease duration, aging, or sex, understanding the association between S-equol levels, microbiota population, and lifestyle among humans with and without cardiovascular diseases should be encouraged to elucidate the preventive or therapeutic effects of S-equol as a nutraceutical or pharmaceutical agent against cardiovascular diseases.
INDOXYL SULFATE
Indoxyl sulfate is a protein-bound uremic toxin that has deleterious effects on the vascular system. Dietary protein-derived tryptophan is metabolized to indole by tryptophanase, which is produced by intestinal bacteria, such as Escherichia coli. Indole is then absorbed into the blood from the intestine, metabolized to indoxyl sulfate in the liver, and normally excreted into the urine.59 During uremia, however, the reduced renal clearance of indoxyl sulfate results in elevated circulating levels, as observed in patients with chronic kidney disease (CKD).21–24,60
Organic anion transporters (OATs) are involved in the cellular uptake of indoxyl sulfate and play a role in the impairment of endothelial and smooth muscle functions.61 Among the OATs related to indoxyl sulfate uptake, OAT1, and OAT3 are expressed in ECs61 and VSMCs.62–65 Studies have shown that the aryl hydrocarbon receptor (AhR) is an intracellular receptor for indoxyl sulfate.66,67 The AhR is a ligand-activated transcriptional factor that mediates adaptive and toxic responses in cells.67,68 Indoxyl sulfate induces a number of inflammation-related substances in ECs and VSMCs via OATs and AhR. Using the small interfering RNA technique, indoxyl sulfate-induced interleukin-6 (IL-6) expression in both HUVECs and HASMCs, which were suppressed by OAT3 small interfering RNA, AhR small interfering RNA, and nuclear factor-κB (NF-κB) subunit p65 small interfering RNA. This suggests that indoxyl sulfate induces IL-6 expression in both ECs and VSMCs via the OAT3/AhR/NF-κB pathway.62 Given that IL-6 plays an important role in the initiation and amplification of inflammation, OAT3/AhR/NF-κB pathway suppression can be effective in preventing indoxyl sulfate-induced inflammation. Indoxyl sulfate not only induced the expression inflammatory substances, including cytokines, but also amplified cytokine-induced responses in ECs and VSMCs. Using EC-specific AhR knockout mouse, Ito et al.68 demonstrated that indoxyl sulfate could enhance tumor necrosis factor (TNF)-α-induced leukocyte–endothelial interactions due to activator protein-1 transcriptional activity through AhR. Morita’s laboratory found that indoxyl sulfate activates AhR and increases oxidative stress in HUVECs via Nox4 (a component of nicotinamide adenine dinucleotide phosphate oxidase), resulting in enhanced monocyte chemoattractant protein-1 expression.69 In addition, indoxyl sulfate-induced monocyte chemoattractant protein-1 expression was related to indoxyl sulfate uptake via OATs in human ECs.61 These evidences strongly suggest that indoxyl sulfate plays a key role in the development of atherosclerosis and that manipulation of indoxyl sulfate-related molecules (e.g., OATs and AhR) may be a potential approach against atherosclerosis.
Tissue factor is the primary initiator of blood coagulation in vivo and has been implicated in the pathogenesis of cardiovascular disorders and development of atherosclerotic diseases. Studies have shown that indoxyl sulfate increased tissue factor production in ECs and peripheral blood mononuclear cells, which are two cellular sources of tissue factor in the blood, via AhR activation.70 This increase in tissue factor expression was associated with increased procoagulant activity.70 Thus, given that indoxyl sulfate may be an initiation factor that increases thrombotic events, suppression of indoxyl sulfate-mediated signaling may prevent thrombotic complications associated with atherosclerosis.
Several reports have suggested an association between indoxyl sulfate and cellular senescence in ECs. Indoxyl sulfate suppresses Sirt1 activity in association with a reduction in intracellular nicotinamide phosphoribosyltransferase activity and NAD+ content, leading to the acceleration of cellular senescence due to oxidative stress, with cellular senescence in HUVECs being mediated by AhR.71 In addition, indoxyl sulfate promoted cellular senescence in HUVECs by increasing ROS production and p53 activity.72 Considering the involvement of vascular senescence in the development of cardiovascular diseases, these evidences suggest that suppressing the indoxyl sulfate–AhR signaling pathway to regulate cellular senescence may be a novel approach against cardiovascular diseases.
EC apoptosis has been an important pathological feature in the development of vascular disease. Indoxyl sulfate downregulated microRNA-214 (miR-214) consistent with enhanced apoptosis in mouse aortic ECs, while cyclooxygenase-2 (COX-2) had been determined to be a target gene of miR-214, the inhibition of which reduced indoxyl sulfate-induced ECs apoptosis along with the suppression of prostaglandin E2 (PGE2) secretion.73 Thus, miR-214 plays a protective role against indoxyl sulfate-induced EC apoptosis by direct downregulation of cyclooxygenase-2/prostaglandin E2 signaling, making it a potential target for indoxyl sulfate-induced EC injury.
Extracellular vesicles released by different cells, including ECs, have been closely associated with vascular dysfunction.74 Indoxyl sulfate increases the release of extracellular vesicles from ECs, which promotes VSMC proliferation by inducing transforming growth factor-β production.75,76 Although mechanisms underlying indoxyl sulfate-induced release of extracellular vesicles remain unclear, the aforementioned data suggest that indoxyl sulfate may be a causative factor in the pathogenesis of vascular access stenosis.
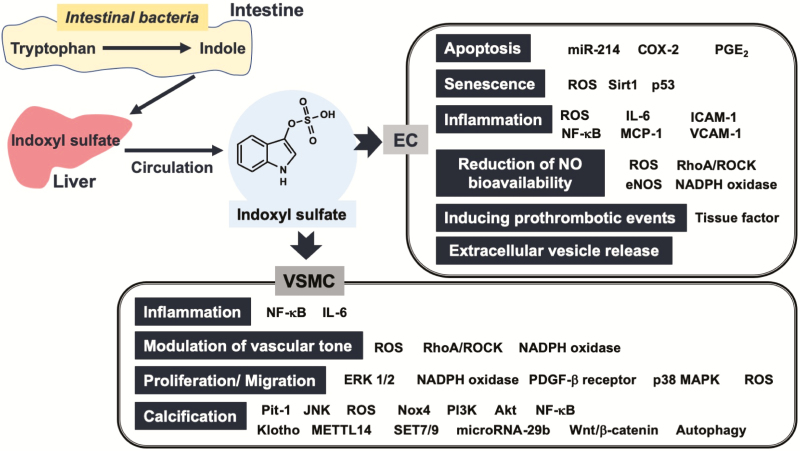
Indoxyl sulfate affects many cellular functions in not only ECs but also VSMCs77,78 (Figure 2) and induces the proliferation and migration of VSMCs through platelet-derived growth factor-β receptors and ROS generation.79
Figure 2.
Effects of indoxyl sulfate on vascular endothelial and smooth muscle cells. Indoxyl sulfate induces apoptosis, senescence, prothrombotic events, reduction of nitric oxide bioavailability, and release of extracellular vesicle in endothelial cells and inflammation, proliferation and/or migration, calcification, and modulation of vascular tone in vascular smooth muscle cells. Details are provided in the text. Abbreviations: COX-2, cyclooxygenase-2; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinase; ICAM-1, intercellular adhesion molecule-1; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; METTL14, methyltransferase-like 14; miR-214, microRNA-214; NF-κB, nuclear factor-kappa B; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; Pit-1, phosphate transporter 1; ROCK, Rho-associated protein kinase; ROS, reactive oxygen species; SET7/9, lysine methyltransferase 7/9; VCAM-1, vascular cell adhesion molecule-1.
Vascular calcification is an independent risk factor for the development of cardiovascular diseases and a prognostic indicator of end-stage renal disease.78,80 Phosphorus (Pi) is an important regulator of vascular calcification, with Pi transport via type-III sodium (Na)–Pi cotransporters, PiT1, and PiT-2, being a crucial step in calcification.81 Indoxyl sulfate promoted Pit-1 expression in part by activating the c-Jun N-terminal kinase pathway related to the mechanism of indoxyl sulfate-induced osteoblastic differentiation and matrix mineralization.82 Indoxyl sulfate-induced ROS generation via Nox4 upregulation and the expression of osteoblast-specific proteins, including core binding factor 1, alkaline phosphatase, and osteopontin in HASMCs.83 The activation of the phosphoinositide-3-kinase/Akt/NF-κB pathway was also related to VSMC calcification induced by indoxyl sulfate.84 Several reports have suggested an association between indoxyl sulfate, calcification, and epigenetic regulators in VSMCs. Indoxyl sulfate increased CpG hypermethylation of the Klotho gene, decreased Klotho expression in HASMCs, and potentiated calcification in HASMCs.85 Methyltransferase-like 14-dependent m6A methylome in VSMCs is also related to the development of indoxyl sulfate-induced calcification.86 Indoxyl sulfate promoted osteoblastic differentiation and calcification of VSMCs and reduced the expression of lysine methyltransferase 7/9, one of the important histone methyltransferases.87 In addition, indoxyl sulfate was able to activate autophagy, with the inhibition of autophagy partly suppressing the stimulating effect of indoxyl sulfate on the expression of both runt-related transcription factor 2 and calcium deposition.87 In HASMCs, indoxyl sulfate accelerates calcification through miRNA-29b-dependent regulation of Wnt/β-catenin signaling.88
Studies have shown that acutely exposing indoxyl sulfate to normal control vessels decreases ACh-induced endothelium-dependent relaxation in the thoracic aorta of mice89 and rats90, and reduces in the superior mesenteric artery of rats.91 Such acute effects of indoxyl sulfate on endothelium-dependent relaxation may be attributed to decreased NO bioavailability. ACh-induced endothelium-dependent relaxation is largely mediated by NO in the thoracic aorta, with EDHF also contributing to superior mesenteric artery relaxation in addition to NO.92 We demonstrated that the indoxyl sulfate-mediated reduction in ACh-induced relaxation within the rat superior mesenteric artery was still preserved following COX inhibition by indomethacin or COX plus EDHF signaling inhibition by indomethacin plus small (SKCa)- and intermediate (IKCa)-conductance calcium-activated K+ channels.91 However, the difference in ACh-induced relaxation between vehicle and indoxyl sulfate treated groups was eliminated by NOS inhibition or NOS/COX inhibition.91 Therefore, acute exposure of rat superior mesenteric artery to indoxyl sulfate impaired ACh-induced endothelium-dependent relaxation due to the reduction in NO signaling rather than alterations in other EDRFs, such as EDHF and prostacyclin. In addition to acute treatment with indoxyl sulfate, prolonged in vitro treatment with indoxyl sulfate impaired ACh-induced relaxation in the aorta of female wild-type mice whereas AST-120 (an oral charcoal adsorbent, described below) improved relaxation and prevented indoxyl sulfate-induced EC loss (assess using CD31 expression) and intercellular adhesion molecule-1/vascular cell adhesion molecule-1 upregulation.89 Furthermore, in vivo AST-120 treatment in a mice model of CKD (i) improved ACh-induced aortic relaxation, (ii) reduced aortic expressions of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, (iii) decreased aorta systolic expansion rate, and (iv) prevented the increase in pulse wave velocity.89 Moreover, treatment with indoxyl sulfate impaired vascular responses, such as increased phenylephrine-induced contraction and decreased ACh-induced relaxation of the aorta, in 5/6 nephrectomized rats, all of which were improved by ROS scavengers or RhoA/Rho kinase (ROCK) pathway blockade.93
Despite the presence of complicating mechanisms underlying indoxyl sulfate-induced EC and VSMC dysfunction (Figure 2), the manipulation of the aforementioned signalings may constitute an effective strategy. AST-120 adsorption of indoxyl sulfate can also be an effective strategy for blocking the deleterious effects of indoxyl sulfate. Indeed, AST-120 treatment has been shown to reduce oxidative stress94,95 and improve flow-mediated vasodilation in patients with CKD94 and endothelium-dependent relaxation in uremic rat arteries95 while exerting protective effects against the progression of atherosclerosis.96 Considering the aforementioned evidence suggesting that indoxyl sulfate is undoubtedly a causative factor for the development of vascular dysfunction, decreasing its levels (e.g., through AST-120) may be an effective and novel therapeutic strategy for the treatment of cardiovascular diseases.
Indoxyl sulfate is excreted from the circulating blood into the urine by healthy kidneys.97 Generally, indoxyl sulfate is metabolized by dietary tryptophan.69,97 Thus, a high-protein diet and gut microflora influence the increase in circulating indoxyl sulfate levels among patients with mild renal dysfunction or without CKD.98,99 However, indoxyl sulfate can easily accumulate in patients with renal dysfunction, especially those with impaired renal tubular excretory function.97 Patients with advanced CKD have higher levels of circulating indoxyl sulfate than those without CKD. In addition, the increased accumulation of indoxyl sulfate has been associated with future risk.100,101 On the other hand, an increased plasma indoxyl sulfate levels had been associated with increased carotid intima-media thickness among patients with chronic coronary artery disease with preserved renal function.102 Also, a recent study using a comprehensive metabolomic profiling of plasma in patients with type 2 diabetes to explore metabolites associated with atherosclerosis found that plasma levels of inositol and indoxyl sulfate were associated with carotid maximal intima-media thickness and/or flow-mediated vasodilation.103 Moreover, subjects with coronary artery disease had significantly higher plasma levels of inositol and indoxyl sulfate than those without an apparent history of cardiovascular disease.103 These findings suggest that increased plasma indoxyl sulfate levels can accelerate atherosclerosis not only in patients with severe renal dysfunction but also in those with early nephropathy or normal renal function. Thus, impaired renal function is not the sole reason for high concentrations of circulating indoxyl sulfate. Given that diabetes profoundly alters the gut microenvironment and is associated with a distinct gut microbial composition and metabolism,104,105 it could be also related to elevated circulating indoxyl sulfate levels. Nonetheless, further investigations on the balance between generation and excretion of indoxyl sulfate in each cardiovascular disease will be required.
TRIMETHYLAMINE N-OXIDE
Dietary betaine, choline, l-carnitine, and other choline-containing compounds, which are the principal nutrient precursors of TMAO, are metabolized to trimethylamine (TMA) by gut microbiota and several enzymes. TMAO is a compound generated by the liver via a flavin-monooxygenase 3 oxidation of gut microbiota-derived TMA, which is absorbed in the intestines and delivered to the liver via the portal circulation.16,18,106 Dietary choline intake has been mechanistically linked to atherosclerotic plaque formation by increasing circulating TMAO levels derived from gut microbiome metabolism of choline to TMA.21,107
A growing body of evidence has suggested that TMAO has various adverse effects at the EC level.106 In HUVEC and aortas from ApoE knockout mouse, TMAO exerted proinflammatory effects through nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome activation partly due to the inhibition of the sirtuin 3-superoxide dismutase 2-mitochondrial ROS signaling pathway.108 TMAO was able to induce the expression of inflammatory markers in both primary human aortic ECs (i.e., cyclooxygenase-2, IL-6, E-selectin, and intercellular adhesion molecule-1) and VSMCs (cyclooxygenase-2, IL-6, tumor necrosis factor-α, and intercellular adhesion molecule-1), increase leukocyte adhesion to ECs, and activate mitogen-activated protein kinases (p38 mitogen-activated protein kinase and ERK1/2) and NF-κB.109 These evidences suggest that TMAO has proinflammatory abilities in both ECs and VSMCs. Decreased self-repair capacity in ECs may play a role in the initiation of atherosclerosis. TMAO impaired the self-repair capacity of HUVECs and increased monocyte adhesion partly due to the activation of the protein kinase C/NF-κB/vascular cell adhesion molecule-1 pathway.110 Another study showed that TMAO increased senescence in HUVECs through suppression of SIRT1 expression, increased oxidative stress, and p53/p21/Rb pathway activation.111 Activation of the ROS-thioredoxin interacting protein-NLRP3 inflammasome contributed to TMAO-induced inflammation (i.e., increased production of IL-1β and IL-18) and endothelial dysfunction (i.e., reduced eNOS expression and NO production).112 These evidences indicate that TMAO is a novel positive regulator of endothelial dysfunction. Although the receptors for TMAO have not yet to be identified, the aforementioned signaling molecules may provide information on suppressing TMAO-related endothelial dysfunction and atherogenesis.
Several reports have investigated the relationship between TMAO and vascular tone regulation (Table 1). Accordingly, an association between elevated circulating TMAO levels and endothelial dysfunction, including decreased eNOS-derived NO bioavailability in the aorta, had been observed in Fischer-344 rats.113 In a reduced uterine perfusion pressure rat model of preeclampsia, increased levels of circulating TMAO, increased superoxide production, and proinflammatory cytokines in the aorta, reduced aortic relaxation, and hypertension had been observed, all of which were normalized following TMAO inhibitor treatment (3,3-dimethyl-1-butanol).114 In a rat model of CKD, 3,3-dimethyl-1-butanol treatment normalized ACh-induced endothelium-dependent relaxation and eNOS activity and reduced superoxide production and proinflammatory cytokine (i.e., tumor necrosis factor-α and IL-6) expressions in the aorta.115 These evidences suggest that TMAO is a causative factor for the development of endothelial dysfunction in aging, preeclampsia, and CKD and that targeting TMAO may be a novel strategy for the prevention and treatment of patients with cardiovascular disease.
Table 1.
Evidence of the relationship between vascular tone and TMAO
| Conditions | Responses | References |
|---|---|---|
| Direct exposure of TMAO to vessels | ||
| Adipose arterioles from healthy volunteers [1 µmol/l for 4 h] | No effect on ACh-induced relaxation | Malik et al.116 |
| Superior mesenteric artery of rat [300 µmol/l for 1 h] | No effect on ACh-induced relaxation | Matsumoto et al.117 |
| Femoral artery of rat [300 µmol/l for 1 h] | Impaired ACh-induced EDHF-type relaxation | Matsumoto et al.117 |
| Treatment with DMB, an inhibitor of trimethylamine formation to reduce TMAO levels | ||
| Aorta of aged rat [male Fisher-344 rat (~22 months old), with vs. without DMB] | Improvement of ACh-induced relaxation | Li et al.113 |
| No effect on SNP-induced relaxation | ||
| Aorta of CKD model rat [5/6 nephrectomy rat, with vs. without DMB] | Improvement of ACh-induced relaxation | Li et al.115 |
| No effect on SNP-induced relaxation | ||
| Aorta of RUPP model rat [with vs. without DMB] | Improvement of ACh-induced relaxation | Chen et al.114 |
| No effect on SNP-induced relaxation |
Abbreviations: ACh, acetylcholine; CKD, chronic kidney disease; DMB, 3,3-dimethyl-1-butanol (an inhibitor of trimethylamine formation); EDHF, endothelium-derived hyperpolarizing factor; RUPP, reduced uterine perfusion pressure; SNP, sodium nitroprusside; TMAO, trimethylamine N-oxide.
However, only a few direct evidences have been available regarding the relationship between TMAO and vascular function (Table 1). Intraluminal exposure to TMAO (1 µmol/l for 4 hours) had no effect on ACh-induced relaxation in adipose arterioles from healthy volunteers.116 In our recent study, acute exposure to TMAO (300 µmol/l for 1 hour) specifically impaired EDHF-type relaxation in rat femoral arteries but not superior mesenteric arteries.117 In that study, we found that ACh-induced femoral arterial relaxation was similar between the control and TMAO-exposed groups, whereas ACh-induced femoral arterial relaxation observed in the presence of Nω-nitro-l-arginine (l-NNA, a NOS inhibitor) plus indomethacin (a COX inhibitor) was greatly impaired in the TMAO-treated group. In addition, under indomethacin treatment (i.e., preserved NO and EDHF components), ACh-induced femoral arterial relaxation was slightly weaker in the TMAO-exposed group than in the control group. We also found that ACh-induced NO-mediated femoral artery relaxation was similar between the control and TMAO-exposed groups under treatment with indomethacin and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34, IKCa inhibitor) and apamin (SKCa inhibitor) (two KCa channel inhibitors related to the source of EDHF). Furthermore, ACh-induced femoral arterial relaxation was considerably small and similar between both two groups under three EDRFs blockades established via treatment with l-NNA, indomethacin, TRAM-34, and apamin. Finally, we showed that ACh-induced relaxation was similar between the control and TMAO-exposed groups under (i) intact, (ii) l-NNA plus indomethacin, and (iii) l-NNA, indomethacin, TRAM-34, and apamin conditions in the rat superior mesenteric arteries. Therefore, the aforementioned findings suggest that TMAO could specifically affect endothelial function with variations among different vessels.
A growing body of evidence has suggested that TMAO affects the cardiovascular system, exerting both harmful or beneficial effects.118,119 These discrepancies may have resulted from the limited number of studies investigating the effects of TMAO at concentrations close to physiological levels in mammals. In fact, chronic, low-dose, oral TMAO treatment could reduce diastolic dysfunction in the pressure-overloaded heart of hypertensive rats.120 On the other hand, Jaworska et al.121 observed that older rats presented higher levels of plasma TMA, which is associated with alterations in gut bacteria composition, structural, and functional changes in the colon, and increased penetration of TMA from the colon to portal blood. The same authors found that close to physiological concentrations of TMA reduced proliferation and viability of human VSMCs, and that TMAO did not exert cytotoxic effects at concentrations exceeding its physiological levels by 1,000-fold.121 Thus, understanding the role of not only TMAO but also its precursor TMA in the initiation and development of vascular dysfunction is necessary.
A comprehensive understanding of the direct effects of TMAO on vascular function and their molecular mechanisms could provide a potentially novel therapeutic target for the treatment of vascular diseases.
Evidence from experimental and clinical investigations has confirmed the role of the gut microbiota in TMAO metabolism, with recent seminal reviews focusing on the relationship between gut microbiota and TMAO in cardiovascular diseases.106,122 For example, the association between gut microbiota dysbiosis and increased circulating TMAO levels had been observed in several pathophysiological states, such as atherosclerosis,123 preeclampsia,124 and CKD.125 Given that microbiota-derived TMA is an important precursor of TMAO generation, the regulation of TMA production, including gut microbiota remodeling (e.g., antibiotics, synbiotics, probiotic functional products, and some natural molecules), and blocking of microbiota TMA lyases (e.g., 3,3-dimethyl-1-butanol126) can be strategies in the regulation of circulating TMAO. In addition, inhibiting hepatic flavin-monooxygenase 3 activity (e.g., trigonelline, a compound from Trigonella foenum-graecum127 and guggulsterone, a nuclear factor farnesoid X receptor antagonist128) to inhibit the conversion from TMA to TMAO can also be a beneficial strategy. Thus, a theoretical basis for controlling the gut microbiota to regulate TMAO levels will be beneficial for preventing and/or treating cardiovascular diseases.106,107,129 However, one should note that reducing TMAO may also have adverse effects. Therefore, we believe that a new TMAO-targeting therapeutic approach against cardiovascular diseases will be established in the near future.
The number of publications on gut-derived substances and host homeostasis has been growing rapidly. This review mainly focused on the effects of S-equol, indoxyl sulfate, and TMAO on vascular functions. Notably, other substances, such as bile acids and short-chain fatty acids, also play a role in the regulation of vascular functions.16–18,20,21 Alterations in vascular tone regulation, including the generation of several endogenous vasoactive substances and responsiveness thereto, and their signaling pathways by gut-derived substances may depend on vessel type, exposure duration (e.g., acute or chronic), and host status (sex, age, or disease). Despite the ongoing questions as mentioned previously, we believe that further knowledge on the manipulation of gut-derived substances will lead to new approaches in the prevention and treatment of vascular diseases.
ACKNOWLEDGMENTS
We thank Enago (www.enago.jp) for the English language review.
FUNDING
This work was supported in part by grants Japan Society for the Promotion of Science (JSPS) KAKENHI grant numbers JP18K06861 (to TM), JP17K08318 (to KT), and JP18K06974 (to TK).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Schiffrin EL; Canadian Institutes of Health Research Multidisciplinary Research Group on Hypertension Beyond blood pressure: the endothelium and atherosclerosis progression. Am J Hypertens 2002; 15:115S–122S. [DOI] [PubMed] [Google Scholar]
- 2. Martinez-Quinones P, McCarthy CG, Watts SW, Klee NS, Komic A, Calmasini FB, Priviero F, Warner A, Chenghao Y, Wenceslau CF. Hypertension induced morphological and physiological changes in cells of the arterial wall. Am J Hypertens 2018; 31:1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pereira CA, Carneiro FS, Matsumoto T, Tostes RC. Bonus effects of antidiabetic drugs: possible beneficial effects on endothelial dysfunction, vascular inflammation and atherosclerosis. Basic Clin Pharmacol Toxicol 2018; 123:523–538. [DOI] [PubMed] [Google Scholar]
- 4. Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res 2018; 114:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 2017; 97:1555–1617. [DOI] [PubMed] [Google Scholar]
- 6. Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC. Vascular smooth muscle contraction in hypertension. Cardiovasc Res 2018; 114:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Qian M, Kyler K, Xu J. Endothelial-vascular smooth muscle cells interactions in atherosclerosis. Front Cardiovasc Med 2018; 5:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barton M. Obesity and aging: determinants of endothelial cell dysfunction and atherosclerosis. Pflugers Arch 2010; 460:825–837. [DOI] [PubMed] [Google Scholar]
- 9. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol 2017; 219:22–96. [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto T, Goulopoulou S, Taguchi K, Tostes RC, Kobayashi T. Constrictor prostanoids and uridine adenosine tetraphosphate: vascular mediators and therapeutic targets in hypertension and diabetes. Br J Pharmacol 2015; 172:3980–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nava E, Llorens S. The local regulation of vascular function: from an inside-outside to an outside-inside model. Front Physiol 2019; 10:729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Félétou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol 2011; 164:894–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiffrin EL. Vascular endothelin in hypertension. Vasc Pharmacol 2005; 43:19–29. [DOI] [PubMed] [Google Scholar]
- 14. Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal 2018; 52:48–64. [DOI] [PubMed] [Google Scholar]
- 15. Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 2018; 114:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brial F, Le Lay A, Dumas ME, Gauguier D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell Mol Life Sci 2018; 75:3977–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmad AF, Dwivedi G, O’Gara F, Caparros-Martin J, Ward NC. The gut microbiome and cardiovascular disease: current knowledge and clinical potential. Am J Physiol Heart Circ Physiol 2019; 317:H923–H938. [DOI] [PubMed] [Google Scholar]
- 18. Battson ML, Lee DM, Weir TL, Gentile CL. The gut microbiota as a novel regulator of cardiovascular function and disease. J Nutr Biochem 2018; 56:1–15. [DOI] [PubMed] [Google Scholar]
- 19. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019; 129:4050–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin M, Qian Z, Yin J, Xu W, Zhou X. The role of intestinal microbiota in cardiovascular disease. J Cell Mol Med 2019; 23:2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 2017; 120:1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond) 2018; 132:509–522. [DOI] [PubMed] [Google Scholar]
- 23. Plata C, Cruz C, Cervantes LG, Ramírez V. The gut microbiota and its relationship with chronic kidney disease. Int Urol Nephrol 2019; 51:2209–2226. [DOI] [PubMed] [Google Scholar]
- 24. Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL, Arthur JM. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol 2019; 316:F1211–F1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayo B, Vazquez L, Florenz AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients 2019; 11:E2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 2015; 5:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackman KA, Woodman OL, Sobey CG. Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr Med Chem 2007; 14:2824–2830. [DOI] [PubMed] [Google Scholar]
- 28. Hazim S, Curtis PJ, Schär MY, Ostertag LM, Kay CD, Minihane AM, Cassidy A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double-blind randomized controlled trial. Am J Clin Nutr 2016; 103:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin D, Song J, Mark C, Eyster K. Understanding the cardiovascular actions of soy isoflavones: potential novel targets for antihypertensive drug development. Cardiovasc Hematol Disord Drug Targets 2008; 8:297–312. [DOI] [PubMed] [Google Scholar]
- 30. Dubey RK, Gillespie DG, Imthum B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension 1999; 33:177–182. [DOI] [PubMed] [Google Scholar]
- 31. Kamiyama M, Kishimoto Y, Tani M, Utsunomiya K, Kondo K. Effects of equol on oxidized low-density lipoprotein-induced apoptosis in endothelial cells. J Atheroscler Thromb 2009; 16:239–249. [DOI] [PubMed] [Google Scholar]
- 32. Chung JE, Kim SY, Jo HH, Hwang SJ, Chae B, Kwon DJ, Lew YO, Lim YT, Kim JH, Kim EJ, Kim JH, Kim MR. Antioxidant effects of equol on bovine aortic endothelial cells. Biochem Biophys Res Commun 2008; 375:420–424. [DOI] [PubMed] [Google Scholar]
- 33. Joy S, Siow RC, Rowlands DJ, Becker M, Wyatt AW, Aaronson PI, Coen CW, Kallo I, Jacob R, Mann GE. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem 2006; 281:27335–27345. [DOI] [PubMed] [Google Scholar]
- 34. Rowlands DJ, Chapple S, Siow RC, Mann GE. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: roles for F-actin and GPR30. Hypertension 2011; 57:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jackman KA, Woodman OL, Chrissobolis S, Sobey CG. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res 2007; 1141:99–107. [DOI] [PubMed] [Google Scholar]
- 36. Ohkura Y, Obayashi S, Yamada K, Yamada M, Kubota T. S-equol partially restored endothelial nitric oxide production in isoflavone-deficient ovariectomized rats. J Cardiovasc Pharmacol 2015; 65:500–507. [DOI] [PubMed] [Google Scholar]
- 37. Gimenez I, Lou M, Vargas F, Alvarez-Guerra M, Mayoral JA, Martinez RM, Garay RP, Alda JO. Renal and vascular actions of equol in the rat. J Hypertens 1997; 15:1303–1308. [DOI] [PubMed] [Google Scholar]
- 38. Yu W, Wang Y, Song Z, Zhao LM, Li GR, Deng XL. Equol increases cerebral blood flow in rats via activation of large-conductance Ca(2+)-activated K(+) channels in vascular smooth muscle cells. Pharmacol Res 2016; 107:186–194. [DOI] [PubMed] [Google Scholar]
- 39. Kim JY, Lee MY, Park HM. The effect of eqoul, a metabolite of isoflavone, on endothelial cell-independent vasodilatation of human uterine artery In Vitro. J Bone Metab 2015; 22:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deng XL, Wang Y, Xiao GS. Effects of equol on multiple K+ channels stably expressed in HEK 293 cells. PLoS One 2017; 12:e0183708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsumoto T, Takayanagi K, Kobayashi S, Kojima M, Taguchi K, Kobayashi T. Effect of equol on vasocontractions in rat carotid arteries treated with high insulin. Biol Pharm Bull 2019; 42:1048–1053. [DOI] [PubMed] [Google Scholar]
- 42. Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Viña J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J 2005; 19:1755–1757. [DOI] [PubMed] [Google Scholar]
- 43. Liu TH, Tsai TY. Effects of equol on deoxycorticosterone acetate salt-induced hypertension and associated vascular dementia in rats. Food Funct 2016; 7:3444–3457. [DOI] [PubMed] [Google Scholar]
- 44. Zhang T, Hu Q, Shi L, Qin L, Zhang Q, Mi M. Equol attenuates atherosclerosis in apolipoprotein E-deficient mice by inhibiting endoplasmic reticulum stress via activation of Nrf2 in endothelial cells. PLoS One 2016; 11:e0167020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 2005; 81:1072–1079. [DOI] [PubMed] [Google Scholar]
- 46. Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr 2010; 140:1363S–1368S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006; 136:2188–2193. [DOI] [PubMed] [Google Scholar]
- 48. Sakane N, Kotani K, Tsuzaki K, Takahashi K, Usui T, Uchiyama S, Fujiwara S. Equol producers can have low leptin levels among prediabetic and diabetic females. Ann Endocrinol (Paris) 2014; 75:25–28. [DOI] [PubMed] [Google Scholar]
- 49. Törmälä RM, Appt S, Clarkson TB, Tikkanen MJ, Ylikorkala O, Mikkola TS. Individual differences in equol production capability modulate blood pressure in tibolone-treated postmenopausal women: lack of effect of soy supplementation. Climacteric 2007; 10:471–479. [DOI] [PubMed] [Google Scholar]
- 50. Törmälä R, Appt S, Clarkson TB, Groop PH, Rönnback M, Ylikorkala O, Mikkola TS. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis 2008; 198:174–178. [DOI] [PubMed] [Google Scholar]
- 51. Liu ZM, Ho SC, Chen YM, Liu J, Woo J. Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women. PLoS One 2014; 9:e87861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong JM, Kendall CW, Marchie A, Liu Z, Vidgen E, Holmes C, Jackson CJ, Josse RG, Pencharz PB, Rao AV, Vuksan V, Singer W, Jenkins DJ. Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am J Clin Nutr 2012; 95:564–571. [DOI] [PubMed] [Google Scholar]
- 53. Acharjee S, Zhou JR, Elajami TK, Welty FK. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism 2015; 64:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clerici C, Setchell KD, Battezzati PM, Pirro M, Giuliano V, Asciutti S, Castellani D, Nardi E, Sabatino G, Orlandi S, Baldoni M, Morelli O, Mannarino E, Morelli A. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr 2007; 137:2270–2278. [DOI] [PubMed] [Google Scholar]
- 55. Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr 2005; 81:189–195. [DOI] [PubMed] [Google Scholar]
- 56. Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft HJ, Ferrari M, Branca F, Dadd T, Talbot D, Powell J, Minihane AM, Cassidy A, Nilsson M, Dahlman-Wright K, Gustafsson JA, Williams CM. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr 2006; 83:592–600. [DOI] [PubMed] [Google Scholar]
- 57. Thorp AA, Howe PR, Mori TA, Coates AM, Buckley JD, Hodgson J, Mansour J, Meyer BJ. Soy food consumption does not lower LDL cholesterol in either equol or nonequol producers. Am J Clin Nutr 2008; 88:298–304. [DOI] [PubMed] [Google Scholar]
- 58. Ahuja V, Miura K, Vishnu A, Fujiyoshi A, Evans R, Zaid M, Miyagawa N, Hisamatsu T, Kadota A, Okamura T, Ueshima H, Sekikawa A. Significant inverse association of equol-producer status with coronary artery calcification but not dietary isoflavones in healthy Japanese men. Br J Nutr 2017; 117:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med 1994; 124:96–104. [PubMed] [Google Scholar]
- 60. Gao H, Liu S. Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci 2017; 185:23–29. [DOI] [PubMed] [Google Scholar]
- 61. Favretto G, Souza LM, Gregório PC, Cunha RS, Maciel RAP, Sassaki GL, Toledo MG, Pecoits-Filho R, Souza WM, Stinghen AEM. Role of organic anion transporters in the uptake of protein-bound uremic toxins by human endothelial cells and monocyte chemoattractant protein-1 expression. J Vasc Res 2017; 54:170–179. [DOI] [PubMed] [Google Scholar]
- 62. Adelibieke Y, Yisireyili M, Ng HY, Saito S, Nishijima F, Niwa T. Indoxyl sulfate induces IL-6 expression in vascular endothelial and smooth muscle cells through OAT3-mediated uptake and activation of AhR/NF-κB pathway. Nephron Exp Nephrol 2014; 128:1–8. [DOI] [PubMed] [Google Scholar]
- 63. Yisireyili M, Saito S, Abudureyimu S, Adelibieke Y, Ng HY, Nishijima F, Takeshita K, Murohara T, Niwa T. Indoxyl sulfate-induced activation of (pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS One 2014; 9:e109268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant 2009; 24:2051–2058. [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, Kusano E. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 2006; 69:1780–1785. [DOI] [PubMed] [Google Scholar]
- 66. Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 2010; 49:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bock KW. Human AHR functions in vascular tissue: pro- and anti-inflammatory responses of AHR agonists in atherosclerosis. Biochem Pharmacol 2019; 159:116–120. [DOI] [PubMed] [Google Scholar]
- 68. Ito S, Osaka M, Edamatsu T, Itoh Y, Yoshida M. Crucial role of the Aryl Hydrocarbon Receptor (AhR) in indoxyl sulfate-induced vascular inflammation. J Atheroscler Thromb 2016; 23:960–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Watanabe I, Tatebe J, Namba S, Koizumi M, Yamazaki J, Morita T. Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells. Circ J 2013; 77:224–230. [DOI] [PubMed] [Google Scholar]
- 70. Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat-George F, Burtey S. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 2013; 84:733–744. [DOI] [PubMed] [Google Scholar]
- 71. Koizumi M, Tatebe J, Watanabe I, Yamazaki J, Ikeda T, Morita T. Aryl hydrocarbon receptor mediates indoxyl sulfate-induced cellular senescence in human umbilical vein endothelial cells. J Atheroscler Thromb 2014; 21:904–916. [DOI] [PubMed] [Google Scholar]
- 72. Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 2012; 22:86–89. [DOI] [PubMed] [Google Scholar]
- 73. Li S, Xie Y, Yang B, Huang S, Zhang Y, Jia Z, Ding G, Zhang A. MicroRNA-214 targets COX-2 to antagonize indoxyl sulfate (IS)-induced endothelial cell apoptosis. Apoptosis 2020;25:92–104. [DOI] [PubMed] [Google Scholar]
- 74. Taguchi K, Narimatsu H, Matsumoto T, Kobayashi T. ERK-containing microparticles from a diabetic mouse induce endothelial dysfunction. J Endocrinol 2019; 241:221–233. [DOI] [PubMed] [Google Scholar]
- 75. Ryu JH, Park H, Kim SJ. The effects of indoxyl sulfate-induced endothelial microparticles on neointimal hyperplasia formation in an ex vivo model. Ann Surg Treat Res 2017; 93:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ryu JH, Jeon EY, Kim SJ. Indoxyl sulfate-induced extracellular vesicles released from endothelial cells stimulate vascular smooth muscle cell proliferation by inducing transforming growth factor-beta production. J Vasc Res 2019; 56:129–138. [DOI] [PubMed] [Google Scholar]
- 77. Guo J, Lu L, Hua Y, Huang K, Wang I, Huang L, Fu Q, Chen A, Chan P, Fan H, Liu ZM, Wang BH. Vasculopathy in the setting of cardiorenal syndrome: roles of protein-bound uremic toxins. Am J Physiol Heart Circ Physiol 2017; 313:H1–H13. [DOI] [PubMed] [Google Scholar]
- 78. Henaut L, Mary A, Chillon JM, Kamel S, Massy ZA. The impact of uremic toxin on vascular smooth muscle cell function. Toxins 2018; 10:E218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shimizu H, Hirose Y, Nishijima F, Tsubakihara Y, Miyazaki H. ROS and PDGF-beta receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am J Physiol Cell Physiol 2009; 297:C389–C396. [DOI] [PubMed] [Google Scholar]
- 80. Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N; European Uremic Toxin Work Group Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 2005; 20:1048–1056. [DOI] [PubMed] [Google Scholar]
- 81. Gonzalez M, Martínez R, Amador C, Michea L. Regulation of the sodium-phosphate cotransporter Pit-1 and its role in vascular calcification. Curr Vasc Pharmacol 2009; 7:506–512. [DOI] [PubMed] [Google Scholar]
- 82. Wu Y, Han X, Wang L, Diao Z, Liu W. Indoxyl sulfate promotes vascular smooth muscle cell calcification via the JNK/Pit-1 pathway. Ren Fail 2016; 38:1702–1710. [DOI] [PubMed] [Google Scholar]
- 83. Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant 2009; 247:2051–2058. [DOI] [PubMed] [Google Scholar]
- 84. He X, Jiang H, Gao F, Liang S, Wei M, Chen L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-κB signaling pathway. Microsc Res Tech 2019; 82:2000–2006. [DOI] [PubMed] [Google Scholar]
- 85. Chen J, Zhang X, Zhang H, Liu T, Zhang H, Teng J, Ji J, Ding X. Indoxyl sulfate enhance the hypermethylation of Klotho and promote the process of vascular calcification in chronic kidney disease. Int J Biol Sci 2016; 12:1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen J, Ning Y, Zhang H, Song N, Gu Y, Shi Y, Cai J, Ding X, Zhang X. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci 2019; 239:117034. [DOI] [PubMed] [Google Scholar]
- 87. Chen J, Gu Y, Zhang H, Ning Y, Song N, Hu J, Cai J, Shi Y, Ding X, Zhang X. Amelioration of uremic toxin indoxyl sulfate-induced osteoblastic calcification by SET domain containing lysine methyltransferase 7/9 protein. Nephron 2019; 141:287–294. [DOI] [PubMed] [Google Scholar]
- 88. Zhang H, Chen J, Shen Z, Gu Y, Xu L, Hu J, Zhang X, Ding X. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/β-catenin signaling. Toxicol Lett 2018; 284:29–36. [DOI] [PubMed] [Google Scholar]
- 89. Six I, Gross P, Rémond MC, Chillon JM, Poirot S, Drueke TB, Massy ZA. Deleterious vascular effects of indoxyl sulfate and reversal by oral adsorbent AST-120. Atherosclerosis 2015; 243:248–256. [DOI] [PubMed] [Google Scholar]
- 90. Matsumoto T, Takayanagi K, Kojima M, Taguchi K, Kobayashi T. Acute exposure to indoxyl sulfate impairs endothelium-dependent vasorelaxation in rat aorta. Int J Mol Sci 2019; 20:E338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Matsumoto T, Takayanagi K, Kojima M, Katome T, Taguchi K, Kobayashi T. Direct impairment of the endothelial function by acute indoxyl sulfate through declined nitric oxide and not endothelium-derived hyperpolarizing actor or vasodilator prostaglandins in the rat superior mesenteric artery. Biol Pharm Bull 2015; 42:1236–1242. [DOI] [PubMed] [Google Scholar]
- 92. Matsumoto T, Kobayashi T, Kamata K. Alterations in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol 2003; 285:H283–H291. [DOI] [PubMed] [Google Scholar]
- 93. Chu S, Mao X, Guo H, Wang L, Li Z, Zhang Y, Wang Y, Wang H, Zhang X, Peng W. Indoxyl sulfate potentiates endothelial dysfunction via reciprocal role for reactive oxygen species and RhoA/ROCK signaling in 5/6 nephrectomized rats. Free Radic Res 2017; 51:237–252. [DOI] [PubMed] [Google Scholar]
- 94. Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 2011; 6:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Namikoshi T, Tomita N, Satoh M, Sakuta T, Kuwabara A, Kobayashi S, Higuchi Y, Nishijima F, Kashihara N. Oral adsorbent AST-120 ameliorates endothelial dysfunction independent of renal function in rats with subtotal nephrectomy. Hypertens Res 2009; 32:194–200. [DOI] [PubMed] [Google Scholar]
- 96. Nakada Y, Onoue K, Nakano T, Ishihara S, Kumazawa T, Nakagawa H, Ueda T, Nishida T, Soeda T, Okayama S, Watanabe M, Kawakami R, Saito Y. AST-120, an oral carbon absorbent, protects against the progression of atherosclerosis in a mouse chronic renal failure model by preserving sFlt-1 expression levels. Sci Rep 2019; 9:15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, Takayama F, Aoyama I, Nakamura S, Endou H, Niwa T. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol 2002; 13:1711–1720. [DOI] [PubMed] [Google Scholar]
- 98. Huć T, Nowinski A, Drapala A, Konopelski P, Ufnal M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol Res 2018; 130:172–179. [DOI] [PubMed] [Google Scholar]
- 99. Konopelski P, Ufnal M. Indoles—gut bacteria metabolites of tryptophan with pharmacotherapeutic potential. Curr Drug Metab 2018; 19:883–890. [DOI] [PubMed] [Google Scholar]
- 100. Aoki K, Teshima Y, Kondo H, Saito S, Fukui A, Fukunaga N, Nawata T, Shimada T, Takahashi N, Shibata H. Role of indoxyl sulfate as a predisposing factor for atrial fibrillation in renal dysfunction. J Am Heart Assoc 2015; 4:e002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Watanabe I, Tatebe J, Fujii T, Noike R, Saito D, Koike H, Yabe T, Okubo R, Nakanishi R, Amano H, Toda M, Ikeda T, Morita T. Prognostic utility of indoxyl sulfate for patients with acute coronary syndrome. J Atheroscler Thromb 2019; 26:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sato B, Yoshikawa D, Ishii H, Kikuchi R, Arima T, Takeshita K, Inoue Y, Suzuki S, Tanaka M, Kumagai S, Matsumoto M, Hayashi M, Ando H, Amano T, Matsubara T, Niwa T, Murohara T. Indoxyl sulfate, a uremic toxin, and carotid intima-media thickness in patients with coronary artery disease. Int J Cardiol 2013; 163:214–216. [DOI] [PubMed] [Google Scholar]
- 103. Omori K, Katakami N, Arakawa S, Yamamoto Y, Ninomiya H, Takahara M, Matsuoka TA, Tsugawa H, Furuno M, Bamba T, Fukusaki E, Shimomura I. Identification of plasma inositol and indoxyl sulfate as novel biomarker candidates for atherosclerosis in patients with Type 2 diabetes. Findings from metabolome analysis using GC/MS. J Atheroscler Thromb 2020. doi:10.5551/jat.52506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E. Intestinal microbiota in Type 2 diabetes and chronic kidney disease. Curr Diab Rep 2017; 17:16. [DOI] [PubMed] [Google Scholar]
- 105. Sato J, Kanazawa A, Ikeda F, Yoshihara T, Goto H, Abe H, Komiya K, Kawaguchi M, Shimizu T, Ogihara T, Tamura Y, Sakurai Y, Yamamoto R, Mita T, Fujitani Y, Fukuda H, Nomoto K, Takahashi T, Asahara T, Hirose T, Nagata S, Yamashiro Y, Watada H. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014; 37:2343–2350. [DOI] [PubMed] [Google Scholar]
- 106. Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, Tian L, Li X, Liu L, Xing Y, Wu M. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol 2019; 10:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc 2017; 6:e006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 2016; 5:e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, Chen B. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep 2017; 37:BSR20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, Shi X, Cheng S, Pan B, Zheng L, Hong H. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med 2018; 116:88–100. [DOI] [PubMed] [Google Scholar]
- 112. Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 2016; 481:63–70. [DOI] [PubMed] [Google Scholar]
- 113. Li T, Chen Y, Gua C, Li X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol 2017; 8:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen H, Li J, Li N, Tang J. Increased circulating trimethylamine N-oxide plays a contributory role in the development of endothelial dysfunction and hypertension in the RUPP rat model of preeclampsia. Hypertens Pregnancy 2019; 38:96–104. [DOI] [PubMed] [Google Scholar]
- 115. Li T, Gua C, Wu B, Chen Y. Increased circulating trimethylamine N-oxide contributes to endothelial dysfunction in a rat model of chronic kidney disease. Biochem Biophys Res Commun 2018; 495:2071–2077. [DOI] [PubMed] [Google Scholar]
- 116. Malik M, Suboc TM, Tyagi S, Salzman N, Wang J, Ying R, Tanner MJ, Kakarla M, Baker JE, Widlansky ME. Lactobacillus plantarum 299v supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res 2018; 123:1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Matsumoto T, Kojima M, Takayanagi K, Taguchi K, Kobayashi T. Trimethylamine-N-oxide specifically impairs endothelium-derived hyperpolarizing factor-type relaxation in rat femoral artery. Biol Pharm Bull 2020; 43:569–573. [DOI] [PubMed] [Google Scholar]
- 118. Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins 2016; 8:E326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab 2017; 28:121–130. [DOI] [PubMed] [Google Scholar]
- 120. Huc T, Drapala A, Gawrys M, Konop M, Bielinska K, Zaorska E, Samborowska E, Wyczalkowska-Tomasik A, Pączek L, Dadlez M, Ufnal M. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am J Physiol Heart Circ Physiol 2018; 315:H1805–H1820. [DOI] [PubMed] [Google Scholar]
- 121. Jaworska K, Konop M, Hutsch T, Perlejewski K, Radkowski M, Grochowska M, Bielak-Zmijewska A, Mosieniak G, Sikora E, Ufnal M. Trimethylamine but not Trimethylamine Oxide increases with age in rat plasma and affects smooth muscle cells viability. J Gerontol A Biol Sci Med Sci 2020; 75:1276–1283. [DOI] [PubMed] [Google Scholar]
- 122. Janeiro MH, Ramirez MJ, Milagro FI, Martinez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 2018; 10:E1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang J, Gu X, Yang J, Wei Y, Zhao Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front Cell Infect Microbiol 2019; 9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, Nie J, Zhou HW, Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep 2017; 7:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015; 163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Anwar S, Bhandari U, Panda BP, Dubey K, Khan W, Ahmad S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J Pharm Biomed Anal 2018; 159:100–112. [DOI] [PubMed] [Google Scholar]
- 128. Gautam A, Paudel YN, Abidin S, Bhandari U. Guggulsterone, a farnesoid X receptor antagonist lowers plasma trimethylamine-N-oxide levels: an evidence from in vitro and in vivo studies. Hum Exp Toxicol 2019; 38:356–370. [DOI] [PubMed] [Google Scholar]
- 129. Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 2014; 124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]