Abstract
Background: Thyroglobulin (TG) is a key autoantigen in autoimmune thyroid diseases (AITD). Several single nucleotide polymorphisms (SNPs) in the TG locus were shown to be strongly associated with disease susceptibility in both humans and mice, and autoimmune response to TG is the earliest event in the development of thyroid autoimmunity in mice. The classical model of experimental autoimmune thyroiditis (EAT) is induced by immunizing mice with TG protein together with an adjuvant to break down immune tolerance. The classical EAT model has limited utility in genetic studies of TG since it does not allow testing the effects of TG sequence variants on the development of autoimmune thyroiditis. In this study, we have immunized CBA-J mice, an EAT-susceptible strain, with an adenovirus vector encoding the full-length human TG (hTG) to generate a model of EAT in which the TG sequence can be manipulated to test AITD-associated TG SNPs.
Methods: We immunized CBA-J mice with hTG-expressing adenovirus following the well-recognized experimental autoimmune Graves' disease protocol that also uses an adenovirus vector to deliver the immunogen.
Results: After hTG adenovirus immunizations, mice developed higher T cell proliferative and cytokine responses to hTG and TG2098 (a major T cell epitope in AITD) and higher titers of TG and thyroperoxidase autoantibodies compared with mice immunized with control LacZ-expressing adenovirus. The mice, however, did not develop thyroidal lymphocytic infiltration and hypothyroidism.
Conclusions: Our data describe a novel murine model of autoimmune thyroiditis that does not require the use of adjuvants to break down tolerance and that will allow investigators to test the effects of hTG variants in the pathoetiology of Hashimoto's thyroiditis.
Keywords: human thyroglobulin, adenovirus, immunization, experimental autoimmune thyroiditis
Introduction
Autoimmune thyroid diseases (AITD), including Hashimoto's thyroiditis (HT) and Graves' disease (GD), are common autoimmune diseases that arise from a complex interaction between genetic susceptibility, epigenetic alterations, and environmental factors (1,2). HT, the most frequent AITD, is a T cell-mediated disease characterized by thyroidal infiltration of lymphocytes reactive to thyroidal antigens that lead to thyroid cell death, accompanied by production of anti-thyroglobulin (TG) and anti-thyroperoxidase (TPO) antibodies that are likely biomarkers of disease but not pathogenic. Thyroid cell death in HT ultimately results in clinical hypothyroidism (3).
TG, the most abundant protein in the thyroid, is believed to be the major autoantigen triggering HT as 75% of AITD patients develop high titers of anti-TG autoantibodies (1), TG autoantibodies appear before TPO antibodies in mouse models (4), and TG is a major AITD susceptibility gene (3,5). Several TG single nucleotide polymorphisms (SNPs) were shown to be strongly associated with both GD and HT (5–8); however, the underlying downstream mechanisms by which these polymorphisms contribute to AITD etiology are yet to be fully understood. To better understand the role of these SNPs in the etiology of AITD, a mouse model is needed where the sequence of TG can be manipulated to express the susceptible and resistant variants.
In this study, we developed a novel murine model of autoimmune thyroiditis by immunization of an experimental autoimmune thyroiditis (EAT)-susceptible strain of mice with adenovirus expressing full-length human TG (hTG) complementary DNA (cDNA), employing the same protocol used to induce experimental autoimmune Graves' disease (EAGD) in which mice are immunized with adenovirus containing human thyrotropin receptor (TSHR) cDNA. Our new mouse model showed T cell responses against hTG and production of TG autoantibodies without the aid of adjuvants, which will allow us and others to investigate the role of different hTG sequence variations in the pathoetiology of autoimmune thyroiditis.
Materials and Methods
Ethical approval
All procedures were performed in accordance with the care and use guidelines established by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine under the protocol 00001023.
Animals
Female CBA-J mice (The Jackson Laboratory, Bar Harbor, ME), 5–6 weeks old, were used in this study. All mice were maintained under 12–12 hours light–dark cycles (beginning at 7 AM and 7 PM), and the room temperature was held at 22°C. Standard rodent chow and water were provided ad libitum. At the end of the experiment, mice were sacrificed; the spleens and thyroids were removed for splenocyte assays and hematoxylin and eosin (H&E) staining, respectively; and blood samples were obtained for measurements of serum total thyroxine (T4) and thyrotropin (TSH).
Classical EAT
Mice were injected subcutaneously with hTG (Cell Sciences, Newburyport, MA) in Complete Freund's Adjuvant (CFA; Sigma-Aldrich, St. Louis, MO) to induce classical EAT as previously described (9). Mice were immunized with hTG on day 0, boosted on day 7, and sacrificed on day 21.
Adenovirus immunization protocol
The full-length hTG cDNA (10) was synthesized by Thermo Fisher Scientific (Waltham, MA) and packaged into an adeno-associated virus vector by Vector BioLabs (Malvern, PA). Mice were immunized with hTG-expressing adenovirus according to the published EAGD protocol (11) with the modifications by Rapoport and colleagues (12,13) and Huber et al. (14). Briefly, mice were injected intramuscularly with 5.0 × 109 particles of adenoviral vector containing full-length hTG (Ad-TG) or control LacZ (Ad-LacZ) cDNA (Viraquest, Inc., North Liberty, IA) in 50 μL of 10% glycerol (in phosphate-buffered saline or PBS) in the thigh muscle. A series of two intramuscular injections were given on day 0, day 21 and mice were sacrificed on day 35. To examine whether the mice develop lymphocytic infiltration at a later time point, a second group of mice received intramuscular injections on day 0, day 21, and day 42 and mice were sacrificed on day 63.
Splenocytes isolation
Mice spleens were harvested in complete RPMI 1640 (Corning, NY) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1 mM sodium pyruvate (Sigma-Aldrich). They were cut and pressed in circular motion by using a 10-mL syringe plunger. The suspension was filtered through a 100-μm cell strainer twice and centrifuged at 200g for 10 minutes. The pellet was washed with RPMI followed by centrifugation at 200g for 10 minutes. To remove non-lymphocytic cells, 5 mL of Ammonium-Chloride-Potassium (ACK) lysis buffer was added to the cell pellet. Cells were incubated with ACK lysis buffer for 5 minutes at room temperature with occasional shaking and then centrifuged at 200g for 10 minutes. The remaining pellet was resuspended in 10 mL of complete RPMI for counting and plating.
T cell stimulation and carboxyfluorescein diacetate, succinimidyl ester analysis
Splenocytes (2 × 106 cells) were resuspended in 0.1% bovine serum albumin/PBS and were labeled with 1.5 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE) (Thermo Fisher Scientific). After incubation for 10 minutes at 37°C, the CFSE staining was terminated by the addition of 4 volumes of ice-cold complete RPMI. After 5 minutes of incubation on ice, the cells were washed three times with fresh RPMI and resuspended in fresh medium for counting and plating. The cells were treated with: (1) medium; (2) scrambled apopeptide (APO) as an unrelated negative control (NC) peptide (20 μg/mL; GenScript, Piscataway, NJ); (3) mouse CD3/CD28 beads (Thermo Fisher Scientific) as positive control; (4) hTG (40 μg/mL; Cell Sciences); and (5) five hTG peptides (TG2098, TG202, TG726, TG1951, and TG1571) (20 μg/mL; GenScript) (Supplementary Table S1). After 5 days, cells were collected, and T cell proliferation was analyzed by flow cytometry analysis. The results were analyzed by using Flowjo (Tree Star, Ashland, OR). Assays were performed in quadruplicate, and data are expressed as stimulation index. We calculated the stimulation index by using the following formula: stimulation index = [% proliferating lymphocytes (peptide or mitogen-treated)]/[% proliferating lymphocytes (medium-treated)]. Proliferation index ≥1.5 was considered as a positive response to the stimulant.
Cytokine assays
Interferon gamma (IFN-γ), interleukin (IL)-2, IL-4, and IL-10 levels were measured by using a Milliplex mouse cytokines/chemokine magnetic panel (catalog no. MCYTOMAG-70K; EMD Millipore Corporation, Burlington, MA) following the manufacturer's instructions. Assays were read by using Luminex 200 with xPONENT software (Luminex, Austin, TX).
Anti-TG antibody detection by enzyme-linked immunosorbent assay
Serum levels of hTG and mouse TG (mTG) autoantibodies were measured by enzyme-linked immunosorbent assay (ELISA) using sera from individual mice as previously described (15). The signal was developed by using freshly prepared para-nitrophenylphosphate substrate (Sigma-Aldrich), and the data are presented as optical density at 405 nm.
Anti-mTPO antibody detection by flow cytometry
Anti-mouse TPO (mTPO) antibodies were measured by flow cytometry using Chinese hamster ovary (CHO) cells stably expressing mTPO as previously described (4). Flow cytometry was performed (10,000 events) by using BD Accuri C6 (BD Biosciences, Woburn, MA), and the results were analyzed by using Flowjo (Tree Star). TPO binding data were reported as the geometric mean (Geo mean).
Hormone measurements
The serum total concentrations of T4 were determined by ELISA according to the manufacturer's instructions (Alpha Diagnostics, San Antonio, TX). Serum TSH levels were measured by radioimmunoassay in Dr. Samuel Refetoff's laboratory (University of Chicago) as previously described (16).
H&E staining
The thyroids were fixed in 10% neutral-buffered formalin and then embedded in paraffin. Sections (4 μm thick) were prepared and stained with H&E by the Histology and Comparative Pathology Core at Albert Einstein College of Medicine. The images were acquired by using a 3DHistec Panoramic 250 Flash II slide scanner at the Analytical Imaging Facility at Albert Einstein College of Medicine.
Statistical analyses
Results are reported as means ± standard error and represent data from a minimum of three independent experiments. The number of animals is indicated in the results. One-way analysis of variance (ANOVA) followed by Student–Newman–Keuls multiple-comparison test, two-way ANOVA followed by Bonferroni's multiple-comparison test, or Student t-test for unpaired data (GraphPad Prism version 5.0; GraphPad Software) were used for analyzing differences between the groups for the different variables measured. Differences were considered to be significant at p < 0.05.
Results
Mice immunized with adenovirus containing hTG develop T cell proliferative responses to hTG and pathogenic hTG peptides
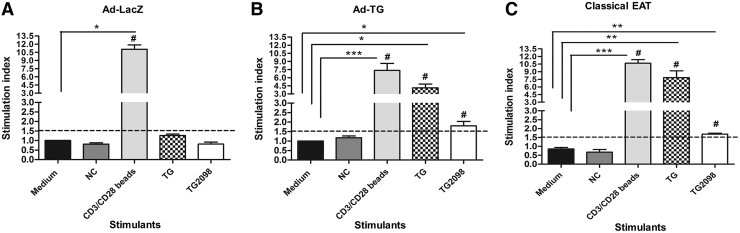
Female CBA-J (H2k) mice were used in this experiment, because they are a well-characterized EAT-susceptible strain (17,18). Mice were immunized with either adenovirus expressing hTG (Ad-TG) or adenovirus expressing Lac-Z (Ad-LacZ) as negative control or with hTG protein in CFA as a positive control. Splenocytes of immunized mice were stimulated with hTG and hTG peptide TG.2098 that was previously shown to be a major T cell epitope in autoimmune thyroiditis (15,19,20). Splenocytes from mice immunized with Ad-LacZ (Fig. 1A) did not show proliferation responses to TG and TG2098, while splenocytes from mice immunized with Ad-TG (Fig. 1B) showed significantly higher proliferation to TG (p < 0.05) and TG.2098 (p < 0.05) when compared with medium. Eighty-eight percent of the mice immunized with Ad-TG responded to TG stimulation (stimulation index ≥1.5), while 70% of the mice responded to TG.2098 stimulation (Fig. 1B). In addition, analysis by two-way ANOVA showed a higher proliferation index in response to TG in mice immunized with Ad-TG (p < 0.001) compared with mice immunized with Ad-LacZ (Fig. 1A). As expected, proliferation index in Ad-TG immunized mice (Fig. 1B) was not as high as that in mice induced with classical EAT (Fig. 1C). Proliferation in response to TG was 2.5 × higher (p < 0.001) in mice induced with classical EAT than in mice immunized with Ad-TG. We also observed positive T cell proliferative responses (proliferation index ≥1.5) to four other previously identified immunogenic TG peptides (15,20) in mice immunized with Ad-TG (Supplementary Fig. S1), suggesting that these thyroidogenic epitopes are conserved in humans and mice.
FIG. 1.
Mice immunized with adenovirus containing hTG develop T cell proliferative responses to hTG and TG2098. (A) Average proliferation indexes of T cells to stimulation with TG and TG2098 from mice immunized with control Ad-LacZ. (B) Average proliferation indexes of T cells to stimulation with TG and TG2098 from mice immunized with Ad-TG. (C) Average proliferation indexes of T cells to stimulation with TG and TG2098 from mice induced with classical EAT. Stimulation with scrambled APO was used as NC, and stimulation with CD3/CD28 beads was used as a positive control. Proliferation index ≥1.5 was considered as a positive response to the stimulant. The pound sign (#) indicates stimulation index ≥1.5. The data are shown as means ± SE. Statistical analysis was performed by using two-way ANOVA followed by Bonferroni's multiple-comparison test or unpaired t-test. *p < 0.05, **p < 0.01 ***p < 0.001 versus medium (Ad-LacZ, n = 13; Ad-TG, n = 17; EAT, n = 7). ANOVA, analysis of variance; APO, apopeptide; EAT, experimental autoimmune thyroiditis; hTG, human TG; NC, negative control; SE, standard error; TG, thyroglobulin.
Th1 and Th2 cytokine responses in mice immunized with Ad-TG
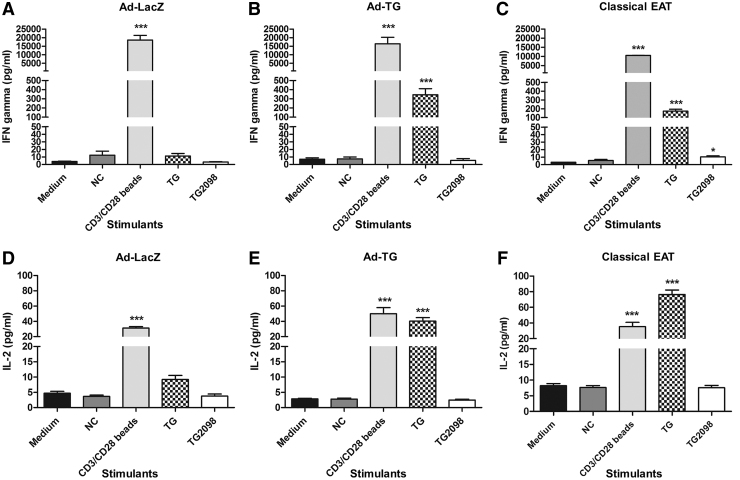
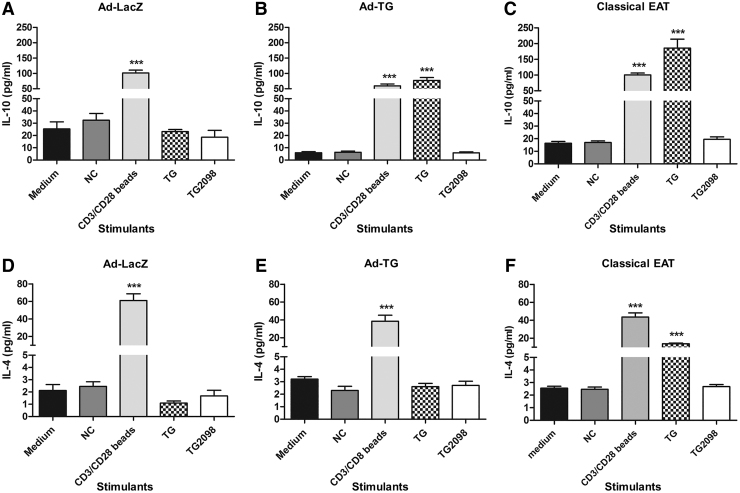
To investigate whether immunization with Ad-TG resulted in a Th1 or Th2 polarization of the anti-hTG T cell responses, we further evaluated the T cell responses by examining T cell cytokine production. Mice immunized with control Ad-LacZ did not show significant Th1 (Fig. 2A, D) and Th2 (Fig. 3A, D) cytokine production. On stimulation with hTG, splenocytes from mice immunized with Ad-TG secreted IFN-γ (Fig. 2B), IL-2 (Fig. 2E), and IL-10 (Fig. 3B), but not IL-4 (Fig. 3E). These data suggest that the EAT induced by Ad-TG immunization is driven mostly by Th1 responses (IFN-γ and IL-2), but that Th2 responses (IL-10) also play a role.
FIG. 2.
Mice immunized with adenovirus containing hTG develop Th1 cytokine responses. (A) Concentration of IFN-γ secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-LacZ. (B) Concentration of IFN-γ secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-TG. (C) Concentration of IFN-γ secreted by T cells in response to stimulation with TG and TG2098 when mice were induced with classical EAT. (D) Concentration of IL-2 secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-LacZ. (E) Concentration of IL-2 secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-TG. (F). Concentration of IL-2 secreted by T cells in response to stimulation with TG and TG2098 when mice were induced with classical EAT. Stimulation with scrambled APO was used as NC, and stimulation with CD3/CD28 beads was used as a positive control. The data are shown as means ± SE. Statistical analysis was performed by using unpaired t-test. *p < 0.05, ***p < 0.001 versus medium (Ad-LacZ, n = 8; Ad-TG, n = 8 EAT, n = 6). IFN-γ, interferon gamma; IL, interleukin.
FIG. 3.
Mice immunized with adenovirus containing hTG have higher levels of Th2 cytokine IL-10 but not IL-4. (A) Concentration of IL-10 secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-LacZ. (B) Concentration of IL-10 secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-TG. (C) Concentration of IL-10 secreted by T cells in response to stimulation with TG and TG2098 when mice were induced with classical EAT. (D) Concentration of IL-4 secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-LacZ. (E) Concentration of IL-4 secreted by T cells in response to stimulation with TG and TG2098 when mice were immunized with Ad-TG. (F). Concentration of IL-4 secreted by T cells in response to stimulation with TG and TG2098 when mice were induced with classical EAT. Stimulation with a scrambled APO peptide was used as NC, and stimulation with CD3/CD28 beads was used as a positive control. The data are shown as means ± SE. Statistical analysis was performed by using unpaired t-test. ***p < 0.001 versus medium (Ad-LacZ, n = 8; Ad-TG, n = 8 EAT, n = 6).
Mice immunized with Ad-TG develop anti-hTG, anti-mTG, and anti-mTPO antibodies
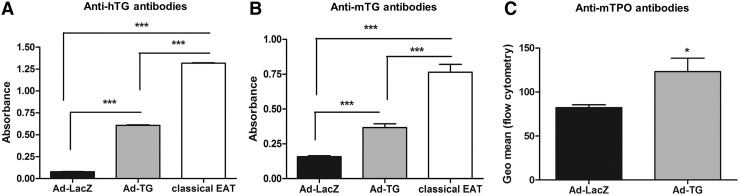
Humoral responses to TG are key markers of EAT development. Indeed, 100% of mice immunized with Ad-TG were positive for hTG antibodies (Fig. 4A; Supplementary Fig. S2A). Serum levels of anti-hTG antibodies were 5 × higher in Ad-TG-immunized mice compared with Ad-LacZ-immunized mice (Fig. 4A). Mice immunized with Ad-TG also had significantly higher levels of mTG (Fig. 4B; Supplementary Fig. S2B) and mTPO (Fig. 4C; Supplementary Fig. S2C) autoantibodies compared with Ad-LacZ-immunized mice, demonstrating the development of de novo autoimmune thyroiditis.
FIG. 4.
Mice immunized with adenovirus containing hTG develop hTG, mTG, and mTPO autoantibodies. (A) Anti-hTG antibodies produced by mice immunized with Ad-LacZ, Ad-TG and induced with classical EAT. (B) Anti-mTG antibodies produced by mice immunized with Ad-LacZ, Ad-TG and induced with classical EAT. (C) Anti-mTPO antibodies produced by mice immunized with Ad-LacZ and Ad-TG. The data are shown as means ± SE. Statistical analysis was performed by using one-way ANOVA followed by Student–Newman–Keuls multiple-comparison test or unpaired t-test. *p < 0.05, ***p < 0.001 (Ad-LacZ, n = 10; Ad-TG, n = 13; EAT, n = 7). mTG, mouse TG; mTPO, mouse thyroperoxidase.
Thyroid functions in mice immunized with Ad-TG
There were no significant differences in serum total T4 levels and TSH levels between mice immunized with Ad-TG and control Ad-LacZ immunized mice (Supplementary Fig. S3).
Thyroid histology and immunohistochemistry
H&E staining (Supplementary Fig. S4) and immunostaining for CD45 (data not shown) of thin sections from thyroid glands of mice sacrificed 35 days after initiation of immunization protocol did not show mononuclear cell infiltration of the thyroid. To test whether thyroid infiltration may appear at a later time point, some mice were followed up to 63 days after the first hTG immunization. Mice sacrificed 63 days after the first immunization also did not develop thyroidal cell infiltration (data not shown).
Discussion
To study the etiology of HT, different experimental approaches have been employed to induce EAT, a mouse model of HT (1). The first EAT model was achieved by immunization of susceptible mice with TG, a known thyroid autoantigen, in combination with an adjuvant to break down immune tolerance. To this date, this classical EAT model is still widely used to study the pathoetiology of HT (1,18). This model, similar to HT, is characterized by lymphocytic infiltration of the thyroid, follicular destruction, T cell proliferative responses against TG, and production of anti-TG autoantibodies (18,21,22). Although this is an excellent model for HT, it has limited utility in testing the functional role of TG variants on the induction of EAT. Therefore, to better understand the contribution of TG variants to thyroid autoimmunity, we have now created a mouse model that is easily amenable to modifications of the TG sequence. In our new model, an adenovirus vector carrying full-length hTG cDNA is used to induce B cell and T cell responses against TG in the absence of adjuvants.
In the past, our group developed a cDNA-based EAT model in which hTG cDNA immunization combined with electroporation induced T cell proliferative responses to TG, IFN-γ secretion, and autoantibodies in C3H/Hen mice (23). Our current model, however, elicited superior immune responses to TG with the advantage of being less laborious than electroporation by utilizing a replication-defective adenovirus to achieve higher transgene expression in the muscle.
We believe that our new EAT model has at least three advantages compared with the conventional TG protein/adjuvant-based models. The first advantage of our model is that hTG is presented to B cells in the correct conformation since it is expressed de novo in cells generating an immune response that is closer to human autoimmune thyroiditis. In our model, similar to the EAGD model (11), we used a recombinant adenovirus vector to induce high hTG expression in the muscle. Using this method, hTG is expressed endogenously in myocytes. The myocytes are capable of presenting TG due to the de novo synthesis of the major histocompatibility complex class II (MHC II) and chemokines driven by the immunostimulatory nature of the adenovirus plasmid, leading to long-term cellular and humoral responses in our mice. Indeed, mice immunized with adenovirus-expressing TPO showed high titers of anti-TPO antibodies with an epitope recognition pattern similar to the anti-TPO antibodies found in HT patients' sera (24). In addition, mice-induced EAGD by TSHR-adenovirus immunization developed goiter, hyperthyroidism, and anti-TSHR antibodies that recognized similar epitopes to the autoantibodies in GD patients (11,25).
The second advantage of our model is that it elicits an immune responses against TG without the need for an adjuvant to break down tolerance to TG, supporting the notion, first demonstrated in the 1980s, that anti-TG reactive T cells can escape central tolerance (26,27). Kong et al. (26) observed thyroid lymphocytic infiltration and T cells and B cells reactive to mTG in mice immunized repeatedly with high doses of mTG or mouse thyroid extract. Notably, our mice developed both T cell and B cell responses to hTG, as evidenced by T cell proliferative responses, IFN-γ and IL-2 secretion, and production of autoantibodies. In addition, mice developed autoantibodies to mTG and mTPO, demonstrating the development of de novo autoimmune responses to the mouse thyroid. Additionally, mice did not produce autoantibodies to glutamate decarboxylase (data not shown) confirming that the immune response was specific to the mouse thyroid. Interestingly, even though the mice developed antibodies to TG and TPO, they did not produce TSHR autoantibodies (data not shown), suggesting that this model is similar to human HT in which patients rarely develop TSHR autoantibodies (1).
Interestingly, there was no lymphocytic infiltration in our mice, similar to what is observed in the TSHR adenovirus-based model of EAGD (11). This is possibly due to insufficient cross-reaction between the hTG used in immunizations and mTG to induce reactivity to mTG that is necessary to drive thyroidal lymphocytic infiltration and hypothyroidism despite the 73% amino acid homology between human and mTG. Another potential explanation is that the production of non-iodinated hTG in the myocytes (where the iodination system is absent) caused the lack of lymphocytic infiltration. Given that our mice developed T cell and B cell responses against non-iodinated hTG, it is likely that iodine is not essential for TG antigenicity, as previously reported (28). However, it is possible that the non-iodinated hTG did not cross-react with the iodinated mTG, or that iodination of TG might be necessary for stimulation of cytotoxic T cells and subsequent lymphocytic infiltration of the thyroid. Indeed, studies by Roitt and associates suggested a role for iodination of TG in activating a population of thyroid autoreactive T cells (29). Of note, our mice showed mononuclear cell infiltrates at the site of injection in the muscle (data not shown).
The third and most important advantage of our model is that it enables modifications to the sequence of Ad-TG used in the immunizations to study the functional effects of TG SNPs that are associated with AITD. TG is a soluble 660-KDa iodinated glycoprotein comprising two 330-KDa monomers that serve as a precursor for thyroid hormones T4 and triiodothyronine, and as a storehouse for iodine (30). Whole-genome linkage scans have reported that the TG gene-locus on chromosome 8q24 was linked to AITD (7,31), and association studies have identified a number of SNPs in the TG locus that were strongly associated with AITD in humans (5,8,32–35). However, the mechanisms by which these TG variants trigger thyroid autoimmunity are not yet known. In contrast to the conventional EAT model, our novel model will allow investigators to test the functional effects of hTG sequence variants on TG immunogenicity, cathepsin-generated TG peptide profiles, and disease induction. Moreover, our new model will also be useful when studying interactions between different TG variants and human leucocyte antigen (HLA) class II molecules, as our group has previously demonstrated that specific sequences of the MHC class II peptide-binding pocket confer susceptibility to both HT and EAT (19).
In summary, for the first time we have developed a mouse model of HT by immunization of EAT-susceptible mice with adenovirus containing full-length hTG cDNA. Since the hTG cDNA is amenable to genetic modifications, it will allow future investigations on the functional role of different hTG sequence variants in the pathoetiology of HT.
Supplementary Material
Acknowledgments
The authors thank Dr. Sandra McLachlan (Cedars-Sinai Medical Center) for generously providing them with the CHO-mTPO cells. They also thank Drs. Glaucia Furtado and Sergio Lira (Icahn School of Medicine at Mount Sinai) for helping them with the immunostaining protocol. They are grateful to Dr. Samuel Refetoff (University of Chicago) for the serum TSH levels measurements. The authors thank Hillary Guzik and Andrea Briceno from the Analytical Imaging Facility (Albert Einstein College of Medicine) for the assistance with the 3DHistec Panoramic 250 Flash II slide scanner (SIG #1S10OD019961-01). They thank the Histology and Comparative Pathology Core (Albert Einstein College of Medicine) for the assistance with H&E staining and pathology report.
Author Disclosure Statement
Yaron Tomer was previously (1/2015–6/2017) the PI on a basic research project jointly funded by the Juvenile Diabetes Research Foundation and Pfizer. The current article is not related to that research project. All other authors have no potential conflict of interest to declare.
Funding Information
The Analytical Imaging Facility and the Histology and Comparative Pathology Core (Albert Einstein College of Medicine) were funded by NCI Cancer Center Support Grant P30CA013330. This work was supported in part by grants DK067555 and DK073681 from NIDDK.
Supplementary Material
References
- 1. McLachlan SM, Rapoport B. 2014. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev 35:59–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee H, Li C, Hammerstad S, Stefan M, Tomer Y. 2015. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun 64:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomer Y. 2014. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol 9:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen CR, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, Rapoport B, McLachlan SM. 2010. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology 151:4583–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stefan M, Jacoboson EM, Huber AK, Greenberg DA, Li CW, Skrabanek L, Conception E, Fadlalla M, Ho K, Tomer Y. 2011. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon alpha-modulated mechanism. J Biol Chem 286:31168–31179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomer Y, Greenberg DA, Concepcion E, Ban Y, Davies TF. 2002. Thyroglobulin is a thyroid specific gene for the familial autoimmune thyroid diseases. J Clin Endocrinol Metab 87:404–407 [DOI] [PubMed] [Google Scholar]
- 7. Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, Davies TF. 2003. Common and unique susceptibility loci in graves and hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet 73:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. 2003. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA 100:15119–15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kong YM. 2007. Experimental autoimmune thyroiditis in the mouse. Curr Protoc Immunol 15:1–21 [DOI] [PubMed] [Google Scholar]
- 10. van de Graaf, Ris-Stalpers C, Pauws E, Mendive FM, Targovnik HM, de Vijlder JJ. 2001. Up to date with human thyroglobulin. J Endocrinol 170:307–321 [DOI] [PubMed] [Google Scholar]
- 11. Nagayama Y, Kita-Furuyama M, Ando T, Nakao K, Mizuguchi H, Hayakawa T, Eguchi K, Niwa M. 2002. A novel murine model of Graves' hyperthyroidism with intramuscular injection of adenovirus expressing the thyrotropin receptor. J Immunol 168:2789–2794 [DOI] [PubMed] [Google Scholar]
- 12. Chen CR, Pichurin P, Nagayama Y, Latrofa F, Rapoport B, McLachlan SM. 2003. The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim. J Clin Invest 111:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CR, Pichurin P, Chazenbalk GD, Aliesky H, Nagayama Y, McLachlan SM, Rapoport B. 2004. Low-dose immunization with adenovirus expressing the thyroid-stimulating hormone receptor A-subunit deviates the antibody response toward that of autoantibodies in human Graves' disease. Endocrinology 145:228–233 [DOI] [PubMed] [Google Scholar]
- 14. Huber AK, Finkelman FD, Li CW, Concepcion E, Smith E, Jacobson E, Latif R, Keddache M, Zhang W, Tomer Y. 2012. Genetically driven target tissue overexpression of CD40: a novel mechanism in autoimmune disease. J Immunol 189:3043–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li CW, Menconi F, Osman R, Mezei M, Jacobson EM, Concepcion E, David CS, Kastrinsky DB, Ohlmeyer M, Tomer Y. 2016. Identifying a small molecule blocking antigen presentation in autoimmune thyroiditis. J Biol Chem 291:4079–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrara AM, Liao XH, Gil-Ibáñez P, Marcinkowski T, Bernal J, Weiss RE, Dumitrescu AM, Refetoff S. 2013. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology 154:2533–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esquivel PS, Rose NR, Kong YC. 1977. Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J Exp Med 145:1250–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong YC. 2007. Experimental autoimmune thyroiditis in the mouse. Curr Protoc Immunol 15:7. [DOI] [PubMed] [Google Scholar]
- 19. Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW, Tomer Y. 2008. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA 105:14034–14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobson EM, Yang H, Menconi F, Wang R, Osman R, Skrabanek L, Li C, Fadlalla M, Gandhi A, Chaturvedi V, Smith EP, Schwemberger S, Osterburg A, Babcock GF, Tomer Y. 2009. Employing a recombinant HLA-DR3 expression system to dissect major histocompatibility complex II-thyroglobulin peptide dynamism: a genetic, biochemical, and reverse immunological perspective. J Biol Chem 284:34231–34243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alimi E, Huang S, Brazillet MP, Charreire J. 1998. Experimental autoimmune thyroiditis (EAT) in mice lacking the IFN-gamma receptor gene. Eur J Immunol 28:201–208 [DOI] [PubMed] [Google Scholar]
- 22. Simon LL, Krco CJ, David CS, Kong YM. 1985. Characterization of the in vitro murine T-cell proliferative responses to murine and human thyroglobulins in thyroiditis-susceptible and -resistant mice. Cell Immunol 94:243–253 [DOI] [PubMed] [Google Scholar]
- 23. Jacobson EM, Concepcion E, Ho K, Kopp P, Toniolo JV, Tomer Y. 2011. cDNA immunization of mice with human thyroglobulin generates both humoral and T cell responses: a novel model of thyroid autoimmunity. PLoS One 6:e19200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo J, Pichurin P, Nagayama Y, Rapoport B, McLachlan SM. 2003. Insight into antibody responses induced by plasmid or adenoviral vectors encoding thyroid peroxidase, a major thyroid autoantigen. Clin Exp Immunol 132:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagayama Y, McLachlan SM, Rapoport B, Niwa M. 2003. A major role for non-MHC genes, but not for micro-organisms, in a novel model of Graves' hyperthyroidism. Thyroid 13:233–238 [DOI] [PubMed] [Google Scholar]
- 26. Kong YC, Rose NR, Elrehewy M, Michaels R, Giraldo AA, Accavitti MA, leon MA. 1980. Thyroid alloantigens in autoimmunity. Transplant Proc 12:129–134 [PubMed] [Google Scholar]
- 27. ElRehewy M, Kong YM, Giraldo AA, Rose NR. 1981. Syngeneic thyroglobulin is immunogenic in good responder mice. Eur J Immunol 11:146–151 [DOI] [PubMed] [Google Scholar]
- 28. Kong Y, McCormick DJ, Wan Q, Motte RW, Fuller BE, Giraldo AA, David CS. 1995. Primary hormonogenic sites as conserved autoepitopes on thyroglobulin in murine autoimmune thyroiditis. Secondary role of iodination. J Immunol 155:5847–5854 [PubMed] [Google Scholar]
- 29. Champion BR, Page KR, Parish N, Rayner DC, Dawe K, Biswas-Hughes G, Cooke A, Geysen M, Roitt IM. 1991. Identification of a thyroxine-containing self-epitope of thyroglobulin which triggers thyroid autoreactive T cells. J Exp Med 174:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeso BD, Arvan P. 2016. Thyroglobulin from molecular and cellular biology to clinical endocrinology. Endocr Rev 37:2–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakai K, Shirasawa S, Ishikawa N, Ito K, Tamai H, Kuma K, Akamizu T, Tanimura M, Furugaki K, Yamamoto K, Sasazuki T. 2001. Identification of susceptibility loci for autoimmune thyroid disease to 5q31-q33 and Hashimoto's thyroiditis to 8q23-q24 by multipoint affected sib-pair linkage analysis in Japanese. Hum Mol Genet 10:1379–1386 [DOI] [PubMed] [Google Scholar]
- 32. Hsiao JY, Hsieh MC, Tien KJ, Hsu SC, Shin SJ, Lin SR. 2007. Association between a C/T polymorphism in exon 33 of the thyroglobulin gene is associated with relapse of Graves' hyperthyroidism after antithyroid withdrawal in Taiwanese. J Clin Endocrinol Metab 92:3197–3201 [DOI] [PubMed] [Google Scholar]
- 33. Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y. 2004. Association of a thyroglobulin gene polymorphism with Hashimoto's thyroiditis in the Japanese population. Clin Endocrinol 61:263–268 [DOI] [PubMed] [Google Scholar]
- 34. Varela V, Rizzo L, Domené S, Bruno OD, Tellechea ML, Rivolta CM, Targovnik HM. 2010. Association of the TGrI29 microsatellite in thyroglobulin gene with autoimmune thyroiditis in a Argentinian population: a case-control study. Endocrine 38:320–327 [DOI] [PubMed] [Google Scholar]
- 35. Collins JE, Heward JM, Carr-Smith J, Daykin J, Franklyn JA, Gough SC. 2003. Association of a rare thyroglobulin gene microsatellite variant with autoimmune thyroid disease. J Clin Endocrinol Metab 88:5039–5042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.