Abstract
Background: Iodine supplementation is recommended to pregnant women in iodine-deficient populations, but the impact in moderate iodine deficiency is uncertain. We assessed the effect of an iodine-containing prenatal multiple micronutrient (MMN) supplement in a rural Gambian population at risk of moderate iodine deficiency.
Materials and Methods: This study uses data and samples collected as a part of the randomized controlled trial Early Nutrition and Immune Development (ENID; ISRCTN49285450) conducted in Keneba, The Gambia. Pregnant women (<20 weeks gestation) were randomized to either a daily supplement of MMNs containing 300 μg of iodine or an iron and folic acid (FeFol) supplement. Randomization was double blinded (participants and investigators). The coprimary outcomes were maternal urinary iodine concentration (UIC) and serum thyroglobulin (Tg), assessed at baseline and at 30 weeks' gestation. Secondary outcomes were maternal serum thyrotropin (TSH), total triiodothyronine (TT3), total thyroxine (TT4) (assessed at baseline and at 30 weeks' gestation), breast milk iodine concentration (BMIC) (assessed at 8, 12, and 24 weeks postpartum), infant serum Tg (assessed at birth [cord], 12, and 24 weeks postpartum), and serum TSH (assessed at birth [cord]). The effect of supplementation was evaluated using mixed effects models.
Results: A total of 875 pregnant women were enrolled between April 2010 and February 2015. In this secondary analysis, we included women from the MMN (n = 219) and FeFol (n = 219) arm of the ENID trial. At baseline, median (interquartile range or IQR) maternal UIC and Tg was 51 μg/L (33–82) and 22 μg/L (12–39), respectively, indicating moderate iodine deficiency. Maternal MMN supplement increased maternal UIC (p < 0.001), decreased maternal Tg (p < 0.001), and cord blood Tg (p < 0.001) compared with FeFol. Maternal thyroid function tests (TSH, TT3, TT4, and TT3/TT4 ratio) and BMIC did not differ according to maternal supplement group over the course of the study. Median (IQR) BMIC, maternal UIC, and infant Tg in the MMN group were 51 μg/L (35–72), 39 μg/L (25–64), and 87 μg/L (59–127), respectively, at 12 weeks postpartum, and did not differ between supplement groups.
Conclusions: Supplementing moderately iodine-deficient women during pregnancy improved maternal iodine status and reduced Tg concentration. However, the effects were not attained postpartum and maternal and infant iodine nutrition remained inadequate during the first six months after birth. Consideration should be given to ensuring adequate maternal status through pregnancy and lactation in populations with moderate deficiency.
Keywords: iodine, thyroid, pregnancy, lactation, infancy
Introduction
Iodine is an essential substrate for the production of thyroid hormone and adequate iodine nutrition is especially important during the first 1000 days of life, when the risk of deficiency for the fetus and infant is high. Infants are particularly sensitive to iodine deficiency because they have the highest production of thyroid hormones per kilogram body weight, and are born with minimal thyroidal iodine stores (1). Exclusively breastfed infants rely on iodine from breast milk alone to cover their high rates of thyroid hormone production (2).
Severe iodine deficiency during pregnancy may result in maternal and fetal hypothyroidism, increased risk for infant and perinatal mortality, pregnancy loss, maternal and fetal goiter, and growth retardation (2). Thyroid hormone is critical for fetal and infant neurodevelopment, and severe iodine deficiency during pregnancy is associated with neurologic deficits and cretinism in children (3,4). Mild-to-moderate iodine deficiency may affect maternal and fetal thyroid function (5), but the impact on neurodevelopmental outcomes in offspring remains uncertain (4,6,7). Iodine deficiency during infancy may also result in altered thyroid function and impaired brain development, but data are limited (8).
Salt iodization is the primary intervention strategy to prevent iodine deficiency in the general population (9). However, poor coverage of iodized salt or use of noniodized alternatives may increase the risk for mild-to-moderate iodine deficiency during the first 1000 days (from conception to the child's second birthday) when dietary iodine requirements are high (10). In The Gambia, the coverage of adequately iodized salt is poor, and a recent nationally representative cross-sectional survey found rural pregnant women to be iodine deficient (11).
Iodine supplementation of pregnant and lactating women is recommended in iodine-deficient populations wherein salt iodization is insufficient (12). Iodine supplementation during pregnancy in mildly iodine-deficient women improves maternal iodine status, thyroid volume, and thyroid indices (5,7). However, studies conducted in moderately iodine-deficient populations are small and did not follow women and infants after delivery (4,5,13,14). A growing body of evidence demonstrates the beneficial effects of prenatal multiple micronutrient (MMN) supplements, particularly in women entering pregnancy with a poor nutritional status (15–17). Iodine is commonly added to MMN supplements, but to our knowledge, the specific impact of iodine delivered in an MMN supplement on iodine status and thyroid function has not been evaluated in moderately iodine-deficient pregnant and lactating women and infants.
The aim of this study was to investigate the effect of an iodine-containing MMN supplement given during pregnancy (providing 300 μg of iodine) in a rural Gambian population exposed to moderate iodine deficiency. We hypothesized that iodine supplementation would improve maternal iodine status and thyroid function in pregnant women and that the impact would last postpartum and improve breast milk iodine concentration (BMIC) and infant iodine status.
Materials and Methods
The current analysis used data and samples collected as part of the Early Nutrition and Immune Development (ENID) trial (ISRCTN49285450), a randomized trial conducted in The Gambia between April 2010 and February 2015.
The objective of the main trial was to assess the effect of combined prenatal and infant nutritional supplementation on infant immune development (18). Full details of the ENID trial have been described in detail in the published trial protocol (18). In brief, pregnant women (aged 18–45 years) from the rural West Kiang region of The Gambia were enrolled. Exclusion criteria were gestational age at enrolment ≥20 weeks, multiple pregnancy, severe anemia (hemoglobin <7 g/dL), or confirmed as HIV positive. When scheduled for prenatal care, pregnant women were randomized to four intervention groups of prenatal dietary supplements: (a) MMNs, (b) iron and folic acid (FeFol = standard care), (c) protein energy (PE), and (d) PE + MMN. Supplementation continued until delivery. In this secondary analysis, we included participants from the two prenatal tablet arms (a) MMN and (b) FeFol). This decision was made on the basis of evidence of differential adherence between the tablet and the lipid-based nutritional supplement (LNS) groups, with significantly lower adherence to supplementation in the LNS groups (19). In ENID, infants were further supplemented with daily LNS or LNS + micronutrients after six months of age. However, the infant intervention arms will not be described here as the present analyses stops at six months after delivery.
The protocol of the original ENID trial was approved by the joint Gambia Government/Medical Research Council (MRC) Unit, The Gambia Ethics Committee (Project No. SCC1126v2). Written informed consent was obtained from all women before enrolment into the trial. The trial observed good clinical practice standards and the current version of the Helsinki Declaration.
Randomization
The women included in this subanalysis of the ENID trial were randomized to one of the two following intervention arms:
-
1.
MMNs, a combination of 15 micronutrients, specifically designed for use during pregnancy as formulated by the World Health Organization (WHO), United Nations University, and United Nations Children's Fund (20), and containing twice the recommended daily allowance for all contained micronutrients, with the exception of FeFol that was set at Gambian Government Guidelines (Supplementary Table S1). The MMN supplement contained 300 μg iodine as potassium iodide.
-
2.
FeFol, representing the usual standard of care during pregnancy as per Gambian Government Guidelines (iron 60 mg/day, folic acid 400 μ/day), with no iodine.
The MMN and FeFol supplements were formulated as tablets and manufactured by Scanpharm, Birkerød, Denmark. The iodine content of the MMN supplement was not verified by independent laboratory testing.
Randomization into the trial was performed in blocks of 8, using an automated system, with the 8 groups reflecting the 8 combinations of prenatal and infancy supplements. The prenatal arm of the full ENID trial was partly open, as it was not possible to blind the field assistants or the women to the supplement type (tablet vs. LNS). However, for the purpose of this analysis, wherein only the two prenatal tablet arms are considered, the trial can be considered as double blinded as the tablets were identical.
Procedures
Clinical visits were performed at baseline, 20 and 30 weeks' gestation, at birth, and 1, 8, 12, 24, and 52 weeks postpartum. Women received an MMN tablet containing 300 μg iodine or a FeFol tablet without iodine, taken once daily at baseline (<20 weeks' gestation) until delivery. Field assistants provided the prenatal supplements on a weekly basis. Compliance was assessed through a count of remaining tablets at the end of each week, and an average weekly compliance was calculated to assess study mean compliance for each group. We assessed side effects by use of a questionnaire at these weekly visits. Serious adverse events were defined as death or hospital admission of either mother or infant for a cause other than delivery. At baseline, a structured questionnaire was administered to collect data on general subject characteristics, race/ethnicity, and educational level.
At baseline, participants' height and weight were measured and gestational age was determined by ultrasound. Body mass index (BMI) was calculated by bodyweight (kg) divided by height (m) squared. A venous blood sample collected from the women after an overnight fast at baseline (<20 weeks' gestation) and 30 weeks' gestation, for measurement of maternal thyroid function, was used in this analysis. The blood samples were immediately put on ice, and then centrifuged, aliquoted, and stored at −80°C. An 24-hour urine sample was collected for measurement of maternal urinary iodine concentration (UIC) at baseline, at 30 weeks' gestation, and 12 weeks postpartum. A field worker visited the women's home every four hours during the day to collect the urine samples (which were stored on ice), and transported the samples to the MRC Keneba field station where they were refrigerated. At the end of 24 hours, the urine samples from each individual woman were pooled, aliquoted, and stored at −20°C.
Immediately after delivery, the placenta was passed to an attending field assistant and a blood sample collected from the umbilical vein. If the woman delivered at home, the sample was put on ice and transported to the MRC Keneba field station. On arrival in the laboratory, samples were centrifuged, aliquoted, and stored at −80°C until processing. Infant birth weight and length were obtained within 72 hours after delivery, by using electronic scales (Seca 336) and length boards (Seca 417), which were precise to 10 g and 1 mm, respectively. Head circumference was also measured at birth, using a circumference measuring tape (Seca 201). Low birth weight is defined as weight at birth of <2500 g irrespective of gestational age (21), preterm birth as gestational age at birth of <37 completed weeks, stunting as height-for-age >2 standard deviations (SDs) below the WHO Child Growth Standards median (22), and wasting as weight-for-length < −2 SDs.
At 8, 12, and 24 weeks postpartum, the women provided a 5 mL breast milk sample from each breast. The samples from right and left breasts were pooled for analysis. The breast milk sample was not collected during a feed or standardized according to the infant's last feed, and was, therefore, a mixture of hind- and foremilk. The breast milk sample was manually expressed between ∼9 and 11 a.m. at the MRC Keneba field station, and immediately put on ice, and stored at −80°C. The majority of the women were fasting when the milk sample was collected, as breakfast (provided at the MRC clinic) was served after the last sample collection. Throughout the trial, participants were asked weekly about breastfeeding practices and introduction of complementary foods. Exclusive breastfeeding (EBF) was defined according to the WHO definition: no other foods or liquids consumed than breast milk with the exception of medicines, or essential vitamins or minerals.
A venous blood sample from each participating infant at 12 and 24 weeks postpartum was used in this analysis. Samples of infant blood were collected by venipuncture, and immediately put on ice before being centrifuged, aliquoted, and stored at −80°C.
The coprimary outcomes in this analysis were maternal UIC and serum thyroglobulin (Tg) concentration (baseline and 30 weeks' gestation). Secondary outcomes were maternal UIC at 12 weeks postpartum, serum thyrotropin (TSH), total triiodothyronine (TT3), total thyroxine (TT4), TT3/TT4 ratio (baseline and 30 weeks' gestation), thyroglobulin antibodies (TgAbs) (baseline), and BMIC (weeks 8, 12, and 24 postpartum) as well as infant serum Tg concentration (birth [cord], 12, and 24 weeks postpartum) and serum TSH in cord blood.
Sample analysis
UIC was measured using inductively coupled plasma mass spectrometry (ICP-MS) (23), at the Human Nutrition Laboratory of Eidgenössische Technische Hochschule (ETH) Zurich (Zurich, Switzerland). WHO criterion based on the median UIC was used to classify adequate iodine intake for pregnant women (≥150 μg/L) (9).
BMIC was measured by ICP-MS at MRC Elsie Widdowson Laboratory (Cambridge, UK). Breast milk samples were first diluted (1:50) with a solution of ultragrade tetramethylammonium hydroxide (TMAH) containing tellurium as internal standard (0.5% TMAH, 20 μg/L tellurium). The samples were then analyzed by ICP-MS along with external matrix-matched calibration standards (commercially sourced pooled breast milk; Sera Laboratories International, Ltd.). Serum and whole blood (RECIPE Chemicals+, Instruments GmbH and Sero AS) were used as quality controls.
Tg was measured in maternal and infant serum using a sandwich serum-Tg enzyme-linked immunosorbent assay (ELISA) (24), at the Human Nutrition Laboratory of ETH Zurich. Liquicheck™ Tumor Marker Control (Bio-Rad Laboratories AG, Cressier, Switzerland; LOT. 19990 and LOT. 19970) was used as the standard. Elevated Tg concentrations during pregnancy, indicating iodine deficiency, is defined as Tg >43.5 μg/L (24,25).
Serum TSH, TT3, TT4, and cord blood TSH were measured by immunoassay (IMMULITE; Siemens Healthcare Diagnostics, UK) at the Human Nutrition Laboratory of ETH Zurich using analyte-specific kits and controls. For TSH during pregnancy, we used trimester-specific reference ranges: 0.1–2.5 mIU/L for the first trimester, 0.2–3.0 mIU/L for the second trimester, and 0.3–3.0 mIU/L for the third trimester (26). For TT4 until gestational week 6, we used the reference range of 58–161 nmol/L; from week 7, we increased the upper reference range by 5% per week until week 15; from week 16 until delivery, we multiplied the nonpregnancy reference range by 1.5 and used the resulting range of 87.0–241.5 nmol/L as a reference (26). For TT3, we used the manufacturer's reference ranges of 1.3–2.6 nmol/L.
Subclinical hypothyroidism was defined as a high TSH and a normal TT4, overt hypothyroidism was defined as a high TSH and a low TT4, overt hyperthyroidism was defined as a low TSH and a high TT4, subclinical hyperthyroidism was defined as low TSH and normal TT4, and isolated hypothyroxinemia was defined as a normal TSH and a low TT4.
Maternal TgAb concentrations were analyzed in baseline samples using a serum ELISA (TgAb ELISA, version 2; RSR, Cardiff, UK). The manufacturer cutoff for TgAb positivity is ≥65 U/mL.
The interassay variability for all analyses are reported in Supplementary Data.
Statistical analysis
Full details of the power calculations applied are provided in the published trial protocol (18). A post hoc power calculation was made based on maternal Tg concentrations from a recent trial of mildly iodine-deficient pregnant women (7), using an SD of 7.5 μg/L. In this study, a total of 175 samples in each supplement arm give 96% power to detect a difference of 3 μg/L or more between the MMN and FeFol arm.
The data were analyzed using STATA version 15 and R (27). Descriptive statistics were applied for all variables. Outliers were identified and removed after visual inspection of box plots stratified by group and time point. Values in the text and tables are presented as mean (SD) for normally distributed data, median (interquartile range or IQR) for non-normal data, and number (%) for prevalence. Baseline characteristics of the study population according to maternal supplement groups were assessed by unpaired t-tests for parametric data, Mann–Whitney U-test for nonparametric data, and Fisher's exact test for categorical dependent variables.
We assessed the intervention effect by fitting individual linear mixed effects models to continuous dependent variables using maximum likelihood procedure for the estimation of variance components. For each variable, an individual mixed effects model was derived with time (two visits for maternal UIC, Tg, TSH, TT3, TT4, infant Tg, and three visits for BMIC), coded as a categorical variable, and maternal supplementation group (MMN or FeFol) as fixed effects. An interaction between time and supplementation was included in the mixed effects models. Between-individual variation was modeled using random effects. For categorical dependent variables, mixed effects logistic regression models were used, using R. For some of the categorical variable analyses, prevalence of thyroid disorders was nonexisting at one or several time points and, therefore, time was not included in these mixed effects logistic regression models.
The residuals were tested for normality and homogeneity of variance using residual plots, and non-normally distributed data were log-transformed and then reanalysed. Outliers were defined as data with residuals >3 SDs from the mean in the linear mixed effects models and were excluded from the models (UIC n = 5 data points removed, maternal Tg n = 3, maternal TSH n = 13, TT3 n = 4, TT4 n = 5, BMIC n = 4, infant Tg n = 5). These outliers were not excluded from the mixed effects logistic regression models.
Interactions between time and supplementation for the linear mixed effects model on BMIC were assessed by likelihood-ratio tests between two nested linear mixed effects models, one model with and the other without the interaction terms. The overall supplementation effect for BMIC independent of time was assessed by a likelihood-ratio test comparing two nested mixed effects models, one with maternal supplement group (and its time interaction) and the other without maternal supplement group (i.e., with fixed effects for time only). The likelihood-ratio test tests whether the model including maternal supplement group as a predictor gave a significantly better fit to the data than that without.
The Mann–Whitney U-test was used to assess group differences in Tg and TSH at birth (cord blood), and maternal UIC at 12 weeks postpartum. Estimated daily maternal iodine intake was calculated using daily iodine excretion at baseline (using UIC and measured urine volume of the 24-hour urine excretion) and assuming an average iodine bioavailability of 90% (28).
Statistical significance was set at p < 0.05.
Results
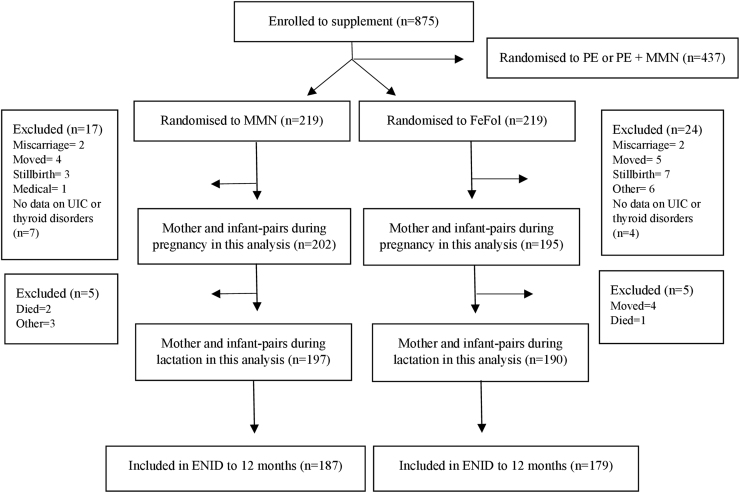
A total of 2798 women consented to the ENID study and 875 pregnant women were eligible. In this study, we only included pregnant women randomly assigned from the MMN (n = 219) and FeFol arms (n = 219). For the analyses conducted during pregnancy, 397 mother–infant pairs were included and 387 mother–infant pairs for the analyses during lactation (Fig. 1).
FIG. 1.
Trial profile for the ENID MMN and FeFol supplement groups and data included in this analysis. ENID, Early Nutrition and Immune Development; FeFol, iron and folic acid; MMN, multiple micronutrient; PE, protein energy; UIC, urinary iodine concentration.
At baseline, mean age of the participating women was 29.5 years (SD 6.7) and the mean gestational age was 13.7 weeks (3.4) (Table 1). The mean BMI at baseline was 21.1 kg/m2 (3.5), and 20% (88 of 437) of the women were underweight (BMI <18.5 kg/m2) and 10% (45 of 437) were overweight (BMI ≥25 kg/m2). The majority (77%, 329 of 430) of the participating women had received no formal Arabic or English schooling. The study population had a mean parity of 4.1 (2.7). There were no differences in baseline characteristics between women from the two supplement groups. A small, but significant (1.79 cm, p = 0.04), difference was observed in height between women who were initially randomized to supplementation, and those who were lost to follow-up, but no other differences in baseline characteristics were observed (data not presented). Compliance rates for women receiving the MMN and FeFol were 93.1% and 95.7%, respectively (19).
Table 1.
Characteristics of Women at Baseline and Infants at Birth
| N | MMN | FeFol | |
|---|---|---|---|
| Maternal age (years) | 438 | 29.1 (6.7) | 29.9 (6.7) |
| Maternal weight (kg) | 437 | 55.4 (9.8) | 55.0 (9.0) |
| Maternal height (cm) | 438 | 162.1 (5.7) | 161.6 (6.2) |
| Maternal BMI (kg/m2) | 437 | 21.1 (3.8) | 21.0 (3.2) |
| Gestational age at baseline (weeks) | 436 | 13.7 (3.4) | 13.8 (3.4) |
| Parity | 431 | ||
| Primiparous | 27 (13%) | 22 (10%) | |
| Multiparous (≥1 previous pregnancy) | 189 (88%) | 193 (90%) | |
| Maternal educationa | 430 | ||
| No education | 159 (73%) | 170 (80%) | |
| Low (1–7 years) | 28 (13%) | 25 (12%) | |
| Medium (8–14 years) | 31 (14%) | 17 (8%) | |
| Still birth | 438 | 3 (1.4%) | 7 (3.2%) |
| Gestational age at birth | 389 | 40.3 (1.6) | 40.1 (1.7) |
| Gestational age at birth categories | 389 | ||
| <37 weeks | 7 (4%) | 6 (3%) | |
| 37–40 weeks | 70 (36%) | 82 (43%) | |
| >40 weeks | 120 (61%) | 104 (54%) | |
| Infant birth weight | 328 | 3.010 (0.4) | 2.992 (0.4) |
| Birth weight categories | |||
| Low birth weight (<2.5 kg) | 15 (9%) | 16 (10%) | |
| Normal birth weight (2.5–3.9 kg) | 155 (91%) | 140 (89%) | |
| High birth weight (≥4.0 kg) | 1 (1%) | 1 (1%) | |
| Infant birth length (cm) | 340 | 49.5 (2.0) | 49.6 (1.8) |
| WAZ at birth | 328 | −0.62 (0.9) | −0.65 (0.9) |
| LAZ at birth | 340 | −0.10 (1.05) | −0.08 (1.0) |
| WLZ at birth | 320 | −0.90 (1.3) | −1.02 (1.2) |
| Infant head circumference at birth | 339 | 33.2 (1.4) | 33.4 (1.4) |
Data are n (%) or mean (SD).
Maternal education was defined as completed years of either English or Arabic schooling.
BMI, body mass index; FeFol, iron and folic acid; LAZ, length-for-age z-score; MMN, multiple micronutrient; SD, standard deviation; WAZ, weight-for-age z-score; WLZ, weight-for-length z-score.
Infants were born with a mean birth weight of 3002 g (0.4) and 9.5% (31 of 328) were born with a low birth weight (<2500 g) (Table 1). Infant mean weight-for-age z-score, length-for-age z-score (LAZ), and weight-for-length z-score (WLZ) at birth were −0.64 (0.9), −0.09 (1.0), and −0.96 (1.3) with no difference according to maternal supplement group (Table 1). Mean infant head circumference was 33.3 cm (1.4) at birth. The majority of infants (93%, 359 of 385) were exclusively breastfed to 3 months of age and 31% (121 of 385) to 6 months of age. The mean age of discontinuation of EBF was 5.2 (1.3) months. Age of discontinuation of EBF did not differ between maternal supplement groups (data not presented). Infants were growth faltering, with 23% (81 of 347) stunting (LAZ < −2) and 14% (50 of 347) wasting (WLZ < −2) at 2 years of age.
Maternal median UIC at baseline was 51 μg/L (IQR 33–82), and the estimated median iodine intake was 71 μg/day (44–104), indicating moderate iodine deficiency. Maternal MMN supplementation during pregnancy significantly improved maternal UIC compared with FeFol (p < 0.001; Table 2). Maternal median UIC at 12 weeks postpartum was 34 μg/L (22–52) and 39 μg/L (25–64) for the FeFol and MMN groups, respectively (p = 0.08). Between 30 weeks' gestation and 12 weeks postpartum, maternal UIC decreased in both supplement groups (p < 0.001 for both groups).
Table 2.
Maternal Iodine Status and Thyroid Function and Disorders During Pregnancy According to Maternal Supplement Group
| N | Baseline | N | 30 weeks' gestation | p* | |
|---|---|---|---|---|---|
| UIC (μg/L) | |||||
| MMN | 171 | 56 (29–89) | 159 | 90 (45–177) | |
| FeFol | 167 | 48 (35–80) | 156 | 41 (28–74) | <0.001 |
| Tg (μg/L) | |||||
| MMN | 186 | 20.8 (11.3–41.6) | 191 | 16.8 (8.6–32.8) | |
| FeFol | 180 | 21.8 (12.6–38.2) | 184 | 24.4 (13.1–41.2) | <0.001 |
| TSH (mIU/L) | |||||
| MMN | 170 | 0.7 (0.3–1.2) | 182 | 1.1 (0.7–1.6) | |
| FeFol | 162 | 0.7 (0.4–1.1) | 182 | 1.2 (0.8–1.6) | 0.3 |
| TT3 (nmol/L) | |||||
| MMN | 153 | 2.5 (0.6) | 166 | 3.1 (0.6) | |
| FeFol | 147 | 2.5 (0.7) | 169 | 3.1 (0.6) | 0.9 |
| TT4 (nmol/L) | |||||
| MMN | 156 | 142.9 (35.6) | 171 | 149.2 (24.9) | |
| FeFol | 151 | 138.0 (42.0) | 172 | 146.0 (26.4) | 0.5 |
| TT3/TT4 ratio | |||||
| MMN | 152 | 0.018 (0.004) | 166 | 0.021 (0.005) | |
| FeFol | 147 | 0.019 (0.005) | 169 | 0.021 (0.005) | 0.4 |
| Elevated Tg | |||||
| MMN | 186 | 23.7% (44) | 191 | 13.1% (25) | |
| FeFol | 180 | 20.6% (37) | 184 | 22.3% (41) | <0.001 |
| Positive TgAb | |||||
| MMN | 135 | 2.2% (3) | — | ||
| FeFol | 136 | 4.4% (6) | — | — | |
| Subclinical hypothyroidism | |||||
| MMN | 153 | 0.0 (0) | 170 | 2.9% (5) | |
| FeFol | 145 | 2.1% (3) | 172 | 2.9% (5) | 0.8** |
| Overt hypothyroidism | |||||
| MMN | 153 | 0.0 (0) | 170 | 0.0 (0) | |
| FeFol | 145 | 0.0 (0) | 172 | 0.0 (0) | — |
| Subclinical hyperthyroidism | |||||
| MMN | 153 | 12.4% (19) | 170 | 5.9% (10) | |
| FeFol | 145 | 9.0% (13) | 172 | 2.3% (4) | 0.6 |
| Overt hyperthyroidism | |||||
| MMN | 153 | 3.4% (5) | 170 | 0.0 (0) | |
| FeFol | 145 | 2.1% (3) | 172 | 0.0 (0) | 0.5** |
| Isolated hypothyroxinemia | |||||
| MMN | 153 | 1.3% (2) | 170 | 0.0 (0) | |
| FeFol | 145 | 2.8% (4) | 172 | 0.0 (0) | 0.4** |
Data are median (IQR) (non-normally distributed data), means (SD), or % (n) derived from raw data. Non-normally distributed data were log-transformed before analysis. Continuous dependent variables were analyzed using linear mixed effects models and categorical dependent variables were analyzed using mixed effects logistic regression models. Subclinical hypothyroidism is defined as high TSH and normal TT4 (relative to gestational age specific cutoffs), overt hypothyroidism is defined as high TSH and low TT4, subclinical hyperthyroidism is defined as low TSH and normal TT4, overt hyperthyroidism is defined as low TSH and high TT4, and isolated hypothyroxinemia is defined as normal TSH and low TT4.
The p-value tests time by supplement interaction.
This p-value is derived without time included in the mixed effects model.
IQR, interquartile range; Tg, thyroglobulin; TgAbs, thyroglobulin antibodies; TSH, thyrotropin; TT3, total triiodothyronine; TT4, total thyroxine; UIC, urinary iodine concentration.
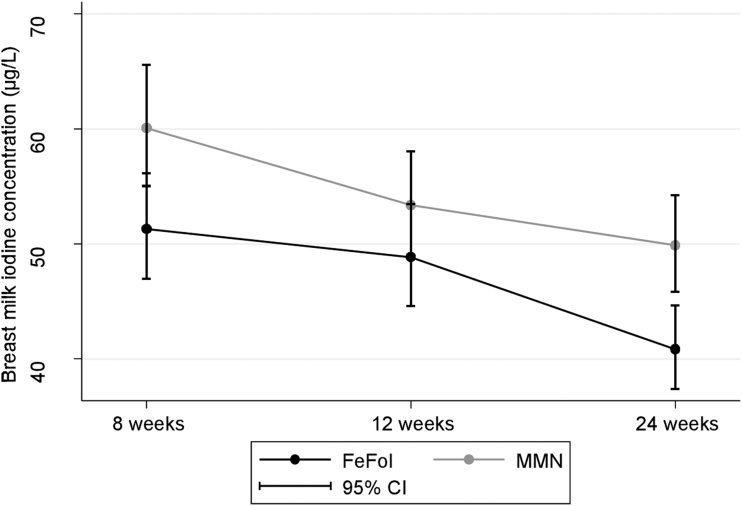
Median BMIC at 8 weeks postpartum was 54 μg/L (37–79), with 57 μg/L (41–83) and 51 μg/L (35–74) for the MMN and FeFol groups, respectively (Table 3). There were no difference in BMIC between supplement groups over the course of the study (p = 0.3; Table 3); however, there was a significant difference in BMIC independent of time (p = 0.006), with a higher BMIC in the MMN group (Fig. 2).
Table 3.
Breast Milk Iodine Concentration and Infant Thyroglobulin Concentration According to Maternal Supplement Group
| N | 8 Weeks postpartum | N | 12 Weeks postpartum | N | 24 Weeks postpartum | p* | |
|---|---|---|---|---|---|---|---|
| Breast milk | |||||||
| BMIC (μg/L) | |||||||
| MMN | 160 | 57 (41–83) | 175 | 51 (35–72) | 175 | 51 (32–74) | |
| FeFol | 154 | 51 (35–74) | 153 | 44 (33–73) | 157 | 39 (30–57) | 0.3 |
| Infants | |||||||
| Tg (μg/L) | |||||||
| MMN | — | 163 | 87 (59–127) | 152 | 67 (42–95) | ||
| FeFol | 165 | 87 (58–124) | 159 | 70 (47–91) | 0.9 | ||
Data are median (IQR) derived from raw data. Data were log-transformed before analysis. Data were analyzed using linear mixed effects models.
The p-value tests time by supplement interaction.
BMIC, breast milk iodine concentration.
FIG. 2.
Breast milk iodine concentration (μg/L) (geometric means) according to supplement group at 8, 12, and 24 weeks postpartum. CI, confidence interval.
At baseline, maternal median Tg concentration was 22 μg/L (12–39), and 22% (81 of 366) of women had elevated Tg (>43.5 μg/L). Maternal MMN supplementation during pregnancy significantly decreased maternal Tg concentration compared with FeFol (p < 0.001; Table 2), and significantly decreased the prevalence of elevated Tg (p < 0.001; Table 2). Only 3.3% (9 of 271) of the pregnant women tested positive for TgAb at baseline, and with no difference between the supplement groups (p = 0.3; Table 2).
There were no differences in the mean or median concentrations between supplement groups in any of the other maternal thyroid function tests (TSH, TT3, TT4, and TT3/TT4 ratio) during pregnancy (Table 2). At baseline, 1.0% (3 of 298) of the mothers had subclinical hypothyroidism and none (0 of 298) had overt hypothyroidism.
At baseline, 2.7% (8 of 298) had overt hyperthyroidism, 2.0% (6 of 298) were hypothyroxinemic, and 10.7% (32 of 298) were affected by subclinical hyperthyroidism, but the prevalence was reduced in both groups at 30 weeks' gestation with no significant overall difference between groups (Table 2).
Maternal MMN supplementation during pregnancy had an effect on cord blood Tg (p < 0.001), with lower cord blood Tg concentration in the MMN group. The median cord blood Tg concentration was 100 μg/L (51–140) in the MMN group (n = 121) and 127 μg/L (81–191) in the FeFol group (n = 108). Furthermore, maternal Tg at 30 week's gestation was associated with cord blood Tg (β coefficient = 0.295 [confidence interval 0.077–0.512], p = 0.008).
Maternal MMN supplementation during pregnancy did not have an effect on infant serum Tg concentration postpartum (p = 0.9; Table 3). Infant Tg concentrations significantly decreased between 12 and 24 weeks postpartum for both maternal supplement groups (p < 0.001).
Median infant TSH concentration in cord blood did not differ between the two supplement groups: 5 mIU/L (4–9) in the MMN group (n = 114) versus 6 mIU/L (4–10) in the FeFol group (n = 105, p = 0.2).
A subanalysis was performed investigating the relationship between gestational age at baseline and maternal Tg, TSH, and TT4 at 30 weeks' gestation in the MMN group; however, no associations were found (data not shown).
Discussion
Our study shows that supplementing moderately iodine-deficient pregnant women with an MMN supplement containing 300 μg/day of iodine versus FeFol improved maternal iodine status and reduced maternal Tg concentration at 30 weeks' gestation, but had negligible impact on maternal thyroid hormone production. Our results further show that prenatal iodine supplementation alone is not sufficient to ensure adequate iodine status in mothers and infants after delivery.
The estimated maternal iodine intake at baseline was 71 μg/day, which falls far below the recommended intake of 250 μg/day (9). Compliance with supplementation regimen was high and although the median UIC increased at 30 weeks' gestation in the group receiving MMN, the median UIC concentration remained below the recommended threshold of 150 μg/L (9). This threshold is, however, based on UIC from spot urine, which has been shown to have a higher UIC than the 24-hour urine samples (29). The finding is in agreement with earlier studies (5,30) and is perhaps not surprising, considering this study was conducted in rural Gambia where pregnant women are at risk of iodine deficiency (11). The women likely had largely depleted thyroid iodine stores when entering pregnancy, and most of the ingested iodine was taken up by the thyroid for both production of thyroid hormone and rebuilding stores, resulting in a lower fraction excreted in the urine.
The median maternal Tg concentration at baseline (22 μg/L) was elevated above the assay-specific target median of 10 μg/L typically observed in a iodine sufficient population (25). The turnover and excretion of Tg from the thyroid are increased during iodine deficiency as thyroid activity increases to adapt to low iodine intakes (31). The elevated Tg concentration thus suggest thyroid stress, that is increased thyroid activity to produce adequate thyroid hormone in the face of limited iodine supply. The improvement in iodine status in the MMN group decreased the Tg concentration and reduced thyroid stress. Tg is a sensitive biomarker of iodine status throughout the life cycle (25,32). Our results agree well with previous studies on maternal Tg concentration of prenatal iodine supplementation in mild and moderate iodine deficiency (5,7), and findings confirm the sensitivity of Tg to assess changes in thyroid stress in response to changes in iodine intake during pregnancy (33). In mild-to-moderate iodine deficiency, increased thyroid activity can compensate for low iodine intake and maintain euthyroidism in most individuals (34). This is confirmed in our study by TSH, TT3, and TT4 concentrations within the normal reference ranges (26) and low prevalence of maternal hypothyroidism and hypothyroxinemia: the prevalence of subclinical hyperthyroidism was 10.7% in the mothers at baseline, but the prevalence of overt hyperthyroidism was lower at 2.7%. We observed no effect of prenatal iodine supplementation on maternal thyroid function.
Our findings on maternal iodine and thyroid status agree with earlier intervention studies conducted in mild-to-moderately iodine-deficient populations (5,30) and a recent randomized controlled trial of prenatal iodine supplementation (200 μg/day) in mildly iodine-deficient pregnant women (7). The latter study reported improved maternal iodine status and reduced thyroid stress, but no effect on maternal thyroid function, and no long-term benefits on development were observed in children at 5–6 years. It is uncertain how low the iodine intake can be without affecting circulating thyroxine and triiodothyronine concentrations. Our data suggest that thyroid adaptation maintains euthyroidism also at moderately deficient iodine status. Therefore, the lack of effect of iodine supplementation on thyroid hormone concentrations is not surprising. Iodine supplementation of pregnant women is recommended in populations with mild-to-moderate maternal iodine deficiency, particularly where the coverage of iodized salt is low (12), as in our study population (11). The supplemental dose of 300 μg iodine slightly exceeds the recommended dietary intake of 250 μg, but is appropriate and safe considering the degree of iodine deficiency in our population. A high dose of iodine given to chronically iodine-deficient adults may transiently induce hyperthyroidism (35,36), but this was not observed in our study. The 8 cases of overt hyperthyroidism observed at baseline resolved over the course of the study and no cases were observed at 30 weeks' gestation. Furthermore, we observed no decrease in TT4 in supplemented women, as previously reported in a cross-sectional study conducted in a moderately iodine-deficient population (37).
BMIC is strongly associated with the iodine intake of the mother (38), and is the most accurate biomarker of iodine status during lactation (39). BMIC did not differ according to supplement group over the course of the study and the median concentration was more than three times lower than reported in lactating women in iodine replete populations (39). Furthermore, the median UIC in the women at 12 weeks postpartum did not differ between the groups and was below the WHO threshold of 100 μg/L (9). Our data suggest that in a population with persistently low dietary iodine intakes postpartum, prenatal supplemental iodine has minimal long-term effect on excretion in breast milk. In areas of iodine deficiency, maternal postnatal iodine supplementation may be justified to ensure adequate maternal iodine status during lactation, to maintain adequate BMIC and infant iodine status (12).
The estimated iodine intake in the breastfeeding infants in our study was 42 μg/day [assuming a breast milk intake of 0 · 78 L (40)], only half of the dietary iodine requirements (41,42). The elevated Tg concentration observed in the infants from both supplement groups at 12 and 24 weeks postpartum suggests deficient iodine intakes, although no reference range has been established for this age group for the assay used. Circulating Tg levels are typically high in early infancy but fall over the first year of life, likely stabilizing by about 6 months to 2 years of age (43), and the Tg concentrations in the infants in our study followed this pattern. We observed no group differences in the infant Tg concentrations, and thus no long-term effect of maternal prenatal iodine supplementation in the infants. At birth, the cord blood Tg concentration was lower in the MMN group than in the FeFol group, at a ratio comparable with the maternal serum Tg concentration at 30 weeks' gestation. The TSH concentration in cord blood was comparable between the two supplement groups. These findings add to previous observational data and controlled studies reporting associations between Tg, TSH, and thyroid hormone concentrations measured in cord blood and maternal thyroid function (5,7,44,45).
Our finding would support the need for iodine supplementation or the inclusion of iodine in MMN supplements in moderately iodine-deficient populations to improve the iodine intake during pregnancy. However, universal salt iodization is the primary intervention strategy to prevent iodine deficiency in the general population (9). Recent data demonstrate that adequately iodized salt at high coverage meets the requirements of all population groups, including pregnant and lactating women (46). The developing fetal brain is especially vulnerable during the first trimester when the fetus relies on maternal thyroid hormone supply (3). Universal salt iodization ensures adequate iodine intake and sufficient maternal iodine stores when the mother enters pregnancy to maintain optimal fetal thyroid hormone supply at a critical time window when targeted supplementation unlikely is introduced yet. The current WHO position recommending targeted iodine supplementation to pregnant and lactating women primarily in populations with poor coverage of iodized salt remains valid. For this rural Gambian population, important prevention strategies are to ensure that locally produced salt is iodized adequately or that MMN supplementation during pregnancy is standard care rather than FeFol supplementation (47).
The strengths of this study are the randomized design of the ENID trial conducted in a moderately deficient population of pregnant and lactating women, with multiple pre- and postnatal measures of iodine status and thyroid function parameters, along with BMIC during the first six months of lactation. Few well-powered studies have been conducted in areas with moderate iodine deficiency, and even fewer have studied pregnancy, lactation, and infancy and measured the range of biomarkers as done in this study. The drop-out rate was overall low, with only 10% dropouts between birth and 1 year follow-up; furthermore, attrition was low and balanced between study arms. Furthermore, ICP-MS was used to measure UIC and BMIC, the gold standard method for these markers (39,48). Moreover, a 24-hour UIC sample was used rather than a spot UIC and we estimated the iodine intake using the daily iodine excretion obtained from the urine volume measured in the 24-hour urine collection. A limitation of this study is that the intervention of focus was a MMN, and not a trial of an iodine supplement in isolation. Furthermore, we did not measure selenium status in these women. We recognize that the results obtained could be influenced by known or unknown interactions between micronutrients. However, the potential interaction of iron deficiency and folate status was accounted for as the same FeFol dose was used in the two groups and thereby accounted for a possible confounder in this iron-deficient population (49). Infant thyroid hormones were not investigated longitudinally, and infant UIC was not measured, as infant urine was not collected as a part of ENID. This could have improved the interpretation of infant iodine status in this population. Lastly, there were no data available regarding serum TPO levels, thyroid-, or antithyroid medication use in this study population.
In conclusion, we observed that in this moderately iodine-deficient population, supplementation during pregnancy with an iodine-containing MMN improved maternal iodine status. Despite markedly inadequate iodine intake, pregnant women were overall euthyroid and supplemental iodine had limited impact on maternal thyroid hormone production. Our data suggest that prenatal iodine supplementation does not ensure optimal postnatal maternal iodine status, BMIC, and infant iodine status during the first six months after birth. Universal salt iodization should remain the main strategy to prevent iodine deficiency during pregnancy, lactation, and early infancy. If the coverage of iodized salt is poor and prenatal supplementation is required, maternal iodine supplementation should be continued through lactation to increase maternal iodine status, BMIC, and infant iodine status.
Supplementary Material
Acknowledgments
We thank the women and their infants of West Kiang who patiently participated in the study. We acknowledge the enthusiastic work of the ENID study team, especially the fieldworkers, village assistants, midwives, clinical staff, and laboratory technicians who tirelessly collected the data and samples. We acknowledge the support of Mr. Bakary Sonko, and colleagues in the Keneba data office, for the development and management of the ENID database. We are grateful for the advice and input of Dr. Will Johnson and Prof. Ann Prentice in the statistical design of this study.
Authors' Contributions
S.E.M. conceived and implemented the ENID trial. K.G.E., M.A., M.B.Z., and S.E.M. conceived this add-on study to the ENID trial. S.H. and K.G.E. conducted sample analysis. K.G.E. conducted the statistical analyses, and wrote the first draft of the article. All authors contributed to the final version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was jointly funded by the MRC and the Department for International Development (DFID) under the MRC/DFID Concordat agreement (MRC Programmes MC_UP_1005/1 and MR/P012019/1).
Supplementary Material
References
- 1. Delange F. 1998. Screening for congenital hypothyroidism used as an indicator of the degree of iodine deficiency and of its control. Thyroid 8:1185–1192 [DOI] [PubMed] [Google Scholar]
- 2. Zimmermann MB. 2009. Iodine deficiency. Endocr Rev 30:376–408 [DOI] [PubMed] [Google Scholar]
- 3. de Escobar GM, Obregon MJ, del Rey FE. 2007. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr 10:1554–1570 [DOI] [PubMed] [Google Scholar]
- 4. Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. 2016. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr 104(Suppl. 3):918s–923s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor PN, Okosieme OE, Dayan CM, Lazarus JH. 2014. Therapy of endocrine disease: impact of iodine supplementation in mild-to-moderate iodine deficiency: systematic review and meta-analysis. Eur J Endocrinol 170:R1–R15 [DOI] [PubMed] [Google Scholar]
- 6. Bath SC. 2019. The effect of iodine deficiency during pregnancy on child development. Proc Nutr Soc 78:150–160 [DOI] [PubMed] [Google Scholar]
- 7. Gowachirapant S, Jaiswal N, Melse-Boonstra A, Galetti V, Stinca S, Mackenzie I, Thomas S, Thomas T, Winichagoon P, Srinivasan K, Zimmermann MB. 2017. Effect of iodine supplementation in pregnant women on child neurodevelopment: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 5:853–863 [DOI] [PubMed] [Google Scholar]
- 8. Bougma K, Aboud FE, Harding KB, Marquis GS. 2013. Iodine and mental development of children 5 years old and under: a systematic review and meta-analysis. Nutrients 5:1384–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (WHO), United Nations Children's Fund, International Council for Control of IDD 2007 Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Pogramme Managers Third edition. WHO, Geneva, Switzerland [Google Scholar]
- 10. Iodine Global Network. Global scorecard of iodine nutrition in 2017. Available at www.ign.org/cm_data/IGN_Global_Scorecard_AllPop_and_PW_May20171.pdf (accessed March6, 2019)
- 11. Petry N, Jallow B, Sawo Y, Darboe MK, Barrow S, Sarr A, Ceesay PO, Fofana MN, Prentice AM, Wegmüller R, Rohner F, Phall MC, Wirth JP. 2019. Micronutrient deficiencies, nutritional status and the determinants of anemia in children 0–59 months of age and non-pregnant women of reproductive age in the Gambia. Nutrients 11:2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersson M, de Benoist B, Delange F, Zupan J. 2007. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr 10:1606–1611 [DOI] [PubMed] [Google Scholar]
- 13. Zhou SJ, Anderson AJ, Gibson RA, Makrides M. 2013. Effect of iodine supplementation in pregnancy on child development and other clinical outcomes: a systematic review of randomized controlled trials. Am J Clin Nutr 98:1241–1254 [DOI] [PubMed] [Google Scholar]
- 14. Censi S, Watutantrige-Fernando S, Groccia G, Manso J, Plebani M, Faggian D, Mion MM, Venturini R, Andrisani A, Casaro A, Vita P, Avogadro A, Camilot M, Scaroni C, Bertazza L, Barollo S, Mian C. 2019. The effects of iodine supplementation in pregnancy on iodine status, thyroglobulin levels and thyroid function parameters: results from a randomized controlled clinical trial in a mild-to-moderate iodine deficiency area. Nutrients 11:2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haider BA, Bhutta ZA. 2017. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 4:CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith ER, Shankar AH, Wu LS, Aboud S, Adu-Afarwuah S, Ali H, Agustina R, Arifeen S, Ashorn P, Bhutta ZA, Christian P, Devakumar D, Dewey KG, Friis H, Gomo E, Gupta P, Kaestel P, Kolsteren P, Lanou H, Maleta K, Mamadoultaibou A, Msamanga G, Osrin D, Persson LA, Ramakrishnan U, Rivera JA, Rizvi A, Sachdev HPS, Urassa W, West KP Jr., Zagre N, Zeng L, Zhu Z, Fawzi WW, Sudfeld CR. 2017. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Global Health 5:e1090–e1100 [DOI] [PubMed] [Google Scholar]
- 17. Prado EL, Sebayang SK, Apriatni M, Adawiyah SR, Hidayati N, Islamiyah A, Siddiq S, Harefa B, Lum J, Alcock KJ, Ullman MT, Muadz H, Shankar AH. 2017. Maternal multiple micronutrient supplementation and other biomedical and socioenvironmental influences on children's cognition at age 9–12 years in Indonesia: follow-up of the SUMMIT randomised trial. Lancet Global Health 5:e217–e228 [DOI] [PubMed] [Google Scholar]
- 18. Moore S, Fulford A, Darboe M, Jobarteh M, Jarjou L, Prentice A. 2012. A randomized trial to investigate the effects of pre-natal and infant nutritional supplementation on infant immune development in rural Gambia: the ENID trial: Early Nutrition and Immune Development. BMC Pregnancy Childbirth 12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore SE, Fulford AJC, Sosseh F, Nshe P, Darboe MK, Prentice AM. 2019. Thymic size is increased by infancy, but not pregnancy, nutritional supplementation in rural Gambian children: a randomized clinical trial. BMC Med 17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization, United Nations University, United Nations Children's Fund (UNICEF) 1999. Composition of a Multi-micronutrient Supplement to be Used in Pilot Programs among Pregnant Women in Developing Countries. UNICEF, New York [Google Scholar]
- 21. United Nations Children's Fund (UNICEF), World Health Organization (WHO) 2004. Low Birthweight: Country, Regional and Global Estimates. UNICEF and WHO, New York [Google Scholar]
- 22. World Health Organization (WHO) 2009 Multicentre Growth Reference Study Group. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. WHO, Geneva, Switzerland
- 23. Caldwell KL, Maxwell CB, Makhmudov A, Pino S, Braverman LE, Jones RL, Hollowell JG. 2003. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem 49:1019–1021 [DOI] [PubMed] [Google Scholar]
- 24. Stinca S, Andersson M, Erhardt J, Zimmermann MB. 2016. Development and validation of a new low-cost enzyme-linked immunoassay for serum and dried blood spot thyroglobulin. Thyroid 25:1297–1305 [DOI] [PubMed] [Google Scholar]
- 25. Stinca S, Andersson M, Weibel S, Herter-Aeberli I, Fingerhut R, Gowachirapant S, Hess SY, Jaiswal N, Jukic T, Kusic Z, Mabapa NS, Nepal AK, San Luis TO, Zhen JQ, Zimmermann MB. 2017. Dried blood spot thyroglobulin as a biomarker of iodine status in pregnant women. J Clin Endocrinol Metab 102:23–32 [DOI] [PubMed] [Google Scholar]
- 26. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 27:315–389 [DOI] [PubMed] [Google Scholar]
- 27. R Core Team 2019 R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria
- 28. Zimmermann MB, Andersson M. 2012. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70:553–570 [DOI] [PubMed] [Google Scholar]
- 29. Perrine CG, Cogswell ME, Swanson CA, Sullivan KM, Chen TC, Carriquiry AL, Dodd KW, Caldwell KL, Wang CY. 2014. Comparison of population iodine estimates from 24-hour urine and timed-spot urine samples. Thyroid 24:748–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harding KB, Pena-Rosas JP, Webster AC, Yap CM, Payne BA, Ota E, De-Regil LM. 2017. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev 3:CD011761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Citterio CE, Targovnik HM, Arvan P. 2019. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 15:323–338 [DOI] [PubMed] [Google Scholar]
- 32. Ma ZF, Skeaff SA. 2014. Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid 24:1195–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo Y, Ishido Y, Hiroi N, Ishii N, Suzuki K. 2014. The emerging roles of thyroglobulin. Adv Endocrinol 2014:7 [Google Scholar]
- 34. Zimmermann MB, Boelaert K. 2015. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3:286–295 [DOI] [PubMed] [Google Scholar]
- 35. Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, Braverman LE, Medeiros-Neto G. 1998. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid 8:83–100 [DOI] [PubMed] [Google Scholar]
- 36. Delange F, de Benoist B, Alnwick D. 1999. Risks of iodine-induced hyperthyroidism after correction of iodine deficiency by iodized salt. Thyroid 9:545–556 [DOI] [PubMed] [Google Scholar]
- 37. Abel MH, Korevaar TIM, Erlund I, Villanger GD, Caspersen IH, Arohonka P, Alexander J, Meltzer HM, Brantsaeter AL. 2018. Iodine intake is associated with thyroid function in mild to moderately iodine deficient pregnant women. Thyroid 28:1359–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dror DK, Allen LH. 2018. Iodine in human milk: a systematic review. Adv Nutr 9:347s–357s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dold S, Zimmermann MB, Aboussad A, Cherkaoui M, Jia Q, Jukic T, Kusic Z, Quirino A, Sang Z, San Luis TO, Vandea E, Andersson M. 2017. Breast milk iodine concentration is a more accurate biomarker of iodine status than urinary iodine concentration in exclusively breastfeeding women. J Nutr 147:528–537 [DOI] [PubMed] [Google Scholar]
- 40. da Costa TH, Haisma H, Wells JC, Mander AP, Whitehead RG, Bluck LJ. 2010. How much human milk do infants consume? Data from 12 countries using a standardized stable isotope methodology. J Nutr 140:2227–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Institute of Medicine 2001 Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic Boron, Chromium Copper, Iodine Iron, Manganese Molybdenum, Nickel Silicon, Vanadium and Zinc National Academy Press, Washington, DC [Google Scholar]
- 42. Dold S, Zimmermann MB, Baumgartner J, Davaz T, Galetti V, Braegger C, Andersson M. 2016. A dose-response crossover iodine balance study to determine iodine requirements in early infancy. Am J Clin Nutr 104:620–628 [DOI] [PubMed] [Google Scholar]
- 43. Sobrero G, Munoz L, Bazzara L, Martin S, Silvano L, Iorkansky S, Bergoglio L, Spencer C, Miras M. 2007. Thyroglobulin reference values in a pediatric infant population. Thyroid 17:1049–1054 [DOI] [PubMed] [Google Scholar]
- 44. Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VV, Hofman A, Hooijkaas H, Steegers EA, Tiemeier H, Bongers-Schokking JJ, Visser TJ. 2012. Maternal early pregnancy and newborn thyroid hormone parameters: the Generation R study. J Clin Endocrinol Metab 97:646–652 [DOI] [PubMed] [Google Scholar]
- 45. Korevaar TI, Chaker L, Jaddoe VW, Visser TJ, Medici M, Peeters RP. 2016. Maternal and birth characteristics are determinants of offspring thyroid function. J Clin Endocrinol Metab 101:206–213 [DOI] [PubMed] [Google Scholar]
- 46. Dold S, Zimmermann MB, Jukic T, Kusic Z, Jia Q, Sang Z, Quirino A, San Luis TOL, Fingerhut R, Kupka R, Timmer A, Garrett GS, Andersson M. 2018. Universal salt iodization provides sufficient dietary iodine to achieve adequate iodine nutrition during the first 1000 days: a cross-sectional multicenter study. J Nutr 148:587–598 [DOI] [PubMed] [Google Scholar]
- 47. Bourassa MW, Osendarp SJM, Adu-Afarwuah S, Ahmed S, Ajello C, Bergeron G, Black R, Christian P, Cousens S, de Pee S, Dewey KG, Arifeen SE, Engle-Stone R, Fleet A, Gernand AD, Hoddinott J, Klemm R, Kraemer K, Kupka R, McLean E, Moore SE, Neufeld LM, Persson L-Å, Rasmussen KM, Shankar AH, Smith E, Sudfeld CR, Udomkesmalee E, Vosti SA. 2020. Antenatal multiple micronutrient supplementation: call to action for change in recommendation. Ann N Y Acad Sci 1465:5–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huynh D, Zhou SJ, Gibson R, Palmer L, Muhlhausler B. 2015. Validation of an optimized method for the determination of iodine in human breast milk by inductively coupled plasma mass spectrometry (ICPMS) after tetramethylammonium hydroxide extraction. J Trace Elements Med Biol 29:75–82 [DOI] [PubMed] [Google Scholar]
- 49. Bah A, Pasricha SR, Jallow MW, Sise EA, Wegmuller R, Armitage AE, Drakesmith H, Moore SE, Prentice AM. 2017. Serum hepcidin concentrations decline during pregnancy and may identify iron deficiency: Analysis of a longitudinal pregnancy cohort in the Gambia. J Nutr 147:1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.