Abstract
Immunogenic, methionine copper-induced response had proven to be precedent in providing resistance against certain diseases in fish. This study allocates the fitness strategy for Oreochromis niloticus by introducing and incorporating the well-designed, stabilized, and biocompatible N-carbamoyl-methionine copper (NCM-Cu) as a Cu potent source in diet that enhances the bioavailability and fitness. The synchronized NCM-Cu complex was characterized by directing ultraviolet and visible spectrophotometry (UV–vis), Fourier-transform infrared (FTIR), X-ray diffractometry (XRD), thermogravimetric analysis (TGA), and single-crystal X-ray diffraction. Results revealed blue columnar crystalline, NCM-Cu complex with an empirical formula as C12H30CuN4O10S2. Anonymously, the overall growth performance of the fish remained unaltered with NCM-Cu adjunct feed. NCM-Cu significantly raised the Cu accumulation in the fish muscles, liver, gill, and intestine in contrast to the basic Cu-rich feed. The serum antioxidant enzyme activity elevated up to (ceruloplasmin: 19.38 U/L) and the lowest liver malondialdehyde (MDA) content (8.81 nmol/mg prot.) and triglyceride content (0.39 nmol/g prot.) were observed in the NCM-Cu group as compared to the basic Cu and CuSO4 groups, suggesting that NCM-Cu promoted antioxidative responses and alleviated lipid peroxidation of O. niloticus. Overweening, the synthesized complex, NCM-Cu significantly regulated the expression levels of lysozyme, immunoglobulin M, complement 4, and complement 3 up to 10.93 U/mL, 0.72, 0.77, and 1.18 mg/mL in serum, respectively. Thus, such endorsed results reveal the preeminence of NCM-Cu-supplemented diet for the fitness in O. niloticus.
Introduction
Methionine (Met) is an essential sulfur amino acid and is generally considered as the primary limiting amino acid in aquafeeds. Dietary Met deficiency would affect the uptake of essential nutrients by alteration of gene expression of amino acid transporters, which results in the health problem of animals.1,2 In biological systems, Met is involved in five metabolic pathways, including transmethylation, transculturation, remethylation, aminopropylation, and salvage.3 However, Met was found to be sensitive to oxidative damage caused by a variety of oxidants, which can result in loss of structural integrity and disruption of protein functions.4 To minimize this shortcoming, a potential strategy for stabilizing Met against oxidative damage is to functionalize it by adding a nonoxidizable group such as a N-carbamoyl group.5 Indeed, N-carbamoyl amino acids are considered as very stable compounds, the hydrolysis of which requires drastic basic conditions or the use of enzymes.6 Since most fish digest feed through the gastrointestinal tract, we only discuss the behavior of an N-carbamoyl-methionine copper (NCM-Cu) sample within two differential pH environments (i.e., acid and neutral).7 It has been reported that a chelated metal complex could be dissolved by stomach acid (acid environment), but its dissolution rate is much lower than that of inorganic salts.8,9 Additionally, some previous in vitro bioavailability experiments displayed that the chelated metal complex was slowly dissolved in the succus entericus (neutral environment) and was kept relatively constant.10,11 Therefore, the prepared NCM-Cu sample theoretically has good gastrointestinal stability as compared to inorganic salt. Interestingly, N-carbamoyl-methionine (NCM) can be reconverted to Met in the body. In prokaryotes, NCM is also the starting amino acid for biological proteins.12
Cu is an essential trace mineral element for aquatic animals, which is the primary component of key enzymes in biological processes such as lysyl oxidase, cytochrome c oxidase, ferroxidase, and tyrosinase.13,14 Adequate dietary Cu levels had a positive effect on growth performance, antioxidant status, and immune response.15,16 Therefore, Cu should be supplemented to diets for maintaining the normal growth in fish. Whereas, the use of Cu supplements requires careful consideration of its levels in feed diet and may lead to negative impacts on the environment. On the one hand, signs of impaired growth, increased mortality, oxidative injury, and immune barrier malfunctions may occur in fish fed on Cu deficiency diet.17,18 On the other hand, unnecessarily high additions of Cu in fish can cause toxicity in the tissue structure, leading to changes in osmotic response and acid–base regulatory system.19,20 In an aquaculture system, dietary intake is the major way of Cu acquisition for fish.21,22 Accordingly, it is necessary for aquaculture producers to give priority to Cu sources with higher bioavailability in the intensification of the breeding system.
In biological systems, the presence of protons (H+) could facilitate the dissolution of dietary Cu due to the interactions of endogenous inhibitors (e.g., phytate and tricalcium phosphate) in the feed; dissolved Cu2+ reprecipitates to form insoluble or indigestible complexes and reduce the bioavailability of dietary Cu.15 Over presence of HCO3– (>50 mmol/L) limits the bioavailability of Fe2+ (precipitation of Fe (HCO3)2), which may also be applicable for Cu2+. Interestingly, studies found that the chelated trace elements may protect metal ions against antinutritional factors present in practical diets.23,24 Moreover, the bioavailability of organic chelated Cu is higher than Cu inorganic salts in fish such as Oncorhynchus mykiss and Epinephelus malabaricus.25 It has been reported that chelated Cu as an organic Cu supplement had great benefits for animal growth performance, antioxidant status, and immunity.26Therefore, using N-carbamoyl-methionine copper (NCM-Cu) as the Cu source may be more beneficial to meet the nutritional needs of aquatic animals.
Nile tilapia (Oreochromis niloticus L.) is the most important aquaculture products economically, being farmed in more than 135 countries, with a global creation around 5.9 million tones.27 In China, Nile tilapia aquaculture production has increased significantly to provide a valuable source of protein for the increasing market demand. In this study, the NCM-Cu complex was fabricated and a new organic Cu source was used in the feed to improve the overall fitness of O. niloticus. The molecular structure and interactions of the NCM-Cu complex were determined by a variety of physicochemical methods including ultraviolet and visible spectrophotometry (UV–vis), Fourier-transform infrared (FTIR), the X-ray diffractometry (XRD), thermogravimetric analysis (TGA), and single-crystal X-ray diffraction. Furthermore, the efficacy of NCM-Cu on the growth performance, tissue mineralization, antioxidant status, and immune response of a freshwater fish, O. niloticus was evaluated.
Results and Discussion
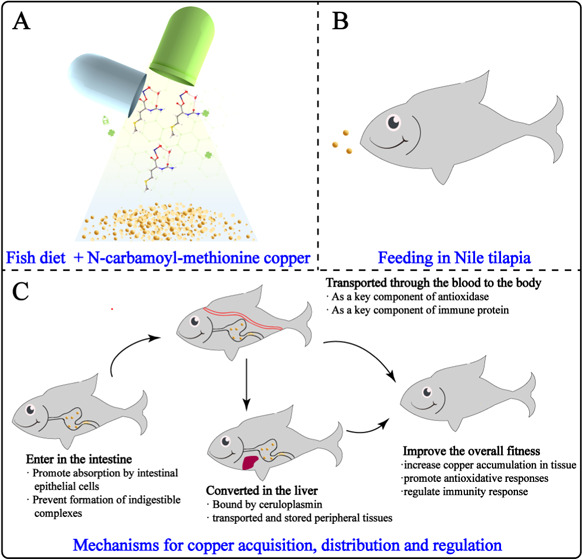
Present work was focused on the synthesis and efficacy of N-carbamoyl-methionine copper on the growth performance, tissue mineralization, immunity, and enzymatic antioxidant capacity of Nile tilapia (O. niloticus). The overall illustration of intake of N-carbamoyl-methionine copper as diet and their biological effects on Nile tilapia (O. niloticus) are shown in Figure 1.
Figure 1.
Illustration of the synthesis of N-carbamoyl-methionine copper (a) intake as diet (b) and their biological effects on Nile tilapia (O. niloticus) (c).
Both methionine (Met) and Cu element are essential substances in maintaining the fish’s normal life activities.1,13 Besides, it has been reported that N-carbamoyl-methionine (NCM) can be reconverted to Met in the body.12 In prokaryotes, NCM is also the starting amino acid for biological proteins. Thus, the NCM-Cu supplements are nontoxic and safe for fish. However, studies have shown that the excessive accumulation of Cu in fish can cause changes in the tissue structure, induce antioxidation, damage and stress response in tissues and organs, lead to changes in osmotic pressure and the acid–base regulatory system, induce immunotoxicity, hinder the cell circulation of immune organs, promote apoptosis, and affect the growth, development, and reproduction of fish.19 Therefore, the use of NCM-Cu supplements requires careful consideration of Cu levels in feed diet.
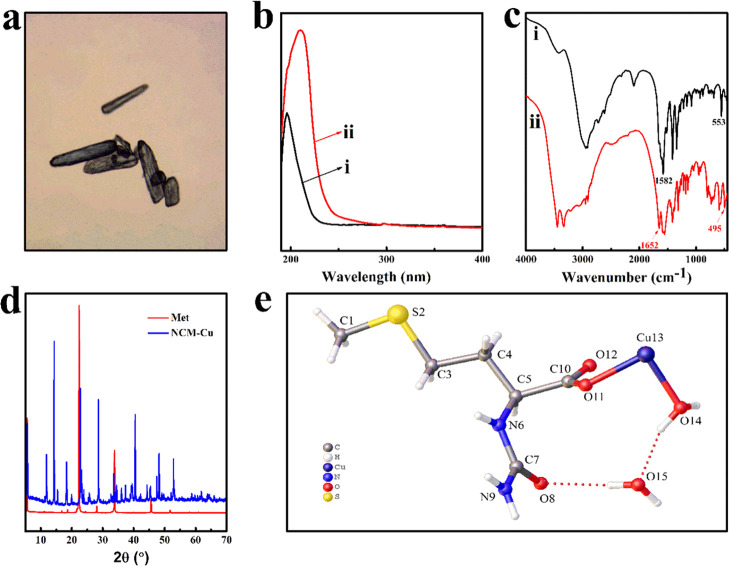
The NCM-Cu complex was successfully synthesized, which presents a stable crystal form for the intake of fish. An optical microscope image (Figure 2a) indicated that the solid product of the NCM-Cu complex had a blue columnar morphology. For the sake of confirming the successful complexation of the NCM-Cu complex, UV–vis spectroscopy and FT-IR spectroscopy were applied. For the UV–vis spectra (Figure 2b), a bathochromic shift of the NCM-Cu complex was obviously observed, which may be due to the extension of the conjugated system with complexation.28 For the FT-IR spectra (Figure 2c), two characteristic peaks of Met were detected at 1582 and 553 cm–1 corresponding to −NH2 bending vibration and to C–S–C stretching vibration, respectively.29,30 In contrast, the appearance of new absorption peak at 1652 and 495 cm–1 indicated the existence of −OH and Cu–O in the NCM-Cu complex, respectively.31,32 The existence of these characteristic peak confirmed that the NCM-Cu complex was formed. In addition, X-ray diffraction (XRD) was further employed to characterize the phase composition of the NCM-Cu complex (Figure 2d). Compared to Met, the appearance of a new and special diffraction pattern confirmed that a new crystal structure of the NCM-Cu complex was formed.33 Besides, a series of intense and sharp peaks of XRD patterns displayed that the NCM-Cu complex was a crystalline compound. To further corroborate this notion, the chemical states of the NCM-Cu complex were investigated by the single-crystal X-ray diffraction. The results (Table 1) revealed that the NCM-Cu complex was crystallized in the monoclinic system and the P21/c space group. Moreover, the empirical formula of NCM-Cu was identified as C12H30CuN4O10S2.
Figure 2.
Structural characterization. (a) Optical microscope image of the NCM-Cu complex. (b) UV–vis spectra: (i) Met and (ii) NCM-Cu. (c) FTIR spectra: (i) Met and (ii) NCM-Cu. (d) XRD patterns. (e) Molecular structure of NCM-Cu.
Table 1. Crystallographic Data and Structure Refinement of N-carbamoyl-methionine copper.
| parameter | values |
|---|---|
| empirical formula | C12H30CuN4O10S2 |
| formula weight (g/mol) | 518.07 |
| T (°C) | 149.99 |
| crystal system | monoclinic |
| space group | P21/c |
| a (Å) | 15.8914(2) |
| b (Å) | 4.65130(10) |
| c (Å) | 15.2181(3) |
| volume (Å3) | 1094.89(4) |
| Z | 2 |
| final R indices | 0.0476/0.1329 |
| R indices (all data) | 0.0491/0.1365 |
As depicted in Figure 2e, the aminocarbonyl (O=C–NH2) group was coordinated with secondary amine (−NH) in the NCM-Cu complex. Simultaneously, the Cu atom in the NCM-Cu complex was coordinated with the carboxyl groups of N-carbamoyl methionine ligands via a Cu–O bond. Besides, two crystallization water was strongly trapped in the crystal lattice. These results were consistent with other analysis data. Noteworthy, the crystallization behavior may be affected by the central atom, which directly affected the packing of the structure.9,34 The content of Cu of the NCM-Cu complex was 14.28% (w/w) as measured by inductively coupled plasma spectrometer (ICP).
TGA–DTA analysis is useful for evaluating the composition, thermal stability, and degradation behavior of metal complexes. In the preparation and processing of feed, heating at high temperatures may create a large change in the chemical properties of feed additives.35 The change of chemical properties of feed additives can influence the stability and physiological efficacy of NCM-Cu samples.36 The poor thermal stability of feed additives has an adverse effect on its development and zoological research.37 Thus, it is important to evaluate the NCM-Cu samples prepared in this work. In another experiment, the thermal stability of Met and NCM-Cu over a range of 40–600 °C was tested (Figure 3). The TG curve of Met showed mass loss within the temperature range of 214.01–340.47 °C with DTG peaks at 279.17 °C. In contrast, the first mass loss of NCM-Cu (8.0%) was related to the loss of crystallization water.38 Besides, the second stage mass loss of NCM-Cu at 134.56–427.10 °C was 61.2%. The difference in the thermal stability of the NCM-Cu complex might be caused by the insertion of metal, leading to reduction in the number of hydrogen bonding.32 Interestingly, the residual amount of Met was smaller than that of the NCM-Cu complex, further proving the occurrence of complexation between N-carbamoyl methionine and Cu ions.
Figure 3.
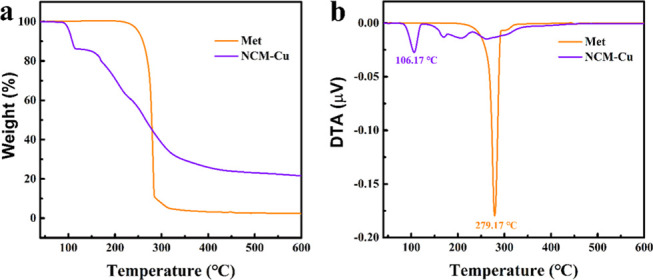
Thermal stability analysis. (a) Thermogravimetry (TG) curves; (b) derivative thermogravimetry (DTA) curves.
In summary, the NCM-Cu complex was successfully synthesized, with the formula NCM-Cu as C12H30CuN4O10S2. Multiple structural analysis indicated that the Cu atom in the NCM-Cu complex was coordinated with carboxyl groups of N-carbamoyl methionine ligands via a Cu–O bond. Besides, the original amino group in Met was substituted with the aminocarbonyl group such that the NCM-Cu compound did not undergo Strecker degradation.39 This stable structure provided the possible idea that the NCM-Cu complex may have better bioavailability and biocompatibility in the body.
CCDC 1945764 contains the supplementary crystallographic data for this paper. These data can be obtained basic of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Results of the growth, feed utilization, and morphological parameters of O. niloticus by feeding the experimental diet with various forms of Cu are displayed in Table 2. According to one-way ANOVA analysis, there were no significant differences in weight gain (WG), feeding efficiency (FE), condition factor (CF), viscerosomatic index (VSI), hepatosomatic index (HSI), and survival of O. niloticus among all treatments (p > 0.05). Based on the results, the growth performance and feed utilization of O. niloticus fed on three experimental diet were comparable. However, the basic Cu diet did not affect the fish feeding and growth performance of O. niloticus. Similar experimental results were found in O. niloticus and Cyprinus carpio.40,41
Table 2. Growth Performance and Feed Utilization of O. niloticus by Feeding Experimental Diet with Various Forms of Cu for 60 Daysa.
| experimental diets | WG (%) | FE (%) | CF (g/cm3) | VSI (%) | HSI (%) | Survival (%) |
|---|---|---|---|---|---|---|
| Cu-basic diet | 234.1 ± 50.5 | 85.8 ± 18.5 | 2.05 ± 0.12 | 8.46 ± 0.78 | 1.94 ± 0.41 | 100 |
| CuSO4 diet | 237.9 ± 37.1 | 85.5 ± 13.3 | 1.96 ± 0.19 | 8.87 ± 0.90 | 2.11 ± 0.41 | 100 |
| NCM-Cu diet | 225.0 ± 34.8 | 82.2 ± 12.6 | 2.01 ± 0.16 | 8.65 ± 0.95 | 2.16 ± 0.53 | 100 |
WG: weight gain; FE: feeding efficiency; CF: condition factor; VSI: viscerosomatic index; HSI: hepatosomatic index.
Besides, it had been reported that varying concentrations of Cu did not significantly affect the weight gain and feed efficiency of Salmo salar.42 Some authors have suggested that the difference in fish growth performance was not only affected by chemical forms of Cu but also related to species, age of fish, and dietary factors. Therefore, the results indicated that O. niloticus was less susceptible to dietary Cu than other fish species.
Concentrations of four metals in tissues of the fish fed on three experiment diets for 60 days are shown in Figure 4 (the values are shown in Tables S1–S4). Obviously, diets supplemented with NCM-Cu increased the Cu absorption and deposition efficiency in tilapia tissues. Specifically, Cu concentrations in the muscle (4.99 mg/kg), liver (154.3 mg/kg), gill (7.18 mg/kg), and intestine (8.48 mg/kg) in the NCM-Cu group were significantly higher than those of the Cu-basic group. Simultaneously, a significant increase in the Cu concentration in the muscle and gill was also observed in fish fed on the NCM-Cu diet when compared to that of fish fed on a CuSO4 diet, as shown in Figure 4a,c. The highest Cu concentration was observed in the liver, suggesting that the liver was the target for Cu accumulation. It has been reported that organic forms of Cu were more effective than CuSO4 in some aquatic animals such as O. mykiss and Litopenaeus vannamei.43,44 In the gastrointestinal tract, Cu ions were easily precipitated by the interactions of the endogenous inhibitors. However, due to its stable structure, the possibility for chelated Cu to form insoluble or indigestible complexes was reduced. Some authors suggested that chelating minerals can be absorbed in an intact form by amino acid transporters. Therefore, tissue mineralization data also showed that the NCM-Cu complex had a higher bioavailability in O. niloticus than the inorganic form.
Figure 4.
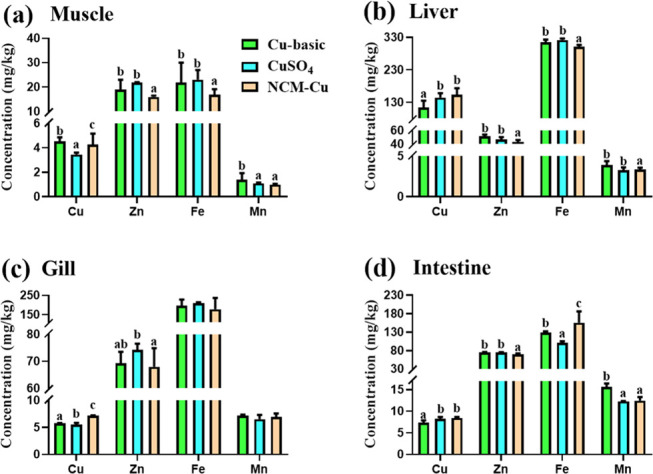
Effects of different Cu sources as the experimental diets on metal elemental concentration changes in tissues of O. niloticus: (a) muscle, (b) liver, (c) gill, and (d) intestine. Bars sharing the different alphabets are significantly different (p < 0.05).
On the other hand, NCM-Cu diet might not be good for Zn accumulation in tilapia tissues. For example, Zn concentration in the muscle, liver, and intestine in the NCM-Cu group was significantly lower than the Cu-basic and CuSO4 groups, as shown in Figure 4a,b,d. Due to similarities in the physiochemical attributes of Cu and other cations, there was an interaction between these minerals, which affected the absorption of the gastrointestinal tract.45 Therefore, the type of the mineral source and the concentration also affected the minimal dietary inclusion levels and the absorption of other minerals. Evidence of competition between Cu and Zn was also found in Larimichthys croceus.46 In addition, the Fe content in the fish muscle and liver in the NCM-Cu group was significantly lower than those in the Cu-basic and CuSO4 groups, as shown in Figure 4a,b, but the opposite result was detected in the intestine, as shown in Figure 4d. Besides, Mn accumulation in tilapia tissues was significantly affected among three treatments. Mn content in the muscle, liver, and intestine in the NCM-Cu group was significantly lower than in the Cu-basic group. Interestingly, the gill appeared to be least affected by different sources of Cu supply. Fe and Mn contents in tilapia gill were comparable when fed on three different diets.
The liver antioxidant parameters and serum ceruloplasmin activity are shown in Table 3. The total superoxide dismutase (T-SOD) and Cu-Zn SOD activities in the NCM-Cu group were comparable when compared to the Cu-basic or the CuSO4 group. However, biological antioxidant potential (CP) recorded the highest value (19.38 U/L) in the NCM-Cu group as compared to the Cu-basic (16.16 U/L) and the CuSO4 groups (16.26 U/L) (p < 0.05). Simultaneously, the content of malondialdehyde (MDA) (8.81 nmol/mg prot.) and TG (0.39 mmol/g prot.) in tilapia liver in the NCM-Cu group was significantly lower than those in the Cu-basic and CuSO4 groups. Moreover, fish fed on diets supplemented with NCM-Cu had significantly lower T-CHO content (0.26 mmol/g prot.) when compared with the Cu-basic group. Similarly, previous results displayed that methionine Cu had higher antioxidant capacity than fish fed on the same Cu content in the CuSO4 diet. It has been reported that adequate dietary Cu levels improved the nonspecific immune responses or antioxidation abilities of Acipenser gueldenstaedtii.47 Noteworthy, a Cu ion is an active center of Cu-Zn SOD and CP and to its catalytic action. It has been reported that an increase in the hepatic Cu pool resulted in a sustained increase in the Cu-Zn SOD and CP concentrations in the liver. Thus, the activity of Cu-Zn SOD and CP enzymes is not determined by enzyme protein but also determined by the Cu2+ content. On the contrary, Cu deficiency would affect the antioxidant defense and lipid metabolism. Therefore, our results suggested that NCM-Cu as a Cu additive alleviated the oxidative stress and lipid peroxidation.48
Table 3. Content of Total Superoxide Dismutase (T-SOD), Cu-Zn Superoxide Dismutase (Cu-Zn SOD), Malondialdehyde (MDA), Triglyceride (TG), Total Cholesterol (T-CHO), and Ceruloplasmin (CP) in the Serum of O. niloticus Fed on Experimental Diets with Different Sources of Cu for 60 Daysa.
| experimental diets | T-SOD (U/mg prot.) | Cu-Zn SOD (U/mg prot.) | CP (U/L) | MDA (nmol/mg prot.) | TG (mmol/g prot.) | T-CHO (mmol/g prot.) |
|---|---|---|---|---|---|---|
| Cu-basic diet | 35.48 ± 1.06 | 33.77 ± 2.97a | 16.16 ± 1.49a | 11.47 ± 1.14b | 0.54 ± 0.06b | 0.39 ± 0.07b |
| CuSO4 diet | 35.12 ± 0.79 | 36.06 ± 0.20b | 16.26 ± 1.58a | 10.54 ± 1.17b | 0.47 ± 0.07b | 0.27 ± 0.03a |
| NCM-Cu diet | 36.07 ± 0.43 | 35.61 ± 0.91ab | 19.38 ± 0.50b | 8.81 ± 1.38a | 0.39 ± 0.06a | 0.26 ± 0.08a |
Data in the same column with different superscript letters are significantly different (p < 0.05).
The activities of serum LZM, IgM, C3, and C4 of O. niloticus fed on three different experimental diets are presented in Table 4. The best immune response in tilapia was observed in the NCM-Cu treatment. Fish fed on diets supplemented with NCM-Cu had a significantly higher content of IgM (0.72 mg/mL), C3 (0.77 mg/mL), and C4 (1.18 mg/mL) when compared to the fish fed on diets supplemented with Cu-basic. Simultaneously, compared to CuSO4, a significant increase in the activity of LZM (10.93 U/mL) and IgM (0.72 mg/mL) of the fish fed on the NCM-Cu diet was observed, as shown in Table 4. This result denoted the positive effect of the NCM-Cu complex on the immunity response of O. niloticus, which may be related to Cu accumulation in tissues. A recent study also demonstrated that Russian sturgeon fed with methionine Cu displayed significantly higher immune responses and resistance to bacterial infection than CuSO4 treatment. Because Cu was the key component of functional protein (e.g., albumin) in an immunity system, the improved bioavailability of dietary Cu might stimulate the innate immune response. On the contrary, Cu deficiency had an adverse impact on immune responses and decreased the resistance in the fish.49 Generally, the first consideration for aquaculture producers is to obtain the best fish production. There are many factors that influence fish populations including aquatic ecosystems conditions, species interactions, disease, and feed supplements.50,51 From the science-based knowledge we have accumulated to-date, these factors can be enacted to mitigate, minimize, or reverse by some suitable management actions. Specifically, the use of fortification of foods or dietary supplements can increase the overall fish fitness and reduce the environmental pollution.52 It is remarkable that aquaculture sustainability must balance the socioeconomic needs and ecological protection. Therefore, choosing fish feed additives with higher bioavailability and bioactivity will be beneficial for the development of the entire aquaculture industry.
Table 4. Activity of Lysozyme (LZM), Immunoglobulin M (IgM), Complement 4 (C4), and Complement 3 (C3) Values in the Serum of O. niloticus, Fed on Experimental Diet Supplemented with Various Forms of Cua.
| experimental diets | LZM (U/mL) | IgM (mg/mL) | C4 (mg/mL) | C3 (mg/mL) |
|---|---|---|---|---|
| Cu-basic diet | 10.48 ± 0.70ab | 0.25 ± 0.10a | 0.50 ± 0.12a | 0.84 ± 0.16a |
| CuSO4 diet | 9.91 ± 0.46a | 0.32 ± 0.05a | 0.75 ± 0.17b | 0.94 ± 0.27ab |
| NCM-Cu diet | 10.93 ± 0.38b | 0.72 ± 0.11b | 0.77 ± 0.10b | 1.18 ± 0.19b |
Data in the same column with different superscript letters are significantly different (p < 0.05).
Conclusions
In the present study, a promising N-carbamoyl-functionalized NCM-Cu complex was synthesized, and its stable structure protected itself against oxidative damage. Besides, multiples structure analysis indicated that the aminocarbonyl group was coordinated with secondary amine and the Cu atom was chelated to the carboxyl groups of the NCM-Cu complex. Single-crystal X-ray diffraction result clearly confirmed the NCM-Cu complex as C12H30CuN4O10S2. Anonymously, the overall growth performance of the fish remained unaltered with NCM-Cu adjunct feed. NCM-Cu significantly raised the Cu accumulation in the fish muscles (4.99 mg/kg), liver (154.3 mg/kg), gill (7.18 mg/kg), and intestine (8.48 mg/kg) in contrast to the basic Cu-rich feed. The serum antioxidant enzyme activity elevated up to (ceruloplasmin: 19.38 U/L) and the lowest liver MDA content (8.81 nmol/mg prot.) and triglyceride content (0.39 nmol/g prot.) were observed in the NCM-Cu group as compared to the basic Cu and CuSO4 groups, suggesting that NCM-Cu promoted antioxidative responses and alleviated lipid peroxidation of O. niloticus. This exhibits the nurturing of the antioxidative reflexes and the alleviated lipid peroxidation in O. niloticus in response to an NCM-Cu adjutant. Overweening, the synthesized complex, NCM-Cu significantly regulated the expression levels of lysozyme, immunoglobulin M, complement 4, and complement 3 up to 10.93 U/mL, 0.72, 0.77, and 1.18 mg/mL in serum, respectively, proclaiming the commendatory immunogenic effects of the NCM-Cu adjutant. Thus, such endorsed results reveal the preeminence of the NCM-Cu-supplemented diet for the fitness in O. niloticus. These findings pointed out the potential use of NCM-Cu as a new Cu supplementation source in the aquaculture industry since its clear molecular structure would facilitate further studies on the mechanism of action in the body. Furthermore, the biological experiment displayed that the NCM-Cu complex had a good efficacy on Cu accumulation, antioxidant stress, alleviated lipid peroxidation, and immunity response of O. niloticus.
Materials and Methods
Methionine (Met), sodium cyanate (NaCNO), and CuSO4 (CuSO4·5H2O) were supplied by Aladdin Co., Ltd. Juvenile tilapia of O. niloticus was bought from a commercial farm in Guangzhou, China. All of the diagnostic kits for measuring the enzymatic capacity and immune indices were purchased from Nanjing Jiancheng Bioengineering Institute, China.
Preparation of NCM-Cu
Briefly, 121.19 g of Met and 67.11 g of NaCNO were accurately weighed and added to 350 mL of deionized water at 90 °C with 90 min of stirring. Subsequently, the mixture solution was naturally cooled to a solution temperature of 40 °C. The pH of the above mixture was adjusted to 3 using dilute sulfuric acid and further stirred for 60 min. The reaction solution was separated after standing for 60 min. To obtain pure N-carbamoyl methionine (NCM), the upper solution was collected and dried at 50 °C for 5 h in a hot air oven.
Five grams of NCM and 13.02 g of CuSO4·5H2O were accurately weighed and dissolved in 20 mL of deionized water, and the pH was adjusted to 4.5 using a 0.1 mol/L NaOH solution. The mixture was then magnetically stirred for 60 min at 60 °C, and the target solution was obtained after filtering. To obtain pure NCM-Cu, the target solution was collected and dried at 50 °C for 4 h in a hot air oven.
Structural Characterization of NCM-Cu
For structural characterization, UV–vis spectra of Met and NCM-Cu were recorded on a TU-1810 spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., China) in the range of 190–400 nm. Besides, Met and NCM-Cu were measured by Fourier-transform infrared spectrometry (Spectrum 100, PerkinElmer Inc.) using a KBr tablet method. Moreover, the crystal phases of Met and NCM-Cu were investigated by an X-ray powder diffractometer (X’Pert PRO MPD, Panalytical, Holland) with Cu Kα radiation in the 2θ range of 5–70° at a scan rate of 1°/min. Furthermore, the structure data of NCM-Cu were collected via single-crystal X-ray diffraction (SMART APEX II, Bruker, Germany) and solved by direct methods using SHELXS, and refinement was done against F2 using SHELXL. Simultaneous thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were performed for Met and NCM-Cu. The curves were obtained using TG-DTA modulus (TGA2, Mettler Toledo, Switzerland) under a nitrogen atmosphere from 40 to 600 °C with a heating rate of 10 °C/min.
Experimental Diet Preparation
To meet all known nutritional requirements for O. niloticus juveniles, the basal diet was prepared. The formulation and proximate composition of the diets are shown in Table 5. Specifically, fish meal, soybean meal, and sesame meal were used as protein sources; wheat flour was used as the carbohydrate source; and soybean oil was used as soybean oil. To evaluate the bioactivities in O. niloticus of supplementing diet with NCM-Cu (synthesized by this studies), the control (as basic Cu) and CuSO4 groups were formulated. The additional Cu sources in the basic diets of both CuSO4and NCM-Cu groups were 30 mg/kg. The dietary Cu concentration was analyzed by inductively coupled plasma spectrometer (ICP-OES 2100, PerkinElmer), as shown in Table 5. The ingredients of the experiment diet were weighed and thoroughly mixed in a food mixer, then put in a pelletizer and cut into uniform sizes. The molded feeds were dried in a mechanical convection oven and then stored at 4 °C for use.
Table 5. Formulation and Proximate Composition of the Basic Diets.
| ingredient (g/kg) | basic Cu diet | CuSO4 diet | NCM-Cu diet |
|---|---|---|---|
| fish meal | 180 | 180 | 180 |
| soybean meal | 230 | 230 | 230 |
| sesame meal | 240 | 240 | 240 |
| DDGS | 50 | 50 | 50 |
| wheat flour | 230 | 230 | 230 |
| soybean oil | 25 | 25 | 25 |
| monocalcium phosphate | 15 | 15 | 15 |
| choline chloride | 10 | 10 | 10 |
| vitamin premixa | 10 | 10 | 10 |
| mineral premix, Cu-basicb | 10 | 10 | 10 |
| proximate composition | |||
| crude protein (%) | 39.38 | 39.31 | 37.50 |
| crude lipid (%) | 7.82 | 7.83 | 7.66 |
| ash (%) | 10.22 | 10.08 | 9.97 |
| moisture (%) | 5.49 | 6.60 | 7.80 |
| final actual concentration of Cu (mg/kg) | 10.43 | 40.14 | 41.88 |
Vitamin premix (mg/kg diet): vitamin B1, 12; vitamin B12, 0.05; vitamin B6, 8; nicotinic acid, 30; vitamin D3, 5; vitamin C, 100; vitamin E, 50; pantothenic acid, 40; biotin, 0.8; folic acid, 5; vitamin A, 25; vitamin K3, 8; riboflavin, 12; inositol, 100.
Mineral premix, zinc-basic (mg/kg diet): FeSO4·H2O, 400; KCl, 200; MnSO4·H2O, 150; KI 60; Na2SeO3·5H2O, 65; ZnSO4·7H2O, 40.
Bioactivity Experiments
Juveniles of O. niloticus in the study were purchased from a commercial farm in Guangzhou, China. Upon arrival, the fish were acclimated in 400 L laboratory tanks for 2 weeks and fed with the basal diets. After acclimation, a total of 180 individuals of O. niloticus with an average initial weight of 77.27 ± 0.15 g were randomly distributed into three groups, each group containing three replicates (20 fish/replicate). The first group served as the control group fed on a Cu basal diet. The second and third groups were fed on a CuSO4 diet and NCM-Cu diet, respectively. The experiment lasted for 60 days and was under ambient temperature and natural light and dark cycle. Besides, each tank was equipped with water inlet and outlet, and continuously aerated to maintain the dissolved oxygen concentration higher than 6 mg/L. O. niloticus was fed two times a day (daily ration was about 4% of biomass) at 9:00 am and 5:00 pm. During the feeding trial, O. niloticus were fed closely to satiation and the uneaten feed after feeding 30 min was collected, dried, and weighed to calculate the feed intake. Moreover, the fish were weighed every 2 weeks and the daily ration diet was adjusted accordingly. Water temperature, dissolved oxygen, total ammonia–nitrogen, and pH were monitored daily by YSI Proplus (YSI, Yellow Springs, Ohio). The mean water quality parameters were recorded as follows: dissolved oxygen was ≥6 mg/L, total ammonia–nitrogen was 0.055 ± 0.012 mg/L, pH was 7.2 ± 0.20, and the water temperature ranged from 27 °C to 32 °C.
Sample Collection
At the end of the feeding period, all fish in each tank were fasted for 24 h prior to final sampling. Subsequently, all of the fish from each replicate were randomly collected and weighed for the analysis of survival and growth performance including the weight gain (WG, %), feeding efficiency (FE, %), and condition factor (CF, g/cm3). Parameters were calculated according to the following formulae:
Then, blood samples from three fish of each tank were collected as quickly as possible from the caudal vein using sterilized syringes and pooled (three pooled samples per tank) [9, 25]. The serum was separated after being stored at 4 °C for 4 h and after that, centrifugation occurred at 3500 rpm for 15 min. The supernatant was collected to evaluate the antioxidant and immunological parameters in O. niloticus including lysozyme (LZM), immunoglobulin M (IgM), complement 4 (C4), complement 3 (C3), and ceruloplasmin (CP). In addition, viscera and liver were dissected out from 10 fish from each aquarium, weighed individually to get the viscerosomatic index (VSI) and hepatosomatic index (HSI). Moreover, muscles, liver, gill, and intestine were collected from three fish each tank to estimate the tissue mineralization (Cu, Zn, Fe, and Mn contents) of O. niloticus. Meanwhile, a small part of the liver from three fish of each tank was collected and pooled (three pooled samples per tank) for the measurement of the contents of total superoxide dismutase (T-SOD), Cu-Zn superoxide dismutase (Cu-Zn SOD), malondialdehyde (MDA), triglyceride (TG), and total cholesterol (T-CHO). Enzymatic activity and immune parameters were analyzed using an automatic biochemical analyzer (Sysmex-800, Sysmex Corporation, Kobe, Japan) and followed the methods and instructions of the specific kits. VSI and HSI were calculated according to the following formulae:
Statistical Analysis
The data (means ± SEM, standard error) in this study were statistically calculated using SPSS 25.0 statistical software and then analyzed by one-way ANOVA method. A p value of <0.05 was judged to be statistically significant. Tukey’s multiple comparison test was used when there was homogeneity of variances.
Acknowledgments
The authors express their gratitude for the financial support from the Guangdong-Hong Kong Cooperation Project (2017A050506055), the Guangdong Provincial Education Department Project (Natural Science, 2017KZDXM045), the Agriculture and Rural Department Project of Guangdong Province, the Guangzhou Foreign Cooperation Project (201907010033), the Graduate Technology Innovation Fund (KJCX2019004), and the Undergraduate Innovation and Entrepreneurship Training Program (S201911347028).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03220.
Tissue mineralization analysis; raw data for Zn, Cu, Fe, and Mn concentrations in the fish tissue (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Garcia-Organista A. A.; Mata-Sotres J. A.; Viana M. T.; Rombenso A. N. The Effects of High Dietary Methionine and Taurine Are Not Equal in Terms of Growth and Lipid Metabolism of Juvenile California Yellowtail (Seriola Dorsalis). Aquaculture 2019, 512, 734304 10.1016/j.aquaculture.2019.734304. [DOI] [Google Scholar]
- Song B.; Zeng Q.; Liu Y.; Wu B. Effect of Methionine Deficiency on the Apoptosis and Cell Cycle of Kidney in Broilers. Res. Vet. Sci. 2019, 10.1016/j.rvsc.2019.09.013. [DOI] [PubMed] [Google Scholar]
- Fagundes N. S.; Milfort M. C.; Williams S. M.; Da Costa M. J.; Fuller A. L.; Menten J. F.; Rekaya R.; Aggrey S. E. Dietary Methionine Level Alters Growth, Digestibility, and Gene Expression of Amino Acid Transporters in Meat-Type Chickens. Poultry Sci. 2020, 99, 67–75. 10.3382/ps/pez588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger Chien H. C.; Hsu C. L.; Hu H. Y.; Wang W. C.; Hsu W. H. Enhancing Oxidative Resistance of Agrobacterium Radiobacter N-Carbamoyl D-Amino Acid Amidohydrolase by Engineering Solvent-Accessible Methionine Residues. Biochem. Biophys. Res. Commun. 2002, 297, 282–287. 10.1016/S0006-291X(02)02184-8. [DOI] [PubMed] [Google Scholar]
- Kim Y. H.; Berry A. H.; Spencer D. S.; Stites W. E. Comparing the Effect on Protein Stability of Methionine Oxidation versus Mutagenesis: Steps toward Engineering Oxidative Resistance in Proteins. Protein Eng. 2001, 14, 343–347. 10.1093/protein/14.5.343. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Shao D.; Wang Q.; Xiao Y.; Zhao X.; Shen Y.; Zhang S.; Tong H.; Shi S. Effects of Dietary N-Carbamylglutamate Supplementation on Growth Performance, Tissue Development and Blood Parameters of Yellow-Feather Broilers. Poultry Sci. 2019, 98, 2241–2249. 10.3382/ps/pey591. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wang A.; Wang W. X. Evaluation of Nano-ZnOs as a Novel Zn Source for Marine Fish: Importance of Digestive Physiology. Nanotoxicology 2017, 11, 1026–1039. 10.1080/17435390.2017.1388865. [DOI] [PubMed] [Google Scholar]
- Miret S.; Tascioglu S.; Van Der Burg M.; Frenken L.; Klaffke W. In Vitro Bioavailability of Iron from the Heme Analogue Sodium Iron Chlorophyllin. J. Agric. Food Chem. 2010, 58, 1327–1332. 10.1021/jf903177q. [DOI] [PubMed] [Google Scholar]
- Luo F.; Wang M.; Huang L.; Wu Z.; Wang W.; Zafar A.; Tian Y.; Hasan M.; Shu X. Synthesis of Zinc Oxide Eudragit FS30D Nanohybrids: Structure, Characterization, and Their Application as an Intestinal Drug Delivery System. ACS Omega 2020, 5, 11799–11808. 10.1021/acsomega.0c01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.; Li H.; Min W. Preparation, Characterization and Bioactivities of Athelia Rolfsii Exopolysaccharide-Zinc Complex (AEPS-Zinc). Int. J. Biol. Macromol. 2018, 113, 20–28. 10.1016/j.ijbiomac.2018.01.223. [DOI] [PubMed] [Google Scholar]
- Dang H.; Meng M. H. W.; Zhao H.; Iqbal J.; Dai R.; Deng Y.; Lv F. Luteolin-Loaded Solid Lipid Nanoparticles Synthesis, Characterization, & Improvement of Bioavailability, Pharmacokinetics in Vitro and Vivo Studies. J. Nanopart. Res. 2014, 16, 2347 10.1007/s11051-014-2347-9. [DOI] [Google Scholar]
- Brosnan J. T.; Brosnan M. E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- Cao J.; Miao X.; Xu W.; Li J.; Zhang W.; Mai K. Dietary Copper Requirements of Juvenile Large Yellow Croaker Larimichthys Croceus. Aquaculture 2014, 346–350. 10.1016/j.aquaculture.2014.05.032. [DOI] [Google Scholar]
- El Basuini M. F.; El-Hais A. M.; Dawood M. A. O.; Abou-Zeid A. E. S.; EL-Damrawy S. Z.; Khalafalla M. M. E. S.; Koshio S.; Ishikawa M.; Dossou S. Effect of Different Levels of Dietary Copper Nanoparticles and Copper Sulfate on Growth Performance, Blood Biochemical Profiles, Antioxidant Status and Immune Response of Red Sea Bream (Pagrus Major). Aquaculture 2016, 455, 32–40. 10.1016/j.aquaculture.2016.01.007. [DOI] [Google Scholar]
- Yuan Y.; Jin M.; Xiong J.; Zhou Q. Effects of Dietary Dosage Forms of Copper Supplementation on Growth, Antioxidant Capacity, Innate Immunity Enzyme Activities and Gene Expressions for Juvenile Litopenaeus Vannamei. Fish Shellfish Immunol. 2019, 84, 1059–1067. 10.1016/j.fsi.2018.10.075. [DOI] [PubMed] [Google Scholar]
- Lin Y. H.; Shih C. C.; Kent M.; Shiau S. Y. Dietary Copper Requirement Reevaluation for Juvenile Grouper, Epinephelus Malabaricus, with an Organic Copper Source. Aquaculture 2010, 310, 173–177. 10.1016/j.aquaculture.2010.10.004. [DOI] [Google Scholar]
- Ergaz Z.; Shoshani-Dror D.; Guillemin C.; Neeman-azulay M.; Fudim L.; Weksler-Zangen S.; Stodgell C. J.; Miller R. K.; Ornoy A. The Effect of Copper Deficiency on Fetal Growth and Liver Anti-Oxidant Capacity in the Cohen Diabetic Rat Model. Toxicol. Appl. Pharmacol. 2012, 265, 209–220. 10.1016/j.taap.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Chen Q. L.; Luo Z.; Liu C. X.; Zheng J. L. Differential Effects of Dietary Cu Deficiency and Excess on Carnitine Status, Kinetics and Expression of CPT I in Liver and Muscle of Yellow Catfish Pelteobagrus Fulvidraco. Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol. 2015, 188, 24–30. 10.1016/j.cbpb.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Yu Z.; Zheng Y. guang.; Du H. L.; Li H. J.; Wu L. F. Bioflocs Protects Copper-Induced Inflammatory Response and Oxidative Stress in Rhynchocypris Lagowski Dybowski through Inhibiting NF-KB and Nrf2 Signaling Pathways. Fish Shellfish Immunol. 2020, 98, 466–476. 10.1016/j.fsi.2020.01.048. [DOI] [PubMed] [Google Scholar]
- Meng F.; Li M.; Tao Z.; Yuan L.; Song M.; Ren Q.; Xin X.; Meng Q.; Wang R. Effect of High Dietary Copper on Growth, Antioxidant and Lipid Metabolism Enzymes of Juvenile Larger Yellow Croaker Larimichthys Croceus. Aquacult. Rep. 2016, 3, 131–135. 10.1016/j.aqrep.2016.02.001. [DOI] [Google Scholar]
- Kim B. E.; Nevitt T.; Thiele D. J. Mechanisms for Copper Acquisition, Distribution and Regulation. Nat. Chem. Biol. 2008, 4, 176–185. 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- Qasim S.; Zafar A.; Saif M. S.; Ali Z.; Nazar M.; Waqas M.; Haq A. U.; Tariq T.; Hassan S. G.; Iqbal F.; Shu X. G.; Hasan M. Green Synthesis of Iron Oxide Nanorods Using Withania Coagulans Extract Improved Photocatalytic Degradation and Antimicrobial Activity. J. Photochem. Photobiol., B 2020, 204, 111784 10.1016/j.jphotobiol.2020.111784. [DOI] [PubMed] [Google Scholar]
- Rider S. A.; Davies S. J.; Jha A. N.; Clough R.; Sweetman J. W. Bioavailability of Co-Supplemented Organic and Inorganic Zinc and Selenium Sources in a White Fishmeal-Based Rainbow Trout (Oncorhynchus Mykiss) Diet. J. Anim. Physiol. Anim. Nutr. 2010, 94, 99–110. 10.1111/j.1439-0396.2008.00888.x. [DOI] [PubMed] [Google Scholar]
- Akbar S.; Haleem K. S.; Tauseef I.; Rehman W.; Ali N.; Hasan M. Raphanus Sativus Mediated Synthesis, Characterization and Biological Evaluation of Zinc Oxide Nanoparticles. Nanosci.. Nanotechnol. Lett. 2017, 9, 2005–2012. 10.1166/nnl.2017.2550. [DOI] [Google Scholar]
- Apines-Amar M. J. S.; Satoh S.; Caipang C. M. A.; Kiron V.; Watanabe T.; Aoki T. Amino Acid-Chelate: A Better Source of Zn, Mn and Cu for Rainbow Trout, Oncorhynchus Mykiss. Aquaculture 2004, 240, 345–358. 10.1016/j.aquaculture.2004.01.032. [DOI] [Google Scholar]
- Wang H.; Zhu H.; Wang X.; Li E.; Du Z.; Qin J.; Chen L. Comparison of Copper Bioavailability in Copper-Methionine, Nano-Copper Oxide and Copper Sulfate Additives in the Diet of Russian Sturgeon Acipenser Gueldenstaedtii. Aquaculture 2018, 482, 146–154. 10.1016/j.aquaculture.2017.09.037. [DOI] [Google Scholar]
- Awad A.; Zaglool A. W.; Ahmed S. A. A.; Khalil S. R. Transcriptomic Profile Change, Immunological Response and Disease Resistance of Oreochromis niloticus Fed with Conventional and Nano-Zinc Oxide Dietary Supplements. Fish Shellfish Immunol. 2019, 93, 336–343. 10.1016/j.fsi.2019.07.067. [DOI] [PubMed] [Google Scholar]
- Bukhari S. B.; Memon S.; Mahroof-Tahir M.; Bhanger M. I. Synthesis, Characterization and Antioxidant Activity Copper-Quercetin Complex. Spectrochim. Acta, Part A 2009, 71, 1901–1906. 10.1016/j.saa.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Laban B.; Ralević U.; Petrović S.; Leskovac A.; Vasić-Anićijević D.; Marković M.; Vasić V. Green Synthesis and Characterization of Nontoxic L-Methionine Capped Silver and Gold Nanoparticles. J. Inorg. Biochem. 2020, 204, 110958 10.1016/j.jinorgbio.2019.110958. [DOI] [PubMed] [Google Scholar]
- Ma F.; Li P.; Zhang B.; Wang Z. The Facile Synthesis of a Chitosan Cu(II) Complex by Solution Plasma Process and Evaluation of Their Antioxidant Activities. Int. J. Biol. Macromol. 2017, 103, 501–507. 10.1016/j.ijbiomac.2017.04.082. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Shi Y.; Lei F.; Dai L. A Novel and Green Cellulose-Based Schiff Base-Cu (II) Complex and Its Excellent Antibacterial Activity. Carbohydr. Poly. 2020, 230, 115671 10.1016/j.carbpol.2019.115671. [DOI] [PubMed] [Google Scholar]
- Manimohan M.; Pugalmani S.; Sithique M. A. Biologically Active Water Soluble Novel Biopolymer/Hydrazide Based O-Carboxymethyl Chitosan Schiff Bases: Synthesis and Characterisation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3658–3676. 10.1007/s10904-020-01487-9. [DOI] [Google Scholar]
- Porkodi J.; Raman N. Synthesis, Characterization and Biological Screening Studies of Mixed Ligand Complexes Using Flavonoids as Precursors. Appl. Organomet. Chem. 2018, 32, e4030 10.1002/aoc.4030. [DOI] [Google Scholar]
- Pan C.; Ou M.; Cheng Q.; Zhou Y.; Yu Y.; Li Z.; Zhang F.; Xia D.; Mei L.; Ji X. Z-Scheme Heterojunction Functionalized Pyrite Nanosheets for Modulating Tumor Microenvironment and Strengthening Photo/Chemodynamic Therapeutic Effects. Adv. Funct. Mater. 2019, 30, 1906466 10.1002/adfm.201906466. [DOI] [Google Scholar]
- Zhang K.; Zhang Y.; Yan D.; Zhang C.; Nie S. Enzyme-Assisted Mechanical Production of Cellulose Nanofibrils: Thermal Stability. Cellulose 2018, 25, 5049–5061. 10.1007/s10570-018-1928-7. [DOI] [Google Scholar]
- Tang S. H.; Li R.; Tan J.; Wang Y.; Jiang Z. T. One Pot Synthesis of Water-Soluble Quercetin Derived Multifunctional Nanoparticles with Photothermal and Antioxidation Capabilities. Colloids Surf., B 2019, 183, 110429 10.1016/j.colsurfb.2019.110429. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Zhao H.; Shen Y.; Wang Y.; Zhao Z.; Zhang Y. Preparation, Characterization and Antioxidant Activity Evaluation in Vitro of Fritillaria Ussuriensis Polysaccharide-Zinc Complex. Int. J. Biol. Macromol. 2020, 146, 462–474. 10.1016/j.ijbiomac.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Mahmoud M. A.; Zaitone S. A.; Ammar A. M. Binary and Ternary Cu(II) Complexes of Pregabalin with Excitatory and Inhibitory Neurotransmitters and Their Antiepileptic Effect. Mater. Sci. Eng. C 2020, 110, 110650 10.1016/j.msec.2020.110650. [DOI] [PubMed] [Google Scholar]
- Damico R. An Investigation of N-Substituted Methionine Derivatives for Food Supplementation. J. Agricult. Food Chem. 1975, 23, 31–33. 10.1021/jf60197a025. [DOI] [PubMed] [Google Scholar]
- Nguyen L.; Kubitza F.; Salem S. M. R.; Hanson T. R.; Allen Davis D. Comparison of Organic and Inorganic Microminerals in All Plant Diets for Nile TilapiaOreochromis Niloticus. Aquaculture 2019, 498, 297–304. 10.1016/j.aquaculture.2018.08.034. [DOI] [Google Scholar]
- Al-Akel A. S.; Al-Balawi H. F. A.; Al-Misned F.; Mahboobab S.; Ahmad Z.; Suliman E. M. Effects of Dietary Copper Exposure on Accumulation, Growth, and Hematological Parameters in Cyprinus Carpio. Toxicol. Environ. Chem. 2010, 92, 1865–1878. 10.1080/02772248.2010.486230. [DOI] [Google Scholar]
- Lorentzen M.; Maage A.; Julshamn K. Supplementing Copper to a Fish Meal Based Diet Fed to Atlantic Salmon Parr Affects Liver Copper and Selenium Concentrations. Aquacult. Nutr. 1998, 4, 67–72. 10.1046/j.1365-2095.1998.00046.x. [DOI] [Google Scholar]
- Apines M. J. S.; Satoh S.; Kiron V.; Watanabe T.; Aoki T. Availability of Supplemental Amino Acid-Chelated Trace Elements in Diets Containing Tricalcium Phosphate and Phytate to Rainbow Trout, Oncorhynchus Mykiss. Aquaculture 2003, 225, 431–444. 10.1016/S0044-8486(03)00307-7. [DOI] [Google Scholar]
- Hasan M.; Zafar A.; Yousaf M.; Gulzar H.; Mehmood K.; Hassan S. G.; Saeed A.; Yousaf A.; Mazher A.; Rongji D.; Mahmood N. Synthesis of Loureirin B-Loaded Nanoliposomes for Pharmacokinetics in Rat Plasma. ACS Omega 2019, 4, 6914–6922. 10.1021/acsomega.9b00119. [DOI] [Google Scholar]
- Huang F.; Jiang M.; Wen H.; Wu F.; Liu W.; Tian J.; Yang C. Dietary Zinc Requirement of Adult Nile Tilapia (Oreochromis Niloticus) Fed Semi-Purified Diets, and Effects on Tissue Mineral Composition and Antioxidant Responses. Aquaculture 2015, 439, 53–59. 10.1016/j.aquaculture.2015.01.018. [DOI] [Google Scholar]
- Wei Z.; Deng K.; Zhang W.; Mai K. Interactions of Dietary Vitamin C and Proline on Growth Performance, Anti-Oxidative Capacity and Muscle Quality of Large Yellow Croaker Larimichthys Crocea. Aquaculture 2020, 528, 735558 10.1016/j.aquaculture.2020.735558. [DOI] [Google Scholar]
- Moazenzadeh K.; Rajabi Islami H.; Zamini A.; Soltani M. Quantitative Dietary Copper Requirement of Juvenile Siberian Sturgeon, Acipenser Baerii, and Effects on Muscle Composition and Some Enzymatic Activities. Aquacult. Nutr. 2020, 26, 1108–1118. 10.1111/anu.13068. [DOI] [Google Scholar]
- Huang Q. C.; Wang E. L.; Dong X. H.; Tan B. P.; Chi S. Y.; Yang Q. H.; Zhang S.; Liu H. Y.; Yang Y. zhi. Investigations on Zinc Bioavailability of Different Sources and Dietary Zinc Requirement in Juvenile Grouper Epinephelus Coioides. Aquacult. Res. 2018, 49, 2763–2773. 10.1111/are.13737. [DOI] [Google Scholar]
- Nayak A. K.; Das B. K.; Kohli M. P. S.; Mukherjee S. C. The Immunosuppressive Effect of α-Permethrin on Indian Major Carp, Rohu (Labeo Rohita Ham.). Fish Shellfish Immunol. 2004, 16, 41–50. 10.1016/S1050-4648(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Li P. H.; Liang Y. L.; Su Y. L.; Jiang Y. F.; Chen B.; Chen S. Y.; Huang Y. H.; Wei J. G.; Huang X. H.; Qin Q. W.; Sun H. Y. Molecular Characterization and Function Analysis of Epinephelus Coioides Hsp22 Response to SGIV and Vribro Alginolyticus Infection. Fish Shellfish Immunol. 2020, 97, 125–134. 10.1016/j.fsi.2019.11.069. [DOI] [PubMed] [Google Scholar]
- Link J. S.; Huse G.; Gaichas S.; Marshak A. R. Changing How We Approach Fisheries: A First Attempt at an Operational Framework for Ecosystem Approaches to Fisheries Management. Fish Fish. 2020, 21, 393–434. 10.1111/faf.12438. [DOI] [Google Scholar]
- Wang Q.; Shen J.; Yan Z.; Xiang X.; Mu R.; Zhu P.; Yao Y.; Zhu F.; Chen K.; Chi S.; Zhang L.; Yu Y.; Ai T.; Xu Z.; Wang Q. Dietary Glycyrrhiza Uralensis Extracts Supplementation Elevated Growth Performance, Immune Responses and Disease Resistance against Flavobacterium Columnare in Yellow Catfish (Pelteobagrus Fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. 10.1016/j.fsi.2019.12.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.