Abstract
Objectives
To assess the incidence and the impact of carbapenem-resistant Acinetobacter baumannii (CRAB) intestinal carriage on subsequent CRAB infection and to study risk factors of acquiring CRAB intestinal carriage among patients in intensive care unit (ICU).
Design
Observational study including a case–control study and a retrospective cohort study.
Setting
A 50-bed general ICU of a university hospital, China.
Methods
From May 2017 to April 2018, an observational study was conducted in a 50-bed general ICU of a university hospital in China. Rectal swabs were collected from ICU patients on admission and thereafter weekly. A case–control study was performed to analyse risk factors of the acquisition of CRAB intestinal carriage in ICU using multiple logistic regression. A retrospective cohort study was performed to address whether intestinal CRAB carriage could lead to an increased likelihood of subsequent CRAB infection using subdistribution hazard model regarding death in the ICU as a competing risk event.
Results
CRAB intestinal carriage was detected in 6.87% (66/961; 95% CI 5.27% to 8.47%) of patients on ICU admission, whereas 11.97% (115/961; 95% CI 9.91% to 14.02%) of patients acquired CRAB intestinal carriage during the ICU stay. Pancreatitis (OR 2.16, 95% CI 1.28 to 3.67), haematological disease (OR 2.26, 95% CI 1.42 to 3.58), gastric tube feeding (OR 3.35, 95% CI 2.03 to 5.51) and use of carbapenems (OR 1.84, 95% CI 1.11 to 3.07) were independent risk factors for acquiring CRAB intestinal carriage. The incidence of subsequent CRAB infection was 2.24-fold in patients with CRAB intestinal carriage compared with that in patients without (95% CI 1.48 to 3.39, p<0.001).
Conclusion
More patients acquired CRAB intestinal carriage during their ICU stay than had on admission. Severity of illness, acute pancreatitis, tube feeding and use of carbapenems were independent risk factors of acquisition of CRAB intestinal carriage. Patients with CRAB intestinal carriage are more likely to develop CRAB infection.
Keywords: epidemiology, adult intensive & critical care, infection control
Strengths and limitations of this study.
This observational study contains a combination of a case–control study for analysing risk factors of the acquisition of carbapenem-resistant Acinetobacter baumannii (CRAB) intestinal carriage in intensive care unit (ICU) and a retrospective cohort study to address whether intestinal CRAB carriage was associated with subsequent CRAB infection.
The competing risk of death in ICU was considered using a well-established model.
This is a single-unit study and the findings may not necessarily generalise well to other settings.
A culture-based method was used to screen CRAB, which is less sensitive than PCR-based methods.
Only rectal swabs were collected for screening CRAB and some CRAB carriers might have been missed.
Background
Acinetobacter baumannii is one of the most common nosocomial pathogens in Asia and South America.1 A systematic review has revealed that A. baumannii accounted for 11.28% of nosocomial infections in general hospitals in China, making it the third most common nosocomial pathogen,2 and carbapenem-resistant A. baumannii (CRAB) has emerged worldwide. As early as 2013, the US Centers for Disease Control and Prevention listed multidrug-resistant A. baumannii (MDRAB) including CRAB as a serious threat,3 and the WHO listed CRAB as one of the three most critical threats in a global drug-resistant warning in 2017.4 The prevalence of A. baumannii and its resistance to carbapenems varies from country to country. For instance, the European Bacterial Resistance Surveillance Report shows that the rate of Acinetobacter resistant to carbapenem in Europe in 2017 was 33.4% (95% CI 32% to 35%), but it was as high as 96.2% in Croatia (95% CI 92% to 98%).5 In the USA, 49.5% of A. baumannii is resistant to carbapeems, while in Singapore, India and Pakistan, it is 50%, 85% and 62%–100%, respectively.6 7 The prevalence of CRAB is also very high in China. The surveillance data released by China Antimicrobial Surveillance Network (http://chinets.com/Chinet), a national network in China, have shown that 77.1% and 78.1% of A. baumannii isolates resistant to imipenem and meropenem, respectively.8
Infections caused by CRAB can lead to serious consequences. A previous study has demonstrated that patients with CRAB infection had longer average length of stay (LOS) in intensive care unit (ICU) (13.1 vs 10.5 days) and US$11 359 higher average in-hospital costs than those with carbapenem-susceptible A. baumannii (CSAB) infection.9 Another previous study has found that the mortality rate of patients with CRAB infection is 2.22-fold that of patients with CSAB infection.10 A case–control study conducted by our team have also shown that the 28 day survival rate of patients with bloodstream CRAB infection was 66.17%, lower than the 96.95% of those with bloodstream CSAB infection.11
It is well known that A. baumannii including CRAB may colonised in the respiratory tract of hospitalised patients, in particular those with mechanical ventilation.12 13 The colonisation of CRAB in the respiratory tract has been found as a major risk factor for subsequent CRAB infection.14 However, ICU patients may carry CRAB in intestine on admission or acquire CRAB during the ICU stay.15 Patients with intestinal carriage of multidrug resistant organisms (MDRO), in particular carbapenem-resistant Enterobacteriaceae (CRE), may sever as a reservoir for further dissemination in ICU16 and could be associated with an increased risk of subsequent MDRO infections.17 Therefore, active screening the carriage of CRE, which is usually performed using rectal swabs, has been recommended as a core component of the infection control bundle.7 However, by contrast to CRE, the prevalence of CRAB intestinal carriage among ICU patients is much less studied and the risk factors of acquisition of CRAB intestinal carriage remain largely unknown. In addition, it remains to be determined whether CRAB intestinal carriage leads to increased risks of subsequent CRAB infection. To address these questions, we therefore conducted this study.
Methods
Study settings
An observational study was conducted in a 50-bed general ICU of a 4300-bed university hospital in China. From May 2017 to April 2018, all patients admitted to the ICU were subjected to collecting a rectal swab within 48 hours of admission and thereafter weekly. For patients hospitalised for less than 3 days, a rectal swab was collected only once within 48 hours of admission.
Inclusion and exclusion criteria
Inclusion criteria: This study included all patients who were ≥18 years of age, admitted to the ICU and underwent collection of rectal swabs.
Exclusion criteria: (1) patients who did not receive a rectal swab within 48 hours of admission to ICU; or (2) patients who were eligible for weekly follow-up collection of rectal swabs but did not receive subsequent sampling or (3) patients with CRAB infection on admission.
Definitions
Patients with CRAB intestinal carriage were defined as those with CRAB isolated from a rectal swab, while patient without CRAB intestinal carriage referred to those whose swabs were all negative for CRAB during the ICU stay. Patients with CRAB isolated from a rectal swab collected within 48 hours of ICU admission were defined as those with CRAB intestinal carriage on ICU admission. The acquisition of CRAB intestinal carriage referred to a patient who had a CRAB-negative rectal swab collected within 48 hours of ICU admission but had CRAB from a swab collected after 48 hours. CRAB infection was defined as the growth of CRAB from clinical specimens in the presence of clinical manifestations of infection.18 Subsequent CRAB infection referred to CRAB infection developed after the collection of a CRAB-positive rectal swab for patients with CRAB intestinal carriage and CRAB infection developed after 48 hours admission to the ICU for patients without CRAB intestinal carriage.
Screening for CRAB by rectal swabs
For collecting rectal swabs, ready-to-use transport medium swabs (HBPT004; Hopebio Biotechnology, Qingdao, China) was inserted about 2–3 cm into the patient’s anus and then gently rotated. After sampling, the swab was inserted into the ready-to-use transport medium and transported to the laboratory within 2 hour. Rectal swabs were inoculated onto modified CHROMagar Acinetobacter colorimetric plates (Chromagar; Paris, France) containing 2 mg/L meropenem using the partition-and-streaking method.19 20 Plates were then cultured at 37°C for 18–24 hours.20
Data collection and statistical analysis
In this study, the patient’s demographic data, underlying diseases, invasive procedures, medical orders and use of antimicrobial agents were retrieved from the electronic medical record system. Two professional statisticians collaborated to clean the data.
We performed two types of comparison. First, a case–control study was performed to analyse risk factors of the acquisition of CRAB intestinal carriage in ICU. Patients with ICU acquisition of CRAB intestinal carriage were assigned to the case group, while those without CRAB intestinal carriage during their ICU stay were assigned to the control group. All potential factors were initially subjected to the univariate analysis. Quantitative data were described by the median (IQR) and were then analysed using a rank-sum test. Qualitative data were described by number of cases (composition ratio) and were then analysed using the χ2 test or Fisher exact probability method when applied. All variables showing p value less than 0.2 in the univariate analysis were then included into the multiple logistic regression using the forward selection stepwise regression method.21 22 OR and 95% CI were calculated. The Hosmer-Lemeshow method was used to test the goodness-of-fit of the multiple logistic model.23
Second, a retrospective cohort study was performed to address whether intestinal CRAB carriage could lead to an increased likelihood of subsequent CRAB infection. In this cohort study, the exposed group comprised patients with CRAB intestinal carriage either detected on ICU admission or acquired during the ICU stay, while the non-exposed group consisted of those without CRAB intestinal carriage. As the impact of CRAB intestinal carriage on subsequent infection may also be influenced by other factors such as patient demographics, underlying diseases, antimicrobial use and medical operations, we included these factors for analysis instead of evaluating CRAB carriage alone. Survival curves (probability of CRAB infection) in patients with and without CRAB intestinal carriage were mapped using the Fine and Gray model regarding death in the ICU as a competing risk event.24 25 After introducing the interaction term of time and each variable (X*ln (T)) into the Cox model,24 25 the proportional hazards hypothesis was tested, and the results showed no statistical significance (p<0.05). Therefore, subdistribution hazard model was used to obtain subdistribution HRs (SDHRs) and to explore whether CRAB intestinal carriage was a risk factor for subsequent CRAB infection for competing events (R package ‘cmprsk’). The Akaike information criteria were used to select the multivariate model.26 We also performed subgroup analyses to investigate whether CRAB intestinal carriage on ICU admission and that acquired in ICU had different impacts on subsequent CRAB infection using the same statistical method as described above. For the subgroup analysis, patients with CRAB intestinal carriage on ICU admission and those with ICU acquisition of CRAB intestinal carriage were assigned to two exposed subgroups, respectively, while those without CRAB intestinal carriage were assigned to the non-exposed group.
All statistical analyses were performed using SPSS V.21.0 (IBM–SPSS, Armonk, New York, USA) and R V.3.5.3 with a 0.05 two-sided test level.
Patient and public involvement
Patients were not involved in this study.
Results
Some patients (6.87%) had CRAB intestinal carriage on ICU admission and more (12.85%) acquired in ICU
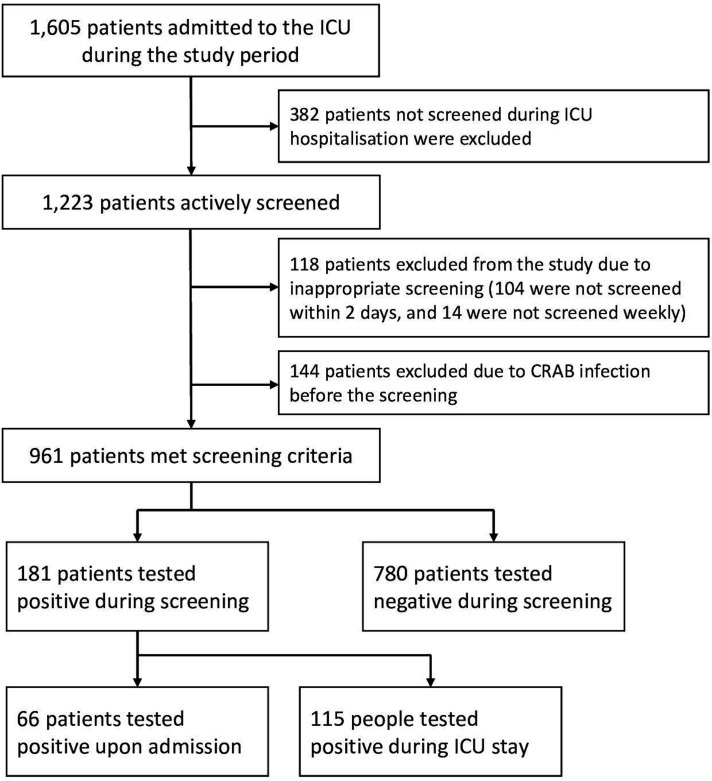
From 1 May 2017 to 30 April 2018, a total of 1605 patients were admitted to the ICU, of which 382 (23.8%) were not screened during their hospital stay. Of which the 382 patients, 323 (84.55%) stayed in the ICU for no more than 2 days, while the other 59 (15.45%) patients were missed for sampling. In addition, 118 patients (118/1605, 7.4%) were excluded due to inappropriate or incomplete sampling including 104 patients whose first rectal swab was collected 48 hours after admission and 14 patients who were not screened weekly. A total of 144 (144/1605, 8.97%) had CRAB infection on ICU admission and were therefore also excluded. Taken together, a total of 961 patients (620 men, 64.52% and 341 women, 35.48%) were included in the analysis, with an average age of 54 (44–68) years (figure 1).
Figure 1.
Patient selection flow algorithm. CRAB, carbapenem-resistant Acinetobacter baumannii;ICU, intensive care unit.
Among the 961 patients, 66 (6.87%, 95% CI 5.27% to 8.47%) had CRAB intestinal carriage on ICU admission. For the remaining 895 patients, 115 acquired (12.85%, 95% CI 10.66% to 15.04%) CRAB intestinal carriage during their ICU stay with an average age of 51 (40–70) and a 1.61 men/women ratio (71 men and 44 women).
Multiple risk factors of acquiring CRAB intestinal carriage were identified
The univariate analysis showed that Acute Physiology and Chronic Health Evaluation II (APACHE II) score (the patient’s disease severity), respiratory failure, renal dysfunction, haematological disease, acute pancreatitis, indwelling central venous catheter, gastric tube feeding, nebulisation and use of vancomycin, aminoglycosides, carbapenems, tigecycline and antifungal agents are risk factors for the acquisition of CRAB intestinal carriage in the ICU. Multiple logistic regression including all variables with p<0.2 in the univariate analysis showed that APACHE II score, pancreatitis, haematological diseases, gastric tube feeding and use of carbapenems were independent risk factors for acquiring CRAB intestinal carriage during the ICU stay (table 1). For APACHE II score, the model estimated that the increase of the score by 1 point would lead to a 4% increase of the risk of acquiring CRAB intestinal carriage in the ICU. Hosmer-Lemeshow test generated a 0.73 p value (χ2=5.25, df=8), suggesting adequate goodness-of-fit of the multiple logistic model.
Table 1.
Risk factors for the acquisition of CRAB intestinal carriage during the ICU stay
| Characteristics | Patients with acquiring CRAB intestinal carriage | Univariate analysis | Multivariate analysis | |||
| Yes (n=115) | No (n=780) | OR (95% CI) | P | OR (95% CI) | P | |
| Demographics | ||||||
| Sex, male | 71 (61.74%) | 502 (64.36%) | 1.12 (0.75 to 1.68) | 0.59 | ||
| Ethnicity, Han Chinese | 108 (93.91%) | 712 (91.28%) | 1.47 (0.66 to 3.29) | 0.34 | ||
| Age (median) | 51 (40–70) | 56 (45–68) | / | 0.21 | ||
| Underlying disease | ||||||
| Myocardial infarction | 1 (0.87%) | 4 (0.51%) | 1.7 (0.19 to 15.36) | 0.50 | ||
| Peripheral vascular disease | 11 (9.57%) | 62 (7.95%) | 1.22 (0.62 to 2.40) | 0.55 | ||
| Cerebrovascular disease | 4 (3.48%) | 36 (4.62%) | 0.74 (0.26 to 2.13) | 0.58 | ||
| Dementia | 1 (0.87%) | 0 (0%) | / | 0.13 | ||
| Connective tissue disease | 1 (0.87%) | 12 (1.54%) | 0.56 (0.07 to 4.36) | 0.89 | ||
| Peptic ulcer | 5 (4.35%) | 25 (3.21%) | 1.37 (0.51 to 3.66) | 0.72 | ||
| Haemiplegia | 0 (0%) | 1 (0.13%) | / | 1.00 | ||
| Hypertension | 36 (31.30%) | 180 (23.08%) | 1.52 (0.99 to 2.33) | 0.05 | ||
| Tuberculosis | 1 (0.87%) | 12 (1.54%) | 0.56 (0.07 to 4.36) | 0.89 | ||
| COPD | 10 (8.70%) | 54 (6.92%) | 1.28 (0.63 to 2.59) | 0.49 | ||
| Respiratory failure | 40 (34.78%) | 163 (20.90%) | 2.02 (1.33 to 3.07) | 0.001 | ||
| Kidney failure | 11 (9.57%) | 31 (3.97%) | 2.56 (1.25 to 5.24) | 0.01 | ||
| Heart failure | 7 (6.09%) | 19 (2.44%) | 2.99 (1.28 to 7.01) | 0.06 | ||
| Diabetes | 21 (18.26%) | 102 (13.08%) | 1.48 (0.89 to 2.49) | 0.13 | ||
| Liver dysfunction | 5 (4.35%) | 37 (4.74%) | 0.91 (0.35 to 2.37) | 0.85 | ||
| Haematological disease | 71 (61.74%) | 268 (34.36%) | 3.08 (2.06 to 4.62) | <0.001 | 2.26 (1.42 to 3.58) | 0.001 |
| Pancreatitis | 35 (30.43%) | 77 (9.87%) | 3.99 (2.52 to 6.34) | <0.001 | 2.16 (1.28 to 3.67) | 0.004 |
| Medical operation | ||||||
| Surgery | 82 (71.30%) | 645 (82.69%) | 0.52 (0.33 to 0.81) | 0.004 | 0.40 (0.24 to 0.68) | 0.001 |
| CVC | 78 (67.83%) | 424 (54.36%) | 1.77 (1.17 to 2.68) | 0.01 | ||
| Ventilator | 101 (87.83%) | 666 (85.38%) | 1.23 (0.68 to 2.23) | 0.49 | ||
| Indwelling catheter | 110 (95.65%) | 742 (95.13%) | 1.13 (0.43 to 2.92) | 0.81 | ||
| Tube feeding | 84 (73.04%) | 280 (35.90%) | 4.84 (3.13 to 7.49) | <0.001 | 3.35 (2.03 to 5.51) | <0.001 |
| Nebuliser fibroptic | 73 (63.48%) | 368 (47.18%) | 1.95 (1.30 to 2.92) | 0.001 | ||
| Bronchoscope | 1 (0.87%) | 21 (2.69%) | 0.32 (0.04 to 2.38) | 0.39 | ||
| Antimicrobial use | ||||||
| Cephalosporin | 35 (30.43%) | 312 (40.00%) | 0.66 (0.43 to 1.00) | 0.05 | 0.59 (0.37 to 0.95) | 0.03 |
| Vancomycin | 13 (11.30%) | 32 (4.10%) | 2.98 (1.51 to 5.86) | 0.001 | ||
| Aminoglycosides | 12 (10.43%) | 31 (3.97%) | 2.81 (1.40 to 5.65) | 0.002 | ||
| Carbapenems | 82 (71.30%) | 295 (37.82%) | 4.09 (2.66 to 6.27) | <0.001 | 1.84 (1.11 to 3.07) | 0.02 |
| Fluoroquinolones | 26 (22.61%) | 137 (17.56%) | 1.37 (0.85 to 2.20) | 0.19 | ||
| Antifungal agents | 49 (42.61%) | 138 (17.69%) | 3.45 (2.29 to 5.22) | <0.001 | ||
| Cephamycins | 16 (13.91%) | 253 (32.44%) | 0.34 (0.19 to 0.58) | <0.001 | ||
| Lincomycin | 3 (2.61%) | 61 (7.82%) | 0.32 (0.10 to 1.02) | 0.04 | ||
| Tigecycline | 19 (16.52%) | 69 (8.85%) | 2.04 (1.18 to 3.54) | 0.01 | ||
| APACHE II | 21.5 (17–26) | 17 (12–22) | / | <0.001 | 1.04 (1.01 to 1.07) | 0.01 |
| Charlson score | 2 (1–5) | 3 (2–4) | / | 0.06 | ||
| Sharing room with other patients with CRAB intestinal carriage | 20 (17.39%) | 153 (19.62%) | 0.86 (0.52 to 1.44) | 0.57 | ||
Variables with p<0.05 in the multiple logistic analysis are highlighted in bold.
APACHE II, Acute Physiology and Chronic Health Evaluation II; COPD, chronic obstructive pulmonary disease; CRAB, carbapenem-resistant Acinetobacter baumannii; CVC, central venous catheter; ICU, intensive care unit.
CRAB intestinal carriage led to increased risks of subsequent CRAB infection
During the study period, 112 of the 961 patients (11.65%, 95% CI 9.63% to 13.68%) developed CRAB infections during the ICU stay. As for the infection type, lower respiratory tract infections were the most common (n=82, 73.21%), followed by bloodstream infections (n=9, 8.04%), surgical site infection (n=8, 7.14%), while 13 patients (11.61%) had infections at other sites. CRAB intestinal carriage was a risk factor for subsequent CRAB infection (HR 2.82, 95% CI 1.94 to 4.09; p<0.001; figure 2). The 90-day cumulative probability of no CRAB infection in patients with and without CRAB intestinal carriage was 69.5.0% (95% CI 43.5% to 95.5%) and 22.3% (95% CI 14.7% to 29.9%), respectively (p<0.001). In the univariate analysis, CRAB intestinal carriage, APACHE II score, respiratory failure, liver dysfunction, haematological disease, pancreatitis, mechanical ventilation, placement of a central venous catheter, gastric tube feeding and the use of carbapenems were identified as risk factors for subsequent CRAB infection. In the Cox multivariate analysis, CRAB intestinal carriage was also found to be an independent risk factor for subsequent CRAB infection (HR 2.24, 95% CI 1.48 to 3.39; table 2). Omnibus test showed a log-likelihood difference of 79.82 and generated a less than 0.001 p value, suggesting adequate goodness-of-fit of the Cox model.
Figure 2.
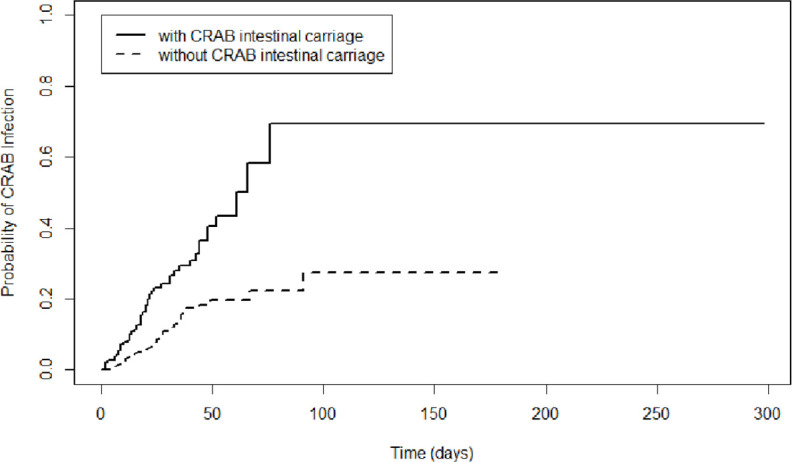
Survival curves of patients with and without CRAB intestinal carriage. CRAB, carbapenem-resistant Acinetobacter baumannii
Table 2.
Variables associated with developing subsequent CRAB infection during the ICU stay using subdistribution hazard model
| Item | Subsequent CRAB infection during the ICU stay | Univariate analysis | Multivariate analysis | |||
| Yes (n=112) | No (n=849) | SDHR (95% CI) | P | SDHR (95% CI) | P | |
| CRAB intestinal carriage | 51 (45.54%) | 130 (15.31%) | 2.82 (1.94 to 4.09) | <0.001 | 2.24 (1.48 to 3.39) | <0.001 |
| Demographics | ||||||
| Sex, male | 72 (64.29%) | 548 (64.55%) | 1.03 (0.70 to 1.52) | 0.87 | ||
| Ethnicity, Han Chinese | 106 (94.64%) | 774 (91.38%) | 1.62 (0.70 to 3.74) | 0.26 | ||
| Age (median) | 53 (42–67) | 55 (44–68) | 1.00 (0.99 to 1.01) | 0.71 | ||
| APACHE II | 21 (17–26) | 17 (12–22) | 1.05 (1.03 to 1.07) | <0.001 | ||
| Charlson score | 3 (1–5) | 3 (1.5–4) | 0.98 (0.88 to 1.08) | 0.66 | ||
| Underlying disease | ||||||
| Peripheral vascular disease | 13 (11.61%) | 66 (7.77%) | 1.30 (0.72 to 2.34) | 0.38 | ||
| Cerebrovascular disease | 4 (3.57%) | 36 (4.24%) | 0.89 (0.34 to 2.33) | 0.81 | ||
| Connective tissue disease | 1 (0.89%) | 12 (1.41%) | 0.60 (0.08 to 4.48) | 0.62 | ||
| Peptic ulcer | 4 (3.57%) | 27 (3.18%) | 1.11 (0.46 to 2.71) | 0.81 | ||
| Hypertension | 28 (25.00%) | 199 (23.44%) | 1.02 (0.67 to 1.57) | 0.92 | ||
| Tuberculosis | 2 (1.79%) | 12 (1.41%) | 1.22 (0.28 to 5.27) | 0.79 | ||
| COPD | 11 (9.82%) | 55 (6.48%) | 1.35 (0.70 to 2.59) | 0.37 | ||
| Respiratory failure | 47 (41.96%) | 170 (20.02%) | 2.02 (1.38 to 2.96) | <0.001 | ||
| Kidney failure | 9 (8.04%) | 42 (4.95%) | 1.42 (0.73 to 2.75) | 0.30 | ||
| Heart failure | 4 (3.57%) | 27 (3.18%) | 1.22 (0.48 to 3.11) | 0.68 | ||
| Diabetes | 15 (13.39%) | 118 (13.90%) | 0.87 (0.50 to 1.49) | 0.61 | ||
| Liver dysfunction | 17 (15.18%) | 34 (4.00%) | 3.15 (1.86 to 5.35) | <0.001 | 2.33 (1.30 to 4.17) | 0.005 |
| Haematological disease | 60 (53.57%) | 314 (36.98%) | 1.61 (1.11 to 2.34) | 0.012 | ||
| Pancreatitis | 29 (25.89%) | 107 (12.60%) | 1.94 (1.29 to 2.92) | 0.002 | ||
| Medical operation | ||||||
| Surgery | 83 (74.11%) | 711 (83.75%) | 0.70 (0.45 to 1.07) | 0.099 | ||
| CVC | 83 (74.11%) | 470 (55.36%) | 1.85 (1.21 to 2.81) | 0.004 | ||
| Ventilator | 103 (91.96%) | 719 (84.69%) | 2.02 (1.04 to 3.93) | 0.038 | ||
| Indwelling catheter | 109 (97.32%) | 808 (95.17%) | 1.84 (0.62 to 5.52) | 0.27 | ||
| Tube feeding | 80 (71.43%) | 332 (39.10%) | 2.44 (1.62 to 3.69) | <0.001 | ||
| Nebulizer fiberoptic | 72 (64.29%) | 413 (48.65%) | 1.18 (0.80 to 1.73) | 0.40 | ||
| Bronchoscope | 5 (4.46%) | 18 (2.12%) | 1.44 (0.59 to 3.52) | 0.43 | ||
| Antimicrobial use | ||||||
| Cephalosporin | 20 (17.86%) | 236 (27.80%) | 0.50 (0.31 to 0.81) | 0.005 | 0.45 (0.28 to 0.73) | 0.001 |
| Vancomycin | 3 (2.68%) | 35 (4.12%) | 0.68 (0.21 to 2.15) | 0.51 | ||
| Aminoglycosides | 1 (0.89%) | 23 (2.71%) | 0.24 (0.03 to 1.71) | 0.15 | ||
| Carbapenems | 82 (73.21%) | 351 (41.34%) | 2.84 (1.87 to 4.32) | <0.001 | 2.21 (1.40 to 3.49) | <0.001 |
| Fluoroquinolones | 32 (28.57%) | 154 (18.14%) | 1.04 (0.69 to 1.56) | 0.84 | ||
| Antifungal agents | 23 (20.54%) | 157 (18.49%) | 0.96 (0.61 to 1.5) | 0.85 | ||
| Cephamycins | 16 (14.29%) | 196 (23.09%) | 0.51 (0.30 to 0.86) | 0.011 | 0.53 (0.31 to 0.90) | 0.018 |
| Lincomycin | 5 (4.46%) | 35 (4.12%) | 1.01 (0.41 to 2.48) | 0.99 | ||
| Tigecycline | 13 (11.61%) | 65 (7.66%) | 1.33 (0.76 to 2.34) | 0.32 | ||
Variables with p<0.05 in the multivariate Cox analysis are highlighted in bold.
APACHE II, Acute Physiology and Chronic Health Evaluation II; COPD, chronic obstructive pulmonary disease; CRAB, carbapenem-resistant Acinetobacter baumannii; CVC, central venous catheter; ICU, intensive care unit; SDHR, subdistribution HR.
To evaluate whether CRAB intestinal carriage on admission and that acquired during the ICU stay has different impact on subsequent CRAB, we performed subgroup analyses. In the subgroup Cox multivariate analysis, both CRAB intestinal carriage on admission and that acquired during the ICU stay were an independent risk factor for subsequent CRAB infection (HR 3.42, 95% CI 1.88 to 6.22 for carriage on admission, online supplementary table S1; HR 1.81, 95% CI 1.15 to 2.86 for acquired carriage, online supplementary table S2). Omnibus test showed log-likelihood difference of 66.06 and 74.18, respectively, and generated a less than 0.001 p value in the subgroup analysis, suggesting adequate goodness-of-fit of the Cox model.
bmjopen-2019-035893supp001.pdf (115.8KB, pdf)
In addition to CRAB intestinal carriage, liver dysfunction (HR 2.33, 95% CI 1.30 to 4.17) and the use of carbapenems (HR 2.21, 95% CI 1.40 to 3.49) were also identified as independent risk factors of subsequent CRAB infection, while the use of cephalosporins (HR 0.45, 95% CI 0.28 to 0.73) and cephamycins (HR 0.53, 95% CI 0.31 to 0.90) were protective factors (table 2).
Discussion
In this study, we found that in a region with a high CRAB prevalence, 6.87% of patients (83.3% of those patients were transferred from other hospitals and 25.8% of them were stayed in emergency ICU before admitted to the ICU) admitted to the ICU had CRAB intestinal carriage on ICU admission, while an additional 11.97% of patients acquired CRAB intestinal carriage during the ICU stay. The overall CRAB intestinal carriage rate was therefore 18.84%. This rate was similar with a study conducted in Thailand, in which 5.45% (15/275) of patients had intestinal carriage on ICU admission and 13.59% (28/206) patients acquired CRAB during their ICU stay15 and with another study in Italy,27 in which 18.92% (74/391) of patients carried CRAB during ICU stay. However, the rate was significantly higher than those in Turkey (7.22%, 55/762),28 Brazil (13.23%, 43/325),29 USA (13.46%, 49/364)30 and South Korea (15.06%, 168/1115),14 although other sites such as respiratory secretions were also screened in these studies. This difference may be related to the local CRAB prevalence.
Interestingly, we found that gastric tube feeding is a risk factor for both acquiring CRAB intestinal carriage of CRAB in ICU, which is consistent with the findings of Kiddee et al,15 in which tube feeding was also a high risk factor for carriage of Gram-negative bacilli. This may suggest an entry point of CRAB into human intestine. In this study, 73.0% (84/115) of patients who acquired CRAB intestinal carriage using tube feeding. During the study, we performed a 1-day snapshot sampling of the feeding tubes (at the tube port), feeding contents and containers for preparing feeding contents in the ICU and found the presence of CRAB in the tube feeding content (24.0%, 6/25), at the tube port (33.3%, 3/9) and the tube feeding containers (7.1%, 1/14), indicating contamination. This may be a key point for intervention in the ICU.
We also found that patients with CRAB intestinal carriage were more likely to develop subsequent CRAB infection than those without carriage. The survival curve in this study showed that the cumulative infection rates in 90 days in patients with and without CRAB intestinal carriage were 69.5% and 22.3%, respectively, similar to those reported in other studies.30 However, the HR was 2.24, which is much lower than those in the previous studies.15 30 31 This may be due to the fact that healthcare-associated infections in our ICU were mainly caused by lower respiratory infections, which accounting for more than 70% of infections, while we only screened the colonisation of the intestines. Interestingly, we found that the use of cephalosporins and cephamycins led to lower risks of subsequent CRAB infection, while carbapenem use led to increased risks. The association between CRAB and carbapenem use has been documented before.30 32 CRAB is usually resistant to cephalosporins and cephamycins. The use of cephalosporins and cephamycins may reflect the fact that patients did not receive carbapenems and could therefore result in reduced selection pressure for CRAB.
There are a few limitations in this study. First, this is a single centre study and the findings may not be generalised. Second, we used a modified CHROMagar Acinetobacter chromogenic plate to screen CRAB from rectal swabs. Not all screened CRABs were confirmed using Vitek II or other methods and there may be false-negative results. Nonetheless, at the beginning of this study, we confirmed that the 58 CRAB strains grown on the chromogenic medium were indeed all A. baumannii by Matrix-Assisted Laser Desorption/ Ionization Time of Flight Mass (MALDI–TOF–MS) and were all non-susceptible to imipenem or meropenem as determined using the agar dilution method recommended by the Clinical and Laboratory Standards Institute.20 Third, we only collected the patients’ rectal swabs for investigating CRAB carriage. Studies have shown concurrent swab collection of skin, oropharyngeal, and airway secretions in addition to rectal swabs, may improve sensitivity. However, the sample sizes in these studies were small with only 21 and 34 cases, respectively.12 33 Nonetheless, for practical reasons and the aim to study CRAB intestinal carriage, we only collected rectal swabs. Fourth, due to the poor sensitivity of rectal swabbing, a single negative test result could overlook carriers. Moreover, no molecular strain typing was performed. Though reasonable, it was not proven that CRAB isolated from intestinal colonisation and site of nosocomial infection were identical. At last, this study failed to collect for the first rectal swab specimen within 48 hours of ICU admission from 23.8% of the patients. Nonetheless, 84.55% of these patients stayed in the ICU for less than 48 hours.
In conclusion, some patients had CRAB intestinal carriage but more acquired during their ICU stay. Severity of illness, acute pancreatitis, tube feeding and use of carbapenems were independent risk factors of the acquisition of CRAB intestinal carriage. Patients with CRAB intestinal carriage were more likely to have subsequent CRAB infection than those without.
Supplementary Material
Acknowledgments
We would like acknowledge the physicians and allied health staff of the ICU and the Department of Electronic Medical Record system of West China Hospital.
Footnotes
Contributors: FQ, ZZ and CT contributed to study conception and design. SZ and YK contributed to acquisition of data. LC collected rectal swabs and transported to the laboratory. FQ, WH and SG analysed and interpreted data. LW inoculated rectal swabs onto plates cultured for 18–24 hours. FQ, ZZ and CT drafted the manuscript. All authors revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding: The work was supported by grants from the National Natural Science Foundation of China (project number 81772233, 81661130159 and 81861138055), West China Hospital of Sichuan University (1.3.5 project for disciplines of excellence, project number ZYYC08006) and the Newton Advanced Fellowship, Royal Society, UK (NA150363).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This project was approved by the Ethics Committee of West China Hospital of Sichuan University. We confirm that consents were not obtained from the patients. First, active screening is part of the routine care for ICU patients in our hospital. In other words, no matter whether we analysed the data, the patients would receive the screening. Second, this is a retrospective study, in which we looked back the patients' data and did not perform any interventions. Third, before we performed this study, we have obtained ethical approval from the Ethical Committee and inform consents were waived due to the retrospective nature of this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The datasets during the current study available from the corresponding author on reasonable request.
References
- 1.Wong D, Nielsen TB, Bonomo RA, et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017;30:409–47. 10.1128/CMR.00058-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Liu F, Tartari E, et al. The prevalence of healthcare-associated infections in mainland China: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2018;39:701–9. 10.1017/ice.2018.60 [DOI] [PubMed] [Google Scholar]
- 3.CDC The biggest antibiotic-resistant threats in the US. centers for disease control and prevention, 2019. Available: https://www.cdc.gov/drugresistance/biggest_threats.html [Accessed 7 Aug 2019].
- 4.Global priority list of antibiotic-resistant bacteria to guide research, discovery and development of new antibiotics. Available: http://apps.who.int/medicinedocs/en/m/abstract/Js23171en/ [Accessed 7 Aug 2019].
- 5.European Centre for Disease Prevention and Control Surveillance of antimicrobial resistance in Europe – annual report of the European antimicrobial resistance surveillance network (EARS-Net) 2017. Stockholm: ECDC, 2018. [Google Scholar]
- 6.Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist 2018;11:1249–60. 10.2147/IDR.S166750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities, 2017. Available: http://www.who.int/infection-prevention/publications/guidelines-cre/en/ [Accessed 7 Aug 2019]. [PubMed]
- 8.Cloud CD. Available: http://chinets.com/Chinet [Accessed 7 Aug 2019].
- 9.Lemos EV, de la Hoz FP, Alvis N, et al. Impact of carbapenem resistance on clinical and economic outcomes among patients with Acinetobacter baumannii infection in Colombia. Clin Microbiol Infect 2014;20:174–80. 10.1111/1469-0691.12251 [DOI] [PubMed] [Google Scholar]
- 10.Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect 2014;20:416–23. 10.1111/1469-0691.12363 [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Qiao F, Wang Y, et al. Risk factors and prognosis of patients with bloodstream infection due to carbapenem-resistant Acinetobacter baumannii. Chinese Journal of Infection Control 2015;14:668–71. [Google Scholar]
- 12.Nutman A, Lerner A, Schwartz D, et al. Evaluation of carriage and environmental contamination by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect 2016;22:949.e5–949.e7. 10.1016/j.cmi.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 13.Cheng VCC, Chen JHK, So SYC, et al. Use of fluoroquinolones is the single most important risk factor for the high bacterial load in patients with nasal and gastrointestinal colonization by multidrug-resistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 2015;34:2359–66. 10.1007/s10096-015-2489-4 [DOI] [PubMed] [Google Scholar]
- 14.An JH, Kim Y-H, Moon J-E, et al. Active surveillance for carbapenem-resistant Acinetobacter baumannii in a medical intensive care unit: can it predict and reduce subsequent infections and the use of colistin? Am J Infect Control 2017;45:667–72. 10.1016/j.ajic.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 15.Kiddee A, Assawatheptawee K, Na-Udom A, et al. Risk factors for gastrointestinal colonization and acquisition of carbapenem-resistant gram-negative bacteria among patients in intensive care units in Thailand. Antimicrob Agents Chemother 2018;62:e00341. 10.1128/AAC.00341-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tischendorf J, de Avila RA, Safdar N. Risk of infection following colonization with carbapenem-resistant Enterobactericeae: a systematic review. Am J Infect Control 2016;44:539–43. 10.1016/j.ajic.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McConville TH, Sullivan SB, Gomez-Simmonds A, et al. Carbapenem-Resistant Enterobacteriaceae colonization (CRE) and subsequent risk of infection and 90-day mortality in critically ill patients, an observational study. PLoS One 2017;12:e0186195. 10.1371/journal.pone.0186195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JY, Wu YH, Cai M, et al. Point-Prevalence survey of healthcare-associated infections in Beijing, China: a survey and analysis in 2014. J Hosp Infect 2016;93:271–9. 10.1016/j.jhin.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 19.Song W, Lee J, Kim T-K, et al. Modified CHROMagar Acinetobacter medium for direct detection of multidrug-resistant Acinetobacter strains in nasal and rectal swab samples. Ann Lab Med 2013;33:193–5. 10.3343/alm.2013.33.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei L, Qiao F, Lin J, et al. Modified CHROMagar Acinetobacter chromogenic culture combined with MALDI-TOF-MS for rapid screening of carbapenem-resistant Acinetobacter baumannii from human gut. Chin J Nosocomiol 2018;28:2893–7. [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied logistic regression, second edition. 2nd edn New York, NY: John Wiley & Sons, 2000. https://onlinelibrary.wiley.com/doi/book/ [Google Scholar]
- 22.Esterly JS, Griffith M, Qi C, et al. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother 2011;55:4844–9. 10.1128/AAC.01728-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DW, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Commun Stat Theory Methods 1980;9:1043–69. 10.1080/03610928008827941 [DOI] [Google Scholar]
- 24.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007;40:381–7. 10.1038/sj.bmt.1705727 [DOI] [PubMed] [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling survival data: extending the COX model. 2000 edition Springer, 2000. [Google Scholar]
- 26.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 2010;45:1388–95. 10.1038/bmt.2009.359 [DOI] [PubMed] [Google Scholar]
- 27.Mammina C, Bonura C, Vivoli AR, et al. Co-colonization with carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii in intensive care unit patients. Scand J Infect Dis 2013;45:629–34. 10.3109/00365548.2013.782614 [DOI] [PubMed] [Google Scholar]
- 28.Karaaslan A, Soysal A, Altinkanat Gelmez G, et al. Molecular characterization and risk factors for carbapenem-resistant gram-negative bacilli colonization in children: emergence of NDM-producing Acinetobacter baumannii in a newborn intensive care unit in turkey. J Hosp Infect 2016;92:67–72. 10.1016/j.jhin.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 29.DalBen MF, Basso M, Garcia CP, et al. Colonization pressure as a risk factor for colonization by multiresistant Acinetobacter spp and carbapenem-resistant Pseudomonas aeruginosa in an intensive care unit. Clinics 2013;68:1128–33. 10.6061/clinics/2013(08)11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latibeaudiere R, Rosa R, Laowansiri P, et al. Surveillance cultures growing carbapenem-resistant Acinetobacter baumannii predict the development of clinical infections: a retrospective cohort study. Clin Infect Dis 2015;60:415–22. 10.1093/cid/ciu847 [DOI] [PubMed] [Google Scholar]
- 31.Tseng W-P, Chen Y-C, Chen S-Y, et al. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant gram-negative bacteria colonization or infection. Antimicrob Resist Infect Control 2018;7:93. 10.1186/s13756-018-0388-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco N, Harris AD, Rock C, et al. Risk factors and outcomes associated with multidrug-resistant Acinetobacter baumannii upon intensive care unit admission. Antimicrob Agents Chemother 2018;62:e01631–17. 10.1128/AAC.01631-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchaim D, Navon-Venezia S, Schwartz D, et al. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol 2007;45:1551–5. 10.1128/JCM.02424-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035893supp001.pdf (115.8KB, pdf)