Abstract
Aim
No study elucidated the role of fasting blood glucose (FBG) level in the prognosis of coronavirus disease 2019 (COVID-19).
Methods
This cohort study was conducted in a single center at Renmin Hospital of Wuhan University, Wuhan, China. Clinical laboratory, and treatment data of inpatients with laboratory-confirmed COVID-19 were collected and analyzed. Outcomes of patients with and without pre-existing diabetes were compared. The associations of diabetes history and/or FBG levels with mortality were analyzed. Multivariate cox regression analysis on the risk factors associated with mortality in patients with COVID-19 was performed.
Results
A total of 941 hospitalized patients with COVID-19 were enrolled in the study. There was a positive relationship between pre-existing diabetes and the mortality of patients who developed COVID-19 (21 of 123 [17.1%] vs 76 of 818 [9.3%]; P = 0.012). FBG ≥7.0 mmol/L was an independent risk factor for the mortality of COVID-19 regardless of the presence or not of a history of diabetes (hazard ratio, 2.20 [95% CI, 1.21–4.03]; P = 0.010).
Conclusions
We firstly showed FBG ≥7.0 mmol/L predicted worse outcome in hospitalized patients with COVID-19 independent of diabetes history. Our findings indicated screening FBG level is an effective method to evaluate the prognosis of patients with COVID-19.
Keywords: Coronavirus disease 2019, Mortality, Fasting blood glucose, Diabetes
1. Introduction
The COVID-19 pandemic, which caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, is a novel and serious global health threat and dramatically spreads worldwide [1].
Diabetes has been uniformly reported to be associated with poor prognosis in coronavirus infections. One retrospective analysis suggested that a known history of diabetes and ambient hyperglycemia were independent predictors for death and morbidity in SARS patients [2]. Diabetes was prevalent in approximately 50% of the patients with MERS-CoV in a systematic analysis of 637 cases [3]. Recent clinical studies confirmed diabetes as a major comorbidity of COVID-19 [4], [5], [6], [7]. Guo et al. [8] and Dong et al. [9] had investigated the correlation between diabetes history and prognosis of COVID-19, which analyzed 174 and 193 patients with COVID-19 respectively. However, no study focused on the direct correlation between fasting blood glucose (FBG) levels and the prognosis of COVID-19. Furthermore, whether diabetes history or blood glucose level is more important for mortality of patients with COVID-19 remains unclear. In the present research, we investigated the clinical characteristics of patients with coexistence of COVID-19 infection and diabetes, and examined the associations of diabetes history and/or FBG levels with the mortality of COVID-19 in a selected cohort of patients in Wuhan, China.
2. Materials and methods
2.1. Study design and participants
This is a retrospective, single center study at Renmin Hospital of Wuhan University in Wuhan, China. The hospital had assigned responsibility for the treatment of patients with severe COVID-19 by the Wuhan government. All enrolled patients were definitively diagnosed based on the criteria for COVID-19 (trial version 6) established by the Chinese National Health Commission. Exclusion criterions included: (1) Mild cases, which defined as mild clinical manifestation and no obvious chest CT findings for COVID-19; (2) Duplicated cases; (3) No available core medical information. Patients were consecutive admitted to hospital from January 20 to February 20, 2020. All included cases were shared with WHO. The final date of follow-up was March 3, 2020. This study was approved by the National Health Commission of China and the institutional review board at Renmin Hospital of Wuhan University. Written informed consent was waived by the ethics commission of the designated hospital for patients with emerging infectious diseases.
2.2. Data collection
We obtained clinical, laboratory, radiological, treatment, and outcome data from patients’ electronic medical records for hospitalized patients. All data were reviewed by two investigators (F.Y. and B.Y.). Patients’ diabetes history information was collected, and FBG levels were measured on admission. The normal reference range of FBG in Renmin hospital of Wuhan University is 3.9–6.1 mmol/L. As FBG ≥ 7.0 mmol/l is one of diagnostic criterions of diabetes, we took 7.0 mmol/l as a cut-point for the FBG levels. Clinical data included symptoms and comorbidities. Laboratory assessments included complete blood count, c-reactive protein (CRP), procalcitonin (PCT), electrolytes, liver and renal function values. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [10]. Acute kidney injury was identified according to the Kidney Disease: Improving Global Outcomes definition [11].
To confirm COVID-19, the Viral Nucleic Acid Kit (Health, Ningbo, China) was used to extract nucleic acids from throat swab, nose swab, anal swab, or phlegm samples according to the kit instructions. A 2019-nCoV detection kit (Bioperfectus, Taizhou, China) was used to detect the ORF1ab gene (nCovORF1ab) and the N gene (nCoV-NP) according to the manufacturer’s instructions using real-time RT-PCR [12]. An infection was considered as laboratory-confirmed if the nCovORF1ab and nCoV-NP both showed positive results.
2.3. Statistical analysis
Descriptive statistics were obtained for all study variables. All categorical variables were compared for the study outcome by using the Fisher's exact test, and continuous variables were compared using the Student t test or the Mann-Whitney U test, if appropriate. Continuous data are expressed as mean ± standard deviation (SD) or median (interquartile ranger, IQR). Categorical data were expressed as the counts and proportion. Survival curves were plotted using the Kaplan-Meier method. Univariate and multivariate cox regression models were used to determine the independent risk factors for death during hospitalization, with a time from admission to the end of follow-up. The cases missing biomarker data were excluded listwise with statistics software. Data were analyzed using SPSS 25.0 (IBM, Chicago, IL). Statistical charts were generated using Excel 2016, Graphpad Prism (Version 5.0, GraphPad Software) or Minitab (Version 18, Minitab statistical Software). For all the statistical analyses, p < 0.05 was considered significant.
3. Results
3.1. Patients Characteristics
Supplementary Fig. 1 depicts a flowchart for patient recruitment. Briefly, a total of 2157 patients with confirmed COVID-19 were screened initially, from January 20, 2020, to February 20, 2020, 1074 mild cases, 142 duplicated cases and cases without available medical information were excluded. 941 cases (death, 97; survival, 844) with COVID-19 were included in final analysis. Of these patients, 123 patients (13.1%) had a history of diabetes and 818 patients (86.9%) did not have pre-existing diabetes; 242 patients (25.7%) had FBG equal or greater than 7 mmol/L and 699 patients (74.3%) had FBG lower than 7 mmol/L. The median age was 57 years (range, 18–98 years), and 487 (51.8%) were female. Among these patients, the most common symptom at onset of illness was fever (687 patients [73.0%]). Less common symptoms were cough (315 patients [33.5%]), shortness of breath (232 patients [24.7%]), and fatigue (133 patients [14.1%]). Sputum production 40 patients (4.3%), chest pain (34 patients [3.6%]), diarrhea (27 patients [2.9%]), and headache (20 patients [2.1%]) were rare. Comorbidities were present in 586 patients. Other than diabetes, hypertension was the most common comorbidity (272 patients [28.9%]), followed by coronary heart disease (74 patients [7.9%]) and cerebrovascular disease (64 patients [6.8%]). The proportion of chronic renal failure, chronic obstructive pulmonary disease (COPD), cancer, chronic heart failure, hepatitis B infection pregnancy and pregnancy was 4.7% (44 patients), 3.7% (35 patients), 3.0% (28 patients), 2.9% (27 patients), 2.6% (24 patients), and 1.9% (18 patients), respectively (Table 1 ).
Table 1.
Baseline Characteristics, Laboratory and Radiographic findings of 941 Patients with COVID-19.
| Characteristics | All (n = 941) | Diabetes (n = 123) | Non-diabetes (n = 818) | P value | FBG ≥ 7.0 mmol/l (n = 242) | FBG < 7.00 mmol/l (n = 699) | P value |
|---|---|---|---|---|---|---|---|
| Age (yrs), Median (range) | 57(18–98) | 69(27–99) | 56(18–95) | <0.001 | 64 (31–98) | 56 (18–95) | <0.001 |
| Female, n (%) | 487(51.8) | 62(50.4) | 425(52.0) | 0.772 | 119 (49.2) | 368 (52.6) | 0.351 |
| Signs and symptoms at admission, n (%) | |||||||
| Fever | 687(73.0) | 87(70.7) | 600(73.3) | 0.586 | 183 (75.6) | 504(72.1) | 0.288 |
| Cough | 315(33.5) | 43(35.0) | 272(33.3) | 0.759 | 90 (37.2) | 225 (32.2) | 0.155 |
| Shortness of breath | 232(24.7) | 40(32.5) | 192(23.5) | 0.033 | 87 (36.0) | 145 (20.7) | <0.001 |
| Fatigue | 133(14.1) | 18(14.6) | 115(14.1) | 0.89 | 46 (19.0) | 87 (12.4) | 0.012 |
| Sputum production | 40(4.3) | 6(4.9) | 34(4.2) | 0.636 | 12 (5.0) | 28 (4.0) | 0.527 |
| Chest pain | 34(3.6) | 1(0.8) | 33(4.0) | 0.113 | 4 (1.7) | 30 (4.3) | 0.058 |
| Diarrhea | 27(2.9) | 3(2.4) | 24(2.9) | 1.000 | 4 (1.7) | 23 (3.3) | 0.188 |
| Headache | 20(2.1) | 2(1.6) | 18(2.2) | 1.000 | 4 (1.7) | 16 (2.3) | 0.554 |
| Chronic medical illness, n (%) | |||||||
| Hypertension | 272(28.9) | 66(53.7) | 206(25.2) | <0.001 | 108 (44.6) | 164 (23.5) | <0.001 |
| Coronary heart disease | 74(7.9) | 19(15.4) | 55(6.7) | 0.002 | 29 (12.0) | 45 (6.4) | 0.006 |
| Cerebrovascular disease | 64(6.8) | 10(8.1) | 54(6.6) | 0.563 | 18 (7.4) | 46 (6.6) | 0.648 |
| Chronic renal failure | 44(4.7) | 16(13.0) | 28(3.4) | <0.001 | 22 (9.1) | 22 (3.1) | <0.001 |
| COPD | 35(3.7) | 6(4.9) | 29(3.5) | 0.444 | 12 (5.0) | 23 (3.3) | 0.237 |
| Cancer | 28(3.0) | 5(4.1) | 23(2.8) | 0.398 | 9 (3.6) | 19 (2.7) | 0.492 |
| Chronic heart failure | 27(2.9) | 11(8.9) | 16(2.0) | <0.001 | 17 (7.0) | 10 (1.4) | <0.001 |
| Hepatitis B infection | 24(2.6) | 4(3.3) | 20(2.4) | 0.542 | 10 (4.1) | 14 (2.0) | 0.070 |
| Pregnancy | 18(1.9) | 1(0.8) | 17(2.1) | 0.495 | 0 (0) | 18 (2.6) | 0.012 |
| Laboratory findings at admission, median (IQR) | |||||||
| Leucocytes (×109/L) | 6(4.3–8.8) | 6.2(4.6–9.2) | 5.9(4.2–8.8) | 0.254 | 7.1 (5.0–11.0) | 5.4 (4.0–8.1) | <0.001 |
| Lymphocytes (%) | 15.7(8.6–23.6) | 12.2(7.9–20.7) | 16(8.7–23.9) | 0.033 | 10.3 (5.9–15.9) | 18.5 (11.1–25.5) | <0.001 |
| Platelets (×109/L) | 207(149–280) | 189(126–262) | 211(152–281) | 0.095 | 194 (133–261) | 211.5 (155–285) | 0.034 |
| Erythrocytes (×1012/L) | 3.9(3.5–4.3) | 3.8(3.4–4.2) | 3.9(3.5–4.3) | 0.432 | 3.9 (3.5–4.3) | 3.9 (3.5–4.3) | 0.863 |
| Haemoglobin (g/L) | 121(109–132) | 120(109–130) | 121(110–132) | 0.941 | 122 (111–133) | 120 (108–131) | 0.145 |
| C-reactive protein (mg/L) | 44.3(15.4–81.4) | 57.7(22.6–86.9) | 42(14.4–80.8) | 0.039 | 68.2 (27.4–114.2) | 36.6 (12.4–66.9) | <0.001 |
| Procalcitonin (ng/mL) | 0.07(0.04–0.16) | 0.1(0.05–0.32) | 0.06(0.04–0.14) | 0.001 | 0.13 (0.05–0.46) | 0.06 (0.03–0.09) | <0.001 |
| ALT (U/L) | 33(20–61) | 34(21–56) | 33(20–62) | 0.747 | 35 (21–68) | 32 (19–69) | 0.142 |
| AST (U/L) | 33(22–51) | 31(23–48) | 33(22–51) | 0.596 | 38 (24–60) | 31 (21–47) | 0.001 |
| Creatinine (μmol/L) | 59(49–74) | 61(51–89) | 59(48–73) | 0.214 | 61 (51–87) | 57 (48–71) | 0.014 |
| FBG (mmol/L) | 6.5(5.2–8.3) | 9.5(7.3–14.8) | 6.1(5.1–7.6) | <0.001 | 9.0 (7.8–11.9) | 5.4 (4.9–6.1) | <0.001 |
| Potassium (mmol/L) | 3.9(3.5–4.3) | 4.1(3.5–4.7) | 3.9(3.4–4.2) | 0.036 | 3.9 (3.4–4.6) | 3.9 (3.5–4.2) | 0.800 |
| Sodium (mmol/L) | 140(137–143) | 139(136–143) | 140(137–144) | 0.064 | 139 (135–143) | 140 (137–144) | 0.005 |
| Chest X-ray and CT findings, n (%) | |||||||
| Unilateral pneumonia | 400(42.5) | 37(30.1) | 363(44.4) | 0.003 | 64 (26.4) | 336 (48.1) | <0.001 |
| Bilateral pneumonia | 541(57.5) | 86(69.9) | 455(55.6) | 178 (73.6) | 363 (51.9) | ||
| Multiple mottling and ground-glass opacity | 224(23.8) | 37(30.1) | 187(22.9) | 0.088 | 61 (25.2) | 163 (23.3) | 0.552 |
yrs, years; IQR, interquartile range; COPD, Chronic obstructive pulmonary disease; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; FBG, fasting blood glucose; CT, computed tomography.
Compared with patients without pre-existing diabetes, patients with diabetes were in older ages (median [range], 69[27–99] years vs 56 [18–95] years; P < 0.001), and more likely to have shortness of breath (40 [32.5%] vs 192 [23.5%]). Moreover, patients with diabetes had more underlying comorbidities, including hypertension (66 [53.7%] vs 206 [25.2%]), coronary heart disease (19 [15.4%] vs 55 [6.7%]), chronic renal failure (16 [13.0%] vs 28 [3.4%]) and chronic heart failure (11 [8.9%] vs 16 [2.0%]) (Table 1).
Compared with patients with FBG < 7.0 mmol/L, patients with FBG ≥ 7.0 mmol/l were older (median [range], 64 [31–98] years vs 56 [18–95] years; P < 0.001), and more likely to have shortness of breath (87 [36.0%] vs 145 [20.7%]) and feel fatigue (46 [19.0%] vs 87 [12.4%]. Patients with hyperglycemia showed more comorbidities, including hypertension (108 [44.6%] vs 164 [23.5%], coronary heart disease (29 [12.0%] vs 45 [6.4%]), chronic renal failure (22 [9.1%] vs 22 [3.1%]) and chronic heart failure (17 [7.0%] vs 10 [1.4%]). Additionally, the FBG levels of all pregnancy patients were lower than 7 mmol/L. (Table 1).
3.2. Laboratory findings
On admission, in the overall study population of 941 patients, median (IQR) levels of FBG (6.5 [5.2–8.3] mmol/L) and CRP (44.3 [15.4–81.4] mg/L) were elevated whereas the proportion of lymphocytes was declined (15.7 [8.6–23.6]%). The median values of other laboratory indicators were within the normal range, such as counts of leucocytes, platelets, erythrocytes and the levels of hemoglobin, alanine aminotransferase, aspartate aminotransferase, creatinine and electrolyte. The proportion of patients with bilateral pneumonia was 57.5% (541 patients) according to chest radiography and computed tomography findings. Also, 224 patients (23.8%) had multiple mottling and ground-glass opacity (Table 1).
Compared with patients who did not have history of diabetes, patients with diabetes showed higher median (IQR) levels of FBG (9.5[7.3–14.8] mmol/L vs 6.1[5.1–7.6] mmol/L), CRP (57.7 [22.6–86.9] mg/L vs 42 [14.4–80.8] mg/L), PCT (0.1 [0.05–0.32] ng/mL vs 0.06 [0.04–0.14] ng/mL), potassium (4.1[3.5–5.7] mmol/L vs 3.9[3.4–4.2] mmol/L), but a lower proportion of lymphocytes (12.2[7.9–20.7]% vs 16[8.7–23.9]%). With regards to radiologic findings, bilateral pneumonia in patients with diabetes was more prevalent than in patients without diabetes (86 of 123 [69.9%] vs 455 of 818 [55.6%]) (P = 0.003, Table 1).
Compared with patients with FBG < 7.0 mmol/L, patient with FBG ≥ 7.0 mmol/L had higher median (IQR) levels of FBG (9.0 [7.8–11.9] mmol/L vs 5.4 [4.9–6.1] mmol/L), leucocytes (7.1 [5.0–11.0] × 109/L vs 5.4 [4.0–8.1] × 109/L), CRP (68.2 [27.4–114.2] mg/L vs 36.6 [12.4–66.9] mg/L), PCT (0.13 [0.05–0.46] ng/mL vs 0.06 [0.03–0.09] ng/mL), AST (38 [24–60] U/L vs 31 [21–47] U/L), creatinine (61 [51–87] μmol/L vs 57 [48–71] μmol/L), but a lower proportion of lymphocytes (10.3 [5.9–15.9]% vs 18.5 [11.1–25.5]%), lower levels of platelets (194 [133–261] × 109/L vs 211.5 [155–285] × 109/L), sodium (139 [135–143] mmol/L vs 140 [137–144] mmol/L). With regards to radiologic findings, bilateral pneumonia in patients with FBG ≥ 7.0 mmol/L was more prevalent than in patients with FBG < 7.0 mmol/L (178 of 242 [73.6%] vs 363 of 699 [51.9%]) (P < 0.001, Table 1).
3.3. Treatment and complications
All patients were treated in isolation. The median duration from symptom onset to admission was 10 (IQR, 7–14) days. A total of 919 patients (97.7%) were treated with oxygen. The percentages of use of oxygen inhalation, noninvasive ventilation, and invasive mechanical ventilation were 85.0% (800 patients), 8.4% (79 patients), and 4.3% (40 patients), respectively. The proportion of antiviral therapy use was the highest (813 [86.4%]), followed by antibiotic therapy (455 [48.4%]), glucocorticoids (363 [38.6%]), and intravenous immunoglobulin therapy (151 [16%]). Only 2 patients (0.2%) among all participants were given continuous kidney therapy. Overall, 185 patients (19.7%) had ARDS, 130 patients had electrolyte disturbance (13.8%), and 79 patients (8.4%) had acute respiratory failure during hospitalization. Other common complications included hypoproteinemia (80 [8.5%]), acute kidney injury (40 [4.3%]), and acute heart failure (29 [3.1%]) (Table 2 ).
Table 2.
Treatment, Complications and Clinical Outcomes of 941 Patients with COVID-19.
| Characteristics | All (n = 941) | Diabetes (n = 123) | Non-diabetes (n = 818) | P value | FBG ≥ 7.0 mmol/l (n = 242) | FBG < 7.00 mmol/l (n = 699) | P value |
|---|---|---|---|---|---|---|---|
| Time from symptom onset to admission (days), Median (IQR) | 10(7–14) | 10(7–15) | 10(7–14) | 0.216 | 10 (7–14) | 10 (7–13) | 0.050 |
| Treatment, n (%) | |||||||
| Oxygen inhalation | 800(85.0) | 83(67.5) | 717(87.7) | <0.001 | 168 (69.4) | 632 (90.4) | <0.001 |
| Non-invasive ventilation | 79(8.4) | 27(22.0) | 52(6.4) | <0.001 | 34 (14.0) | 45 (6.4) | <0.001 |
| Invasive mechanical ventilation | 40(4.3) | 11(8.9) | 29(3.5) | 0.013 | 25 (10.3) | 15 (2.1) | <0.001 |
| Continuous renal replacement therapy | 2(0.2) | 1(0.8) | 1(0.1) | 0.244 | 2 (0.8) | 0 (0) | 0.016 |
| Antiviral treatment | 813(86.4) | 105(85.4) | 708(86.6) | 0.675 | 211 (87.2) | 602 (86.1) | 0.676 |
| Glucocorticoids | 363(38.6) | 50(40.7) | 313(38.3) | 0.62 | 102 (42.1) | 261 (37.3) | 0.185 |
| Intravenous immunoglobulin therapy | 151(16.0) | 20(16.3) | 131(16.0) | 0.896 | 49 (20.2) | 102 (14.6) | 0.039 |
| Antibiotic treatment | 455(48.4) | 58(47.2) | 397(48.5) | 0.847 | 126 (52.1) | 329 (47.1) | 0.180 |
| Complications, n (%) | |||||||
| ARDS | 185(19.7) | 47(38.2) | 138(16.9) | <0.001 | 71 (29.3) | 114 (16.3) | <0.001 |
| Acute respiratory failure | 79(8.4) | 13(10.6) | 66(8.1) | 0.382 | 43 (17.8) | 36 (5.2) | <0.001 |
| Acute kidney injury | 40(4.3) | 10(8.1) | 30(3.7) | 0.03 | 18 (7.4) | 22 (3.1) | 0.004 |
| Acute heart failure | 29(3.1) | 8(6.5) | 21(2.6) | 0.043 | 20 (8.3) | 9 (1.3) | <0.001 |
| Electrolyte disturbance | 130(13.8) | 18(14.6) | 112(13.7) | 0.779 | 50 (20.7) | 80 (11.4) | <0.001 |
| Hypoproteinemia | 80(8.5) | 21(17.1) | 59(7.2) | 0.001 | 32 (13.2) | 48 (6.9) | 0.002 |
ARDS, acute respiratory distress syndrome;
Compared with those without history of diabetes, patients with diabetes required more noninvasive ventilation (27 [22.0%] vs 52 [6.4%]; P < 0.001) and invasive mechanical ventilation (11 [8.9%] vs 29 [3.5%]; P < 0.013) (Table 2). With regards to complications, patients with diabetes were more likely to develop ARDS (47 [38.2%] vs 138 [16.9%]; P < 0.001), acute kidney injury (10 [8.1%] vs 30 [3.7%]; P = 0.03) and acute heart failure (8 [6.5%] vs 21 [2.6%]; P = 0.043) than those without diabetes (Table 2).
Compared with patients with FBG < 7.0 mmol/L, patients with FBG ≥ 7.0 mmol/L required more noninvasive ventilation (34 [14.0%] vs 45 [6.4%]; P < 0.001), invasive mechanical ventilation (25 [10.3%] vs 15 [2.1%]; P < 0.001), continuous renal replacement therapy (2 [0.8%] vs 0 [0%]; P = 0.016), and intravenous immunoglobulin therapy (49 [20.2%] vs 102 [14.6%]; P < 0.001) (Table 2). With regards to complications, patients with hyperglycemia were more likely to develop ARDS (71 [29.3%] vs 114 [16.3%]; P < 0.001), acute respiratory failure (43 [17.8%] vs 36 [5.2%]; P < 0.001), acute kidney injury (18 [7.4%] vs 22 [3.1%]; P = 0.004), acute heart failure (20 [8.3%] vs 9 [1.3%]; P < 0.001), electrolyte disturbance (50 [20.7%] vs 80 [11.4%]; P < 0.001) and hypoproteinemia (32 [13.2%] vs 48 [6.9%]; P = 0.002) than those with FBG < 7.0 mmol/L (Table 2).
3.4. Diabetes, FBG and mortality
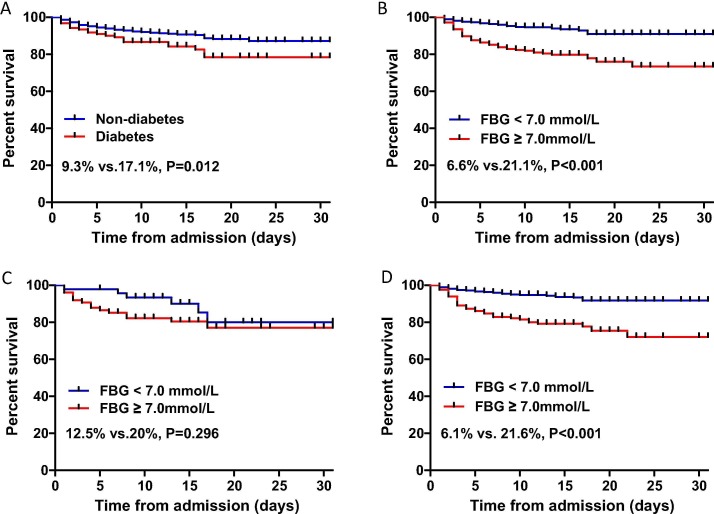
On Kaplan-Meier analysis, the mortality in patients with pre-existing diabetes was higher than that in patients without pre-existing diabetes (21/123 [17.1%] vs 76/818 [9.3%]; P = 0.012) (Fig. 1A). The mortality difference was more pronounced between patients with FBG higher and lower than 7 mmol/L (/51/245[20.1%] vs 46/696[6.6%]; P < 0.001) (Fig. 1B).
Fig. 1.
Kaplan-Meier survival curves of survival probability in hospitalized patients with COVID-19. A. Mortality was higher in patients with diabetes. B. Mortality was significantly higher in patients with FBG ≥ 7.0 mmol/l. C. Among diabetes subjects, mortality was higher in patients with FBG ≥ 7.0 mmol/L, there was no statistical difference after log-rank test. D. Among non-diabetes subjects, the mortality was significantly higher in patients with FBG ≥ 7.0 mmol/L. FBG, fasting blood glucose.
Among diabetes subjects, the mortality in patients with FBG ≥ 7.0 mmol/L (15/75 [20%]) was higher than that in patients with FBG < 7.0 mmol/L (6/48 [12.5%]), however, there was no significant difference after log-rank test (P = 0.296) (Fig. 1C). Among non-diabetes subjects, the mortality in patients with FBG ≥ 7.0 mmol/L was remarkably higher than that in patients with FBG < 7.0 mmol/L (36/167 [21.6%] vs 40/651[6.1%]; P < 0.001) (Fig. 1D).
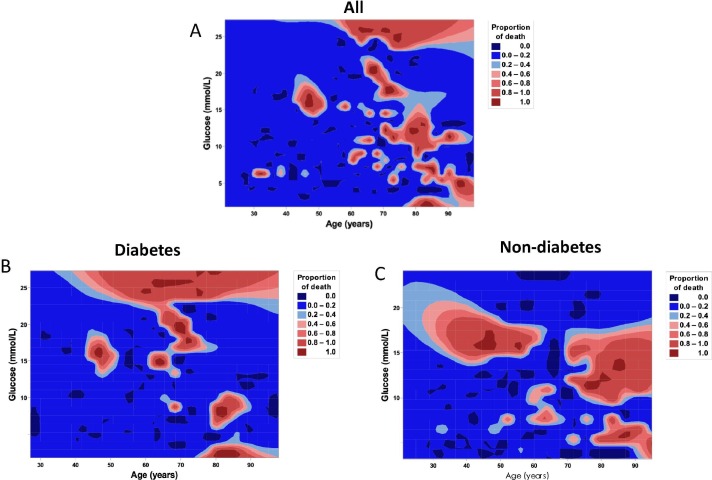
We performed a contour plot to determine the relationship between levels of FBG or age and mortality. Overall, the death rate of older patients was higher, especially for the age was greater than 60 years old (Fig. 2 A). For patients with history of diabetes, high mortality was much closely correlated with hyperglycemia (Fig. 2B). For patients without diabetes, higher mortality was also, to certain extent, related with hyperglycemia (Fig. 2C).
Fig. 2.
Contour plot of survival probability in hospitalized patients with COVID-19. A. Mortality was higher in patients with senior age. B. In diabetes group, mortality was higher in patients with hyperglycemia. C. In non-diabetes group, mortality was higher in patients with hyperglycemia and senior age. FBG, fasting blood glucose.
After adjusting age, sex, most common comorbidities of COVID-19, inflammatory indicators and FBG levels, the univariate adjusted cox proportional hazard regression model revealed a significantly higher risk of death in COVID-19 patients with senior age, hypertension, diabetes, coronary heart disease, cerebrovascular disease, chronic renal failure, COPD, chronic heart failure, high levels of CRP and PCT, FBG ≥ 7 mmol/L. In multivariable cox regression analysis, age (hazard ratio [HR], 1.04; 95% confidence interval [CI], 1.01–1.06), hypertension (HR, 1.89; 95%CI, 1.04–3.45), coronary heart disease (HR, 0.42; 95%CI, 0.18–0.96), chronic renal failure (HR, 3.17; 95%CI,1.48–6.80), chronic heart failure (HR, 3.44; 95%CI, 1.52–7.83), CRP (HR, 1.01; 95%CI, 1.00–1.01), and FBG ≥ 7.0 mmol/L (HR, 2.20; 95%CI, 1.21–4.03) were independent risk factors of mortality in patients with COVID-19 (Table 3 ).
Table 3.
Univariate and multivariate cox regression analysis on the risk factors associated with mortality in patients with COVID-19.
| Characteristics | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Male | 0.81 | 0.55–1.21 | 0.312 | 0.90 | 0.50–1.63 | 0.729 |
| Age | 1.07 | 1.05–1.08 | <0.001 | 1.04 | 1.01–1.06 | 0.004 |
| Hypertension | 2.76 | 1.85–4.12 | <0.001 | 1.89 | 1.04–3.45 | 0.037 |
| Diabetes | 1.78 | 1.10–2.89 | 0.019 | 0.67 | 0.32–1.39 | 0.280 |
| Coronary heart disease | 3.05 | 1.88–4.95 | <0.001 | 0.42 | 0.18–0.96 | 0.041 |
| Cerebrovascular disease | 0.20 | 0.11–0.36 | <0.001 | 0.75 | 0.30–1.87 | 0.534 |
| Chronic renal failure | 4.17 | 2.36–7.35 | <0.001 | 3.17 | 1.48–6.80 | 0.003 |
| Chronic obstructive pulmonary disease | 0.24 | 0.13–0.43 | <0.001 | 0.40 | 0.15–1.06 | 0.066 |
| Chronic heart failure | 7.47 | 4.30–12.97 | <0.001 | 3.44 | 1.52–7.83 | 0.003 |
| C-reactive protein (mg/L) | 1.01 | 1.01–1.02 | <0.001 | 1.01 | 1.00–1.01 | <0.001 |
| Procalcitonin (ng/mL) | 1.10 | 1.05–1.15 | <0.001 | 1.02 | 0.96–1.09 | 0.506 |
| FBG ≥ 7.0 mmol/L | 3.07 | 2.06–4.57 | <0.001 | 2.20 | 1.21–4.03 | 0.010 |
HR, hazard ratio; CI, confidence interval; FBG, fasting blood glucose.
4. Discussion
In this retrospective analysis, we found that there was a positive relationship between pre-existing diabetes and the mortality of patients who developed COVID-19, however, the diabetes history was not an independent risk factor of death rate in patients with COVID-19. More importantly, the present study was the first to show that FBG ≥ 7.0 mmol/L was a predictor of mortality in patients with COVID-19 independent of diabetes history. Besides that, patients with diabetes revealed senior age and much severer inflammatory responses, as well as increased incidences of comorbidities and complications compared with those without diabetes. When grouped by FBG levels, in addition to above difference, patients with FBG ≥ 7.0 mmol/L required more treatment than patients with FBG < 7.0 mmol/L.
It was reported that glucose control is associated with different outcomes in patients with COVID-19 and pre-existing diabetes [13]. A recent study, which included 47 patients, showed FBG associated with mortality rate in COVID-19 patients with diabetes [14]. Notably, the present study found that, not only among known diabetes patients, but also in all the in-hospital COVID-19 patients, FBG ≥ 7.0 mmol/L was an independent risk factor of mortality in patients with COVID-19. Specifically, in patients without diabetes history, the death rate was elevated significantly in response to hyperglycemia. The reasons why hyperglycemia but not diabetes history serves as an independent factor of death rate in COVID-19 patients appear to be due to not only the mechanisms concerning the effects of hyperglycemia on inflammation and angiotensin-converting enzyme 2 (ACE2) expression, but also the effects of diabetes-associated multifactorial factors on contributing to death in COVID-19 patients. For instance, patients with diabetes and COVID-19 were older, and had more severe inflammation and comorbidities, including hypertension, coronary heart disease, chronic renal failure and chronic heart failure, which also affected the mortality rate of COVID-19. Given this, it is very likely that the role of diabetes history as an independent risk factor for the mortality of COVID-19 was superimposed by other factors. In addition, dissociation of hyperglycemia from diabetes history likely also negated the role of diabetes as an independent factor for death rate in COVID-19 patients. As supporting evidence, certain patients without diabetes history had developed acute stress hyperglycemia after infection of COVID-19 [15]. Our findings indicated that screening FBG level is an effective and simple method to evaluate the prognosis of patients with COVID-19, and intervention should be taken in time when patients with FBG ≥ 7.0 mmol/L regardless of the presence or not of a history of diabetes.
We did not analyze the exact relationship between hypoglycemia and mortality due to the patients with FBG < 3.9 mmol/L were rare in the present study. Actually, in both diabetes and non-diabetes groups, the death rates were high at a lower level of FBG. It was worth noting that patients with hypoglycemia were very old, and probably had a few comorbidities. It demonstrated that hypoglycemia appeared to reflect a poor general health condition, rather than a direct cause of death [16].
There are some mechanisms that FBG ≥ 7.0 mmol/L might play a role in COVID-19 infection and poor prognosis. Since immunity is the first line of defense against SARS-CoV-2, it appears that the disturbed immunity in patients with hyperglycemia allowed unhindered proliferation of the pathogen within the host [17]. Hyperglycemia inhibits neutrophil chemotaxis, decreases phagocytosis by neutrophils, macrophages, and monocytes, and impairs innate cell-mediated immunity [18]. In patients with COVID-19, the proportion of proinflammatory Th17 CD4 + T cells and cytokine levels were elevated whereas peripheral counts of CD4 + and CD8 + T cells were decreased [19]. As such, patients with hyperglycemia may display impaired anti-viral IFN responses and delayed activation of Th1/Th17, which contributed to hyperinflammatory response [19]. On the other hand, it has been reported that elevated blood glucose levels could directly increase glucose concentrations in airway secretion, which may undermine the defensive ability of airway epithelia [20]. Consistently, in the present study, the proportion of lymphocytes was declined and the CRP levels were increased in patients with diabetes. As substantial evidence, we revealed that the CRP level was a risk factor of the mortality in COVID-19. Moreover, CRP and PCT levels were elevated more significantly in patients with diabetes who developed COVID-19. The study by Guo et al also revealed that the serum levels of inflammation-related biomarkers such as IL-6 and CRP were significantly higher in COVID-19 patients with diabetes [8]. Accumulating evidence suggests that patients with severe COVID-19 have increased incidence of a cytokine storm syndrome, which would cause mortality [6], [21]. Due to exaggerated inflammatory responses, patients with hyperglycemia were more susceptible to cytokine storm, which appeared to lead to rapid deterioration of COVID-19.
As the cellular receptor for SARS-CoV-2 [22] ACE2 may also account for the association between diabetes and COVID-19. ACE2 is abundant in the type I and II alveolar epithelial cells, endothelial cells, kidney tubular epithelium, heart and pancreas [19]. Latest study confirmed human pancreatic beta cells are highly permissive to SARS-CoV-2 infection [23]. Furthermore, patients with diabetes were often treated with the pioglitazone and liraglutide to control glucose levels, which could upregulate ACE2 levels and facilitate SARS-CoV-2 uptake [24], [25]. This may explain, in part, increased risk of severe infection in patients with hyperglycemia. In addition, the clearance of SARS-CoV-2 was delayed in patients with hyperglycemia [26]. Because of this, it is plausible that patients with hyperglycemia are susceptible to SARS-CoV-2 infection.
Although this study included a large number of patients, some limitations existed. Firstly, because of the logistical restriction at the onset of these emerging infections in Wuhan, some data were lacking from clinical examinations of patients in isolation ward or ICU, such as glycosylated hemoglobin levels and records of monitoring blood glucose during in-patients. Secondly, diabetes was not subtyped when patients admitted to hospital, and this may make the results less comprehensive. Finally, the number of COVID-19 infection worldwide is increasing rapidly, the current sample is relative small, and it is only a single center study. In order to avoid statistical bias, data from larger populations and multiple centers are warranted to further confirm the results of the present study.
Although diabetes history was associated with the mortality of patients hospitalized with COVID-19, FBG ≥ 7.0 mmol/l was an independent risk factor for the death of COVID-19. Our findings presented here highlight the role for FBG screening and glycemic control in COVID-19 management regardless of the presence or not of a history of diabetes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Funding: This work was supported by the Natural Science Foundation of Hubei Province [grant numbers 2018CFB140, 2017CFB204]; and the National Natural Science Foundation of China [grant number 81800447]. The funding sources had no part in study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions
Yuli Cai, Shaobo Shi, Zhongyuan Wen contributed to the conception of the work. Yuli Cai, Shaobo Shi, Fan Yang, Bo Yi, Xiaolin Chen, Junfeng Li contributed to the data collection. Yuli Cai, Shaobo Shi, Fan Yang contributed to the data analysis. Yuli Cai, Shaobo Shi, Zhongyuan Wen drafted the article. All authors contributed to the interpretation of data and critical revision of the Article. All authors gave final approval of the version to be published.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108437.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhu N.a., Zhang D., Wang W., Li X., Yang B.o., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., Sun G.Z., Yang G.R., Zhang X.L., Wang L., Xu X., Xu X.P., Chan J.C.N. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 3.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. International Journal of Infectious Diseases. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.-J., Ni Z.-y., Hu Y.u., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-l., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L.i., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y.i., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S., Qin M.u., Shen B.o., Cai Y., Liu T., Yang F., Gong W., Liu X.u., Liang J., Zhao Q., Huang H.e., Yang B.o., Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed]
- 9.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diab Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Stevens P.E., Levin A., Disease K. Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Wu Q, Xu W, Qiao B, Wang J, Zheng H et al. Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv preprint doi: https://doi.org/10.1101/2020.02.12.20022327.
- 13.Zhu L., She Z.-G., Cheng X.u., Qin J.-J., Zhang X.-J., Cai J., Lei F., Wang H., Xie J., Wang W., Li H., Zhang P., Song X., Chen X.i., Xiang M., Zhang C., Bai L., Xiang D.a., Chen M.-M., Liu Y., Yan Y., Liu M., Mao W., Zou J., Liu L., Chen G., Luo P., Xiao B., Zhang C., Zhang Z., Lu Z., Wang J., Lu H., Xia X., Wang D., Liao X., Peng G., Ye P., Yang J., Yuan Y., Huang X., Guo J., Zhang B.-H., Li H. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong W., Zhang J., Xu Y., Li L.i., Li Q., Yang L.i., Wang H., Hu Y.u., Chen L., Pan A.n., Zeng T. Letter to the Editor: Fasting plasma glucose associated with mortality rate in T2DM patients with COVID-19 infection. Metabolism. 2020;108:154255. doi: 10.1016/j.metabol.2020.154255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumdar S.R., Hemmelgarn B.R., Lin M., McBrien K., Manns B.J., Tonelli M. Hypoglycemia Associated With Hospitalization and Adverse Events in Older People: Population-based cohort study. Diabetes Care. 2013;36(11):3585–3590. doi: 10.2337/dc13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma R.C.W., Holt R.I.G. COVID‐19 and diabetes. Diabet. Med. 2020;37(5):723–725. doi: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. American Journal of Physiology-Endocrinology and Metabolism. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philips B.J., Meguer J.-X., Redman J., Baker E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29(12):2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 21.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L.i. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan R., Zhang Y., Li Y., Xia L.u., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;In Press. [DOI] [PMC free article] [PubMed]
- 24.Romani-Perez M, Outeirino-Iglesias V, Moya CM, Santisteban P, Gonzalez-Matias LC, Vigo E et al. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology. 2015;156(10):3559-69. doi:10.1210/en.2014-1685. [DOI] [PubMed]
- 25.Zhang W., Xu Y.-Z., Liu B.o., Wu R., Yang Y.-Y., Xiao X.-Q., Zhang X. Pioglitazone Upregulates Angiotensin Converting Enzyme 2 Expression in Insulin-Sensitive Tissues in Rats with High-Fat Diet-Induced Nonalcoholic Steatohepatitis. The Scientific World Journal. 2014;2014:1–7. doi: 10.1155/2014/603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Hu W, Ling J, Mo P, Zhang Y, Jiang Q et al. Hypertension and Diabetes Delay the Viral Clearance in COVID-19 Patients. medRxiv preprint doi: https://doi.org/10.1101/2020.03.22.20040774.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.