ABSTRACT
Since the early 2000s, pneumococcal conjugate vaccines (PCVs) have been shown to be effective in the prevention of pneumonia and invasive pneumococcal diseases. In 2011, the Galician region incorporated PCV in the routine infant immunization, the very first stable program in Spain. We aim to assess direct and indirect benefits of PCV vaccination on all-cause pneumonia in the region across different age groups using an ecological study design. For this, we calculated the annual hospitalization rates using a hospital-based disease registry. We identified all-cause pneumonia, pneumococcal pneumonia and pneumococcal invasive diseases within the registry. Hospitalization rates were computed and compared across three study periods: pre-vaccination (1998–2003), early-vaccination (2005–2009) and routine-vaccination (2011–2015). Across Northern Spain, we identified 114,873 all-cause pneumonia hospitalizations, of which 24,808 were further diagnosed with pneumococcal pneumonia. The majority were elderly > 64 years (67.3%). Hospitalizations from all-cause pneumonia had a net increase from 20.6 (pre-PCV) and 21.4/10,000 (early) to 28.4/10,000 (routine) (+32.7%, p < .0001), this is attributed to the huge number of cases in the elderly age group. In contrast, a net reduction of incidence of hospitalized pneumococcal pneumonia was observed from 6.3/10,000 (pre-PCV) and 5.7/10,000 (early) to 2.4/10,000 (routine) cases (−57.9%, p < .0001). Thus, routine infant vaccination may have resulted to an overall decline of pneumococcal pneumonia in infants, as well as in elderly age groups. However, a paradoxical increase on all-cause pneumonia was observed in Galicia, mostly attributed to the growing number of cases in the elderly population.
KEYWORDS: Conjugate vaccines, pneumococcal vaccines, invasive pneumococcal disease, pneumonia, pneumococcus, Streptococcus pneumoniae, vaccination, Spain
Introduction
Streptococcus pneumoniae, or pneumococcus, is one of the most common causes of vaccine-preventable bacterial disease in the world.1 Children and elderly age-group are particularly at high risk for pneumococcal disease. Lower respiratory infection, or pneumonia, is its most common disease manifestation, with 81% of the childhood deaths accounted from the pathogen.1-3 Pneumococcal meningitis and sepsis, although much less frequent, carry high fatality rates and high risk for lifelong disability. In 2000, the US Food and Drugs Authority licensed the use of the first pneumococcal conjugate vaccine for infants, PCV7 (7 valent-pneumococcal conjugate vaccine). This vaccine had been proven to be safe and effective in preventing pneumonia and invasive disease (i.e., sepsis and meningitis).4 Most of the first adopters of the vaccine were high-income countries, and significant declines in IPD and pneumonia were observed after routine immunization.5,6 Since then, higher-valency PCVs (i.e., PCV10 and PCV13) have been licensed and replaced the use of PCV7 to respond to the increasing diseases caused by non-vaccine serotypes.7-10
Pneumococcal conjugate vaccines (PCVs) were first introduced in Spain in June 2001 after the marketing authorization of PCV7.11 In 2009, newer generation PCVs, which cover more serotypes (PCV10 and PCV13), have received marketing approval in Europe. However, only PCV13 had gained a recommendation from the Spanish Health Ministry on June 2010. In 2011, the Galician region included PCV13 in their routine infant immunization,12 the first in Spain to adopt a routine immunization for PCV13. In the interim, PCV reached relatively high coverage based on the Spanish private market for regions where no routine immunization program was started. Nationwide routine immunization in Spain was started only in 2015 after a favorable decision released by the Technical Working group for PCV13.11,13,14
The significant impact of conjugate vaccines is on its capacity to confer herd protection as it extends its beneficial effects on the whole population.15 The countries that have routinely given the vaccines in children saw a concomitant decline in pneumococcal diseases in the non-targeted population, specifically the adults and elderly.16-18 It is worth noting that Galicia has an aging population, and the children who are the targets of the current immunization program comprise 4% of the total population. Our study aims to assess the direct and indirect benefits of PCV from a region with a routine PCV immunization program. The peculiar age-distribution in Galicia offers a good case study on the vaccine impact in a different population. Also, this information is crucial for the immunization program managers and policymakers for monitoring the immunization program and guiding the vaccination policies for PCV in Spain.
Methods
Study design
This is an ecological study designed to evaluate the incidence rates of all-cause pneumonia, pneumococcal pneumonia, and pneumococcal invasive diseases hospitalizations in all age-groups in the region of Galicia, comparing three vaccination periods: pre-vaccination (1998–2003), early vaccination period (2005–2009) and routine vaccination period (2011–2015).
Study setting
Galicia is one of the autonomous regions found in the northwest of the Iberian Peninsula covering 29,574.80 km2 land area (Figure 1). The region has 4 major provinces, A Coruña, Lugo, Orense, and Pontevedra, with the regional capital, Santiago de Compostela. The region has a total population of 2,732,347 in 2015, which represents 5.9% of the whole population in Spain. The population has a crude birth rate of 7.02 (per 1000 population, 8.41 average in whole Spain) and a life expectancy of 83.3 years (83.1 years average in whole Spain).19,20 The healthcare system (Servizio Galego de Saude, SERGAS) is composed of 7 tertiary referral hospitals and 7 other secondary healthcare units which serve the entire region (Figure 1).19-21
Figure 1.
The region of Galicia and the disease reporting units.
Galicia has a predominantly aging population. From the household census conducted in 2011 in the region, elderly (> 64 years) comprised 19% of the population, with more than 47% of the population aged 45 years and older. Children < 5 years old only comprise 3.8% of the total population.19
Conjunto Minimo Basico Datos (CMBD) is the national disease registry of Spain which is maintained by the Spanish Health Ministry. All health facilities in the country submit hospital admissions and outpatient clinic visits to this registry. This system uses clinical codes from the Clinical Modification-9th International Classification of Diseases ICD-9-CM (Spanish version: Modificación Clínica Clasificación Internacional de Enfermedades; CIE-9-MC). Detailed characteristics of the database were described in separate publications.22,23
Although not included in the routine immunization program, PCV7 was available in Galicia in the private market in 2004, after marketing authorization in June 2001 in the country.11,12 The vaccine was given using a three-dose primary series at 2-4-6 months and a booster dose at 12 months following the recommendations from the Spanish Pediatric Association.24 In 2011, PCV13 was included in the routine infant immunization program of Galicia,12,25,26 and is one of the very first regions in Spain to do so. The decision was made based on the epidemiologic profile of invasive disease with vaccine serotypes covering 90% of children < 2 years old and to alleviate the socioeconomic inequity hindering the vaccine access of more than 40% of infants in the region.12 The vaccine was given at 2 months and 4 months, plus a booster dose at 12 months of age (2p+1) with a catch-up immunization of children aged </ = 2 years of age. High-risk infants received more doses at 2-4-6 months of age and a booster dose at 12 months (3p+1).
Case identification
We identified hospitalized cases of all-cause pneumonia, pneumococcal pneumonia, and pneumococcal invasive diseases from the CMBD using specific ICD-9 codes shown in Box 1. We collected cases submitted to the disease registry from January 1, 1998, to December 31, 2015, by hospitals within Galicia (SERGAS). Furthermore, we collected the age and disaggregated into <5 years (young children), 5–14 years (older children), 15–24 years (young adult), 24–44 years (middle adult), 45–64 years (older adult), and >64 years (elderly).
Box 1.
ICD 9 codes of cases used in identifying cases.
| All-cause pneumonia |
| 480 Viral pneumonia |
| 481 Pneumococcal pneumonia (Streptococcus pneumoniae, pneumonia) |
| 482 Other bacterial pneumonia |
| 483 Pneumonia due to other specified organism |
| 484 Pneumonia in infectious diseases classified elsewhere |
| 485 Bronchopneumonia, organism unspecified |
| 486 Pneumonia, organism unspecified |
| 487 Influenza |
| 487.0 Influenza with pneumonia, any form |
| Pneumococcal pneumoniae |
| 481 Pneumococcal pneumonia [Streptococcus pneumoniae pneumonia] |
| Invasive Pneumococcal disease |
| 320.1 Pneumococcal meningitis |
| 038.2 Pneumococcal septicemia [Streptococcus pneumoniae septicemia] |
| 481 Pneumococcal pneumonia [Streptococcus pneumoniae pneumonia] |
Statistical analysis
Annual hospitalization rates were calculated using the official surveillance system for hospitalization (CMBD) and compared among three study periods: pre-PCV vaccination (1998–2003), early vaccination (using PCV7 released in private market in 2005–2009) and routine vaccination (using PCV13 in 2011–2015). We omitted the years 2004 and 2010 as a transition period for PCV7 and PCV13 introduction, respectively. We used one-way ANOVA to compare between study periods. We performed all the analysis using R Software, Version 3.0.2 (http://www.r-project.org). A p-value of < 0.05 is considered significant.
Furthermore, we also collected the vaccination coverage of the target population from the PCV introduction from 2004 to 2015 from the Epidemiologic Reports in Galicia (Boletin Epidemiológico de Galicia) published by the General Directorate of Innovation and Public Health Management of the Galician Health Department.27-29
Ethical considerations
The information contained in CMBD is aggregated and includes no data that could identify patients, doctors or centres, ensuring full confidentiality of the database. CMBD meets the requirements of Law 15/1999 (adopted on December 13th) on Biomedical Research regarding the protection of personal data and privacy. The Galician Research Ethics Committee granted permission for the study (2016/142).
Results
Vaccination coverage of PCV7 (2004-2009) and PCV13 (2010-2015)
From 2004 to 2010, PCV7 was available in the private market of Galicia. The Galician Health system released an estimate on the vaccine coverage of PCV7 in the private market using sales figures in the pharmacies within the region.27 This is a rough estimate of the PCV7 coverage in children < 2 years of age. From 2004 to 2010, there was a moderate vaccine coverage (> 50% of the birth cohort) which slowly increased up to 67% in 2010. Starting 2011, and upon the introduction of PCV13 in the market, high vaccination coverage was reached, starting at 87%, up to 100% in 2015.27-29
All-cause pneumonia hospitalizations
In over 18 years of the observation period (1998–2015), a total of 114,873 cases were hospitalized for all-cause pneumonia in the Galician region. Among these, 24,808 were further diagnosed as pneumococcal pneumonia. The majority of the cases were documented in the elderly population (>64 years) with 77,299 cases (67.3%), followed by 16,212 cases (14.1%) in adults 45–64 years old. Infants and younger children (<5 years) comprised 8.2% of total cases (n = 9,418). The average number of cases hospitalized due to all-cause pneumonia increased from 5,639 cases/year (pre-PCV) and 5,937 cases/year (early vaccination), to 7,834 cases/year (routine vaccination) (+32.0%, p < .0001) (Table 1) after routine introduction of the vaccine.
Table 1.
Average number of hospitalization across different vaccination period (pre-PCV, early vaccination using PCV7, and routine vaccination using PCV13), disaggregated into different age groups.
| Period | Pre-PCV 1998-2003 |
Early 2005-2009 |
Routine 2011-2015 |
% change* | p value** |
|---|---|---|---|---|---|
| All-cause pneumonia | |||||
| POPULATION | 5639 | 5937 | 7834 | +32.0% | *** |
| < 5 | 596 | 596 | 373 | −37.4% | *** |
| 5-14 | 193 | 206 | 152 | −26.2% | 0.061 |
| 15-24 | 110 | 81 | 53 | −34.6% | 0.061 |
| 24-44 | 398 | 422 | 368 | −12.8% | 0.095 |
| 45-64 | 817 | 875 | 1048 | +19.8% | *** |
| > 64 | 3525 | 3759 | 5840 | +55.4% | *** |
| Pneumococcal pneumonia | |||||
| POPULATION | 1734 | 1594 | 671 | −57.9% | *** |
| < 5 | 222 | 130 | 23 | −82.3% | *** |
| 5-14 | 63 | 48 | 12 | −75.0% | *** |
| 15-24 | 27 | 22 | 6 | −72.7% | *** |
| 24-44 | 112 | 114 | 43 | −43.0% | *** |
| 45-64 | 220 | 228 | 112 | −50.9% | *** |
| > 64 | 1090 | 1051 | 476 | −54.7 | *** |
| Pneumococcal invasive diseases | |||||
| POPULATION | 1778 | 1666 | 741 | −55.5% | **** |
| < 5 | 227 | 137 | 25 | −81.8% | *** |
| 5-14 | 65 | 49 | 13 | −73.5% | *** |
| 15-24 | 28 | 24 | 6 | −75.0% | *** |
| 24-44 | 118 | 124 | 51 | −73.0% | *** |
| 45-64 | 230 | 245 | 130 | −46.9% | *** |
| > 64 | 1110 | 1086 | 515 | −52.5% | *** |
*% change comparing private market use and routine immunization.
**p-value according to one-way ANOVA
***p < 0.0001
Using population-based estimates, there is an increasing trend of hospitalized all-cause pneumonia in the region (Figure 2(a)). Hospitalizations increased from 20.6/10,000 (pre-PCV) and 21.4/10,000 (early vaccination) to 28.4/10,000 population (routine vaccination) with a 32.7% increase (p < .0001) (Table 2). Children < 5 years of age had the largest reduction of incidence (−40.2%, p < .0001), while adults 45–64 and > 64 years old had an increase in the average number of cases (+13.9%, p < .0001; +44.7%, p < .0001).
Figure 2.
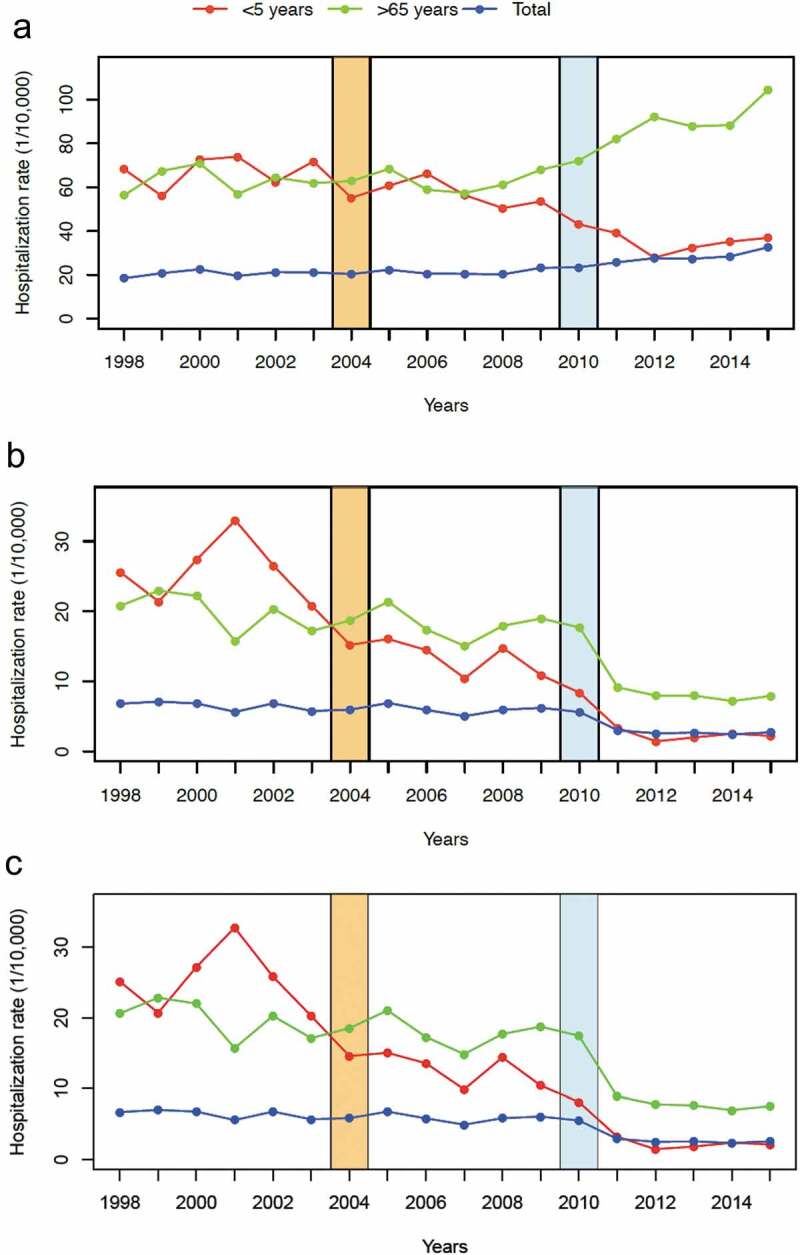
All-cause pneumonia (a), pneumococcal pneumonia (b) and pneumococcal invasive diseases (c) hospitalizations in all age groups according to vaccination periods: pre-vaccination (1998–2003), early vaccination period, PCV7 (2005–2009) and routine vaccination period, PCV13 (2011–2013).
Table 2.
Average hospitalization rates (case/10,000) across different vaccination period (pre-PCV, early vaccination using PCV7, and routine vaccination using PCV13), disaggregated into different age groups.
| Period | Pre-PCV 1998-2003 |
Early 2005-2009 |
Routine 2011-2015 |
% change* | p value** |
|---|---|---|---|---|---|
| All cause pneumonia | |||||
| POPULATION | 20.6 | 21.4 | 28.4 | +32.7% | *** |
| < 5 year | 67.5 | 57.4 | 34.3 | −40.2% | *** |
| 5-14 | 8.0 | 9.8 | 7.0 | −28.6% | 0.031 |
| 15-24 | 2.8 | 2.7 | 2.2 | −18.5% | 0.103 |
| 24-44 | 5.0 | 4.9 | 4.6 | −6.1% | 0.246 |
| 45-64 | 12.6 | 12.2 | 13.9 | +13.9% | 0.018 |
| > 64 | 63.0 | 62.8 | 90.9 | +44.7% | *** |
| Pneumococcal pneumonia | |||||
| POPULATION | 6.3 | 5.7 | 2.4 | −57.9% | *** |
| < 5 | 25.2 | 12.6 | 2.1 | −83.3% | *** |
| 5-14 | 2.6 | 2.3 | 0.5 | −78.3% | *** |
| 15-24 | 0.7 | 0.8 | 0.2 | −75.0% | *** |
| 24-44 | 1.4 | 1.3 | 0.5 | −61.5% | *** |
| 45-64 | 3.4 | 3.2 | 1.5 | −53.1% | *** |
| > 64 | 19.5 | 17.6 | 7.4 | −58.0% | *** |
| Invasive diseases | |||||
| POPULATION | 6.5 | 6.0 | 2.7 | −55.0% | *** |
| < 5 | 25.8 | 13.3 | 2.3 | −82.7% | *** |
| 5-14 | 2.7 | 2.3 | 0.6 | −73.9% | *** |
| 15-24 | 0.7 | 0.8 | 0.3 | −62.5% | *** |
| 24-44 | 1.5 | 1.5 | 0.6 | −60.0% | *** |
| 45-64 | 3.5 | 3.4 | 1.7 | −50.0% | *** |
| > 64 | 19.9 | 18.1 | 8.0 | −55.8% | *** |
*% change comparing private market use and routine immunization.
**p-value according to one-way ANOVA
***p < 0.0001
Pneumococcal pneumonia hospitalizations
A total of 24,808 pneumococcal pneumonia were hospitalized from 1998 to 2015. As previously noted, most of the cases belonged to elderly aged > 64 years (65.7%). There is a net decrease in the average number of cases from 1,734 cases/year (pre-PCV) and 1,594 cases/year (early vaccination), to 671 cases/year (routine vaccination) after routine vaccine introduction (−57.9%, p < .0001) (Table 1). Comparing population-based estimate across the years, a reduction of the incidence of hospitalized pneumococcal pneumonia was observed from 6.3/10,000 (pre-PCV) and 5.7/10,000 (early vaccination) to 2.4/10,000 population (routine vaccination) (−57.9%, p < .0001). A general declining trend was also observed (Figure 2(b)). Children < 5 years has a maximum reduction of incidence (−83.3%, p < .0001). Adults 45-64 years and elderly > 64 years had a reduction at −53.1% and −58.0%, respectively (Table 2).
Pneumococcal invasive diseases
In total, 25,095 pneumococcal invasive diseases were hospitalized in the observation period (Table 1). The largest burden of cases is on the elderly group (> 64 years old) with 16,858 cases or 65.1%, and in older adults (45–64 years old) with 3,687 cases or 14.2% of the total. Children < 5 years with 2,314 cases (9.3%). There is a general declining trend of cases across the years (Figure 2(c)). Using population-based estimates of hospitalization, there is 55.0% reduction from 6.0 case/10,000 population (early vaccination) compared to 2.7 case/10,000 population (routine vaccination) (p < .0001) (Table 2). The largest reduction of incidence was observed in children < 5 years old (−82.7%, p < .0001). Elderly age-group similarly has a significant reduction of hospitalization (−55.8%, p < .0001)
Discussion
The high and sustained coverage with PCV confers direct and indirect benefits among individuals and the community, respectively. Our results showed a significant decline in pneumococcal pneumonia hospitalizations for both the target population (infants and children) and the older age group (>65 years old). This offers supportive evidence on the substantial direct and indirect benefits (herd protection) of the vaccine using a population-based hospital surveillance data from a region of Spain with a mature PCV vaccination program.
Since the introduction of PCV13 into the routine childhood vaccination schedule in 2010 in Galicia, pneumococcal pneumonia declined globally by 57.9%. More notably, children <5 years (target population) has a reduction of hospitalized pneumococcal pneumonia by 82.3% after routine vaccination of PCV13. The continuous decline in childhood pneumonia has also been observed in multiple countries where PCVs have been introduced.30-37 However, the assessment of the full benefit from PCV has always been underestimated, partly because much of its protective effects were seen through herd protection of unvaccinated adults.30-33,38,39 In our study, a decline in pneumococcal pneumonia was similarly seen in non-vaccine targets (older children up to the elderly) (See supplementary figures). The largest decline in the number of pneumococcal pneumonia was seen in the elderly from an average of 1,051 cases/year to 476 cases/year after routine vaccination, which amounts to an average decline of 575 cases/year. Case-control and prospective cohort studies can only measure the direct benefits but cannot assess the indirect (herd) effects of the vaccine. In contrast, our analysis provides an assessment of both direct and indirect effects of the vaccination program. We report a significant portion of the hospital admissions for all-cause pneumonia and pneumococcal pneumonia averted by PCV13 were in the elderly, suggesting herd protection in the community conferred by the PCV program in children.
In our study, we observed a paradoxical net increase in the total burden of all-cause pneumonia in the population. Although all-cause pneumonia declined in the younger segments of the population, this was grossly obliterated by the huge number of cases of pneumonia in older people. There are numerous reasons for this occurrence. First, the primary cause of pneumonia in the population may have shifted to etiologies not covered by the current program. There are numerous etiologies that may cause pneumonia in the elderly, which includes viral agents (i.e., influenza and metapneumovirus), atypical bacteria (i.e., Legionella and mycoplasma), and anaerobes.40,41 And though PCVs were reported to have off-target effects, we did not see this to impact the total burden of all-cause pneumonia in the elderly in Galicia. Second, Galicia has an aging population. The most recent census and the latest projections estimate that the region is hugely dominated by people aged 64 years and older.19 The aging population could change the infection epidemiology in the community, different from what was usually seen in the literature. In addition, the elderly group tends to have numerous comorbidities, which places them at higher risk for hospitalization.42 Finally, the PCV routine immunization program had targeted only a small subset of the population. Herd effect is a function of the number of vaccinated individuals and their proportion in the population.1,5,43-45 Infants and children only comprise a small percentage of the community, and may not have been sufficient to cut the transmission within the region. Although pneumococcal pneumonia was observed to decline, it is premature to conclude that the remaining burden is that of vaccine-serotype pneumococcus.
Most of the studies in Spain on the effect of PCV have focused on areas with limited implementation of the vaccine,46 or used case-control design for the assessment.47 In Madrid, a similar region that introduced routine PCV13 vaccination, an impact study documented a 63% reduction of invasive pneumococcal diseases specific to PCV13-serotypes.45 Our group supports their findings and further extends the vaccine impact on the hospitalization burden from pneumonia of other causes. Furthermore, our results also reinforce the previous assessment on the herd effects of the vaccine in Spain.48-50 Although previous assessments in the country have documented an increasing detection of non-vaccine serotypes causing disease after use of vaccine, its overall burden is yet to be seen from surveillance data in a region after prolonged vaccine-use.51-53
Our study has its limitations. First, this is an ecological study that evaluated the impact of the Galician PCV program in Spain, and thus, are subject to similar biases using a similar study design. Possible sources of bias include changes in admission criteria, changes in management and diagnostic criteria, and population migration that may have occurred across the period of observation. Second, etiology was not determined in most cases. This is another inherent weakness in using a registry as a source of information. Whether the decline in all-cause pneumonia or invasive disease is a secondary (off-target) effects of the vaccine and other preventive health programs, or a change in the epidemiology of another etiologic agent are all plausible reasons for the decline in hospitalized cases. Finally, no serotype information is available to quantify the vaccine effectiveness against individual serotypes. Our analysis cannot separate vaccine-type from non-vaccine-type disease because diagnoses are not serotype-specific. Monitoring the circulating serotype post-PCV introduction is crucial as an increase in non-vaccine serotypes were seen in some countries after routine vaccination.32,54,55 Whether this serotype replacement can also be seen in Spain is beyond the coverage of our study. Continued monitoring of circulating pneumococcal serotypes, especially in mature PCV vaccination programs like in Galicia, is important in this regard.
Mature PCV vaccination program in infants and children can result in the protection of the elderly population, a group that does not respond as well to vaccination because of immune senescence or underlying diseases. Although PCV13-vaccinated children (<5 years) accounted for only ~4% of the Galician population, our study suggests that this was sufficient to reduce the burden of pneumococcal pneumonia substantially across all age groups. These findings are important now that PCV13 vaccination is being used in adults as shown in clinical trials and few impact studies.56-58 However, there is still debate whether indirect protection through infant vaccination is enough for the whole population protection.59 With the aging population in Galicia (and in Spain), and the significant remaining burden of pneumococcal pneumonia despite the shown herd benefits, immunization of elderly is an equally valid strategy alongside other preventive measures for the holistic control of respiratory infections in the population.60 July 2017, Galicia has introduced PCV13 in the elderly population (>/ = 65 years old).25 The impact of this strategy in the region is yet unknown, but it is necessary to evaluate the added value of adult vaccination with PCV13 to determine the most efficient vaccination schedule.
Conclusion
The vaccines have shown direct and indirect benefits against pneumococcal disease in Northern Spain, considering that the vaccine was given only to children, and a big fraction of the community are non-vaccine targets (adults and elderly). Despite the global impact of infant pneumococcal conjugate vaccination, the remaining burden of pneumococcal disease in elderly is huge, and adult immunization of PCV seems a promising strategy in an aging population. However, pneumococcal diseases have a dynamic epidemiology, and in other countries, an increase in non-vaccine serotype diseases has been documented. We have also shown that the Spanish hospital-based national registry (Conjunto Minimo Basico Datos, CMBD) could be used to closely monitor these changes and ensure the continued benefit of the population from the vaccine. The data coming from a mature PCV program as that of Galicia with a background of an aging population, could provide a sentinel in predicting the pneumococcal epidemiology in Spain.
Supplementary Material
Acknowledgments
We would like to thank the assistance of Raquel Vaquero Rodrigo from the Admission and Clinical Documentation section at Hospital Clínico Universitario de Santiago de Compostela for the data acquisition process and the specific requests regarding the CMBD registry on pneumococcal diseases. We also thank Alberto Gomez Carballa of the Departamento de Anatomía Patológica y Ciencias Forenses - Universidad de Santiago de Compostela for drafting the images used in this paper.
Funding Statement
This study received support from the Instituto de Salud Carlos III (Intensificación Actividad Investigadora and Proyecto de Investigación en Salud, Acción Estratégica en Salud): PI07/0069, PI10/00540, PI16/01478 and PI16/01569) and 2016-PG071 Consolidación e Estructuración REDES 2016GI-1344 G3VIP (Grupo Gallego de Genética Vacunas Infecciones y Pediatría, ED341D R2016/021) This study has been partially sponsored by an unrestricted grant from Pfizer to the Healthcare Research Institute of Santiago de Compostela. The sponsor had no role in the design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and was ultimately responsible for the decision to submit this work for publication; Instituto de Salud Carlos III [PI07/0069, PI10/00540, PI16/01478 and PI16/01569].
Disclosure of potential conflicts of interest
FMT has received research grants and/or honoraria as a consultant/advisor and/or speaker and for conducting vaccine trials from GlaxoSmithKline, Sanofi Pasteur MSD, Merck, Sanofi Pasteur, Pfizer, Novartis, and MedImmune Inc.
IRC has received research grants and honoraria as an advisor and speaker, and for attending conferences and practical courses from GlaxoSmithKline, Sanofi Pasteur MSD, Merck, Sanofi Pasteur, Novartis, and Pfizer.
None declared for other authors.
Supplementary Material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2019.1690884.
References
- 1.Saadatian-Elahi M, Horstick O, Breiman RF, Gessner BD, Gubler DJ, Louis J, Parashar UD, Tapia R, Picot V, Zinsou J-A, et al. Beyond efficacy: the full public health impact of vaccines. Vaccine. 2016;34(9):1139–47. doi: 10.1016/j.vaccine.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudan I. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408–16. doi: 10.2471/BLT.00.000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 4.Lucero MG, Dulalia VE, Nillos LT, Williams G, Parreno RA, Nohynek H, Riley, ID and Makela, H. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database of Syst Rev. 2009;(4):Cd004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramani RRH, William N, Boulton M, Johnson DR, Zhu B-P. Impact of PCV7 on invasive pneumococcal disease among children younger than 5 years: a population-based study. Am J Pub Health. 2004;94(6):958–59. doi: 10.2105/AJPH.94.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO position on pneumococcal vaccines: the polysaccharide vaccine. Wkly Epid Rev 2003;14:110–20. [Google Scholar]
- 7.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis 2007;196(9):1346–54. doi: 10.1086/524219. [DOI] [PubMed] [Google Scholar]
- 8.Lipsitch M. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis 1999;5(3):336–45. doi: 10.3201/eid0503.990304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller E, Andrews NJ, Waight PA, Slack MPE, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011;11(10):760–68. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 10.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011;378(9807):1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grupo de Trabajo de la Ponencia de Registro y Program de Vacunas . Enfermedad invasora por Streptococcus pneumoniae: implicación de la vacunación con la vacuna conjugada heptavalente. Abril 2006. Madrid (España): Ministerio de Sanidad y Consumo; 2006. [Google Scholar]
- 12.de Xaude C. Dirrección Xeral de Innovación e Xestión da Saúde Pública. Vacunación frente al neumococo (estudio piloto). Santiago de Compostela (España): Conselleria de Xaude; 2010. [Google Scholar]
- 13.Grupo de Trabajo de Neumococo . Nuevas vacunas antineumococicas conjugadas. Madrid (Espana): Ministerio de Sanidad y Politica Social; 2009. [Google Scholar]
- 14.Grupo de Trabajo Vacunación frente a neumococo . Vacunación frente a neumococo en grupos de riesgo, Mayo de 2015. Madrid (España): Ministerio de Sanidad, Servicios Sociales e Igualidad; 2015. [Google Scholar]
- 15.Goldblatt D. Conjugate vaccines. Clin Exp Immunol. 2000;119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention . Direct and indirect effects of routine vaccination of children with7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease — United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;16(54):893–97. [PubMed] [Google Scholar]
- 17.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–55. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger DM, Grant LR, Weatherholtz RC, Warren JL, O’Brien KL, Hammitt LL. Relating pneumococcal carriage among children to disease rates among adults before and after the introduction of conjugate vaccines. Am J Epidemiol. 2016;183(11):1055–62. doi: 10.1093/aje/kwv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Instituto Galego de Estatistica . Indicadores demograficos anos 2016 e 2017. Santiago de Compostela (España): Xunta de Galicia; 2018. http://www.ige.eu/web/avisolegal.jsp?idioma=gl. [Google Scholar]
- 20.Instituto Nacional de Estadistica . Indicadores demográficos - Definitivos año 2017 Madrid (España): Instituto Nacional de Estadistica; 2018. https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177003&menu=ultiDatos&idp=1254735573002. [Google Scholar]
- 21.Conselleria de Sanidad . Estrategia SERGAS 2020. Santiago de Compostela (Espana): Xunta de Galicia; 2014. [Google Scholar]
- 22.Gil-Prieto R, Garcia-Garcia L, Alvaro-Meca A, Mendez C, Garcia A, de Miguel AG. The burden of hospitalisations for community-acquired pneumonia (CAP) and pneumococcal pneumonia in adults in Spain (2003-2007). Vaccine. 2011;29(3):412–16. doi: 10.1016/j.vaccine.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Ministerio de Sanidad Consumo y Bienestar Social . Conjunto Minimo Basico Datos. Portal Estadistico-Area de Inteligencia de Gestion. Madrid (Espana): Ministerio de Sanidad, Consumo y Bienestar Social (MSCBS); 2010. [Google Scholar]
- 24.Comité Asesor de Vacunas . Actualizacion en vacunacion antineumococica 2010: recomendaciones del Comite Asesor de Vacunas. Madrid (España): Asociación Española de Pediatría; 2010. [Google Scholar]
- 25.Conselleria de Xaude - Dirección Salud Publica . Vacinación antipneumocócica en adultos, Xullo 2017. Santiago de Compostela (España): Xunta de Galicia; 2017. [Google Scholar]
- 26.Conselleria de Xaude . Dirección Xeral de Innovación e Xestión de Saúde Pública. Uso vacina antipneumocócica conxugada grupos risco, Xullo 2014. Santiago de Compostela (España): Xunta de Galicia; 2014. [Google Scholar]
- 27.Boletín Epidemiolóxico de Galicia - Xuño 2012 . A Vacinación Infantil Coa Vc-13 En Galicia: O Estudo Piloto. Santiago de Compostela (Spain): Dirección xeral de innovación e xestión da saúde pública – DXIXSP; 2012. [Google Scholar]
- 28.Boletín Epidemiolóxico de Galicia - Xulio 2013 . A Vacinación Infantil Coa Vc-13 En Galicia: Duos Años de Estudo Piloto. Santiago de Compostela (Spain): Dirección xeral de innovación e xestión da saúde pública – DXIXSP; 2013. [Google Scholar]
- 29.Boletín Epidemiolóxico de Galicia - Xulio 2015 . A Vacinación Infantil Coa Vc-13 En Galicia: Os Catro Anos De Estudo Piloto. Santiago de Compostela (Spain): Dirección xeral de innovación e xestión da saúde pública – DXIXSP; 2015. [Google Scholar]
- 30.Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010;29(7):607–12. doi: 10.1097/INF.0b013e3181d7d09c. [DOI] [PubMed] [Google Scholar]
- 31.Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997-2008. Thorax. 2010;65(9):770–74. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- 32.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger DM, Givon-Lavi N, Shemer-Avni Y, Bar-Ziv J, Alonso WJ, Greenberg D, Dagan R. Influence of pneumococcal vaccines and respiratory syncytial virus on alveolar pneumonia, Israel. Emerg Infect Dis. 2013;19(7):1084–91. doi: 10.3201/eid1907.121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, Minodier P, Dommergues MA, Hees L, Gillet Y, et al. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis. 2014;58(7):918–24. doi: 10.1093/cid/ciu006. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg D, Givon-Lavi N, Ben-Shimol S, Ziv JB, Dagan R. Impact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 years. Vaccine. 2015;33(36):4623–29. doi: 10.1016/j.vaccine.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Shimol S, Dagan R, Givon-Lavi N, Avital D, Bar-Ziv J, Greenberg D. Use of chest radiography examination as a probe for PCV impact on lower respiratory tract infections in young children. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz768. [DOI] [PubMed] [Google Scholar]
- 37.Ouldali N, Levy C, Minodier P, Morin L, Biscardi S, Aurel M, Dubos F, Dommergues MA, Mezgueldi E, Levieux K, et al. Long-term association of 13-valent pneumococcal conjugate vaccine implementation with rates of community-acquired pneumonia in children. JAMA Pediatr. 2019;173(4):362–70. doi: 10.1001/jamapediatrics.2018.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig A, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648599. [DOI] [PubMed] [Google Scholar]
- 39.Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP, Low DE. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2(1):e00309–10. doi: 10.1128/mBio.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrie TJ. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31:1066–78. doi: 10.1086/cid.2000.31.issue-4. [DOI] [PubMed] [Google Scholar]
- 41.Stupka JE, Mortensen EM, Anzueto A, Restrepo MI. Community-acquired pneumonia in elderly patients. Aging Health. 2009;5(6):763–74. doi: 10.2217/ahe.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivero-Calle I, Pardo-Seco J, Aldaz P, Vargas DA, Mascaros E, Redondo E, Díaz-Maroto JL, Linares-Rufo M, Fierro-Alacio MJ, Gil A, et al. Incidence and risk factor prevalence of community-acquired pneumonia in adults in primary care in Spain (NEUMO-ES-RISK project). BMC Infect Dis. 2016;16(1):645. doi: 10.1186/s12879-016-1974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. 2013;32(1):133–45. doi: 10.1016/j.vaccine.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Pilishvili T, editor. Impact of PCV13 on Invasive Pneumococcal Disease (IPD) Burden and the Serotype Distribution in the U.S. Advisory Committee on Immunization Practices October 2018 Meeting; 2018; Atlanta, GA: US Centers for Disease Control and Prevention. [Google Scholar]
- 45.Ruiz-Contreras J, Picazo J, Casado-Flores J, Baquero-Artigao F, Hernandez-Sampelayo T, Otheo E, Méndez C, Del Amo M, Balseiro C. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in children. Vaccine. 2017;35(35):4646–51. doi: 10.1016/j.vaccine.2017.06.070. [DOI] [PubMed] [Google Scholar]
- 46.Moraga-Llop F, Garcia-Garcia JJ, Diaz-Conradi A, Ciruela P, Martinez-Osorio J, Gonzalez-Peris S, Hernández S, de Sevilla MF, Uriona S, Izquierdo C, et al. Vaccine failures in patients properly vaccinated with 13-valent pneumococcal conjugate vaccine in Catalonia, a region with low vaccination coverage. Pediatr Infect Dis J. 2016;35(4):460–63. doi: 10.1097/INF.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 47.Latasa P, Ordobas M, Garrido-Estepa M, Gil de Miguel A, Sanz JC, Barranco MD, Insúa E, García-Comas L. Effectiveness of different vaccine schedules for heptavalent and 13-valent conjugate vaccines against pneumococcal disease in the Community of Madrid. Vaccine. 2017;35(40):5381–87. doi: 10.1016/j.vaccine.2017.07.089. [DOI] [PubMed] [Google Scholar]
- 48.Ardanuy C, de la Campa AG, Garcia E, Fenoll A, Calatayud L, Cercenado E, Pérez-Trallero E, Bouza E, Liñares J. Spread of Streptococcus pneumoniae serotype 8-ST63 multidrug-resistant recombinant Clone, Spain. Emerg Infect Dis. 2014;20(11):1848–56. doi: 10.3201/eid2011.131215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calbo E, Diaz A, Canadell E, Fabrega J, Uriz S, Xercavins M, Morera MA, Cuchi E, Rodríguez-Carballeira M, Garau J, et al. Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12(9):867–72. doi: 10.1111/j.1469-0691.2006.1502_1.x. [DOI] [PubMed] [Google Scholar]
- 50.Camara J, Marimon JM, Cercenado E, Larrosa N, Quesada MD, Fontanals D, Cubero M, Pérez-Trallero E, Fenoll A, Liñares J, et al. Decrease of invasive pneumococcal disease (IPD) in adults after introduction of pneumococcal 13-valent conjugate vaccine in Spain. PLoS One. 2017;12(4):e0175224. doi: 10.1371/journal.pone.0175224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ercibengoa M, Arostegi N, Marimón JM, Alonso M, Pérez-Trallero E. Dynamics of pneumococcal nasopharyngeal carriage in healthy children attending a day care center in Northern Spain. Influence of detection techniques on the results. BMC Infect Dis. 2012;12:69. doi: 10.1186/1471-2334-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46(2):174–82. doi: 10.1086/524660. [DOI] [PubMed] [Google Scholar]
- 53.Rolo D, Fenoll A, Ardanuy C, Calatayud L, Cubero M, de la Campa AG, Linares J. Trends of invasive serotype 6C pneumococci in Spain: emergence of a new lineage. J Antimicrob Chemother. 2011;66(8):1712–18. doi: 10.1093/jac/dkr193. [DOI] [PubMed] [Google Scholar]
- 54.Brandileone MC, Almeida SCG, Minamisava R, Andrade AL. Distribution of invasive Streptococcus pneumoniae serotypes before and 5years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. 2018;36(19):2559–66. doi: 10.1016/j.vaccine.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi: 10.1016/S1473-3099(18)30052-5. [DOI] [PubMed] [Google Scholar]
- 56.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention . Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012;61(21):394–95. [PubMed] [Google Scholar]
- 58.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–25. [PMC free article] [PubMed] [Google Scholar]
- 59.Wiese AD, Griffin MR, Grijalva CG. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert Rev Vaccines. 2019;18(4):327–41. doi: 10.1080/14760584.2019.1582337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teresa Aguado M, Barratt J, Beard JR, Blomberg BB, Chen WH, Hickling J, Hyde TB, Jit M, Jones R, Poland GA, et al. Report on WHO meeting on immunization in older adults: Geneva, Switzerland, 22-23 March 2017. Vaccine. 2018;36(7):921–31. doi: 10.1016/j.vaccine.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


