Abstract
Objective:
Previous research suggests recovery from cannabis-related deficits in verbal learning and memory functioning after periods of cannabis abstinence in adolescents. Here we examine how cannabis cessation affects cognitive performance over two weeks of monitored abstinence compared to controls in adolescents and young adults.
Method:
Seventy-four participants (35 cannabis users) aged 16-26 ceased all cannabis, alcohol and other illicit substance consumption for a two-week period; abstinence was monitored via weekly urinalysis, breath, and sweat patch testing. Starting at baseline, participants completed weekly abbreviated neuropsychological batteries. Measures included tests of attention, inhibition, verbal working memory and learning. Repeated measures assessed within and between subject effects for time and group status, while controlling for past year alcohol and nicotine use.
Results:
Cannabis users showed increased performance compared to controls on sustained attention tasks after two weeks of cannabis use.
Conclusions:
Deficits in attention, but not verbal learning and memory, recovered after two-week of monitored abstinence. This differs from previous literature, suggesting that other cognitive domains may show signs of recovery after periods of cannabis cessation in adolescents and young adults.
Keywords: Cannabis Cessation, Marijuana, Neurocognition, Adolescents, Emerging Adulthood, Abstinence
Adolescent cannabis use has remained relatively stable at high levels over the past several years, as 36% of high school seniors reported using within the past year (Johnston et al., 2019). The relative popularity of cannabis within adolescents and young adults makes it of interest to parse out the long-term effects of cannabis use, specifically in neurocognition. Adolescence and emerging adulthood is a time of great neurodevelopmental growth, including in the endocannabinoid system (Giedd et al., 1999; Jernigan, Trauner, Hesselink, & Tallal, 1991). Therefore, adolescents may be more susceptible to the effects of repeated and early cannabis use (Ehrenreich et al., 1999; Lisdahl, Wright, Medina-Kirchner, Maple, & Shollenbarger, 2014).
Regular cannabis use during adolescence and early adulthood has been previously associated with neurocognitive deficits (Lisdahl, Gilbart, Wright, & Shollenbarger, 2013; Lisdahl et al., 2014). Specifically, cannabis use during this period have been associated with deficits in processing speed (Gruber, Dahlgren, Sagar, Gönenc, & Killgore, 2012; Lisdahl & Price, 2012; Medina, Hanson, et al., 2007), verbal working memory (Medina, Hanson, et al., 2007; Solowij et al., 2011; Tait, Mackinnon, & Christensen, 2011; Thoma et al., 2011), and executive functioning (Gonzalez et al., 2012; Gruber, Dahlgren, et al., 2012; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Lisdahl & Price, 2012; Mathias et al., 2011; Medina, Hanson, et al., 2007; Wade, Wallace, Swartz, & Lisdahl, 2019). Animal models have revealed that chronic cannabis use leads to downregulation of endocannabinoid receptors located within the central nervous system (De Fonseca, Gorriti, Fernandez-Ruiz, Palomo, & Ramos, 1994). This downregulation of receptors may play a role in the observable neurocognitive deficits highlighted above. As adolescence marks a time of great neurodevelopmental growth, teens may be more vulnerable to the effects of cannabis in these receptors as the brain is still undergoing maturation (Casey, Getz, & Galvan, 2008). This point has been emphasized by work investigating early age of onset of cannabis use, which suggests that earlier use is associated with neurocognitive deficits (Fontes et al., 2011; Gruber, Dahlgren, et al., 2012).
While differences in neurocognition have been reported across cannabis using samples, the persistence of these deficits following cessation of use has been questioned. Some research suggests that cognitive deficits found in cannabis users may no longer be observed following even short periods of abstinence (e.g., 72 hours), suggesting that some effects may be due to residual intoxication (Schreiner & Dunn, 2012; Scott et al., 2018). This residual intoxication is attributed to the fat soluble properties of delta-9-tetrahydrocannabinol (THC), allowing for it to stay within the human body and slowly release over a period of months (Grotenhermen, 2003). Further, there is some evidence that the downregulation of endocannabinoid receptors may by reversible after monitored abstinence (Hirvonen et al., 2012). However, it is notable that meta-analyses examining the impact of length of abstinence on neurocognitive outcomes include almost exclusively cross-sectional studies that also mix a wide variety of traditional cognitive and MRI based tasks; thus, the heterogeneity of methodologies may obscure real differences. Further, longitudinal functional studies provide evidence showing differences in cerebral blood flow after cannabis use that persists up to seven days post-cannabis use compared to controls, suggesting residual effects from cannabis use may be more long lasting (Sneider et al., 2008). Cross-sectional neuroimaging studies examining cannabis users after a month of abstinence have shown structural (Jacobus, Squeglia, Sorg, Nguyen-Louie, & Tapert, 2014; McQueeny et al., 2011; Medina et al., 2009; Medina, Nagel, & Tapert, 2010) and functional differences (Padula, Schweinsburg, & Tapert, 2007; Tapert et al., 2007) suggesting neural changes remain even after cannabis cessation. This conflicting literature points to a need for more longitudinal studies examining the effects of cannabis use following periods of abstinence.
In an attempt to address these concerns, studies examining how the length of abstinence impacts cognitive performance have gained attention (Ganzer, Broning, Kraft, Sack, & Thomasius, 2016). Only three known longitudinal studies have examined the impact of monitored sustained abstinence from cannabis use in adolescents and young adults. Hanson and colleagues (2010) examined neuropsychological function between users and non-users over three weeks of abstinence, assessing verbal learning, verbal working memory, and attention on a weekly basis. Schuster and colleagues (2018) randomized cannabis users into an abstinence or continuous-using group and examined the impact of sustained abstinence over a four-week period on memory and attention outcomes measured weekly. Roten and colleagues (2015) examined cannabis cessation in treatment seeking adolescents in a placebo controlled trial comparing cognitive performance of abstinent and non-abstinent adolescents at four and eight weeks. Findings across these three studies suggest that abstinence from cannabis results in verbal learning and memory recovery after one-to-two weeks of abstinence and psychomotor speed after a month of abstinence, while deficits in sustained and selective attention and inhibition endured for at least three to four weeks (Hanson et al., 2010; Roten, Baker, & Gray, 2015; Schuster et al., 2018). It is worth noting that the effects of continuously monitored cannabis cessation within young adult cannabis users compared to controls has not yet been investigated, as the only study that included young adults (Schuster et al., 2018) compared abstinent users to still using cannabis users without a healthy control group. Taken together, findings suggest that some cognitive deficits from cannabis use may be from acute intoxication or resolve with abstinence. However, deficits in attention may represent a long-term chronic effect of cannabis use (Hanson et al., 2010; Pope & Yurgelun-Todd, 1996; Schuster et al., 2018). Notably, these studies often neglect to account for length of abstinence leading up to their enrollment in the study, despite the fact that this initial abstinence period may play an important role in the return of cognitive functioning (Ganzer et al., 2016). In order to better address how monitored sustained cannabis abstinence impacts neurocognition in both adolescents and young adults while also taking into considerations for pre-enrollment cannabis abstinence, we have proposed the following study.
The current longitudinal study aimed to investigate how continuous abstinence from cannabis use affects cognitive performance, including measures of attention, inhibition, verbal working memory and learning in adolescents and young adults. Participants were monitored over a period of two weeks, with toxicology-confirmed abstinence. We hypothesized that adolescent and young adult cannabis users would experience improved performance on cognitive tests measuring verbal working memory and learning after one week of monitored sustained abstinence, but would continue to exhibit deficits in sustained attention compared to controls across the entire two-week period of monitored abstinence.
Method
Participants
Participants included seventy-four 16-26 year olds (35 Cannabis Users and 39 controls) balanced for gender (53% male) and predominantly Caucasian (66%). Cannabis users were current users (nearly weekly; (Lisdahl & Price, 2012; Medina, Hanson, et al., 2007; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007) who smoked over 44 times in the last year, over 100 times in their lifetime, and demonstrated negative results or downward trends in THC levels in sweat patch analyses for the period of monitored abstinence. Controls smoked no more than five times in the past year and no more than 20 times in their lifetime (Wade, Wallace, et al., 2019; Wallace, Wade, Hatcher, & Lisdahl, 2019). Controls who had used cannabis in the past 30 days on the Timeline Follow-back (TLFB) or had positive toxicology screens at session one (baseline) were excluded from the study. Participants were excluded from the study if they were left handed, possessed magnetic resonance imaging (MRI) contraindications (claustrophobia, pregnancy, or mental in body), had major medical or neurological conditions (such as seizures, migraines, tumor, chemotherapy exposure, multiple sclerosis, movement disorders), past head trauma where they experienced unconsciousness for longer than two minutes, independent Axis I mood, anxiety, and attention disorders, prenatal substance use exposure or complications (gestations <35 weeks), or excessive illicit substance use (>20 lifetime uses across all major drug categories). Inclusion and exclusion criteria were selected based off previous neurocognitive and neuroimaging studies done with cannabis using adolescents and young adults (Lisdahl & Price, 2012). Four participants (three cannabis users and one control) dropped out during the first three weeks of the study. Rationale for why participants dropped out was not obtained.
Procedures
Screening.
Participants were recruited through the local community and university and were required to call in if interested in the study. Interested participants completed initial phone screening wherein they were asked basic questions to verify eligibility for the study. Participants that met initial eligibility requirements were invited to complete written consent (assent for participants under the age of 18) via mail and then underwent a more detailed screening. During detailed screening participants were given the Mini International Neuropsychiatric Interview (MINI (Sheehan et al., 1998)) and adolescents under 18 and their parents were separately given the MINI-Kid (Sheehan et al., 2010). Further, participants were given the Customary Drinking and Drug Use Record (CDDR) to assess for current and lifetime substance use history. All participants were compensated for completing the detailed screening and participants who met full study inclusion criteria were invited to complete the remainder of the study. Ineligible participants were not informed of study eligibility criteria.
Study Sessions.
Participants enrolled into the larger study were required to come in weekly for a brief battery of neuropsychological testing and questionnaires, once a week for three weeks (creating a two-week monitored abstinence period for the current study). During the last visit of the brief neuropsychological battery, participants were given the TLFB to assess for substance use over the past year. Participants were asked to remain abstinent over the course of the study from all substances for the entire study duration, except for nicotine use. Participants were asked to abstain from nicotine use one hour prior to testing, so as not to confound results with effects from nicotine withdrawal (Ashare, Falcone, & Lerman, 2014). Abstinence was monitored via weekly urinalysis (sessions one, two, and three) drug testing as well as continuous drug sweat patch testing (administered at study sessions one, two, and three); thus, abstinence was confirmed for a minimum of two weeks between study sessions one and three. One week after the last brief neuropsychological testing visit, participants went on to complete an expanded neuropsychological battery as well as volume oxygen maximum (VO2 max) testing (aerobic fitness) and MRI scan (data described in more detail in Wade, Wallace, et al. (2019)). Visits were scheduled approximately seven days from their previous visit in order to attain at least 14 days of abstinence between study sessions one and three. Examiners were not blinded to the status of participant’s drug use status, in part due to the fact that participants had to report on the date of their last substance use episodes. All data was collected in accordance and with oversight of the local Institutional Review Board.
Materials
MINI Psychiatric Interview.
The MINI is a semi-structured clinical interview and was utilized during the detailed screener to identify comorbid Axis-I diagnoses (Sheehan et al., 1998). Participants under the age of 18 completed the MINI-Kid separately from their parents, who also completed the MINI-Kid (Sheehan et al., 2010).
Substance Use
Customary Drinking and Drug Use Record. During the detailed screen, participants completed the CDDR, a valid self-report measure for assessing adolescent substance use, to collect age of first use and regular use (defined as using cannabis once a week) as well as the frequency and quantity of the participant’s lifetime usage (Brown et al., 1998).
Timeline Follow-back. The TLFB (Sobell & Sobell, 1992) was used to measure past year use (including the period of monitored abstinence). Trained research assistants utilized holidays and memorable events to the participants (e.g. birthdays, family gatherings, parties, etc.) as anchor points to help the participant remember exact substance use over the past year. Substances were measured by standard units and included alcohol (in number of standard drinks), nicotine (in number of cigarettes and hits of chew/snuff/pipe/cigar/hookah), cannabis [in number of joints (number of cannabis concentrates converted to joints (based on estimated 0.5 gram= 1 joint))] and other illicit substances including stimulants, sedatives, gamma-hydroxybutyric acid (GHB), ketamine, hallucinogens, ecstasy, methylenedioxy-methamphetamine (MDMA), opioids, prescription opiates, and inhalants. Last date of use were also collected for each substance.
Substance Use Toxicology Measurement. Continuous Sweat Patch. All participants (cannabis users and controls) wore a PharmChek drug patch throughout the course of the study. PharmChek drug patches capture sweat excreted across multiple days, which allows detection of substances that may not be found in weekly urinalysis tests. This form of testing has proven to be a valid and accurate way of detecting substances within users across various substances including THC (Gentili, Mortali, Mastrobattista, Berretta, & Zaami, 2016; Saito et al., 2004). PharmChek drug patches allowed for the quantitative detection of cocaine, benzoylecgonine, heroin, 6-monoacetylmorphine, morphine, codeine, amphetamines, methamphetamine, THC, and phencyclidine (PCP) at a minimum cut off of 0.5ng/mL for THC, 7.5 ng/mL for PCP, and 10 ng/ML for all other drugs tested. Alcohol Breathalyzer. Participants were required to undergo breathalyzer testing for detection of recent alcohol use before every study session. Urine Toxicology. Weekly urine samples were collected and drug toxicology was tested via ACCUTEST SplitCup 10 panel drug tests. Substances that were screened included (minimum cut-offs included) amphetamines (1,000 ng/mL), barbiturates (300 ng/mL), benzodiazepines (300 ng/mL), cocaine (300 ng/mL), ecstasy (500 ng/mL), methadone (300 ng/mL), methamphetamines (500 ng/mL), opiates (300 ng/mL), PCP (25 ng/mL), THC (15,000 ng/mL), and THC-COOH (50 ng/mL) as well as nicotine usage through additional testing via Nicalert cotinine urinalysis test strips.
Abstinence Decision-Making.
Since THC can be detected in the body for several weeks after cessation of cannabis use, participants were allowed to test positive for THC in their system via PharmChek as long as THC levels continued to drop between each study session (session one to two and two to three). If participants tested positive for any other substances, had a breath alcohol concentration higher than .000, had an increase in their THC levels during study participation, or self-reported the use of cannabis throughout the two-week abstinence period, participants were excluded from the present analyses.
Neuropsychological testing.
The neuropsychological tests were selected based off previous research indicating they are sensitive to the effects of cannabis use, are associated with aberrant brain structure results related to cannabis use, and have been used in prior investigations of the role of sustained cannabis abstinence in adolescent users (Hanson et al., 2010; Lisdahl et al., 2013; Lisdahl et al., 2014; Medina, Hanson, et al., 2007). The CVLT-II Forced Choice trial was used as an embedded validity measure during the fourth testing session (Wade, Wallace, et al., 2019). As no participants were below cutoff scores, participants are believed to have given appropriate effort on testing measures. Examiners were not blinded to whether participants were within the control or cannabis use group.
The Stroop task was used to measure inhibition and cognitive control (Golden & Freshwater, 1978). Participants were required to correctly identify the ink color of words while suppressing the response to read the word, which was written as an opposing word color. The number of correct responses and incorrect responses within a 60 second time period were recorded.
WAIS-III Letter-Number Sequencing (LNS) was used to measure verbal working memory (Wechsler, Coalson, & Raiford III, 1997). Participants were asked to remember a series of letters and numbers and repeat them back in alphabetical and serial order. The length of letters and numbers participants were required to remember slowly grew with subsequent trials. Total raw correct responses were measured.
The Hopkins Verbal Learning Task (HVLT) measures verbal learning and verbal working memory (Brandt & Benedict, 1991). Participants were read a list of twelve words and required to freely recall as many of the words as they can remember. After three trials of being read and asked to recall said list, participants were given a 20-25 minute delay before being asked to recall the list again. Alternative forms were used every week of testing.
The Ruff 2&7 measures selective and sustained attention (Ruff & Allen, 1996). Participants were asked to scan across a series of lines containing target and distractor stimuli. Participants were asked to mark the target stimuli (2 & 7) as quickly and accurately as possible while ignoring distractor stimuli (other numbers or letters). Participants were timed on how long it takes to complete each of the twenty given trials. Scores reported are summed T-values.
Statistical Analyses
A series of repeated measures analyses of covariance (ANCOVA) were run to examine the interaction between time (three study sessions) and group (cannabis users and controls). The number of past year alcohol and nicotine uses (as measured by the TLFB) were used as separate covariates within the repeated measures model to control for impact of comorbid substance use on recovery rates. Cognitive measures that were used for outcomes included the number of correct responses made during the Stroop task, total score from LNS, total score from immediate free recall and delayed free recall from the HVLT, and total accuracy and total response time from the Ruff 2&7. All statistical differences were made at a p-value less than .05.
Results
Demographics
Cannabis users and controls did not significantly differ by age, race, gender or years of education. See Table 1 for more demographic information.
Table 1.
Demographics and Group Descriptors
| Cannabis Users M (SD) [range] |
Controls M (SD) [range] |
|
|---|---|---|
| Age | 21.5 (2.2) [17.0-26.0] | 20.8 (2.8) [16.0-25.0] |
| Gender (Male) | 62.8% | 43.6% |
| Race (Caucasian) | 62.8% | 69.2% |
| Years of Education | 14.0 (1.6) [11.0-18.0] | 14.2 (2.3) [9.0-19.0] |
| Past Year Alcohol Use | 347.4 (298.0) [0.0-1120.5] | 100.9 (171.2) [0.0-698.5] |
| Past Year Nicotine Use | 220.5 (489.5) [0.0-1867.0] | 0.5 (2.0) [0.0-12.0] |
| Past Year Cannabis Use | 353.2 (292.9) [44.7-1394.0] | 0.2 (0.8) [0.0-4.8] |
| Lifetime Cannabis Use | 1210.4 (1380.7) [101.0-6000.0] | 1.3 (2.8) [0.0-12.5] |
| Length of Abstinence | 20.7 (10.1) [12.0-48.0] | 153.5 (118.9) [39.0-262.0] |
| Age of First Regular Use | 17.4 (1.5) [15.0-21.0] | N/A |
Note. M=Mean, SD=Standard Deviation. Past year and lifetime substance uses reflect total standard substance uses in the past year (e.g., alcohol in number of standard drinks; nicotine in number of cigarettes and hits of chew/snuff/pipe/cigar/hookah; and cannabis in number of joints). More detail of how this information was collected is reflected in the Method section under TLFB.
Substance Use
As expected, cannabis users had significantly more cannabis use in the past year (t(34)=−7.1, p<.001) as well as lifetime uses (t(34)=−5.2, p<001). On average, cannabis users had been abstinent from cannabis for 20.7 days by the third session. In the control group, four participants had used cannabis within the past year and their length of abstinence on average was 153.5 days; there was no reported use during the period of the study. Cannabis users had significantly more past year alcohol (t(53)=−4.3, p<.001), nicotine use (t(34)=−2.7, p=0.01), and session three cotinine levels (t(41)=−2.7, p=.01) compared to controls. Due to these differences, past year alcohol and nicotine use was included in the model to account for their potential influence in outcomes. See Table 1 for full substance use information by group.
Repeated Measures
Time.
Repeated measures ANCOVAs revealed a significant main effect by time with improved performance over time for LNS performance (F(1, 69)= 11.73, p<.001, ηP2=0.15), Ruff 2&7 Total Time (F(1, 69)=74.03, p<.001, ηP2=0.52), Ruff 2&7 Total Accuracy (F(1, 69)=37.41, p<.001, ηP2=.35), and total Stroop performance (F(1, 69)=16.24, p<.001, ηP2=0.19).
Group Effects.
There were no significant effects between cannabis group, past year alcohol use, and past year nicotine use on neuropsychological performance. See Table 2 for mean neuropsychological performance by cannabis group for each session.
Table 2.
Mean Neuropsychological Scores across Sessions
| Cannabis Users | Controls | |||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 1 | Session 2 | Session 3 | |
| Letter-Number-Span | 11.5(3.1) | 12.3(2.9) | 13.1(3.1) | 11.4(2.4) | 12.3(2.4) | 12.8(2.4) |
| Ruff 2&7 Total Speed | 94.6(19.3) | 104.7(17.4) | 112.1(20.4) | 101.0(19.9) | 110.5(22.3) | 116.8(23.7) |
| Ruff 2&7 Total Accuracy | 80.6(18.7) | 90.4(18.2) | 98.7(12.3) | 82.7(22.6) | 88.7(18.1) | 94.6(13.7) |
| Stroop | 51.6(7.7) | 55.6(7.2) | 58.6(10.3) | 52.7(10.2) | 55.9(13.3) | 60.2(11.4) |
| HVLT-Total Recall | 28.4(3.7) | 28.1(3.1) | 27.9(3.9) | 28.8(3.9) | 28.7(4.2) | 28.8(4.1) |
| HVLT-Delayed Recall | 9.8(1.7) | 9.8(1.8) | 10.1(2.2) | 10.1(1.8) | 10.2(2.1) | 10.2(2.0) |
Note. Values include means and standard deviation in the format of mean(standard deviation).
Interaction Effects.
There was a significant interaction between time and cannabis group on Ruff 2&7 Total Accuracy (F(1, 69)=6.47, p=.01, ηP2=0.09), with cannabis users demonstrating significant improvements in accuracy compared to controls (see Figure 1). There was a significant interaction between time and past year alcohol use on Ruff 2&7 Total Accuracy (F(1, 69)=12.22, p=.001, ηP2=0.15) and HVLT Immediate Free Recall (F(1, 69)=4.31, p=.04, ηP2=0.06) with heavier alcohol users showing increased performance with more time. There were no significant interaction with past year nicotine use.
Figure 1. Ruff 2&7 Total Accuracy across Sessions between Cannabis Users and Controls.
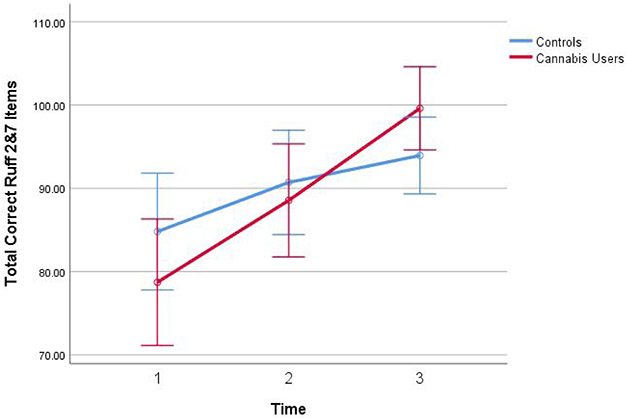
Note. Figure highlights the interaction between cannabis use group and time on improved Ruff 2&7 accuracy across all three sessions. Covariates appearing in the model are evaluated at the following values: Past year alcohol use = 212.80, Past year nicotine use = 105.95. Error bars: 95% Confidence interval. Past year alcohol use and past year nicotine use were measured from the TLFB. More information about how these measures were collected can be found in the Method section.
Discussion
We predicted that cannabis users would have significant deficits in cognitive performance compared to controls at baseline, but that domains of verbal working memory and verbal learning would recover with one week of monitored sustained abstinence, but would continue to exhibit deficits in sustained attention compared to controls. However, our findings suggested that cannabis users were not significantly different from controls at baseline on these brief measures [although this same sample did demonstrate some deficits on a more expansive battery at a later time point (Wade, Wallace, et al., 2019)], nor did they demonstrate improved performance in verbal working memory and learning tests compared to controls. However, the adolescent and young adult cannabis users in this study did show significant improvement in their sustained attention performance following the two-week period of monitored abstinence.
While previous research has suggested sustained deficits in attention following three weeks and one month of monitored abstinence in a sample of adolescents (Hanson et al., 2010) and a sample of adolescents and young adults (Schuster et al., 2018), respectively, our findings indicate that attentional deficits may continue to recover with continued abstinence in adolescents and young adults. It is also important to note that although our period of monitored abstinence was a minimum of two weeks, our cannabis users reported that they had on average an additional six and a half days of abstinence prior to enrolling the study, suggesting a total of at least three weeks of abstinence prior to the final date of testing. Thus, it is possible that subtle deficits in verbal learning and working memory had already recovered by the first day of testing and could explain the lack of significant neurocognitive differences between cannabis users and controls at any time point. This is consistent with Schuster et al. (2018) who found that these cognitive domains recovered within the first week. However, Hanson et al. (2010) demonstrated at least two weeks of sustained abstinence for cognitive recovery in these areas in a younger sample of adolescents. The age difference in the included cannabis user samples (Hanson et al. (2010) mean age = 18.1; present study’s mean age = 21.5) may indicate that adolescents may require more time to experience cognitive recovery than their young adult counterparts. Further, the cannabis users within our sample had relatively late onset of regular cannabis use (M=17.4 years old), which may explain a lack of significant differences in cognitive functioning at baseline due to both later onset of cannabis use and potentially fewer years of cannabis exposure (mean age of regular use was 15.6 for Hanson et al. (2010)). Consistent with this, age of regular cannabis use and duration of use have been implicated as important factors in attention deficits (Ehrenreich et al., 1999; Solowij, Michie, & Fox, 1995). Taken together, these studies highlight the importance of measuring date of last cannabis use as the length of sustained abstinence depending on age may determine rates and domains of neurocognitive recovery from chronic cannabis use. Further, years of chronic cannabis use may also moderate the above effects. Accordingly, future studies should examine these factors more closely. Finally, it is notable that we did observe cognitive deficits in primarily the same sample following three-weeks of monitored abstinence on other neuropsychological tasks measuring psychomotor processing speed and sustained attention, including the CPT-2 and D-KEFS Trail Making Test (Wade, Wallace, et al., 2019; Wallace et al., 2019). In addition, cannabis users demonstrated impaired working memory on the letter number sequencing task after controlling for aerobic fitness level and low-fit cannabis users had impaired verbal memory performance on the CVLT-2 (Wade, Wallace, et al., 2019). Thus, it is also possible that the tests utilized in the mini-NP battery were not sufficiently sensitive to detect cognitive deficits.
The underlying mechanism explaining recovery of cognitive functioning with continued abstinence from cannabis is most likely due to recovery within the endogenous endocannabinoid system (Hirvonen et al., 2012). Continued cannabis exposure has been shown to downregulate CB1 receptors, which are found largely within frontal and limbic regions of the brain (Howlett et al., 2004). Animal models have shown that this CB1 receptor downregulation may exhibit recovery comparable to controls after seven days within the striatum and 14 days within the hippocampus after cannabis cessation (Sim-Selley et al., 2006). Due to the timeline and areas of CB1 recovery, this mechanism provides a potential explanation for the relatively rapid recovery of certain domains of cognitive functioning. Still, these findings must first be replicated within human populations. Additional research examining the direct relationship between these mechanism and cognition needs to be done.
Overall, these findings provide a message of hope that cannabis cessation could lead to the recovery of attentional deficits in adolescents and young adults. As research has shown that youth are at particular risk for harm from cannabis use (Turner, Spithoff, & Kahan, 2014), treatment models for adolescents engaging in cannabis use have been established (Diamond et al., 2002). While these programs seem promising, continuous abstinence rates within youth remain low (Budney, Roffman, Stephens, & Walker, 2007). Thus, additional research is needed to encourage continuous abstinence in adolescents and young adults, which may help to attenuate more long-term cognitive deficits that may occur with continued use and as the brain continues to mature.
Interestingly, past year alcohol also significantly predicted improved performance on attention and verbal working memory tasks over the period of abstinence. Recent studies have suggested that co-use of alcohol and cannabis can be an important consideration when disentangling the effects of substance use on cognition (Jacobus et al., 2014; Ramaekers et al., 2011; Wade, Thomas, et al., 2020). Our findings highlight that abstinence from concurrent cannabis and alcohol use in co-users may play a role in the recovery of neuropsychological functioning. Patterns of substance use and co-use should be more closely investigated within future studies examining the impact of cannabis on cognition. Though these two issues are entangled in the present design, future studies may want to consider different groups abstaining from alcohol and cannabis separately.
There are limitations to consider within the context of our study. Cannabis users reported at least a week of abstinence from cannabis prior to entering the study; this may have resulted in some recovery of function prior to the monitored abstinence period. Further, we were unable to disentangle the effects of abstaining from alcohol or cannabis use in the current design. Of other note, one study reported that toxicology testing with the sweat patch may miss small doses of edible cannabis (Huestis et al., 2008); however, none of our participants reported recent edible use in the past month, edible use during the past year was very low, and we also conducted urine toxicology analyses weekly. Examiners were not blind to participant drug status, which may have impacted results as recent research has emphasized that neuropsychologists have intrinsic biases on the cognitive effects of chronic cannabis users (Hirst, Watson, Rosen, & Quittner, 2019). Further, no specific validity testing was done until session four. So while appropriate effort was given at session four, we can only infer valid effort during previous neuropsychological testing sessions leading up to session four. Our brief neuropsychological battery did not include measures of psychomotor speed or visual learning and memory, which have also been shown to be impaired in cannabis users (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Lisdahl & Price, 2012; Wade, Wallace, et al., 2019; Winward, Hanson, Tapert, & Brown, 2014). Further, we did not conduct repeated neuroimaging and it is notable that previous studies have reported subtle deficits in brain function, connectivity, and structure following 2-4 weeks of monitored abstinence (Jacobus et al., 2012; Jacobus et al., 2014; Maple, Thomas, Kangiser, & Lisdahl, 2019; Price et al., 2015; Schweinsburg et al., 2010; Shollenbarger, Price, Wieser, & Lisdahl, 2015; Tapert et al., 2007); thus, additional longitudinal studies utilizing more sensitive measures of neuroimaging are needed before it can be concluded that full recovery is obtained following abstinence.
Research on the impact of sustained abstinence from cannabis on neuropsychological outcomes is understudied. Our findings add to this literature by demonstrating significant recovery in sustained attention deficits in regular adolescent and young adult cannabis users following two weeks of monitored abstinence. Further investigations into confirming the impact of sustained abstinence in samples with an earlier age of cannabis use onset and examining other outcomes, especially neuroimaging, are needed. The implications of these findings are hopeful and suggest that sustained abstinence in late adolescent and young adult regular users can improve cognition in the domain of sustained attention. Thus, programs that promote abstinence in young users should remain a high public health priority.
Acknowledgements
Disclosure of Funding: This work was supported by the National Institute on Drug Abuse (NIDA) Grant [R01DA030354] to K.L. During manuscript preparation, that authors were supported by NIDA [U01DA041025; PI: K.L.].
Footnotes
Conflicts of Interest: No conflicts to declare.
References
- Ashare RL, Falcone M, & Lerman C (2014). Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology, 76, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, & Cadet J (2002). Dose-related neurocognitive effects of marijuana use. Neurology, 59(9), 1337–1343. [DOI] [PubMed] [Google Scholar]
- Brandt J, & Benedict R (1991). Hopkins Verbal Learning Test-Revised (HVLT-R). Lutz, FL: Psychological Assessment Resources: Inc. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, & Vik PW (1998). Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of studies on alcohol, 59(4), 427–438. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, & Walker D (2007). Marijuana dependence and its treatment. Addiction science & clinical practice, 4(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, & Galvan A (2008). The adolescent brain. Developmental Review, 28(1), 62–77. doi: 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fonseca FR, Gorriti M, Fernandez-Ruiz J, Palomo T, & Ramos J (1994). Downregulation of rat brain cannabinoid binding sites after chronic Δ9-tetrahydrocannabinol treatment. Pharmacology Biochemistry and Behavior, 47(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Diamond G, Godley SH, Liddle HA, Sampl S, Webb C, Tims FM, & Meyers R (2002). Five outpatient treatment models for adolescent marijuana use: a description of the Cannabis Youth Treatment Interventions. Addiction, 97, 70–83. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, … Hoehe MR (1999). Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology, 142(3), 295–301. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, … Lacerda AL (2011). Cannabis use before age 15 and subsequent executive functioning. The British Journal of Psychiatry, 198(6), 442–447. [DOI] [PubMed] [Google Scholar]
- Ganzer F, Bröning S, Kraft S, Sack P-M, & Thomasius R (2016). Weighing the evidence: a systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychology review, 26(2), 186–222. [DOI] [PubMed] [Google Scholar]
- Gentili S, Mortali C, Mastrobattista L, Berretta P, & Zaami S (2016). Determination of different recreational drugs in sweat by headspace solid-phase microextraction gas chromatography mass spectrometry (HS-SPME GC/MS): Application to drugged drivers. Journal of pharmaceutical and biomedical analysis, 129, 282–287. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience, 2(10), 861. [DOI] [PubMed] [Google Scholar]
- Golden CJ, & Freshwater SM (1978). Stroop color and word test.
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, & Diviak KR (2012). Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical Experimental Neuropsychology, 34(9), 962–976. doi: 10.1080/13803395.2012.703642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F (2003). Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical pharmacokinetics, 42(4), 327–360. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, & Killgore WD (2012). Age of onset of marijuana use impacts inhibitory processing. Neuroscience letters, 511(2), 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, & Lukas SE (2012). Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors, 26(3), 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, & Tapert SF (2010). Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive behaviors, 35(11), 970–976. doi: 10.1016/j.addbeh.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst RB, Watson J, Rosen AS, & Quittner Z (2019). Perceptions of the cognitive effects of cannabis use: A survey of neuropsychologists’ beliefs. Journal of clinical and experimental neuropsychology, 41(2), 133–146. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin R, Li C-T, Terry G, Zoghbi S, Morse C, … Innis R (2012). Reversible and regionally selective downregulation of brain cannabinoid CB 1 receptors in chronic daily cannabis smokers. Molecular psychiatry, 17(6), 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, & Porrino LJ (2004). Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology, 47, 345–358. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Scheidweiler KB, Saito T, Fortner N, Abraham T, Gustafson RA, & Smith ML (2008). Excretion of Δ9-tetrahydrocannabinol in sweat. Forensic science international, 174(2-3), 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, & Tapert SF (2012). Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology, 222(4), 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, & Tapert SF (2014). Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. Journal of studies on alcohol and drugs, 75(5), 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, & Tallal PA (1991). Maturation of human cerebrum observed in vivo during adolescence. Brain, 114(5), 2037–2049. [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O'Malley PM, Bachman JG, Scheulenberg JE, & Patrick ME (2019). Monitoring the Future national survey results on drug use, 1975-2018: Overview, key findings on adolescent drug use. Retrieved from Ann Arbor: Institute for Social Research: [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, & Shollenbarger S (2013). Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in Psychiatry, 4, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, & Price JS (2012). Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society, 18(4), 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Medina-Kirchner C, Maple KE, & Shollenbarger S (2014). Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Current addiction reports, 1(2), 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple KE, Thomas AM, Kangiser MM, & Lisdahl KM (2019). Anterior cingulate volume reductions in abstinent adolescent and young adult cannabis users: Association with affective processing deficits. Psychiatry Research: Neuroimaging, 288, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CW, Blumenthal TD, Dawes MA, Liguori A, Richard DM, Bray B, … Dougherty DM (2011). Failure to sustain prepulse inhibition in adolescent marijuana users. Drug and alcohol dependence, 116(1-3), 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, & Tapert SF (2011). Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural brain research, 224(1), 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, & Tapert SF (2007). Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society, 13(5), 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, & Tapert SF (2009). Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addiction biology, 14(4), 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, & Tapert SF (2010). Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research: Neuroimaging, 182(2), 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, & Tapert SF (2007). Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and teratology, 29(1), 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, & Tapert SF (2007). Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors, 21(4), 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, & Yurgelun-Todd D (1996). The residual cognitive effects of heavy marijuana use in college students. Jama, 275(7), 521–527. [PubMed] [Google Scholar]
- Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, & Lisdahl KM (2015). Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology, 232(16), 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Theunissen EL, De Brouwer M, Toennes SW, Moeller MR, & Kauert G (2011). Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology, 214(2), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roten A, Baker NL, & Gray KM (2015). Cognitive performance in a placebo-controlled pharmacotherapy trial for youth with marijuana dependence. Addictive behaviors, 45, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RM, & Allen CC (1996). Ruff 2 & 7 selective attention test: professional manual Psychological Assessment Resources. [Google Scholar]
- Saito T, Wtsadik A, Scheidweiler KB, Fortner N, Takeichi S, & Huestis MA (2004). Validated gas chromatographic–negative ion chemical ionization mass spectrometric method for δ9-tetrahydrocannabinol in sweat patches. Clinical chemistry, 50(11), 2083–2090. [DOI] [PubMed] [Google Scholar]
- Schreiner AM, & Dunn ME (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Experimental and clinical psychopharmacology, 20(5), 420. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Gilman J, Schoenfeld D, Evenden J, Hareli M, Ulysse C, … Evins AE (2018). One Month of Cannabis Abstinence in Adolescents and Young Adults Is Associated With Improved Memory. The Journal of clinical psychiatry, 79(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, & Tapert SF (2010). The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs, 42(3), 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, & Gur RC (2018). Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA psychiatry, 75(6), 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, … Wilkinson B (2010). Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). The Journal of clinical psychiatry. [DOI] [PubMed] [Google Scholar]
- Shollenbarger SG, Price J, Wieser J, & Lisdahl K (2015). Poorer frontolimbic white matter integrity is associated with chronic cannabis use, FAAH genotype, and increased depressive and apathy symptoms in adolescents and young adults. NeuroImage: Clinical, 8, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, & Selley DE (2006). Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Molecular pharmacology, 70(3), 986–996. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Pope HG Jr, Silveri MM, Simpson NS, Gruber SA, & Yurgelun-Todd DA (2008). Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. European neuropsychopharmacology, 18(8), 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back Measuring alcohol consumption (pp. 41–72): Springer. [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, … Yücel M (2011). Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology, 216(1), 131–144. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, & Fox AM (1995). Differential impairments of selective attention due to frequency and duration of cannabis use. Biological psychiatry, 37(10), 731–739. [DOI] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, & Christensen H (2011). Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction, 106(12), 2195–2203. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, & Frank LR (2007). Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl), 194(2), 173–183. doi: 10.1007/s00213-007-0823-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma RJ, Monnig MA, Lysne PA, Ruhl DA, Pommy JA, Bogenschutz M, … Yeo RA (2011). Adolescent substance abuse: the effects of alcohol and marijuana on neuropsychological performance. Alcoholism: Clinical and Experimental Research, 35(1), 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SD, Spithoff S, & Kahan M (2014). Approach to cannabis use disorder in primary care: Focus on youth and other high-risk users. Canadian Family Physician, 60(9), 801–808. [PMC free article] [PubMed] [Google Scholar]
- Wade NE, Thomas AM, Gruber SA, Tapert SF, Filbey FM, & Lisdahl KM (2020). Binge and cannabis co-use episodes in relation to white matter integrity in emerging adults. Cannabis and Cannabinoid Research, 5(1), 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade NE, Wallace AL, Swartz AM, & Lisdahl KM (2019). Aerobic fitness level moderates the association between cannabis use and executive functioning and psychomotor speed following abstinence in adolescents and young adults. Journal of the International Neuropsychological Society, 25(2), 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AL, Wade NE, Hatcher KF, & Lisdahl KM (2019). Effects of Cannabis Use and Subclinical ADHD Symptomology on Attention Based Tasks in Adolescents and Young Adults. Archives of Clinical Neuropsychology, 34(5), 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Coalson D, & Raiford S (1997). WAIS-III: Wechsler adult intelligence scale: Psychological Corporation. San Antonio, TX. [Google Scholar]
- Winward JL, Hanson KL, Tapert SF, & Brown SA (2014). Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. Journal of the International Neuropsychological Society, 20(8), 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]


