Abstract
IMPORTANCE
Obesity in adolescence has reached epidemic proportions around the world, with the prevalence of severe obesity increasing at least 4-fold over the last 35 years. Most youths with obesity carry their excess adiposity into adulthood, which places them at increased risk for developing obesity-driven complications, such as type 2 diabetes and cardiovascular disease, and negatively affects social and emotional health. Given that adolescence is a unique transition period marked by significant physiologic and developmental changes, obesity-related complications can also negatively affect adolescent growth and developmental trajectories.
OBSERVATIONS
Provision of evidence-based treatment options that are tailored and appropriate for the adolescent population is paramount, yet complex. The multifactorial etiology of obesity along with the significant changes that occur during the adolescent period increasingly complicate the treatment approach for adolescent obesity. Treatment practices discussed in this review include an overview of evidence supporting currently available behavioral, pharmacologic, surgical, and device interventions for obesity. However, it is important to note that these practices have not been effective at reducing adolescent obesity at the population level.
CONCLUSIONS AND RELEVANCE
Because adolescent obesity requires lifelong treatment, effectively addressing this disease will require significant resources, scientific rigor, and the provision of access to quality care similar to other chronic health conditions. Effective and less invasive therapies, effective adjuncts, and comprehensive centers that offer specialized treatment are critical. This considerable need for increased attention to obesity care calls for dedicated resources in both education and research for treatment of obesity in youths.
Adolescent obesity is a global health problem, particularly in high-income countries where more than 20% of children have obesity.1,2 Prevalence of severe obesity3 in adolescence is rising,1,2 increasing at least 4-fold since 1985.2 Adolescents with obesity experience emotional, social, and physical health ramifications,4 including impaired growth and developmental trajectories.4 Most adolescents with obesity carry excess adiposity into adulthood,5 placing them at increased cardiometabolic risk and at risk for some types of cancer.6,7
Adolescence, defined by the American Academy of Pediatrics as ages 11 to 21 years,8 is a unique period of psychologic change, characterized by increased autonomy, decreased receptivity to adult input, and reduced self-regulatory skills.8,9 Specifically, executive function changes can affect risk-taking behaviors and impulsivity relevant to development of obesity.10 Even within this relatively narrow window, variation exists by age in mental and emotional development as youths progress though early (ages 11–14 years), middle (ages 15–17 years), and late (ages 18–21 years) adolescence.8 Taken together, these distinct features help explain why working with adolescents can be challenging and requires different approaches.
Identifying effective treatment strategies for adolescent obesity is paramount but complicated by the multifactorial etiology of obesity (Figure 1).2,4,11–18 Moreover, adolescent obesity is strongly linked to low socioeconomic status11 and differs by race and ethnicity.12 It is unlikely a single treatment regimen will address the multifactorial etiologies underlying obesity and be effective across populations. Given that appropriate treatment approaches for adolescent obesity must account for age, sex, pubertal status, severity of obesity, underlying etiology, obesity-related complications, psychosocial factors, and patient and family preferences, we provide an overview of behavioral, pharmacologic, surgical, and device intervention options to guide treatment. Importantly, practices have not led to effective reduction of adolescent obesity on a population level; hence, we also propose future directions that may inspire novel strategies to reduce development and severity of obesity among adolescents.
Figure 1. The Multifactor Development of Obesity.
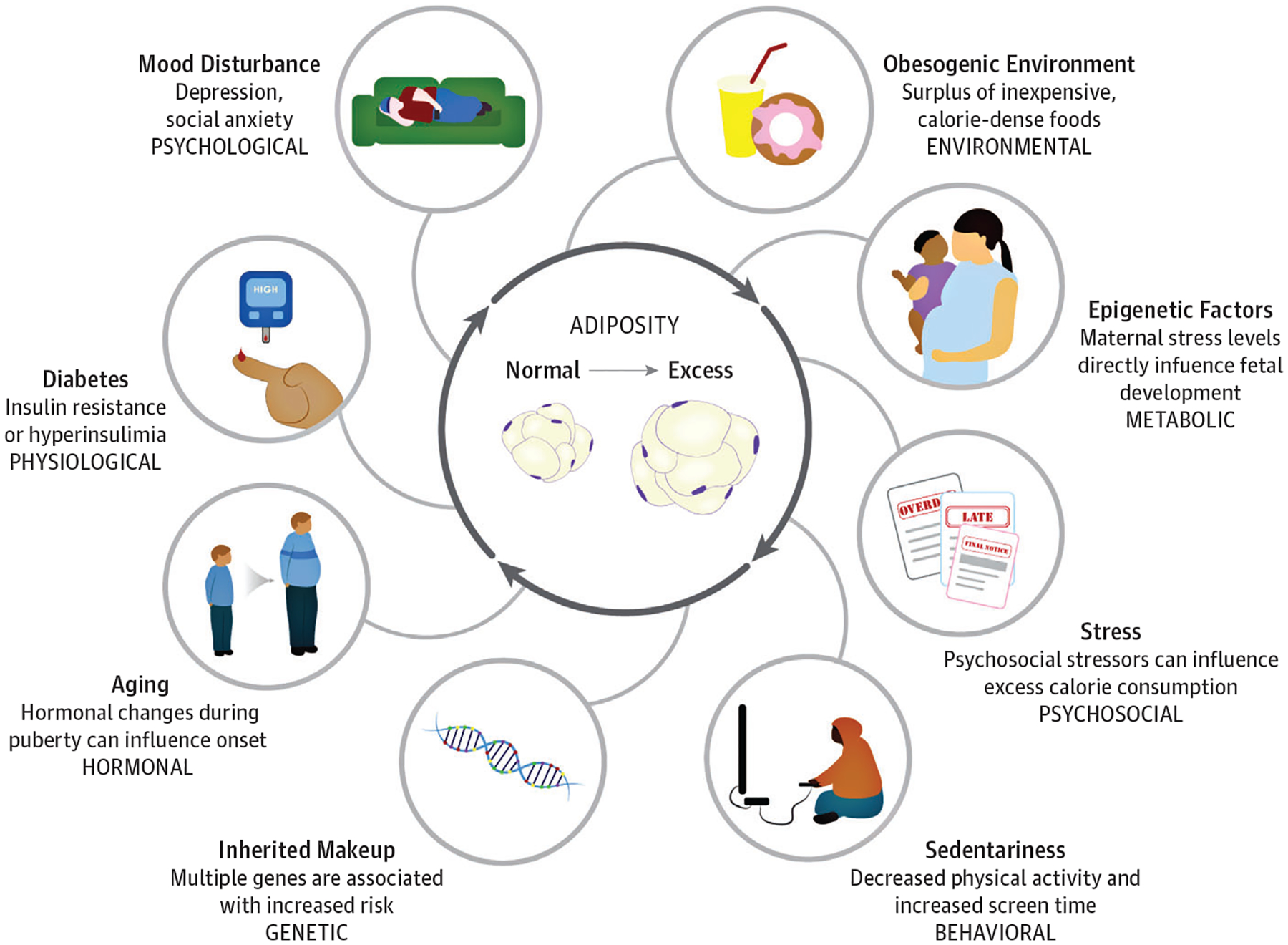
This figure demonstrates the multifactorial and complex nature of the development of obesity.
Adolescent Obesity Treatment
Diagnosing Adolescent Obesity: Clinical Guidelines
Obesity is a complex chronic disease, both multifactorial in cause and multivariable in manifestation,19 making it diagnostically challenging, especially in adolescence.20 The terms overweight and obesity have been defined using various methods aimed at quantifying or estimating degree of body fat and its effect on disease risk, morbidity, and mortality.21 This concept is further complicated by an incomplete understanding of and lack of definition for healthy vs unhealthy degrees of body fat. Fat is an essential body compartment both for physiologic functioning and evolutionary survival; however, the transition point from being physiologic necessity to liability is not clearly defined, particularly in pediatric populations during development.
Body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) is easily obtained and widely used but is only an indirect measure of fatness because it cannot differentiate between lean body mass and adipose tissue. Youths (≥2 years) are diagnosed as being overweight if BMI is at least 85th percentile but less than 95th percentile for sex and age, with obesity if BMI is at least at 95th percentile, and with severe obesity if BMI is at least 120% of the 95th percentile or at least 35, whichever is lower.3,21 Health care clinicians should plot and view BMI on a chart regularly but be aware of variations in body composition owing to race and ethnicity22 and increased lean mass contributing to higher BMI.
A comprehensive history, medication review, physical examination, and laboratory evaluation should be conducted to assess contributors to obesity and obesity-related complications.23 Specifically, this should include weight history and previous weight loss attempts, social factors, barriers, and facilitators to weight loss, lifestyle factors, medical history, medications, and family history.
Obesity-Related Complications
Adolescents with obesity rarely outgrow their excess adiposity,5 and obesity may result in more than 29 complications that impair normal growth, development, and health.4 Specifically, adolescent obesity is associated with hypertension, dyslipidemia, and impaired glucose metabolism, all clustering within the definition of metabolic syndrome,4 and may increase risk of some types of cancer.7 Beyond physical ramifications, adolescents with obesity often display emotional and social complications, including depression, eating disorders, and poor self-esteem, likely owing in part to stigma, discrimination, and bullying.4 A detailed review4 of obesity-related complications was published in 2018.
Clinical Efficacy of Weight Loss Interventions
No standard or agreed-on definition of weight loss success exists for pediatric populations4 nor for weight maintenance or regain. The Endocrine Society defines a BMI decrease of 1.5 to have modest but significant effects on adolescents with obesity, while also stating that at least 7% weight loss is recommended for youths with severe obesity.21 The Pediatric Obesity Weight Evaluation Registry, an ongoing prospective study collecting data from 31 pediatric weight management programs across the United States, has defined success as at least 5% BMI reduction after 6 to 12 months of intervention.24 Within the past 5 years, a movement away from use of BMI z score in children and adolescents aged 2 to 19 years has occurred based on data indicating this metric is a poor indicator of adiposity among youths with obesity, particularly those with severe obesity.25 Thus, among adolescents with obesity, it has been recommended that BMI should be expressed relative to the US Centers for Disease Control and Prevention 95th percentile (eg, as %BMIp95, which expresses the BMI as a percentage of the sex- and age-specific 95th percentile) or ΔBMIp95 (BMI-95th percentile), which is the distance (in kilograms per meters squared) from the 95th percentile.25 When possible, examination of changes in adiposity, rather than weight, is warranted clinically and across studies.26 However, others suggest changes in cardiometabolic risk factors may be more clinically meaningful than weight or BMI changes.4
Discussing Obesity Status and Treatment With Adolescents and Their Families
Weight-related discussions are sensitive; thus, clinicians should communicate with compassion and respect. Adolescents do not want clinicians to use the words “fat,” “large,” or “obese” because these terms are believed to perpetuate stigmatization.27 Rather, “weight problem” or “BMI” are rated among the most preferred for clinician use.27 Moreover, people-first language is recommended, referring to individuals as “persons with obesity” rather than “obese persons.” This is an important difference because individuals often do not wish to be defined by their disease state.
Multicomponent Behavioral Interventions
For some adolescents with obesity, multicomponent, intensive lifestyle modification programs can be effective for weight reduction, with long-term weight loss maintenance possible.4,21,28 Multicomponent, behavioral interventions ideally include caregiver involvement and trained case managers who can serve as the consistent liaison between the family and various health care clinicians. The physician and case managers should rely on an interdisciplinary team of experts including registered dietitians, exercise physiologists, psychologists, and others as needed.21,28 Behavioral strategies common among adolescent obesity interventions include diet and physical activity modification and behavioral counseling.4,29,30 Multicomponent, behavioral interventions are the foundation of all treatments for adolescent weight loss, and must be an integral part of obesity treatment across treatment modalities.31,32
Dietary Modification
Modifying dietary intakes, both quantity and quality of food, is routinely incorporated into behavioral interventions. The goal of such efforts is to decrease energy intake while improving nutritional quality of foods consumed. The most commonly used approach is the Traffic Light Diet,33 a family-friendly method of categorizing foods according to traffic light colors. Green foods, or “almost anytime foods,” are low calorie and nutrient dense and include fruits and vegetables. Yellow foods, or “sometimes foods,” are high in nutrients but calorie dense. Red foods, or “once-in-a-while foods,” include ultra-processed foods such as desserts, sugar-sweetened beverages, and fried foods. Using the Traffic Light Diet, a statistically significant decrease in adolescent weight (ranging from BMI decrease of 0.18–2.6) was observed in interventions ranging from 6 months to 2 years.34 Importantly, in terms of safety, structured pediatric obesity treatment interventions with a dietary component are associated with reduced eating disorder prevalence, risk, and symptoms and appear to mildly improve symptoms of depression and anxiety.35,36
Other interventions have focused on altering macronutrient distribution of the diet, including “low fat” or “low carbohydrate.” Similar to results observed in adults,37 longer-term studies of greater than 12 months demonstrate weight loss can be achieved in adolescents irrespective of macronutrient distribution in a reduced-calorie diet.38 Shorter-term studies (8–12 weeks)4,39 suggest that a very low-calorie diet(VLCD), generally consisting of approximately 800 kcal per day, can safely result in rapid weight loss and potentially reverse new-onset type 2 diabetes in adolescents. However, the study was small (n = 8) and had no control group; thus, more evidence is needed.39
Physical Activity and Sedentary Behavior Modification
Modifying energy expenditure is focused on decreasing sedentary behavior and increasing moderate-to-vigorous physical activity. However, a systematic review and meta-analysis found that diet-only and diet-plus-exercise interventions resulted in improved metabolic profiles and weight loss in youths,40 with no significant differences in weight loss between them. Regardless, increasing physical activity has the potential to improve cardiometabolic outcomes40 and is predictive of sustained weight change 10 years after engagement in a weight loss intervention in adolescents.41 Thus, prescribed physical activity should include 30 minutes of moderate-intensity physical activity per day 5 days per week, or 20 minutes of vigorous physical activity 3 days per week, and physical inactivity should be reduced by limiting nonacademic screen time and other sedentary activities to less than 2 hours per day.4,21,28,42
Behavioral Counseling
Behavioral strategies are often based in cognitive behavioral therapy (CBT), assisting participants in modifying their dietary, physical activity, and sleep behaviors through skill development in self-monitoring, goal-setting, and stimulus control.30,43 These interventions help participants manage negative emotional states and encourage changing negative thoughts (eg, certain food cravings) through cognitive reframing and distraction techniques.30,43
Family-Based Behavioral Interventions
Family-based behavioral weight loss treatment is a multicomponent intervention targeting parents and children and is the most extensively studied behavioral interventionforyouths.29,30,33,41 Family-based behavioral weight loss treatment is effective at improving child weight status in the short and longterm.29,30,33,41 However, behavioral interventions to date appear to be more effective in children than adolescents,30 with family-based behavioral weight loss treatments (both parent-only and family involvement) having been deemed as “possibly efficacious treatments” for adolescents.29
Overall Assessment of Multicomponent, Behavioral Adolescent Weight Loss Interventions
Multicomponent, intensive lifestyle modification programs are the foundation of any obesity treatment among adolescents and can be moderately effective for reducing excess weight, with long-term weight loss maintenance possible.4,21,28 Specifically, BMI z score change ranges from −0.06 to 0.13 following treatment in adolescents,44–47 which equates to a body weight change of −2.7% to 1.9%.44–46,48 The harms of treatment are noted as small to none28 and may even decrease eating disorder risk and prevalence and mildly improve depression and anxiety symptoms.35,36 Although even a modest decrease in BMI z score or percentage body fat may potentially improve cardiometabolic risk markers,4 efforts are needed to improve the effectiveness of multicomponent, behavioral weight management interventions among adolescents.
Pharmacologic Interventions
Pharmacologic interventions have been proposed for youths with obesity who respond suboptimally to multicomponent, intensive behavioral therapy.21,49 However, options are extremely limited. Orlistat, a lipase inhibitor, is the only medication approved by the US Food and Drug Administration (FDA) for long-term pediatric obesity treatment (≥12 years) and is not approved by the European Medicines Agency.50 Clinical use is believed to be limited because of the modest efficacy (approximately 3% BMI reduction over 12 months) and adverse effects such as oily spotting and flatus with discharge.50 Phentermine, a norepinephrine reuptake inhibitor, is also approved by the FDA (not the European Medicines Agency) for use in older adolescents; however, it is recommended for only short-term use (generally interpreted as 12 weeks) and limited to adolescents older than 16 years (often interpreted as 17 years and older) per FDA-approved labeling. Although randomized, placebo-controlled studies are lacking, phentermine appears to have relatively modest effects on weight reduction in youth (approximately 4% BMI reduction in the clinical setting).51 Potential adverse effects of phentermine are not well studied but appear similar to other stimulant-type medications (eg, methylphenidate and amphetamine derivatives) for treatment of attention-deficit/hyperactivity disorder.51 The Endocrine Society suggests discontinuing pharmacotherapy if, at full dose, BMI/BMI z score is not reduced by 4% after 12 weeks.21 Other off-label medications have been evaluated for pediatric obesity.52–56
Surgical and Device Interventions
Adolescents with severe obesity and/or serious comorbid conditions may be candidates for metabolic and bariatric surgery (MBS) or device therapy. However, data addressing safety and effectiveness of these approaches are scarce compared with behavioral interventions. Metabolic and bariatric surgery is widely considered the most effective obesity treatment for adolescents, as prospective studies of the Roux-en-Y gastric bypass and the vertical sleeve gastrectomy have demonstrated body weight loss from 26% to 40% up to 8 years following surgery and reductions in obesity-related complications.57–60 Yet about half of adolescents who meet clinical indications for MBS are denied insurance benefits for the procedure on first request,61 likely affecting long-term health outcomes.60 Although adolescents and adults who undergo MBS have similar marked weight loss 5 years following surgery, adolescents are significantly more likely than adults to have remission of type 2 diabetes (86% vs 53%) and hypertension (68% vs 41%).60 Mortality within 5 years of MBS does not differ between adults and adolescents; however, adolescents are more likely to need abdominal re-operations and have low ferritin levels.60 Metabolic and bariatric surgery also leads to clinically meaningful improvements in numerous obesity-related complications and cardiometabolic risk factors as well as reduced musculoskeletal pain and enhanced functional mobility.57,62,63 Pediatric clinical practice guidelines discuss eligibility criteria for MBS and highlight important risks and special considerations.64 Widespread uptake of MBS is hindered by potential referral bias, insufficient number of specialized programs resulting in lack of access, irreversibility, and potential long-term risks such as micronutrient deficiencies. Device therapy studies for obesity in adolescents are limited to a few small, uncontrolled pilot studies of the intragastric balloon, either placed endoscopically or swallowed by the patient, designed to occupy space in the stomach to reduce hunger and increase satiety. Intragastric balloon studies conducted among adolescents with obesity have demonstrated mean weight losses ranging from 0% to 16% with short-term treatment (3–6 months), with gastric pain and cramping reported as the most common adverse events.65–68
Recommendations for Care, Emerging Therapies, and Future Directions of Adolescent Obesity
Integrated Continuum of Care in Obesity Treatment
We recommend that treatment options for adolescent obesity involving behavioral, pharmacologic, and/or surgical therapy be viewed as an integrated continuum of care (Figure 2) involving overlapping rather than chronological stages.69 As with other chronic diseases, all options should be presented by the clinician and discussed with the patient and family. Choice of treatment(s) should be guided by the patient’s age, sex, pubertal status, severity of obesity, psychosocial factors, obesity-related complications, anticipated adaptations to fat mass, and patient and family preferences.21,69 Comprehensive, behavioral lifestyle modification targeting behavior change is essential and must serve as key component across treatment modalities. To improve the likelihood of adolescent weight loss, caregivers should be involved in treatment, encouraged to make changes to the home food environment, and model healthy behaviors.70 This would maximize treatment potential by modifying the shared family environment and should be encouraged across treatment modalities. Additionally, behavioral and pharmacologic interventions should be integrated such that multicomponent, behavioral treatments may amplify the action of medication(s). Figure 2 outlines the continuum of care we believe will be necessary for successful treatment of adolescent obesity overtime. Additionally, interventions should include trained case managers in pediatric patient-centered medical homes who can serve as the consistent liaison between the family, patient, and various health care clinicians, particularly as patients navigate across behavioral, pharmacologic, and surgical treatment modalities. As adolescents with obesity reach adulthood, coordinated planning is needed for effective transitioning from pediatric to adult care clinicians. To date, such transition programs for obesity are limited, and research is needed to develop and evaluate these clinical pathways.
Figure 2. Continuum of Integrated Care for Obesity.
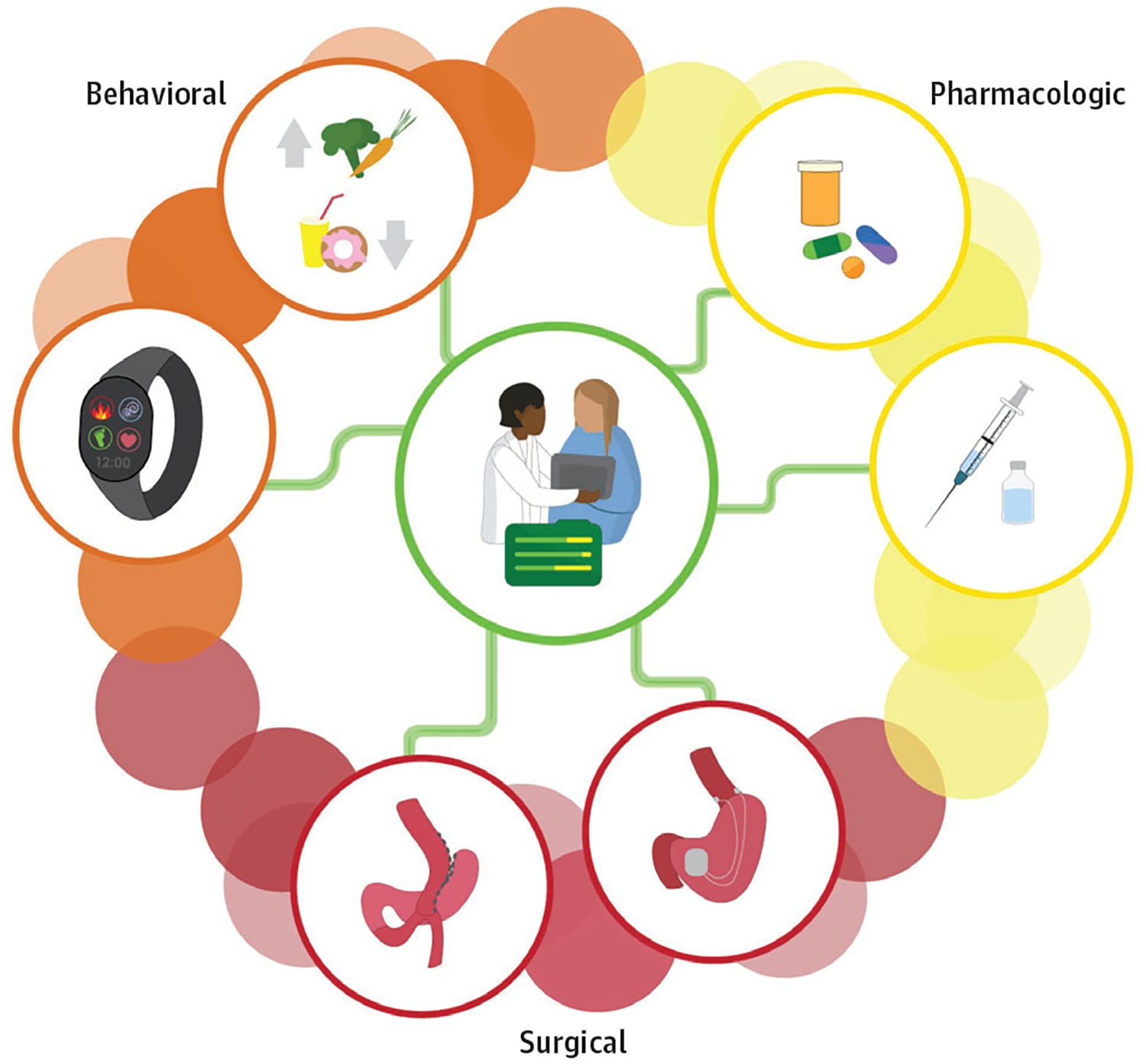
Treatment of obesity may include behavioral, pharmacologic, device, and/or surgical treatment modalities. These treatments should be viewed as an integrated continuum of care involving overlapping rather than chronological stages and begin with the least invasive yet appropriately intensive treatment. Choice of treatment(s) should be guided by the patient’s age, sex, pubertal status, severity of obesity, psychosocial factors, obesity-related complications, anticipated adaptations to fat mass, and patient and family preferences.
Future Directions for Multicomponent, Behavioral Interventions
Treatments have traditionally been grounded in CBT, where participants are encouraged to change thoughts and manage negative emotions with cognitive reframing and distraction.43 However, studies in adults suggest acceptance-based therapy (ABT), which helps align diet and physical activity changes to larger life values and emphasizes skills such as willingness to engage in values-consistent behavior, self-regulation, and mindfulness, may be more effective for weight loss than traditional behavioral therapy based in CBT.71 Given the adolescent period is uniquely characterized by changes in emotional and mental development that inhibit self-regulation and executive function,8 skills learned with ABT may be particularly relevant for adolescents. Future research should explore the effectiveness of ABT interventions for adolescent weight loss.
Moreover, given that significant variation exists by age in mental and emotional development as youths progress though early (ages 11–14years), middle (ages 15–17years), and late (ages 18–21 years) adolescence,8 the same intervention is unlikely to be effective across adolescence. Thus, interventions may be improved by addressing executive function changes that can affect risk-taking behaviors, self-regulation factors, and impulsivity.10 Other factors to consider in tailoring interventions can include social factors and the need for independence and autonomy8,9 It is difficult to compare effectiveness across studies because there is a lack of consistency in measures and outcome variables for adolescent weight loss interventions. In adults, standardization of measures is being encouraged by the National Institutes of Health Accumulating Data to Optimally Predict Obesity Treatment core measures project,72 and we recommend creating a similar initiative for pediatric populations. Additionally, relative to other chronic diseases, few treatment strategies for adolescent obesity exist. Many treatments remain underfunded by insurance payers and underused by health care clinicians. One way to address these issues is to shift research priorities to include implementation science methods to rigorously test interventions that have demonstrated effectiveness in randomized clinical trials but subsequently fail when implemented in clinical or community-based settings. However, to date, there has been little merging of implementation science in treatment of obesity With the use of implementation science and a focus on sustainable and easily disseminated obesity prevention and treatment programs, progress could accelerate toward addressing the obesity epidemic and subsequent effects of associated comorbidities.
Future Directions for Pharmacologic Interventions
Despite a lull in development of pharmacologic therapies for pediatric obesity during the last decade, it is possible at least 2 new medications will be available for pediatric use (ages 12 to 18 years) within the next 5 years. High-dose liraglutide (3 mg/d), a glucagonlike peptide-1 receptor agonist, is being evaluated in a large safety and efficacy clinical trial among youths with obesity (goal for regulatory review in 2020), while lower-dose liraglutide has already been approved for adolescents with type 2 diabetes. The combination of phentermine and topiramate is also being evaluated for safety and efficacy in adolescents with obesity in a clinical trial launched in 2019. Phentermine and topiramate did not receive approval by the European Medicines Agency for treatment of adult obesity so the intent of this ongoing pediatric trial is to obtain FDA approval. Both liraglutide and phentermine/topiramate have demonstrated a degree of weight reduction considered clinically meaningful in adults,73 which bodes well for potential effectiveness in adolescents. Two additional interventions are FDA approved for use in adults with obesity but remain untested in youths, including lorcaserin (selective serotonin receptor agonist) and the combination of naltrexone (opioid antagonist) and bupropion (norepinephrine and dopamine reuptake inhibitor). Another notable medication, owing to its superior efficacy is semaglutide (glucagonlike peptide-1 receptor agonist), which is currently in adult phase III trials. Adult phase II trials reported mean weight loss of approximately 10%.74
Future Directions for Surgical and Device Interventions
Although MBS is the most effective treatment for adolescent obesity in terms of weight reduction, it will likely remain reserved for the most severely affected patients having serious complications. It is estimated that at least 6 million adolescents in the US would meet clinical guidelines for MBS, yet fewer than 1000 get MBS each year.12,60,61 The percentage of pediatricians willing to refer patients to MBS and the number of patients with obesity (and their caregivers) opting to undergo MBS will likely remain low until substantial data on long-term safety and effectiveness has been generated. Insurers are often reluctant to pay for MBS for individuals younger than 18 years,61 which is partially driven by the relative paucity of long-term outcomes data. Yet the message from emerging data are clear: it is best to intervene on adolescents with severe obesity early in hopes to improve remission rates of type 2 diabetes and hypertension.60
Device therapy for pediatric obesity is potentially attractive owing to its reversibility Device interventions in the pediatric pipeline include the intragastric balloon, vagal blockade, and aspiration therapy As discussed earlier, studies using the intragastric balloon in adolescents have been small and uncontrolled,65–68 and more research is needed before recommendations can be made. Although, to our knowledge, no pediatric studies have been conducted using intra-abdominal vagal blockade, adult trials have demonstrated modest weight loss efficacy (control-subtracted weight loss of approximately 3%).75 Vagal blockade uses an implanted device to activate the vagus nerve to enhance satiety and decrease food intake,76 with the most common adverse effects being infection at the neuroregulator site, heartburn and dyspepsia, and abdominal pain. Aspiration therapy uses percutaneous gastrostomy to evacuate a portion of the stomach contents (approximately 30%) shortly following consumption of meals. To our knowledge, no pediatric trials have been reported, but the largest adult study demonstrated body weight reduction of approximately 8% (control-subtracted) at 1 year.77 The most commonly reported adverse effect was peristomal pain, but importantly, there were no significant changes in electrolyte and mineral levels or increased incidence of disordered eating behaviors. If the risk to benefit balance is deemed appropriate with future research, there may be a role for device therapy in certain patients.
Precision Obesity Medicine
Precision medicine seeks to identify factors that predict responses to various treatments, synthesize the information, and apply it to select tailored therapies designed to achieve optimal health outcomes while also minimizing patient risks. Although in its infancy, the field of precision obesity medicine could fundamentally change the outlook for millions of youths worldwide affected by obesity and its complications. In general, BMI reduction with all types of pediatric obesity interventions demonstrates a high degree of heterogeneity,24,78 yet some predictive factors are beginning to emerge. For example, early weight loss success, as soon as within 1 month, has been shown to differentiate youths who are more likely to experience clinically meaningful BMI reduction at 6 months and 1 year.24 Similarly, baseline disordered eating pathology and self-reported appetite have been identified as potential predictors of response to lifestyle modification and pharmacotherapy inyouths.79,80 In Figure 3, we identify potential opportunities for optimizing precision medicine in obesity care.
Figure 3. Precision Medicine for Obesity Care.
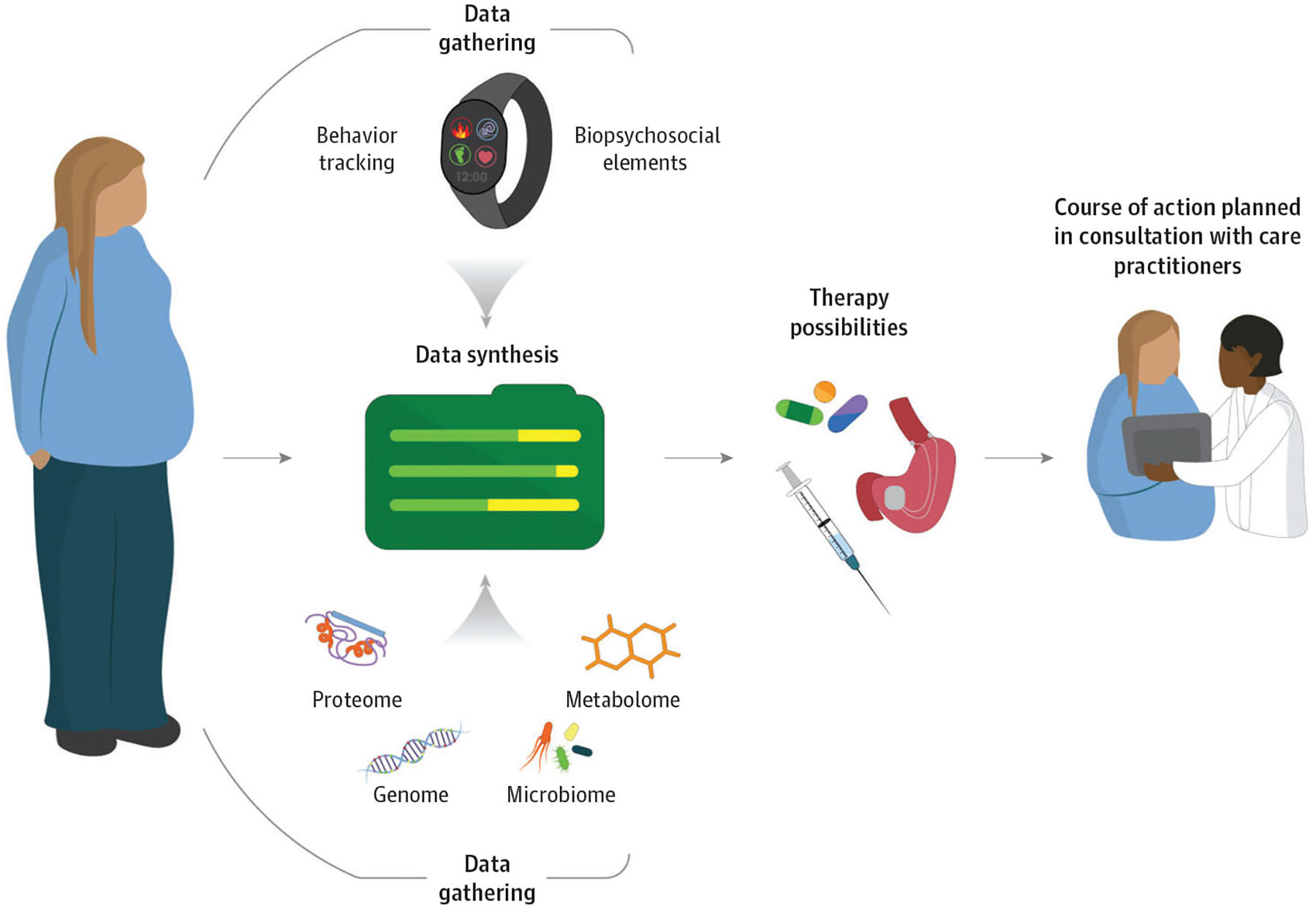
This figure outlines gaps and opportunities for future research aimed at developing more effective and targeted treatments for adolescents with obesity, based on a 2017 National Institutes of Health-sponsored workshop devoted to this topic.81 Numerous areas were identified as potentially strategic opportunities, including achieving consensus on appropriate body mass index metrics, development of valid measures of phenotypes and predictors, characterization of mechanisms associated with development of severe obesity, discovery of novel treatments informed by biologically and psychosocially plausible mechanisms, identification of biopsychosocial phenotypes predicting treatment response, standardization of outcome measures, and improving clinical care.81 An important goal will be to characterize physiologic information (eg, genetics, metabolomics, and microbiome) with biopsychosocial elements (eg, behavior, psychological factors, and social factors such as socioeconomic status), to synthesize the data into meaningful predictors to identify and maximize treatments with the most potential for a particular individual. As the knowledge base grows in these areas, tailored treatment strategies may be developed, rigorously evaluated, and ultimately translated to the clinical setting to enhance care delivery for adolescents living with obesity.
Conclusions
There are almost 15 million adolescents with overweight and obesity in the United States alone.1 There is no cure for obesity and, similar to other chronic diseases, it requires lifelong treatment.69 Thus, adolescent obesity is one of the most significant public health problems of our time. We recognize prevention and treatment of obesity on a global scale will not be possible in clinical settings alone. Changes must be made to social and cultural norms, across sectors (government, education, health care, marketing, and food and beverage industries), and in various settings (schools, worksites, and community). However, these recommendations are outside the scope of this review.
Adolescent obesity deserves the same resources, scientific rigor, and access to quality, lifelong care provided to other chronic diseases. Dedicated resources for development of effective and less invasive therapies and effective adjuncts (eg, pharmacotherapy and lifestyle programs) are critically needed. Additionally, an insufficient number of comprehensive centers offering specialized pediatric obesity treatment, limited obesity medicine specialists, and only a handful of accredited bariatric surgery centers in pediatric hospitals exist. Adolescent obesity care will likely need to be provided, in part, within primary care settings wherein clinicians offer diagnostic expertise, identify and treat complications of obesity, and, when appropriate, prescribe or refer for pharmacologic or surgical treatment options. However, trained case managers and specialists who can deliver behavioral interventions, such as registered dietitians and psychologists, will need to be a part of the medical team within primary care pediatric practices to implement behavioral intervention and assist with issues related to parenting, eating disorders, smoking and drug use, and medication adherence. Together, this underscores the critical need for additional research and obesity medicine training for all physicians in medical school and dedicated resources for behavior specialists to implement multicomponent interventions to treat adolescent obesity. As mounting evidence highlights the deleterious effects of excess adiposity during adolescence and beyond, it is our ethical duty to provide lifelong access to quality, evidence-based treatment for obesity.
Funding/Support:
Funding for research efforts related to preparation of the manuscript are provided by the National Institutes of Health (K01HL141535 to Dr Cardel; P01 AI42288to Dr Atkinson; UL1TR001427), the American Diabetes Association, and the Jeffrey Keene Family Professorship.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Kelly receives research support (drug and placebo) from AstraZeneca Pharmaceuticals and serves as a consultant for Novo Nordisk, Orexigen, Vivus Pharmaceuticals, and WW but does not accept personal or professional income for these activities. Dr Holm declares that Dr Holm Health Ltd and Dr Holm App Ltd provide education, prevention, and treatment of obesity.
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health, American Diabetes Association, or the Jeffrey Keene Family Professorship.
REFERENCES
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garnett SP, Baur LA, Jones AMD, Hardy LL. Trends in the prevalence of morbid and severe obesity in Australian children aged 7–15 years, 1985–2012. PLoS One. 2016;11(5):e0154879. doi: 10.1371/journal.pone.0154879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly AS, Barlow SE, Rao G, et al. ; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young, Council on Nutrition, Physical Activity and Metabolism, and Council on Clinical Cardiology. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3 [DOI] [PubMed] [Google Scholar]
- 4.Steinbeck KS, Lister NB, Gow ML, Baur LA. Treatment of adolescent obesity. Nat Rev Endocrinol. 2018;14(6):331–344. doi: 10.1038/s41574-018-0002-8 [DOI] [PubMed] [Google Scholar]
- 5.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365 (20):1876–1885. doi: 10.1056/NEJMoa1010112 [DOI] [PubMed] [Google Scholar]
- 6.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–1325. doi: 10.1056/NEJMoa1006992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger NA. Young adult cancer: influence of the obesity pandemic. Obesity (Silver Spring). 2018; 26(4):641–650. doi: 10.1002/oby.22137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan JF, Shaw JS, Duncan PM. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. Frove Village, IL: American Academy of Pediatrics; 2017. [Google Scholar]
- 9.Haynos AF, O’Donohue WT. Universal childhood and adolescent obesity prevention programs: review and critical analysis. Clin Psychol Rev. 2012; 32(5):383–399. doi: 10.1016/j.cpr.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 10.Braet C, Claus L, Verbeken S, Van Vlierberghe L. Impulsivity in overweight children. Eur Child Adolesc Psychiatry. 2007;16(8):473–483. doi: 10.1007/s00787-007-0623-2 [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Fakhouri TH, et al. Prevalence of obesity among youths by household income and education level of head of household: United States 2011–2014. MMWR Morb Mortal Wkly Rep. 2018;67(6):186–189. doi: 10.15585/mmwr.mm6706a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the united states, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. ; NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113): 2627–2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardel M, Lemas DJ, Lee AM, et al. D2 dopamine receptor (DRD2) Taq1a allele is associated with eating behavior and greater total and visceral adiposity in a racially diverse sample of children. Pediatric Obesity. 2012. [Google Scholar]
- 16.Cardel M, Willig AL, Dulin-Keita A, Casazza K, Beasley TM, Fernández JR. Parental feeding practices and socioeconomic status are associated with child adiposity in a multi-ethnic sample of children. Appetite. 2012;58(1):347–353. doi: 10.1016/j.appet.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardel MI, Johnson SL, Beck J, et al. The effects of experimentally manipulated social status on acute eating behavior: a randomized, crossover pilot study. Physiol Behav. 2016;162:93–101. doi: 10.1016/j.physbeh.2016.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardel MI, Tong S, Pavela G, et al. Youth subjective social status (SSS) is associated with parent SSS, income, and food insecurity but not weight loss among low-income Hispanic youth. Obesity (Silver Spring). 2018;26(12):1923–1930. doi: 10.1002/oby.22314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB. Obesity as a disease: the Obesity Society 2018 Position Statement. Obesity (Silver Spring). 2019;27(1):7–9. doi: 10.1002/oby.22378 [DOI] [PubMed] [Google Scholar]
- 20.Reinehr T Long-term effects of adolescent obesity: time to act. Nat Rev Endocrinol. 2018;14(3): 183–188. doi: 10.1038/nrendo.2017.147 [DOI] [PubMed] [Google Scholar]
- 21.Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–757. doi: 10.1210/jc.2017-00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardel M, Higgins PB, Willig AL, et al. African genetic admixture is associated with body composition and fat distribution in a cross-sectional study of children. Int J Obes (Lond). 2011;35(1):60–65. doi: 10.1038/ijo.2010.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong S, Lazorick S, Hampl S, et al. Physical examination findings among children and adolescents with obesity: an evidence-based review. Pediatrics. 2016;137(2):e20151766. doi: 10.1542/peds.2015-1766 [DOI] [PubMed] [Google Scholar]
- 24.Gross AC, Kaizer AM, Kelly AS, et al. ; POWER Work Group. Long and short of it: early response predicts longer-term outcomes in pediatric weight management. Obesity (Silver Spring). 2019;27(2): 272–279. doi: 10.1002/oby.22367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman DS, Butte NF, Taveras EM, et al. BMI z-scores are a poor indicator of adiposity among 2-to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring). 2017;25(4):739–746. doi: 10.1002/oby.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente-Rodríguez G, Rey-López JP, Mesana MI, et al. ; HELENA Study Group. Reliability and intermethod agreement for body fat assessment among two field and two laboratory methods in adolescents. Obesity (Silver Spring). 2012;20(1):221–228. doi: 10.1038/oby.2011.272 [DOI] [PubMed] [Google Scholar]
- 27.Puhl RM, Himmelstein MS. Adolescent preferences for weight terminology used by health care providers. Pediatr Obes. 2018;13(9):533–540. doi: 10.1111/ijpo.12275 [DOI] [PubMed] [Google Scholar]
- 28.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. ; US Preventive Services Task Force. Screening for obesity in children and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(23):2417–2426. doi: 10.1001/jama.2017.6803 [DOI] [PubMed] [Google Scholar]
- 29.Altman M, Wilfley DE. Evidence update on the treatment of overweight and obesity in children and adolescents. J Clin Child Adolesc Psychol. 2015; 44(4):521–537. doi: 10.1080/15374416.2014.963854 [DOI] [PubMed] [Google Scholar]
- 30.Wadden TA, Bray GA. Handbook of Obesity Treatment. 2nd ed New York, New York: The Guillard Press; 2018. [Google Scholar]
- 31.Kornet-van der Aa DA, Altenburg TM, van Randeraad-van der Zee CH, Chinapaw MJM. The effectiveness and promising strategies of obesity prevention and treatment programmes among adolescents from disadvantaged backgrounds: a systematic review. Obes Rev. 2017; 18(5):581–593. doi: 10.1111/obr.12519 [DOI] [PubMed] [Google Scholar]
- 32.Thomason DL, Lukkahatai N, Kawi J, Connelly K, Inouye J. A systematic review of adolescent self-management and weight loss. J Pediatr Health Care. 2016;30(6):569–582. doi: 10.1016/j.pedhc.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 33.Epstein LH, Squires S. The Stop light Diet for Children: An Eight-Week Program for Parents and Children. New York, NY: Little, Brown; 1988. [Google Scholar]
- 34.Ho M, Garnett SP, Baur L, et al. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130(6): e1647–e1671. doi: 10.1542/peds.2012-1176 [DOI] [PubMed] [Google Scholar]
- 35.Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, Lister NB. Treatment of obesity, with a dietary component, and eating disorder risk in children and adolescents: a systematic review with meta-analysis. Obes Rev. 2019;20:1287–1298. doi: 10.1111/obr.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jebeile H, Gow ML, Baur LA, Garnett SP, Paxton SJ, Lister NB. Association of pediatric obesity treatment, including a dietary component, with change in depression and anxiety: a systematic review and meta-analysis. JAMA Pediatr. 2019: e192841. doi: 10.1001/jamapediatrics.2019.2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner CD, Trepanowski JF, Del Gobbo LC, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–679. doi: 10.1001/jama.2018.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brock DW, Chandler-Laney PC, Alvarez JA, Gower BA, Gaesser GA, Hunter GR. Perception of exercise difficulty predicts weight regain in formerly overweight women. Obesity (Silver Spring). 2010;18 (5):982–986. doi: 10.1038/oby.2009.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gow ML, Baur LA, Johnson NA, Cowell CT, Garnett SP. Reversal of type 2 diabetes in youth who adhere to a very-low-energy diet: a pilot study. Diabetologia. 2017;60(3):406–415. doi: 10.1007/s00125-016-4163-5 [DOI] [PubMed] [Google Scholar]
- 40.Ho M, Garnett SP, Baur LA, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167(8):759–768. doi: 10.1001/jamapediatrics.2013.1453 [DOI] [PubMed] [Google Scholar]
- 41.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373–383. doi: 10.1037/0278-6133.13.5.373 [DOI] [PubMed] [Google Scholar]
- 42.Medicine ACoS. ACSM’s Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2017. [Google Scholar]
- 43.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011; 34(4):841–859. doi: 10.1016/j.psc.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baxter KA, Ware RS, Batch JA, Truby H. Predicting success: factors associated with weight change in obese youth undertaking a weight management program. Obes Res Clin Pract. 2013;7 (2):e147–e154. doi: 10.1016/j.orcp.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Carraway ME, Lutes LD, Crawford Y, et al. Camp-based immersion treatment for obese, low socioeconomic status, multi-ethnic adolescents. Child Obes. 2014;10(2):122–131. doi: 10.1089/chi.2013.0111 [DOI] [PubMed] [Google Scholar]
- 46.Germann JN, Kirschenbaum DS, Rich BH, O’Koon JC. Long-term evaluation of multi-disciplinary treatment of morbid obesity in low-income minority adolescents: La Rabida Children’s Hospital’s FitMatters program. J Adolesc Health. 2006;39(4):553–561. doi: 10.1016/j.jadohealth.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 47.Rudolf M, Christie D, McElhone S, et al. WATCH IT: a community based programme for obese children and adolescents. Arch Dis Child. 2006;91 (9):736–739. doi: 10.1136/adc.2005.089896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resnicow K, Yaroch AL, Davis A, et al. GO GIRLS!: results from a nutrition and physical activity program for low-income, overweight African American adolescent females. Health Educ Behav. 2000;27(5):616–631. doi: 10.1177/109019810002700507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl4):S164–S192. doi: 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 50.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873–2883. doi: 10.1001/jama.293.23.2873 [DOI] [PubMed] [Google Scholar]
- 51.Ryder JR, Kaizer A, Rudser KD, Gross A, Kelly AS, Fox CK. Effect of phentermine on weight reduction in a pediatric weight management clinic. Int J Obes (Lond). 2017;41(1):90–93. doi: 10.1038/ijo.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherafat-Kazemzadeh R, Yanovski SZ, Yanovski JA. Pharmacotherapy for childhood obesity: present and future prospects. Int J Obes (Lond). 2013;37(1):1–15. doi: 10.1038/ijo.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. Int J Obes (Lond). 2016;40(7): 1043–1050. doi: 10.1038/ijo.2016.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly AS, Fox CK. Pharmacotherapy in the management of pediatric obesity. Curr Diab Rep. 2017;17(8):55. doi: 10.1007/s11892-017-0886-z [DOI] [PubMed] [Google Scholar]
- 55.Ryder JR, Fox CK, Kelly AS. Treatment options for severe obesity in the pediatric population: current limitations and future opportunities. Obesity (Silver Spring). 2018;26(6):951–960. doi: 10.1002/oby.22196 [DOI] [PubMed] [Google Scholar]
- 56.Srivastava G, Fox CK, Kelly AS, et al. Clinical considerations regarding the use of obesity pharmacotherapy in adolescents with obesity Obesity (Silver Spring). 2019;27(2):190–204. doi: 10.1002/oby.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inge TH, Courcoulas AP, Jenkins TM, et al. ; Teen-LABS Consortium. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. 2016;374(2):113–123. doi: 10.1056/NEJMoa1506699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inge TH, Jenkins TM, Xanthakos SA, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017;5(3):165–173. doi: 10.1016/S2213-8587(16)30315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5(3):174–183. doi: 10.1016/S2213-8587(16)30424-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inge TH, Courcoulas AP, Jenkins TM, et al. ; Teen-LABS Consortium. Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med. 2019;380(22):2136–2145. doi: 10.1056/NEJMoa1813909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inge TH, Boyce TW, Lee M, et al. Access to care for adolescents seeking weight loss surgery. Obesity (Silver Spring). 2014;22(12):2593–2597. doi: 10.1002/oby.20898 [DOI] [PubMed] [Google Scholar]
- 62.Kelly AS, Ryder JR, Marlatt KL, Rudser KD, Jenkins T, Inge TH. Changes in inflammation, oxidative stress and adipokines following bariatric surgery among adolescents with severe obesity. Int J Obes (Lond). 2016;40(2):275–280. doi: 10.1038/ijo.2015.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryder JR, Edwards NM, Gupta R, et al. Changes in functional mobility and musculoskeletal pain after bariatric surgery in teens with severe obesity: teen-Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Pediatr. 2016;170(9):871–877. doi: 10.1001/jamapediatrics.2016.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pratt JSA, Browne A, Browne NT, et al. ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis. 2018;14(7):882–901. doi: 10.1016/j.soard.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Peppo F, Caccamo R, Adorisio O, et al. The Obalon swallowable intragastric balloon in pediatric and adolescent morbid obesity. Endosc Int Open. 2017;5(1):E59–E63. doi: 10.1055/s-0042-120413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fittipaldi-Fernandez RJ, Guedes MR, Galvao Neto MP, Klein MRST, Diestel CF. Efficacy of intragastric balloon treatment for adolescent obesity. Obes Surg. 2017;27(10):2546–2551. doi: 10.1007/s11695-017-2699-1 [DOI] [PubMed] [Google Scholar]
- 67.Nobili V, Della Corte C, Liccardo D, et al. Obalon intragastric balloon in the treatment of paediatric obesity: a pilot study. Pediatr Obes. 2015;10(5):e1–e4. doi: 10.1111/ijpo.268 [DOI] [PubMed] [Google Scholar]
- 68.Reece LJ, Sachdev P, Copeland RJ, Thomson M, Wales JK, Wright NP. Intra-gastric balloon as an adjunct to lifestyle support in severely obese adolescents; impact on weight, physical activity, cardiorespiratory fitness and psychosocial well-being. Int J Obes (Lond). 2017;41(4):591–597. doi: 10.1038/ijo.2016.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardel MI, Jastreboff AM, Kelly AS. Treatment of adolescent obesity in 2020. JAMA. 2019. doi: 10.1001/jama.2019.14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26(5):521–532. doi: 10.1037/0278-6133.26.5.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forman EM, Butryn ML, Manasse SM, et al. Acceptance-based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity (Silver Spring). 2016;24(10):2050–2056. doi: 10.1002/oby.21601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacLean PS, Rothman AJ, Nicastro HL, et al. The Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) core measures project: rationale and approach. Obesity (Silver Spring). 2018;26(S2)(suppl 2):S6–S15. doi: 10.1002/oby.22154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016; 315(22):2424–2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649. doi: 10.1016/S0140-6736(18)31773-2 [DOI] [PubMed] [Google Scholar]
- 75.Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312(9):915–922.doi: 10.1001/jama.2014.10540 [DOI] [PubMed] [Google Scholar]
- 76.Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143(6):723–731. doi: 10.1016/j.surg.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 77.Thompson CC, Abu Dayyeh BK, Kushner R, et al. Percutaneous gastrostomy device for the treatment of class ii and class iii obesity: results of a randomized controlled trial. Am J Gastroenterol. 2017;112(3):447–457.doi: 10.1038/ajg.2016.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryder JR, Kaizer AM, Jenkins TM, Kelly AS, Inge TH, Shaibi GQ. Heterogeneity in response to treatment of adolescents with severe obesity: the need for precision obesity medicine. Obesity (Silver Spring). 2019;27(2):288–294. doi: 10.1002/oby.22369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balantekin KN, Hayes JF, Sheinbein DH, et al. Patterns of eating disorder pathology are associated with weight change in family-based behavioral obesity treatment. Obesity (Silver Spring). 2017;25(12):2115–2122.doi: 10.1002/oby.22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nathan BM, Rudser KD, Abuzzahab MJ, et al. Predictors of weight-loss response with glucagon-like peptide-1 receptor agonist treatment among adolescents with severe obesity. Clin Obes. 2016;6(1):73–78. doi: 10.1111/cob.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly AS, Marcus MD, Yanovski JA, Yanovski SZ, Osganian SK. Working toward precision medicine approaches to treat severe obesity in adolescents: report of an NIH workshop. Int J Obes (Lond). 2018; 42(11):1834–1844. doi: 10.1038/s41366-018-0231-x [DOI] [PMC free article] [PubMed] [Google Scholar]


