Abstract
Rationale:
Chronic exposure to hypoxia is associated with elevated sympathetic nervous activity (SNA) and reduced vascular function in lowlanders, and Andean highlanders suffering from excessive erythrocytosis (EE); however, the mechanistic-link between chronically elevated SNA and hypoxia-induced vascular dysfunction has not been determined.
Objective:
To determine the impact of heightened SNA on resistance artery endothelial-dependent dilation (EDD), and endothelial-independent dilation (EID), in lowlanders and Andean highlanders with and without EE.
Methods and Results:
We tested healthy lowlanders (n=9) at sea-level (344m) and following 14–21 days at high-altitude (4300 m), and permanent Andean highlanders with (n=6) and without (n=9) EE at high-altitude. Vascular function was assessed using intra-arterial infusions (three progressive doses) of acetylcholine (ACh; EDD) and sodium nitroprusside (SNP; EID) before and after local α+β adrenergic receptor blockade (phentolamine and propranolol). Intra-arterial blood pressure, heart rate, and simultaneous brachial artery diameter and blood velocity were recorded at rest and during drug infusion. Changes in forearm vascular conductance (ΔFVC) were calculated. The main findings were: 1) chronic hypoxia reduced EDD in lowlanders (ΔFVC from sea-level: ACh1: −52.7±19.6%, ACh2: −25.4±38.7%, ACh3: −35.1±34.7%, all P≤0.02); and in Andeans with EE compared to non-EE (ΔFVC at ACh3: −36.4%, P=0.007). Adrenergic blockade fully restored EDD in lowlanders at high-altitude, and normalized EDD between EE and non-EE Andeans. 2) Chronic hypoxia had no effect on EID in lowlanders, and no differences were detected between EE and non-EE Andeans; however, EID was increased in the non-EE Andeans after adrenergic blockade (P=0.012), but this effect was not observed in the EE Andeans.
Conclusions:
These data indicate that chronic hypoxia reduces EDD via heightened α-adrenergic signaling in lowlanders and in Andeans with EE. These vascular mechanisms have important implications for understanding the physiological consequences of acute and chronic high-altitude adaptation.
Keywords: Hypoxia, altitude acclimatization, endothelial function, sympathetic nervous system, chronic mountain sickness, autonomic nervous system, endothelium
Subject Terms: Autonomic Nervous System, Vascular Disease
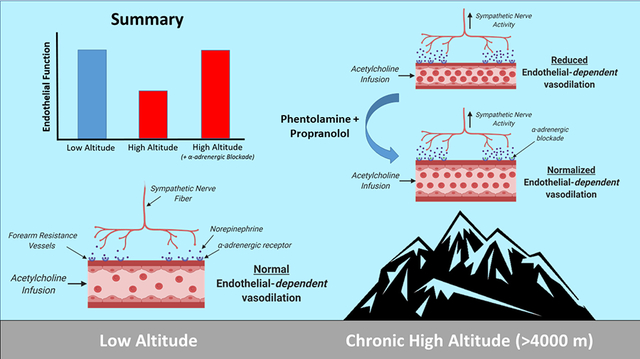
Graphical Abstract
Brachial artery vascular function was quantified at sea level, and after acclimatization to high altitude (4300 m; Cerro de Pasco, Peru), using forearm intra-arterial infusions of vasoactive drugs (acetylcholine, endothelial-dependent vasodilation; sodium nitroprusside, endothelial-independent vasodilation) before and after forearm adrenergic blockade (phentolamine, non-selective α-adrenergic blockade; propranolol, non-selective β-adrenergic blockade). The primary main finding was that high altitude exposure elevated muscle sympathetic nervous activity and reduced endothelial-dependent function, which was fully restored after adrenergic blockade in lowlanders (n=9). The same protocol was repeated in Andean highlanders with (n=6) and without (n=9) excessive erythrocytosis (defined as hemoglobin >21 g/dl in males). Andeans with excessive erythrocytosis had elevated muscle sympathetic nervous activity and reduced endothelial-dependent function compared to their healthy counterparts, but vascular function was normalized between groups after forearm adrenergic blockade. These data establish the direct-link between heightened neural activity and reduced endothelial-dependent function at high altitude.
INTRODUCTION
Exposure to acute hypoxia induces several vascular and autonomic-related adjustments resulting in increased signaling of local vasodilatory substances1, 2, and increased sympathetic nervous activity3, 4. Local vasodilatory signaling including the production of nitric oxide and prostaglandins5, ATP6, 7, and the activation of inwardly-rectifying potassium channels8, serve to increase blood flow and oxygen delivery to metabolically active tissues. At the same time, the dramatic rise in sympathetic nervous system activity counteracts and restrains the local vasodilatory response to hypoxia. The complex interplay between local vasodilatory signaling and sympathetic nervous activity signalling results in mild systemic vasodilation that serves to ensure proper blood flow and oxygen delivery to peripheral tissues. However, chronic exposure to severe hypoxia (e.g. high altitude, >3000 m) results in sustained increases in sympathetic nervous activity9–11, reduced conduit artery endothelial-dependent dilation10, 12, 13, and reduced conduit artery endothelial-independent dilation12. Importantly, exaggerated sympathetic nervous activity and impaired endothelial function are primary pathophysiological characteristics of many cardiopulmonary diseases characterized by sustained or intermittent hypoxia, such as: heart failure14, chronic obstructive pulmonary disease15, and sleep apnea16.
The key mechanism(s) responsible for the development of arterial dysfunction with long-term exposure to severe hypoxia are not well understood, but is likely multifactorial and includes elevated oxidative stress17–19, changes in hemodynamics such as shear stress and blood pressure13, 20, 21, and elevations in sympathetic nervous activity22. Investigations on the mechanism(s) of chronic hypoxemia induced arterial dysfunction have been conducted almost exclusively in large elastic arteries which serve as structural conduits for blood flow, as opposed to resistance arteries which are most directly responsible for sensing alterations in oxygen availability and sympathetic vasoconstrictor signaling23, 24. To date, limited evidence indicates that acute increases in sympathetic nervous activity are associated with reduced conduit artery endothelial-dependent dilation at sea level22, 25, 26; however, the mechanistic link between chronically elevated sympathetic nervous activity and endothelial-dependent dilation in lowlanders exposed to chronic hypoxemia, and in highlander residents, remains uncertain.
Considerably less is known regarding the impact of permanent living at high altitude on vascular function, but it is well established that indigenous populations (e.g. Tibetans, Peruvians, Ethiopians) are at risk of developing high altitude illness. The Andean plateau has been populated for at least 7,000 years27, 28, and up to ~30% of long-term high altitude male residents in the Cerro de Pasco region, Peru, are prone to suffering from chronic mountain sickness29, 30. Although chronic mountain sickness is prevalent within other high altitude populations such as Han Chinese, a recent meta-analysis demonstrated that Andean highlanders have consistently higher concentrations of hemoglobin and hematocrit31. Chronic mountain sickness is characterized by an excessive production of red blood cells [i.e. excessive erythrocytosis (i.e. EE)], which potentially leads to more severe hypoxemia and greater cardiovascular disease risk32, 33. A previous investigation conducted in Andean highlanders demonstrated that Andeans with EE have impaired conduit artery endothelial-dependent dilation (i.e. reduced brachial artery flow-mediated dilation) compared to healthy Andean highlanders, despite preserved endothelial-independent dilation (assessed via sublingual nitroglycerin)34. However, no studies have examined the underlying mechanism(s) related to microvascular endothelial-dependent dilation in Andeans with and without EE. Interestingly, Andean highlanders with EE have exaggerated plasma norepinephrine compared to non-EE Andeans – indicating that sympathetic nervous activity is elevated compared to their healthy counterparts35, but this has yet to be confirmed via microneurography (i.e. direct recording of muscle sympathetic nervous activity). It is currently unknown whether the observed reductions in endothelial-dependent dilation in EE Andean highlanders are mediated via exaggerated sympathetic nervous activity.
The primary purpose of the current investigation was to determine if vascular dysfunction induced by chronic hypoxemia is due to heightened adrenergic signalling in lowlanders, and in Andean highlanders with EE. We hypothesized that: 1) resistance artery endothelial-dependent dilation, would be reduced at high altitude in lowlanders compared to sea level, and Andean highlanders with EE compared to healthy Andeans, and this impairment would be ameliorated after forearm adrenergic blockade; and 2) resistance artery endothelial-independent dilation would be reduced in lowlanders at high altitude compared to sea level, and there would be no differences observed between EE and non-EE Andeans. To test these hypotheses, we administered graded intra-arterial infusions of acetylcholine (ACh; endothelium-dependent vasodilator) and sodium nitroprusside (SNP; endothelium-independent vasodilator) before and after local forearm adrenergic blockade (phentolamine and propranolol).
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical approval.
The Clinical Research Ethics Board at the University of British Columbia (ethics H17–02687 and H18–01404), and the Universidad Peruana Cayetano Heredia Comité de Ética (ethics #101686) approved all experimental procedures and protocols in adherence with the principles of the Declaration of Helsinki (except registration in a database). All participants provided written informed consent before participation in this study. Andean participants were provided with a translated consent form and a Spanish translator thoroughly explained the experimental protocol prior to consent. This investigation was part of a larger research expedition conducted between March and July 2018. Therefore, all participants underwent extensive physiological assessments at the Unviersity of British Columbia (Kelowna, British Columbia; 344 m) and during ~three weeks at a high altitude laboratory located in Cerro de Pasco, Peru (4300 m), but at least eight hours of recovery (and total drug washout if applicable; i.e. five half-lives) from other assessments was required prior to participation in this study.
Participants.
Lowlander participants (n=11; all male) were normotensive (systolic blood pressure <140 and diastolic blood pressure <90 mmHg) at rest, and were asked to complete a medical history questionnaire. Because female research volunteers were prioritized for participation in separate studies focused on sex differences and altitude acclimatization, and chronic mountain sickness preferentially affects Andean males32, all participants in this investigation were male. Participants with history of smoking, cardiovascular, cerebrovascular, or respiratory disease were avoided. One lowlander participant was excluded from data analysis due to equipment malfunction at sea level, and a second lowlander declined to participate in the experiment at high altitude. All lowlander participants arrived in Cerro de Pasco, Peru (4300 m) within a three-day period, and were tested between 14–21 days after arrival. Upon ascent, all participants refrained from prophylactic use of oral acetazolamide (i.e., Diamox); however, medical personnel instructed two participants to take acetazolamide briefly for the treatment of acute mountain illness symptoms within the first week of arrival in Cerro de Pasco. In these two cases, experimentation was completed outside of the drug elimination time of acetazolamide. In addition, participants were asked to avoid using aspirin, non-steroidal anti-inflammatory, and phosphodiesterase-5 inhibitors (e.g. sildenafil) for at least 24 hours prior to experimentation, and no participants in this study were administered corticosteroids (e.g. dexamethasone). Andean participants who were born, permanently living in the Cerro de Pasco region, and have no history of working in the mining industry were recruited for the study using a pre-existing database. Andean participants (n=15; 6 diagnosed with EE) were not taking any medications, and after consent was obtained, a venous blood sample was utilized to diagnose EE, which was defined as having a hemoglobin [Hb] >21.0 g/dl. Chronic mountain sickness was determined using the Qinghai chronic mountain sickness score36. A score of zero (i.e. absent), to three (i.e. severe), was assigned for the following signs and symptoms: breathlessness/palpitations, sleep disturbance, cyanosis, venodilation, paresthesia, headache, and tinnitus. The sum of the score for each symptom and EE defines chronic mountain sickness severity as absent (0–5), mild (6–10), moderate (11–14), and severe (≥ 15)36. Blood pressure data during the intra-arterial ACh and SNP infusion was absent in two Andean participants (one EE, and one non-EE) due to equipment malfunction; therefore, an estimation of the blood pressure response within these two individuals to ACh and SNP was calculated based on the group mean average. Interpolating these data points did not alter our study findings.
Experimental design.
To address our research questions, we conducted two protocols: Protocol A: lowlanders at sea level vs. high altitude, and Protocol B: healthy Andean highlanders vs. Andeans with EE (see figure 1). Prior to each experiment, all participants avoided alcohol, caffeine, and tea (e.g. regular and coca tea) for at least 12-hours. Additionally, participants were asked to consume a light meal at least two-hours prior to experimentation.
Figure 1:
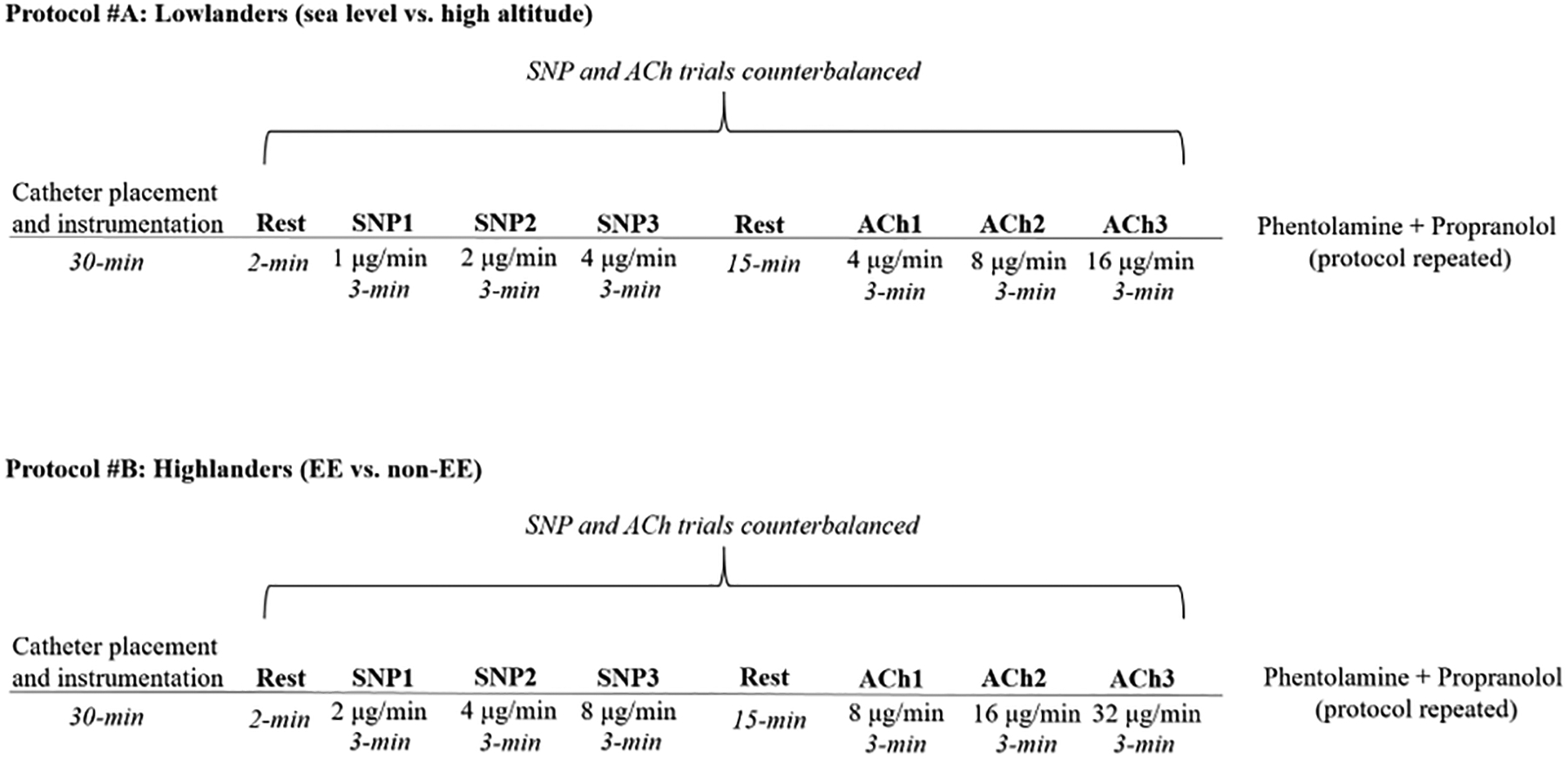
Schematic of experimental design.
General procedures.
After local anesthesia (2% lidocaine), a 20-gauge, 7.6 cm catheter (Arrow International, Reading, PA) was inserted retrograde into the brachial artery using ultrasound guidance and a modified Seldinger technique. The catheter was connected to an arterial blood sampling kit (VAMP Adult, Edwards Lifescience, Irvine, CA), for repeated blood sampling and flushing with 0.9% saline. The VAMP blood sampling kit was connected to a blood pressure amplifier (ADInstruments, FE117, Colorado Springs, USA), and was calibrated (i.e. zeroed) at the level of the fourth intercostal space for continuous blood pressure recording. Participants were also instrumented with a three-lead electrocardiogram connected to an amplifier to calculate heart rate (i.e. HR; ADInstruments, FE231, Colorado Springs, USA). Resting arterial blood samples (~1.5 ml) were collected in preheparanized syringes (safePICO syringes, Radiometer, Copenhagen, Denmark), and blood analysis (PaO2, PaCO2, SaO2) was performed within 30 minutes of blood sampling using an analyzer (ABL90 FLEX, Radiometer). The blood analyzer was calibrated at a minimum of every eight hours using manufacturer’s standard internal quality checks. Participants were resting in a semi-recumbent position throughout testing, and after brachial artery catheterization, ~30 minutes of quiet rest was completed to ensure any positional related shifts in blood volume were complete prior to experimentation.
Microneurography.
Muscle sympathetic nerve activity was obtained in the radial nerve by inserting a Tungsten microelectrode into a muscle nerve fascicle of a sympathetic nerve bundle, and a reference electrode subcutaneously 2–3 cm from the recording electrode37. This technique was conducted by the same experienced microneurographer (G.M.). Neural signals were collected using commercially available recording systems (Neuro AMP EX FE185, ADInstruments, Colorado Springs, CO, USA). The nerve signals were amplified (gain 70 000–160 000), band-pass filtered (700–2000 Hz), full-wave rectified, and integrated with a resistance-capacitance circuit (time constant 0.1 s). Criteria for adequate Muscle sympathetic nerve activity recording included: (1) pulse synchrony, (2) facilitation during the hypotensive phase of the Valsalva maneuver, and suppression during the hypertensive overshoot after release, (3) increases in response to breath holding, and (4) insensitivity to a gentle skin touch or a loud shout38. Earphones were used during microneurography searching to reduce distraction related to ambient noise. High-quality microneurography signals were obtained in seven lowlanders at sea level and high altitude (same participants at each time-point), nine non-EE Andean highlanders, and five EE Andean highlanders.
Vasoactive drug infusion.
Heart rate, blood pressure, and brachial artery diameter and blood velocity were measured at rest and during graded intra-arterial infusions of either ACh (to assess endothelial-dependent dilation) or SNP (to assess endothelial-independent dilation; see the Major Resources Table in the Supplemental Materials for information on these drugs). The infusions were counterbalanced across subjects and administered for three-minutes at three separate doses using 60 cc syringes secured onto a syringe pump (Harvard apparatus, PHD Ultra, Holliston, MA, USA). In lowlanders (i.e. protocol A), ACh was infused at 4.0, 8.0, 16.0 μg/100 ml of forearm tissue/min, and SNP at 1.0, 2.0, 4.0 μg/100 ml forearm tissue/min. In Andean highlanders (i.e. protocol B) ACh was infused at 8.0, 16.0, 32.0 μg/100 ml of forearm tissue/min, and SNP at 2.0, 4.0, 8.0 μg/100 ml forearm tissue/min (see figure 1). Participants had a 15-minute recovery period for drug washout between ACh and SNP trials. After the ACh and SNP trials were completed, phentolamine and propranolol [1000 μg over 5-minutes (loading dose), and 50 μg/min for 10-minutes (maintenance dose)] were infused to achieve local forearm adrenergic blockade. Heightened β-adrenergic activity was not expected to impair endothelial function or contribute dramatically to ACh/SNP mediated vasodilation, but was eliminated to avoid norepinephrine-mediated β-adrenergic vasodilation39, 40.
Experimental measurements.
Forearm blood flow and conductance.
The participants left arm was extended perpendicular and was fixed into position on a table at the level of the heart. Brachial artery image acquisition was obtained using a linear array probe attached to a high-resolution ultrasound machine (Vivid 7, General Electric, Milwaukee, WI, USA) to determine brachial artery mean blood velocity and diameter as previously described41. All brachial artery images were collected by the same experienced ultrasonographer42–44 (C.M.H; intra-observer coefficient of variability <3%), and all analysis was performed off-line and the conditions were blinded to the observer. The probe insonation angle was maintained at <60 degrees and frequency was set at five MHz. Brachial artery diameter was measured in triplicate during end-diastole during the last 30 seconds of each drug stage. Forearm blood flow was calculated as: forearm blood flow (ml/min) = mean blood velocity (cm/s) × π [brachial artery diameter (cm) / 2]^2 × 60. Forearm vascular conductance was calculated as [forearm blood flow (ml/min) / mean arterial pressure (MAP)] × 100, and expressed as ml/min/100 mmHg.
Statistics.
All statistical analyses were performed using SigmaStat V13 (Systat, Chicago, IL) and were reported as means ± SD. Statistical significance was set at P<0.05. Data normality was confirmed using a Shapiro-Wilks test. Paired and un-paired t-tests were used to detect changes in resting variables in lowlanders (sea level vs. high altitude) and highlanders (non-EE vs EE; see table 1 and figure 2), respectively. Two-way repeated measures (lowlanders: Drug dose x Pre/Post blockade, and Drug dose x Altitude) and two-way mixed (highlanders: Drug dose x Pre/Post blockade, and Drug dose x EE/non-EE) analysis of variance were used to detect any differences in systemic and forearm hemodynamics (see tables 2 and 3, and figures 3 and 4). Two separate two-way analysis of variance designs were utilized for both lowlanders and highlanders due to the large shift in resting forearm blood flow after adrenergic blockade. An analysis of covariance was utilized to assess whether the significant differences in body mass index between Andeans with and without EE influenced the forearm conductance response to ACh. When significant F-ratios were detected, post-hoc comparisons were made using a Tukey test. Appropriate adjustment for multiple comparisons was only conducted for within-test models.
Table 1:
Lowlander and highlander participant characteristics
| LL Sea level (n=9) | LL High altitude (n=9) | Non-EE Highlanders (n=9) | EE Highlanders (n=6) | |
|---|---|---|---|---|
| Age (yrs) | 26.3 ± 2.9 | - | 43.8 ± 15.3 | 47.0 ± 7.8 |
| Height (cm) | 177.3 ± 6.1 | - | 158.8 ± 4.4 | 164.2 ± 3.4┼ |
| Weight (kg) | 76.0 ± 9.2 | - | 62.2 ± 6.8 | 80.2 ± 7.0┼ |
| Body mass index (kg/m2) | 24.1 ± 1.9 | - | 24.7 ± 2.9 | 29.8 ± 2.5┼ |
| Forearm volume (ml) | 871.4 ± 158.9 | - | 791.0 ± 146.8 | 1040.8 ± 118.3┼ |
| Hb (g/dl) | 15.1 ± 0.8 | 17.8 ± 0.6* | 18.7 ± 1.5 | 22.5 ± 1.2┼ |
| PaO2 (mmHg) | 96.6 ± 5.4 | 56.1 ± 5.0* | 48.0 ± 7.4 | 46.0 ± 2.4 |
| PaCO2 (mmHg) | 37.8 ± 1.8 | 27.0 ± 3.6* | 29.2 ± 3.9 | 32.3 ± 1.0 |
| SaO2 (%) | 98.0 ± 0.4 | 89.7 ± 2.2* | 86.0 ± 3.5 | 81.3 ± 2.3┼ |
| CMS Score | - | - | 1.6 ± 1.0 | 6.0 ± 1.8┼ |
List of Abbreviations: cm, centimeters; CMS, chronic mountain sickness; dl, deciliter; EE, excessive erythrocytosis; g, grams; kg, kilograms; LL, lowlander; ml, milliliter; mmHg, millimeters of mercury; PaCO2, partial pressure of arterial carbon dioxide; PaO2, partial pressure of arterial oxygen; SaO2, arterial hemoglobin saturation of oxygen; yrs, years.
P<0.05, high altitude vs. sea-level.
P<0.05, EE vs. non-EE. Paired and un-paired t-tests were used to detect changes in resting variables in lowlanders (sea level vs. high altitude) and highlanders (non-EE vs EE)
Figure 2: Muscle sympathetic nervous activity in lowlanders at sea level and high altitude, and Andean highlanders at high altitude.
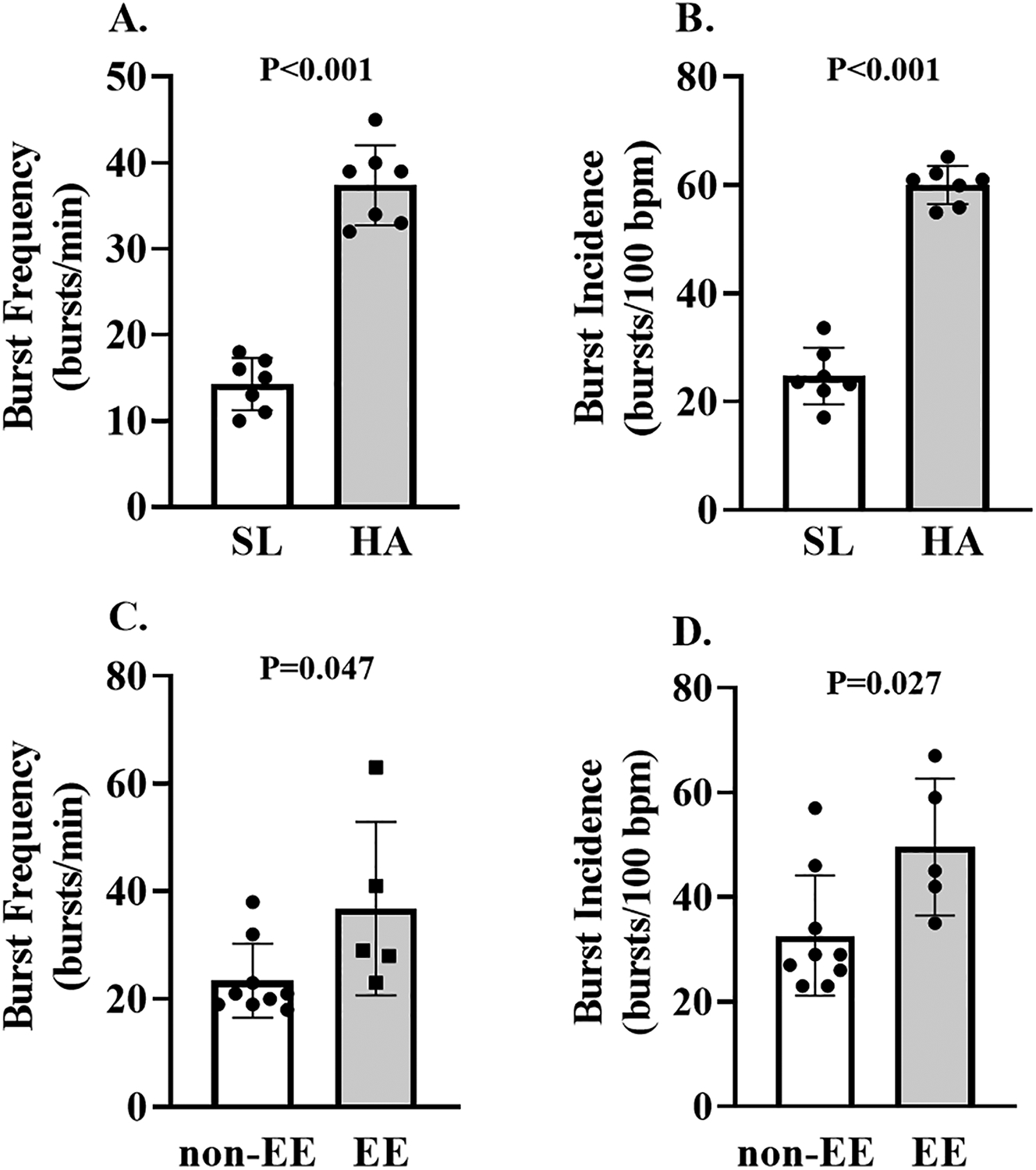
Individual and mean data displaying the impact of high altitude on resting muscle sympathetic burst frequency and incidence in lowlanders are presented on panels A and B. Individual and mean data comparing resting muscle sympathetic nerve burst frequency and incidence between EE and non-EE Andeans are presented on panels C and D. P-values are displayed on each figure panel. Paired and un-paired t-tests were used to detect changes in resting variables in lowlanders (sea level vs. high altitude) and highlanders (non-EE vs EE).
Table 2:
Systemic hemodynamics in lowlanders at sea level and high altitude.
| Control | Adrenergic Blockade | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | SNP1 | SNP2 | SNP3 | Rest | SNP1 | SNP2 | SNP3 | ||
| LL Sea level | MAP (mmHg) | 89.3 ± 11.8 | 86.1 ± 9.6 | 85.0 ± 9.1* | 81.5 ± 7.8* | 89.9 ± 8.5 | 88.2 ± 10.6 | 85.9 ± 10.7* | 82.2 ± 11.8* |
| HR (bpm) | 64.9 ± 11.2 | 66.0 ± 12.7 | 67.5 ± 12.0 | 69.3 ± 10.9* | 55.2 ± 9.1┼ | 57.4 ± 10.3┼ | 57.4 ± 12.2┼ | 63.5 ± 10.8┼* | |
| LL High Altitude | MAP (mmHg) | 94.1 ± 9.2 | 89.7 ± 9.2 | 90.4± 8.2 | 87.8 ± 7.7* | 92.3 ± 13.9 | 93.5 ± 12.1 | 92.3 ± 11.3 | 91.8 ± 9.4 |
| HR (bpm) | 75.2 ± 12.7 | 75.9 ± 15.0 | 75.3 ± 14.2 | 76.8 ± 12.4 | 66.3 ± 8.9┼ | 69.5 ± 9.4┼ | 71.2 ± 10.2┼ | 68.9 ± 13.6┼ | |
| Rest | ACH1 | ACH2 | ACH3 | Rest | ACH1 | ACH2 | ACH3 | ||
| LL Sea level | MAP (mmHg) | 88.8 ± 9.1 | 87.2 ± 8.0 | 88.2 ± 8.5 | 87.2 ± 8.3 | 88.8 ± 8.8 | 87.3 ± 9.8 | 86.8± 8.3 | 86.6 ± 9.1 |
| HR (bpm) | 66.0 ± 12.7 | 67.5 ± 12.7 | 67.8 ± 11.5 | 70.4 ± 11.3* | 55.3 ± 12.8┼ | 55.9 ± 12.2┼ | 55.8 ± 11.4┼ | 59.9 ± 9.5┼* | |
| LL High Altitude | MAP (mmHg) | 94.1 ± 9.0 | 93.2 ± 9.3 | 91.7 ± 7.8 | 89.6 ± 8.2* | 91.8 ± 12.3 | 90.0 ± 13.9 | 92.2 ± 11.9 | 89.5 ± 11.6* |
| HR (bpm) | 78.6 ± 7.5 | 78.3 ± 12.4 | 81.2 ± 15.4 | 79.9 ± 10.3 | 66.1 ± 8.7┼ | 63.7 ± 9.1┼ | 67.6 ± 11.8┼ | 69.7 ± 9.0┼ | |
List of Abbreviations: ACH, acetylcholine; HR, heart rate; LL, lowlander; MAP, mean arterial pressure; SNP, sodium nitroprusside.
P<0.05, vs Rest.
P<0.05, vs control. Two-way repeated measures (lowlanders: Drug dose x Pre/Post blockade, and Drug dose x Altitude) analysis of variance were used to detect any differences in systemic and forearm hemodynamics.
Table 3:
Systemic hemodynamics in highlanders at high altitude.
| Control | Adrenergic Blockade | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | SNP1 | SNP2 | SNP3 | Rest | SNP1 | SNP2 | SNP3 | ||
| EE | MAP (mmHg) | 91.4 ± 4.5 | 87.8 ± 5.0 | 83.0 ± 4.0* | 76.5 ± 4.4* | 87.5 ± 10.4 | 83.2 ± 7.7 | 83.6 ± 6.6 | 82.5 ± 7.0 |
| HR (bpm) | 81.0 ± 13.5 | 80.9 ± 14.4 | 86.3 ± 12.4* | 91.3 ± 9.0* | 71.9 ± 11.8┼ | 75.1 ± 11.5┼ | 82.2 ± 10.0┼* | 84.0 ± 8.5┼* | |
| Non-EE | MAP (mmHg) | 95.5 ± 12.3 | 91.2 ± 9.6* | 88.0 ± 11.4* | 85.4 ± 8.1* | 95.9 ± 12.1 | 91.3 ± 11.9* | 88.3 ± 11.0* | 84.9 ± 10.7* |
| HR (bpm) | 79.6 ± 11.3 | 81.3 ± 12.1 | 85.0 ± 11.2* | 87.5 ± 11.9* | 69.1 ± 12.5┼ | 70.4 ± 11.1┼ | 76.6 ± 14.2┼* | 78.8 ± 15.1┼* | |
| Rest | ACH1 | ACH2 | ACH3 | Rest | ACH1 | ACH2 | ACH3 | ||
| EE | MAP (mmHg) | 91.5 ± 3.8 | 90.9 ± 4.6 | 91.6 ± 3.0 | 90.0 ± 2.9 | 86.0 ± 9.6 | 89.1 ± 10.1 | 88.0 ± 10.5 | 88.3 ± 10.1 |
| HR (bpm) | 81.6 ± 10.7 | 81.0 ± 10.4 | 79.9 ± 11.3 | 78.6 ± 10.6* | 70.6 ± 9.0┼ | 73.1 ± 9.9┼* | 72.1 ± 10.6┼ | 71.1 ± 12.1┼ | |
| Non-EE | MAP (mmHg) | 96.3 ± 12.6 | 95.1 ± 12.9 | 95.3 ± 13.6 | 94.2 ± 10.1 | 96.3 ± 11.9 | 96.1 ± 12.9 | 95.7 ± 12.3 | 96.5 ± 11.5 |
| HR (bpm) | 82.1 ± 11.2 | 78.4 ± 10.9* | 80.6 ± 10.4* | 79.3 ± 10.2* | 70.8 ± 10.7┼ | 69.9 ± 11.1┼* | 68.0 ± 10.8┼* | 68.0 ± 11.4┼* | |
List of Abbreviations: : ACH, acetylcholine; EE, Excessive erythrocytosis; HR, heart rate; MAP, mean arterial pressure; SNP, sodium nitroprusside.
P<0.05, vs Rest.
P<0.05, vs control. Two-way mixed (highlanders: Drug dose x Pre/Post blockade, and Drug dose x EE/non-EE) analysis of variance were used to detect any differences in systemic and forearm hemodynamics.
Figure 3: Resistance artery endothelial-dependent and -independent dilation in lowlanders at sea level and high altitude.
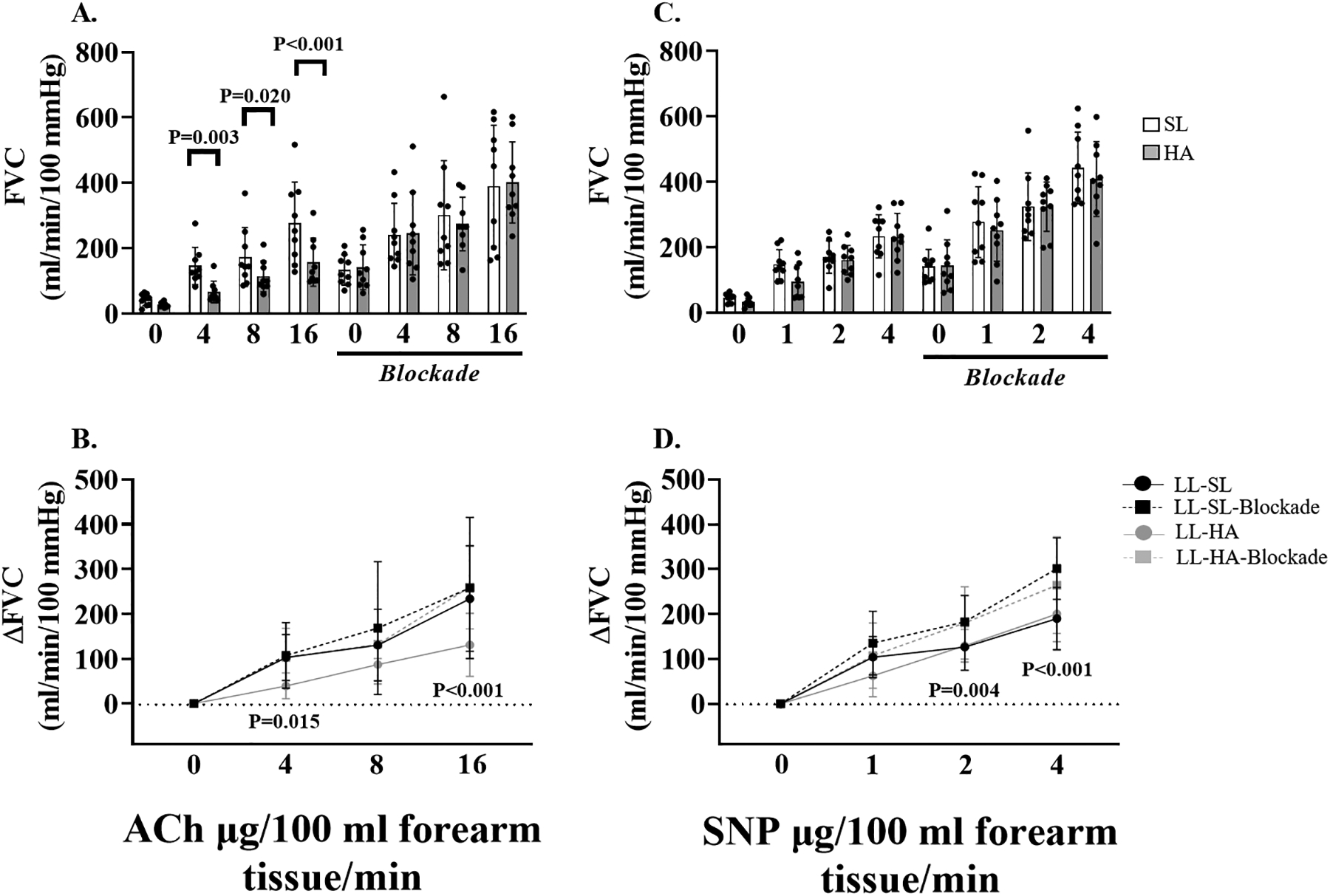
Individual and mean data displaying the impact of adrenergic blockade at sea level and high altitude on forearm vascular conductance during acetylcholine (ACh) infusion (panel A), and sodium nitroprusside (SNP) infusion (panel C). Mean summary data (±SD) highlighting the impact of adrenergic blockade on forearm vascular conductance at sea level and high altitude in response to ACh (panel B), and SNP (panel D). Forearm vascular conductance in response to ACh was reduced at high altitude compared to sea level in lowlanders (panel A). Adrenergic blockade increased forearm vascular conductance to ACh at high altitude in lowlanders (panel B). Adrenergic blockade increased forearm vascular conductance to SNP at sea level in lowlanders (panel C). P-values are displayed on each figure panel. Two-way repeated measures (lowlanders: Drug dose x Pre/Post blockade, and Drug dose x Altitude) analysis of variance were used to detect any differences in systemic and forearm hemodynamics.
Figure 4: Resistance artery endothelial-dependent and -independent dilation in Andean highlanders at high altitude.
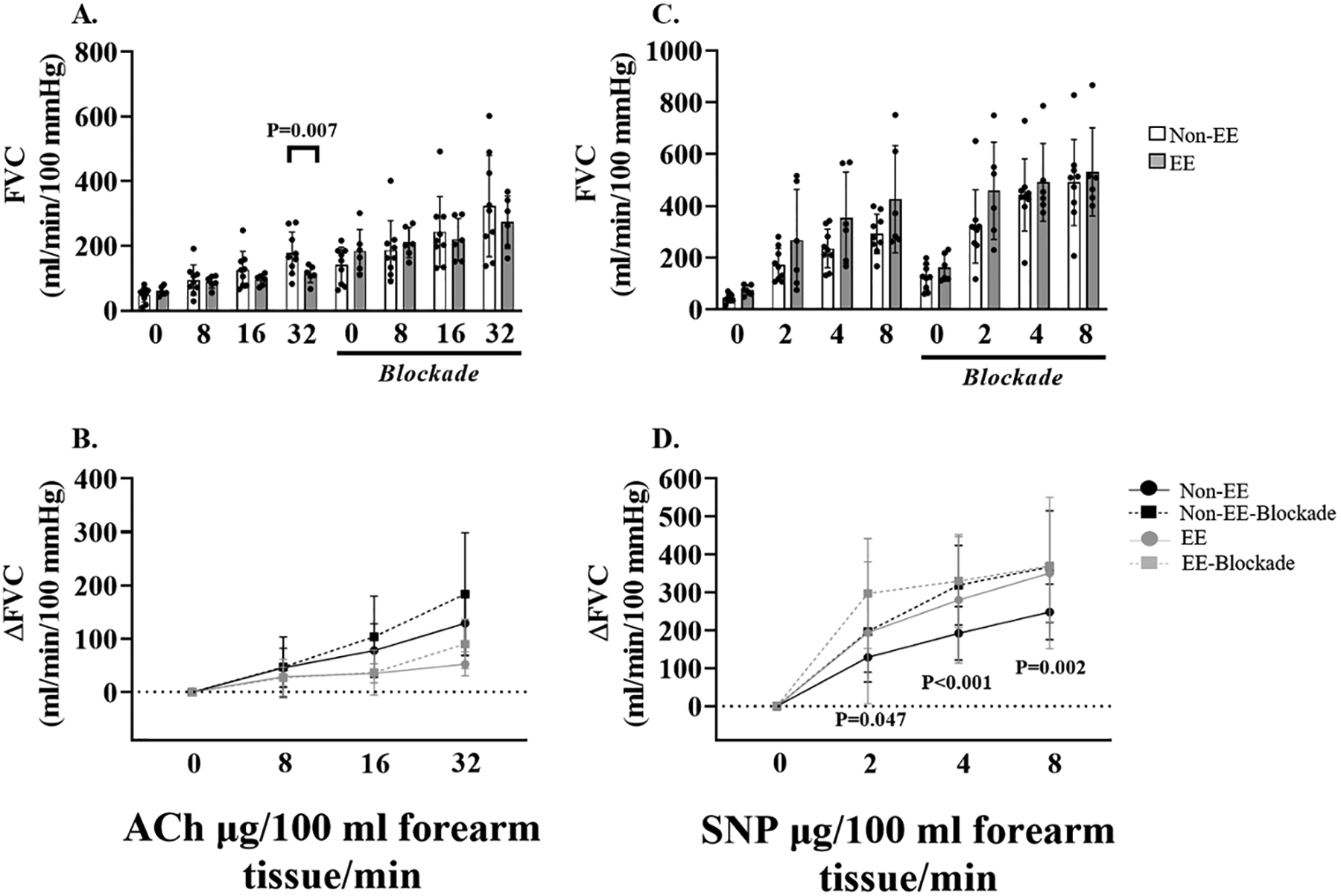
Individual and mean data displaying the impact of adrenergic blockade on forearm vascular conductance during acetylcholine (ACh) infusion (panel A), and sodium nitroprusside (SNP) infusion (panel C) in Andeans with and without EE. Mean summary data (±SD) highlighting the impact of adrenergic blockade on forearm vascular conductance in response to ACh (panel B), and SNP (panel D), in Andeans with and without EE. Forearm vascular conductance to ACh was reduced in Andeans with EE compared to non-EE Andeans (panel A). Adrenergic blockade increased forearm vascular conductance to SNP in non-EE Andeans (Panel D). P-values are displayed on each figure panel. Two-way mixed (highlanders: Drug dose x Pre/Post blockade, and Drug dose x EE/non-EE) analysis of variance were used to detect any differences in systemic and forearm hemodynamics.
RESULTS
Participants.
Lowlander and Andean highlander participant characteristics are presented in table 1. In lowlanders, PaO2, PaCO2, and SaO2 were reduced, and [Hb] was elevated at high altitude compared to sea level (all P<0.001). Between EE and non-EE Andean highlanders, there were no differences in age (P=0.92) or PaO2 (P=0.65); however, Andeans with EE were taller (P=0.03), had a higher body mass index (P<0.01), had a lower forearm volume (P=0.02), and were heavier (P=0.01). Andeans with EE also had reduced SaO2 (P=0.02), higher PaCO2 (P=0.06), elevated [Hb] (22.5 vs 18.7 g/dl; P<0.001), and elevated chronic mountain sickness scores (P<0.001).
Protocol A: Lowlanders
Systemic hemodynamic and neural responses.
During the higher doses of SNP (SNP2 and SNP3) there was a mild, but consistent decrease in MAP, which was associated with an increase in HR – these effects were observed at both sea level and high altitude (table 2). During the ACh infusion both MAP and HR stayed relatively constant at both sea level and high altitude (table 2). These hemodynamic responses are similar in magnitude to previous investigations45. In lowlanders, both muscle sympathetic nerve burst frequency and burst incidence were elevated at high altitude compared to sea level (both P<0.001; Figure 2A and B).
Resistance artery endothelial-dependent and -independent dilation in lowlanders
As expected, during both SNP and ACh infusion protocols forearm blood flow (see supplemental data), and forearm vascular conductance increased from rest in a stepwise manner (Figure 3A and C). After 14–21 days of exposure to chronic hypoxia, resistance artery endothelial-dependent dilation was reduced compared to sea level at ACh1 (−52.7 ± 19.6%; P=0.003), ACh2 (−25.4 ± 38.7%; P=0.020), and ACh3 (−35.1 ± 34.7%; P<0.001). There was no effect of adrenergic blockade on the forearm vascular conductance response to ACh at sea level (P=0.228); however, it was increased following adrenergic blockade at high altitude ACh1 (P=0.015), and ACh3 (P<0.001), and was no longer different from sea level (main effect: P=0.988 sea level blockade vs. high altitude blockade; Figure 3B). There was no effect of high altitude on resistance artery endothelial-independent dilation (main effect P=0.397). Adrenergic blockade increased the Δ forearm vascular conductance response at SNP2 (P=0.004), and SNP3 at sea level (P<0.001). The same trend towards increased resistance artery endothelial-independent dilation during adrenergic blockade was apparent at high altitude but did not reach statistical significance (main effect: P=0.069; Figure 3D).
Protocol B: Highlanders
Systemic hemodynamic and neural responses
In EE and non-EE Andean highlanders, there was a consistent decrease in MAP and an associated increase in HR during the SNP infusion trials. In contrast, during the ACh infusion, MAP and HR stayed relatively stable in both EE and non-EE participants (table 3). Andean highlanders with EE had elevated muscle sympathetic nerve burst frequency and burst incidence (P=0.047 and P=0.027, respectively), compared to non-EE Andeans (Figure 2C and D).
Resistance artery endothelial-dependent and –independent dilation in Andean highlanders
In both EE and non-EE Andean highlanders SNP and ACh infusion increased forearm blood flow (see supplemental data), and forearm vascular conductance increased in a stepwise manner (figure 4A and B). Andean highlanders with EE had reduced forearm vascular conductance at ACh3 (−36.4%; P=0.007), compared to non-EE Andean highlanders, which persisted after accounting for body mass index as a covariate (P=0.007). After adrenergic blockade, there was no difference in the absolute forearm vascular response during ACh infusion between EE and non-EE Andean highlanders (main effect: P=0.971; see figure 4A). Adrenergic blockade yielded no changes in the forearm vascular response to ACh within EE (main effect: P=0.251), and non-EE Andean highlanders (main effect: P=0.145; see figure 4B). No differences in absolute forearm vascular conductance in response to SNP were detected between non-EE and EE Andeans before (P=0.085), and after blockade (P=0.326; see figure 4C). Forearm vascular conductance in response to SNP was elevated in non-EE Andeans after adrenergic blockade at SNP1 (P=0.047), SNP2 (P<0.001), and SNP3 (P=0.002; see figure 4D). In contrast, adrenergic blockade had no effect on the forearm vascular conductance response to SNP in EE Andeans (main effect: P=0.276).
DISCUSSION
The primary novel findings were the following: 1) lowlanders exposed to chronic hypoxia, and Andean highlanders with EE compared to non-EE, have impaired resistance artery endothelial-dependent dilation that was improved after local adrenergic blockade; and 2) chronic hypoxia had no effect on endothelial-independent dilation in lowlanders, and no differences were detected between EE and non-EE Andean highlanders. Furthermore, despite administering a two-fold greater dose of ACh in the Andean highlanders, non-EE Andeans had a similar, and EE Andeans had a reduced, forearm vascular conductance response compared to lowlanders prior to adrenergic blockade (i.e. control trial).
Effect of chronic hypoxia on vascular function in lowlanders.
Previous reports investigating the effects of chronic hypoxia on endothelial function in lowlanders are inconsistent and often limited by methodological challenges and considerations that preclude direct comparison of investigations. For example, conduit artery endothelial-dependent dilation has been shown to be unaltered at altitude in some [ascent to 3842 m via cable car46; ascent via automobile to 3800 m47, 48], and reduced in others [ascent to 3700 m49, 4371 m13, and 5050 m by trekking10, 12, 13]. Of note, one study investigated the effects of different altitude exposure on conduit artery endothelial-dependent dilation (3440 m, 4371m, and 5050 m) in healthy lowlanders, and demonstrated that brachial artery flow-mediated dilation was not reduced at 3440 m, but was reduced at 4371 m and 5050 m13. Collectively, these previous reports indicate that there is an altitude threshold of ~3500–4000 m where vascular function becomes impaired. It is noteworthy, however, that all of these previous studies were conducted using non-invasive flow-mediated dilation as an index of conduit artery endothelial function10, 12, 13, 46–49, and only one of these studies investigated smooth muscle function via sublingual nitroglycerin12.
A strength of the current study is the use of direct pharmacological assessment of both endothelial-dependent dilation (via ACh) and endothelial-independent dilation (via SNP) microvascular function in a step-wise controlled manner in order to definitively isolate the effects of chronic hypoxia on endothelial function per se. The data in the current study confirmed previous reports that muscle sympathetic nervous activity is elevated9–11, and resistance artery endothelial-dependent dilation is impaired, in lowlanders >4000 m. However, in opposition to previous findings12, chronic hypoxia did not impact resistance artery endothelial-independent dilation in lowlanders. These findings indicate that the observed reduction in resistance artery endothelial-dependent dilation at 4300m in lowlanders is specifically due to endothelial-dependent dilation. The disparity between these two studies is likely attributable to the differences in methodologies employed to assess vascular function (conduit vs. resistance arteries), and/or the difference in altitude severity (5050 m vs 4300 m).
Furthermore, the results from this investigation are the first to mechanistically link exaggerated adrenergic signaling with impaired resistance artery endothelial-dependent dilation after acclimatization to chronic hypoxia. Under resting conditions, tonic sympathetic nervous activity actively restraints skeletal muscle blood flow and vasodilatory signaling via α-adrenergic mechanisms40. Interestingly, at sea level, adrenergic blockade had little impact on ACh-mediated vasodilation, whereas SNP-mediated dilation was significantly increased (Figure 3B and D). This is likely related to the signalling mechanisms between endothelium-dependent and -independent vasodilators. Specifically, previous investigations in animals and humans have demonstrated a unique resistance of endothelial-dependent dilation to sympathetic restraint, while nitric oxide-mediated endothelial-independent dilation is typically more susceptible to modulation by sympathetic nervous activity under normal conditions42, 50–52. However, basal restraint of SNP-mediated signaling was not altered at high altitude in lowlanders (P=0.069), indicating that tonic restraint of endothelial-independent dilation by sympathetic signaling that is not consistently affected by chronic hypoxia.
In contrast, the impairment in resistance artery endothelial-dependent dilation was strikingly improved after adrenergic blockade at high altitude (see figure 3B), highlighting the interaction between chronic adrenergic signaling and endothelial-dependent dilation during exposure to chronic hypoxia. In animal models, acute activation of α-adrenergic activity significantly reduces the microvascular vasodilatory response to ACh by attenuating endothelial-derived hyperpolarization and subsequent conducted vasodilation53. In addition, endogenous nitric oxide bioavailability may be lower in chronic hypoxia due to reduced endothelial-derived nitric oxide synthase expression or activity54, and elevated oxidative stress12, while leaving the vascular response to exogenous nitric oxide unaltered. Further research is needed to identify the precise vasodilatory mechanisms that are attenuated by persistent α-adrenergic vasoconstrictor signaling.
Effect of chronic hypoxia on vascular function in Andean highlanders.
In the town of Cerro de Pasco, Peru, chronic mountain sickness is a severe health crisis that occurs in young and aging males. The disease is characterized by EE, exacerbated hypoxemia, pulmonary hypertension, elevated 24-hour blood pressure33, increased arterial stiffness and intima-media thickness34, which can contribute to the development of cardiovascular disease (e.g. heart failure)32. Interestingly, Andeans with and without EE share similar relative maximum oxygen uptake during exercise55, suggesting that although several markers of cardiovascular disease are elevated, exercise performance is maintained. Surprisingly, only few investigations have assessed vascular function in Andean highlanders with EE compared to healthy Andean highlanders18, 34, 56. Each of these studies demonstrated that conduit artery endothelial function, assessed via non-invasive flow-mediated dilation, was impaired in Andeans diagnosed with EE. These studies, however, were unable to account for differences in blood viscosity between the healthy and non-healthy Andean cohorts, which directly impacts the flow-mediated dilation response through differences in shear stress. Therefore, it remains unclear if differences in endothelial function between EE and non-EE Andeans exist due to impaired endothelial-dependent dilation related signaling mechanisms, or are related to alterations in shear stress. A recent study demonstrated that endothelial-dependent dilation (assessed via brachial artery flow-mediated dilation) was improved after hemodilution in Andean highlanders with EE57; however, the Andeans with EE were not compared against a control group of healthy Andeans, and sympathetic nervous activity was not assessed, thus, cannot be ruled out as mechanism contributing to endothelial dysfunction. The present study directly measured sympathetic nervous activity via radial nerve microneurography (see figure 2), and demonstrated that muscle sympathetic nerve burst frequency and incidence were elevated in Andeans with EE compared to non-EE. However, these data should be cautiously interpreted due to our small sample size, and since radial nerve SNA may not be an accurate representative of global SNA in this population.
In the current study, resistance artery endothelial-dependent dilation was impaired in Andeans with EE compared to non-EE at the highest dose of ACh, and this impairment was no longer present after administration of local adrenergic blockade. These data indicate that adrenergic signaling contributes to endothelial-dependent dilation dysfunction in Andeans with EE, but it is likely not the primary contributor to the magnitude of observed endothelial dysfunction since endothelial-dependent dilation was not completely restored. Although not a primary research objective due to the age difference (~15–20 years) between lowlander and Andean participants, Andean highlanders (EE and non-EE) had similar vascular conductance responses to ACh despite receiving a two-fold higher drug dose. This observation indicates that Andean highlanders have impaired endothelial-dependent dilation function compared to lowlanders, likely due to the combination of aging and vascular alterations that occur with lifelong exposure to hypoxia58. The inability of adrenergic blockade to normalize the resistance artery endothelial-dependent dilation between EE and non-EE Andeans indicates that other chronic signalling mechanisms in addition to adrenergic signaling, such as reactive oxygen species related nitric oxide scavenging59, endothelin-1 signaling, or angiotensin receptor signalling, may also contribute to endothelial impairment in the Andeans suffering from EE. An unexpected finding was that non-EE Andeans had increased endothelial-independent dilation after adrenergic blockade, similar to lowlanders. These results indicate that normal sympathetic nervous activity mediated vascular restraint of exogenous nitric oxide mediated vasodilation in lowlanders and healthy Andeans is absent in Andeans with EE. This may reflect pathological alterations in neural and vascular signaling mechanisms in Andeans with EE; however, more investigations are needed to confirm these findings.
Methodological considerations.
There are some methodological considerations that warrant further comment. First, in lowlanders the doses for ACh, SNP, and local adrenergic blockade (i.e. phentolamine and propranolol) were kept the same between sea level and high altitude. It is unknown if the drug efficacy of these substances are similar between altitudes, however, in a subset of subjects the efficacy of the blockade was challenged with intra-arterial infusion of phenylephrine (α1-adrenergic agonist) with similar efficacy noted at sea level and high altitude (>90% effective blockade).
Second, the Andean highlanders had a greater (i.e. double) drug dosage of ACh and SNP compared to the lowlanders. The experimental rationale for this approach was two-fold: 1) we anticipated an older Andean cohort compared to the recruited lowlanders; therefore, direct comparison between lowlander and highlander groups was not a primary research objective; 2) due to the effect of aging on endothelial function58, and on previous research conducted in Andean highlanders18, 34, we expected to observe reduced endothelial function in the Andeans compared to lowlanders; therefore, the drug doses were increased in order to avoid a “basement” effect; 3) conversely, healthy lowlanders would likely show robust vasodilatory responses to ACh and SNP in control conditions that would likely reach a “ceiling” effect when performed in addition to the ~3x increase in blood flow expected with local adrenergic blockade.
Third, forearm volume was not measured at high altitude in lowlander participants. Although very high altitude exposure (>5000 m) results in muscle wasting60, it is unlikely that substantial muscle wasting occurred within 14–21 days at altitude (especially in Cerro de Pasco where there is high food availability). Hypothetically, if forearm volume was reduced at altitude, it would potentially result in a greater relative drug dose, and would underestimate the impact of chronic hypoxia on endothelial function. Despite this, we still observed profound endothelial dysfunction that was abolished after local adrenergic blockade in lowlanders.
Fourth, Andeans with EE have been shown to have a higher prevalence of sleep disordered breathing compared to their healthy counterparts61, which is associated with heightened SNA, vascular dysfunction, and elevated oxidative stress. Unfortunately, we were not equipped to perform sleep studies to confirm the presence or absence of sleep disordered breathing in lowlander and Andean highlander participants. Caution is warranted in this regard when interpreting our data.
Lastly, although it would have been valuable to additionally test other mechanism(s) related to vascular health such as the role of nitric oxide and prostaglandins5, ATP6, 7, and asymmetric dimethylarginine62, it was not feasible due to the technical demands and restraints imposed by high altitude field research. The current investigation focused on well-defined hypotheses related to heightened adrenergic signaling and endothelial dysfunction.
Significance and Perspective.
Our data demonstrates that that chronic severe hypoxia exposure results in endothelial dysfunction in lowlanders that is mediated through heightened adrenergic vasoconstrictor signaling. In addition, Andean highlanders with excessive erythrocytosis have reduced endothelial function compared to healthy highlanders and, similar to lowlanders, this impairment was also mediated in part through adrenergic vasoconstrictor signaling. Collectively, our data provides the direct mechanistic link between altitude-mediated sympatho-excitation and endothelial impairment.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
The complex interplay between local vasodilatory signaling and sympathetic nervous activity signalling results in mild systemic vasodilation that serves to ensure proper blood flow and oxygen delivery to peripheral tissues.
However, chronic exposure to severe hypoxia (e.g. high altitude, >3000 m) results in sustained increases in sympathetic nervous activity and reduced endothelial-independent dilation and may be associated with many cardiopulmonary diseases characterized by sustained or intermittent hypoxia, such as: heart failure and sleep apnea
In lowlanders, hypoxemia results in reduced endothelial-dependent function.
Andean highlanders, whom have life-long exposure to hypobaric hypoxia, are prone to suffering from excessive erythrocytosis, which has been shown to have been associated with reduced endothelial-dependent function.
Elevated sympathetic nervous activity is proposed as a key pathway responsible for reduced endothelial-dependent function at high altitude, but this has not been tested.
What New Information Does This Article Contribute?
In lowlanders, ~14 days of acclimatization to 4300 m resulted in a reduction in brachial artery endothelial-dependent function, which was abolished after administration of adrenergic blockade.
Andean highlanders suffering from excessive erythrocytosis had reduced brachial artery endothelial-dependent function compared to their healthy counterparts; however, endothelial-dependent function was normalized between groups after adrenergic blockade.
These data are the first to directly-link heightened sympathetic nervous activity and reduced endothelial-dependent function at high altitude.
ACKNOWLEDGEMENTS
This study was performed within the framework of the Global REACH International Research Expedition to Peru. We would like to thank the research laboratory staff for friendly accommodation and all Andean highlander participants for their valuable time. We are grateful to the members of Global REACH for invaluable help with organization and implementation of this research study.
SOURCES OF FUNDING
This study was supported by a Canada Research Chair (P.N.A.), Natural Sciences and Engineering Research Council of Canada (P.N.A.), the Canadian Foundation for Innovation and a Canada Research Chair (P.N.A). Michael M. Tymko was supported by a Natural Sciences and Engineering Research Council of Canada Doctoral CGS award. Christopher M. Hearon Jr. was funded by a National Research Service Award from the National Heart Lung and Blood Institute (NIH 1F32HL137285-0). Gilbert Moralez was funded by a Wilderness Medical Society grant
Nonstandard Abbreviations and Acronyms:
- ACh
acetylcholine
- EE
excessive erythrocytosis
- Hb
hemoglobin
- HR
heart rate
- MAP
mean arterial pressure
- PaCO2
partial pressure of arterial oxygen
- PaO2
partial pressure of arterial oxygen
- SaO2
arterial saturation of oxyhemoglobin
- SNP
sodium nitroprusside
Footnotes
CONFLICT OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the authors.
SUPPLEMENTAL MATERIALS
REFERENCES
- 1.Jia L, Bonaventura C, Bonaventura J and Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–6. [DOI] [PubMed] [Google Scholar]
- 2.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd and Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. [DOI] [PubMed] [Google Scholar]
- 3.Saito M, Mano T, Iwase S, Koga K, Abe H and Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol (1985). 1988;65:1548–52. [DOI] [PubMed] [Google Scholar]
- 4.Somers VK, Mark AL, Zavala DC and Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985). 1989;67:2101–6. [DOI] [PubMed] [Google Scholar]
- 5.Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF and Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol. 2011;589:1979–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crecelius AR, Kirby BS and Dinenno FA. Intravascular ATP and the regulation of blood flow and oxygen delivery in humans. Exerc Sport Sci Rev. 2015;43:5–13. [DOI] [PubMed] [Google Scholar]
- 7.Sprague RS and Ellsworth ML. Erythrocyte-derived ATP and perfusion distribution: role of intracellular and intercellular communication. Microcirculation. 2012;19:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Racine ML, Crecelius AR, Luckasen GJ, Larson DG and Dinenno FA. Inhibition of Na(+) /K(+) -ATPase and KIR channels abolishes hypoxic hyperaemia in resting but not contracting skeletal muscle of humans. J Physiol. 2018;596:3371–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen J and Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tymko MM, Tremblay JC, Steinback CD, Moore JP, Hansen AB, Patrician A, Howe CA, Hoiland RL, Green DJ and Ainslie PN. UBC-Nepal Expedition: acute alterations in sympathetic nervous activity do not influence brachial artery endothelial function at sea level and high altitude. J Appl Physiol (1985). 2017;123:1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duplain H, Vollenweider L, Delabays A, Nicod P, Bartsch P and Scherrer U. Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation. 1999;99:1713–8. [DOI] [PubMed] [Google Scholar]
- 12.Lewis NCS, Bailey DM, duManoir GR, Messinger L, Lucas SJE, Cotter JD, Donnelly J, McEneny J, Young IS, Stembridge M, Burgess KR, Basnet AS and Ainslie PN. Conduit artery structure and function in lowlanders and native highlanders: relationships with oxidative stress and role of sympathoexcitation. J Physiol. 2014;592:1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremblay JC, Hoiland RL, Carter HH, Howe CA, Stembridge M, Willie CK, Gasho C, MacLeod DB, Pyke KE and Ainslie PN. UBC-Nepal expedition: upper and lower limb conduit artery shear stress and flow-mediated dilation on ascent to 5,050 m in lowlanders and Sherpa. Am J Physiol Heart Circ Physiol. 2018;315:H1532–h1543. [DOI] [PubMed] [Google Scholar]
- 14.Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, Colucci WS, Sutton MG, Selwyn AP, Alexander RW and et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–9. [DOI] [PubMed] [Google Scholar]
- 15.Moro L, Pedone C, Scarlata S, Malafarina V, Fimognari F and Antonelli-Incalzi R. Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology. 2008;59:357–64. [DOI] [PubMed] [Google Scholar]
- 16.Carlson JT, Rangemark C and Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14:577–84. [DOI] [PubMed] [Google Scholar]
- 17.Bailey DM, Brugniaux JV, Filipponi T, Marley CJ, Stacey B, Soria R, Rimoldi SF, Cerny D, Rexhaj E, Pratali L, Salmon CS, Murillo Jauregui C, Villena M, Smirl JD, Ogoh S, Pietri S, Scherrer U and Sartori C. Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J Physiol. 2019;597:611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey DM, Rimoldi SF, Rexhaj E, Pratali L, Salinas Salmon C, Villena M, McEneny J, Young IS, Nicod P, Allemann Y, Scherrer U and Sartori C. Oxidative-nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest. 2013;143:444–451. [DOI] [PubMed] [Google Scholar]
- 19.Rimoldi SF, Sartori C, Rexhaj E, Bailey DM, de Marchi SF, McEneny J, Arx R, Cerny D, Duplain H, Germond M, Allemann Y and Scherrer U. Antioxidants improve vascular function in children conceived by assisted reproductive technologies: A randomized double-blind placebo-controlled trial. Eur J Prev Cardiol. 2015;22:1399–407. [DOI] [PubMed] [Google Scholar]
- 20.Birk GK, Dawson EA, Batterham AM, Atkinson G, Cable T, Thijssen DH and Green DJ. Effects of exercise intensity on flow mediated dilation in healthy humans. Int J Sports Med. 2013;34:409–14. [DOI] [PubMed] [Google Scholar]
- 21.Lamping KG and Dole WP. Acute hypertension selectively potentiates constrictor responses of large coronary arteries to serotonin by altering endothelial function in vivo. Circ Res. 1987;61:904–13. [DOI] [PubMed] [Google Scholar]
- 22.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ and Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–8. [DOI] [PubMed] [Google Scholar]
- 23.Fronek K and Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol. 1975;228:791–6. [DOI] [PubMed] [Google Scholar]
- 24.Shoemaker JK, Badrov MB, Al-Khazraji BK and Jackson DN. Neural Control of Vascular Function in Skeletal Muscle. Compr Physiol. 2015;6:303–29. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson CL, Lewis NCS, Carter HH, Thijssen DHJ, Ainslie PN and Green DJ. Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. J Physiol. 2015;593:5145–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijssen DH, Atkinson CL, Ono K, Sprung VS, Spence AL, Pugh CJ and Green DJ. Sympathetic nervous system activation, arterial shear rate, and flow-mediated dilation. J Appl Physiol (1985). 2014;116:1300–7. [DOI] [PubMed] [Google Scholar]
- 27.Aldenderfer MS. High elevation foraging societies In Handbook of South American Archaeology , ed Silverman HG, Isbell WH , pp 131.– New York: Springer; 2008. [Google Scholar]
- 28.Haas R, Stefanescu IC, Garcia-Putnam A, Aldenderfer MS, Clementz MT, Murphy MS, Llave CV and Watson JT. Humans permanently occupied the Andean highlands by at least 7 ka. R Soc Open Sci. 2017;4:170331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monge C, Leon-Velarde F and Arregui A. Increasing prevalence of excessive erythrocytosis with age among healthy high-altitude miners. N Engl J Med. 1989;321:1271. [DOI] [PubMed] [Google Scholar]
- 30.Leon-Velarde F, Sanchez J, Bigard AX, Brunet A, Lesty C and Monge C. High altitude tissue adaptation in Andean coots: capillarity, fibre area, fibre type and enzymatic activities of skeletal muscle. J Comp Physiol B. 1993;163:52–8. [DOI] [PubMed] [Google Scholar]
- 31.Gassmann M, Mairbaurl H, Livshits L, Seide S, Hackbusch M, Malczyk M, Kraut S, Gassmann NN, Weissmann N and Muckenthaler MU. The increase in hemoglobin concentration with altitude varies among human populations. Ann N Y Acad Sci. 2019;1450:204–220. [DOI] [PubMed] [Google Scholar]
- 32.Villafuerte FC and Corante N. Chronic Mountain Sickness: Clinical Aspects, Etiology, Management, and Treatment. High Alt Med Biol. 2016;17:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corante N, Anza-Ramirez C, Figueroa-Mujica R, Macarlupu JL, Vizcardo-Galindo G, Bilo G, Parati G, Gamboa JL, Leon-Velarde F and Villafuerte FC. Excessive Erythrocytosis and Cardiovascular Risk in Andean Highlanders. High Alt Med Biol. 2018;19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimoldi SF, Rexhaj E, Pratali L, Bailey DM, Hutter D, Faita F, Salinas Salmon C, Villena M, Nicod P, Allemann Y, Scherrer U and Sartori C. Systemic vascular dysfunction in patients with chronic mountain sickness. Chest. 2012;141:139–146. [DOI] [PubMed] [Google Scholar]
- 35.Gamboa A, Gamboa JL, Holmes C, Sharabi Y, Leon-Velarde F, Fischman GJ, Appenzeller O and Goldstein DS. Plasma catecholamines and blood volume in native Andeans during hypoxia and normoxia. Clin Auton Res. 2006;16:40–5. [DOI] [PubMed] [Google Scholar]
- 36.Leon-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G and Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. 2005;6:147–57. [DOI] [PubMed] [Google Scholar]
- 37.Mano T, Iwase S and Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol. 2006;117:2357–84. [DOI] [PubMed] [Google Scholar]
- 38.Delius W, Hagbarth KE, Hongell A and Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972;84:82–94. [DOI] [PubMed] [Google Scholar]
- 39.Torp KD, Tschakovsky ME, Halliwill JR, Minson CT and Joyner MJ. beta-Receptor agonist activity of phenylephrine in the human forearm. J Appl Physiol (1985). 2001;90:1855–9. [DOI] [PubMed] [Google Scholar]
- 40.Richards JC, Luckasen GJ, Larson DG and Dinenno FA. Role of alpha-adrenergic vasoconstriction in regulating skeletal muscle blood flow and vascular conductance during forearm exercise in ageing humans. J Physiol. 2014;592:4775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crecelius AR, Kirby BS, Voyles WF and Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol. 2010;299:H1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hearon CM Jr., Kirby BS, Luckasen GJ, Larson DG and Dinenno FA. Endothelium-dependent vasodilatory signalling modulates alpha1 -adrenergic vasoconstriction in contracting skeletal muscle of humans. J Physiol. 2016;594:7435–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hearon CM, Jr., Richards JC, Racine ML, Luckasen GJ, Larson DG and Dinenno FA. Amplification of endothelium-dependent vasodilatation in contracting human skeletal muscle: role of KIR channels. J Physiol. 2019;597:1321–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hearon CM Jr., Richards JC, Racine ML, Luckasen GJ, Larson DG, Joyner MJ and Dinenno FA. Sympatholytic effect of intravascular ATP is independent of nitric oxide, prostaglandins, Na(+) /K(+) -ATPase and KIR channels in humans. J Physiol. 2017;595:5175–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirby BS, Crecelius AR, Voyles WF and Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol. 2010;588:4017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruno RM, Ghiadoni L and Pratali L. Vascular adaptation to extreme conditions: The role of hypoxia. Artery Research. 2016;14:15–21. [Google Scholar]
- 47.Tremblay JC, Thom SR, Yang M and Ainslie PN. Oscillatory shear stress, flow-mediated dilatation, and circulating microparticles at sea level and high altitude. Atherosclerosis. 2017;256:115–122. [DOI] [PubMed] [Google Scholar]
- 48.Tymko MM, Tremblay JC, Hansen AB, Howe CA, Willie CK, Stembridge M, Green DJ, Hoiland RL, Subedi P, Anholm JD and Ainslie PN. The effect of alpha1 -adrenergic blockade on post-exercise brachial artery flow-mediated dilatation at sea level and high altitude. J Physiol. 2017;595:1671–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakker E, Engan H, Patrician A, Schagatay E, Karlsen T, Wisloff U and Gaustad SE. Acute dietary nitrate supplementation improves arterial endothelial function at high altitude: A double-blinded randomized controlled cross over study. Nitric Oxide. 2015;50:58–64. [DOI] [PubMed] [Google Scholar]
- 50.Engelke KA, Williams MM, Dietz NM and Joyner MJ. Does sympathetic activation blunt nitric oxide-mediated hyperemia in the human forearm? Clin Auton Res. 1997;7:85–91. [DOI] [PubMed] [Google Scholar]
- 51.VanTeeffelen JW and Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z and Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurjiaka DT and Segal SS. Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circ Res. 1995;76:885–91. [DOI] [PubMed] [Google Scholar]
- 54.McQuillan LP, Leung GK, Marsden PA, Kostyk SK and Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol. 1994;267:H1921–7. [DOI] [PubMed] [Google Scholar]
- 55.Swenson ER. Normal exercise capacity in chronic mountain sickness: how high can the hematocrit go without consequence? Chest. 2012;142:823–825. [DOI] [PubMed] [Google Scholar]
- 56.Rexhaj E, Rimoldi SF, Pratali L, Brenner R, Andries D, Soria R, Salinas C, Villena M, Romero C, Allemann Y, Lovis A, Heinzer R, Sartori C and Scherrer U. Sleep-Disordered Breathing and Vascular Function in Patients With Chronic Mountain Sickness and Healthy High-Altitude Dwellers. Chest. 2016;149:991–8. [DOI] [PubMed] [Google Scholar]
- 57.Tremblay JC, Hoiland RL, Howe CA, Coombs GB, Vizcardo-Galindo GA, Figueroa-Mujica RJ, Bermudez D, Gibbons TD, Stacey BS, Bailey DM, Tymko MM, MacLeod DB, Gasho C, Villafuerte FC, Pyke KE and Ainslie PN. Global REACH 2018: High Blood Viscosity and Hemoglobin Concentration Contribute to Reduced Flow-Mediated Dilation in High-Altitude Excessive Erythrocytosis. Hypertension. 2019;73:1327–1335. [DOI] [PubMed] [Google Scholar]
- 58.Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y and Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. [DOI] [PubMed] [Google Scholar]
- 59.Meli R, Nauser T, Latal P and Koppenol WH. Reaction of peroxynitrite with carbon dioxide: intermediates and determination of the yield of CO3*- and NO2*. J Biol Inorg Chem. 2002;7:31–6. [DOI] [PubMed] [Google Scholar]
- 60.Kayser B. Nutrition and high altitude exposure. Int J Sports Med. 1992;13 Suppl 1:S129–32. [DOI] [PubMed] [Google Scholar]
- 61.Julian CG, Vargas E, Gonzales M, Davila RD, Ladenburger A, Reardon L, Schoo C, Powers RW, Lee-Chiong T and Moore LG. Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis). Respir Physiol Neurobiol. 2013;186:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Millatt LJ, Whitley GS, Li D, Leiper JM, Siragy HM, Carey RM and Johns RA. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation. 2003;108:1493–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


