Abstract
Rationale
Evaluation of the feasibility of [18F]EF5-PET/CT scan in identifying hypoxic lesions in ovarian tumors in prospective clinical setting.
Methods
Fifteen patients with a suspected malignant ovarian tumor were scanned with [18F]EF5 and [18F]FDG-PET/CT preoperatively. The distribution of [18F]EF5-uptake, total intraabdominal metabolic tumor volume (TMTV), and hypoxic subvolume (HSV) were assessed.
Results
[18F]EF5-PET/CT suggested hypoxia in 47% (7/15) patients. The median HSV was 87 cm3 (31% of TMTV). The [18F]EF5-uptake was detected in primary tumors and in four patients also in intra-abdominal metastases. The [18F]EF5-uptake in cancer tissue was low compared to physiological excretory pathways, complicating the interpretation of PET/CT images.
Conclusions
[18F]EF5-PET/CT is not feasible in ovarian cancer imaging in clinical setting due to physiological intra-abdominal [18F]EF5-accumulation. However, it may be useful when used complementarily to FDG-PET/CT.
Keywords: EF5-PET/CT, Ovarian cancer, Hypoxia
Introduction
Ovarian cancer (OC) is the most lethal gynecological malignancy, and the majority of patients are diagnosed at an advanced stage [1]. Although OC is initially chemosensitive, most women experience multiple and finally chemoresistant relapses. The survival odds have not markedly improved despite extensive research, and completeness of surgery is still a major prognostic factor [2].
The presence of hypoxic regions in solid tumors is associated with a poor prognosis for many cancer types [3–5]. Hypoxia-mediated chemoresistance is also the greatest clinical challenge in OC [6, 7].
There is a considerable need for non-invasive imaging of tumor hypoxia since it provides additional information, which could be integrated into strategies of treatment [8]. The method can improve therapeutic outcomes by predicting chemoresistance and selecting potentially treatment-resistant tumors for targeted surgery.
18F-nitroimidazolpentafluoropropylacetamide ([18F]EF5) is one of the extensively investigated and clinically tested tracers of tissue hypoxia [9, 10]. 18F]EF5 belongs to the nitroimidazole group and has considerable membrane permeability and capability to accumulate in viable hypoxic, though not in apoptotic or necrotic cells [11–13].
Since there is no systematic data evaluating the eligibility of hypoxia-imaging among patients with ovarian malignancy, we conducted the prospective clinical study to evaluate the feasibility of [18F]EF5-PET/CT scan in identifying hypoxic lesions in ovarian tumors.
Material and methods
Study population
This prospective non-randomized study was conducted at Turku University Hospital, Finland, between November 2017 and June 2019. Patients between 38 and 79 years of age with ovarian tumor, who were not pregnant, nursing, or had a history of previous malignancies were included. Ethical approval was obtained from the institutional review board (18.10.2016§443), and all subjects signed an informed consent form, ClinicalTrials.gov identifier: NCT04001023.
A whole-body contrast-enhanced [18F]FDG-PET/CT and [18F]EF5-PET/CT of the abdomen were performed on separate days preoperatively. PET/CT images were then evaluated by a nuclear medicine specialist and gynecological oncologist to assess the distribution of the cancer and to determine regions of suspected hypoxia in the intraabdominal tumor load for targeted biopsies for future research.
PET/CT scanning procedure
The PET/CT studies were performed with a digital PET/CT scanner: Discovery MI (General Electric Medical Systems, Milwaukee, WI, USA). It has combined PET/CT-scanners with a 128-slice CT and a 3D PET imaging capability. The PET imaging field of view (FOV) was 70 cm in diameter and 20 cm in axial length. To obtain attenuation correction for 511 keV photon distribution, the transmission scan was performed using a low-dose (noise index 30, automatic 3D current modulation, 10–120 mAs, and 120 kVp) CT protocol.
The patients received an intravenous injection of 370 MBq of 18F-EF5. A static emission scan was acquired 180 min from the tracer injection to cover the entire abdomen (3 bed positions, 7.5 min/bed). The patients voided prior to the scan. The sinogram data was corrected for deadtime, decay, and photon attenuation and reconstructed in a 256 × 256 matrix. Image reconstruction followed the Q.Clear method (a Bayesian-penalized likelihood reconstruction algorithm for PET) incorporating random and scatter correction with β value of 350. The final in-plane FWHM (full-width half-maximum) of the systems was < 5 mm.
A whole-body [18F]FDG-PET/CT with low-dose CT combined with diagnostic contrast-enhanced imaging for anatomical reference was performed following the standard institutional protocol for OC. The details of the protocol used for [18F]FDG-PET/CT are the same as abovementioned 18F]EF-5 PET/CT protocol except the larger scan area starting 60 min post injection from the base of the scull to the middle thigh (6 bed positions, 2 min/bed) with 4 MBq/kg of [18F]FDG.
The scans were performed in a random order depending on the availability of the [18F]EF5 and camera. The mean interval between scans was 2 (range 1–7) days.
Image analyses
Total metabolic tumor volume (MTV), maximal standardized [18F]FDG, and [18F]EF5-uptake values (SUVmax) were assessed with the PET VCAR (Volume Computer-Assisted Reading) program. For [18F]FDG-PET/CT analyses the SUVmax values were corrected for body weight and injected dose. TMTV was defined as the sum volume of all lesions.
A hypoxic voxel was defined using a threshold tumor to gluteus maximus muscle ratio (TMR) for [18F]EF5-uptake of 1.5, based on earlier experience [14]. The hypoxic subvolume (HSV) was defined as the sum volume of all lesions with TMR over 1.5.
Statistical analyses
Statistical analyses were performed using JMP Pro 13 software from SAS. Continuous variables were compared using a Wilcoxon rank-sum test. A non-parametric Spearman rank correlation test was used to evaluate the association between SUVmax values. Two-tailed P values < 0.05 were considered statistically significant.
Results
Fifteen patients were enrolled. EF5-PET/CT suggested hypoxia in 46% (7/15) of the patients. In those patients the mean HSV was 87 [Cl95% (9.5–238)] cm3 and proportionally 31 [Cl95% (2–47)]% of MTV.
Patients’ clinical and imaging characteristics are presented in Table 1. The distribution of [18F]EF5-avid lesions was variable. In 7 of the 15 (46%) patients, [18F]EF5PET/CT was suggestive for hypoxia. All of these patients had [18F]EF5-accumulation inside the primary tumor and 4 also had [18F]EF5-avid metastatic lesions. Seven patients had omental metastases, and 3 had [18F]EF5-accumulation in the omental cake.
Table 1.
Patient characteristics and PET information
| Nr. | Age at diagnosis (years) | Weight (kg) | Histology | Stage | MTV (cm3) | Hypoxia in [18F]EF5 PET/CT | Hypoxic subvolume (cm3, % of MTV) | Injected [18F]EF5 activity (Mbq) |
|---|---|---|---|---|---|---|---|---|
| 1 | 62 | 106 | high grade serous | IIIC | 571 | yes | 364 (64%) | 381 |
| 2 | 56 | 81 | endometrioid | IIB | 250 | yes | 121 (49%) | 367 |
| 3 | 65 | 100 | sarcoma | IIB | 1908 | yes | 42 (2%) | 374 |
| 4 | 38 | 93 | mucinous | IA | 158 | no | 367 | |
| 5 | 73 | 54 | carcinosarcoma | IIIC | 267 | yes | 17 (6%) | 301 |
| 6 | 79 | 68 | high grade serous | IVB | 615 | yes | 202 (33%) | 220 |
| 7 | 67 | 63 | high grade serous | IIIC | 955 | no | 385 | |
| 8 | 56 | 70 | fibroma | - | 224 | no | 377 | |
| 9 | 50 | 103 | clear cell | IIIC | 116 | no | 378 | |
| 10 | 69 | 77 | high grade serous | IVA | 324 | no | 380 | |
| 11 | 45 | 91 | serous BOT | IA | 66 | no | 374 | |
| 12 | 65 | 69 | high grade serous | IIIC | 273 | no | 377 | |
| 13 | 50 | 152 | endometrioid | IIA | 173 | no | 363 | |
| 14 | 68 | 59 | high grade serous | IIIC | 1288 | yes | 87 (7%) | 362 |
| 15 | 64 | 60 | endometrioid | IIA | 346 | yes | 33 (10%) | 177 |
BOT borderline ovarian tumor, MTV metabolic tumor volume, HSV hypoxic subvolume
All of the [18F]EF5-avid tumors also had increased uptake of [18F]FDG; however, only a weak statistical correlation between [18F]EF5 and [18F]FDG-uptake in tumors was detected (Spearman’s rho 0.33, p = 0.057). SUVmax values of EF5 and FDG uptake in the malignant primary tumors and metastases of 13 patients are presented in Fig. 1.
Fig. 1.
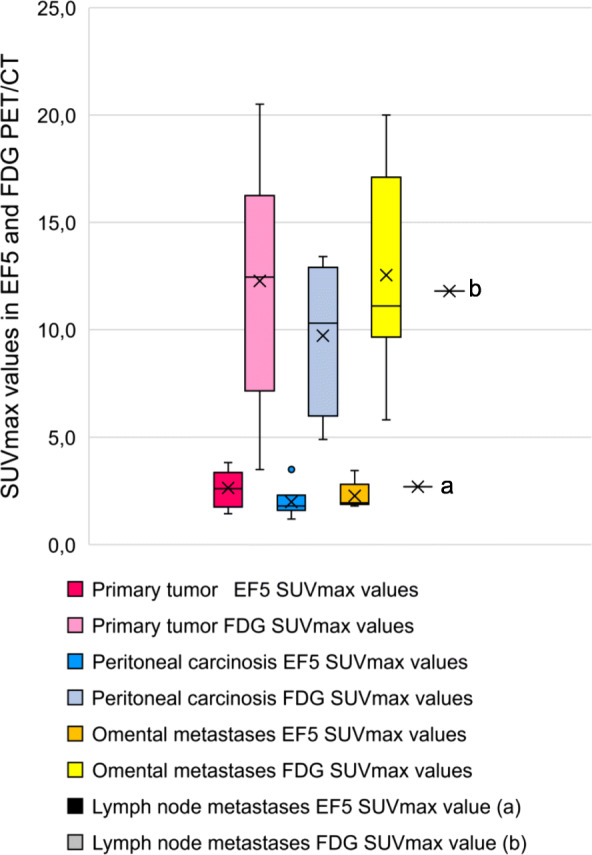
EF5 and FDG SUVmax values in malignant primary tumors and metastases with different anatomical location
Seven patients had FDG-avid peritoneal carcinosis, a finding typical of OC. Only 1 patient had [18F]EF5-accumulation in the peritoneal carcinosis (thickness < 1 cm). The distribution of the disease, MTV, and HSV values are presented in Fig. 2. None of the patients had [18F]EF5-avid metastases when the primary tumor PET-finding was not suggestive of hypoxia. There was no statistical difference between [18F]EF5-uptake in the primary tumor and metastases (p = 0.72).
Fig. 2.
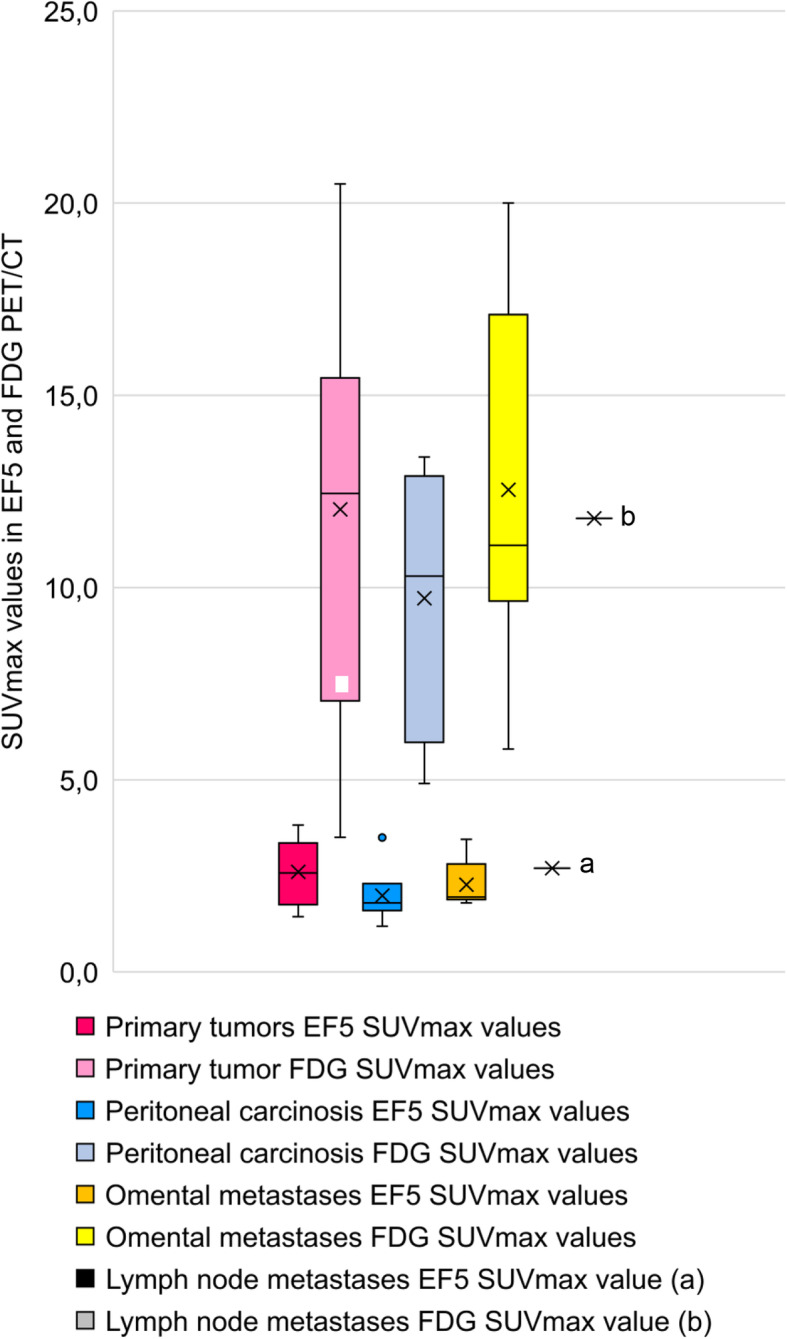
MTV (metabolic tumor volume, cm3) and HSV (hypoxic subvolume, cm3) of a particular patient (nr 1–15). Under the x-axis, the same patient’s distribution of the disease is presented
Our study included two patients with non-malign tumors (patient nr 8 and 11), which presented no [18F]EF5-accumulation.
The physiological uptake of [18F]EF5 in the gall bladder/bile, small intestine, and urinary bladder was notably higher than in the tumors (Fig. 3).
Fig. 3.
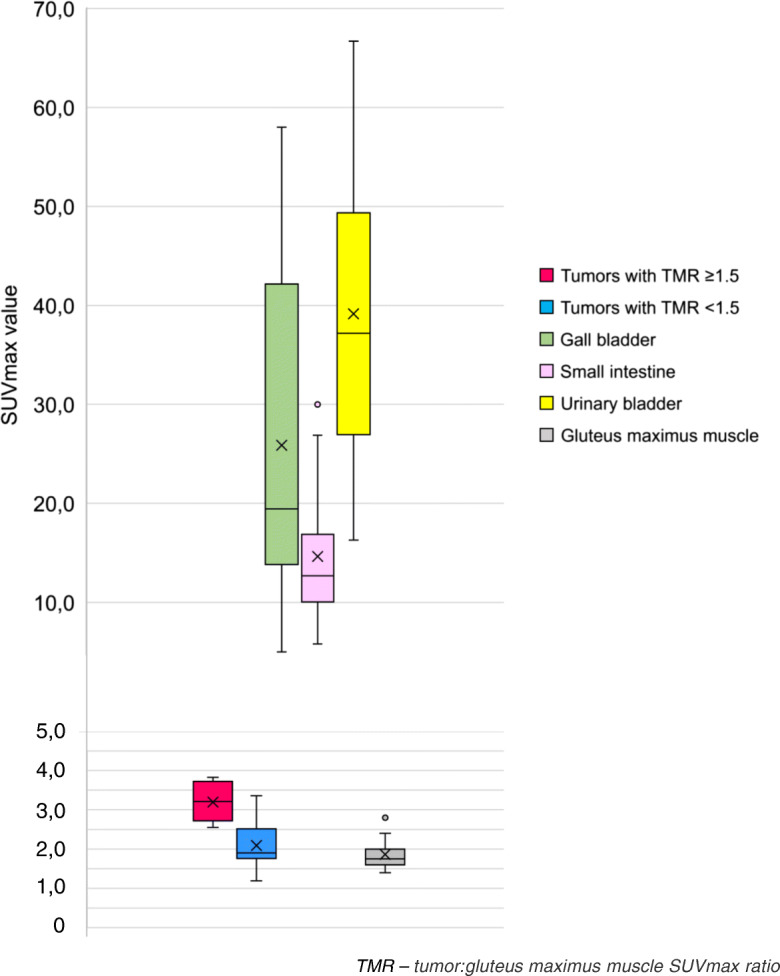
The SUV max values from different anatomical sites in [18F]EF5-PET/CT in patients with ovarian tumor
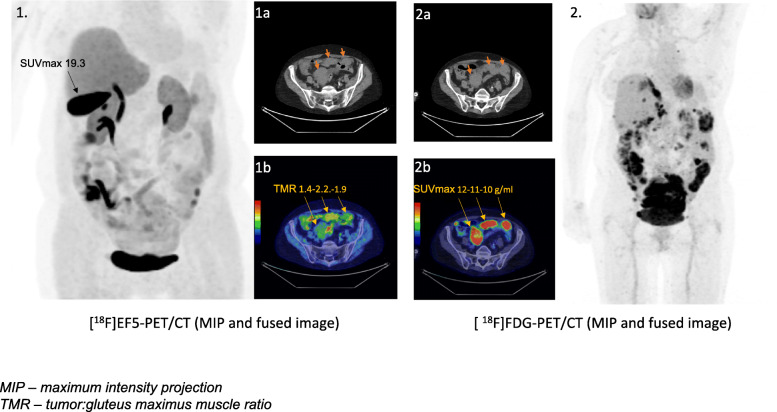
A demonstrative EF5- and FDG-PET/CT images of a patient with advanced ovarian cancer are presented in Fig. 4.
Fig. 4.
An image of a patient with advanced ovarian cancer. All three metastases are FDG-avid (2b) and 2 of them have also accumulated EF5 (1b). The SUVmax-values in [18F]EF5-avid (tumor:gluteus maximus muscle ratio ≥ 1.5) are remarkably lower (1b) compared to the physiological uptake in bile (1.)
Discussion
Hypoxia is a common phenomenon in cancer with 50–60% of solid tumors containing hypoxic regions [15]. While hypoxia has a well-established role in promoting hematogenous metastases of cancer cells [16], the hematogenous spread is rare in OC at the time of diagnosis [17]. It should also be noted that the role of hypoxia in the transcoelomic spread to the peritoneum and omentum (common to OC) has not been widely investigated. On the basis of our study, [18F]EF5-PET/CT suggested hypoxia in half of the patients and the distribution of [18F]EF5-uptake was variable. [18F]EF5-uptake was detected mainly inside the ovarian tumor and less often in metastases. One preclinical study suggested a hypoxic environment to induce omental/peritoneal metastases [18]. Another study [19] which included two OC patients detected EF5-uptake and severe hypoxia in a peritoneal carcinosis biopsied laparoscopically promptly after the injection of EF5. Our cases with widespread peritoneal carcinosis typically had several [18F]FDG-avid areas but only one patient had [18F]EF5-avid peritoneal lesion.
The previous hypoxia imaging studies are conducted mostly on solid and locally advanced tumors [3, 5, 14, 20, 21], while half of our patients had disseminated cancer in the abdominal cavity hampering the comparison of our results to the other studies. However, [18F]EF5-uptake values in our study were comparable to previously reported [18F]EF5 SUVmax values in head and neck tumor studies, where the tumor hypoxia was confirmed with immunohistochemical staining [3, 14, 20]. Our study suggests that hypoxia is not a common phenomenon OC in transcoelomic metastases.
According to our study, the feasibility of [18F]EF5 PET/CT in ovarian cancer imaging in clinical practice appears to be limited. An uptake of [18F]EF5 in tumors was relatively weak and as the tumors were also [18F]FDG-avid, the visual estimation of [18F]-EF5-PET/CT was strongly lead by [18F]FDG-PET/CT. The avidity to both [18F]EF5 and [18F]FDG can be explained with Warburg’s effect [22], where cancer cells rely widely on glycolysis and reduce their respiration regardless of tissue oxygenation level. Unlike OC, head and neck and lung cancer are isolated tumors that are not surrounded by physiologically EF5-affine tissues. Our study is prospective, and two tumors eventually appeared to be benign. Nevertheless, we consider it important to present them especially as they showed no EF5 uptake.
Previously, two excretory paths of highly lipophilic [18F]EF5-tracer have been demonstrated [23, 24]. In the latter, it was assumed that due to slow tracer biliary excretion, only small amounts of activity would be seen in the small intestine. However, our study revealed an excessive [18F]EF5-uptake in the bile and small intestine. In contrast to the supradiaphragmatic tumors, this phenomenon imposes limitations on assessing tumors presenting weak [18F]EF5-uptake. Especially when located near to the intestine, metastases may be easily be mistaken to physiological uptake and remain unnoted.
Conclusion
Non-invasive hypoxia imaging with [18F]EF5-PET/CT is possible, but its clinical use is restrained by the weak tumor uptake of the tracer compared to the non-specific uptake in excretory organs. The potential usefulness of with [18F]EF5-PET/CT in OC could be complementary to FDG-PET/CT with the intent to determine high-risk patients. The role of hypoxia in OC is intensively studied and [18F]EF5-PET/CT forms an attractive tool for patient stratification.
Acknowledgements
Trial registration: ClinicalTrials.gov Identifier: NCT04001023; Registered 27 June 2018; https://clinicaltrials.gov/ct2/show/NCT04001023
Abbreviations
- OC
Ovarian cancer
- [18F]EF5
18F-nitroimidazolpentafluoropropylacetamide
- MTV
Total metabolic tumor volume
- VCAR
Volume Computer-Assisted Reading
- SUVmax
Maximal standardized uptake value
- TMR
Tumor to gluteus maximus muscle ratio
- HSV
Hypoxic subvolume
Authors’ contributions
SH, MS, ML and JH conceptualized the paper; ML managed patient’s diagnostic workup; SF was responsible for the tracer production; MS, TN, and ML analyzed and reported the imaging findings; ML drafted the manuscript; and all the authors revised and commented on the paper and approved the final version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 667403 for HERCULES, Maud Kuistila Memorial Foundation, and South-West Finland Cancer Society.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval was obtained from the review board of Turku University Hospital (18.10.2016§443), and all subjects signed an informed consent form.
Consent for publication
A consent to publish individual persons’ data was obtained from that person.
Competing interests
The authors report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Bristow R, Tomacruz R, Armstrong DK, Trimble E, Montz F. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the paltinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Komar G, Lehtiö K, Seppänen M, Eskola O, Levola H, Lindholm P, et al. Prognostic value of tumour blood flow, [18F]EF5 and [18F]FDG PET/CT imaging in patients with head and neck cancer treated with radiochemotherapy. Eur J Nucl Med Mol Imaging. 2014:2042–50. [DOI] [PubMed]
- 4.Kinoshita T, Fujii H, Hayashi Y, Kamiyama I, Ohtsuka T, Asamura H. Lung cancer prognostic significance of hypoxic PET using 18 F-FAZA and 62 Cu-ATSM in non-small-cell lung cancer. 2016;91:56–66. [DOI] [PubMed]
- 5.Qian Y, Eyben R Von, Liu Y, Chin FT, Miao Z, Apte S, et al. F-EF5 PET-based imageable hypoxia predicts local recurrence in tumors treated with highly conformal radiation therapy. Radiat Oncol Biol. 2018;1–10. Available from: 10.1016/j.ijrobp.2018.03.045. [DOI] [PubMed]
- 6.Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527(7579):S217. doi: 10.1038/527S217a. [DOI] [PubMed] [Google Scholar]
- 7.Shen W, Li H, Liu L, Cheng J. Expression levels of PTEN, HIF-1 α, and VEGF as prognostic factors in ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21:2596–2603. [PubMed] [Google Scholar]
- 8.Wilson WR, Hay MP. Hypoxia influences many aspects of the biology of tumours and their responses to therapy. Nat Publ Gr. 2011;11.
- 9.Ziemer LS, Evans SM, Kachur A V, Shuman AL, Cardi CA, Jenkins WT, et al. Original article noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18 F-EF5. 2003;30(2). [DOI] [PubMed]
- 10.Gaertner FC, Souvatzoglou M, Brix G, Beer AJ. Imaging of hypoxia using PET and MRI. Curr Pharm Biotechnol. 2012 [cited 2019 Dec 19];13(4):552–570. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22214501. [DOI] [PubMed]
- 11.Chapman JD, Franko AJ, Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981;43(4):546–550. doi: 10.1038/bjc.1981.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch CJ, Scheuermann JS, Divgi C, Judy KD, Kachur AV, Freifelder R, et al. Biodistribution and dosimetry of 18F-EF5 in cancer patients with preliminary comparison of 18F-EF5 uptake versus EF5 binding in human glioblastoma. Eur J Nucl Med Mol Imaging. 2010;37(11):2048–2059. doi: 10.1007/s00259-010-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasey JS, Grunbaum Z, Magee S, Nelson NJ, Olive PL, Durand RE, et al. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat Res. 1987;111(2):292. doi: 10.2307/3576986. [DOI] [PubMed] [Google Scholar]
- 14.Komar G, Seppänen M, Eskola O, Lindholm P, Grönroos TJ, Forsback S, et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. J Nucl Med. 2008;49(12):1944–1951. doi: 10.2967/jnumed.108.053785. [DOI] [PubMed] [Google Scholar]
- 15.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007[cited 2019 Sep 16];26(2):225–39. Available from: http://link.springer.com/10.1007/s10555-007-9055-1. [DOI] [PubMed]
- 16.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016 [cited 2019 Sep 16];352(6282):175–180. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27124451. [DOI] [PMC free article] [PubMed]
- 17.Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. [cited 2019 19];13(2):125–129. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12657111. [DOI] [PubMed]
- 18.Natarajan S, Foreman KM, Soriano MI, Rossen NS, Shehade H, Fregoso DR, et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res [Internet]. 2019 [cited 2019 Sep 16];79(9):2271–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30862717. [DOI] [PMC free article] [PubMed]
- 19.Busch TM, Hahn SM, Wileyto EP, Koch CJ, Fraker DL, Zhang P, et al. Hypoxia and photofrin uptake in the intraperitoneal carcinomatosis and sarcomatosis of photodynamic therapy patients. Clin Cancer Res. 2004;10(14):4630–4638. doi: 10.1158/1078-0432.CCR-04-0359. [DOI] [PubMed] [Google Scholar]
- 20.Silvoniemi A, Suilamo S, Laitinen T, Forsback S. Repeatability of tumour hypoxia imaging using [ 18 F ] EF5 PET/CT in head and neck cancer. Eur J Nucl Med Mol Imaging [Internet]. 2017; Available from: 10.1007/s00259-017-3857-3%0AORIGINALARTICLE%0ARepeatability. [DOI] [PMC free article] [PubMed]
- 21.Yapp DTT, Woo J, Kartono A, Sy J, Oliver T, Skov KA, et al. Non-invasive evaluation of tumour hypoxia in the Shionogi tumour model for prostate cancer with 18 F-EF5 and positron emission tomography. BJU Int. 2007 1 [cited 2019 Sep 19];99(5):1154–60. Available from: http://doi.wiley.com/10.1111/j.1464-410X.2007.06761.x. [DOI] [PubMed]
- 22.Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12(50):1131–1137. doi: 10.1007/BF01504608. [DOI] [Google Scholar]
- 23.Eskola O, Grönroos TJ, Forsback S, Tuomela J, Komar G, Bergman J, et al. Tracer level electrophilic synthesis and pharmacokinetics of the hypoxia tracer [ 18F]EF5. Mol Imaging Biol. 2012;14(2):205–212. doi: 10.1007/s11307-011-0484-4. [DOI] [PubMed] [Google Scholar]
- 24.Lin LL, Silvoniemi A, Stubbs JB, Rengan R, Suilamo S, Solin O, et al. Radiation dosimetry and biodistribution of the hypoxia tracer 18 F-EF5 in oncologic patients. Cancer Biother Radiopharm. 2012;27(7):412–419. doi: 10.1089/cbr.2011.1130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.