Abstract
Objective:
The majority of cases of the fatal neurodegenerative disease amyotrophic lateral sclerosis (ALS) are of unknown etiology. A proportion of these cases are likely to be attributable to contaminant exposures, although the specific environmental etiology of ALS remains largely a mystery. Certain forms of the neurotoxic metal mercury readily cross into the central nervous system. Fish is a dietary source of methymercury, but also of beneficial components, such as omega-3 polyunsaturated fatty acids. Prior work using clinic-based studies of toenails and hair as keratinous biomarkers of exposure suggest elevated mercury levels in ALS patients compared with controls. We sought to validate this relationship in a U.S. case-control comparison of mercury levels in nail clippings.
Methods:
We performed trace element analysis using inductively coupled plasma mass spectrometry (ICP-MS) on the nail clippings of n=70 female, geographically representative ALS patients from the National ALS Biorepository and compared them to n=210 age-matched controls from a set of n=1216 nationally distributed controls from the Sister and Two Sister Studies.
Results:
Compared to the lowest quartile of nail mercury, moderate levels were associated with decreased risk of ALS (P=4.18e-6). However, the odds of having nail mercury levels above the 90th percentile were 2.3 fold higher among ALS patients compared with controls (odds ratio (OR)=2.3, 95% confidence interval 1.10 – 4.58, adjusted for age and smoking status).
Conclusions:
This finding suggests that excessive mercury exposure may be associated with the neurodegenerative health of aging populations.
Keywords: biomarker, keratin, nail, mercury, amyotrophic lateral sclerosis, ALS, U.S.
Introduction
In amyotrophic lateral sclerosis (ALS), the selective death of motor neurons in the brain and spinal cord produces debilitating symptoms of progressive weakness, muscle wasting, and spasticity. The onset is most commonly between 55 and 75 years of age; the average life expectancy of a person with ALS is two to five years from the time of diagnosis (1). A simple genetic explanation for sporadic ALS seems unlikely, because a whole-genome sequencing study of ALS phenotypically discordant monozygotic twins found no coding, regulatory nucleotide, or genome-wide structural differences (2). With only 10% of cases attributable to known inherited mutations, researchers have hypothesized that environmental exposures, including toxic chemicals, may play an important role. However, few such environmental risk factors have been identified (3).
Several case-reports of mercury poisoning have demonstrated convincing ALS-like clinical symptoms, leading authors to postulate a causal relationship (4–6). Rats exposed to methylmercury (2 mg/kg/day) had ALS-like symptoms, with nerve conduction velocity abnormalities, tail-flick responses, and inhibited motor equilibrium performance (7). In vitro proteomics studies show that methylmercury treatment causes electron transport chain dysfunction, oxidative stress, and impairs ubiquitin proteasome system protein degradation (8), molecular mechanisms that are thought to be involved in the pathogenesis of ALS.
Methylmercury exposure via fish consumption has been suggested as an ALS risk factor, however evidence from questionnaire-based studies is mixed (9–11). The relationship between ALS and fish consumpution is complex due to beneficial components, such as omega-3 polyunsaturated fatty acids (12). Methylmercury binds to the thiol group of cysteine, mimicking the neutral amino acid methionine, which allows it to easily cross the blood-brain barrier into neurons (13). Inhalation of mercury vapor is another route of exposure, although rare in the U.S. other than in dental occupations. Elemental mercury and methyl mercury are both lipid soluble and easily diffuse into the central nervous system (14). After entering the brain, mercury is converted to the inorganic form, where it is thought to have a half-life of several years to decades (15).
Keratinous nail tissue is a reliable biomarker because the thiol (-SH) groups of the amino acid cysteine bind methylmercury and other trace elements with high affinity (16). Non-invasive nail clippings are thought to represent 4–6 months of exposure occurring approximately 6–12 months before collection and correlate with the level of methylmercury exposure from fish (17, 18). Only 10% of nail mercury represents mercury derived from inorganic sources, such as dental amalgam in non-occupationally exposed populations (19). In our prior clinic-based work in northern New England, we observed higher toenail Hg levels in 46 ALS patients, compared with n=66 controls who did not have neurodegenerative illness (p=0.024) (20). In the present analysis, we sought to validate this mercury finding in an independent U.S. cohort using a case-control comparison. We assessed additional trace elements in the nails to verify that the disease had not broadly affected the element disposition.
Methods
Eligible ALS patients were identified from the National ALS Biorepository of the population-based National ALS Registry project, which is conducted by the U.S. federal Agency for Toxic Substances and Disease Registry (ATSDR), part of the Centers for Disease Control and Prevention (CDC) (21, 22). ALS patients in the National ALS Registry are identified using a two-pronged approach. The first approach identifies cases from three large national administrative databases (Medicare, Veterans Health Administration, and Veterans Benefits Administration) by using an algorithm with elements such as the International Classification of Diseases (ICD) code for ALS, frequency of visits to a neurologist, and prescription drug use. The second approach is a secure web portal that enables persons with ALS to answer a series of validation questions developed by the Veterans Administration in order to enroll in the Registry, thereby identifying cases not Included in the national administrative databases (23, 24). The distribution of patients recruited for the Biorepository included representation from each state in the U.S. in proportion to the state population. A total of 330 patients contributed to the Biorepository between 2013 and 2015 out of 458 patients who were sent biospecimen information packets (74%). Biospecimens, including fingernail clippings (left and right hand pooled), were collected during in-home visits to the patients. Toenails and fingernails are both strong keratinous biomarkers of methylmercury exposure that are highly correlated within individuals (r=0.92, p<0.01) (16). For this study, we assessed metal levels in the Dartmouth Trace Element Analysis Core on the nails from 84 of the randomly selected female participants, who ranged in age from 30 to 72 at diagnosis. Our analysis was restricted to n=70 women under age 67 in order to match the age-range and sex of the Sister and Two Sister Study participants, who served as a unique source of controls with trace elements measured in nails from the same laboratory as the ALS cases.
For a comparison group, we used participants from the Sister Study (25) and Two Sister Study (26) who previously had nail metal levels assessed in the same laboratory as the ALS cases (Dartmouth Trace Element Analysis Core) (27). The eligible controls were U.S. women aged 35–66 years who provided data and large toenail clippings (right and left foot pooled) at a baseline visit (2003–2009) and had a sister diagnosed with young-onset breast cancer (age at diagnosis <50), but had never been diagnosed with breast cancer themselves at enrollment (n=1216) (27). We utilized the R-package “MatchIt” to perform nearest neighbor matching of controls to the cases in a 3:1 ratio based on age at diagnosis (28). With this propensity score matching, we selected a subset of n=210 as a comparison group in our case-control analysis.
All study participants provided informed consent. Study procedures were conducted under the oversight of the Dartmouth Committee for Protection of Human Subjects and Sister Study controls were overseen by the Institutional Review Board of the National Institute of Environmental Health Sciences (NIEHS). Biorepository Pilot Study procedures were conducted under the oversight of Western Institutional Review Board.
Metal levels
Any visible dirt was removed from the nails and they were transferred to a polyethylene vial with 2 ml of acetone for a 20 min ultrasonic bath. Nails were then similarly washed with a 1% solution of Triton X-100, followed by 5 washes with deionized water. This washing procedure removes external contamination (nail polish, dirt etc.) without extracting metals from inside the nails. The washed nail clippings were then acid-digested with HNO3 using a MARS6 microwave digestion unit, accounting for the weight of the nail sample (CEM, Mathews, NC). The Dartmouth Trace Element Analysis Core Facility measured 16 trace-elements in the nail samples (arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), mercury (Hg), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), antimony (Sb), selenium (Se), tin (Sn), vanadium (V), zinc (Zn)). This analysis was performed by inductively-coupled plasma mass spectrometry (ICP-MS) on an Agilent 8800 ICP-QQQ (Santa Clara, CA), following EPA 6020 protocol. Multi-point calibration curves (n ≥ 5) were constructed for each analyte with correlation coefficient criteria >0.995. The calibration was followed by an Initial Calibration Blank, an Initial Calibration Verification (ICV), and Continuing Calibration Verifications (CCVs) after every 10 samples.
To correct for any batch effects or differences in measured levels due to shifts in exposure to metals based on the year of nail collection (29), we applied the ‘ComBat’ procedure to normalize the log10 levels of all 16 elements by the year of nail collection, grouped to include both cases and controls in each batch-group (30). This procedure was implemented in the ‘sva’ package (version 3.28.0) in R software using a parametric empirical Bayes framework to adjust the data for any time-related batch effects (31).
Statistical analysis
We first performed univariate analyses to assess participant characteristics and covariates using chi-square tests for categorical factors and t-tests for continuous variables. We also estimated p-values for the association between log10 continuous levels, as well as ≥90th percentile versus <90th percentile of each of the 10 elements modeled individually (Hg, As, Cd, Co, Cr, Cu, Fe, Mn, Pb and Se) and ALS. These analyses were adjusted for age (cateogorized <50, 50–55, 55–60, 60+) and smoking (ever/never) status. Spline plots were constructed in R-software using the “rms” package, with adjustment for age and smoking status (32). These spline plots revealed a non-linear relationship between nail mercury and ALS, so we used a multivariable unconditional logistic regression model to estimate the odds ratio (OR) and 95% confidence intervals for categories of nail mercury selected based on the inflection points.
Data availability: Data sharing is restricted by human subjects data management restrictions, governed by the institutional committee for protection of human subjects.
Results
The mean age of the ALS patients was 55, similar to that of the matched controls (Table 1). The smoking status, smoking duration, level of education, and racial / ethnic makeup did not significantly differ between ALS patients and the matched controls.
Table 1.
Population characteristics.
| Controls n=210 | ALS patients n=70 | univariate p-value | |
|---|---|---|---|
| Age (mean (range)) | 54.0 (35–66) | 55.5 (30–66) | 0.08 |
| Smoking | n (%) | n (%) | |
| never | 129 (61.4) | 34 (48.6) | 0.13 |
| current | 13 (6.2) | 4 (5.7) | |
| past | 68 (32.4) | 32 (45.7) | |
| Smoked <5 years | 147 (70.0) | 42 (60.0) | 0.16 |
| 5+ years | 63 (30.0) | 28 (40.0) | |
| Education | 0.085 | ||
| High school or less | 32 (15.2) | 12 (17.1) | |
| Technical or some college | 72 (34.3) | 13 (18.6) | |
| College degree | 51 (24.3) | 24 (34.3) | |
| Graduate school | 55 (26.2) | 21 (30.0) | |
| Race / ethnicity | 1 | ||
| White, non-hispanic | 203 (96.7) | 68 (97.1) | |
| Other | 7 (3.3) | 2 (2.9) |
The mean (log10 μg/g) nail mercury levels did not differ significantly between ALS patients and controls (Supplemental Table 1). The spline plots in Figure 1 showed a u-shaped relationship between nail mercury and ALS risk, adjusted for age and smoking. The logistic regression results calculated from this spline model (Table 2) showed decreased risk of ALS at the 25th vs. the 5th percentile for nail mercury, but increased risk at the highest levels of nail mercury (90th vs. 75th percentiles). We also used the inflection points from the spline plot to assess risk in categories. Compared to the lowest quartile of nail mercury, moderate levels of nail mercury (25th – 90th percentile) were associated with a statistically significant decreased risk of ALS (OR 0.21 95%CI 0.11–0.41, p=4.18e-6). In contrast, very elevated levels of Hg exposure were associated with increased risk of ALS. As shown in Table 3, we observed a 2.6-fold increased risk of ALS associated with having nail Hg ≥ 90th percentile (OR 2.64 95%CI 1.12–6.18, p=0.024 adjusted for age, smoking status). This association remains elevated with adjustment for age and level of education (OR 2.54 95%CI 1.05–6.07).
Figure 1.
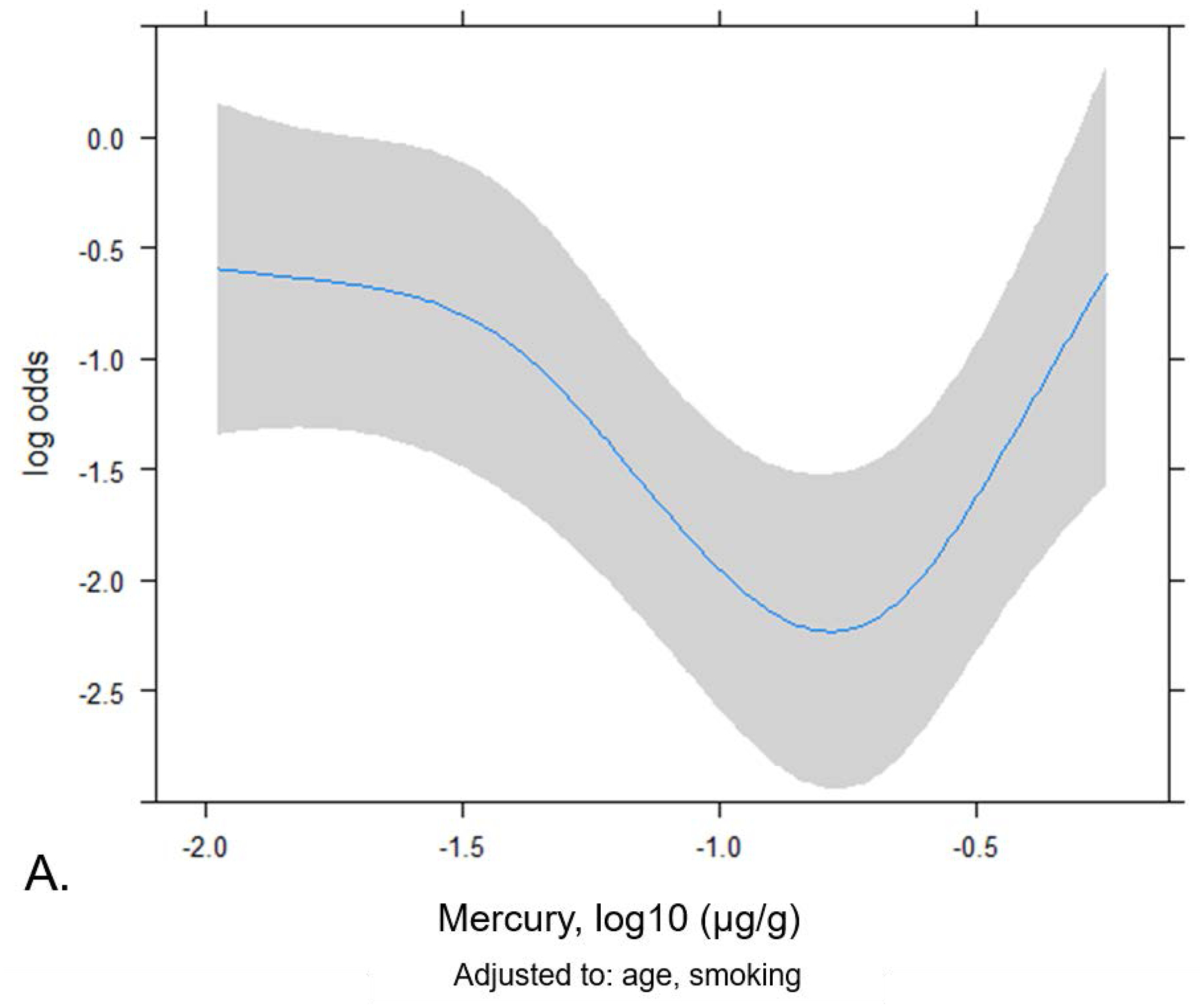
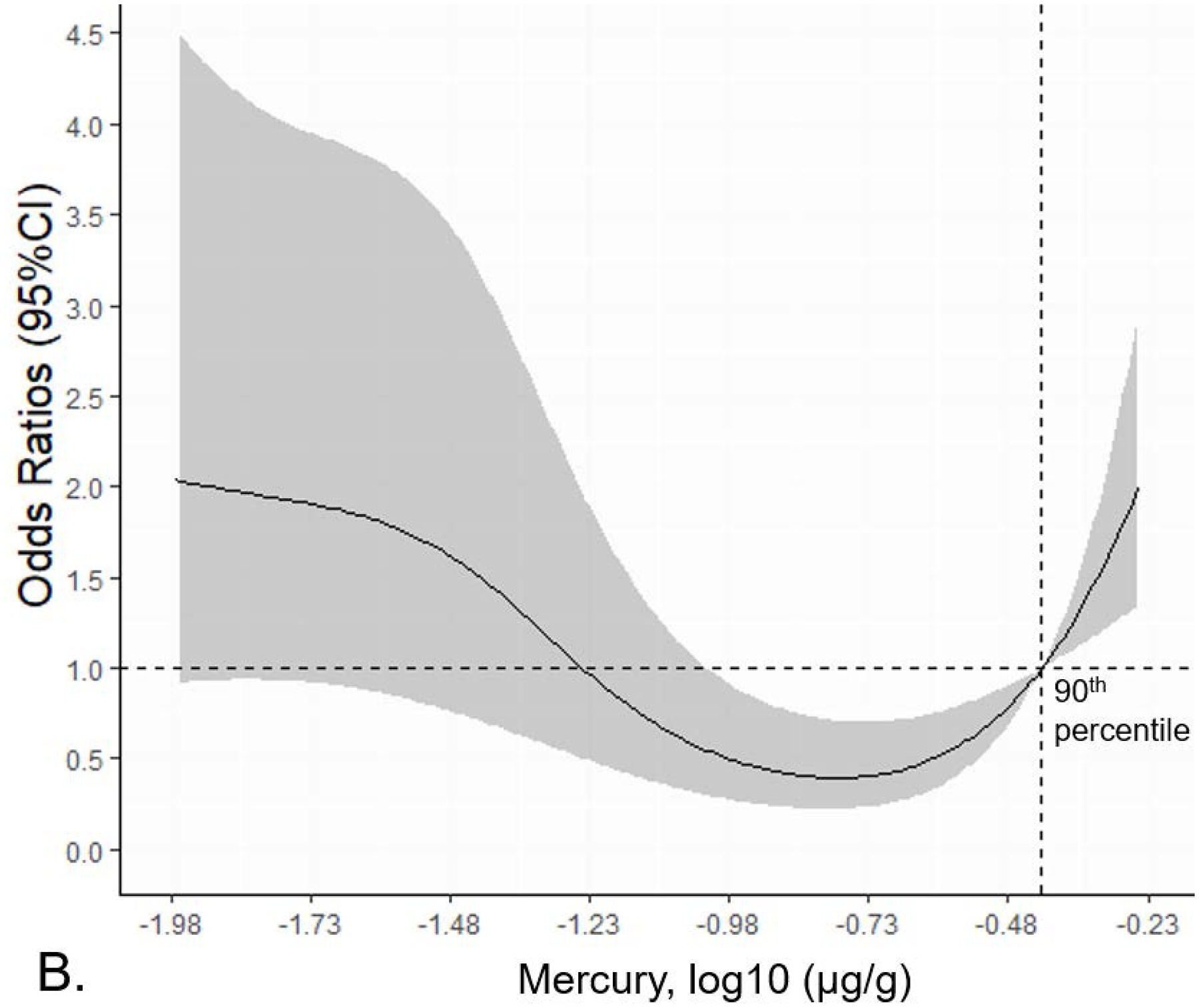
Spline plot showing the non-linear relationship between nail mercury levels and ALS risk. A) Log odds of ALS plotted by nail mercury (log10 (μg/g) adjusted for age and smoking. B) Odds ratios for ALS risk associated by nail mercury (log10 (μg/g).
Table 2.
Logistic regression odds ratios for ALS risk by nail Hg level calculated from spline model
| Percentile | Hg (μg/g) | Multivariable* Odds Ratio | (95% CI) |
|---|---|---|---|
| 25th vs. 5th | 0.041 vs. 0.016 | 0.53 | (0.37–0.76) |
| 75th vs. 25th | 0.21 vs. 0.041 | 0.8 | (0.55–1.15) |
| 90th vs. 75th | 0.38 vs. 0.2 | 1.54 | (1.07–2.22) |
Adjusted for age, smoking status.
Table 3.
ALS risk associated with categorized nail Hg levels.
| Nail mercury level | μg/g | Controls n=210 (%) | ALS patients n=70 (%) | Univariate Odds Ratio | (95% CI) | Multivariable* Odds Ratio | (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| <25th percentile | < 0.042 | 41 (19.5) | 30 (42.9) | 1.0 (ref) | 1.0 (ref) | |||
| 25th–90th percentile | 0.042–0.38 | 154 (73.3) | 27 (38.6) | 0.24 | (0.13–0.45) | 0.21 | (0.11–0.41) | 4.18E–06 |
| < 90th percentile | < 0.38 | 195 (92.9) | 57 (81.4) | 1.0 (ref) | 1.0 (ref) | |||
| ≥ 90th percentile | ≥ 0.38 | 15 (7.1) | 13 (18.6) | 2.96 | (1.32–6.61) | 2.64 | (1.12–6.18) | 0.024 |
Adjusted for age, smoking status.
We also evaluated the levels of the 10 trace elements measured in nails as a biomarker (Hg, As, Cd, Co, Cr, Cu, Fe, Mn, Pb, Se), again with adjustment for age and smoking status, to check for broad, disease-related differences in the distribution. The levels of the assessed elements did not differ among cases and controls (Supplemental Table 1) (20). Comparison of the proportion of cases vs. controls above vs. below the 90th percentile for each trace element showed a significant difference for mercury (chi-square P=0.011), but not for the other elements (Supplemental Table 2).
In order to identify participant characteristics associated with having nail Hg levels higher than the 90th percentile, we also performed an analysis within the full control group (n=1216) (Table 4). Elevated Hg was not related to age (p=0.96) or race / ethnicity (p=0.91). Current smoking was not significantly associated with elevated Hg, compared to never smoking. However, 36.9% of controls with Hg ≥ 90th percentile were past smokers, compared with 24.7% of those with lower Hg levels (multivariable OR 1.97 95%CI 1.29–3.00 for former versus never smokers, adjusted for age and income bracket). The controls with Hg ≥ 90th percentile also had higher levels of education than those with lower exposure (p=0.048). Among controls with Hg ≥ 90th percentile 68.8% had a Bachelors, Masters, or Doctoral degree, compared to 56.1% of those with lower toenail Hg levels. Elevated toenail Hg levels were also strongly related to increasing income. 22% of controls with Hg ≥ 90th percentile had a household income of $200,000 or more, compared to 8% of those with lower Hg levels (multivariable OR 4.05 95%CI 2.32 – 6.96, adjusted for age and smoking status).
Table 4.
Factors associated with high nail Hg levels in controls.
| Analysis restricted to controls: | Toenail Hg by 90th percentile | univariate multivariable* | ||||
|---|---|---|---|---|---|---|
| <90th | ≥90th | p-value | OR | (95%CI) | ||
| n=1094 | n=122 | |||||
| Age (mean (SD)) | 46.81 (6.20) | 46.79 (6.26) | 0.96 | 0.99 | (0.96–1.02) | |
| Smoking (%) | n (%) | n (%) | 0.014 | |||
| never | 737 (67.4) | 68 (55.7) | 1.0 ref | |||
| current | 87 (8.0) | 9 (7.4) | 1.27 | (0.54–2.63) | ||
| past | 270 (24.7) | 45 (36.9) | 1.97 | (1.29–3.00) | ||
| Ever smoker | 357 (32.6) | 54 (44.3) | 0.013 | |||
| Race, ethnicity (%) | 0.91 | |||||
| Non-Hisp. White | 980 (89.7) | 109 (89.3) | ||||
| Other | 113 (10.3) | 13 (10.7) | ||||
| Education level of participant (%) | 0.048 | |||||
| High school or less | 122 (11.2) | 11 (9.0) | ||||
| Some college or technical | ||||||
| degree | 359 (32.8) | 27 (22.1) | ||||
| Bachelor’s degree | 353 (32.3) | 51 (41.8) | ||||
| Master or Doctoral degree | 260 (23.8) | 33 (27.0) | ||||
| Household income per-person (mean (SD)) | $38,263 (23,921) | $56,393 (35,211) | 3.37E–07 | |||
| Income bracket (%) | 1.80E–06 | |||||
| Less than $100,000 | 614 (58) | 45 (39) | 1.0 ref | |||
| $100,000 to $200,000 | 363 (34) | 45 (39) | 1.75 | (1.13–2.71) | ||
| $200,000+ | 89 (8) | 25 (22) | 4.05 | (2.32–6.96) | ||
Model includes age, smoking status, income bracket. Missing race n=1, income n=35.
Discussion
We observed a u-shaped relationship between nail mercury and ALS risk. Moderate levels of nail mercury were associated with decreased risk, while ALS patients had a 2.3-fold increased risk of having nail Hg levels above the 90th percentile, compared to controls. The excess risk, which was estimated using a U.S.-wide case-control study, are consistent with our prior clinic-based findings from a small New England-based sample (20). The prior studies that used keratinous biomarkers of exposure provide further evidence supporting this link. For example, hair levels of mercury were higher among 15 ALS patients in the Nara and Mie regions of Japan, compared with 26 normal controls (p<0.05) (33). The levels of mercury in hair were also higher in ALS patients from Sardinia, Italy (n=34, median 0.68 μg/g, 95th percentile 3.74 μg/g), compared with controls (n=30 median 0.45 μg/g, 95th percentile 1.49 μg/g), although the difference was not statistically significant (34). Similarly, hair Hg was modestly higher in Japanese ALS patients who were not bed-ridden (ALS n=7 2.9±0.82 μg/g vs. controls n=20 2.5±1.29 μg/g, and vs. patients with other neurological diseases n=19 2.40±1.25 μg/g) (35).
The major source of mercury exposure for the U.S. population is the consumption of fish. High-trophic level fish (high in the food chain) bioaccumulate methylmercury, such as swordfish (36, 37). Some fish are also an excellent source of omega-3 polyunsaturated fatty acids, which were protective against ALS in a large prospective study of diet (12). The inverse relationship we observed between moderate nail mercury levels and ALS risk in the present study is not causal for mercury, per se, but rather, the moderate nail mercury denotes the regular fish consumers. In our prior work, we estimated annual methylmercury exposure among finfish and shellfish consumers by cross-referencing self-reported consumption with the corresponding fish fillet mean methylmercury concentrations. We observed that toenail Hg levels in participants from northern New England were positively correlated with estimated fish-derived methylmercury consumption (p=0.018) (20). Participants in that study who reported that they “eat fish at least monthly” had moderately higher levels of mercury in their toenails (mean 0.21 μg/g) than those who do not (mean 0.11 μg/g), p=0.021 (20). Thus, toenail mercury levels are a biomarker for fish consumption. We speculate that the u-shape reflects a benefical effect of consumption of fish that do not have very high levels of mercury, but this finding motivates future work to identify the individual protective dietary components underlying this effect.
In the Sister Study control sample, we observed an association between elevated Hg exposure (≥ 90th percentile) and higher income. This matches the 1999–2010 NHANES data showing that blood Hg concentration of U.S. women was significantly higher when the household income was ≥ $75,000, compared to those with incomes <$20,000. Consumption of fish was also linked to higher household income (p<0.01), and swordfish/shark consumption (p=0.05), which was in turn associated with elevated blood Hg levels (OR 6.05, 95%CI: 1.77 – 20.29) (38). Thus, income seems to be in the causal pathway for high-trophic level fish consumption.
The molecular mechanism for mercury-induced neurodegeneration is not yet established. Methylmercury activates the mitochondrial permeability transition pore and elevates presynaptic Ca2+, leading to enhanced glutamate release and an excitotoxic response in rat neurons (39). Mercury uptake at motor nerve terminals in the muscle and retrograde axonal transport to the cell bodies is thought to deposit mercury in the spinal motor neurons when mice are dosed with HgCl2 (40). Mercury was also found in the central nervous system cells of an exposed human (41). Compared to controls, the locus coeruleus and motor neurons in patients with motor neuron disease had higher levels of silver nitrate autometallographic staining, potentially reflecting the presence of mercury (42).
A gene-environment interaction mechanism is supported by the report that mice with the ALS-associated SOD1 G93A mutation who were exposed to methylmercury in their drinking water (1–3 ppm/day) showed early onset hind limb weakness and shorter time to rotarod failure, compared to unexposed or SOD1 wild-type mice (43).
One limitation of our study is that the nails of the ALS patients were collected more recently than those of the controls. Although NHANES data show a decrease in blood Hg levels in U.S. women since the late 1990s, that trend has plateaued in recent years and Hg levels did not decrease between 2005 – 2010 (38). Correcting our analysis for sample collection year to account for any batch or temporal effects did not change the results (29). The levels we observed in the control group were similar to those of the men in the Normative Aging Study (median Hg 0.34 μg/g, SD 0.44) (44). Another limitation is that the ALS patient nails were collected after diagnosis. Nail tissue is a biomarker that integrates exposure over several months, mitigating the effects of recent behavioral changes. The time from diagnosis to nail collection was also not correlated with the Hg level (p=0.51). The case-control similarity in the mean levels of the 10 trace elements assessed reassured us that there was no disease-related fundamental change in the nails or broad-based effect on distribution of elements in the body. While we did not collect body mass index at the time of nail collection, a prior study showed that the median toenail mercury level was the same for participants with low body mass index (BMI) (median Hg 0.22 μg/g for both BMI <25 and for BMI 25–29) (44). Although the medium assessed was toenails in the cases and fingernails in the controls, Hg levels in matched fingernails and toenails from the same individuals are highly correlated (r=0.923, p<0.01) (16).
In a nationally distributed case-control comparison, we observed that women with ALS were more likely than controls to have very high nail levels of Hg. U.S. public health campaigns to reduce mercury exposure have focused on women of child-bearing age, but our results suggest a need for attention to older individuals, who also may be susceptible to mercury-related neurological health effects.
Supplementary Material
Acknowledgements
Funding for this study was provided by the Diamond Endowment Project at Dartmouth. The Trace Element Shared Analysis Core is supported by NCI Cancer Center Support Grant 5P30CA023108-37 and NIEHS Superfund grant P42 ES007373. We would like to thank the research staff and study participants who made this work possible. This research was supported in part through the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH, under projects ES103086 and ES044005.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of ATSDR, CDC, and/or the HHS.
Financial Disclosure: The authors report no disclosures.
References
- 1.Martin S, Khaleifat AA, Al-Chalabi A. What causes amyotrophic lateral sclerosis? F1000Research. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meltz Steinberg K, Nicholas TJ, Koboldt DC, Yu B, Mardis E, Pamphlett R. Whole genome analyses reveal no pathogenetic single nucleotide or structural differences between monozygotic twins discordant for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:385–92. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JP, Brown RH Jr., Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Praline J, Guennoc AM, Limousin N, Hallak H, de Toffol B, Corcia P. ALS and mercury intoxication: a relationship? Clin Neurol Neurosurg. 2007;109:880–3. [DOI] [PubMed] [Google Scholar]
- 5.Adams CR, Ziegler DK, Lin JT. Mercury intoxication simulating amyotrophic lateral sclerosis. JAMA. 1983;250:642–3. [PubMed] [Google Scholar]
- 6.Schwarz S, Husstedt I, Bertram HP, Kuchelmeister K. Amyotrophic lateral sclerosis after accidental injection of mercury. J Neurol Neurosurg Psychiatry. 1996;60:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuu JJ, Liu SH, Lin-Shiau SY. Differential neurotoxic effects of methylmercury and mercuric sulfide in rats. Toxicol Lett. 2007;169:109–20. [DOI] [PubMed] [Google Scholar]
- 8.Karri V, Ramos D, Martinez JB, Odena A, Oliveira E, Coort SL, et al. Differential protein expression of hippocampal cells associated with heavy metals (Pb, As, and MeHg) neurotoxicity: Deepening into the molecular mechanism of neurodegenerative diseases. J Proteomics. 2018;187:106–25. [DOI] [PubMed] [Google Scholar]
- 9.Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30:761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin Kullmann JA, Pamphlett R. A Comparison of Mercury Exposure from Seafood Consumption and Dental Amalgam Fillings in People with and without Amyotrophic Lateral Sclerosis (ALS): An International Online Case-Control Study. Int J Environ Res Public Health. 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sienko DG, Davis JP, Taylor JA, Brooks BR. Amyotrophic lateral sclerosis. A case-control study following detection of a cluster in a small Wisconsin community. Arch Neurol. 1990;47:38–41. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald KC, O’Reilly EJ, Falcone GJ, McCullough ML, Park Y, Kolonel LN, et al. Dietary omega-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–64. [DOI] [PubMed] [Google Scholar]
- 14.Rice KM, Walker EM Jr., Wu M, Gillette C, Blough ER. Environmental mercury and its toxic effects. J Prev Med Public Health. 2014;47:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney JP. The retention time of inorganic mercury in the brain--a systematic review of the evidence. Toxicol Appl Pharmacol. 2014;274:425–35. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto M, Chan HM, Domingo JL, Oliveira RB, Kawakami S, Murata K. Significance of fingernail and toenail mercury concentrations as biomarkers for prenatal methylmercury exposure in relation to segmental hair mercury concentrations. Environ Res. 2015;136:289–94. [DOI] [PubMed] [Google Scholar]
- 17.Rees JR, Sturup S, Chen C, Folt C, Karagas MR. Toenail mercury and dietary fish consumption. J Expo Sci Environ Epidemiol. 2007;17:25–30. [DOI] [PubMed] [Google Scholar]
- 18.Bergomi M, Vinceti M, Nacci G, Pietrini V, Bratter P, Alber D, et al. Environmental exposure to trace elements and risk of amyotrophic lateral sclerosis: a population-based case-control study. Environ Res. 2002;89:116–23. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Watanabe S, Matsuo N. Comparison of hair with nail as index media for biological monitoring of mercury. Sangyo Igaku. 1989;31:235–8. [DOI] [PubMed] [Google Scholar]
- 20.Andrew AS, Chen CY, Caller TA, Tandan R, Henegan PL, Jackson BP, et al. Toenail mercury Levels are associated with amyotrophic lateral sclerosis risk. Muscle Nerve. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton DK, Kaye W, Wagner L. Integrating a Biorepository Into the National Amyotrophic Lateral Sclerosis Registry. J Environ Health. 2016;79:38–40. [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond J, Oskarsson B, Mehta P, Horton K. Clinical characteristics of a large cohort of US participants enrolled in the National Amyotrophic Lateral Sclerosis (ALS) Registry, 2010–2015. Amyotroph Lateral Scler Frontotemporal Degener. 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antao VC, Horton DK. The National Amyotrophic Lateral Sclerosis (ALS) Registry. J Environ Health. 2012;75:28–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta P, Antao V, Kaye W, Sanchez M, Williamson D, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis - United States, 2010–2011. MMWR Suppl. 2014;63:1–14. [PubMed] [Google Scholar]
- 25.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, et al. The Sister Study Cohort: Baseline Methods and Participant Characteristics. Environ Health Perspect. 2017;125:127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fei C, Deroo LA, Sandler DP, Weinberg CR. Fertility drugs and young-onset breast cancer: results from the Two Sister Study. J Natl Cancer Inst. 2012;104:1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien KM, White AJ, Jackson BP, Karagas MR, Sandler DP, Weinberg CR. Toenail-Based Metal Concentrations and Young-Onset Breast Cancer. Am J Epidemiol. 2019;188:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Ho D, Imai K, King G, Stuart E. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysisi. 2007;15:199–236. [Google Scholar]
- 29.O’Brien KM, White AJ, Sandler DP, Jackson BP, Karagas MR, Weinberg CR. Do Post-breast Cancer Diagnosis Toenail Trace Element Concentrations Reflect Prediagnostic Concentrations? Epidemiology. 2019;30:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27. [DOI] [PubMed] [Google Scholar]
- 31.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrell FJ. rms: Regression Modeling Strategies. R package version 5.1–0. 2017. [Google Scholar]
- 33.Mano Y, Takayanagi T, Ishitani A, Hirota T. [Mercury in hair of patients with ALS]. Rinsho Shinkeigaku. 1989;29:844–8. [PubMed] [Google Scholar]
- 34.Bocca B, Forte G, Oggiano R, Clemente S, Asara Y, Peruzzu A, et al. Level of neurotoxic metals in amyotrophic lateral sclerosis: A population-based case-control study. J Neurol Sci. 2015;359:11–7. [DOI] [PubMed] [Google Scholar]
- 35.Moriwaka F, Satoh H, Ejima A, Watanabe C, Tashiro K, Hamada T, et al. Mercury and selenium contents in amyotrophic lateral sclerosis in Hokkaido, the northernmost island of Japan. J Neurol Sci. 1993;118:38–42. [DOI] [PubMed] [Google Scholar]
- 36.Karimi R, Fisher NS, Meliker JR. Mercury-nutrient signatures in seafood and in the blood of avid seafood consumers. Sci Total Environ. 2014;496:636–43. [DOI] [PubMed] [Google Scholar]
- 37.Gribble MO, Karimi R, Feingold BJ, Nyland JF, O’Hara TM, Gladyshev MI, et al. Mercury, selenium and fish oils in marine food webs and implications for human health. J Mar Biol Assoc UK. 2016;96:43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cusack LK, Smit E, Kile ML, Harding AK. Regional and temporal trends in blood mercury concentrations and fish consumption in women of child bearing Age in the united states using NHANES data from 1999–2010. Environ Health. 2017;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limke TL, Otero-Montanez JK, Atchison WD. Evidence for interactions between intracellular calcium stores during methylmercury-induced intracellular calcium dysregulation in rat cerebellar granule neurons. J Pharmacol Exp Ther. 2003;304:949–58. [DOI] [PubMed] [Google Scholar]
- 40.Arvidson B Accumulation of inorganic mercury in lower motoneurons of mice. Neurotoxicology. 1992;13:277–80. [PubMed] [Google Scholar]
- 41.Pamphlett R, Kum Jew S. Inorganic mercury in human astrocytes, oligodendrocytes, corticomotoneurons and the locus ceruleus: implications for multiple sclerosis, neurodegenerative disorders and gliomas. Biometals. 2018;31:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamphlett R, Kum Jew S. Heavy metals in locus ceruleus and motor neurons in motor neuron disease. Acta Neuropathol Commun. 2013;1:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson FO, Yuan Y, Hajela RK, Chitrakar A, Parsell DM, Atchison WD. Exposure to an environmental neurotoxicant hastens the onset of amyotrophic lateral sclerosis-like phenotype in human Cu2+/Zn2+ superoxide dismutase 1 G93A mice: glutamate-mediated excitotoxicity. J Pharmacol Exp Ther. 2011;338:518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect. 2012;120:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


