Abstract
Purpose of Review:
Despite a call for better understanding of the role of environmental pollutant influences on mental health and the tremendous public health burden of mental health, this issue receives far less attention than many other effects of pollutants. Here we summarize the body of literature on non-occupational environmental pollutant exposures and adult depression, anxiety, and suicide—in PubMed, Embase, Web of Science, and PsychINFO through the end of year 2018.
Recent Findings:
One hundred twelve articles met our criteria for further review. Of these, we found 88 articles on depression, 33 on anxiety, and 22 on suicide (31 articles covered multiple outcomes). The earliest article was published in 1976, and the most frequent exposure of interest was air pollution (n=33), followed by secondhand smoke (n=20), metals (n=18), noise (n=17), and pesticides (n=10). Other exposures studied less frequently included radiation, magnetic fields, persistent organic pollutants (POPs), volatile organic compounds, solvents, and reactive sulfur compounds.
Summary:
The current literature, although limited, clearly suggests many kinds of environmental exposures may be risk factors for depression, anxiety, and suicide. For several pollutants, important limitations exist with many of the studies. Gaps in the body of research include a need for more longitudinal, life-course studies, studies that can measure cumulative exposures as well as shorter-term exposures, studies that reduce the possibility of reverse causation, and mechanistic studies focused on neurotoxic exposures.
Keywords: Mental Health, Environmental Pollutants, Depression, Anxiety, Suicide
Introduction
Depression and anxiety are the two most prominent mental health conditions worldwide [1]. Suicide is perhaps the most dramatic outcome of mental health disorders. Depression is the third most prevalent cause of disease burden worldwide [2], and affects about 300 million people globally [3]. In 2010, depression attributed to the most disability-adjusted life years (DALYs) lost among mental and substance use disorders—accounting for approximately 74.5 million DALYs worldwide [4]. The lifetime prevalence of Major Depressive Disorder (MDD) in the US is 17% [5] and 7% have experienced an MDD in the past year [6, 7]. The personal costs from MDD include significant clinical morbidity, increased mortality from suicide, and loss of quality of life [8]. Financial costs from MDD to employers in the US in 2000 measured $83 billion, with the majority (62% or $52 billion) due to lost workplace productivity [9]. Furthermore, the cost of MDD to employers in lost work days is as great or greater than the cost of many other common medical conditions, including heart disease, diabetes, or back problems [10]. Anxiety disorders are the most common types of psychiatric disorder in the general population [6], and affect around 270 million people worldwide [1]. Approximately 29% of people have an anxiety disorder in their lifetime [5], and 18% have experienced an anxiety disorder in the past year [6]. Anxiety disorders are associated with reduced productivity and increased psychiatric and non-psychiatric medical care, absenteeism, and risk of suicide [11]. The monetary cost of anxiety disorders is also substantial; in the US, the annual direct cost of anxiety disorders in the 1990s has been estimated to be $42.3 billion [12]. In 2012, there was an estimated 804,000 suicide deaths worldwide with an annual age-standardized suicide rate of 11.4 per 100,000 people (15.0 for males, 8.0 for females) [13]. Although suicides accounted for about 1.4% of deaths globally in 2012, it is the second leading cause of death after traffic accidents among young-adults age 15 to 29 years and the fifth leading cause of death among adults age 30 to 49 years [14]. In 2016, suicides accounted for nearly 45,000 deaths (rates increased >30% in half of states since 1999) in the US and was the 2nd leading cause of death among people age 10 to 43 years [14, 15].
Exposure to environmental contaminants may be important modifiable risk factors for depression, anxiety, and suicide—individually and through public policy. Many environmental toxicants can cause oxidative stress that can contribute to such negative effects, but many also have known effects on more specific neural systems that underlie depression and anxiety. For example, effects on synaptic transmission [16, 17], dopaminergic systems [18], and glucocorticoid signaling and the hypothalamic-pituitary axis [19, 20] could be potential mechanisms. Furthermore, in many occupational and other high contaminant exposure settings strong associations between toxicants and mood or psychological distress have been seen [21–23]. Despite a decades-old call by a panel convened by the National Institute for Mental Health to study the link between environmental pollution and mental health, this area of research has received limited attention [24]. Over the last decade, several reviews of epidemiologic literature have addressed various aspects of individual toxicant exposures in relation to mental health outcomes as systematic [25–33], scoping [34–36], and narrative reviews [37–44]. However, some of these reviews only focused on individual metals [30, 31, 44, 45], certain components of air pollution like ozone [28] or particulate matter [46], and particular pesticides like organophosphate (OP) pesticides [33, 37]. Some of these previous reviews also limited included literature to outcomes reported in very specific populations, such as pregnant women [27], subjects in Bangladesh [30], and agricultural workers [33, 42], while others utilized a much broader definition of mental health outcomes that included personality and developmental disorders [28, 34, 43]. This scoping review advances upon these in using multiple data sources to obtain the most comprehensive collection of literature on non-occupational exposures to toxicants and risk of highly prevalent frequently co-occurring mental health outcomes: depression, anxiety, and suicide. As a scoping review, we will not systematically evaluate study quality, but we will describe the research that has been conducted and identified thus far and identify future directions for research in this field.
Methods
We conducted a scoping review [47, 48] by selecting, collecting, and summarizing the existing literature on environmental pollutants and depression, anxiety, and suicide. We defined “environmental” as a person’s surroundings, and “pollutants” as metals, pesticides, particles (air pollution, secondhand smoke), noise, and radiation that pollute a person’s surroundings. Our definition of “pollutants” did not include personal lifestyle factors such as diet, tobacco use, medications, or psychosocial factors, nor did we consider biological agents (e.g., viruses, bacteria, and mold). We searched for English articles on human subjects published through December 31, 2018 utilizing four databases: PubMed (US National Library of Medicine, Bethesda, Maryland), Web of Science (Thomson Reuters Corporation, New York, New York), PsychINFO (American Psychological Association, Washington, D.C.), and Embase (Elsevier, Amsterdam, Netherlands). Table 1 details search terms used in each database.
Table 1.
Journal database search terms utilized in identifying studies.
| Database | Search terms |
|---|---|
| PubMed (n=1642) | “Environmental Pollution”[Mesh] OR Pollut*[tw] OR ((Environment*[tw] OR water[tw] OR Hazardous[tw]) AND (waste[tw] OR radioactive*[tw] OR radiation[tw])) |
| AND | |
| “Depression”[Mesh] OR “Depression”[tiab] OR “Depressions”[tiab] OR “Depressive”[tiab] OR “Anxiety”[Mesh] OR | |
| “Anxiety”[tiab] OR “Catastrophization”[tiab] OR “Hypervigilance”[tiab] OR “Suicide”[Mesh] OR “Suicide”[tiab] OR | |
| “Internalizing”[tiab] OR “externalizing”[tiab] | |
| AND | |
| “cohort studies”[mesh] OR “case-control studies”[mesh] OR “comparative study”[pt] OR “risk factors”[mesh] OR | |
| “cohort”[tw] OR “compared”[tw] OR “groups”[tw] OR “case control”[tw] OR “multivariate”[tw] OR “Epidemiologic | |
| Research Design”[Mesh] OR “Epidemiologic Study Characteristics as Topic”[Mesh] OR | |
| “Depression/epidemiology”[Mesh] OR “Anxiety/epidemiology”[Mesh] | |
| NOT | |
| (“invertebrates”[Mesh] OR “Rodentia”[Mesh]) | |
| Filters: date to Dec. 31, 2018 | |
| Embase (n=1532) | ‘pollution and pollution related phenomena’/exp OR ‘pollut*’:ab,ti OR ((‘environment*’ OR ‘water’ OR ‘hazardous’) AND (‘waste’ OR ‘radioactive*’ OR ‘radiation’)) |
| AND | |
| ‘depression’/exp OR ‘depression’:ab,ti OR ‘depressions’:ab,ti OR ‘depressive’:ab,ti | |
| OR ‘anxiety’/exp OR ‘anxiety disorder’/exp OR ‘anxiety’:ab,ti OR ‘catastrophization’:ab,ti OR ‘hypervigilance’:ab,ti | |
| OR ‘suicide’/exp OR ‘suicidal behavior’/exp OR ‘suicide’:ab,ti OR ‘internalizing’:ab,ti OR ‘externalizing’:ab,ti | |
| AND | |
| ‘clinical article’/exp OR ‘controlled study’/exp OR ‘major clinical study’/exp OR ‘prospective study’/exp OR ‘cohort analysis’/exp OR ‘cohort’:ab,ti OR ‘compared’:ab,ti OR ‘groups’:ab,ti OR ‘case control’:ab,ti OR ‘multivariate’:ab,ti | |
| AND | |
| (1952:py OR 1958:py OR 1959:py OR 1960:py OR 1961:py OR 1962:py OR 1963:py OR 1964:py OR 1965:py OR 1966:py OR 1967:py OR 1969:py OR 1970:py OR 1971:py OR 1972:py OR 1973:py OR 1974:py OR 1975:py OR 1976:py OR 1977:py OR 1978:py OR 1979:py OR 1980:py OR 1981:py OR 1982:py OR 1983:py OR 1984:py OR 1985:py OR 1986:py OR 1987:py OR 1988:py OR 1989:py OR 1990:py OR 1991:py OR 1992:py OR 1993:py OR 1994:py OR 1995:py OR 1996:py OR 1997:py OR 1998:py OR 1999:py OR 2000:py OR 2001:py OR 2002:py OR 2003:py OR 2004:py OR 2005:py OR 2006:py OR 2007:py OR 2008:py OR 2009:py OR 2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py OR 2015:py OR 2016:py) | |
| Web of Science (n=10,145) | TS=(Environmental pollution OR Pollut* OR air pollution OR water pollution OR pesticide* OR environmental exposure* OR environmental monitoring OR radiation OR noise OR waste water OR wastewater OR toxi* OR metal* OR pesticide* OR particle* OR particulate*) |
| OR | |
| TS=((Environment* OR water OR Hazardous OR industrial OR solid OR radioactive) SAME (waste OR radioa ctive* OR radiation)) | |
| AND | |
| TS=(depression OR depressions OR depressive OR anxiety OR catastrophization OR hypervigilance OR hypervigilant OR suicid* OR internalizing OR externalizing) | |
| AND | |
| TS=((cohort NEAR/1 studies) OR (case NEAR/1 control) OR (comparative NEAR/1 study) OR (risk NEAR/1 factors) OR cohort OR compared OR groups OR multivariate OR (cross NEAR/1 sectional)) | |
| Filters: date 1900 – 2018 | |
| PsychINFO (n=1004) | DE “Hazardous Materials” OR DE “Insecticides” OR DE “Teratogens” OR DE “Toxins” OR DE “Toxic Disorders” OR DE “Acute Alcoholic Intoxication” OR DE “Carbon Monoxide Poisoning” OR DE “Drug Induced Congenital Disorders” OR DE “Lead Poisoning” OR DE “Mercury Poisoning” OR DE “Narcosis” OR DE “Neuroleptic Malignant Syndrome” OR DE “Thyrotoxicosis” OR DE “Toxic Encephalopathies” OR DE “Toxic Hepatitis” OR DE “Toxic Psychoses” OR DE “Pollution” OR DE “Passive Smoking” OR DE “Prenatal Exposure” OR DE “Noise Effects” |
| OR | |
| TX (pollut* OR ((Environment* OR water OR Hazardous) AND (waste OR radioactive* OR radiation))) | |
| AND | |
| DE “Major Depression” OR DE “Anaclitic Depression” OR DE “Dysthymic Disorder” OR DE “Endogenous Depression” OR DE “Late Life Depression” OR DE “Postpartum Depression” OR DE “Reactive Depression” OR DE “Recurrent Depression” OR DE “Treatment Resistant Depression” OR DE “Atypical Depression” OR DE “Bipolar Disorder” OR DE “Cyclothymic Personality” OR DE “Depression (Emotion)” OR DE “Euthymia” OR DE “Neurosis” OR DE “Childhood Neurosis” OR DE “Experimental Neurosis” OR DE “Occupational Neurosis” OR DE “Traumatic Neurosis” OR DE “Pseudodementia” OR DE “Seasonal Affective Disorder” OR DE “Anxiety” OR DE “Computer Anxiety” OR DE “Mathematics Anxiety” OR DE “Performance Anxiety” OR DE “Social Anxiety” OR DE “Speech Anxiety” OR DE “Test Anxiety” OR DE “Anxiety Disorders” OR DE “Acute Stress Disorder” OR DE “Castration Anxiety” OR DE “Death Anxiety” OR DE “Generalized Anxiety Disorder” OR DE “Obsessive Compulsive Disorder” OR DE “Panic Disorder” OR DE “Phobias” OR DE “Post-Traumatic Stress” OR DE “Posttraumatic Stress Disorder” OR DE “Separation Anxiety Disorder” OR DE “Anxiety Management” OR DE “Generalized Anxiety Disorder” OR DE “Panic” OR DE “Panic Attack” OR DE “Panic Disorder” OR DE “Externalization” OR DE “Internalization” OR DE “Introjection” OR DE “Suicide” OR DE “Assisted Suicide” OR DE “Attempted Suicide” OR DE “Suicidal Ideation” | |
| OR | |
| TX (depression OR depressions OR Depressive OR anxiety OR catastrophization OR hypervigilance OR suicide OR internalizing OR externalizing) | |
| AND | |
| TX (“case control” OR “comparative study” OR “comparative studies” OR “risk factor” OR “risk factors” OR cohort OR compared OR groups OR multivariate) | |
| Filters: publication year 1936–2018 | |
After removing duplicates, titles and abstracts through 2016 were screened for potential relevance by at least two of three reviewers (M.G.W., A.C.W., and Z.L.) and articles for 2017 and 2018 were screened by two others (A.S.D and J.G.). Questions or discrepancies were resolved by consensus with the lead author (M.G.W.). After the initial title and abstract screening, full texts of articles were further reviewed and information on authors, year, study design, study populations, outcome measurements (i.e. depression, anxiety, and/or suicide), and markers and type of exposure (i.e. metals, air pollution, pesticides, noise, secondhand smoke, or other) were extracted and entered into Excel files. If any papers were thought to potentially be ineligible at this stage, the decision was made by consensus. Additionally, we hand searched reference lists in review papers and journal articles captured by our search terms for additional relevant papers. We excluded articles based on the following criteria: 1) study designs (not human studies, assessments in childhood (≤18 years) without results reported separately for adults); 2) reported exposures (occupational cohorts, did not study environmental pollutants, acute poisonings, exposure due to an environmental health incident or disaster); 3) reported outcomes (did not study depression, anxiety, or suicide independent of other psychological outcomes); and 4) article types (review papers, commentaries, conference abstracts, dissertations, and case studies). After full-text review, any discrepancies about which articles would be included in the scoping review were discussed and, when necessary, adjudicated by the lead author (M.G.W.). We present these articles organized by outcome and subdivided by exposure, and presented in visualized, interactive format using Tableau Public (version 2019.2; Tableau, https://public.tableau.com/authoring/EnvironmentalPollutantsandMentalHealthReview/Dashboard). For each exposure of interest, we summarize the number of studies included, range of sample sizes, countries of origin, and study design.
Results
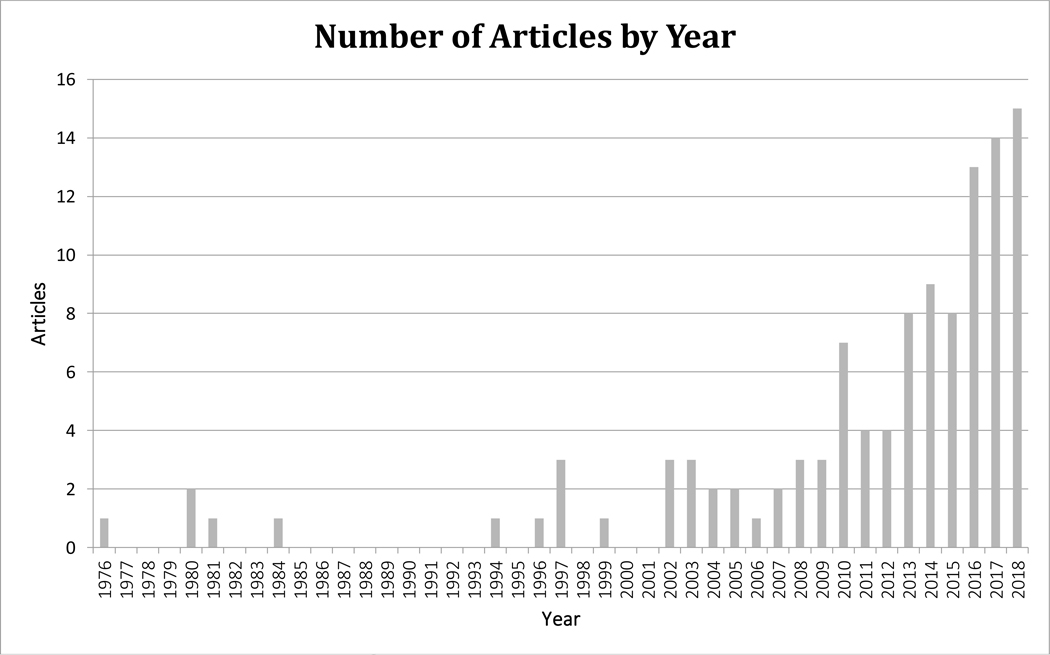
We identified 14,323 articles by searching PubMed (1,642), Embase (1,532), Web of Science (10,145), and PsychINFO (1,004). Of these, there were 13,302 unique articles. Following exclusions and hand searches among references, we ended with 112 relevant articles for further review (Figure 1). Of these, we found 88 articles on depression, 33 on anxiety, and 22 on suicide, with 31 articles covering multiple outcomes. The majority (75 articles) of included articles were published after 2011 with the earliest article published in 1976 (Figure 2). Details on individual study designs, exposures, outcomes, study populations, sample sizes, confounders, and overall results can be found in Excel Table S1 and https://public.tableau.com/profile/aisha.dickerson#!/vizhome/EnvironmentalPollutantsandMentalHealthReview/Dashboard.
Figure 1.
Article search and selection method utilized in reviewing studies on the associations between environmental contaminants/pollutants and depression and anxiety among non-occupational cohorts. Solid lines represent articles included and dashed lines denote excluded articles. The final search included articles published before Dec. 31, 2018.
Figure 2.
Number of articles included in the review by year (112 total articles).
Depression
Of the 88 studies of depression, a large majority (n=60) were cross-sectional studies, followed by 20 cohort, 4 case-crossover, 3 case-control (1 nested) studies, and 1 ecological study. A variety of methods for assessing depression were used across studies, including self-reported depression symptoms or diagnosis via health questionnaires and several validated depression scales, and 8 studies used ICD codes. Of the depression diagnostic tools used, the Centre for Epidemiologic Studies Depression Scale (CES-D) (n=14) and Patient Health Questionnaire (PHQ) (n=10) were most frequently used, while others were also common, including the Composite International Diagnostic Interview (CIDI) (n=5), Generic Depression Scale (GDS) (n=4), Edinburgh Postnatal Depression Scale (EPDS) (n=4), Beck’s Depression Inventory (BDI) (n=3), General Health Questionnaire (GHQ) (n=2), and Hospital Anxiety Depression Scale (HADS) (n=2). Additional study information can be found in the Excel Supplemental Table and https://public.tableau.com/authoring/EnvironmentalPollutantsandMentalHealthReview/Dashboard.
Metals
Sixteen studies examined exposure to metals and depression (Figure 3, Excel Table S1) with sample sizes ranging from 210 to 15,140 subjects. Only 4 of the studies were not conducted in the US, with 2 in Québec [49, 50], 1 in Korea [51], and 1 in India [52]. The most studied metal was lead (Pb) (n=7), followed by cadmium (Cd) (n=5), manganese (Mn) (n=3), mercury (Hg) (n=2) and arsenic (As) (n=2), and strontium (Sr) (n=1), with Shiue et al. (2015) also incorporating antimony, beryllium, cesium, cobalt, molybdenum, selenium, thallium, tin, tungsten, and uranium.
Figure 3.
Heat map of included studies by exposure category and mental health outcome.
The majority of the studies (n=14) were cross-sectional in design with blood concentrations of metals used for exposure assessment, which may not capture the relevant exposure window and can be susceptible to reverse causation. Most of these used National Health and Nutrition Examination Survey (NHANES) data. Six other cross-sectional studies also examined concurrent short-term metal biomarkers in settings other than NHANES. These studies were smaller than the NHANES studies and not representative samples of the populations from which samples were drawn. Blood concentrations of Cd (n=4), Mn (n=2), Pb (n=5), and Hg (n=2) have been examined in such studies. Other markers of exposure to metals included urine (n=3), bone Pb (n=2), and drinking water for As (n=2). Of the 2 cohort studies, 1 was a study of childhood (before age 7) blood Pb and depression at 25–29 years of age [53], while the other assessed bone Pb, a marker of cumulative exposure in adulthood, via K-shell X-Ray Fluorescence (KXRF) [54].
Air Pollution
There were 22 studies on exposure to air pollution and depression (Figure 3, Excel Table S1), with sample ranging from 102 to 124,205 subjects. Although one of these studies was global, [55] most were set in the US (n=5) and China (n=5), followed by Korea (n=4), Canada (n=3), Europe (n=3), and India (n=1). All but one of these involved outdoor exposure estimates independent of the participant—e.g. based on air monitor data or distance to road, which avoids much reverse causation [56]. Most of the studies were cross-sectional (n=10) and the rest were cohort (n=8) and case-crossover (n=4) studies. While 5 of the cohort studies only looked at long-term exposures, 2 only examined short-term exposures [57, 58], and 1 investigated both short-and long-term air pollution exposures [59]. All other studies, except 2 cross-sectional studies [60, 61] focused on short-term exposures. There was a variety of air pollutants covered by the included studies, with the most frequently studied being particulate matter (PM) (i.e. PM2.5, n=12; PM10, n=9), ozone (O3) (n=9), nitrogen oxides (n=12), sulfur dioxide (SO2) (n=10), carbon monoxide (CO) (n=8), black carbon (n=1), smog (n=1), and sulfates (n=1). There was one study of indoor biomass burning, which would be expected to measure much higher levels of exposure, and was the only study that used personal-based exposure monitoring [62].
Pesticides
There were 7 studies of exposure to pesticides and depression (Figure 3, Excel Table S1), with samples ranging from 149 to 29,074 study participants. Many had important limitations, such as self-reported exposures (n=4). Of the 5 studies conducted in the US, 3 were conducted in spouses of agricultural workers [63–65], while 1 was in Turkey [66] and the other was in Brazil [67]. Regarding study design, 3 were cohort studies, 3 were cases-control (1 nested), 1 was cross-sectional, and 1 was an ecological study. Additionally, 1 of the case-control studies was the only to use a biological measure as a proxy for a specific pesticide exposure – red blood cell acetylcholine esterase (AChE) for OP pesticide exposure [66].
Noise
We found 13 studies of exposure to noise and depression (Figure 3, Excel Table S1) with sample sizes ranging from 144 to 77,295 people. Most studies assessed exposure based on where people lived, with 1 case-control study, 1 cohort study, and the rest (11 studies) being cross-sectional. Most studies were set in European countries, including Germany (n=2), the UK (n=2), Serbia (n=1), Italy (n=1), Finland (n=1), and Norway (n=1), while the rest were in Asia, including Japan (n=2), Korea (n=1), India (n=1), and Iran (n=1). Eight articles addressed road traffic noise and depression symptoms as measured by scales [68–75]. Five additional studies considered noise around airfields [76–80]. One study in India investigated indoor noise pollution and neighborhood noise with depression, but not street noise, although no details on how the noise was assessed were provided [74]. Another conducted small randomized trials of ear plugs or sound cancelling headphones in a noisy hospital setting and found no differences in depression scores between any of the trial conditions [73].
Secondhand Smoke
We found 19 studies describing depression and exposure to secondhand smoke (SHS) (Figure 3, Excel Table S1), with sample sizes ranging from 162 to 123,665 people. Eight of these studies took place in the US, with others located in various countries, including Taiwan (n=2), Canada (n=2), China (n=2), Korea (n=3), Germany (n=1), and the Netherlands (n=1). Three of these studies used blood or salivary cotinine as biomarkers of exposure [81–83]. Of 14 studies that determined SHS exposure by questionnaire [84–97], 5 of these were among pregnant women [87–90, 97] and 1 specifically examining post-partum depression [88]. Two of the pregnancy studies were in Taiwan where maternal smoking during pregnancy is illegal, presumably increasing the likelihood that mothers truly did not smoke during pregnancy and so were only exposed to SHS [89, 90]. Three of the studies considered earlier life (in utero and childhood) exposures to SHS and later life depression [94, 96, 97].
Other
There were 13 studies evaluating depression and other environmental pollutants including polyfluorinated compounds (n=1), phthalates (n=4), polychlorinated biphenyls (PCBs) (n=2), volatile organic compounds (VOCs) (n=1), trichloroethylene (TCE), and electromagnetic and gamma radiation (n=3) (Figure 3, Excel Table S1). With sample sizes ranging from 143 to 15,140 people, most (n=10) were cross-sectional in design, and 3 were cohort studies. Of the 7 studies conducted in the US, 3 used NHANES data [98–100]. There were also 2 studies set in Taiwan, while others were distributed to Australia, Egypt, New Zealand, and Korea. Phthalate exposure was primarily determined through urine measurements [99–102]. Three studies used NHANES data and examined contaminants in biosamples collected from participants [98–100]. Three studies considered electromagnetic fields (EMF) [103–105]. One related to living or working near a mobile phone base station antenna and was quite small with a high risk of confounding [103]. The other two examined living near high-voltage power transmission lines [104, 105].
Anxiety
There were 33 papers on anxiety; most of which were cross-sectional studies (n=20). Eight of the studies were cohort studies, 2 randomized experiments, 1 case-crossover, 1 case-control, and 1 time-series study. Two studies used anxiety diagnosis as indicated by ICD codes in hospital records [57, 106], and 3 were based on self-reported diagnosis or symptoms [92, 107, 108]. Other studies used various anxiety measurement scales, including the State-Trait Anxiety Inventory (STAI) (n=6), CIDI (n=5), Brief Symptom Inventory (BSI) (n=2), Crown-Crisp Index (CCI) (n=2), HADS (n=2), Profile of Mood States (POMS) (n=2), Symptom Checklist-90 (SCL-90) scale (n=1), CES-D (n=1), and the World Health Organization Neurobehavioral Core Test Battery (WHO-NCTB) (n=1).
Metals
There were 8 studies that examined exposure to metals and anxiety (Figure 3, Excel Table S1) with sample sizes ranging from 190 to 654 adults. Half of the studies were set in the US (n=4), 2 in Canada, and the others in Australia and India. Most of these were cross-sectional studies (n=5) of Pb, Mn, and As, 2 were cohort investigations of Pb, and 1 case-control study examined Mn. Four studies considered anxiety in association with blood (n=3) and bone (n=2) Pb measures, and one specifically investigated childhood blood levels and anxiety in adulthood [53, 54, 109, 110]. A study in India also examined the association between As concentrations in drinking water and anxiety symptoms [52]. Three studies considered Mn exposure: two assessing exposure via blood Mn levels [49, 50], and the other based on ambient air concentrations [111]. Because blood Mn is under tight homeostatic control, it is possible that it is not as effective at identifying higher Mn exposures as is simply living in the area with higher air Mn concentrations [56].
Air Pollution
There were 7 studies on air pollution and anxiety (Figure 3, Excel Table S1), with sample sizes ranging from 102 to 71,271 study participants. There were 4 cohorts set in the US (n=3) and Canada (n=1), 1 cross-sectional study in Spain, 1 case-crossover study in China, and 1 time-series study in Korea. Two of the cohort studies only assessed long-term exposures [112, 113], 1 only used short-term exposures [57], and 1 examined both short- and long-term air pollution exposures [59]. All other study designs investigated anxiety in relation to short-term air pollution exposures. Different aspects of air pollution were studied: particulate matter (i.e. PM10, n=4; PM2.5, n=5), nitrogen oxides (n=4), CO (n=2), SO2 (n=2), O3 (n=2), and smog (n=1). Two of these considered daily concentrations of several different ambient air pollutants and hospital emergency department (ED) visits for anxiety [57, 106], 4 studies explored individual level anxiety symptoms [59, 112–114], and 1 considered self-reported history of anxiety [108].
Pesticides
The review found no studies on pesticides and anxiety.
Noise
There were 9 studies on noise and anxiety (Figure 3, Excel Table S1) with sample sizes ranging from 48 to 2898 people. Although most (n=6) of the studies were cross-sectional, 2 were randomized experiments and one was a cohort study. Both randomized experiments were conducted in North American (Canada and the US) psychology classes [115, 116], while the only cohort study was conducted in the UK [69]. The remaining cross-sectional studies were distributed among Germany, Italy, Finland, and Norway (each with n=1), while the rest were in Asia, including Japan (n=2), Korea (n=1), India (n=1), and Iran (n=1). Two studies were of noise around airfields [77, 78], 4 others considered road traffic noise [68, 69, 72, 107], and 1 of these also examined exposure to railway noise and overall outdoor noise (i.e. commercial properties, renovations, etc.) [107]. Three studies took an experimental approach to assessing noise and anxiety [73, 115, 116].
Secondhand Smoke
Six studies examined secondhand smoke (SHS) and anxiety (Figure 3, Excel Table S1), with sample sizes ranging from 162 to 49,701 subjects. All studies were cross-sectional studies, except 1 cohort study of pregnant women [89]. Study locations included Taiwan (n=2), Canada, the US, the Netherlands, and Germany. Two studies considered SHS exposure during pregnancy specifically and came to opposite conclusions [89, 90]. Another study considered adult anxiety incidence and SHS exposure during childhood and adulthood separately [96].
Other
Three studies examined other exposures and anxiety (Figure 3, Excel Table S1), with sample sizes ranging from 143 to 540. All were cross-sectional studies examining magnetic field exposure in New Zealand [104], TCE exposure in the US [117], and serum PCB concentration in the US [118].
Suicide
We included 22 papers on environmental exposures and suicide. Of these, 7 were cohort studies, 7 were cross-sectional studies, 6 were case-crossover, 1 was a case-control, and 1 was an ecological study. Over half of these studies were set in Asian populations, including Korea (n=7), Taiwan (n=2), China (n=1), Japan (n=1), and Iran (n=1), while 3 were conducted in US populations, along with 3 in European countries (Belgium, Denmark, and the UK), 2 in South America (Brazil and Columbia), 1 in Australia, and 1 in Turkey. We included studies that evaluated completed suicides (n=15), attempts (n=4), and ideation of suicides (n=3). Completed suicide was determined via ICD codes in mortality records (n=11) and police or medical examiner records (n=3). All ideation was determined through self-report (n=3) while suicide attempts were determined via hospital records (n=2) and self-report (n=2).
Metals
There was 1 study of completed suicides and metals in adults, which was a prospective population-based study in Denmark (Figure 3, Excel Table S1). Specifically, this study examined the association with area lithium concentration in drinking water [119].
Air Pollution
We identified 9 studies on completed suicide and 1 on suicide attempts and air pollution (Figure 3, Excel Table S1), with samples sizes ranging from 1546 to 265,749 people. One study was in the US [120], 1 was in South America [121], 1 was in Europe [122], and the rest were in Asia. Of these, 6 were case-crossover, 2 were cohort studies, 1 was cross-sectional, and 1 was an ecological study. All studies used air pollution exposure estimates based on ambient monitoring data. All studies examined particulate matter (PM10, n=8; PM2.5, n=5) and nitrogen oxides (n=7), followed by SO2 (n=8), O3 (n=4), and CO (n=4). There were also slight differences in timing of exposure examined (preceding days, weeks, or years).
Pesticides
In relation to studies examining exposure to pesticides, there were 3 on completed suicides, 1 focused on suicide ideation, and 1 on suicide attempts with sample sizes ranging from 149 to 81,988 people (Figure 3, Excel Table S1). Regarding study design, 2 were cohort, 1 was case-control, and 2 were cross-sectional. The largest studies were among spouses of participants in the Agricultural Health Study (AHS) [123, 124]. Only 1 study considered a biomarker of exposure, red blood cell AChE activity (a specific molecular target of organophosphate and carbamate pesticides) in relation to attempted suicide [66].
Noise
There was 1 cohort study of outdoor noise and completed suicides, as indicated by ICD-10 codes in the Korean Statistical Office database [125].
Secondhand Smoke
Two cross-sectional studies explored suicide ideation and 1 cohort study examined suicide attempts and secondhand smoke (SHS) (Figure 3, Excel Table S1). These studies took place in Australia, Korea, and Taiwan and ranged from 2736 to 6043 subjects, with the smallest sample belonging to the cohort study [126].
Other
Two cross-sectional studies examined other exposures in relation to suicide, including hydrogen sulfide (H2S) from ambient natural gas [127] and magnetic fields [128].
Discussion
The number of studies on general population environmental exposures and depression, anxiety, or suicide has increased over the last two decades (Figure 2). However, we found this literature to be rather limited for specific exposures of interest. Additionally, the vast majority of studies of depression and anxiety have important limitations, including small sample sizes, comparisons between crude proxies of exposure such as distinct communities, limited control for confounding, and designs that raise concern of reverse causation. Many of the studies were cross-sectional in nature, such as studies based on NHANES data. Because mental health conditions affect behavior, this study design aspect makes it difficult to conclude that the exposures are causally related to the depression, anxiety, or suicide as opposed to the mental health issues affecting measured exposure levels.
Mental health diagnosis
Our review demonstrated that several (n=15) of the studies of depression, anxiety, and suicide were based on ICD diagnosis codes provided in medical records. However, numerous studies have shown differences in likelihood of seeking mental health care as well as disparities in referrals and treatment provided based on gender [129] and socioeconomic status [130, 131]. Thus, studies using ICD codes from medical records may underrepresent depression and/or anxiety in populations considered underserved. However, there were several assessment scales utilized in other studies. Some studies used outcome-specific assessments, while others assessed depression and/or anxiety as a subpart of overall general health. Mental health screens should be provided as part of general healthcare visits, but for the purposes of research, continuous scales suited for assessing severity of depression and anxiety may be more appropriate. Additionally, it is important to note that depression and anxiety are often co-occurring conditions [132], and that suicide is commonly the result of these underlying conditions [133]. For these reasons, the authors suggest future studies of environmental exposures and depression and anxiety should investigate these disorders using both symptoms and scale measures in order to capture preclinical influence of exposures on mental health. Furthermore, although 20 of the discussed studies considered depression and anxiety in conjunction, and 8 considered other co-occurring mental health outcomes, including panic disorder [53, 109], psychotic episodes [49, 50], paranoia and obsessive-compulsive disorder [49], aggression [49, 50, 117], memory and learning [52, 118], executive function and impulse control [50, 118], drug and alcohol abuse [53, 95], and post-traumatic stress disorder [53], we believe that future studies should consider a broader spectrum of outcomes and evaluate comorbidity whenever possible.
Metal exposures
Studies of metal exposures were mostly cross-sectional, many using US NHANES data [98, 100, 109, 134–137]. For most of these, reverse causation is hard to rule out. However, several other studies used exposure assessments that more readily avoid reverse causation, including some related to metals in drinking water [52, 138] and bone measurements [54, 110], while a protective association was seen between water selenium concentrations and depression [139]. Only 3 studies used biomarkers of exposure that more readily captured past exposure—all were biomarkers of Pb. One used child blood Pb levels and found no association with young adult depression, but a nearly significant association with anxiety [53]. Two others used bone Pb in adults as a marker of cumulative exposure and did find associations with worse depression and anxiety [54, 110].
The only studies to not find higher metal concentrations associated with depression were 2 studies of Hg [98, 135], 1 of As [100], and 2 of Pb [98, 100]. Analyses of Hg are complicated because the primary source of Hg exposure is fish consumption, which could have beneficial neurological effects [140, 141]. One of these studies suggested the lack of association with Hg could be related to protection from fish consumption [98], although the other study attempted to control for fish consumption and fish oil intake [135]. Only one study of blood Pb used an assessment tool (the CIDI) to identify a clinical disorder (major depressive disorder [MDD]) as the outcome [109]. The study reported differences among smokers and non-smokers, which could relate to increased exposure from more hand-to-mouth activity among smokers. Smoking could be a form of self-medication to reduce symptoms (and therefore their CIDI score) [142, 143], highlighting the concern of the possibility of reverse causation with such cross-sectional studies. Three studies of depression and metals used exposure metrics that involved measures independent of the participants to estimate individual exposures. Although this approach can result in a less accurate assessment of true exposure—which is likely non-differential and so biases effect estimates to the null—it avoids problems of reverse causation and confounding by personal behaviors [56].
Air Pollution
Studies of air pollution exposures more readily avoid reverse causation than many studies of other exposures because the exposure estimates typically are not affected by personal behaviour [56]. Although each paper reported the primary source of air pollution data, very few mentioned the specific air dispersion models used for these sources, including Gaussian line-source dispersion, inverse distance weighting, and land-use regression modeling. Furthermore, time series and case-crossover designs are commonly used in air pollution studies, and these study designs eliminate concern for confounding by time-invariant factors. The largest group of any type of study examined associations between same day or recent days’ ambient pollutant concentrations and the number of hospital visits for depression or anxiety using a time series or case-crossover design [57, 58, 144–146]. While these study designs help avoid confounding by time-invariant factors, ED visits are a crude method for capturing depression. Different components of air pollution, including PM and metal, have been established as neurotoxic [147]. The mechanisms are not fully understood, but animal and human studies have suggested that this may be through cerebrovascular dysfunction, oxidative stress or inflammation [148, 149]. While 17 of the included studies only looked at short-term exposures, this may only be a reflection of triggered inflammation. Conversely, long-term exposures, examined in 10 of the included studies, are indicators of chronic exposure and are more likely to result in overall poorer health and subsequent mental health decline.
Pesticides
Of work on pesticides and depression, the most compelling studies on non-occupational exposures were in the AHS cohort in part because of its size, ability to control for covariates, and, in one case, a prospective follow-up design. The AHS was established to examine health effects of pesticide exposures primarily among pesticide applicators who presumably would be much better than the general public at recalling past pesticide use and exposures [150], but as an occupational group fall outside the scope of this review. However, the AHS also enrolled spouses of the applicators and we considered AHS studies among these non-workers. Although participants self-reported past exposures, the analyses of prevalent depression could still suffer from reverse causation if reporting of past exposures was influenced by depression. It should be noted that while studies among spouses were not among workers, spouses of workers likely have different exposure routes and profiles than the general public. No studies were identified of pesticide exposures and anxiety.
Other studies of pesticide exposures were more limited. A large study using NHANES data assessed associations between urinary pesticides (including organophosphates) and depression [98], but as a cross-sectional study with a short half-life biomarker of exposure. The only other study to use a biomarker of exposure used red blood cell acetylcholinesterase (AChE) activity as an indicator of exposure to organophosphate (OP) pesticides in a study of only 149 patients with MDD and 64 patients without [66].
Noise
We found several studies of noise with most estimating outdoor noise levels. Assessments of noise was done by various methods including simply living near an airfield or not, residential address combined with different noise maps, or direct noise measurements outside the home. One study assessed indoor and neighbor noise although did not provide details of the approach [74], one considered self-report of noise levels [107], one considered noise reduction through use of earplugs [73], and two delivered white noise experimentally [115, 116]. Several studies also asked about noise sensitivity and considered outcomes relative to that. For these studies, many alternate explanations other than a causal association are hard to rule out, including chance (many studies were small) and confounding by SES. Some study designs make it difficult to ascribe depression or anxiety differences specifically to noise exposure, as higher noise exposure would likely be almost completely co-linear with other aspects of living nearer noisy places such as airports. In addition, noise represents a complex exposure for these types of studies, as individuals are generally aware of these exposures, which is not the case with many other environmental contaminants. As a result, a participants’ responses regarding depression and anxiety could be influenced by recognition of their exposure. At the same time, recognition of exposure could lead to annoyance and stress, which could then lead to biological effects resulting in more depression and/or anxiety. This would nonetheless be a somewhat different causal path to worse mental health than would be postulated for most of the other environmental exposures considered here.
Secondhand smoke
Secondhand smoke (SHS) is an exposure that could conceivably suffer from some of the same issues outlined above for noise since people are generally aware of their exposure to SHS. Nonetheless, there are also more direct biological actions of SHS that could conceivably lead to depression, anxiety, and suicide. Overall, the studies tended to reasonably account for confounding, in particular by SES factors, and many were quite large. The few studies on associations between childhood SHS exposure, or the combination of childhood and adult exposure, and adult depression and anxiety suffer less from the issue of reverse causation or the exposure causing the outcome because of annoyance or stress, since the exposures were very removed in time. In addition, one study avoided these problems in considering SHS indirectly by examining the association between depression and smoking policies both at home and work [151].
Other exposures
Several other contaminants have also been explored, including more recently explored chemicals like polyfluorinated compounds, phthalates, and triclosans, as well as older ones such as PCBs, TCE and different types of EMF. There were analyses of many compounds with shorter half-lives—such as phenols, phthalates, and polyaromatic hydrocarbons in urine. Given the short half-lives of the biomarkers, one measure may not reflect longer-term exposure well, which could add error to the estimation of exposures over relevant time periods and contribute to differences in findings particularly in cross-sectional studies such as NHANES. However, 1 of these studies examined blood polyfluorinated compounds, which can have much longer half-lives [98]. Polychlorinated biphenyls (PCBs) have even longer half-lives in blood than polyfluorinated compounds, so one blood measure is a good cumulative exposure estimate. However, many of these studies also suffered from the kinds of limitations discussed above.
Future directions
Previous reviews of environmental exposures and mental health outcomes restricted inclusion criteria to specific metals, air pollution components, and particularly volatile toxicants. However, our scoping method used multiple data sources to obtain the most comprehensive collection of literature on non-occupational exposures to toxicants. We also provided a thorough synopsis of the studies included, with searchable data on modes of assessment of both exposures and assessment. Though we did summarize and interpret included studies, as this is not a systematic review, we did not appraise overall weight of evidence based on sample size, study design, risk of bias, or other factors commonly evaluated for study quality. The wealth of available data ascertained in our scoping review suggests that a systematic review and/or meta-analysis would be an appropriate next step, though it would need to have more restrictions on publication range. However, we recognize that harmonizing certain outcomes may be difficult due to the variety of methods used in each study. Current literature on pesticides is overall crude and particularly difficult to provide comparative reviews. Thus, the authors believe that future studies of these exposures should assess particular types of pesticides grouped by their potential toxicity and mechanism of action. Additionally, we recommend that future individual studies of environmental exposures and depression, anxiety, and suicide should investigate a broader range of validly measured mental health outcomes using repeated symptom and scale measures in order to mitigate reverse-causation with both cumulative and short-term exposures.
Conclusion
Given the tremendous public health impact of adverse mental health conditions, understanding the contribution of environmental exposures—often modifiable—should be of paramount importance. The current literature, although limited, clearly suggests many kinds of environmental exposures may be risk factors for depression, anxiety, and suicide. Gaps in the body of research include a need for more longitudinal studies, studies that can measure cumulative exposures as well as shorter-term ones, and studies that control well for potential confounders and reduce the possibility of reverse causation. Furthermore, there should be more examination into the potentially mediating impact of pharmacological treatment in these exposure-outcome relationships.
Supplementary Material
Acknowledgments
We thank Scott Lapinski, Digital Resource and Services Librarian at the Francis A. Countway Library of Medicine, for his counsel and constant assistance in honing our literature review. We also thank Julie Goodman for extending the literature search and Maggie Mittleman and Christian Hoover for checking our review for missed articles. No authors received compensation for their participation in this project. All authors declare no financial interest. Drs. Weisskopf, Wu, and Dickerson had full access to all of the references in the review and take responsibility for the integrity and accuracy of the review. This work was supported by NIH grant P30 ES000002. Dr. Wu was supported by the NIOSH ERC training-grant, T42 OH008416. Dr. Dickerson was supported in part by a National Institutes of Health training grant T32 ES007069. Dr. Liew was partly supported by the NIH/NIEHS Pathway to Independence Award (K99ES026729/R00ES026729).
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Aisha S. Dickerson, Alexander C. Wu, Zeyan Liew, and Marc G. Weisskopf declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any data directly collected from human or animal subjects by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Aisha S. Dickerson, Departments of Epidemiology and Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, 02115, USA.
Alexander C. Wu, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, 02115, USA.
Zeyan Liew, Department of Environmental Health Sciences, Center for Perinatal Pediatric, and Environmental Epidemiology, Yale School of Public Health, New Haven, CT, 06510, USA.
Marc G. Weisskopf, Departments of Epidemiology and Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, 02115, USA.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
REFERENCES
- 1.Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers C, Fat DM, Boerma JT, World Health O. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. 2008. vii+146 p. [Google Scholar]
- 3.WHO. WHO | Mental disorders 2017 [updated 2017 Available from: http://www.who.int/mediacentre/factsheets/fs396/en/.
- 4.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382(9904):1575–86. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeves W, Strine T, Pratt L, Thompson W, Ahluwalia I, Dhingra S, et al. Mental Illness Surveillance Among Adults in the United States. Supplement. Atlanta: Centers for Disease Control and Prevention; 2011. 2011. Report No.: 60. [PubMed] [Google Scholar]
- 8.Wang PS, Simon G, Kessler RC. The economic burden of depression and the cost-effectiveness of treatment. Int J Methods Psychiatr Res. 2003;12(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–75. [DOI] [PubMed] [Google Scholar]
- 10.Druss BG, Rosenheck RA, Sledge WH. Health and disability costs of depressive illness in a major U.S. corporation. Am J Psychiatry. 2000;157(8):1274–8. [DOI] [PubMed] [Google Scholar]
- 11.Lépine J-P. The epidemiology of anxiety disorders: prevalence and societal costs. J Clin Psychiatry. 2002;63 Suppl 14:4–8. [PubMed] [Google Scholar]
- 12.Greenberg P, Sisitsky T, Kessler R, Finkelstein S, Berndt E, Davidson J, et al. The economic burden of anxiety disorders in the 1990s. Journal of Clinical Psychiatry. 1999;60(7):427–35. [DOI] [PubMed] [Google Scholar]
- 13.WHO. WHO | Preventing suicide: A global imperative 2014. [updated 2014 Available from: http://www.who.int/mental_health/suicide-prevention/world_report_2014/en/.
- 14.CDC. Suicide rising across the US Atlanta, GA: Centers for Disease Control and Prevention; 2016. [updated June 2018 Available from: https://www.cdc.gov/vitalsigns/pdf/vs-0618-suicide-H.pdf. [Google Scholar]
- 15.CDC CfDCaP-. 10 Leading Causes of Death by Age Group, United States – 2016 Antlanta, GA: Centers for Disease Control and Prevention; 2018. [Available from: https://www.cdc.gov/injury/wisqars/pdf/leading_causes_of_death_by_age_group_2016-508.pdf. [Google Scholar]
- 16.Atchison WD. Effects of neurotoxicants on synaptic transmission: lessons learned from electrophysiological studies. Neurotoxicology and teratology. 1988;10(5):393–416. [DOI] [PubMed] [Google Scholar]
- 17.Kiss T, Osipenko ON. Toxic effects of heavy metals on ionic channels. Pharmacological reviews. 1994;46(3):245–67. [PubMed] [Google Scholar]
- 18.Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochemical pharmacology. 2008;76(5):569–81. [DOI] [PubMed] [Google Scholar]
- 19.Munzel T, Daiber A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxidants & redox signaling. 2018;28(9):735–40. [DOI] [PubMed] [Google Scholar]
- 20.Marketon JI, Sternberg EM. The glucocorticoid receptor: a revisited target for toxins. Toxins. 2010;2(6):1357–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker A, McKune A, Ferguson S, Pyne DB, Rattray B. Chronic occupational exposures can influence the rate of PTSD and depressive disorders in first responders and military personnel. Extreme physiology & medicine. 2016;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beard JD, Umbach DM, Hoppin JA, Richards M, Alavanja MCR, Blair A, et al. Pesticide Exposure and Depression among Male Private Pesticide Applicators in the Agricultural Health Study. Environmental Health Perspectives. 2014;122(9):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisskopf MG, Moisan F, Tzourio C, Rathouz PJ, Elbaz A. Pesticide Exposure and Depression Among Agricultural Workers in France. American Journal of Epidemiology. 2013;178(7):1051–8. [DOI] [PubMed] [Google Scholar]
- 24.Williams J, Leyman E, Karp S, Wilson P. Environmental pollution and mental health. Wash, DC, Information Resources Press, 1973. 1973:136-. [Google Scholar]
- 25.Freire C, Koifman S. Pesticides, depression and suicide: a systematic review of the epidemiological evidence. Int J Hyg Environ Health. 2013;216(4):445–60. [DOI] [PubMed] [Google Scholar]
- 26.*Gladka A, Rymaszewska J, Zatonski T. Impact of air pollution on depression and suicide. International journal of occupational medicine and environmental health. 2018;31(6):711–21.This paper is of importance because it discusses potential mechanisms of air pollution and mental health associations through a review of epidemiological, clinical, and experiemental studies.
- 27.Suzuki D, Wariki WMV, Suto M, Yamaji N, Takemoto Y, Rahman MM, et al. Association of secondhand smoke and depressive symptoms in nonsmoking pregnant Women: A systematic review and meta-analysis. Journal of affective disorders. 2019;245:918–27. [DOI] [PubMed] [Google Scholar]
- 28.Zhao T, Markevych I, Romanos M, Nowak D, Heinrich J. Ambient ozone exposure and mental health: A systematic review of epidemiological studies. Environmental research. 2018;165:459–72. [DOI] [PubMed] [Google Scholar]
- 29.Rautio N, Filatova S, Lehtiniemi H, Miettunen J. Living environment and its relationship to depressive mood: A systematic review. The International journal of social psychiatry. 2018;64(1):92–103. [DOI] [PubMed] [Google Scholar]
- 30.Brinkel J, Khan MH, Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. International journal of environmental research and public health. 2009;6(5):1609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown EE, Gerretsen P, Pollock B, Graff-Guerrero A. Psychiatric benefits of lithium in water supplies may be due to protection from the neurotoxicity of lead exposure. Medical hypotheses. 2018;115:94–102. [DOI] [PubMed] [Google Scholar]
- 32.Clark C, Paunovic K. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Quality of Life, Wellbeing and Mental Health. International journal of environmental research and public health. 2018;15(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi N, Hashizume M. A systematic review of the influence of occupational organophosphate pesticides exposure on neurological impairment. BMJ open. 2014;4(6):e004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buoli M, Grassi S, Caldiroli A, Carnevali GS, Mucci F, Iodice S, et al. Is there a link between air pollution and mental disorders? Environment international. 2018;118:154–68. [DOI] [PubMed] [Google Scholar]
- 35.Pall ML. Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. Journal of chemical neuroanatomy. 2016;75(Pt B):43–51. [DOI] [PubMed] [Google Scholar]
- 36.Ragguett RM, Cha DS, Subramaniapillai M, Carmona NE, Lee Y, Yuan D, et al. Air pollution, aeroallergens and suicidality: a review of the effects of air pollution and aeroallergens on suicidal behavior and an exploration of possible mechanisms. Reviews on environmental health. 2017;32(4):343–59. [DOI] [PubMed] [Google Scholar]
- 37.Jaga K, Dharmani C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Reviews on environmental health. 2007;22(1):57–73. [DOI] [PubMed] [Google Scholar]
- 38.Loganovsky KN, Vasilenko ZL. Depression and ionizing radiation. Problemy radiatsiinoi medytsyny ta radiobiolohii. 2013(18):200–19. [PubMed] [Google Scholar]
- 39.Sinyor M, Tse R, Pirkis J. Global trends in suicide epidemiology. Current opinion in psychiatry. 2017;30(1):1–6. [DOI] [PubMed] [Google Scholar]
- 40.Calderón-Garcidueñas L, Vojdani A, Blaurock-Busch E, Busch Y, Friedle A, Franco-Lira M, et al. Air pollution and children: Neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. Journal of Alzheimer’s Disease. 2015;43(3):1039–58. [DOI] [PubMed] [Google Scholar]
- 41.Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacological reports : PR. 2013;65(6):1632–9. [DOI] [PubMed] [Google Scholar]
- 42.Patel S, Sangeeta S. Pesticides as the drivers of neuropsychotic diseases, cancers, and teratogenicity among agro-workers as well as general public. Environmental science and pollution research international. 2019;26(1):91–100. [DOI] [PubMed] [Google Scholar]
- 43.Sram RJ, Veleminsky M Jr, Veleminsky M, Stejskalova J Sr., The impact of air pollution to central nervous system in children and adults. Neuro endocrinology letters. 2017;38(6):389–96. [PubMed] [Google Scholar]
- 44.Vorvolakos T, Arseniou S, Samakouri M. There is no safe threshold for lead exposure: Alpha literature review. Psychiatrike = Psychiatriki. 2016;27(3):204–14. [DOI] [PubMed] [Google Scholar]
- 45.Dinocourt C, Legrand M, Dublineau I, Lestaevel P. The neurotoxicology of uranium. Toxicology. 2015;337:58–71. [DOI] [PubMed] [Google Scholar]
- 46.Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF. Air Pollution (Particulate Matter) Exposure and Associations with Depression, Anxiety, Bipolar, Psychosis and Suicide Risk: A Systematic Review and Meta-Analysis. Environ Health Perspect. 2019;127(12):126002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32. [Google Scholar]
- 48.Armstrong R, Hall BJ, Doyle J, Waters E. ‘Scoping the scope’ of a cochrane review. J Public Health (Oxf). 2011;33(1):147–50. [DOI] [PubMed] [Google Scholar]
- 49.Sassine MP, Mergler D, Bowler R, Hudnell HK. Manganese accentuates adverse mental health effects associated with alcohol use disorders. Biological psychiatry. 2002;51(11):909–21. [DOI] [PubMed] [Google Scholar]
- 50.Bowler RM, Mergler D, Sassine MP, Larribe F, Hudnell K. Neuropsychiatric effects of manganese on mood. Neurotoxicology. 1999;20(2–3):367–78. [PubMed] [Google Scholar]
- 51.Han C, Lim YH, Hong YC. Does cadmium exposure contribute to depressive symptoms in the elderly population? Occupational and environmental medicine. 2016;73(4):269–74. [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee B, Bindhani B, Saha H, Sinha D, Ray MR. Platelet hyperactivity, neurobehavioral symptoms and depression among Indian women chronically exposed to low level of arsenic. Neurotoxicology. 2014;45:159–67. [DOI] [PubMed] [Google Scholar]
- 53.McFarlane AC, Searle AK, Van Hooff M, Baghurst PA, Sawyer MG, Galletly C, et al. Prospective associations between childhood low-level lead exposure and adult mental health problems: the Port Pirie cohort study. Neurotoxicology. 2013;39:11–7. [DOI] [PubMed] [Google Scholar]
- 54.Eum KD, Korrick SA, Weuve J, Okereke O, Kubzansky LD, Hu H, et al. Relation of cumulative low-level lead exposure to depressive and phobic anxiety symptom scores in middle-age and elderly women. Environ Health Perspect. 2012;120(6):817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin H, Guo Y, Kowal P, Airhihenbuwa CO, Di Q, Zheng Y, et al. Exposure to air pollution and tobacco smoking and their combined effects on depression in six low- and middle-income countries. The British journal of psychiatry : the journal of mental science. 2017;211(3):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.**Weisskopf MG, Webster TF Trade-offs of Personal Versus More Proxy Exposure Measures in Environmental Epidemiology. Epidemiology (Cambridge, Mass). 2017;28(5):635–43.This paper is important because it discusses the risks and benefits of using biological markers of environmental exposures as compared to proxy estimates if exposure in environmental epidemiologic studies.
- 57.Szyszkowicz M. Air pollution and emergency department visits for depression in Edmonton, Canada. International journal of occupational medicine and environmental health. 2007;20:241–5. [DOI] [PubMed] [Google Scholar]
- 58.Szyszkowicz M, Rowe BH, Colman I. Air pollution and daily emergency department visits for depression. International journal of occupational medicine and environmental health. 2009;22:355–62. [DOI] [PubMed] [Google Scholar]
- 59.Pun VC, Manjourides J, Suh H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ Health Perspect. 2017;125(3):342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giovanis E, Ozdamar O. Health status, mental health and air quality: evidence from pensioners in Europe. Environmental science and pollution research international. 2018;25(14):14206–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim KN, Lim YH, Bae HJ, Kim M, Jung K, Hong YC. Long-Term Fine Particulate Matter Exposure and Major Depressive Disorder in a Community-Based Urban Cohort. Environ Health Perspect. 2016;124(10):1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banerjee M, Siddique S, Dutta A, Mukherjee B, Ranjan Ray M. Cooking with biomass increases the risk of depression in pre-menopausal women in India. Social science & medicine (1982). 2012;75(3):565–72. [DOI] [PubMed] [Google Scholar]
- 63.Beard JD, Hoppin JA, Richards M, Alayanja MCR, Blair A, Sandler DP, et al. Pesticide exposure and self-reported incident depression among wives in the Agricultural Health Study. Environmental research. 2013;126:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beseler C, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, et al. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the agricultural health study cohort. J Occup Environ Med. 2006;48(10):1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stallones L. Suicide and potential occupational exposure to pesticides, Colorado 1990–1999. Journal of agromedicine. 2006;11(3–4):107–12. [DOI] [PubMed] [Google Scholar]
- 66.Altinyazar V, Sirin FB, Sutcu R, Eren I, Omurlu IK. The Red Blood Cell Acetylcholinesterase Levels of Depressive Patients with Suicidal Behavior in an Agricultural Area. Indian J Clin Biochem. 2016;31(4):473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer A, Koifman S, Koifman RJ, Moreira JC, Chrisman JD, Abreu-Villaca Y. Mood Disorders Hospitalizations, Suicide Attempts, and Suicide Mortality Among Agricultural Workers and Residents in an Area With Intensive Use of Pesticides in Brazil. J Toxicol Env Health Part A. 2010;73(13–14):866–77. [DOI] [PubMed] [Google Scholar]
- 68.Sygna K, Aasvang GM, Aamodt G, Oftedal B, Krog NH. Road traffic noise, sleep and mental health. Environmental research. 2014;131:17–24. [DOI] [PubMed] [Google Scholar]
- 69.Stansfeld S, Gallacher J, Babisch W, Shipley M. Road traffic noise and psychiatric disorder: prospective findings from the Caerphilly Study. BMJ. 1996;313(7052):266–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belojević G, Jakovljević B, Aleksić O. Subjective reactions to traffic noise with regard to some personality traits. Environment international. 1997;23(2):221–6. [Google Scholar]
- 71.Yoshida T, Osada Y, Kawaguchi T, Hoshiyama Y, Yoshida K, Yamamoto K. Effects of road traffic noise on inhabitants of Tokyo. Journal of Sound and Vibration. 1997;205:517–22. [Google Scholar]
- 72.Park J, Chung S, Lee J, Sung JH, Cho SW, Sim CS. Noise sensitivity, rather than noise level, predicts the non-auditory effects of noise in community samples: a population-based survey. BMC public health. 2017;17(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Czaplik M, Rossaint R, Kaliciak J, Follmann A, Kirfel S, Scharrer R, et al. Psychoacoustic analysis of noise and the application of earplugs in an ICU: A randomised controlled clinical trial. European journal of anaesthesiology. 2016;33(1):14–21. [DOI] [PubMed] [Google Scholar]
- 74.Firdaus G, Ahmad A. Temporal variation in risk factors and prevalence rate of depression in urban population: Does the urban environment play a significant role? International Journal of Mental Health Promotion. 2014;16(5):279–88. [Google Scholar]
- 75.Okokon EO, Yli-Tuomi T, Turunen AW, Tiittanen P, Juutilainen J, Lanki T. Traffic noise, noise annoyance and psychotropic medication use. Environment international. 2018;119:287–94. [DOI] [PubMed] [Google Scholar]
- 76.Miyakita T, Matsui T, Ito A, Tokuyama T, Hiramatsu K, Osada Y, et al. Population-based questionnaire survey on health effects of aircraft noise on residents living around US airfields in the Ryukyus - Part 1: An analysis of 12 scale scores. Journal of Sound and Vibration. 2002;250(1):129–37. [Google Scholar]
- 77.Tamini BK, Pak MM. Comparative study of the effect of aircraft noise on emotional states between airport neighboring and city residents. Shiraz E Medical Journal. 2016;17(12). [Google Scholar]
- 78.Hardoy MC, Carta MG, Marci AR, Carbone F, Cadeddu M, Kovess V, et al. Exposure to aircraft noise and risk of psychiatric disorders: the Elmas survey. Social Psychiatry and Psychiatric Epidemiology. 2005;40:24–6. [DOI] [PubMed] [Google Scholar]
- 79.Seidler A, Hegewald J, Seidler AL, Schubert M, Wagner M, Droge P, et al. Association between aircraft, road and railway traffic noise and depression in a large case-control study based on secondary data. Environmental research. 2017;152:263–71. [DOI] [PubMed] [Google Scholar]
- 80.Tarnopolsky A, Watkins G, Hand DJ. Aircraft noise and mental health: I. Prevalence of individual symptoms. Psychological medicine. 1980;10(4):683–98. [DOI] [PubMed] [Google Scholar]
- 81.Bot M, Vink JM, Willemsen G, Smit JH, Neuteboom J, Kluft C, et al. Exposure to secondhand smoke and depression and anxiety: a report from two studies in the Netherlands. Journal of psychosomatic research. 2013;75(5):431–6. [DOI] [PubMed] [Google Scholar]
- 82.Mbah AK, Salihu HM, Dagne G, Wilson RE, Bruder K. Exposure to environmental tobacco smoke and risk of antenatal depression: application of latent variable modeling. Archives of women’s mental health. 2013;16(4):293–302. [DOI] [PubMed] [Google Scholar]
- 83.Bandiera FC, Arheart KL, Caban-Martinez AJ, Fleming LE, McCollister K, Dietz NA, et al. Secondhand smoke exposure and depressive symptoms. Psychosomatic medicine. 2010;72(1):68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim NH, Choi H, Kim NR, Shim JS, Kim HC. Secondhand smoke exposure and mental health problems in Korean adults. Epidemiology and health. 2016;38:e2016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim NH, Kim HC, Lee JY, Lee JM, Suh I. Association between environmental tobacco smoke and depression among Korean women. BMJ open. 2015;5(6):e007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ye X, Li L, Gao Y, Zhou S, Yang Y, Chen S. Dose-response relations between second-hand smoke exposure and depressive symptoms among middle-aged women. Psychiatry research. 2015;229(1–2):533–8. [DOI] [PubMed] [Google Scholar]
- 87.Tan S, Courtney LP, El-Mohandes AA, Gantz MG, Blake SM, Thornberry J, et al. Relationships between self-reported smoking, household environmental tobacco smoke exposure and depressive symptoms in a pregnant minority population. Maternal and child health journal. 2011;15 Suppl 1:S65–74. [DOI] [PubMed] [Google Scholar]
- 88.Khan S, Arif AA, Laditka JN, Racine EF. Prenatal exposure to secondhand smoke may increase the risk of postpartum depressive symptoms. J Public Health (Oxf). 2015;37(3):406–11. [DOI] [PubMed] [Google Scholar]
- 89.Alibekova R, Huang JP, Lee TSH, Au HK, Chen YH. Effects of smoking on perinatal depression and anxiety in mothers and fathers: A prospective cohort study. Journal of affective disorders. 2016;193:18–26. [DOI] [PubMed] [Google Scholar]
- 90.Weng SC, Huang JP, Huang YL, Lee TS, Chen YH. Effects of tobacco exposure on perinatal suicidal ideation, depression, and anxiety. BMC public health. 2016;16:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patten SB, Williams JVA, Lavorato DH, Woolf B, Wang JL, Bulloch AGM, et al. Major depression and secondhand smoke exposure. Journal of affective disorders. 2018;225:260–4. [DOI] [PubMed] [Google Scholar]
- 92.Asbridge M, Ralph K, Stewart S. Private space second-hand smoke exposure and the mental health of non-smokers: a cross-sectional analysis of Canadian adults. Addictive behaviors. 2013;38(3):1679–86. [DOI] [PubMed] [Google Scholar]
- 93.Gim W, Yoo JH, Shin JY, Goo AJ. Relationship between Secondhand Smoking with Depressive Symptom and Suicidal Ideation in Korean Non-Smoker Adults: The Korean National Health and Nutrition Examination Survey 2010–2012. Korean journal of family medicine. 2016;37(2):97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elmasry H, Goodwin RD, Terry MB, Tehranifar P. Early Life Exposure to Cigarette Smoke and Depressive Symptoms Among Women in Midlife. Nicotine & Tobacco Research. 2014;16(10):1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michal M, Wiltink J, Reiner I, Kirschner Y, Wild PS, Schulz A, et al. Association of mental distress with smoking status in the community: Results from the Gutenberg Health Study. Journal of affective disorders. 2013;146(3):355–60. [DOI] [PubMed] [Google Scholar]
- 96.Taha F, Goodwin RD. Secondhand smoke exposure across the life course and the risk of adult-onset depression and anxiety disorder. Journal of affective disorders. 2014;168:367–72. [DOI] [PubMed] [Google Scholar]
- 97.Talati A, Wickramaratne PJ, Wesselhoeft R, Weissman MM. Prenatal tobacco exposure, birthweight, and offspring psychopathology. Psychiatry research. 2017;252:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berk M, Williams LJ, Andreazza AC, Pasco JA, Dodd S, Jacka FN, et al. Pop, heavy metal and the blues: Secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES national epidemiological survey. BMJ open. 2014;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim KN, Choi YH, Lim YH, Hong YC. Urinary phthalate metabolites and depression in an elderly population: National Health and Nutrition Examination Survey 2005–2012. Environmental research. 2016;145:61–7. [DOI] [PubMed] [Google Scholar]
- 100.Shiue I. Urinary heavy metals, phthalates and polyaromatic hydrocarbons independent of health events are associated with adult depression: USA NHANES, 2011–2012. Environ Sci Pollut Res. 2015;22(21):17095–103. [DOI] [PubMed] [Google Scholar]
- 101.Bai PY, Wittert G, Taylor AW, Martin SA, Milne RW, Jenkins AJ, et al. The association between total phthalate concentration and non-communicable diseases and chronic inflammation in South Australian urban dwelling men. Environmental research. 2017;158:366–72. [DOI] [PubMed] [Google Scholar]
- 102.Lee KS, Lim YH, Kim KN, Choi YH, Hong YC, Lee N. Urinary phthalate metabolites concentrations and symptoms of depression in an elderly population. The Science of the total environment. 2018;625:1191–7. [DOI] [PubMed] [Google Scholar]
- 103.Abdel-Rassoul G, Abou El-Fateh O, Abou Salem M, Michael A, Farahat F, El-Batanouny M, et al. Neurobehavioral effects among inhabitants around mobile phone base stations. Neurotoxicology. 2007;28(2):434–40. [DOI] [PubMed] [Google Scholar]
- 104.Beale IL, Pearce NE, Conroy DM, Henning MA, Murrell KA. Psychological effects of chronic exposure to 50 Hz magnetic fields in humans living near extra-high-voltage transmission lines. Bioelectromagnetics. 1997;18(8):584–94. [PubMed] [Google Scholar]
- 105.McMahan S, Ericson J, Meyer J. Depressive symptomatology in women and residential proximity to high-voltage transmission lines. Am J Epidemiol. 1994;139(1):58–63. [DOI] [PubMed] [Google Scholar]
- 106.Cho J, Choi YJ, Sohn J, Suh M, Cho SK, Ha KH, et al. Ambient ozone concentration and emergency department visits for panic attacks. Journal of Psychiatric Research. 2015;62:130–5. [DOI] [PubMed] [Google Scholar]
- 107.Ma J, Li C, Kwan MP, Chai Y. A Multilevel Analysis of Perceived Noise Pollution, Geographic Contexts and Mental Health in Beijing. International journal of environmental research and public health. 2018;15(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vert C, Sanchez-Benavides G, Martinez D, Gotsens X, Gramunt N, Cirach M, et al. Effect of long-term exposure to air pollution on anxiety and depression in adults: A cross-sectional study. Int J Hyg Environ Health. 2017;220(6):1074–80. [DOI] [PubMed] [Google Scholar]
- 109.Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, et al. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry. 2009;66(12):1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rhodes D, Spiro A, Aro A, Hu H. Relationship of bone and blood lead levels to psychiatric symptoms: the normative aging study. J Occup Environ Med. 2003;45(11):1144–51. [DOI] [PubMed] [Google Scholar]
- 111.Bowler RM, Harris M, Gocheva V, Wilson K, Kim Y, Davis SI, et al. Anxiety affecting parkinsonian outcome and motor efficiency in adults of an Ohio community with environmental airborne manganese exposure. Int J Hyg Environ Health. 2012;215. [DOI] [PubMed] [Google Scholar]
- 112.Power MC, Kioumourtzoglou MA, Hart JE, Okereke OI, Laden F, Weisskopf MG. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350:h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tallon LA, Manjourides J, Pun VC, Salhi C, Suh H. Cognitive impacts of ambient air pollution in the National Social Health and Aging Project (NSHAP) cohort. Environment international. 2017;104:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen C, Liu C, Chen R, Wang W, Li W, Kan H, et al. Ambient air pollution and daily hospital admissions for mental disorders in Shanghai, China. The Science of the total environment. 2018;613–614:324–30. [DOI] [PubMed] [Google Scholar]
- 115.Edsell RD. Anxiety as a function of environmental noise and social interaction. The Journal of psychology. 1976;92(2d Half):219–26. [DOI] [PubMed] [Google Scholar]
- 116.Standing L, Stace G. The effects of environmental noise on anxiety level. The Journal of general psychology. 1980;103(2d Half):263–72. [DOI] [PubMed] [Google Scholar]
- 117.Reif JS, Burch JB, Nuckols JR, Metzger L, Ellington D, Anger WK. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environmental research. 2003;93(3):248–58. [DOI] [PubMed] [Google Scholar]
- 118.Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, et al. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environmental Health Perspectives. 2008;116(2):209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knudsen NN, Schullehner J, Hansen B, Jorgensen LF, Kristiansen SM, Voutchkova DD, et al. Lithium in Drinking Water and Incidence of Suicide: A Nationwide Individual-Level Cohort Study with 22 Years of Follow-Up. International journal of environmental research and public health. 2017;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bakian AV, Huber RS, Coon H, Gray D, Wilson P, McMahon WM, et al. Acute air pollution exposure and risk of suicide completion. Am J Epidemiol. 2015;181(5):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandez-Nino JA, Astudillo-Garcia CI, Rodriguez-Villamizar LA, Florez-Garcia VA. Association between air pollution and suicide: a time series analysis in four Colombian cities. Environmental health : a global access science source. 2018;17(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Casas L, Cox B, Bauwelinck M, Nemery B, Deboosere P, Nawrot TS. Does air pollution trigger suicide? A case-crossover analysis of suicide deaths over the life span. European journal of epidemiology. 2017;32(11):973–81. [DOI] [PubMed] [Google Scholar]
- 123.Blair A, Sandler DP, Tarone R, Lubin J, Thomas K, Hoppin JA, et al. Mortality among participants in the agricultural health study. Annals of epidemiology. 2005;15(4):279–85. [DOI] [PubMed] [Google Scholar]
- 124.Beard JD, Umbach DM, Hoppin JA, Richards M, Alavanja MC, Blair A, et al. Suicide and pesticide use among pesticide applicators and their spouses in the agricultural health study. Environ Health Perspect. 2011;119(11):1610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Min JY, Min KB. Night noise exposure and risk of death by suicide in adults living in metropolitan areas. Depression and anxiety. 2018;35(9):876–83. [DOI] [PubMed] [Google Scholar]
- 126.Mitrou F, Gaudie J, Lawrence D, Silburn SR, Stanley FJ, Zubrick SR. Antecedents of hospital admission for deliberate self-harm from a 14-year follow-up study using data-linkage. BMC psychiatry. 2010;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saadat M, Bahaoddini A, Mohabatkar H, Noemani K. High incidence of suicide by burning in Masjid-i-Sulaiman (southwest of Iran), a polluted area with natural sour gas leakage. Burns. 2004;30(8):829–32. [DOI] [PubMed] [Google Scholar]
- 128.Perry FS, Reichmanis M, Marino AA, Becker RO. Environmental power-frequency magnetic fields and suicide. Health physics. 1981;41(2):267–77. [DOI] [PubMed] [Google Scholar]
- 129.Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychological bulletin. 2017;143(8):783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dorner TE, Mittendorfer-Rutz E. Socioeconomic inequalities in treatment of individuals with common mental disorders regarding subsequent development of mental illness. Soc Psychiatry Psychiatr Epidemiol. 2017;52(8):1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Todd M, Teitler J. Darker days? Recent trends in depression disparities among U.S. adults. The American journal of orthopsychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kircanski K, LeMoult J, Ordaz S, Gotlib IH. Investigating the nature of co-occurring depression and anxiety: Comparing diagnostic and dimensional research approaches. Journal of affective disorders. 2017;216:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cameron S, Brown VJ, Dritschel B, Power K, Cook M. Understanding the relationship between suicidality, current depressed mood, personality, and cognitive factors. Psychology and psychotherapy. 2017;90(4):530–49. [DOI] [PubMed] [Google Scholar]
- 134.Golub NI, Winters PC, van Wijngaarden E. A population-based study of blood lead levels in relation to depression in the United States. International archives of occupational and environmental health. 2010;83(7):771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ng THH, Mossey JM, Lee BK. Total Blood Mercury Levels and Depression among Adults in the United States: National Health and Nutrition Examination Survey 2005–2008. PLoS One. 2013;8(11):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scinicariello F, Buser MC. Blood cadmium and depressive symptoms in young adults (aged 20–39 years). Psychological medicine. 2015;45(4):807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kostrubiak DE, Vacchi-Suzzi C, Smith DM, Meliker JR. Blood cadmium and depressive symptoms: Confounded by cigarette smoking. Psychiatry research. 2017;256:444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. American journal of public health. 2004;94(11):1936–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson LA, Phillips JA, Mauer C, Edwards M, Balldin VH, Hall JR, et al. The impact of GPX1 on the association of groundwater selenium and depression: a project FRONTIER study. Bmc Psychiatry. 2013;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Berger M, Taylor S, Harriss L, Campbell S, Thompson F, Jones S, et al. Cross-sectional association of seafood consumption, polyunsaturated fatty acids and depressive symptoms in two Torres Strait communities. Nutritional neuroscience. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 141.Matsuoka YJ, Sawada N, Mimura M, Shikimoto R, Nozaki S, Hamazaki K, et al. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: a population-based prospective cohort study. Translational psychiatry. 2017;7(9):e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zale EL, Maisto SA, Ditre JW. Anxiety and Depression in Bidirectional Relations Between Pain and Smoking: Implications for Smoking Cessation. Behavior modification. 2016;40(1–2):7–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fluharty M, Taylor AE, Grabski M, Munafo MR. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2017;19(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho J, Choi YJ, Suh M, Sohn J, Kim H, Cho SK, et al. Air pollution as a risk factor for depressive episode in patients with cardiovascular disease, diabetes mellitus, or asthma. Journal of affective disorders. 2014;157:45–51. [DOI] [PubMed] [Google Scholar]
- 145.Szyszkowicz M. Ambient sulfur dioxide and female ED visits for depression. Air Qual Atmos Health. 2011;4(3–4):259–62. [Google Scholar]
- 146.Szyszkowicz M, Tremblay N. Case-crossover design: air pollution and health outcomes. International journal of occupational medicine and environmental health. 2011;24(3):249–55. [DOI] [PubMed] [Google Scholar]
- 147.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicologic pathology. 2008;36(2):289–310. [DOI] [PubMed] [Google Scholar]
- 149.Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. Journal of toxicology. 2012;2012:782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104(4):362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bandiera FC, Caban-Martinez AJ, Arheart KL, Davila EP, Fleming LE, Dietz NA, et al. Secondhand smoke policy and the risk of depression. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2010;39(2):198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.