Abstract
Tears have a vital function to protect and lubricate the ocular surface. Tear production, distribution and clearance is tightly regulated by the lacrimal functional unit (LFU) to meet ocular surface demands. The tear film consists of an aqueous-mucin layer, containing fluid and soluble factors produced by the lacrimal glands and mucin secreted by the goblet cells, that is covered by a lipid layer. The array of proteins, glycoproteins and lipids in tears function to maintain a stable, well-lubricated and smooth optical surface. Tear factors also promote wound healing, suppress inflammation, scavenge free radicals, and defend against microbial infection. Disease and dysfunction of the LFU leads to tear instability, increased evaporation, inflammation, and blurred and fluctuating vision. The function of tear components and the consequences of tear deficiency on the ocular surface are reviewed.
Keywords: Tears, Tear stability, mucin, lipid, growth factor, innate immunity, dry eye, dry eye disease, visual acuity, pain
1. Introduction
The tear film is the interface between the ocular surface epithelium and the environment. Although the precorneal tear thickness is estimated to be 3 microns (King-Smith et al., 2000), it has a highly complex composition containing water, electrolytes, mucins, and an array of proteins and lipids. Indeed, a study surveying human tear fluid using liquid chromatography-mass spectroscopy (LC-MS) reported detection of over 1500 proteins (Zhou et al., 2012). The structure of the tear film continues to evolve, but evidence suggests it consists of a hydrated mucus layer (secretory mucus layer) covered by lipid that moves over the glycocalyx on the surface epithelium (Figure 1) (Yokoi and Georgiev, 2018). Knowledge regarding the biological function of the tears is based on activity of individual constituents (e.g. growth factors), imaging studies and the consequences of tear deficiency. Findings from these studies show tears function to maintain comfort, prevent infection, suppress inflammation, heal traumatic and surgical injuries, clear debris and maintain high quality vision. Evidence in support of these functions are reviewed herein.
Figure 1.
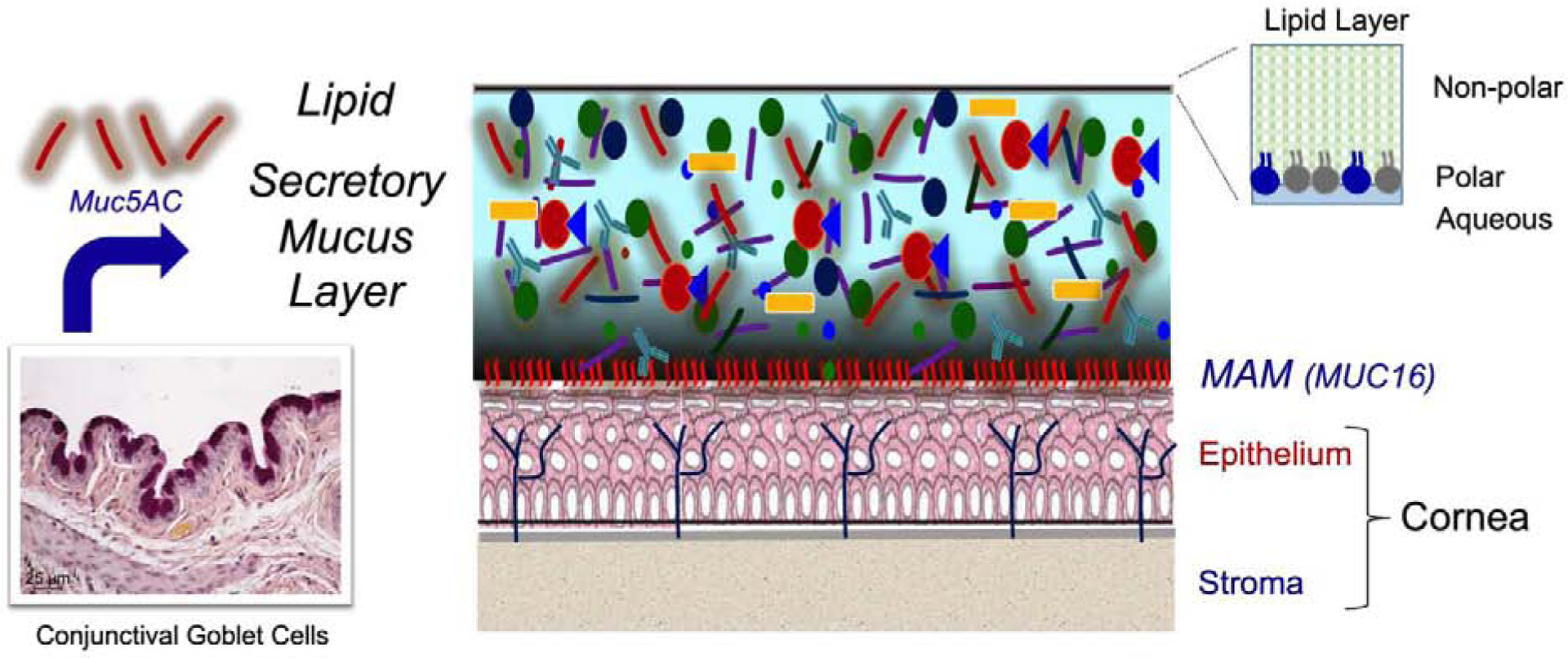
Tear film structure. Evidence suggests the tear film consists of membrane associated mucins (MAM) such as MUC16 that form the glycocalyx on the apical epithelium, a secretory mucus layer containing soluble MUC5AC mucin secreted by the conjunctival goblet cells, aqueous fluid and electrolytes, and proteins secreted by the lacrimal glands. The surface of the tears is covered with a lipid layer with polar lipids adjacent to the aqueous layer and nonpolar lipids interfacing with the air.
2. Methods.
A literature search of clinical and basic studies, and review articles published from 1960 to 2020 was performed in PubMed.gov using major terms tear film, tear function and tear stability and subheadings mucin, lipid, growth factors, cytokines, visual acuity and pain. The bibliographies of references identified by this strategy were also reviewed.
3. Regulation of Tear production
The normal tear film contains a tightly controlled complement of ions, proteins and lipids which allow it to fulfill its basic functions. Perhaps its most important function is the primary optical surface of the eye (Tutt et al., 2000). The tear film assures eye comfort through its lubricative properties which decrease shear forces from the lid margin as it traverses the ocular surface during a blink cycle (Rolando and Zierhut, 2001). Reduced tear volume and altered tear film composition in DED can lead to increased shear force levels capable of causing epitheliopathy of the lid marginal conjunctiva that wipes the ocular surface during blinking (termed lid wiper epitheliopathy), as well as corneal epithelial disease, nociceptor stimulation and pain (Korb et al., 2005). Another function of the normal tear film is protection of the ocular surface epithelium from the environmental insults incurred on a daily basis. These include microbes, pollutants, allergens and adverse environmental conditions, such as low humidity and rapid air movement from wind or inside air handling. This is accomplished through regulated secretion of fluid containing protective factors, including hydrating glycoproteins and antimicrobials (e.g. IgA, lactoferrin, lysozyme and defensins) (Zhou et al., 2007; Zhou et al., 2004). The tear film functions to provide a trophic environment to the ocular surface epithelial tissues. Integrity and secretory function of the epithelium is important to maintain its role as an innate barrier and “seal” over the extensive network of epithelial free nerve endings (Zhou and Beuerman, 2012).
The lacrimal functional unit (LFU) regulates the production, delivery and clearance of tears to maintain a homeostatic environment on the ocular surface (Stern et al., 1998a, b). Anatomically, the LFU includes the tear secreting glands (main and accessory glands lacrimal glands, Meibomian glands, conjunctival goblet cells), the surface epithelium, eye lids, lacrimal drainage system, the glandular and mucosal immune system and the interconnecting innervation. The neural component of the LFU consists of a reflexive loop starting at the highly innervated cornea with afferent traffic to the central nervous system, including the brainstem and cerebral cortex (Figure 2). These afferents along with emotional centers in the brain project to secretory and motor efferent nerves to drive tear production and blinking. The efferent pathways are found to terminate within the main and accessory lacrimal glands, conjunctival goblet cells and the meibomian glands, indicating that secretion of all major components of the tear film are tightly controlled to maintain a normal homeostatic tear composition. Seminal work by Carlos Belmonte and colleagues characterized the types of ocular surface nociceptors and made a critical discovery that the TRPM8 “cold receptor” stimulated by cooling of the corneal surface between blinks, is responsible for driving normal tear flow (Parra et al., 2010).
Figure 2.
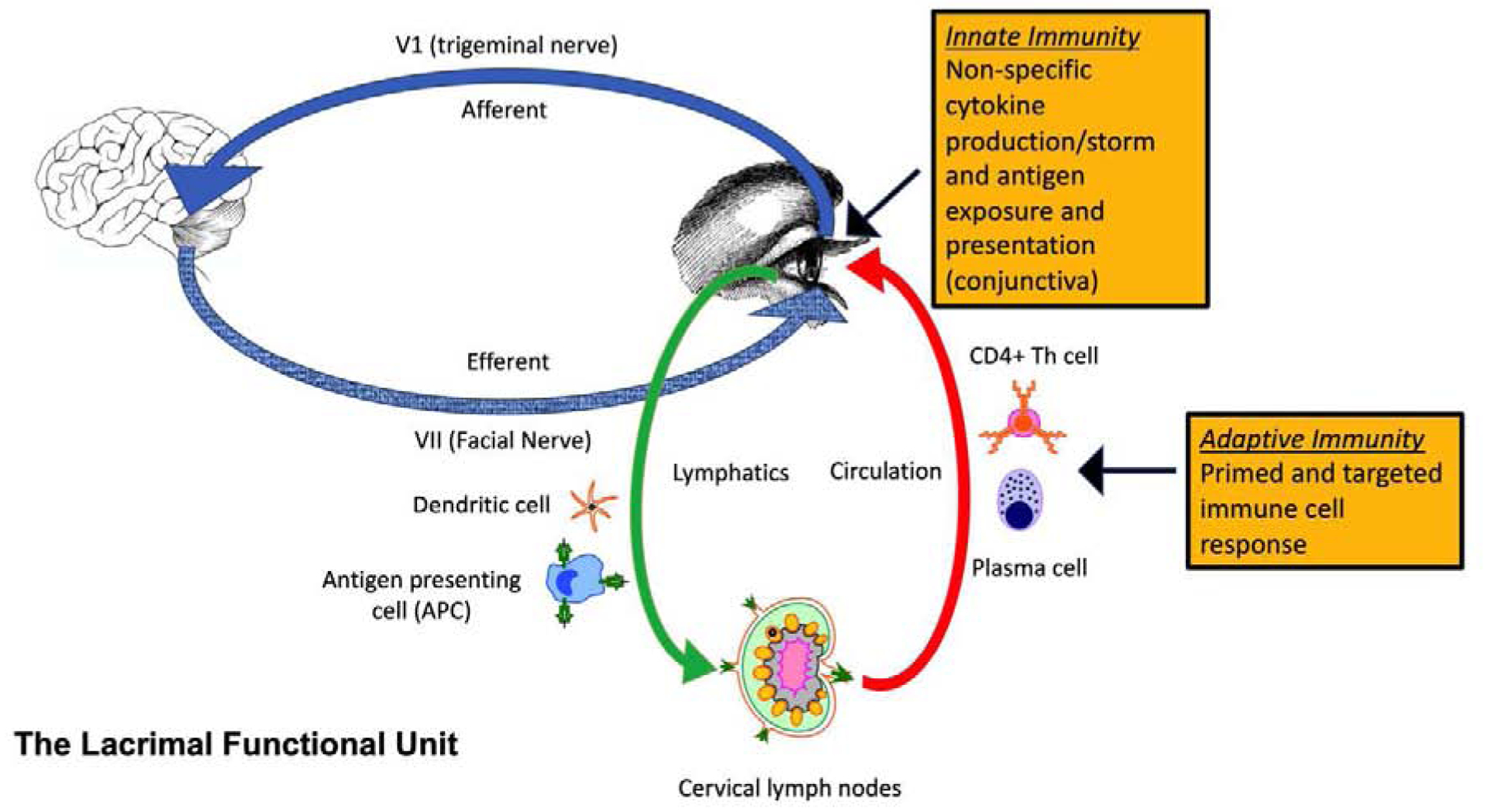
Lacrimal Functional Unit (LFU). The LFU regulates the production, delivery and clearance of tears to maintain a homeostatic environment on the ocular surface. Anatomically, the LFU includes the main and accessory lacrimal glands, Meibomian glands, conjunctival goblet cells, the surface epithelium, eye lids, lacrimal drainage system, the glandular and mucosal immune system and the interconnecting innervation. The neural component of the LFU consists of a reflexive loop starting at the highly innervated cornea surface with afferent traffic to the central nervous system, including the brainstem and cerebral cortex. These afferents project to secretory and motor efferent nerves to drive tear production and blinking. The efferent pathways are found to terminate within the secretory glands. Innate and adaptive inflammatory/immune pathways maintain immune tone to defend the ocular surface from microbial infection. Dysfunction of the LFU stimulates cytokine, chemokine and protease production by ocular surface epithelial and immune cells (cytokine storm) that results in autoantigen release, antigen presenting cell activation and migration to the lymph nodes and priming of effector CD4+ T cells that can traffic to the ocular surface and can provide the cytokines to stimulate autoantibody production by plasma cells. These immune mediators and cells cause ocular surface epithelial disease and can sensitize pain receptors.
Functional denervation of the ocular surface, either as a result of surgery or chronic disease such as diabetes, results in decreased tear secretion and surface epithelial disease with disrupted barrier function. It is now recognized that the dense innervation of the cornea is susceptible to insults that can cause neuropathic pain, including altered tear composition, inflammation and trauma (Rosenthal and Borsook, 2016).
Dysfunction of the LFU results in dry eye disease (DED), also described as Dysfunctional Tear Syndrome by the Delphi Panel, results in an altered tear composition that can’t maintain stability and protect the ocular surface and activates innate inflammatory and adaptive immunity to yet to be determined ocular surface antigens (Figure 2) (Behrens et al., 2006; Pflugfelder and de Paiva, 2017).
4. Stability.
Maintenance of tear stability is essential for maintaining comfort and quality vision. Tear stability requires dynamic interaction between the major tear constituents described below. An unstable tear film is the hallmark of tear dysfunction/deficiency and maintenance of stability is a major goal of therapy.
4.1. Mucins.
Tear mucus, composed of water and mucin glycoproteins serve to maintain barrier function, hydration and wettability of the hydrophobic surface epithelial cell membranes, provide a matrix for lacrimal secreted factors and minimize friction from blinking. The surface epithelial cells on the cornea and conjunctiva produce membrane associated mucins (MAM), including MUC1, MUC4, MUC16 that are the major constituents of the glycocalyx (Gipson, 2004; Pflugfelder et al., 2000) (Gipson et al., 2014). In addition to being expressed on the apical corneal and conjunctival epithelia, MUC16 has also been found on the surface of mucin granules in human conjunctival goblet cells and may participate in expelling gel forming mucin from these cells (Gipson et al., 2016). The goblet cells express the gel-forming mucin genes MUC5AC, MUC5B (in a subpopulation) and MUC2 (Gipson and Inatomi, 1998; Jumblatt et al., 2003; Marko et al., 2014; McKenzie et al., 2000) (Argueso et al., 2002; Spurr-Michaud et al., 2007) (Alam et al., 2020) Tear mucus is composed primarily of the gel forming mucin MUC5AC with minor contributions from membrane associated mucins shed from the surface epithelium (Spurr-Michaud et al., 2007).
MUC16 has the longest ectodomain of the membrane associated mucins and has an important function in maintaining lubricity and wettability by forming H-bonds with water (Georgiev et al., 2019). While there is no evidence that secreted mucins bind to MUC16, there may be chemical attractions between MUC16 and soluble tear mucins. Treatment of the rabbit cornea with N-acetylcysteine (NAC), an agent that disrupts disulfide linkages between cysteine residues was reported to decrease wettability (Tiffany, 1990), while treatment of the rat ocular surface decreased conjunctival microvilli area, increased tear MUC16 (indicating shedding) and corneal fluorescein staining and decreased tear MUC5AC concentration, surface wettability and tear break up time (Li et al., 2018). The gel formed by goblet cell secretory mucus moves over the ocular surface and contributes to tear stability by binding water (Gipson and Argueso, 2003). Secretory mucin also has been found to clear pathogens and debris (Gipson, 2016). Spdef knockout mice that lack goblet cells have increased debris in the tears and did not clear topically applied Pseudomonas bacteria, although they didn’t show increased susceptibility to corneal infection (Gipson, 2016).
Reduced conjunctival goblet cell density and levels of soluble goblet cell MUC5AC and have been reported in DED (Pflugfelder SC, 2015) (Khimani et al., 2020) (Uchino et al., 2014). Goblet cell loss also occurs in systemic inflammatory diseases, such as Sjögren syndrome, Stevens-Johnson syndrome and graft vs. host disease (GVHD) (Nelson and Wright, 1984; Pflugfelder et al., 1997; Ralph, 1975; Wang et al., 2010) Conjunctival goblet cell loss is correlated with severity of irritation symptoms, clinical ocular surface disease and level of ocular surface inflammation in aqueous tear deficiency (Zuazo et al., 2014). A significant inverse correlation was found between categorical severity of Sjögren syndrome associated DED using the Dry Eye Workshop scale and goblet cell density in the temporal and superior bulbar conjunctiva (Pflugfelder et al., 2018). Goblet cell density in the temporal bulbar conjunctiva was found to inversely correlate with Rose Bengal staining score at that site and with staining of the entire exposure zone (Pflugfelder et al., 1997). Goblet cell density was also noted to be inversely correlated with expression of the cytokine interferon gamma (IFN-γ) in the bulbar conjunctiva (Pflugfelder et al., 2015) and with the percentage of HLA-DR positive cells obtained in impression cytology (Pflugfelder et al., 2018). Eyes with significant goblet cell loss due to Stevens-Johnson syndrome and Sjögren syndrome are at risk for developing sight-threatening corneal ulceration and opacification that can occur bilaterally (Bagga et al., 2018; Ormerod et al., 1988; Pflugfelder et al., 1986).
4.2. Lipids
The surface lipid layer of the tear film, primarily derived from the Meibomian glands, serves as the interface between the aqueous layer and the air. Tear film lipid is composed of a thin layer of polar lipids interfacing with the underlying secretory mucus layer and a thicker layer of non-polar lipids at the air interface (Figure 1). The lipid layer functions as a smooth optical surface, reduces surface tension of the tear film, prevents anterior migration of aqueous tears on to the lid margin and retards evaporation (Georgiev et al., 2017) (Cwiklik, 2016). The lipid layer is compressed towards the lower lid during a blink, then spreads upward as the lid opens. Altered spreading and focal thinning of the lipid layer in Meibomian gland disease contributes to tear instability (Braun et al., 2015). Increased tear evaporation and osmolarity in areas of lipid thinning has been proposed to further destabilize the tears (Braun et al., 2014; Braun et al., 2015)
Modeling of tear osmolarity in areas of tear break up predicts that osmolarity could reach levels as high as 800–900 mOsM, much higher than the range measured in the inferior tear meniscus (290–340 mOsm in normal and 305–360 mOsm in DED) (Braun et al., 2015) (Braun et al., 2014; Peng et al., 2014) (Zubkov et al., 2012) (Lemp et al., 2011). The threshold of tear osmolarity stimulating pain sensation by corneal nociceptors is approximately 450mOsm (Liu et al., 2009), and topical application of hypertonic solutions in the range of 800–900 mOsm produced a similar level of irritation that occurs during tear break up (Liu et al., 2009). These findings indicate the focal rise in tear osmolarity could be responsible for the discomfort associated with tear breakup (Braun et al., 2015).
5. Visual performance
The tear film is a critical component of the optical system of the eye. The tears and the anterior surface of the cornea account for approximately 80% of the refractive power for the eye (Rolando and Zierhut, 2001). Deterioration in cornea surface smoothness, reduced contrast sensitivity and increased optical aberrations that degrade retina image quality in eyes with tear film instability highlight the functional role of the tear film in maintaining high quality vision (Rieger, 1992). Studies using the topographic surface regularity index (SRI) developed by Wilson and Klyce have found that reflections from the central cornea/tear film are more irregular in DED (Liu and Pflugfelder, 1999; Wilson and Klyce, 1991) (de Paiva et al., 2003). Furthermore, the timewise increase in SRI from 0–10 seconds after a blink was reported to be higher in DED (Gumus et al., 2011; Kojima et al., 2004). DED has also been found to increase optical scattering and optical aberrations (Diaz-Valle et al., 2012). Differences in tear film thickness during tear film break up increases higher order optical aberrations (Koh, 2016, 2018). These changes in optical properties may be responsible for the reduced low contrast visual acuity and functional visual acuity in DED. (Chotikavanich et al., 2009; Goto et al., 2002; Kaido et al., 2011; Rolando et al., 1998) (Liu et al., 2010; Szczotka-Flynn et al., 2019). These alterations may cause symptoms of visual fatigue, photophobia and stimulate increased blink rate. (Rahman et al., 2015)
6. Trophic/Wound Healing Factors
The aqueous-mucin tear layer contains numerous proteins, including growth and supportive factors. Some of these have a homeostatic function (e.g. suppress inflammation, maintain innervation or barrier), while others participate primarily in epithelial and/or stromal wound healing (Klenkler et al., 2007). The Table lists the most abundant tear growth factors and their function. Certain factors, such as epidermal growth factor (EGF), are secreted by the lacrimal gland into tears (Jones et al., 1997). Others, such as TGF-β are produced by the ocular surface stratified epithelium (TGF-β1 and β2) and goblet cells (TGF-β2) (Contreras-Ruiz and Masli, 2015; Pflugfelder et al., 2008; Torricelli et al., 2016; Yoshino et al., 1996). Concentrations of certain lacrimal gland secreted growth factors, such as EGF, decrease in aqueous tear deficiency (Lam et al., 2009); however, concentration or activity of others, such as NGF and TGF-β1 have been reported to increase in DED. (Lambiase et al., 2011; Zheng et al., 2010)
7. Innate Defense/antimicrobial factors
Since the initial discovery of lysozyme in the tears by Alexander Fleming in 1922, many anti-infective molecules have been found in the normal tear film (Gallo, 2013). They include lysozyme (present at 2.5mg/ml) (Wiesner and Vilcinckas 2010) and lactoferrin (present at 1.5 mg/ml) (Kijlstra et al., 1983; Wiesner and Vilcinskas, 2010). Lactoferrin’s basic anti-bacterial mechanism is through its ability to bind free iron which is necessary for bacterial growth (Flanagan and Willcox, 2009). This molecule, has both anti-infective (suppressing bacterial growth and preventing viral particles from entering cells) and anti-inflammatory (decreasing complement activation and scavenging free radicals) (Flanagan and Willcox, 2009). Like lactoferrin, lipocalin, which is produced and secreted by lacrimal gland acinar cells also exhibits an anti-bacterial function by interfering with free iron uptake (Dartt, 2011; Fluckinger et al., 2004). Prevention of damaging infection and inflammation in the cornea is essential for maintaining its clarity. Unlike the conjunctiva, the cornea poorly tolerates chronic inflammation. (Pers. Comm – J. Niederkorn). Another method by which the ocular surface is protected from pathogen intrusion is through sIgA (secretory immunoglobulin A). This antibody, secreted by plasma cells (terminally differentiated B cells), is taken up by acinar cells and re-secreted in a more stable form complexed with secretory component that can prevent adherence of pathogens to host epithelial cells. This has been shown by in the case of acanthamoeba and Staphylococcus aureus (Campos-Rodriguez et al., 2004; Lan et al., 1997) Use of mass spectrometry (LC-MS/MS) to evaluate protein profiles has elucidated the presence of β-defensins (hBD-2 and hBD-3) in tears. Concentration of β-defensins in normal tears may be sub-antimicrobial; however, they have been found to be upregulated following corneal surgery and in chronic disease processes (Zhou et al., 2007; Zhou et al., 2004). S100 proteins which inhibit bacterial adherence to mucosal epithelial cells have also been found in tears and also increase in chronic inflammation (Garreis et al., 2010; Raquil et al., 2008; Zhou et al., 2009a; Zhou et al., 2009b). A more extensive list of tear antimicrobial proteins is found by a review by Zhou and Beuerman (Zhou and Beuerman, 2012).
7. Anti-inflammatory/antioxidant factors
The tears contain factors that suppress inflammation, such as interleukin 1 receptor antagonist that binds the IL-1 receptor and inhibits IL-1 activity (Solomon et al., 2001), and TGF-β2 and vitamin A and its metabolites that suppress maturation and cytokine production by antigen presenting cells (Contreras-Ruiz and Masli, 2015; Lam et al., 2009; Pflugfelder et al., 2008; Ubels et al., 1986; Xiao et al., 2018) There are a number of antioxidants, including ascorbic acid, lactoferrin and cysteine, that scavenge and protect the ocular surface against damage from free radicals (Ohashi et al., 2006). Tear protease inhibitors include secretory leukocyte protease inhibitor (SLPI) that inhibits serine proteases (e.g. plasmin, elastase, cathepsin G) and tissue inhibitors of matrix metalloproteinases (MMPs). (Corrales et al., 2006; Sathe et al., 1998; Sobrin et al., 2000)
8. Summary
The tear film has a complex structure and composition that protects the cornea, promotes wound healing after injury and maintains eye comfort and high-quality vision. Altered tear composition and stability in DED causes eye irritation, corneal epithelial and nerve disease and blurred vision. The ease of collecting tear fluid, identification of relevant biomarkers in health and disease and more sensitive immunoassays that can be read by smartphones create technological opportunities for developing point of care tear biomarker testing.
Table.
Trophic and Wound Healing Factors
| Factor | Source | Function/Properties |
|---|---|---|
| Transforming growth factor alpha (TGF-α) | Lacrimal glands | Mitogen (van Setten and Schultz, 1994; van Setten et al., 1996) |
| Transforming growth factor-β1 (TGF-β1) | Lacrimal glands, surface epithelium | Inhibits corneal epithelial proliferation, profibrotic (Gupta et al., 1996; Tuominen et al., 2001; Vesaluoma and Tervo, 1998; Yoshino et al., 1996) |
| Transforming growth factor-β2 (TGF-β2) | Conjunctival goblet cells | Suppresses antigen presenting cell maturation (Contreras-Ruiz and Masli, 2015; Kokawa et al., 1996; Pflugfelder et al., 1997) |
| Epidermal growth factor (EGF) | Lacrimal glands | Stimulates corneal epithelial proliferation and migration (Dartt, 2001; Pflugfelder et al., 1999; van Setten et al., 1989) |
| Hepatocyte growth factor (HGF) | Fibroblasts, Lacrimal glands | Stimulates corneal epithelial proliferation and migration, promotes wound healing (Li and Tseng, 1995; Li et al., 1996; Vesaluoma and Tervo, 1998; Wilson et al., 1999a; Wilson et al., 1999b) |
| Keratinocyte growth factor (KGF) | Fibroblasts, Lacrimal glands | Stimulates corneal epithelial proliferation (Li and Tseng, 1995; Wilson et al., 1999a; Wilson et al., 1999b) |
| Basic Fibroblast growth factor (FGF) | Corneal epithelium | Mitogenic, angiogenic and neurotrophic (Sekiyama et al., 2006; van Setten, 1996) |
| Platelet derived growth factor (PDGF BB) | Fibroblast proliferation and migration (Tuominen et al., 2001; Vesaluoma et al., 1997a; Vesaluoma and Tervo, 1998) | |
| Vascular endothelial growth factor (VEGF) | Ocular surface epithelium | Angiogenic (Enriquez-de-Salamanca et al., 2010; Vesaluoma et al., 1997b) |
| Insulin/IGF | Lacrimal glands | Stimulates corneal epithelial proliferation (Patel et al., 2018; Rocha et al., 2002) |
| Substance P | Nerves | Stimulates epithelial growth, wound healing (Fujishima et al., 1997; Varnell et al., 1997; Yamada et al., 2003) |
Highlights.
This review highlights the biological function of the tear film. The tear film has a complex structure and composition that protects the cornea, promotes wound healing after injury and maintains eye comfort and high-quality vision. Altered tear composition and stability in dry eye cause eye inflammation, corneal disease and blurred vision.
Funding:
This work was supported by NIH Grant EY11915 (SCP), NIH Core Grants-EY002520 & EY020799, Pathology Cell Core P30CA125123, Biology of Inflammation Center Baylor College of Medicine, an unrestricted grant from Research to Prevent Blindness, New York, NY (SCP), the Oshman Foundation, Houston, TX (SCP), the William Stamps Farish Fund, Houston, TX (SCP), Hamill Foundation, Houston, TX (SCP), Sid W. Richardson Foundation, Ft Worth, TX (SCP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: None of the authors have any financial or personal relationships to disclose that would cause a conflict of interest regarding this article.
References
- Alam J, de Paiva CS, Pflugfelder SC, 2020. Immune - Goblet Cell Interaction in the Conjunctiva. Ocul Surf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK, 2002. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci 43, 1004–1011. [PubMed] [Google Scholar]
- Bagga B, Motukupally SR, Mohamed A, 2018. Microbial keratitis in Stevens-Johnson syndrome: Clinical and microbiological profile. Ocul Surf 16, 454–457. [DOI] [PubMed] [Google Scholar]
- Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O’Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC, 2006. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 25, 900–907. [DOI] [PubMed] [Google Scholar]
- Braun RJ, Gewecke NR, Begley CG, King-Smith PE, Siddique JI, 2014. A model for tear film thinning with osmolarity and fluorescein. Invest Ophthalmol Vis Sci 55, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RJ, King-Smith PE, Begley CG, Li L, Gewecke NR, 2015. Dynamics and function of the tear film in relation to the blink cycle. Prog Retin Eye Res 45, 132–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Rodriguez R, Oliver-Aguillon G, Vega-Perez LM, Jarillo-Luna A, Hernandez-Martinez D, Rojas-Hernandez S, Rodriguez-Monroy MA, Rivera-Aguilar V, Gonzalez-Robles A, 2004. Human IgA inhibits adherence of Acanthamoeba polyphaga to epithelial cells and contact lenses. Can J Microbiol 50, 711–718. [DOI] [PubMed] [Google Scholar]
- Chotikavanich S, de Paiva CS, Li de Q, Chen JJ, Bian F, Farley WJ, Pflugfelder SC, 2009. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci 50, 3203–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Ruiz L, Masli S, 2015. Immunomodulatory Cross-Talk between Conjunctival Goblet Cells and Dendritic Cells. PLoS One 10, e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC, 2006. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Investigative ophthalmology & visual science 47, 3293–3302. [DOI] [PubMed] [Google Scholar]
- Cwiklik L, 2016. Tear film lipid layer: A molecular level view. Biochim Biophys Acta 1858, 2421–2430. [DOI] [PubMed] [Google Scholar]
- Dartt DA, 2001. Regulation of lacrimal gland secretion by neurotransmitters and the EGF family of growth factors. Exp Eye Res 73, 741–752. [DOI] [PubMed] [Google Scholar]
- Dartt DA, 2011. Tear lipocalin: structure and function. The ocular surface 9, 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Lindsey JL, Pflugfelder SC, 2003. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology 110, 1102–1109. [DOI] [PubMed] [Google Scholar]
- Diaz-Valle D, Arriola-Villalobos P, Garcia-Vidal SE, Sanchez-Pulgarin M, Borrego Sanz L, Gegundez-Fernandez JA, Benitez-Del-Castillo JM, 2012. Effect of lubricating eyedrops on ocular light scattering as a measure of vision quality in patients with dry eye. J Cataract Refract Surg 38, 1192–1197. [DOI] [PubMed] [Google Scholar]
- Enriquez-de-Salamanca A, Castellanos E, Stern ME, Fernandez I, Carreno E, Garcia-Vazquez C, Herreras JM, Calonge M, 2010. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Molecular vision 16, 862–873. [PMC free article] [PubMed] [Google Scholar]
- Flanagan JL, Willcox MD, 2009. Role of lactoferrin in the tear film. Biochimie 91, 35–43. [DOI] [PubMed] [Google Scholar]
- Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B, 2004. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother 48, 3367–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima H, Takeyama M, Takeuchi T, Saito I, Tsubota K, 1997. Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis. Clin Exp Allergy 27, 372–378. [PubMed] [Google Scholar]
- Gallo RL, 2013. The birth of innate immunity. Exp Dermatol 22, 517. [DOI] [PubMed] [Google Scholar]
- Garreis F, Gottschalt M, Paulsen FP, 2010. Antimicrobial peptides as a major part of the innate immune defense at the ocular surface. Dev Ophthalmol 45, 16–22. [DOI] [PubMed] [Google Scholar]
- Georgiev GA, Eftimov P, Yokoi N, 2017. Structure-function relationship of tear film lipid layer: A contemporary perspective. Experimental eye research 163, 17–28. [DOI] [PubMed] [Google Scholar]
- Georgiev GA, Eftimov P, Yokoi N, 2019. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. International journal of molecular sciences 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, 2004. Distribution of mucins at the ocular surface. Exp Eye Res 78, 379–388. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 2016. Goblet cells of the conjunctiva: A review of recent findings. Prog Retin Eye Res 54, 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Argueso P, 2003. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 231, 1–49. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Inatomi T, 1998. Cellular origin of mucins of the ocular surface tear film. Adv Exp Med Biol 438, 221–227. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, 2016. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Exp Eye Res 145, 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB, 2014. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One 9, e100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto E, Yagi Y, Matsumoto Y, Tsubota K, 2002. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol 133, 181–186. [DOI] [PubMed] [Google Scholar]
- Gumus K, Crockett CH, Rao K, Yeu E, Weikert MP, Shirayama M, Hada S, Pflugfelder SC, 2011. Noninvasive assessment of tear stability with the tear stability analysis system in tear dysfunction patients. Invest Ophthalmol Vis Sci 52, 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Monroy D, Ji Z, Yoshino K, Huang A, Pflugfelder SC, 1996. Transforming growth factor beta-1 and beta-2 in human tear fluid. Current eye research 15, 605–614. [DOI] [PubMed] [Google Scholar]
- Jones DT, Monroy D, Pflugfelder SC, 1997. A novel method of tear collection: comparison of glass capillary micropipettes with porous polyester rods. Cornea 16, 450–458. [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE, 2003. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea 22, 41–45. [DOI] [PubMed] [Google Scholar]
- Kaido M, Ishida R, Dogru M, Tsubota K, 2011. The relation of functional visual acuity measurement methodology to tear functions and ocular surface status. Jpn J Ophthalmol 55, 451–459. [DOI] [PubMed] [Google Scholar]
- Khimani KS, Go JA, De Souza RG, Mitchell T, Yu Z, de Paiva CS, Saumur M, Pflugfelder SC, 2020. Regional Comparison of Goblet Cell Number and Area in Exposed and Covered Dry Eyes and Their Correlation with Tear MUC5AC. Sci Rep 10, 2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijlstra A, Jeurissen SH, Koning KM, 1983. Lactoferrin levels in normal human tears. Br J Ophthalmol 67, 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE, Fink BA, Fogt N, Nichols KK, Hill RM, Wilson GS, 2000. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci 41, 3348–3359. [PubMed] [Google Scholar]
- Klenkler B, Sheardown H, Jones L, 2007. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. The ocular surface 5, 228–239. [DOI] [PubMed] [Google Scholar]
- Koh S, 2016. Mechanisms of Visual Disturbance in Dry Eye. Cornea 35 Suppl 1, S83–s88. [DOI] [PubMed] [Google Scholar]
- Koh S, 2018. Irregular Astigmatism and Higher-Order Aberrations in Eyes With Dry Eye Disease. Invest Ophthalmol Vis Sci 59, Des36–des40. [DOI] [PubMed] [Google Scholar]
- Kojima T, Ishida R, Dogru M, Goto E, Takano Y, Matsumoto Y, Kaido M, Ohashi Y, Tsubota K, 2004. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthalmol Vis Sci 45, 1369–1374. [DOI] [PubMed] [Google Scholar]
- Kokawa N, Sotozono C, Nishida K, Kinoshita S, 1996. High total TGF-beta 2 levels in normal human tears. Curr Eye Res 15, 341–343. [DOI] [PubMed] [Google Scholar]
- Korb DR, Herman JP, Greiner JV, Scaffidi RC, Finnemore VM, Exford JM, Blackie CA, Douglass T, 2005. Lid wiper epitheliopathy and dry eye symptoms. Eye Contact Lens 31, 2–8. [DOI] [PubMed] [Google Scholar]
- Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC, 2009. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol 147, 198–205. e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S, 2011. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol 129, 981–986. [DOI] [PubMed] [Google Scholar]
- Lan J, Willcox MD, Jackson GD, 1997. Detection and specificity of anti-Staphylococcus intermedius secretory IgA in human tears. Aust N Z J Ophthalmol 25 Suppl 1, S17–19. [DOI] [PubMed] [Google Scholar]
- Lemp MA, Bron AJ, Baudouin C, Benitez Del Castillo JM, Geffen D, Tauber J, Foulks GN, Pepose JS, Sullivan BD, 2011. Tear osmolarity in the diagnosis and management of dry eye disease. American journal of ophthalmology 151, 792–798.e791. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SC, 1995. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol 163, 61–79. [DOI] [PubMed] [Google Scholar]
- Li Q, Weng J, Mohan RR, Bennett GL, Schwall R, Wang ZF, Tabor K, Kim J, Hargrave S, Cuevas KH, Wilson SE, 1996. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest Ophthalmol Vis Sci 37, 727–739. [PubMed] [Google Scholar]
- Li X, Kang B, Woo IH, Eom Y, Lee HK, Kim HM, Song JS, 2018. Effects of Topical Mucolytic Agents on the Tears and Ocular Surface: A Plausible Animal Model of Mucin-Deficient Dry Eye. Investigative ophthalmology & visual science 59, 3104–3114. [DOI] [PubMed] [Google Scholar]
- Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, Nelson JD, Simpson T, 2009. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci 50, 3671–3679. [DOI] [PubMed] [Google Scholar]
- Liu H, Thibos L, Begley CG, Bradley A, 2010. Measurement of the time course of optical quality and visual deterioration during tear break-up. Invest Ophthalmol Vis Sci 51, 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Pflugfelder SC, 1999. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology 106, 939–943. [DOI] [PubMed] [Google Scholar]
- Marko CK, Tisdale AS, Spurr-Michaud S, Evans C, Gipson IK, 2014. The ocular surface phenotype of Muc5ac and Muc5b null mice. Invest Ophthalmol Vis Sci 55, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie RW, Jumblatt JE, Jumblatt MM, 2000. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci 41, 703–708. [PubMed] [Google Scholar]
- Nelson JD, Wright JC, 1984. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol 102, 1049–1051. [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Dogru M, Tsubota K, 2006. Laboratory findings in tear fluid analysis. Clin Chim Acta 369, 17–28. [DOI] [PubMed] [Google Scholar]
- Ormerod LD, Fong LP, Foster CS, 1988. Corneal infection in mucosal scarring disorders and Sjogren’s syndrome. Am J Ophthalmol 105, 512–518. [DOI] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C, 2010. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 16, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Patel R, Zhu M, Robertson DM, 2018. Shifting the IGF-axis: An age-related decline in human tear IGF-1 correlates with clinical signs of dry eye. Growth Horm IGF Res 40, 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CC, Cerretani C, Braun RJ, Radke CJ, 2014. Evaporation-driven instability of the precorneal tear film. Adv Colloid Interface Sci 206, 250–264. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Bian F, Gumus K, Farley W, Stern ME, De Paiva CS, 2018. Severity of Sjogren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen-Presenting Cells. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, de Paiva CS, 2017. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 124, S4–s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, De Paiva CS, Moore QL, Volpe EA, Li DQ, Gumus K, Zaheer ML, Corrales RM, 2015. Aqueous Tear Deficiency Increases Conjunctival Interferon-gamma (IFN-gamma) Expression and Goblet Cell Loss. Invest Ophthalmol Vis Sci 56, 7545–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME, 2008. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea 27, 64–69. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC DPC, Moore QL, Volpe EA, Li DQ, Gumus K, Zaheer ML, Corrales RM, 2015. Aqueous tear deficiency increases conjunctival interferon-gamma (IFN-γ) expression and goblet cell loss. Investigative ophthalmology & visual science. 56, 7545–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D, 1999. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res 19, 201–211. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Liu Z, Monroy D, Li DQ, Carvajal ME, Price-Schiavi SA, Idris N, Solomon A, Perez A, Carraway KL, 2000. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci 41, 1316–1326. [PubMed] [Google Scholar]
- Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL, 1997. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology 104, 223–235. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Wilhelmus KR, Osato MS, Matoba AY, Font RL, 1986. The autoimmune nature of aqueous tear deficiency. Ophthalmology 93, 1513–1517. [DOI] [PubMed] [Google Scholar]
- Rahman EZ, Lam PK, Chu CK, Moore Q, Pflugfelder SC, 2015. Corneal Sensitivity in Tear Dysfunction and its Correlation With Clinical Parameters and Blink Rate. American journal of ophthalmology 160, 858–866.e855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RA, 1975. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol 14, 299–302. [PubMed] [Google Scholar]
- Raquil MA, Anceriz N, Rouleau P, Tessier PA, 2008. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol 180, 3366–3374. [DOI] [PubMed] [Google Scholar]
- Rieger G, 1992. The importance of the precorneal tear film for the quality of optical imaging. Br J Ophthalmol 76, 157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Cunha DA, Carneiro EM, Boschero AC, Saad MJ, Velloso LA, 2002. Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Invest Ophthalmol Vis Sci 43, 963–967. [PubMed] [Google Scholar]
- Rolando M, Iester M, Macri A, Calabria G, 1998. Low spatial-contrast sensitivity in dry eyes. Cornea 17, 376–379. [DOI] [PubMed] [Google Scholar]
- Rolando M, Zierhut M, 2001. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 45 Suppl 2, S203–210. [DOI] [PubMed] [Google Scholar]
- Rosenthal P, Borsook D, 2016. Ocular neuropathic pain. The British journal of ophthalmology 100, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe S, Sakata M, Beaton AR, Sack RA, 1998. Identification, origins and the diurnal role of the principal serine protease inhibitors in human tear fluid. Curr Eye Res 17, 348–362. [DOI] [PubMed] [Google Scholar]
- Sekiyama E, Nakamura T, Kawasaki S, Sogabe H, Kinoshita S, 2006. Different expression of angiogenesis-related factors between human cultivated corneal and oral epithelial sheets. Exp Eye Res 83, 741–746. [DOI] [PubMed] [Google Scholar]
- Sobrin L, Liu Z, Monroy DC, Solomon A, Selzer MG, Lokeshwar BL, Pflugfelder SC, 2000. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci 41, 1703–1709. [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC, 2001. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci 42, 2283–2292. [PubMed] [Google Scholar]
- Spurr-Michaud S, Argueso P, Gipson I, 2007. Assay of mucins in human tear fluid. Exp Eye Res 84, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC, 1998a. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 17, 584–589. [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC, 1998b. A unified theory of the role of the ocular surface in dry eye. Advances in experimental medicine and biology 438, 643–651. [DOI] [PubMed] [Google Scholar]
- Szczotka-Flynn LB, Maguire MG, Ying GS, Lin MC, Bunya VY, Dana R, Asbell PA, 2019. Impact of Dry Eye on Visual Acuity and Contrast Sensitivity: Dry Eye Assessment and Management Study. Optom Vis Sci 96, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany JM, 1990. Measurement of wettability of the corneal epithelium. I. Particle attachment method. Acta Ophthalmol (Copenh) 68, 175–181. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE, 2016. The corneal fibrosis response to epithelial-stromal injury. Experimental eye research 142, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen IS, Tervo TM, Teppo AM, Valle TU, Gronhagen-Riska C, Vesaluoma MH, 2001. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res 72, 631–641. [DOI] [PubMed] [Google Scholar]
- Tutt R, Bradley A, Begley C, Thibos LN, 2000. Optical and visual impact of tear break-up in human eyes. Invest Ophthalmol Vis Sci 41, 4117–4123. [PubMed] [Google Scholar]
- Ubels JL, Foley KM, Rismondo V, 1986. Retinol secretion by the lacrimal gland. Invest Ophthalmol Vis Sci 27, 1261–1268. [PubMed] [Google Scholar]
- Uchino Y, Uchino M, Yokoi N, Dogru M, Kawashima M, Okada N, Inaba T, Tamaki S, Komuro A, Sonomura Y, Kato H, Argueso P, Kinoshita S, Tsubota K, 2014. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol 132, 985–992. [DOI] [PubMed] [Google Scholar]
- van Setten G, Schultz G, 1994. Transforming growth factor-alpha is a constant component of human tear fluid. Graefes Arch Clin Exp Ophthalmol 232, 523–526. [DOI] [PubMed] [Google Scholar]
- van Setten GB, 1996. Basic fibroblast growth factor in human tear fluid: detection of another growth factor. Graefes Arch Clin Exp Ophthalmol 234, 275–277. [DOI] [PubMed] [Google Scholar]
- van Setten GB, Macauley S, Humphreys-Beher M, Chegini N, Schultz G, 1996. Detection of transforming growth factor-alpha mRNA and protein in rat lacrimal glands and characterization of transforming growth factor-alpha in human tears. Invest Ophthalmol Vis Sci 37, 166–173. [PubMed] [Google Scholar]
- van Setten GB, Viinikka L, Tervo T, Pesonen K, Tarkkanen A, Perheentupa J, 1989. Epidermal growth factor is a constant component of normal human tear fluid. Graefes Arch Clin Exp Ophthalmol 227, 184–187. [DOI] [PubMed] [Google Scholar]
- Varnell RJ, Freeman JY, Maitchouk D, Beuerman RW, Gebhardt BM, 1997. Detection of substance P in human tears by laser desorption mass spectrometry and immunoassay. Curr Eye Res 16, 960–963. [DOI] [PubMed] [Google Scholar]
- Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T, 1997a. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: a potential modulator of corneal wound healing following photorefractive keratectomy. Curr Eye Res 16, 825–831. [DOI] [PubMed] [Google Scholar]
- Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T, 1997b. Release of TGF-beta 1 and VEGF in tears following photorefractive keratectomy. Curr Eye Res 16, 19–25. [DOI] [PubMed] [Google Scholar]
- Vesaluoma MH, Tervo TT, 1998. Tenascin and cytokines in tear fluid after photorefractive keratectomy. Journal of refractive surgery (Thorofare, N.J. : 1995) 14, 447–454. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ogawa Y, Dogru M, Tatematsu Y, Uchino M, Kamoi M, Okada N, Okamoto S, Tsubota K, 2010. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant 45, 1077–1083. [DOI] [PubMed] [Google Scholar]
- Wiesner J, Vilcinskas A, 2010. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1, 440–464. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Chen L, Mohan RR, Liang Q, Liu J, 1999a. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res 68, 377–397. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Klyce SD, 1991. Quantitative descriptors of corneal topography. A clinical study. Arch Ophthalmol 109, 349–353. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Liang Q, Kim WJ, 1999b. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci 40, 2185–2190. [PubMed] [Google Scholar]
- Xiao Y, De Paiva CS, Yu Z, Guimaraes de Souza R, Li DQ, Pflugfelder SC, 2018. Goblet cell produced retinoic acid suppresses CD86 expression and IL-12 production in bone marrow derived cells. Int Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ogata M, Kawai M, Mashima Y, Nishida T, 2003. Substance P in human tears. Cornea 22, S48–54. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Georgiev GA, 2018. Tear Film-Oriented Diagnosis and Tear Film-Oriented Therapy for Dry Eye Based on Tear Film Dynamics. Invest Ophthalmol Vis Sci 59, Des13–des22. [DOI] [PubMed] [Google Scholar]
- Yoshino K, Garg R, Monroy D, Ji Z, Pflugfelder SC, 1996. Production and secretion of transforming growth factor beta (TGF-beta) by the human lacrimal gland. Curr Eye Res 15, 615–624. [DOI] [PubMed] [Google Scholar]
- Zheng X, De Paiva CS, Rao K, Li DQ, Farley WJ, Stern M, Pflugfelder SC, 2010. Evaluation of the transforming growth factor-beta activity in normal and dry eye human tears by CCL-185 cell bioassay. Cornea 29, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, 2012. Tear analysis in ocular surface diseases. Prog Retin Eye Res 31, 527–550. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Ang LP, Chan CM, Li SF, Chew FT, Tan DT, 2009a. Elevation of human alpha-defensins and S100 calcium-binding proteins A8 and A9 in tear fluid of patients with pterygium. Invest Ophthalmol Vis Sci 50, 2077–2086. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D, 2009b. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res 8, 4889–4905. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Huang L, Barathi A, Foo YH, Li SF, Chew FT, Tan D, 2007. Proteomic analysis of rabbit tear fluid: Defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics 7, 3194–3206. [DOI] [PubMed] [Google Scholar]
- Zhou L, Huang LQ, Beuerman RW, Grigg ME, Li SF, Chew FT, Ang L, Stern ME, Tan D, 2004. Proteomic analysis of human tears: defensin expression after ocular surface surgery. Journal of proteome research 3, 410–416. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW, 2012. In-depth analysis of the human tear proteome. J Proteomics 75, 3877–3885. [DOI] [PubMed] [Google Scholar]
- Zuazo F, Lopez-Ponce D, Salinas-Toro D, Valenzuela F, Sans-Puroja J, Srur M, Lopez-Solis RO, Traipe-Castro L, 2014. [Conjunctival impression cytology in patients with normal and impaired OSDI scores]. Arch Soc Esp Oftalmol 89, 391–396. [DOI] [PubMed] [Google Scholar]
- Zubkov VS, Breward CJ, Gaffney EA, 2012. Coupling fluid and solute dynamics within the ocular surface tear film: a modelling study of black line osmolarity. Bull Math Biol 74, 2062–2093. [DOI] [PubMed] [Google Scholar]


