Abstract
Previously, we have shown that Staphylococcus (S) aureus induces a glycolytic response in retinal residential (microglia) and infiltrated cells (neutrophils and macrophages) during endophthalmitis. In this study, we sought to investigate the physiological role of glycolysis in bacterial endophthalmitis using a glycolytic inhibitor, 2-deoxy-glucose (2DG). Our data showed that 2DG treatment attenuated the inflammatory responses of mouse bone marrow-derived macrophages (BMDM) and neutrophils (BMDN) when challenged with either live or heat-killed S. aureus (HKSA). Among the inflammatory mediators, 2DG caused a significant reduction in levels of cytokines (TNFα, IL-1β, IL-6) and chemokines (CXCL1 and CXCL2). Western blot analysis of 2DG treated cells showed downregulation of bacterial-induced MEK/ERK pathways. In vivo, intravitreal administration of 2DG both pre- and post- bacterial infection resulted in a significant reduction in intraocular inflammation in C57BL/6 mouse eyes and downregulation of ERK phosphorylation in retinal tissue. Collectively, our study demonstrates that 2DG attenuates inflammatory response in bacterial endophthalmitis and cultured innate immune cells via inhibition of ERK signaling. Thus glycolytic inhibitors in combination with antibiotics could mitigate inflammation-mediated tissue damage in ocular infections.
Keywords: S. aureus, Endophthalmitis, Retina, Inflammation, Glycolysis, 2DG
1. Introduction
Infectious endophthalmitis is a devastating ocular complication caused by a wide range of microbes and can result in visual impairment or blindnessif not treated promptly. Gram-positive bacteria, specifically Staphylococci, which typically enters the eye during ocular surgeries (e.g., cataract surgery) and/or trauma, are the leading cause of the majority of endophthalmitis cases. The incidence rate of post-operative endophthalmitis ranges from 0.01% to 0.87% while post-traumatic endophthalmitis occurs in 3–17% of cases following open globe injury (Cailegan et al., 1999; Callegan et al., 2002; Sadaka et al., 2012; Talreja et al., 2015; Miller et al., 2019; Thompson et al., 1995). In addition, intravitreal injections, commonly used for delivery of therapeutic modalities in the eyes, increase the risk of developingendophthalmitis (Sadaka et al., 2012; Thompson et al., 1995). Once, inside the eye, retinal residential cells (glial cells) trigger inflammatory signaling as a host defense mechanism to eradicate invading pathogens by increasing the infiltration of myeloid cells, such as neutrophils and monocytes/macrophages (Gupta et al., 2019; Miller et al., 2019; Talreja et al., 2015). If unchecked, this inflammatory cascade can result in inflammation-mediated ocular tissue damage resulting in vision loss or blindness (Callegan et al., 2007; Miller et al., 2019; Talreja et al., 2015). Current treatment regimens use antibiotics and, if severe, vitrectomies are performed. Even with these medical and surgical treatment options, 1/3 of cases will result in decreased visual acuity (Callegan et al., 2002; Callegan et al., 2007; Sadaka et al., 2012; Singh et al., 2014a; Talreja et al., 2015). In an attempt to identify new treatment strategies, our laboratory's interest has been on discovering therapeutic modalities which take into consideration both eradicating invading pathogens and limiting intraocular inflammation (Kumar et al., 2016; Kumar and Kumar, 2015; Kumar et al., 2013; Singh et al., 2014a).
Using transcriptomic and system biology approaches, our prior studies demonstrate that bacterial and viral infections dysregulate several metabolic pathways in retinal cells (Rajamani et al., 2016; Singh et al., 2018). We previously reported Staphylococcus aureus (SA) infection increased glycolysis in both retinal microglia as well as BMDM. Moreover, an AMP-activated protein kinase (AMPK) activator, 5aminoimidazole-4-carboxamide ribonucleoside (AICAR), reduced the glycolytic response and protected the mouse retina from SA induced damage (Kumar et al., 2016). Similarly, AMPK activation was found to restrict Zika virus replication in endothelial cells by inhibiting glycolysis and potentiating innate anti-viral response (Singh et al., 2020). Collectively, these findings from our lab and others highlight the importance of modulating cellular metabolism to improve the outcome of wide range of diseases (Dasgupta et al., 2017; Leung et al., 2012; O'Neill et al., 2016). Furthermore, many immune cells, such as T-cells (Pearce et al., 2009; Shi et al., 2011), NK-cells (Mah et al., 2017) and macrophages (Galvan-Pena and O'Neill, 2014; Kumar et al., 2016), utilize glycolysis during their effector functions by allowing cells to quickly obtain energy and biosynthetic intermediates needed to fight against infection. Because pro-inflammatory cells utilize glycolysis during their effector phase, this pathways could potentially be targeted to limit host driven inflammatory damage.
The glycolytic inhibitor, 2-deoxy-glucose (2DG), has been best characterized in animal models and widely used to investigate the role of glycolysis in several diseases (Brown, 1962; Sun et al., 2016). In cancer cell lines, 2DG slowed down cell proliferation and apoptosis (Cheong et al., 2011; Dasgupta et al., 2017). Similarly, inhibition of T-cell glycolysis prevented graft rejection (Lee et al., 2015) and reduced excessive IFN-γ production in autoreactive CD4+ T cells (Yin et al., 2015). Furthermore, 2DG has been shown to alter macrophage functions such as cytokine production, and phagocytosis (Galvan-Pena and O'Neill, 2014; Michl et al., 1976) and modulation of several infectious diseases (Leung et al., 2012; Varanasi et al., 2017; Wang et al., 2016). However, to our knowledge, the role of glycolysis has not been investigated in ocular bacterial infections.
Since increased inflammation causes retinal tissue damage in bacterial endophthalmitis and infectious/inflammatory conditions have been linked to increased glycolytic activity, we hypothesize that inhibition of glycolysis may exert anti-inflammatory effects. Herein, we investigated the role of a glycolytic inhibitor, 2DG, on innate immune cells and in a mouse model of staphylococcal endophthalmitis.
2. Materials and Methods
2.1. Bacterial strain and Reagents
S. aureus (strain RN6390) was maintained in tryptic soy broth and agar plates (TSB/TSA; Sigma-Aldrich, St. Louis, MO). 2-Deoxy-glucose was purchased from Cayman Chemical (Ann Arbor, MI). For in vitro experiments cells were pretreated with 10mM of 2DG for 1 h followed by indicated infection times. For in vivo applications, the left eyes of each mouse was injected with 25 μg/eye (1μl volume) of 2DG, 14–16 h prior to or 6 h post bacterial infection. The dose of 2DG for intravitreal injection was chosen based on preliminary dose (10–100 μg/eye) titration. Contralateral eyes with sterile PBS injection were used as a controls. An ERK inhibitor, U1260, was purchased from InvivoGen (San Diego, CA) and cells were pretreated for 1 h with 10 μM of U1260. Antiphospho-ERK and ERK antibodies were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX). Anti-HSP90 antibody was purchased from Cell Signaling Technology (Beverly, MA). Secondary horseradish peroxidase (HRP)-conjugated anti-mouse or antirabbit IgG antibodies were purchased from Bio-Rad (Hercules, CA).
2.2. Mice Infection and Ethics Statement
C57BL/6 (wild type [WT]) was purchased from Jackson Laboratory (Bar Harbor, ME). Both male and female mice, 6 to 8 weeks of age, were used in all the experiments. Mice were housed in a restricted-access Division of Laboratory Animal Resources (DLAR) facility, maintained on a 12 h light 12 h dark cycle at 22°C temperature, and fed on LabDiet rodent chow (PicoLab; LabDiet, St. Louis, MO) and water ad libitum. Mice were treated in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University. Endophthalmitis was induced in the mice by intravitreal injection of S. aureus RN6390 (5000 CFU/eye). Eyes injected with sterile PBS served as controls. Following infection at endpoints, eyes were enucleated and subjected to bacterial growth determination, inflammatory cytokine/chemokine, and immunoblot assays.
2.3. Electroretinography
Scotopic electroretinography (ERG) was used to determine retinal function in S. aureus infected and 2DG treated mouse eyes using Celeries ERG system (Diagnosis LLC, Lowell, MA), as described previously (Guest et al., 2018; Singh et. al. 2014a)
2.4. Isolation of bone marrow-derived macrophages (BMDM) and neutrophils (BMDN)
Mice BMDM and BMDN were isolated as described previously (Kumar et al., 2016; Swamydas and Lionakis, 2013). Briefly, femurs and tibias were isolated from euthanized mice. Bone marrow was flushed using a 25-gauge needle and syringe filled with RPMI media containing 10% FBS and 0.2 mM EDTA. Cells were pelleted by centrifugation at 400 x g for 5 min at 4°C. RBCs were lysed by adding a hypotonic solution of 0.2% NaCl for 20 sec, followed by the addition of 1.6% NaCl and centrifugation. Following RBC lysis, cell pellets were washed with RPMI media by centrifugation at 400 x g. Cells were resuspended and seeded in RPMI media supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 10 ng/ml M-CSF for macrophage differentiation at 37°C in 5% CO2. Six days post differentiation ~1×106 BMDM/mL were seeded in 6 well tissue-culture plates for in-vitro experiments.
For BMDN extraction, bone marrow cell pellets were resuspended in 1 ml PBS and overlayed over a histopaque gradient of 1119 and 1077 (Sigma-Aldrich, St. Louis, MO), followed by centrifugation at 600 x g at 25°C for 30 min without centrifuge break. The layer between histopaques 1119 and 1077 was collected and washed two times with RPMI media by centrifugation at 400 x g for 7 min and BMDN cells were cultured as described for macrophages. For in vitro infections, cells were challenged with S. aureus RN6390 at Multiplicity of Infection (MOI) 10:1 for indicated time points. For heat-killed bacteria, similar MOI of S. aureus is boiled for 5 min and used for in vitro experiments.
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of the inflammatory cytokines and chemokines in the in vitro culture supernatant and mice eye lysates (15–20 μg total protein) were determined using commercially available ELISA kits. ELISA was performed for inflammatory cytokines: TNF-α, IL-1β, IL6, and chemokines: CXCL2 (MIP2), and CXCL1 (KC) as per manufacturer's instruction (R&D Systems, Minneapolis, MN, USA). Data were presented as mean ± standard deviation (SD) pg/ml for in vitro conditioned media, and mean ± SD pg/mg of eye lysates.
2.6. RNA Extraction and qRT-PCR Analysis
Total RNA was then extracted from cells using TRIzol reagent, as per the manufacturer’s instruction (Invitrogen, Carlsbad, CA). cDNA was synthesized using 1 μg of total RNA using a Maxima first-strand cDNA synthesis kit, as per the manufacturer’s instructions (ThermoFisher Scientific, Rockford, IL). Quantitative RT-PCR was conducted in a StepOnePlus instrument (Applied Biosystems, Grand Island, NY, USA) using cDNA for pro-inflammatory cytokines and chemokines, Tnf-α, ll-1β, Il6, Cxcl1, and Cxcl2 genes. TaqMan primers were purchased from Integrated DNA Technologies (Coralville, lA). The quantification of gene expression was determined by the comparative ΔΔCT method. Expression levels in the test samples were normalized to those of endogenous reference β-actin levels. Data are mean ± SD fold change.
2.7. Immunoblotting
Following incubation, cells were washed with ice-cold PBS and lysed using RIPA buffer containing protease inhibitor cocktail. Samples were sonicated briefly, followed by centrifugation at 15,000 x g for 20 min. Total protein was quantified using a Micro BCA protein assay kit (ThermoFisher, Rockford, IL). Total protein samples (30 μg) were resolved on a 12% SDS-PAGE in Tris-giycine-SDS buffer and electro-blotted onto a nitrocellulose membrane (BioRad, Hercules, CA). Blots were blocked for 1 h at room temperature with Tris-buffered saline containing 0.05% Tween 20 (TBST) and 5% skimmed milk. The membrane was probed with primary antibodies diluted in 3% BSA in TBST overnight at 4°C. After three washes with TBST, the membrane was incubated with anti-mice/rabbit HRP conjugated secondary antibody diluted in TBST with 5% milk at room temperature for 2 h. Protein bands were developed with SuperSignal West Femto Chemiluminescent Substrate (ThermoFisher Scientific, Rockford, IL), and visualized using iBright FL1500 Imaging Systems (ThermoFisher Scientific, Rockford, IL).
2.8. Statistics
All data have been expressed as means ± SD unless indicated otherwise. All statistical analysis was performed using GraphPad Prism V8 (GraphPad Software, La Jolla, CA). One-way ANOVA was used for group comparisons followed by Tukey’s Multiple Comparison post-hoc test. A P-value of <0.05 was considered statistically significant. All experiments were performed at least three times unless indicated otherwise.
3. Results
3.1. 2DG attenuates SA-induced inflammatory response in macrophages and neutrophils
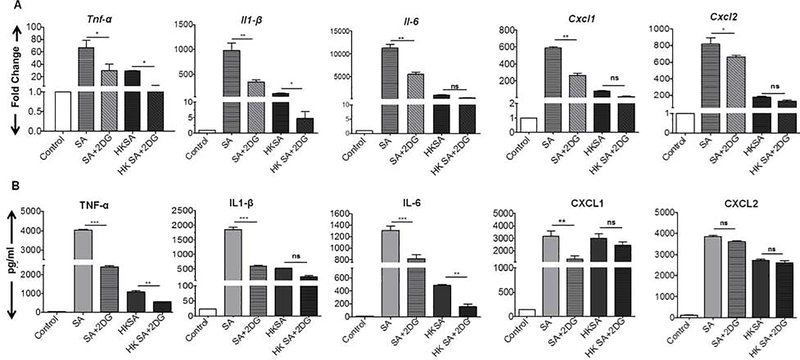
Previously, we have shown that S. aureus infection induced a glycolytic response in retinal microglia and mouse retina (Kumar et al., 2016). To ascertain the role of glycolysis in bacterial-induced inflammation, we assessed the effect of a glycolytic inhibitor, 2DG, in vitro using BMDM (Fig. 1) and BMDN (Fig. 2). In response to live SA challenge, BMDM exhibited induced expression of inflammatory cytokines Tnf-α, ll-1β, 11–6, and chemokines Cxcl1, Cxcl2 mRNA transcripts as compared to control (Fig. 1A). 2DG treatment attenuated the SA- induced expression of all of these cytokines/chemokines {Tnf-α, ll-1β, Il-6, Cxcl1, and Cxcl2) transcripts. Concomitant with mRNA expression, levels of these pro-inflammatory mediators, at protein levels were also increased by live SA infection and reduced by 2DG treatment except CXCL2 protein levels (Fig. 1B). BMDM challenged with HKSA also resulted in induced expression of various inflammatory mediators, however, their levels were relatively low compared to live SA infection. In HKSA challenged BMDM, 2DG treatment attenuated mRNA expression of Tnf-α, and ll-1β but not Il-6, Cxcl1 and Cxcl2. A similar trend was observed at protein levels of these inflammatory mediators with 2DG reducing levels of TNF-α and IL-6 but not IL-1β, CXCL1, and CXCl2.
Figure 1. 2DG treatment attenuates S. aureus induced inflammation in the macrophages.
Bone marrow-derived macrophages (BMDMs) were pretreated with 2DG (10 mM) for 1 h followed by challenge with either live S. aureus (SA) or heat-killed S. aureus (HKSA) for 8 h. Uninfected cells were used as control. Control, SA, and HKSA challenged cells were subjected to qRT-PCR for inflammatory mediators (A). Conditioned media were subjected to ELISA to quantify the cytokine protein levels (B). Data represent mean ± SD from three independent experiments. A one-way ANOVA with Tukey post hoc test was used to determine statistical significance. *p<0.05, **p<0.01, ***p<0.001, ns: not significant.
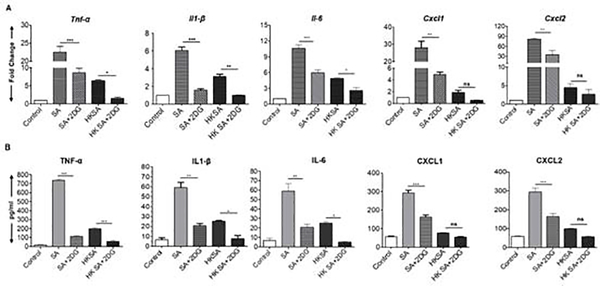
Figure 2. 2DG treatment attenuates SA induced inflammation in the neutrophils.
Bone marrow-derived neutrophils (BMDNs) were pretreated with 2DG (10 mM) for 1 h followed by challenge with either live S. aureus (SA) or heat-killed S. aureus (HKSA) for 8 h. Uninfected cells were used as control. Control, SA, and HKSA challenged cells were subjected to qRT-PCR for inflammatory mediators (A). Conditioned media were subjected to ELISA to quantify the cytokine protein levels (B). Data represent mean ± SD from three independent experiments. A one-way ANOVA with Tukey post hoc test was used to determine statistical significance. *p<0.05, **p<0.01, ***p<0.001, ns: not significant.
To determine whether the anti-inflammatory effects of 2DG are cell-specific, bone marrow-derived neutrophils (BMDN) were challenged with live or HKSA. Likewise, in BMDN, live SA challenge significantly induced the expression of TNF-α, IL-1β, IL-6, CXCL1, and CXCL2 both at transcripts (Fig. 2A) as well as protein levels (Fig. 2B) and 2DG treatment attenuated this response. In BMDN challenged with HKSA, 2DG treatment only reduced the expression of cytokines TNF-α, IL-1β, and IL-6 and had no significant effect on others. Taken together, these data suggest the 2DG exerts anti-inflammatory properties in SA challenged BMDM and BMDN.
3.2. 2DG treatment inhibits SA-induced MEK/ERK signaling
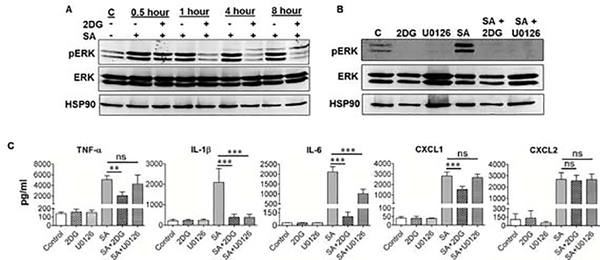
Since we observed the downregulation of inflammatory cytokines upon 2DG treatment, we assessed the effect of 2DG on classical MAPKs (p38, JNK and MEK-ERK), involved in inflammatory signaling (Kumar and Shamsuddin, 2012; Singh and Kumar, 2015). Our results show that S. aureus induced time-dependent phosphorylation of ERK1/2 in BMDMs and 2DG pretreatment reduced this effect (Fig. 3A). However, 2DG had no effect on p38 and JNK signaling (data not shown). To further validate the effect of 2DG on the downregulation of ERK1/2 phosphorylation, we used a specific pharmacological inhibitor of MEK, U0126. We found that both 2DG, as well as U0126, downregulated the ERK phosphorylation (Fig. 3B), indicating that 2DG behaves similarly to MEK inhibitor. To investigate the biological consequence of 2DG and U0126 mediated downregulation of ERK1/2 phosphorylation, we quantified the production of inflammatory cytokines by ELISA. Our data show that while both 2DG and U0126 treatment significantly reduced IL-1β, and IL-6 levels (Fig. 3C), only 2DG was found to reduce the production of TNF-α and CXCL1, whereas U0126 had no effect, indicating the effect of 2DG on other parallel mechanisms independent of ERK inhibition (Fig. 3C). The levels of CXCL2 remained unchanged and were comparable in both of these drug treatments. The drug alone (without SA infection) had no effect on these cytokines/chemokines production as their levels were similar to uninfected controls.
Figure 3. S. aureus induced MEK/ERK pathways is inhibited by 2DG.
BMDMs were pretreated with 2DG (10 mM) for 1 h followed by challenge with S. aureus (SA) for indicated time-points. Cells were harvested and subjected to Western blotting for pERK, ERK and HSP90 proteins (A). BMDMs were pretreated with either 2DG (10 mM) or ERK inhibitor U0126 (10 μM) for 1 h, followed by a challenge with SA for 8 h. Cells were harvested and subjected to Western blotting for pERK, ERK and HSP90 proteins (B). The conditioned media from 2DG and U0126 pretreated cells in the presence and absence of SA were subjected to ELISA (C). Data represent mean ± SD from three independent experiments. A one-way ANOVA with Tukey post hoc test was used to determine statistical significance. **p<0.01, ***p<0.001, ns: not significant.
3.3. 2DG treatment attenuates inflammation in staphylococcal endophthalmitis
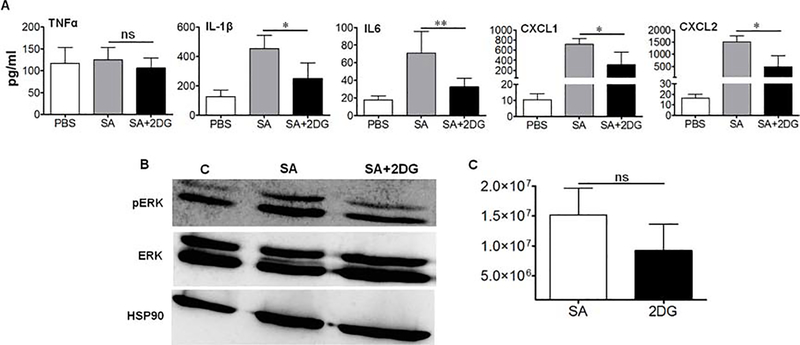
To validate our in vitro findings, we tested the effect of 2DG in a mouse model of S. aureus endophthalmitis (Singh et al., 2014a; Talreja et al., 2015). Since we observed the downregulation of ERK1/2 phosphorylation following 2DG pretreatment in cultured cells, we treated mice with 2DG by intravitreal injections 12–14 h prior to SA infection and investigated its effect on intraocular inflammation. Our results show that 2DG treatment significantly reduced the expression of inflammatory mediators, IL-1β, IL-6, CXCL1, and CXCL2 in SA infected eyes, as measured by ELISA (Fig. 4A). To determine whether 2DG inhibits ERK1/2 phosphorylation in vivo, western blot analysis was performed on retinal tissue lysate which showed that 2DG treated eyes had reduced ERK1/2 phosphorylation (Fig. 4B). Surprisingly, 2DG had no significant effect on bacterial burden in the eye albeit slight reduction (Fig. 4C).
Figure 4. 2DG pre-treatment reduces intraocular inflammation in Staphycocccal endophthalmitis.
WT C57BL/6 mice (n=10) were pretreated overnight with intravitreal injections of 2DG (25 μg/eye) followed by intravitreal injection of S. aureus. 24 h post-infection eyes were enucleated, homogenized and subjected to ELISA for cytokine quantification (A), Western blotting for pERK1/2, ERK1/2 and HSP90 proteins (B), and bacterial burden estimation (C). A one-way ANOVA with Tukey post hoc test was used to determine statistical significance. **p<0.01, ***p<0.001, ns: not significant. Data represent mean ± SD.
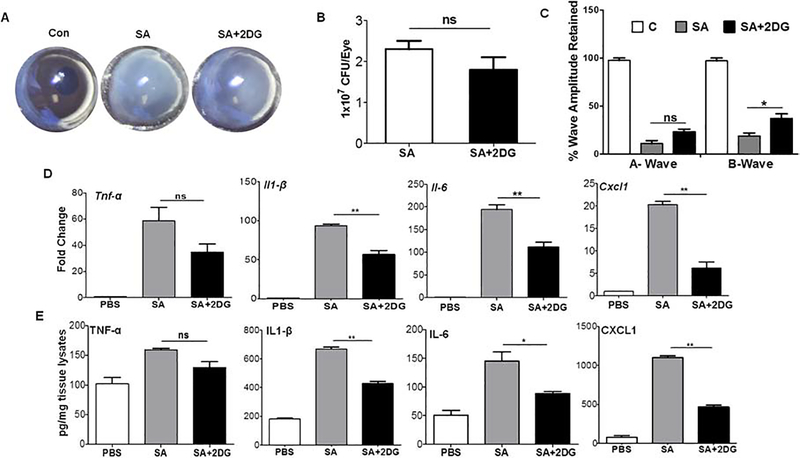
Because our pretreatment (prophylactic) approach was found reduce intraocular inflammation, we next sought whether 2DG can exert anti-inflammatory effects when given post-infection. Indeed, administered of 2DG after 6h of SA challenge was found to reduce inflammation. Our data show that 2DG post-treatment reduced corneal haze and anterior chamber opacity in mouse eyes (Fig. 5A) but had no significant impact on bacteria growth (Fig. 5B). ERG analysis showed reduction of both a- and b-wave amplituteds in SA infected eyes and 2DG treatment was found to better retain b- but not the a-wave amplitude (Fig. 5C). Most importantly, similar to pre-treatment, 2DG post-treatment reduced pro-inflammatory cytokines/chemokines both at transcript (Fig. 5D) as well as protein levels with the exception of TNF-α (Fig. 5E). Collectively, these results indicate that 2DG treatment pre- or post- SA infection attenautes inflammatory response in endophthalmitis.
Figure 5. 2DG post-treatment attenuates S. aureus induced intraocular inflammation in mouse eyes.
WT C57BL/6 mice (n=10) eyes were infected with S. aureus and at 6 h post-infection eyes were treated with 2DG (25 μg/eye) by intravitreal injection. 24 h post-2DG treatment eyes were examined and photomicrograph was taken (A). Eyes were enucleated and subjected to bacterial burden estimation by plate count (B). The retinal function was measured using scotopic ERG (C). The retinal tissue was subjected to qPCR for various pro-inflammatory mediators (D). The eye lysates were subjected to ELISA for cytokine/chemokine quantification (E). A one-way ANOVA with Tukey post hoc test was used to determine statistical significance. *p<0.05, **p<0.01, ns: not significant. Data represent mean ± SD.
4. Discussion
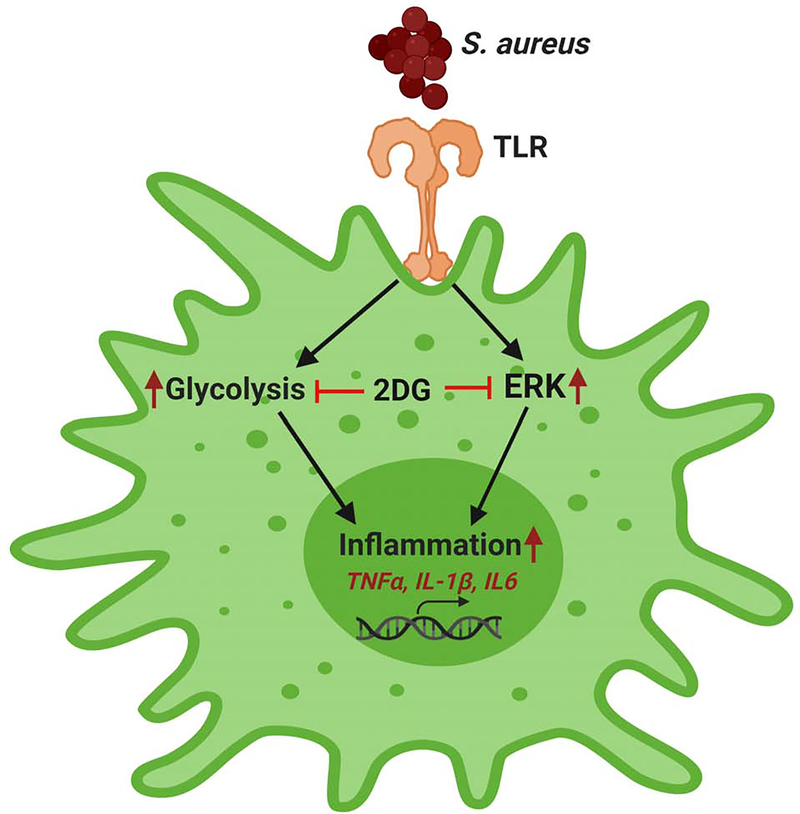
The concept of immunometabolism has greatly evolved in past few years to study the interface between metabolism and immunity as it has provided novel therapeutic targets (O'Neill, L.A., 2016). Because several studies, including those from our laboratory, have implicated the role of glycolysis in promoting inflammation (Hackett et al., 2020; Kumar et al., 2016; Lin et al., 2020; Varanasi et al., 2017; Wei et al., 2020), we investigated the link between glycolytic metabolism and inflammation in intraocular infection, which is currently not known. Here, we demonstrated that inhibition of glycolysis exerts anti-inflammatory effects in macrophages and neutrophils challenged with live or heat killed S. aureus. Additionally, 2DG treatment either pre- or post-bacterial infection resulted in diminished intraocular inflammation in experimental staphylococcal endophthalmitis. Mechanistically, 2DG exerted its anti-inflammatory effects via inhibition of bacterial-induced MEK-ERK1/2 pathways (Fig. 6). Collectively, our study suggests that glycolytic inhibitors such as 2DG might aid in controlling excessive inflammation during intraocular infection and can be used as adjunct therapy with antibiotics.
Figure 6. A schematic of anti-inflammatory mechanisms of 2DG.
During bacterial endophthalmitis, S. aureus is recognized by TLR2 expressed on innate immune cells, leading to activation of glycolytic and inflammatory (ERK) signaling cascade resulting in induced expression of inflammatory mediators. 2DG exerts anti-inflammatory effects by inhibiting these cellular responses. This schematic was created using BioRender software.
The eye is classically viewed as an immune-privileged organ (Guest et al., 2018; Miller et al., 2019; Mursalin et al., 2020). However, this immune-privileged status can be breached by pathogens which causes damage to ocular tissues, potentially leading to vision impairment or blindness. Various pathogens, including bacteria, viruses, and fungi, have been shown to infect the eye through multiple means including trauma, ocular surgeries, or endogenously through the bloodstream (Gupta et al., 2019; Mursalin et al., 2020). Following infection, retinal residential cells viz. astrocytes, microglia, Müller cells-become activated through recognition of PAMPs via PRR/TLRs activation and induced an innate immune response (Kumar et al., 2013; Singh et al., 2014b; Talreja et al., 2015). Cytokines/chemokines produced by these cells promote the permeabilization of the blood-retinal barrier, attract PMNs and macrophages, and clear the invading pathogens (Kumar and Kumar, 2015; Novosad and Callegan, 2010). However, the persistent influx of innate immune cells and intraocular inflammation leads to the destruction of ocular tissues (Miller et al., 2019; Parkunan et al., 2016; Talreja et al., 2015). Therefore, immunomodulatory therapies are needed to minimize the pathological consequences of excessive inflammation (Miller et al., 2019).
The effects of 2DG (glycolysis inhibition) has been primarily studied in cancer and T-cell mediated diseases (Ciavardelli et al., 2014; Zhang et al., 2006), under conditions known to have high metabolic demand (Chang et al., 2013; Chang et al., 2015; O'Neill et al., 2016). Outcomes of glycolytic inhibition as a treatment in infectious disease models have varied depending on organ/tissue infected, as well as the pathogen involved (Ogawa et al., 1994; Sutrina et al., 2016; Varanasi et al., 2017; Wickersham et al., 2017), implying 2DG efficacy is disease dependent. To test whether 2DG modulates innate responses in bacterial endophthalmitis, we first tested its effect on cytokine production using primary bone marrow-derived macrophages and neutrophils, as these particular innate cells infiltrate the eye soon after bacterial invasion and promote intraocular inflammation. Our data shows that 2DG pretreatment significantly attenuates both live and heat-killed S. aureus in BMDM. Our finding corroborated with reports where 2DG has been shown to decrease S. aureus induced TNF-α and IL-1β production in keratinocytes (Wickersham et al., 2017), and LPS induced IL-6 production by human macrophages (Chiba et al., 2017) and microglia (Fodelianaki et al., 2019).
Polymorphonuclear neutrophils (PMN) are also known to rely on glycolysis for their effector functions (Loftus and Finlay, 2016) and are essential for bacterial clearance in endophthalmitis (Coburn et al., 2018; Parkunan et al., 2015; Singh et al., 2014a; Talreja et al., 2015). When we inhibited glycolysis using 2DG, the BMDN demonstrated reduced cytokine production following SA challenge. Similarly, 2DG differentially modulated the expression of inflammatory mediators following the HKSA challenge. These results suggest that immune cells, to varying degrees, utilize glycolysis for pro-inflammatory cytokine secretion that can be reduced when blocking the glycolytic pathway. We also observed a slight discrepancy in the reduction of inflammatory cytokine/ chemokines by 2DG in BMDM and BMDN suggesting glycolysis independent expression of some of these inflammatory mediators. Our findings are further supported by studies showing 2DG significantly reduced LPS-induced release of IL-6 and IL-1β in microglia, although it did not influence TNF-α expression suggesting TNF-α expression is regulated by LPS independently of glycolysis in microglia (Fodelianaki et al., 2019). These differential effects of 2DG specific cytokines or chemokines could be harnessed to modulate the pathogenic versus protective effects of these inflammatory mediators in the context of a disease condition.
To determine the in vivo relevance of our findings, we tested the anti-inflammatory effect of 2DG in a mouse model of bacterial endophthalmitis (Talreja et al., 2015). We observed that intravitreal injection of 2DG followed by infection with S. aureus resulted in a significant reduction in intraocular inflammation by inhibiting the IL-1β, IL-6, CXCL1, and CXCL2 production. We did not observe any effect of 2DG on TNF-α production. Our finding corroborated with a previous study where 2DG treatment was shown to reduce S. aureus induced cytokines production, including IL-1β, but had no effect on bacterial burden or PMN infiltration in a skin infection model (Wickersham et al., 2017). Because we also observed no significant reduction in intraocular bacterial growth, the observed anti-inflammatory effects of 2DG are likely due to its ability to inhibit bacterial-induced inflammatory signaling (MEK/ERK) in infiltrated innate immune cells (e.g., PMNs, macrophages). However, further studies are neede to assess the effect of 2DG on cellular infiltration in bacterial endophthalmitis.
Although 2DG treatment was not found to exhibit significant effect on bacterial burden in infected mouse eyes, some in vitro studies have shown that 2DG attenuates the multiplication of intracellular pathogens such as Legionella pneumophila, in part via inducing autophagy (Matsuda et al., 2009; Ogawa et al., 1994). We also performed RNASeq analysis of BMDMs challenged with SA in presence or absence of 2DG. Our unpublished data shows induced expression of genes related to autophagy and oxidative stress patways. However, further validation and more indepth investigations are needed to draw conclusion on whether 2DG modulates phagocytosis and/or intracellular killing on S. aureus by innate immune cells.
It is well known that in infectious conditions, following recognition of PAMPs via TLRs, induction of multiple signaling pathways occurs, including MAPK/ ERK kinase (MEK) and NF-ĸB resulting production of inflammatory mediators (Fig. 6). Because these pathways has been shown to modulate cellular metabolism, including glycolysis (Sanin et al., 2015; Traves et al., 2012), we assessed the effect of 2DG on MAPK signaling pathways. Similar to a previous study (Chiba et ai,, 2017), we found that 2DG mainly inhibits bacterial-induced ERK pathway and had no significant effect on other MAPKs, such as p38 and JNK (data not shown). Hence our data imply that 2DG exerts its anti-inflammatory effects by inhibiting bacterial-induced ERK resulting in reduced transcription and translation of inflammatory mediators. This observation was further supported by the head-to-head comparison of 2DG with an ERK specific inhibitor, U0126. While our data show that both 2DG and U0126 decreased ERK phosphorylation to similar degrees in BMDMs and 2DG inhibited TNF-α, IL-1β, IL-6, and CXCL1 production, whereas U0126 only inhibited IL-1β and IL-6 production. These findings indicate that 2DG-mediated abrogation of cytokine/chemokine production is not exclusively dependent on ERK inhibition. Recently, we showed that 2DG induces AMPK (Singh et al, 2020), a negative regulator of inflammation, whose activity is reduced in bacterial endophthalmitis (Kumar et al., 2016). Therefore another potential anti-inflammatory mechanism of 2DG is the activation of AMPK.
In summary, our study demonstrates that glycolytic inhibitor, 2DG can limit S. aureus induced inflammation in bacterial endophthalmitis by attenuating inflammatory mediators produced by infiltrating macrophages and neutrophils. Additionally, we show that 2DG exerts its anti-inflammatory effects by inhibiting the ERK signaling. As our study establishes the link between cellular metabolism and innate immune cell effector functions, we propose that metabolic inhibitors can be used as an adjunct therapy along with antibiotics to attenuate excessive inflammation in ocular or non-ocular infectious diseases.
Highlights.
First report investigating the role of glycolysis in S. aureus (SA) endophthalmitis.
Glycolytic inhibitor, 2DG, diminished the SA-induced inflammation in BMDM and BMDNs.
2DG treatment reduced intraocular inflammation in SA-infected mouse eyes.
The anti-inflammatory effects of 2DG were mediated by the MEK/ERK pathway inhibition.
Acknowledgments
This study was supported by NIH grants R01EY026964, R01EY02738, 1R21AI140033, and R21Al135583. Our research is also supported in part by an unrestricted grant to the Kresge Eye Institute/Department of Ophthalmology, Visual, and Anatomical Sciences from Research to Prevent Blindness Inc. The immunology resource core is supported by an NIH center grant P30EY004068. The authors would like to thank Robert Wright and other members of the lab for their helpful discussion and critical editing of the final manuscript. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Footnotes
Disclosure
The authors have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown J, 1962. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism 11, 1098–1112. [PubMed] [Google Scholar]
- Callegan MC, Booth MC, Jett BD, Gilmore MS, 1999. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun 67, 3348–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Engelbert M, Parke DW 2nd, Jett BD, Gilmore MS, 2002. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev 15, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegan MC, Gilmore MS, Gregory M, Ramadan RT, Wiskur BJ, Moyer AL, Hunt JJ, Novosad BD, 2007. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog Retin Eye Res 26, 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL, 2013. Posttranscriptional control ofT cell effector function by aerobic glycolysis. Cell 153, 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tone E, Schreiber RD, Pearce EJ, Pearce EL, 2015. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 162, 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, Hall H, Zhang D, Lu Y, Ravoori M, Kundra V, Ajani J, Lee JS, Ki Hong W, Mills GB, 2011. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther 10, 2350–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Hisamatsu T, Suzuki H, Mori K, Kitazume MT, Shimamura K, Mizuno S, Nakamoto N, Matsuoka K, Naganuma M, Kanai T, 2017. Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol Lett 183, 17–23. [DOI] [PubMed] [Google Scholar]
- Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D'Agostino D, Forli F, D'Aguanno S, Todaro M, Stassi G, Di llio C, De Laurenzi V, Urbani A, 2014. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis 5, el336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn PS, Miller FC, LaGrow AL, Parkunan SM, Blake Randall C, Staats RL, Callegan MC, 2018. TLR4 modulates inflammatory gene targets in the retina during Bacillus cereus endophthalmitis. BMC Ophthalmol 18, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Trucco M, Rainusso N, Bernardi RJ, Shuck R, Kurenbekova L, Loeb DM, Yustein JT, 2017. Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties. Oncotarget 8, 77292–77308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodelianaki G, Lansing F, Bhattarai P, Troullinaki M, Zeballos MA, Charalampopoulos I, Gravanis A, Mirtschink P, Chavakis T, Alexaki VI, 2019. Nerve Growth Factor modulates LPS - induced microglial glycolysis and inflammatory responses. Exp Cell Res 377, 10–16. [DOI] [PubMed] [Google Scholar]
- Galvan-Pena S, O'Neill LA, 2014. Metabolic reprograming in macrophage polarization. Front Immunol 5, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JM, Singh PK, Revankar SG, Chandrasekar PH, Kumar A, 2018. Isavuconazole for Treatment of Experimental Fungal Endophthalmitis Caused by Aspergillus fumigatus. Antimicrob Agents Chemother 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Singh PK, Revankar SG, Chandrasekar PH, Kumar A, 2019. Pathobiology of Aspergillus Fumigatus Endophthalmitis in Immunocompetent and Immunocompromised Mice. Microorganisms 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett EE, Charles-Messance H, OĽeary SM, Gleeson LE, Munoz-Wolf N, Case S, Wedderburn A, Johnston DGW, Williams MA, Smyth A, Ouimet M, Moore KJ, Lavelle EC, Corr SC, Gordon, S.V. Keane J, Sheedy FJ, 2020. Mycobacterium tuberculosis Limits Host Glycolysis and IL-lbeta by Restriction of PFK-M via MicroRNA-21. Cell Rep 30, 124–136 el24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Giri S, Kumar A, 2016. 5-Aminoimidazole-4-carboxamide ribonucleoside-mediated adenosine monophosphate-activated protein kinase activation induces protective innate responses in bacterial endophthalmitis. Cell Microbiol 18,1815–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kumar A, 2015. Role of Staphylococcus aureus Virulence Factors in Inducing Inflammation and Vascular Permeability in a Mouse Model of Bacterial Endophthalmitis. PLoS One 10, e0128423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Pandey RK, Miller LJ, Singh PK, Kanwar M, 2013. Muller glia in retinal innate immunity: a perspective on their roles in endophthalmitis. Critical reviews in immunology 33, 119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Shamsuddin N, 2012. Retinal Muller glia initiate innate response to infectious stimuli via tolllike receptor signaling. PLoS One 7, e29830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CF, Lo YC, Cheng CH, Furtmuller GJ, Oh B, Andrade-Oliveira V, Thomas AG, Bowman CE, Slusher BS, Wolfgang MJ, Brandacher G, Powell JD, 2015. Preventing Allograft Rejection by Targeting Immune Metabolism. Cell Rep 13, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HJ, Duran EM, Kurtoglu M, Andreansky S, Lampidis TJ, Mesri EA, 2012. Activation of the unfolded protein response by 2-deoxy-D-glucose inhibits Kaposi's sarcoma-associated herpesvirus replication and gene expression. Antimicrob Agents Chemother 56, 5794–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Jin P, Shao C, Lu W, Xiang Q, Jiang Z, Zhang Y, Bian J, 2020. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses and protects against endotoxemia in mice by suppressing HIFlalpha-induced glycolysis. Int Immunopharmacol 80, 106150. [DOI] [PubMed] [Google Scholar]
- Loftus RM, Finlay DK, 2016. Immunometabolism: Cellular Metabolism Turns Immune Regulator. J Biol Chem 291, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah AY, Rashidi A, Keppel MP, Saucier N, Moore EK, Alinger JB, Tripathy SK, Agarwal SK, Jeng EK, Wong HC, Miller JS, Fehniger TA, Mace EM, French AR, Cooper MA, 2017. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F, Fujii J, Yoshida S, 2009. Autophagy induced by 2-deoxy-D-glucose suppresses intracellular multiplication of Legionella pneumophila in A/J mouse macrophages. Autophagy. 5(4) 484–93. [DOI] [PubMed] [Google Scholar]
- Michl J, Ohlbaum DJ, Silverstein SC, 1976. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med 144, 1465–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FC, Coburn PS, Huzzatul MM, LaGrow AL, Livingston E, Callegan MC, 2019. Targets of immunomodulation in bacterial endophthalmitis. Prog Retin Eye Res 73, 100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mursalin MH, Livingston ET, Callegan MC, 2020. The cereus matter of Bacillus endophthalmitis. Exp Eye Res 193, 107959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novosad BD, Callegan MC, 2010. Severe bacterial endophthalmitis: towards improving clinical outcomes. Expert review of ophthalmology 5, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Kishton RJ, Rathmell J, 2016. A guide to immunometabolism for immunologists. Nat Rev Immunol 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Yoshida S, Mizuguchi Y, 1994. 2-Deoxy-D-glucose inhibits intracellular multiplication and promotes intracellular killing of Legionella pneumophila in A/J mouse macrophages. Infect Immun 62, 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkunan SM, Randall CB, Astley RA, Furtado GC, Lira SA, Callegan MC, 2016. CXCL1, but not IL-6, significantly impacts intraocular inflammation during infection. J Leukoc Biol 100, 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkunan SM, Randall CB, Coburn PS, Astley RA, Staats RL, Callegan MC, 2015. Unexpected Roles for Toll-Like Receptor 4 and TRIF in Intraocular Infection with Gram-Positive Bacteria. Infect Immun 83, 3926–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y, 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani D, Singh PK, Rottmann BG, Singh N, Bhasin MK, Kumar A, 2016. Temporal retinal transcriptome and systems biology analysis identifies key pathways and hub genes in Staphylococcus aureus endophthalmitis. Sci Rep 6, 21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaka A, Durand ML, Gilmore MS, 2012. Bacterial endophthalmitis in the age of outpatient intravitreal therapies and cataract surgeries: host-microbe interactions in intraocular infection. Prog Retin Eye Res 31, 316–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanin DE, Prendergast CT, Mountford AP, 2015. IL-10 Production in Macrophages Is Regulated by a TLR-Driven CREB-Mediated Mechanism That Is Linked to Genes Involved in Cell Metabolism. J Immunol 195, 1218–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H, 2011. HIFlalpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Donovan DM, Kumar A, 2014a. Intravitreal Injection of the Chimeric Phage Endolysin Plyl87 Protects Mice from Staphylococcus aureus Endophthalmitis. Antimicrob Agents Chemother 58, 4621–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Khatri I, Jha A, Pretto CD, Spindler KR, Arumugaswami V, Giri S, Kumar A, Bhasin MK, 2018. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Sci Rep 8,11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Kumar A, 2015. Retinal Photoreceptor Expresses Toll-Like Receptors (TLRs) and Elicits Innate Responses Following TLR Ligand and Bacterial Challenge. PLoSOne 10, e0119541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Shiha MJ, Kumar A, 2014b. Antibacterial responses of retinal Muller glia: production of antimicrobial peptides, oxidative burst and phagocytosis. J Neuroinflammation 11, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Singh PK, suhail H, Arumugaswami V, pellett PE, Giri S, Kumar A, 2020. AMP-Activated protein kinase restricts Zika virus replication in endothelial cells by potentiating innate antiviral response and inhibiting glycolysis. J Immunol, 204, 1810–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Liu X, Fu H, Zhou W, Zhong D, 2016. 2-Deoxyglucose Suppresses ERK Phosphorylation in LKB1 and Ras Wild-Type Non-Small Cell Lung Cancer Cells. PLoS One 11, e0168793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrina SL, Griffith MSJ, Lafeuillee C, 2016. 2-Deoxy-d-glucose is a potent inhibitor of biofilm growth in Escherichia coli. Microbiology 162, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Swamydas M, Lionakis MS, 2013. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. J Vis Exp, e50586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talreja D, Singh PK, Kumar A, 2015. In Vivo Role of TLR2 and MyD88 Signaling in Eliciting Innate Immune Responses in Staphylococcal Endophthalmitis. Invest Ophthalmol Vis Sci 56, 1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WS, Rubsamen PE, Flynn HW Jr., Schiffman J, Cousins SW, 1995. Endophthalmitis after penetrating trauma. Risk factors and visual acuity outcomes. Ophthalmology 102, 1696–1701. [DOI] [PubMed] [Google Scholar]
- Traves PG, de Atauri P, Marin S, Pimentel-Santillana M, Rodriguez-Prados JC, Marin de Mas I, Selivanov VA, Martin-Sanz P, Bosca L, Cascante M, 2012. Relevance of the MEK/ERK signaling pathway in the metabolism of activated macrophages: a metabolomic approach. J Immunol 188, 1402–1410.. [DOI] [PubMed] [Google Scholar]
- Varanasi SK, Donohoe D, Jaggi U, Rouse BT, 2017. Manipulating Glucose Metabolism during Different Stages of Viral Pathogenesis Can Have either Detrimental or Beneficial Effects. J Immunol 199, 1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R, 2016. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell 166, 1512–1525 el512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Zhang Y, Li C, Ai K, Li K, Li H, Yang J, 2020. The evolutionarily conserved MAPK/Erk signaling promotes ancestral T-cell immunity in fish via c-Myc-mediated glycolysis. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham M, Wachtel S, Wong Fok Lung T, Soong G, Jacquet R, Richardson A, Parker D, Prince A, 2017. Metabolic Stress Drives Keratinocyte Defenses against Staphylococcus aureus Infection. Cell Rep 18, 2742–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L, 2015. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7, 274ra218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P, Schwartz L, Icard P, 2006. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res 26, 3561–3566. [PubMed] [Google Scholar]