Abstract
While aging is the greatest risk factor for the development of neurodegenerative disease, the role of aging in these diseases is poorly understood. In the inherited forms of these diseases, the disease-causing mutation is present from birth but symptoms appear decades later. This indicates that these mutations are well tolerated in younger individuals but not in older adults. Based on this observation, we hypothesized that changes taking place during normal aging make the cells in the brain (and elsewhere) susceptible to the disease-causing mutations. If so, then delaying some of these age-related changes may be beneficial in the treatment of neurodegenerative disease. In this review, we examine the effects of five compounds that have been shown to extend longevity (metformin, rapamycin, resveratrol, N-acetyl-L-cysteine, curcumin) in four of the most common neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis). While not all investigations observe a beneficial effect of these compounds, there are multiple studies that show a protective effect of each of these lifespan-extending compounds in animal models of neurodegenerative disease. Combined with genetic studies, this suggests the possibility that targeting the aging process may be an effective strategy to treat neurodegenerative disease.
Keywords: Aging, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis
Graphical Abstract
1. Introduction
Neurodegenerative diseases are characterized by the progressive dysfunction and degeneration of neurons in the brain and peripheral nervous system. These devastating disorders affect more than 50 million people worldwide. Although genetic and environmental risk factors have been identified for all of these diseases, the pathogenesis of these diseases remains incompletely understood. While symptomatic treatments are available for some of these disorders, there are currently no disease-modifying treatments that can slow disease progression or halt the loss of neurons. As aging is the greatest risk factor for many of these adult-onset neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease, it will be important to determine if interventions that extend lifespan can protect against these devastating disorders.
1.1. Alzheimer’s disease (AD).
AD is the most common neurodegenerative disorder. AD and other dementias affect 40 to 50 million people worldwide (Nichols et al., 2019). While there are rare cases of early-onset familial AD affecting individuals under 65 years of age, AD typically affects individuals over 65 years of age, and over 95% of AD cases are sporadic cases. Moreover, the risk of AD doubles every 5 years after the age of 65. AD is characterized by difficulties with memory, language, problem-solving and cognitive functions. Brains of AD patients are marked by amyloid plaques, which contain amyloid beta (Aβ), and neurofibrillary tangles, which contain hyperphosphorylated tau. Degeneration starts in the medial temporal lobe, spreads to the hippocampus and amygdala, and eventually to other parts of the brain (Lehéricy et al., 1994; Scahill et al., 2002). Decades of research point towards Aβ aggregation as an important contributor to AD pathogenesis. This could be due to either increased production of Aβ or decreased clearance of Aβ. Aβ oligomers are toxic in neuronal culture (Lambert et al., 1998), contribute to memory deficits in mice (Lesne et al., 2006), and correlate with neurodegeneration in AD patients (McLean et al., 1999). However, therapies targeting Aβ have so far failed in clinical trials shifting the field towards studying other contributing factors to AD pathogenesis, such as microbial infection (Fulop et al., 2018), dysfunctional vasculature (Zlokovic, 2011), and environmental pollution (Peters et al., 2019). Currently, AD treatments can treat the symptoms but fail to slow disease progression or cognitive decline.
1.2. Parkinson’s disease (PD).
PD is the second most common neurodegenerative disease, affecting as many as 10 million individuals worldwide. PD is a movement disorder, which is characterized by the degeneration of dopaminergic neurons within the substantia nigra (SN) and the formation of aggregates, called Lewy bodies, which are primarily composed of a protein called α-synuclein. While interventions are available to control the motor symptoms of PD, there are currently no treatments that can prevent the loss of dopamine neurons or halt the progression of this disease. A growing number of genes have been shown to contribute to the development of PD including SNCA, the gene that encodes α-synuclein (Polymeropoulos et al., 1997; Singleton et al., 2003), and LRRK2, which encodes a Leucine-Rich Repeat Kinase (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). Nonetheless, the majority of PD cases are considered to be sporadic, as only ~10% of patients have a direct genetic cause. Although the pathogenesis of PD is incompletely understood, aging is the greatest risk factor for the development of PD (Collier et al., 2011; Driver et al., 2009). Individuals typically do not develop PD until they are 50 years and older. The prevalence of PD is 0.3% in the general population but increases to 1% for those over 60 years, and 4% for those more than 80 years of age. A role for aging in PD is also suggested by the fact that there are many commonalities between PD and normal aging (Rodriguez et al., 2015) including protein aggregation (Tan et al., 2009), increased oxidative stress (Zhou et al., 2008), decreased mitochondrial function (Henchcliffe and Beal, 2008), dysfunction of the proteasome (Cook and Petrucelli, 2009), and impairment of autophagy (Pan et al., 2008).
1.3. Huntington’s disease (HD).
HD is the most common inherited neurodegenerative disorder, affecting approximately one in 10,000 individuals. Unlike more widespread disorders, such as AD and PD, HD is entirely due to a single genetic cause: a trinucleotide CAG repeat expansion in the huntingtin (HTT) gene. HD is an autosomal dominant disorder which causes motor dysfunction, cognitive deficits, and neuropsychiatric abnormalities. In the brain, HD is characterized by selective degeneration of the striatum and the cortex as well as the appearance of mutant Htt aggregates. Following the discovery of the HTT gene, a number of genetic animal models of HD have been generated to study disease mechanisms and treatment (Brignull et al., 2006; Faber et al., 1999; Fernandez-Funez et al., 2000; Gray et al., 2008; Mangiarini et al., 1996; Marsh et al., 2000; Parker et al., 2001; Satyal et al., 2000; Schilling et al., 1999; Slow et al., 2003). Nonetheless, the pathogenesis of HD is not completely understood, and there are currently no approved disease-modifying therapies. An important unanswered question is why HD takes decades to develop when the disease-causing mutation is present in all cells throughout life. The average age of onset for HD is 40 years of age, progressing inevitably to death 10–15 years thereafter. A role for aging in the pathogenesis of HD is supported by the fact that multiple functions that decline with age have also been implicated in the disease including decreased proteasome activity, decreased autophagy, decreased chaperone function, mitochondrial dysfunction, increased oxidative stress, and increased protein aggregation (Bence et al., 2001; Browne and Beal, 2006; DiFiglia et al., 1997; Kitamura et al., 2006; Panov et al., 2002; Ravikumar et al., 2004).
1.4. Amyotrophic lateral sclerosis (ALS).
ALS is the most common adult-onset motor neuron disease, affecting one in 50,000 people in Western countries (Chiò et al., 2013). ALS is caused by degeneration of upper and lower motor neurons, leading to loss of voluntary movement. The disease progresses swiftly, with patients succumbing to the disease within an average of three years after diagnosis (del Aguila et al., 2003). Roughly 95% of ALS cases are sporadic (Byrne et al., 2011), with the largest known risk factor for sporadic ALS being aging. Both sporadic and familial ALS-onset typically occurs between 40–70 years of age (Alonso et al., 2009). The cellular pathogenesis of ALS is unclear, but a cellular hallmark of ALS is cytoplasmic aggregates in the motor neurons of patients. Over 97% of sporadic ALS cases have aggregates containing the RNA/DNA binding proteins TAR DNA-binding protein 43 (TDP-43) or fused in sarcoma (FUS) (Deng et al., 2010; Neumann et al., 2006). Mutations in FUS or the TDP-43-encoding gene, transactive response DNA binding protein (TARDBP), can cause familial ALS (Guerrero et al., 2016), and have been used to generate animal models of the disease. Animal models of ALS have also been generated using aggregation-inducing mutations in superoxidase dismutase 1 (SOD1), as this is a common genetic cause of ALS in humans. However, SOD1 animal models and patients do not exhibit FUS aggregation, making it unclear if SOD1 ALS animals are an accurate model for sporadic ALS (Deng et al., 2010). Work with these ALS models and others have identified defects in RNA regulation, phase-separation, nucleocytoplasmic trafficking, stress granule regulation, selective autophagy, and cytoskeleton dynamics (Kim and Taylor, 2017; Taylor et al., 2016; Van Damme et al., 2017). It is unclear if one of these pathways is the driving cause of ALS or if they each contribute to ALS pathogenesis.
1.5. Targeting aging pathways as a therapeutic strategy for neurodegenerative disease.
While aging was traditionally considered to be a stochastic process of damage accumulation, it is now clear that lifespan is strongly influenced by genetics. Mutations in a single gene out of many thousand genes can increase lifespan in various model organisms including yeast, flies, worms and mice, and are associated with longevity in humans. Importantly, genes and interventions that increase lifespan in one species have been shown to be conserved across species (Friedman and Johnson, 1988; Holzenberger et al., 2003; Kenyon et al., 1993; Suh et al., 2008). Interestingly, these lifespan-extending genes have also been shown to be protective in animal models of neurodegenerative disease including AD (Cohen et al., 2006; Cohen et al., 2009; Freude et al., 2009; Killick et al., 2009), PD (Cooper et al., 2015; Knight et al., 2014), HD (Hsu et al., 2003; Jiang et al., 2012; Morley et al., 2002; Sadagurski et al., 2011), and ALS (Boccitto et al., 2012). This strongly suggests that targeting molecular pathways that modulate aging may be an effective strategy to treat neurodegenerative disease. Despite these exciting observations, it remains a challenge to translate genetic treatments to humans.
As an alternative, it may be possible to develop novel treatments for neurodegenerative diseases using compounds that modulate aging. In addition to identifying a plethora of genes that can affect longevity (https://genomics.senescence.info/genes/), aging research has also identified multiple compounds that extend lifespan in model organisms (http://genomics.senescence.info/drugs/). In this review, we examine the effects of five compounds that have been shown to modulate aging (metformin, resveratrol, rapamycin, N-acetyl-L-cysteine, and curcumin) in four neurodegenerative diseases (AD, PD, HD and ALS). We find that compounds that increase lifespan can be neuroprotective in models of neurodegenerative disease (Figure 1), and thus may represent a novel treatment strategy for these disorders.
Figure 1. Lifespan-extending compounds can be protective in neurodegenerative diseases.
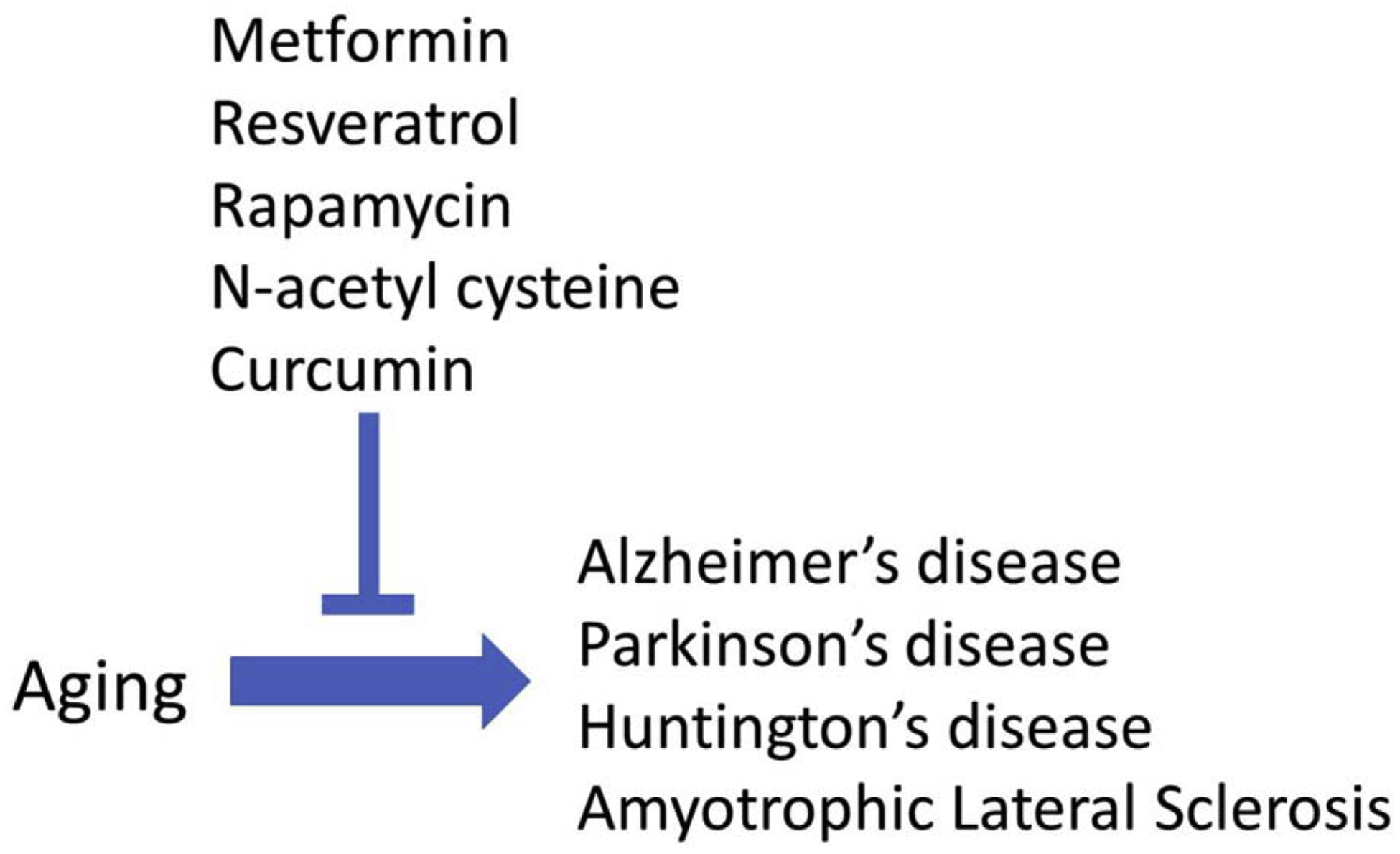
Five compounds that have been shown to increase lifespan in model organisms (metformin, resveratrol, rapamycin, N-acetyl cysteine, curcumin) have all been shown to have beneficial effects in four different neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis).
2. Metformin
Metformin is a promising preventative treatment for age-associated diseases. Metformin is commonly used for treating Type 2 Diabetes Mellitus (T2DM), and retrospective studies discovered that metformin treatment of T2DM patients caused reduced incidents of age-associated diseases such as cardiovascular diseases (Campbell et al., 2017), cancers (Gandini et al., 2014; Wu et al., 2014), and neurodegenerative diseases (Cheng et al., 2014; Ng et al., 2014). In T2DM patients, metformin causes reduced glucose production in the liver, as well as increased insulin sensitivity in the peripheral tissues (Giannarelli et al., 2003). Metformin is delivered orally and disperses throughout the body to the liver, kidney, muscles, and the brain (Gormsen et al., 2016; Kulkarni et al., 2018; Labuzek et al., 2010), which may explain its widespread physiological effects. The specific molecular interactions of metformin are still unclear. However, metformin leads to activation of the highly conserved energy-sensing AMP-activated protein kinase (AMPK) pathway (Fullerton et al., 2013; Zhou et al., 2001). Metformin also independently activates mTORC1, which causes activation of AMPK through a lysosomal pathway (Kalender et al., 2010; Zhang et al., 2016). Activation of the AMPK pathway corrects energy imbalances by increasing lipid metabolism, increasing mitochondrial biogenesis, increasing autophagy, delaying cell cycle progression and decreasing protein production (Hardie et al., 2012). The AMPK pathway also modulates inflammation and inhibits the c-Jun N-terminal kinase (JNK) pathway (Chen et al., 2019; Hu et al., 2016). Therefore, metformin treatment regulates multiple pathways associated with aging.
2.1. Effect of metformin on lifespan
Metformin has mixed effects on lifespan in various model organisms (Table 1), but improves healthspan experimentally in murine models and is associated with decreases in age-related diseases in humans with T2DM. Metformin was first shown to extend lifespan in C. elegans (Onken and Driscoll, 2010), but, interestingly, this effect was due to metabolic changes in the bacterial food source, not the nematodes (Cabreiro et al., 2013). Subsequent studies were unable to demonstrate a beneficial effect of metformin on lifespan in Drosophila (Slack et al., 2012). In mice, metformin was shown to cause a small, but statistically significant, increase in lifespan (Martin-Montalvo et al., 2013). However, a larger study performed at multiple locations with genetically heterogenous mice failed to find a statistically significant increase in lifespan when animals were treated with metformin alone (Strong et al., 2016). Nonetheless, metformin exhibits a more clearly beneficial effect on the healthspan of mice (Feng et al., 2020; Martin-Montalvo et al., 2013). This is consistent with previous research showing that metformin improves cardiovascular function and cognition in aging mice (Campbell et al., 2017; UKPDS, 1998). In retrospective cohort studies, metformin was associated with extended lifespan of T2DM patients (Bannister et al., 2014; Campbell et al., 2017). Based on these promising results, a large randomized controlled study has been planned to determine if metformin affects lifespan and healthspan in healthy humans (Barzilai et al., 2016).
Table 1.
Effect of compounds on lifespan in model organisms
| Compound | Mechanisms | C. elegans | Drosophila | Mouse |
|---|---|---|---|---|
| Metformin | Activates AMPK Activates mTORC1 |
Increase1 | No effect2 | Increase3
No effect4 |
| Resveratrol | Activates SIRT1 Activates AMPK Antioxidant Anti-inflammatory |
Increase5–8 | Increase5, 9 | No effect10
Increase11 (mice on high fat diet) |
| Rapamycin | mTORC1 inhibition | Increase12 | Increase13 | Increase14–18 |
| N-acetyl-L-cysteine | Antioxidant | Increase19 | Increase20 | Increase21
(possibly through dietary restriction) |
| Curcumin | Antioxidant Anti-inflammatory |
Increase22 | Increase23–26 | Increase27
No effect28 |
Onken B, et al. (2010) PloS one 5(1).
Slack C, et al. (2012) PloS one 7(10).
Martin-Montalvo A, et al. (2013) Nat Commun 4.
Strong R, et al. (2016) Aging Cell 15(5).
Wood JG, et al. (2004) Nature 430(7000).
Viswanathan M, et al. (2005) Developmental Cell 9(5).
Bass TM, et al. (2007) Mechanisms of ageing and development 128(10).
Gruber J, et al. (2007) Annals of the New York Academy of Sciences 1100.
Bauer JH, et al. (2004) Proceedings of the National Academy of Sciences of the United States of America 101(35).
Pearson KJ, et al. (2008) Cell Metab 8(2).
Baur JA, et al. (2006) Nature 444(7117).
Robida-Stubbs S, et al. (2012) Cell metabolism 15(5).
Bjedov I, et al. (2010) Cell Metab 11(1).
Harrison DE, et al. (2009) Nature 460(7253).
Miller RA, et al. (2011) J Gerontol A Biol Sci Med Sci 66(2).
Wilkinson JE, et al. (2012) Aging Cell 11(4).
Fok WC, et al. (2014) PLoS One 9(1).
Zhang Y, et al. (2014) J Gerontol A Biol Sci Med Sci 69(2).
Oh S-I, et al. (2015) Clinics 70(5).
Brack C, et al. (1997) Cellular and Molecular Life Sciences CMLS 53(11).
Flurkey K, et al. (2010) Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 65(12).
Liao VH, et al. (2011) Mech Ageing Dev 132(10).
Suckow B, et al. (2006) InternatIonal journal of BIomedIcal scIence 4.
Shen LR, et al. (2013) Age (Dordr) 35(4).
Akinyemi AJ, et al. (2018) Metab Brain Dis 33(2).
Soh JW, et al. (2013) Exp Gerontol 48(2).
Kitani K, et al. (2007) Biogerontology 8(5).
Strong R, et al. (2013) J Gerontol A Biol Sci Med Sci 68(1).
2.2. Metformin and Alzheimer’s disease
In the majority of studies reported, metformin is protective against dementia and AD in model organisms and in humans (Table 2). Treatment with metformin ameliorates phenotypic deficits in AD models in cell culture (Chen et al., 2016), C. elegans (Ahmad and Ebert, 2017), Drosophila (Niccoli et al., 2016), and mice (Chen et al., 2019; Farr et al., 2019; Ou et al., 2018). Metformin treatment after phenotypic onset improved behavioral phenotypes in APP/PS1 mice (Matthes et al., 2018) and in an STZ-induced AD rodent model (Nassar et al., 2018). In contrast, metformin treatment exacerbated motor and behavioral deficits in a P301S mutant human tau mouse model (Barini et al., 2016). Metformin treatment also increased tau phosphorylation in murine AD models expressing human ApoE3 or ApoE4 (Zhang et al., 2019). Interestingly, metformin still improved cognition in the ApoE3 mouse, but not the ApoE4 mice, suggesting that genetic differences can influence the efficacy of metformin in treating AD. Another cautionary note is that metformin can cause increased Aβ production, which is inhibited by insulin (Chen et al., 2009). In patients with T2DM, metformin treatment alone was associated with a decrease in the risk of dementia in multiple retrospective cohort studies (Bohlken et al., 2018; Cheng et al., 2014; Hsu et al., 2011; Orkaby et al., 2017; Shi et al., 2019). However, two studies did find that metformin treatment of T2DM patients was associated with an increased risk of developing AD (Imfeld et al., 2012; Kuan et al., 2017). Metformin has been proposed to be protective against AD by inhibiting the JNK pathway, causing suppression of cell death (Chen et al., 2016). It may also be protective through increasing glucose transport in neurons (Niccoli et al., 2016). While metformin has promising effects on AD models, more research needs to be done to determine why it has detrimental effects in certain systems.
Table 2.
Effect of compounds on Alzheimer’s disease
| Compound | Cell Culture | C. elegans | Drosophila | Mouse | Human |
|---|---|---|---|---|---|
| Metformin | Protective1, 2 | Protective3 | Protective4 | Protective5–10 Deleterious11 |
Protective12–16
Deleterious17, 18 |
| Resveratrol | Protective19–24 | Protective25 | N/A | Protective26–32 | Protective33, 34
No effect35 |
| Rapamycin | Deleterious36, 37 | N/A | Protective38, 39 | Protective40–43 Deleterious44 |
N/A |
| NAC | Protective45 | N/A | N/A | Protective46, 47 | Protective48 |
| Curcumin | Protective49, 50 | Protective51 | Protective52 | Protective53–55 | N/A |
Chen B, et al. (2016) Biomed Res Int 2016.
Li L-X, et al. (2019) Basic Clin Pharmacol Toxicol 125(5).
Ahmad W, et al. (2017) Mol Neurobiol 54(7).
Niccoli T, et al. (2016) Curr Biol 26(17).
Farr SA, et al. (2019) J Alzheimers Dis 68(4).
Ou Z, et al. (2018) Brain Behav Immun 69.
Nassar SZ, et al. (2018) Arch Physiol Biochem.
Chen J-L, et al. (2019) Experimental neurology 311.
Zhang J, et al. (2019) FASEB J 33(6).
Matthes F, et al. (2018) Cell Death Discov 4.
Barini E, et al. (2016) Mol Neurodegener 11.
Shi Q, et al. (2019) BMJ Open 9(7).
Bohlken J, et al. (2018) J Alzheimers Dis 66(2).
Orkaby AR, et al. (2017) Neurology 89(18).
Cheng C, et al. (2014) The journals of gerontology. Series A, Biological sciences and medical sciences 69(10).
Hsu C-C, et al. (2011) J Alzheimers Dis 24(3).
Imfeld P, et al. (2012) J Am Geriatr Soc 60(5).
Kuan Y-C, et al. (2017) Prog Neuropsychopharmacol Biol Psychiatry 79(Pt B).
Marambaud P, et al. (2005) The Journal of biological chemistry 280(45).
Vingtdeux V, et al. (2010) The Journal of biological chemistry 285(12).
Granzotto A, et al. (2011) PloS one 6(6).
Cristòfol R, et al. (2012) Journal of pineal research 52(3).
Feng X, et al. (2013) PloS one 8(3).
Wang H, et al. (2018) Toxicology letters 282.
Regitz C, et al. (2016) European journal of nutrition 55(2).
Kim D, et al. (2007) The EMBO journal 26(13).
Karuppagounder SS, et al. (2009) Neurochemistry international 54(2).
Gong Q-H, et al. (2010) Journal of Health Science 56(6).
Huang T-C, et al. (2011) PloS one 6(12).
Porquet D, et al. (2013) AGE 35(5).
Wang G, et al. (2016) Oncotarget 7(14).
Capiralla H, et al. (2012) J Neurochem 120(3).
Turner RS, et al. (2015) Neurology 85(16).
Moussa C, et al. (2017) Journal of neuroinflammation 14(1).
Zhu CW, et al. (2018) Alzheimer’s & dementia (New York, N. Y.) 4.
Yu WH, et al. (2005) J Cell Biol 171(1).
Lafay-Chebassier C, et al. (2006) J Neurosci Res 84(6).
Berger Z, et al. (2006) Hum Mol Genet 15(3).
Khurana V, et al. (2006) Curr Biol 16(3).
Spilman P, et al. (2010) PLOS ONE 5(4).
Majumder S, et al. (2011) PLoS One 6(9).
Caccamo A, et al. (2010) Journal of Biological Chemistry 285.
Van Skike CE, et al. (2018) Am J Physiol Heart Circ Physiol 314(4).
Zhang S, et al. (2010) Biochem Biophys Res Commun 398(3).
Tardiolo G, et al. (2018) Molecules 23(12).
Costa M, et al. (2016) Chem Biol Interact 253.
Shahidi S, et al. (2017) Brain Res Bull 131.
Adair JC, et al. (2001) Neurology 57(8).
Qian W, et al. (2018) Med Sci Monit 24.
Reddy PH, et al. (2016) J Investig Med 64(8).
Kim BK, et al. (2019) Drug Discov Ther 13(4).
Caesar I, et al. (2012) PLoS One 7(2).
Ahmed T, et al. (2010) Neuroscience 169(3).
Frautschy S, et al. (2001) Neurobiology of Aging
Garcia-Alloza M, et al. (2007) J Neurochem 102(4).
2.3. Metformin and Parkinson’s disease
Metformin may reduce the risk of developing PD (Table 3). Of three retrospective cohort studies, two found that metformin-treated T2DM patients had reduced risk of developing PD (Shi et al., 2019; Wahlqvist et al., 2012), while the other found an increased risk of developing PD (Kuan et al., 2017). Experimentally, metformin is protective in PD models in cell culture (Dulovic et al., 2014; Kang et al., 2017), C. elegans (Saewanee et al., 2019), Drosophila (Ng et al., 2012), and in mice (Kang et al., 2017; Lu et al., 2016). Metformin appears to be protective against PD through the activation of AMPK, though dopaminergic neurons do not need to have functioning AMPK for metformin to be protective (Bayliss et al., 2016). Several downstream targets of AMPK have been identified as possibly delaying PD symptoms, specifically inhibiting microglia overactivation (Lu et al., 2016) and activation of the PGC-1α pathway, which stimulates mitochondrial biogenesis (Kang et al., 2017). Interestingly, while metformin increases autophagy induction (Lu et al., 2016), autophagy is not required for the protective effects of metformin (Dulovic et al., 2014).
Table 3.
Effect of compounds on Parkinson’s disease
| Compound | Cell Culture | C. elegans | Drosophila | Mouse | Human |
|---|---|---|---|---|---|
| Metformin | Protective1–4 | Protective5 | Protective6 | Protective1, 2, 4, 7, 8 | Protective9, 10
Deleterious11 |
| Resveratrol | Protective12–15 | N/A | Deleterious16 | Protective15, 17–23 | N/A |
| Rapamycin | Protective24, 25 | Protective26 | Protective27 | Protective24, 25, 28, 29 | N/A |
| NAC | Protective30 | N/A | N/A | Protective31, 32 | Protective30 |
| Curcumin | Protective33–36 | N/A | Protective37 | Protective38–45 | N/A |
Lu M, et al. (2016) Int J Neuropsychopharmacol 19(9).
Bayliss JA, et al. (2016) PloS one 11(7).
Dulovic M, et al. (2014) Neurobiology of disease 63.
Kang H, et al. (2017) Oncotarget 8(30).
Saewanee N, et al. (2019) Neuroscience Research.
Ng C-H, et al. (2012) J Neurosci 32(41).
Adedeji HA, et al. (2014) Prog Neuropsychopharmacol Biol Psychiatry 48.
Yan Q, et al. (2017) Mol Pharmacol 92(6).
Wahlqvist ML, et al. (2012) Parkinsonism Relat Disord 18(6).
Shi Q, et al. (2019) BMJ Open 9(7).
Kuan Y-C, et al. (2017) Prog Neuropsychopharmacol Biol Psychiatry 79(Pt B).
Alvira D, et al. (2007) Neuroscience 147(3).
Wu Y, et al. (2011) Neuro-Signals 19(3).
Ferretta A, et al. (2014) Biochimica et biophysica acta 1842(7).
Wang ZH, et al. (2015) Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 74.
Bagatini PB, et al. (2011) Invertebrate neuroscience : IN 11(1).
Blanchet J, et al. (2008) Progress in neuro-psychopharmacology & biological psychiatry 32(5).
Jin F, et al. (2008) European journal of pharmacology 600(1–3).
Khan MM, et al. (2010) Brain research 1328.
Gaballah HH, et al. (2016) Chemico-biological interactions 251.
Zhang LF, et al. (2018) Food & function 9(12).
Liu Q, et al. (2019) Behavioural brain research 367.
Xia D, et al. (2019) Journal of cellular biochemistry 120(4).
Malagelada C, et al. (2010) J Neurosci 30(3).
Dehay B, et al. (2010) J Neurosci 30(37).
Tyson T, et al. (2017) Sci Rep 7(1).
Tain LS, et al. (2009) Nat Neurosci 12(9).
Masini D, et al. (2018) Front Neurol 9.
Crews L, et al. (2010) PLoS One 5(2).
Monti DA, et al. (2016) PLoS One 11(6).
Rahimmi A, et al. (2015) Brain Research Bulletin 113.
Clark J, et al. (2010) PloS one 5(8).
van der Merwe C, et al. (2017) Mol Neurobiol 54(4).
Jayaraj RL, et al. (2013) J Mol Neurosci 51(3).
Qualls Z, et al. (2014) Neurotox Res 25(1).
Sang Q, et al. (2018) Cell Physiol Biochem 51(2).
Nguyen TT, et al. (2018) Oxid Med Cell Longev 2018.
Zbarsky V, et al. (2005) Free Radic Res 39(10).
Du XX, et al. (2012) Neurosci Bull 28(3).
Rajeswari A, et al. (2008) Inflammopharmacology 16(2).
Chiu S, et al. (2013) J Complement Integr Med 10.
Pan J, et al. (2012) Translational Neurodegeneration 1.
Jayaraj RL, et al. (2014) Biomed Res Int 2014.
Abbaoui A, et al. (2017) Neurosci Lett 660.
Sharma N, et al. (2018) Inflammopharmacology 26(2).
2.4. Metformin and Huntington’s disease
Metformin consistently suppresses or delays HD phenotypic deficits in cell culture (Jin et al., 2016), C. elegans (Sanchis et al., 2019; Vázquez-Manrique et al., 2016), mice (Arnoux et al., 2018; Sanchis et al., 2019) and humans (Hervás et al., 2017) (Table 4). Most promisingly, diabetic HD patients treated with metformin exhibited improved cognitive functions compared to nondiabetic HD controls (Hervás et al., 2017). This delay of HD symptoms may be due to decreased translation of mutant HTT, the protein which drives HD disease progression (Arnoux et al., 2018; Sanchis et al., 2019). The protective effect of metformin may also be due to its ability to prevent mitochondrial depolarization as shown in mHTT striatal cell culture (Jin et al., 2016).
Table 4.
Effect of compounds on Huntington’s disease
| Compound | Cell Culture | C. elegans | Drosophila | Mouse | Human |
|---|---|---|---|---|---|
| Metformin | Protective1 | Protective2, 3 | N/A | Protective2, 4, 5 | Protective6 |
| Resveratrol | Protective7, 8 | Protective7 | Protective9, 10 | Protective8, 11, 12 | N/A |
| Rapamycin | Protective13, 14 | N/A | Protective15 | Protective15
No effect16 |
N/A |
| NAC | N/A | N/A | N/A | Protective17, 18 | N/A |
| Curcumin | N/A | N/A | Protective19 | Protective20 | N/A |
Jin J, et al. (2016) Neuromolecular Med 18(4).
Sanchis A, et al. (2019) Exp Mol Med 51(6).
Vázquez-Manrique RP, et al. (2016) Hum Mol Genet 25(6).
Arnoux I, et al. (2018) Elife 7.
Ma TC, et al. (2007) Neurosci Lett 411(2).
Hervás D, et al. (2017) PloS one 12(6).
Parker JA, et al. (2005) Nature genetics 37(4).
Naia L, et al. (2017) Molecular neurobiology 54(7).
Pallos J, et al. (2008) Human molecular genetics 17(23).
Maher P, et al. (2011) Human molecular genetics 20(2).
Ho DJ, et al. (2010) Experimental neurology 225(1).
Gerhardt E, et al. (2011) PloS one 6(12).
Ravikumar BD, R.; Rubinsztein, C. (2002) Hum Mol Genet 11.
King MA, et al. (2008) Mol Pharmacol 73(4).
Ravikumar B, et al. (2004) Nat Genet 36(6).
Fox JH, et al. (2010) Mol Neurodegener 5.
Sandhir R, et al. (2012) Neurodegenerative Diseases 9(3).
Wright DJ, et al. (2015) Translational Psychiatry 5(1).
Chongtham A, et al. (2016) Sci Rep 6.
Hickey MA, et al. (2012) Mol Neurodegener 7.
2.5. Metformin and amyotrophic lateral sclerosis
Based on the limited number of studies that have been reported, metformin has either negligible or exacerbating effects on ALS (Table 5). In the single experimental study identified, metformin treatment of SOD1(G93A) male mice caused no effect, while treatment of SOD1(G93A) female mice caused a decreased age of onset and increased rate of disease progression (Kaneb et al., 2011). A retrospective cohort study determined that metformin treatment had no effect on the progression of ALS in T2DM patients (Bond et al., 2020).
Table 5.
Effect of Compounds on Amyotrophic lateral sclerosis
| ALS | Cell Culture | C. elegans | Drosophila | Mouse | Human |
|---|---|---|---|---|---|
| Metformin | N/A | N/A | N/A | Deleterious1 | No effect2 |
| Resveratrol | Protective3 | N/A | N/A | Protective4 | N/A |
| Rapamycin | Protective5 | N/A | Protective6 | Protective7 No effect8 Deleterious9 |
In Progress10 |
| NAC | Protective11 | N/A | N/A | Protective12 | Deleterious13 |
| Curcumin | Protective14, 15 | N/A | N/A | N/A | Protective16, 17 |
Kaneb HM, et al. (2011) PloS one 6(9).
Bond L, et al. (2020) Behavioral Sciences 10(1).
Kim D, et al. (2007) The EMBO journal 26(13).
Mancuso R, et al. (2014) Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 11(2).
Caccamo A, et al. (2009) J Biol Chem 284(40).
Cheng CW, et al. (2015) J Neurogenet 29(2–3).
Wang IF, et al. (2012) Proc Natl Acad Sci U S A 109(37).
Staats KAH, S.; Schonefeldt, S.; Bento-Abreu, A.; Dooley, J.; Van Damme, P.; Liston, A.; Robberecht, W.; Van Den Bosch, L. (2013) Mol Neurodegener 8.
Zhang X, et al. (2011) Autophagy 7(4).
Mandrioli J, et al. (2018) Medicine (Baltimore) 97(24).
Beretta S, et al. (2003) Neurobiology of disease 13(3).
Andreassen OA, et al. (2000) Neuroreport 11(11).
Deepmala, et al. (2015) Neuroscience & Biobehavioral Reviews 55.
Lu J, et al. (2012) Brain Res Bull 89(5–6).
Dong H, et al. (2014) Neuroscience 272.
Ahmadi M, et al. (2018) Neurotherapeutics 15(2).
Chico L, et al. (2018) CNS Neurol Disord Drug Targets 17(10).
3. Resveratrol
Resveratrol (3,5,4’-trihydroxystilbene) is a polyphenol found in purple grapes, red wine and many plants. Its potential benefits were first manifested through the “French Paradox”, which associated red wine consumption with decreased risk of cardiovascular diseases (Renaud and de Lorgeril, 1992). Although red wine content in resveratrol is insufficient to provide significant pharmacological effects (Vitaglione et al., 2005; Walle et al., 2004), resveratrol has been associated with many benefits such as anti-carcinogenic (Jang and Pezzuto, 1999), cardioprotective (Ungvari et al., 2007) and neuroprotective effects (Bastianetto et al., 2015).
The mechanism underlying its protection is not completely understood. The conserved NAD-dependent deacetylase sirtuin-1, SIRT1, and its invertebrate homologue SIR-2 were initially believed to mediate the lifespan-extending effect and protection conferred by resveratrol (Howitz et al., 2003; Viswanathan et al., 2005; Wood et al., 2004). However, these findings have been debated considering that sirtuin-independent lifespan extension was observed (Bass et al., 2007; Zhang, 2006) and that resveratrol did not directly affect SIRT1 activity (Beher et al., 2009; Kaeberlein et al., 2005; Pacholec et al., 2010). Furthermore, resveratrol activates AMP-activated kinase (AMPK) in both SIRT-1 dependent and independent manner (Dasgupta and Milbrandt, 2007; Price et al., 2012; Suchankova et al., 2009) Whether SIRT-1 is involved in the protective effects of resveratrol is yet to be confirmed. Other reported activities of resveratrol include reducing oxidative damage by acting as an anti-oxidant (Li et al., 2014a), reducing neuroinflammation through inhibition of the NFκB pro-inflammatory pathway (Das and Das, 2007; Manna et al., 2000; Zhang et al., 2010b), decreasing neuronal apoptosis via acetylation of p53 pro-apoptotic factor (Kim et al., 2007; Luo et al., 2001; Vaziri et al., 2001) and improving mitochondrial functions through PGC-1α activation (Naia et al., 2017; Price et al., 2012).
3.1. Effect of resveratrol on lifespan
Resveratrol was first observed to extend lifespan in yeast (Howitz et al., 2003) and the same effect was later demonstrated in C. elegans (Bass et al., 2007; Gruber et al., 2007; Viswanathan et al., 2005; Wood et al., 2004), Drosophila (Bauer et al., 2004; Wood et al., 2004) and honey bees (Rascón et al., 2012) (Table 1). Lifespan extension by resveratrol in vertebrates was only observed in the fish N. furzeri (Valenzano et al., 2006), as resveratrol failed to extend lifespan in mice (Strong et al., 2013), except in mice that were placed on a high calorie diet (Baur et al., 2006). However, evidence from rodent studies suggest that resveratrol can increase neuronal cell viability (Albani et al., 2009) and delay onset of age-related diseases (Pearson et al., 2008). The mechanism underlying the lifespan-extending effect of resveratrol is mainly unknown, although some studies showed that this relies on AMPK activation and related changes in metabolism (Apfeld et al., 2004; Greer et al., 2007). While resveratrol increased lifespan in simple animal models, additional research will be required to determine why this effect was not observed in mammals.
3.2. Resveratrol and Alzheimer’s disease
Resveratrol improves AD pathology in many studies (Table 2), but its mechanisms of action are unclear. Both rodent and cell line models of AD treated with resveratrol showed decreased amyloid-β aggregation and reduced cytotoxicity (Capiralla et al., 2012; Huang et al., 2011; Karuppagounder et al., 2009; Marambaud et al., 2005; Porquet et al., 2013; Vingtdeux et al., 2010; Zhao et al., 2015). The same effect was also observed in C. elegans (Regitz et al., 2016). Moreover, tau hyperphosphorylation was found to be reduced by resveratrol treatment (Lin et al., 2018; Porquet et al., 2013; Schweiger et al., 2017), along with reduced levels of oxidative damage (Granzotto and Zatta, 2011; Huang et al., 2011; Lin et al., 2018). These effects appear to be mediated by an increased production of antioxidants such as heme oxygenase-1 (HO-1) and glutathione (GSH) through the Nrf2 pathway (Chen et al., 2005; Huang et al., 2011; Kong et al., 2019; Sharma and Gupta, 2002). Additionally, SIRT1 activation is believed to mediate many of the beneficial effects of resveratrol on AD impairments (Cristòfol et al., 2012; Porquet et al., 2013) and might act through reducing neuronal apoptosis (Feng et al., 2013; Kim et al., 2007). Resveratrol can also promote Aβ clearance through the AMPK pathway (Porquet et al., 2013; Vingtdeux et al., 2010). Furthermore, resveratrol treatment decreased neuroinflammation in rodent models (Capiralla et al., 2012; Gong et al., 2010), as well as in a recent clinical trial conducted on AD patients (Moussa et al., 2017). However, other human studies have shown mixed results (Moussa et al., 2017; Turner et al., 2015; Zhu et al., 2018). Together, these findings show a protective effect of resveratrol in AD models, while further human clinical trials will be necessary to determine the efficacy of resveratrol for AD treatment.
3.3. Resveratrol and Parkinson’s disease
Resveratrol improves PD-associated impairments in various PD rodent models (Table 3), including the 6-OHDA rat model (Jin et al., 2008; Khan et al., 2010) and the MPTP-treated mouse model (Blanchet et al., 2008; Liu et al., 2019; Wang et al., 2015; Xia et al., 2019). A decrease in α-synuclein aggregation, dopamine loss and oxidative stress was observed after resveratrol treatment (Gaballah et al., 2016; Khan et al., 2010; Zhang et al., 2018), along with reduced neuroinflammation (Jin et al., 2008; Liu et al., 2019; Lofrumento et al., 2014). The specific mechanism by which resveratrol exerts its effects in PD is still unknown. However, resveratrol was shown to act through enhanced autophagy (Ferretta et al., 2014; Wu et al., 2011), decreased apoptosis (Alvira et al., 2007; Xia et al., 2019) and mitochondrial biogenesis (Ferretta et al., 2014). These improvements require AMPK and SIRT1 activation (Ferretta et al., 2014; Wu et al., 2011), as well as the SIRT1 target PGC-1α (Ferretta et al., 2014). Combined these studies demonstrate a potential protective role of resveratrol in PD.
3.4. Resveratrol and Huntington’s disease
HD-associated phenotypes such as neuronal degeneration were reduced by resveratrol treatment in many HD animal models, including C. elegans (Parker et al., 2005), Drosophila (Maher et al., 2011; Pallos et al., 2008) and multiple rodent models (Ho et al., 2010; Naia et al., 2017) (Table 4). Similar to PD models, resveratrol treatment in HD models is believed to act through SIRT1 to increase neuronal viability (Ho et al., 2010; Naia et al., 2017; Parker et al., 2005). Furthermore, SIRT1 activation by resveratrol may increase PGC-1α expression (Ho et al., 2010; Naia et al., 2017). PGC-1α is involved in mitochondrial biogenesis and functions, and its reduced expression may contribute to the mitochondrial defects in HD (Naia et al., 2017). Therefore, PGC-1α might mediate some of the protective effects of resveratrol in HD models.
3.5. Resveratrol and amyotrophic lateral sclerosis
Research on the effects of resveratrol in ALS is scarce. A first report in ALS cell lines and SOD1 mouse models showed increased neuronal survival and decreased neurodegeneration after treatment with resveratrol (Kim et al., 2007; Mancuso et al., 2014). Additionally, activation of SIRT1 and reduced acetylation of p53 were observed (Kim et al., 2007; Mancuso et al., 2014), as well as the activation of AMPK (Mancuso et al., 2014). These findings indicate that resveratrol may be protective in ALS (Table 5), through the same mechanisms that are proposed to contribute to protection in other neurodegenerative diseases.
4. Rapamycin
Rapamycin, also known as sirolimus, is an inhibitor of the evolutionarily conserved target of rapamycin (TOR). It is an anti-fungal metabolite produced by Streptomyces hygroscopicus (Li et al., 2014b). Rapamycin inhibits TOR activity by acting as a “lynchpin” in a molecular interaction between TOR and FKBP12 that impedes TOR from phosphorylating its downstream targets (Choi et al., 1996; Stan, 1994; Stanfel et al., 2009). TOR is active in target of rapamycin complex 1 and 2 (TORC1 and TORC2), which together act as key regulators of cellular metabolism and growth (Stanfel et al., 2009). It is thought that the benefits of short-term doses of rapamycin are due to TORC1 inhibition (Stanfel et al., 2009), resulting in several changes including inhibition of protein synthesis and stimulation of autophagy (Kapahi et al., 2010; Stanfel et al., 2009).
4.1. Effect of rapamycin on lifespan
Rapamycin treatment increases lifespan in C. elegans (Robida-Stubbs et al., 2012), Drosophila (Bjedov et al., 2010), and mice (Fok et al., 2014; Harrison et al., 2009; Miller et al., 2011; Wilkinson et al., 2012; Zhang et al., 2014) (Table 1). Interestingly, rapamycin has also been reported to increase immune function in older humans and it has been suggested that it could be used to ameliorate immunosenescence in the elderly population (Mannick et al., 2014; Mannick, 2018). Increased mTOR activity has been implicated in multiple hallmarks of aging, including deregulated protein synthesis and decreased autophagy (Johnson et al., 2013; Kapahi et al., 2010; Laplante and Sabatini, 2012). One way in which rapamycin is thought to slow aging is by reducing protein synthesis via TORC1 inhibition (Lamming et al., 2013). Inhibition or loss of the TORC1 effector, ribosomal S6 kinase (S6K), has been shown to enhance longevity by reducing translation initiation in model organisms (Hansen et al., 2007; Kapahi et al., 2004; Pan et al., 2007; Selman et al., 2009). Furthermore, studies in C. elegans show that inhibition of the cap-dependent eukaryotic translation initiation factor 4E (eIF4E) plays a significant role in increasing lifespan through TORC1 inhibition (Hansen et al., 2007; Pan et al., 2007; Syntichaki et al., 2007). Rapamycin treatment may therefore reduce cap-dependent translation, thereby increasing cap-independent translation, which may allow for expression of stress-response genes (Bjedov et al., 2010). Rapamycin is also thought to slow aging by stimulating autophagy (Lamming et al., 2013). TORC1 inhibition results in increased levels of AMPK-activated ULK1 and ATG13 (Laplante and Sabatini, 2009), which, together, initiate autophagosome formation and allow for clearance of accumulated material in the cell (Ganley et al., 2009; Kim et al., 2011).
4.2. Rapamycin and Alzheimer’s disease
Activation of the downstream effectors of TOR is increased in AD models, suggesting that over-active TORC1 could be contributing to pathology in AD brains (An, 2003; Griffin et al., 2005; Li, 2004; Pei et al., 2006). In Drosophila AD models, rapamycin treatment results in decreased levels of tau toxicity, apoptotic neurons, neurodegenerative markers and increased survival (Berger et al., 2006; Khurana et al., 2006). Studies in both PDAPP and 3xTg-AD mouse models have also reported reduced Aβ and tau toxicity as well as amelioration of cognitive deficits in response to rapamycin treatment (Caccamo et al., 2010; Majumder et al., 2011; Spilman et al., 2010). Additionally, in hAPP(J20) mice, rapamycin protected the blood brain barrier, which is often critically disrupted in AD (Van Skike et al., 2018). However, studies with cells expressing human APP and studies with Tg2576 mice suggest that inhibition of mTORC1 by rapamycin can also exacerbates Aβ toxicity (Yu et al., 2005; Zhang et al., 2010c). Together, these findings suggest that rapamycin may be able to attenuate AD phenotypes (Table 2), but further studies are required to determine why, in certain AD models, rapamycin may augment Aβ toxicity.
4.3. Rapamycin and Parkinson’s disease
Both increased mTOR activity and reduced autophagy are seen in mouse PD models and in patients with dementia with Lewy bodies (DLB) (Crews et al., 2010). The effects of rapamycin in PD models have largely been linked to reduced translation initiation via S6K and eIF4E as well as recovery of autophagy levels, both mediated by TORC1 inhibition (Bove et al., 2011). Treatment with rapamycin improves neurodegenerative phenotypes seen in PD models of Drosophila (Table 3), including climbing ability and survival of dopaminergic neurons. It was also shown in Drosophila that 4E-binding protein (4E-BP), a downstream effector of TOR and inhibitor of translation initiation factor eIF4E, is required for rapamycin protection (Tain et al., 2009). Furthermore, rapamycin reduces α-synuclein accumulation in neurons, improves lysosomal function and reduces neurodegeneration in C57BL/6 mice expressing human α-synuclein (D-line tg mice) (Crews et al., 2010). Additionally, in mice and human dopaminergic neuroblastoma cells treated with parkinsonian toxins, rapamycin enhanced autophagy and protected against death of neurons (Dehay et al., 2010; Malagelada et al., 2010). Rapamycin also improved memory deficits and depressive and anxiety-like behaviours in murine models (Masini et al., 2018), illustrating its ability to rescue both molecular and behavioural markers of PD.
4.4. Rapamycin and Huntington’s disease
The putative role of mTOR in HD is demonstrated by the observations that overexpression of mutant Htt enhances mTORC1 activity in mouse striatal cells and HEK293 cells, and that enhanced mTORC1 activity in the striatum of HD mice results in premature death and severe motor defects (Pryor et al., 2014). Rapamycin reduces mutant Htt aggregates in Drosophila, in the N171–82Q mouse model, and in mammalian cells expressing exon 1 of the mutant HD gene with disease-length polyglutamine expansion (King et al., 2008; Ravikumar et al., 2004; Ravikumar, 2002). Both Drosophila and mouse models of HD also show improved motor function with rapamycin treatment (Ravikumar et al., 2004) (Table 4). Proposed mechanisms of action include both induction of autophagy and reduction of protein synthesis (King et al., 2008; Ravikumar et al., 2004; Ravikumar, 2002). However, other studies suggest that while rapamycin may reduce protein synthesis and improve motor function, it may also have no effect on mutant Htt levels and may not be neuroprotective in R6/2 mouse models of HD (Fox et al., 2010). Therefore, while rapamycin acted as a promising therapeutic in many HD models, further studies should be conducted to distinguish between rapamycin’s effects on motor function and mutant Htt in mouse models.
4.5. Rapamycin and amyotrophic lateral sclerosis
Rapamycin increases survival and suppresses locomotor deficits in TDP-43 Drosophila models of ALS (Cheng et al., 2015) (Table 5). Similarly, in murine TDP-43 models of ALS, rapamycin treatment also reduced neuronal death, reduced accumulation of abnormal TDP-43 and reduced loss of motor functions (Wang et al., 2012). Studies in TDP-43 cell lines also report that rapamycin reduces abnormal TDP-43 (Caccamo et al., 2009). Notably, in all TDP-43 animal models of ALS, there is strong evidence that the activation of autophagy mediates the therapeutic effects seen with rapamycin treatment (Caccamo et al., 2009; Cheng et al., 2015; Wang et al., 2012). In contrast, in SOD1 murine models of ALS, it has been observed that rapamycin does not reduce neuronal death, nor does it increase survival (Staats, 2013; Zhang et al., 2011). It is important to note that SOD1 mouse models do not display TDP-43 aggregation (Turner et al., 2008) and that TDP-43 aggregates are seen in most sporadic ALS cases (Prasad et al., 2019), suggesting that the SOD1 mouse model may not be representative of the pathology seen in the majority of ALS cases (Turner et al., 2008). Rapamycin has been recognized as a promising therapeutic for ALS patients and currently, a clinical trial in Italy is investigating rapamycin and riluzole as a potential combination therapy (Mandrioli et al., 2018).
5. N-acetyl cysteine
N-acetyl-L-cysteine (NAC) is a thiol, a mucolytic agent and an antioxidant. Most commonly known as a treatment for acetaminophen toxicity, NAC has a wide range of clinical applications primarily due to its ability to act as an antioxidant (Dekhuijzen, 2004). Despite its widespread use, how NAC acts as an antioxidant is still unclear. While it was originally believed that NAC scavenges oxidants such as hydrogen peroxide (H2O2) and superoxide (O2.-) by using its thiol group to reduce them, it has been suggested that the rate constants of these reactions are too low for this to be likely (Ezerina et al., 2018). Instead, it has been proposed that NAC derives most of its antioxidant properties from being a cysteine precursor, resulting in elevated synthesis of glutathione (GSH), a critical and ubiquitous thiol antioxidant (Arakawa and Ito, 2007). GSH contributes to enzymatic and non-enzymatic antioxidant systems and is thus important for protection against many toxins (Bump and Brown, 1990). NAC also protects cells from apoptosis by activating extracellular signal-related kinase pathways (Zafarullah et al., 2003). Thus, NAC acts as an antioxidant through its capacity as a free radical scavenger and as a cysteine precursor, elevating intracellular concentrations of GSH.
5.1. Effect of N-acetyl cysteine on lifespan
NAC has been shown to increase longevity in various model organisms (Table 1). In C. elegans, NAC treatment at 5 mM increases mean lifespan by up to 30.5% (Oh et al., 2015), while increased concentrations of 10 mM cause a smaller increase of mean lifespan (Yang and Hekimi, 2010). In Drosophila, NAC significantly increases median lifespan by up to 26.6% at 10 mg/ml concentrations (Brack et al., 1997), while higher concentrations are toxic leading to a significant decrease in lifespan (Niraula and Kim, 2019). The effect of NAC treatment in mice is sex-dependent, as it increases mean lifespan in males but not in females (Flurkey et al., 2010). It is important to note however that mice treated with NAC exhibit reduced food intake which is also shown to increase lifespan in mice (Flurkey et al., 2010). Hence, it is possible that dietary restriction contributes to NAC’s effect on lifespan in mice.
5.2. N-acetyl cysteine and Alzheimer’s Disease
NAC protects against Aβ‐induced apoptosis in cultured cortical neurons by stimulating p35/Cdk5-mediated synaptic plasticity (Hsiao et al., 2008) and reducing JNK activity (Xu et al., 2009) (Table 2). NAC may also help protect against the neuro-inflammatory aspect of AD by increasing intracellular concentrations of GSH in glial cells and astrocytes (Tardiolo et al., 2018). In Aβ-injected and streptozotocin-induced rodent AD models, NAC treatment alleviates AD-induced cognitive deficits (Costa et al., 2016; Shahidi et al., 2017) by restoring the cortical and hippocampal cholinergic system and alleviating of oxidative stress (Huang et al., 2010; Prakash et al., 2015; Tchantchou et al., 2005). Clinical trials of NAC as a treatment for AD have yielded insignificant and mixed results (Deepmala et al., 2015). The largest controlled trial reports favourable changes in almost all cognitive outcome measures when patients were treated with NAC; however, only a few changes were significant (Adair et al., 2001). Additional trials with greater sample sizes would be needed to determine if NAC can act as an AD treatment.
5.3. N-acetyl cysteine and Parkinson’s Disease
NAC is protective in both animal models of PD, and PD patients (Table 3). PD is associated with decreased GSH concentration in the substantia nigra, potentially due to mitochondrial dysfunction and oxidative stress (Bavarsad Shahripour et al., 2014). NAC treatment significantly increases the survival of iPSC-derived dopaminergic neurons treated with rotenone to model PD (Monti et al., 2016). NAC treatment of rotenone-induced PD murine models protects against motor deficits and loss of dopaminergic neurons (Rahimmi et al., 2015). In murine models, it is thought that protection of dopaminergic neurons is mediated by the antioxidant capacity of NAC. Thus, increasing GSH synthesis may be protecting against α-synuclein toxicity (Clark et al., 2010). In preliminary human trials, NAC treatment caused a decrease in clinical symptoms of PD and an increase in dopamine transporter binding, which is impaired in PD (Monti et al., 2016).
5.4. N-acetyl cysteine and Huntington’s Disease
Preliminary data suggests that NAC treatment is protective in HD models due to its ability to counter oxidative stress and mitochondrial dysfunction, which both contribute significantly to HD pathogenesis (Li et al., 2010; Sandhir et al., 2012; Stack et al., 2008) (Table 4). Chronic NAC administration is protective in the R6/1 mouse model of HD. Treatment delays the onset of HD-associated motor deficits, and, through cysteine supplementation, improves the glutamatergic dysfunction that underlies depressive behavior in HD (Wright et al., 2015).
5.5. N-acetyl cysteine and amyotrophic lateral sclerosis
NAC is protective in certain models of ALS, however, it has no significant effect in human ALS patients (Table 5). In human neuroblastoma SH-SY5Y cells expressing mutant SOD1 (G93A), increased cytosolic and mitochondrial ROS production is observed (Beretta et al., 2003). NAC treatment reverses the observed mitochondrial impairment (Beretta et al., 2003). Furthermore, NAC treatment of SOD1 transgenic mice improved survival and delayed onset of motor deficits (Andreassen et al., 2000). Despite promising results in ALS models, clinical trials testing NAC as a potential treatment for ALS have been less successful. ALS patients show no significant difference in neuronal survival and/or disease progression with NAC treatment (Deepmala et al., 2015).
6. Curcumin
Curcumin is an extract from the spice turmeric, which belongs to the ginger family. Curcumin has been commonly used in herbal medicine in Asia for its anti-inflammatory, antioxidant, and analgesic properties (Hewlings and Kalman, 2017). Therefore, it has been studied as a potential therapeutic for various diseases and conditions (Gupta et al., 2013). Curcuminoids, which includes curcumin and its related compounds such as tetrahydroxycurcumin, demethoxycurcumin and bis-demethoxycurcumin have also been tested as longevity-promoting compounds. Curcumin acts through various mechanisms; it decreases levels of reactive oxygen species (ROS) (Dai et al., 2018; Maugeri et al., 2018; Tapia et al., 2014) and has a variety of immunomodulatory activities, such as regulating B-cells, T cells and macrophage activities (Churchill et al., 2000; Yadav et al., 2005). Curcumin also reduces pro-inflammatory cytokines (Jin et al., 2007; Panahi et al., 2016), potentially through inhibition of the NF-κB inflammatory pathway (Singh and Aggarwal, 1995). Additionally, curcumin also affects epigenetic regulation of gene expression by methods such as inhibiting DNA methylation (Liu et al., 2009) and increasing histone acetylation (Chen et al., 2007; Liu et al., 2005). While studies investigating the lifespan and neuroprotective effects of curcumin show a beneficial effect, it is important to note that these studies are relatively sparse compared to studies on other compounds discussed, and further research needs to be conducted.
6.1. Effect of curcumin on lifespan
Curcumin generally increases lifespan in the animal models tested (Table 1). In C. elegans, curcumin increases lifespan as well as increases resistance to oxidative stress (Liao et al., 2011). A recent study using a synthetic curcumin derivative, Cur2004–8, was also found to increase lifespan and increase resistance to oxidative stress to an even greater extent than curcumin. Curcumin also extends the lifespan of Drosophila (Chandrashekara et al., 2014; Lee et al., 2010; Shen et al., 2013; Soh et al., 2013; Suckow and Suckow, 2006), potentially due to the increased resistance to oxidative stress, heat stress and irradiation (Chen et al., 2018; Lee et al., 2010; Seong et al., 2015). The curcumin derivative tetrahydrocurcumin has been tested in mice and showed mixed results; one smaller study showed increased lifespan when treatment was given at 13 months of age whereas a subsequent larger study using a lower concentration showed no effect on lifespan when treatment was started at 4 months of age (Kitani et al., 2007; Strong et al., 2013). Because many parameters differed between the two studies (dose, age of intervention, strain of mice, site), it is unclear why the latter study failed to reproduce the effect on lifespan.
6.2. Curcumin and Alzheimer’s disease
Curcumin is protective in the AD models tested (Table 2). Curcumin treatment protects against Aβ-induced toxicity and increases oxidative stress in neuroblastoma, endothelial and rat PC12 culture (Kim et al., 2001; Qian et al., 2018). Curcumin can also affect Aβ formation (Ono et al., 2004) and metabolism (Zhang et al., 2010a), as well as reduce levels of Aβ by attenuating the maturation of the precursor protein (Zhang et al., 2010a). Additionally, curcumin decreases activation of microglia and astrocytes (Liu et al., 2016) which is another hallmark of AD. Curcumin treatment on peripheral blood mononuclear cells (PBMCs) derived from AD patients showed a decrease in Aβ levels compared to PBMCs without curcuminoid treatment (Gagliardi et al., 2018). In a recent study, a synthetic curcumin derivative, Cur2004–8, decreased Aβ-induced toxicity in a C. elegans model of AD (Kim et al., 2019). In a Drosophila AD model, various curcuminoids rescue eye morphology and locomotor defects (Wang et al., 2014). Similarly, in rodents, curcumin decreases Aβ levels and plaque burden (Garcia-Alloza et al., 2007; Hamaguchi et al., 2009; Lim et al., 2001; Yang et al., 2005). Oxidative damage is also reduced with curcumin treatment of rodent models of AD, as indicated by a decrease in oxidized proteins (Lim et al., 2001). Curcumin also reduces pro-inflammatory cytokines and reduces activation of microglia and astrocytes in rodent models of AD (Begum et al., 2008; Liu et al., 2016; Sundaram et al., 2017). Cognitive performance in rodents was also improved with curcumin (Cheng et al., 2013; Frautschy et al., 2001; Ma et al., 2009). Curcumin and curcuminoid treatment were found to supress the loss of synapses in AD rodent models (Ahmed et al., 2010; Frautschy et al., 2001; Garcia-Alloza et al., 2007). To date, only a few clinical trials have examined the effect of curcumin on AD patients, two of which found no changes in serum Aβ levels or improvement in cognitive ability (Baum et al., 2008; Ringman et al., 2012).
6.3. Curcumin and Parkinson’s disease
Curcumin is generally protective in the PD models tested (Table 3). Oxidative stress is strongly associated with PD. Curcumin protects against oxidative stress through reducing lipid oxidation and reducing levels of malondialdehyde (Rascón et al.), H2O2 and ROS in a glutathione-depleted dopaminergic cell line and in a human neuroblastoma cell line (Harish et al., 2010; van der Merwe et al., 2017). Importantly, curcumin pre-treatment rescued cell viability and reduced apoptosis in a human neuroblastoma cell line, possibly through inhibiting caspase-3 (van der Merwe et al., 2017). Curcumin was also found to reduce toxicity by inhibiting apoptosis (Qualls et al., 2014; Sang et al., 2018). Lastly, curcumin and curcuminoids can increase α-synuclein degradation by preventing α-synuclein fibrillization and reducing α-synuclein aggregation (Gadad et al., 2012; Jiang et al., 2013). In various Drosophila models of PD, curcumin improved locomotor activity, reduced oxidative stress, and rescued dopaminergic neurons in the brain (Nguyen et al., 2018; Pandareesh et al., 2016; Siddique et al., 2014). In a rodent 6-hydroxydopamine (6-OHDA) model of PD, curcumin treatment protected against loss of TH+ cells and dopamine levels (Du et al., 2012; Zbarsky et al., 2005). Other models such as the Park7 (DJ-1) knockout model, the copper intoxication model, and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model also found that curcumin protected against neuronal apoptosis and improved motor deficits (Abbaoui et al., 2017; Chiu et al., 2013; Pan et al., 2012). Other studies in rodent PD models support the antioxidant role (Khatri and Juvekar, 2016; Wang et al., 2017) and anti-inflammatory role (Sharma and Nehru, 2018) of curcumin.
6.4. Curcumin and Huntington’s disease
Curcumin is beneficial in models of HD (Table 4). Curcumin protects against polyQ-mediated photoreceptor neuron degeneration and locomotor dysfunction in Drosophila models of HD (Chongtham and Agrawal, 2016; Ringman et al., 2012). In a CAG140 knockin mouse model of HD, curcumin protects the striatum, and rescues rearing behaviour (Hickey et al., 2012). In an R6/2 mouse model of HD, curcumin also protects the striatum, reduces mHtt levels and improves motor function (Elifani et al., 2019).
6.5. Curcumin and amyotrophic lateral sclerosis
Curcumin is protective in ALS models (Table 5). Using a motor neuron-like cell model of ALS (NCS-34 transfected with TDP-43), curcumin treatment rescued the abnormal excitability in the cells, such as higher firing frequency and lower threshold of action potential (Dong et al., 2014). In a clinical study, curcumin prevented the decline of motor functions as measured by the ALS Functional Rating Scale (ALS-FRS-r). The treatment group also showed improved ability to handle oxidative stress (Chico et al., 2018). Lastly, curcumin treatment significantly improved survival in patients who were also taking riluzole, compared to patients who were only taking riluzole alone (Ahmadi et al., 2018). However, other functional measures such as ALSFRS-R score, and muscle strength did not differ between the groups (Ahmadi et al., 2018).
7. Discussion
Metformin, resveratrol, rapamycin, NAC, and curcumin all increase lifespan in C. elegans, and all but metformin also increase lifespan in Drosophila (Table 1). In mice, the effect of these compounds on lifespan is more complicated, except for rapamycin, which has the most reproducibly beneficial effect on mouse longevity. While both metformin and curcumin have been shown to increase lifespan in mice, other studies have failed to reproduce this finding. In the case of NAC, it may be acting indirectly by decreasing food consumption thereby leading to dietary restriction. Resveratrol has only been shown to increase lifespan in mice maintained on a high fat diet. While examining the effects of these compounds on lifespan in mice has produced mixed results, the fact that at least one study has observed a significant increase in lifespan for each compound demonstrates that all of these compounds can increase lifespan in mammals, but, in some cases only under certain experimental conditions (dose, environment, strain, diet, etc.). Additional studies would be required to determine the precise reasons why the compounds increase lifespan in one experiment but not another, but these studies are time consuming and costly to perform in mice. Currently, it remains unknown whether these compounds can affect longevity in healthy humans.
Similar to their effect on longevity, the effect of these lifespan-extending compounds in models of neurodegenerative disease is more complicated in mammals than in simpler model organisms. Metformin, resveratrol, rapamycin, NAC and curcumin have all been shown to improve deficits in cellular, C. elegans and Drosophila models of neurodegenerative diseases (Tables 2–5). Although many studies support the beneficial effects of these compounds in rodents, and in some cases humans, other studies fail to show a benefit. In cases where a compound fails to show a benefit, the reasons for failure are often unclear. It is possible that a difference in one or more of the experimental conditions can account for the contradictory results. However, without further experimentation, it is hard to determine which factors if any are responsible for the differing outcomes. When an experiment does not reproduce a beneficial effect observed by others, it could simply be that the experiment failed for one of the several possible reasons when a compound and a complex organism are involved (e.g. wrong dose of compound, compound doesn’t reach target tissue, compound delivery began at the wrong time point, experimenter error etc.). At the same time, it is also possible that neutral or negative studies are not published leading to a reporting bias.
To better understand why differing results are sometimes obtained, it may be important to elucidate the precise mechanisms by which the lifespan-extending compounds are providing protection. One important question would be to determine how the neuroprotective effects of these compounds are related to their effects on aging. While we have hypothesized a simple relationship in which aging contributes to neurodegeneration and these compounds are neuroprotective because they delay aging, it is also possible that these compounds impact aging and neurodegeneration independently. Another possibility is that neurodegeneration is part of the aging process such that any impact that these compounds have on aging will necessarily also affect neurodegeneration.
The fact that each of the lifespan-extending compounds reviewed here can be protective in multiple different neurodegenerative diseases suggests the possibility that by targeting the aging process it may be possible to delay or prevent multiple diseases simultaneously. The idea of targeting aging as a cause of disease rather than studying individual diseases is the focus of the growing field of geroscience (Kennedy et al., 2014). As in the neurodegenerative diseases discussed here, aging is the main risk factor for a multitude of diseases, including cardiovascular disease and many forms of cancer. The goal of geroscience is to gain insight into the aging process and use that knowledge to delay or prevent changes that take place during normal aging. By intervening in the aging process, a single treatment may have a beneficial effect on a wide range of diseases in which aging contributes to disease pathogenesis.
8. Conclusions
In this review, we have shown that five different compounds that increase lifespan can be protective in four different neurodegenerative diseases. This suggests the possibility that compounds that increase lifespan may provide novel treatment strategies for these devastating disorders. For this to happen, it will be important to determine why these compounds are neuroprotective in some studies but not others, and why the beneficial effects have not, for the most part, translated into humans. In addition to the compounds reviewed here, there are a number of other compounds that have been shown to extend longevity, many of which have also been shown to be protective in models of neurodegenerative disease. Screening these compounds in cell culture and invertebrate models of neurodegenerative disease would allow for a rapid and cost-effective means to prioritize lifespan-extending compounds for further study in mammals.
Highlights.
Aging is the greatest risk factor for the development of neurodegenerative disease
Multiple compounds have been shown to extend lifespan in model organisms
These lifespan-extending compounds have been shown to be neuroprotective in animal models of disease
Targeting the aging process may be an effective strategy to treat neurodegeneration
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canadian Foundation for Innovation (CFI), the Fonds de Recherche Quebec Santé (FRQS), and the National Institutes of General Medical Sciences (NIGMS).
Abbreviations
- 4E-BP
4E-binding protein
- 6-OHDA
6-hydroxydopamine
- AD
Alzheimer’s disease
- Aβ
Amyloid beta
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated protein kinase
- APP
Amyloid precursor protein
- ApoE3
Apolipoprotein E3
- ApoE4
Apolipoprotein E4
- ATG13
Autophagy Related 13
- DLB
Dementia with Lewy bodies
- eIF4E
Eukaryotic Translation Initiation Factor 4E
- FKBP12
12 kDa FK506-binding protein
- FUS
Fused in sarcoma
- GSH
Glutathione
- HD
Huntington’s disease
- HO-1
heme oxygenase-1
- Htt
huntingtin
- JNK
c-Jun N-terminal kinase
- LRRK2
Leucine-Rich Repeat Kinase
- MDA
malondialdehyde
- MPP+1-methyl-4-phenylpyridinium
MPTP1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl-L-cysteine
- PCG-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- S6K
Ribosomal protein S6 kinase
- SIRT1
sirtuin-1
- SN
substantia nigra
- SNCA
synuclein alpha
- SOD1
Superoxide dismutase 1
- T2DM
Type 2 Diabetes Mellitus
- TDP-43
TAR DNA-binding protein 43
- TOR
target of rapamycin
- TORC1
Target of rapamycin complex 1
- TORC2
Target of rapamycin complex 2
- ULK1
Unc-51 Like Autophagy Activating Kinase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbaoui A, Chatoui H, El Hiba O, Gamrani H, 2017. Neuroprotective effect of curcumin-I in copper-induced dopaminergic neurotoxicity in rats: A possible link with Parkinson’s disease. Neurosci Lett 660, 103–108. [DOI] [PubMed] [Google Scholar]
- Adair JC, Knoefel JE, Morgan N, 2001. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology 57, 1515–1517. [DOI] [PubMed] [Google Scholar]
- Ahmad W, Ebert PR, 2017. Metformin Attenuates Aβ Pathology Mediated Through Levamisole Sensitive Nicotinic Acetylcholine Receptors in a C. elegans Model of Alzheimer’s Disease. Mol Neurobiol 54, 5427–5439. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Agah E, Nafissi S, Jaafari MR, Harirchian MH, Sarraf P, Faghihi-Kashani S, Hosseini SJ, Ghoreishi A, Aghamollaii V, Hosseini M, Tafakhori A, 2018. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 15, 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Enam SA, Gilani AH, 2010. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience 169, 1296–1306. [DOI] [PubMed] [Google Scholar]
- Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G, 2009. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by α-synuclein or amyloid-β (1–42) peptide. Journal of Neurochemistry 110, 1445–1456. [DOI] [PubMed] [Google Scholar]
- Alonso A, Logroscino G, Jick SS, Hernan MA, 2009. Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study. Eur J Neurol 16, 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvira D, Yeste-Velasco M, Folch J, Verdaguer E, Canudas AM, Pallàs M, Camins A, 2007. Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience 147, 746–756. [DOI] [PubMed] [Google Scholar]
- An WLC,R; Li L; Braak H; Alafuzoff I; Iqbal K; Iqbal IG; Winblad B; Pei JJ , 2003. Up-Regulation of Phosphorylated/Activated p70 S6 Kinase and Its Relationship to Neurofibrillary Pathology in Alzheimer’s Disease. American Journal of Pathology 163, 591–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Dedeoglu A, Klivenyi P, Beal MF, Bush AI, 2000. N-acetyl-L-cysteine improves survival and preserves motor performance in an animal model of familial amyotrophic lateral sclerosis. Neuroreport 11, 2491–2493. [DOI] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R, 2004. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes & development 18, 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M, Ito Y, 2007. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. The Cerebellum 6, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoux I, Willam M, Griesche N, Krummeich J, Watari H, Offermann N, Weber S, Narayan Dey P, Chen C, Monteiro O, Buettner S, Meyer K, Bano D, Radyushkin K, Langston R, Lambert JJ, Wanker E, Methner A, Krauss S, Schweiger S, Stroh A, 2018. Metformin reverses early cortical network dysfunction and behavior changes in Huntington’s disease. Elife 7, e38744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister CA, Holden SE, Jenkins-Jones S, Morgan CL, Halcox JP, Schernthaner G, Mukherjee J, Currie CJ, 2014. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab 16, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Barini E, Antico O, Zhao Y, Asta F, Tucci V, Catelani T, Marotta R, Xu H, Gasparini L, 2016. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener 11, 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a Tool to Target Aging. Cell metabolism 23, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L, 2007. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mechanisms of ageing and development 128, 546–552. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Ménard C, Quirion R, 2015. Neuroprotective action of resveratrol. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL, 2004. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America 101, 12980–12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum L, Lam C, Cheung S, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu H, Goggins W, Zee B, Cheng K, Fong C, Wong A, Mok H, Chow M, Ho P, Ip S, Ho C, Yu X, Lai C, Chan M, Szeto S, Chan I, Mok V, 2008. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients With Alzheimer Disease. Journal of Clinical Psychopharmacology 1. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA, 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavarsad Shahripour R, Harrigan MR, Alexandrov AV, 2014. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and Behavior 4, 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss JA, Lemus MB, Santos VV, Deo M, Davies JS, Kemp BE, Elsworth JD, Andrews ZB, 2016. Metformin Prevents Nigrostriatal Dopamine Degeneration Independent of AMPK Activation in Dopamine Neurons. PloS one 11, e0159381–e0159381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA, 2008. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 326, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M, 2009. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chemical biology & drug design 74, 619–624. [DOI] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR, 2001. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555. [DOI] [PubMed] [Google Scholar]
- Beretta S, Sala G, Mattavelli L, Ceresa C, Casciati A, Ferri A, Carrì MT, Ferrarese C, 2003. Mitochondrial dysfunction due to mutant copper/zinc superoxide dismutase associated with amyotrophic lateral sclerosis is reversed by N-acetylcysteine. Neurobiology of disease 13, 213–221. [DOI] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O’Kane CJ, Rubinsztein DC, 2006. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 15, 433–442. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L, 2010. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet J, Longpré F, Bureau G, Morissette M, DiPaolo T, Bronchti G, Martinoli MG, 2008. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Progress in neuro-psychopharmacology & biological psychiatry 32, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Boccitto M, Lamitina T, Kalb RG, 2012. Daf-2 signaling modifies mutant SOD1 toxicity in C. elegans. PloS one 7, e33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlken J, Jacob L, Kostev K, 2018. Association Between the Use of Antihyperglycemic Drugs and Dementia Risk: A Case-Control Study. J Alzheimers Dis 66, 725–732. [DOI] [PubMed] [Google Scholar]
- Bond L, Bowen G, Mertens B, Denson K, Jordan K, Vidakovic B, Mitchell SC, 2020. Associations of Patient Mood, Modulators of Quality of Life, and Pharmaceuticals with Amyotrophic Lateral Sclerosis Survival Duration. Behavioral Sciences 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, Martinez-Vicente M, Vila M, 2011. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci 12, 437–452. [DOI] [PubMed] [Google Scholar]
- Brack C, Bechter-Thüring E, Labuhn M, 1997. N-Acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cellular and Molecular Life Sciences CMLS 53, 960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Moore FE, Tang SJ, Morimoto RI, 2006. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. J Neurosci 26, 7597–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Beal MF, 2006. Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal 8, 2061–2073. [DOI] [PubMed] [Google Scholar]
- Bump EA, Brown JM, 1990. Role of glutathione in the radiation response of mammalian cells invitro and in vivo. Pharmacology & Therapeutics 47, 117–136. [DOI] [PubMed] [Google Scholar]
- Byrne S, Walsh C, Lynch C, Bede P, Elamin M, Kenna K, McLaughlin R, Hardiman O, 2011. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 82, 623–627. [DOI] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung K-Y, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene NDE, Gems D, 2013. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Deng JJ, Bai Y, Thornton FB, Oddo S, 2009. Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability. J Biol Chem 284, 27416–27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S, 2010. Molecular Interplay between Mammalian Target of Rapamycin (mTOR), Amyloid-β, and Tau. Journal of Biological Chemistry 285, 13107–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JM, Bellman SM, Stephenson MD, Lisy K, 2017. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Research Reviews 40, 31–44. [DOI] [PubMed] [Google Scholar]
- Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P, 2012. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem 120, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekara KT, Popli S, Shakarad MN, 2014. Curcumin enhances parental reproductive lifespan and progeny viability in Drosophila melanogaster. Age 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Teng Y, Zhang X, Lv X, Yin Y, 2016. Metformin Alleviated Aβ-Induced Apoptosis via the Suppression of JNK MAPK Signaling Pathway in Cultured Hippocampal Neurons. Biomed Res Int 2016, 1421430–1421430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Jang JH, Li MH, Surh YJ, 2005. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochemical and biophysical research communications 331, 993–1000. [DOI] [PubMed] [Google Scholar]
- Chen J-L, Luo C, Pu D, Zhang G-Q, Zhao Y-X, Sun Y, Zhao K-X, Liao Z-Y, Lv A-K, Zhu S-Y, Zhou J, Xiao Q, 2019. Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Experimental neurology 311, 44–56. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu X, Jiang C, Liu L, Ordovas JM, Lai CQ, Shen L, 2018. Curcumin supplementation increases survival and lifespan in Drosophila under heat stress conditions. Biofactors 44, 577–587. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shu W, Chen W, Wu Q, Liu H, Cui G, 2007. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol 101, 427–433. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou K, Wang R, Liu Y, Kwak Y-D, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao F-F, 2009. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proceedings of the National Academy of Sciences of the United States of America 106, 3907–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Lin C-H, Tsai Y-W, Tsai C-J, Chou P-H, Lan T-H, 2014. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. The journals of gerontology. Series A, Biological sciences and medical sciences 69, 1299–1305. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Lin MJ, Shen CK, 2015. Rapamycin alleviates pathogenesis of a new Drosophila model of ALS-TDP. J Neurogenet 29, 59–68. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L, 2013. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J 15, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico L, Ienco EC, Bisordi C, Lo Gerfo A, Petrozzi L, Petrucci A, Mancuso M, Siciliano G, 2018. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol Disord Drug Targets 17, 767–779. [DOI] [PubMed] [Google Scholar]
- Chiò A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, White LA, 2013. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology 41, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S, Terpstra KJ, Bureau Y, Hou J, Raheb H, Cernvosky Z, Badmeav V, Copen J, Husni M, Woodbury-Farina M, 2013. Liposomal-formulated curcumin [Lipocurc] targeting HDAC (histone deacetylase) prevents apoptosis and improves motor deficits in Park 7 (DJ-1)-knockout rat model of Parkinson’s disease: implications for epigenetics-based nanotechnology-driven drug platform. J Complement Integr Med 10. [DOI] [PubMed] [Google Scholar]
- Choi J, Chen J, Schreiber SL, Clardy J, 1996. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 273, 239–242. [DOI] [PubMed] [Google Scholar]
- Chongtham A, Agrawal N, 2016. Curcumin modulates cell death and is protective in Huntington’s disease model. Scientific reports 6, 18736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM, 2000. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res 89, 169–175. [DOI] [PubMed] [Google Scholar]
- Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK, 2010. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PloS one 5, e12333–e12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A, 2006. Opposing activities protect against age-onset proteotoxicity. Science 313, 1604–1610. [DOI] [PubMed] [Google Scholar]
- Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A, 2009. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 139, 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH, 2011. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nature reviews. Neuroscience 12, 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Petrucelli L, 2009. A critical evaluation of the ubiquitin-proteasome system in Parkinson’s disease. Biochim Biophys Acta 1792, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JF, Dues DJ, Spielbauer KK, Machiela E, Senchuk MM, Van Raamsdonk JM, 2015. Delaying aging is neuroprotective in Parkinson’s disease: a genetic analysis in C. elegans models. NPJ Parkinson’s disease 1, 15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Bernardi J, Fiuza T, Costa L, Brandao R, Pereira ME, 2016. N-acetylcysteine protects memory decline induced by streptozotocin in mice. Chem Biol Interact 253, 10–17. [DOI] [PubMed] [Google Scholar]
- Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E, 2010. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One 5, e9313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cristòfol R, Porquet D, Corpas R, Coto-Montes A, Serret J, Camins A, Pallàs M, Sanfeliu C, 2012. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. Journal of pineal research 52, 271–281. [DOI] [PubMed] [Google Scholar]
- Dai J, Gu L, Su Y, Wang Q, Zhao Y, Chen X, Deng H, Li W, Wang G, Li K, 2018. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-kappaB pathways. Int Immunopharmacol 54, 177–187. [DOI] [PubMed] [Google Scholar]
- Das S, Das DK, 2007. Anti-inflammatory responses of resveratrol. Inflammation & allergy drug targets 6, 168–173. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J, 2007. Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences of the United States of America 104, 7217–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepmala Slattery, J., Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R, 2015. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neuroscience & Biobehavioral Reviews 55, 294–321. [DOI] [PubMed] [Google Scholar]
- Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M, 2010. Pathogenic lysosomal depletion in Parkinson’s disease. J Neurosci 30, 12535–12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekhuijzen PN, 2004. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J 23, 629–636. [DOI] [PubMed] [Google Scholar]
- del Aguila MA, Longstreth WT Jr., McGuire V, Koepsell TD, van Belle G, 2003. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology 60, 813–819. [DOI] [PubMed] [Google Scholar]
- Deng HX, Zhai H, Bigio EH, Yan J, Fecto F, Ajroud K, Mishra M, Ajroud-Driss S, Heller S, Sufit R, Siddique N, Mugnaini E, Siddique T, 2010. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol 67, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N, 1997. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993. [DOI] [PubMed] [Google Scholar]
- Dong H, Xu L, Wu L, Wang X, Duan W, Li H, Li C, 2014. Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neuroscience 272, 141–153. [DOI] [PubMed] [Google Scholar]
- Driver JA, Logroscino G, Gaziano JM, Kurth T, 2009. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 72, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XX, Xu HM, Jiang H, Song N, Wang J, Xie JX, 2012. Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson’s disease. Neurosci Bull 28, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulovic M, Jovanovic M, Xilouri M, Stefanis L, Harhaji-Trajkovic L, Kravic-Stevovic T, Paunovic V, Ardah MT, El-Agnaf OMA, Kostic V, Markovic I, Trajkovic V, 2014. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiology of disease 63, 1–11. [DOI] [PubMed] [Google Scholar]
- Elifani F, Amico E, Pepe G, Capocci L, Castaldo S, Rosa P, Montano E, Pollice A, Madonna M, Filosa S, Calogero A, Maglione V, Crispi S, Di Pardo A, 2019. Curcumin dietary supplementation ameliorates disease phenotype in an animal model of Huntington’s disease. Hum Mol Genet 28, 4012–4021. [DOI] [PubMed] [Google Scholar]
- Ezerina D, Takano Y, Hanaoka K, Urano Y, Dick TP, 2018. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2 S and Sulfane Sulfur Production. Cell Chem Biol 25, 447–459.e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber PW, Alter JR, MacDonald ME, Hart AC, 1999. Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc Natl Acad Sci U S A 96, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Roesler E, Niehoff ML, Roby DA, McKee A, Morley JE, 2019. Metformin Improves Learning and Memory in the SAMP8 Mouse Model of Alzheimer’s Disease. J Alzheimers Dis 68, 1699–1710. [DOI] [PubMed] [Google Scholar]
- Feng X, Liang N, Zhu D, Gao Q, Peng L, Dong H, Yue Q, Liu H, Bao L, Zhang J, Hao J, Gao Y, Yu X, Sun J, 2013. Resveratrol inhibits β-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PloS one 8, e59888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Pan J, Li J, Zeng C, Qi W, Shao Y, Liu X, Liu L, Xiao G, Zhang H, Bai X, Cai D, 2020. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging 12, 1087–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Funez P, Nino-Rosales ML, de Gouyon B, She WC, Luchak JM, Martinez P, Turiegano E, Benito J, Capovilla M, Skinner PJ, McCall A, Canal I, Orr HT, Zoghbi HY, Botas J, 2000. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408, 101–106. [DOI] [PubMed] [Google Scholar]
- Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, Cocco T, 2014. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. Biochimica et biophysica acta 1842, 902–915. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Astle CM, Harrison DE, 2010. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 65, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH 3rd, Zhang Y, Becker KG, Perez VI, Richardson A, 2014. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One 9, e83988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Connor T, Chopra V, Dorsey K, Kama JA, Bleckmann D, Betschart C, Hoyer D, Frentzel S, Difiglia M, Paganetti P, Hersch SM, 2010. The mTOR kinase inhibitor Everolimus decreases S6 kinase phosphorylation but fails to reduce mutant huntingtin levels in brain and is not neuroprotective in the R6/2 mouse model of Huntington’s disease. Mol Neurodegener 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller S, Chu T, Harris-White ME, Cole GM, 2001. Phenolic anti-inflammatory antioxidant reversal of Aβ-induced cognitive deficits and neuropathology. Neurobiology of Aging 22, 993–1005. [DOI] [PubMed] [Google Scholar]
- Freude S, Schilbach K, Schubert M, 2009. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer’s disease: from model organisms to human disease. Current Alzheimer research 6, 213–223. [DOI] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE, 1988. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen Z-P, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JRB, van Denderen BJ, Kemp BE, Steinberg GR, 2013. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19, 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Witkowski JM, Bourgade K, Khalil A, Zerif E, Larbi A, Hirokawa K, Pawelec G, Bocti C, Lacombe G, Dupuis G, Frost EH, 2018. Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer’s Disease? Front Aging Neurosci 10, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballah HH, Zakaria SS, Elbatsh MM, Tahoon NM, 2016. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chemico-biological interactions 251, 10–16. [DOI] [PubMed] [Google Scholar]
- Gadad B, Subramanya P, Pullabhatla S, Shantharam I, Rao KS, 2012. Curcumin-glucoside, A Novel Synthetic Derivative of Curcumin, Inhibits -Synuclein Oligomer Formation: Relevance to Parkinson’s Disease. Current Pharmaceutical Design 18, 76–84. [DOI] [PubMed] [Google Scholar]
- Gagliardi S, Franco V, Sorrentino S, Zucca S, Pandini C, Rota P, Bernuzzi S, Costa A, Sinforiani E, Pansarasa O, Cashman JR, Cereda C, 2018. Curcumin and Novel Synthetic Analogs in Cell-Based Studies of Alzheimer’s Disease. Front Pharmacol 9, 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E, 2014. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 7, 867–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X, 2009. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem 284, 12297–12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ, 2007. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem 102, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Giannarelli R, Aragona M, Coppelli A, Del Prato S, 2003. Reducing insulin resistance with metformin: the evidence today. Diabetes & Metabolism 29, 6S28–26S35. [DOI] [PubMed] [Google Scholar]
- Gong Q-H, Li F, Jin F, Shi J-S, 2010. Resveratrol Attenuates Neuroinflammation-mediated Cognitive Deficits in Rats. Journal of Health Science 56, 655–663. [Google Scholar]
- Gormsen LC, Sundelin EI, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, Hougaard Christensen MM, Brøsen K, Frøkiær J, Jessen N, 2016. In Vivo Imaging of Human 11C-Metformin in Peripheral Organs: Dosimetry, Biodistribution, and Kinetic Analyses. Journal of Nuclear Medicine 57, 1920–1926. [DOI] [PubMed] [Google Scholar]
- Granzotto A, Zatta P, 2011. Resveratrol acts not through anti-aggregative pathways but mainly via its scavenging properties against Aβ and Aβ-metal complexes toxicity. PloS one 6, e21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW, 2008. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci 28, 6182–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A, 2007. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol 17, 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C, 2005. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem 93, 105–117. [DOI] [PubMed] [Google Scholar]
- Gruber J, Tang SY, Halliwell B, 2007. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Annals of the New York Academy of Sciences 1100, 530–542. [DOI] [PubMed] [Google Scholar]
- Guerrero EN, Wang H, Mitra J, Hegde PM, Stowell SE, Liachko NF, Kraemer BC, Garruto RM, Rao KS, Hegde ML, 2016. TDP-43/FUS in motor neuron disease: Complexity and challenges. Prog Neurobiol 145-146, 78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Kismali G, Aggarwal BB, 2013. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors 39, 2–13. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Ono K, Murase A, Yamada M, 2009. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am J Pathol 175, 2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C, 2007. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6, 95–110. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA, 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish G, Venkateshappa C, Mythri RB, Dubey SK, Mishra K, Singh N, Vali S, Bharath MM, 2010. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: Implications for Parkinson’s disease. Bioorg Med Chem 18, 2631–2638. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA, 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchcliffe C, Beal MF, 2008. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4, 600–609. [DOI] [PubMed] [Google Scholar]
- Hervás D, Fornés-Ferrer V, Gómez-Escribano AP, Sequedo MD, Peiró C, Millán JM, Vázquez-Manrique RP, 2017. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PloS one 12, e0179283–e0179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlings SJ, Kalman DS, 2017. Curcumin: A Review of Its’ Effects on Human Health. Foods 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, Maiti P, Frautschy SA, Zeitlin S, Levine MS, Chesselet MF, 2012. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol Neurodegener 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF, 2010. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Experimental neurology 225, 74–84. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y, 2003. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421, 182–187. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA, 2003. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196. [DOI] [PubMed] [Google Scholar]
- Hsiao YH, Chen PS, Yeh SH, Lin CH, Gean PW, 2008. N-acetylcysteine prevents β-amyloid toxicity by a stimulatory effect on p35/cyclin-dependent kinase 5 activity in cultured cortical neurons. Journal of neuroscience research 86, 2685–2695. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C, 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145. [DOI] [PubMed] [Google Scholar]
- Hsu C-C, Wahlqvist ML, Lee M-S, Tsai H-N, 2011. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 24, 485–493. [DOI] [PubMed] [Google Scholar]
- Hu M, Ye P, Liao H, Chen M, Yang F, 2016. Metformin Protects H9C2 Cardiomyocytes from High-Glucose and Hypoxia/Reoxygenation Injury via Inhibition of Reactive Oxygen Species Generation and Inflammatory Responses: Role of AMPK and JNK. J Diabetes Res 2016, 2961954–2961954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Aluise CD, Joshi G, Sultana R, St Clair DK, Markesbery WR, Butterfield DA, 2010. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J Neurosci Res 88, 2618–2629. [DOI] [PubMed] [Google Scholar]
- Huang T-C, Lu K-T, Wo Y-YP, Wu Y-J, Yang Y-L, 2011. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PloS one 6, e29102–e29102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld P, Bodmer M, Jick SS, Meier CR, 2012. Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 60, 916–921. [DOI] [PubMed] [Google Scholar]
- Jang M, Pezzuto JM, 1999. Cancer chemopreventive activity of resveratrol. Drugs under experimental and clinical research 25, 65–77. [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, Seredenina T, Arbez N, Zhu S, Sommers K, Qian J, Zhang J, Mori S, Yang XW, Tamashiro KL, Aja S, Moran TH, Luthi-Carter R, Martin B, Maudsley S, Mattson MP, Cichewicz RH, Ross CA, Holtzman DM, Krainc D, Duan W, 2012. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nature medicine 18, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD, 2013. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol 8, 356–369. [DOI] [PubMed] [Google Scholar]
- Jin CY, Lee JD, Park C, Choi YH, Kim GY, 2007. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol Sin 28, 1645–1651. [DOI] [PubMed] [Google Scholar]
- Jin F, Wu Q, Lu YF, Gong QH, Shi JS, 2008. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. European journal of pharmacology 600, 78–82. [DOI] [PubMed] [Google Scholar]
- Jin J, Gu H, Anders NM, Ren T, Jiang M, Tao M, Peng Q, Rudek MA, Duan W, 2016. Metformin Protects Cells from Mutant Huntingtin Toxicity Through Activation of AMPK and Modulation of Mitochondrial Dynamics. Neuromolecular Med 18, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Rabinovitch P, Kaeberlein M, 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK, 2005. Substrate-specific activation of sirtuins by resveratrol. The Journal of biological chemistry 280, 17038–17045. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G, 2010. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metabolism 11, 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneb HM, Sharp PS, Rahmani-Kondori N, Wells DJ, 2011. Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice. PloS one 6, e24189–e24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Khang R, Ham S, Jeong GR, Kim H, Jo M, Lee BD, Lee YI, Jo A, Park C, Kim H, Seo J, Paek SH, Lee Y-S, Choi J-Y, Lee Y, Shin J-H, 2017. Activation of the ATF2/CREB-PGC-1α pathway by metformin leads to dopaminergic neuroprotection. Oncotarget 8, 48603–48618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L, 2010. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab 11, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S, 2004. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE, 2009. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochemistry international 54, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F, 2014. Geroscience: linking aging to chronic disease. Cell 159, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Khan MM, Ahmad A, Ishrat T, Khan MB, Hoda MN, Khuwaja G, Raza SS, Khan A, Javed H, Vaibhav K, Islam F, 2010. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain research 1328, 139–151. [DOI] [PubMed] [Google Scholar]
- Khatri DK, Juvekar AR, 2016. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacol Biochem Behav 150-151, 39–47. [DOI] [PubMed] [Google Scholar]
- Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB, 2006. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol 16, 230–241. [DOI] [PubMed] [Google Scholar]
- Killick R, Scales G, Leroy K, Causevic M, Hooper C, Irvine EE, Choudhury AI, Drinkwater L, Kerr F, Al-Qassab H, Stephenson J, Yilmaz Z, Giese KP, Brion JP, Withers DJ, Lovestone S, 2009. Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochemical and biophysical research communications 386, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BK, Kim SA, Baek SM, Lee EY, Lee ES, Chung CH, Ahn CM, Park SK, 2019. Cur2004–8, a synthetic curcumin derivative, extends lifespan and modulates age-related physiological changes in Caenorhabditis elegans. Drug Discov Ther 13, 198–206. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH, 2007. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO journal 26, 3169–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Park S, Kim J, 2001. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from Aβ(1–42) insult. Neuroscience Letters 303, 57–61. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Taylor JP, 2017. Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases. Neuron 96, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL, 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Hands S, Hafiz F, Mizushima N, Tolkovsky AM, Wyttenbach A, 2008. Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol Pharmacol 73, 1052–1063. [DOI] [PubMed] [Google Scholar]
- Kitamura A, Kubota H, Pack CG, Matsumoto G, Hirayama S, Takahashi Y, Kimura H, Kinjo M, Morimoto RI, Nagata K, 2006. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat Cell Biol 8, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Kitani K, Osawa T, Yokozawa T, 2007. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology 8, 567–573. [DOI] [PubMed] [Google Scholar]
- Knight AL, Yan X, Hamamichi S, Ajjuri RR, Mazzulli JR, Zhang MW, Daigle JG, Zhang S, Borom AR, Roberts LR, Lee SK, DeLeon SM, Viollet-Djelassi C, Krainc D, O’Donnell JM, Caldwell KA, Caldwell GA, 2014. The glycolytic enzyme, GPI, is a functionally conserved modifier of dopaminergic neurodegeneration in Parkinson’s models. Cell metabolism 20, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Yan Y, He XY, Yang H, Liang B, Wang J, He Y, Ding Y, Yu H, 2019. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed research international 2019, 8983752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan Y-C, Huang K-W, Lin C-L, Hu C-J, Kao C-H, 2017. Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry 79, 77–83. [DOI] [PubMed] [Google Scholar]
- Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP, Barzilai N, 2018. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B, 2010. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 62, 956–965. [DOI] [PubMed] [Google Scholar]
- Lambert M, Barlow A, Chromy B, Edwards C, Freed R, Liosatos M, Morgan T, Rozovsky I, Trommer S, Viola K, Wals P, Zhang C, Finch C, Krafft G, Klein W, 1998. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95, 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA, 2013. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 123, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2009. mTOR signaling at a glance. J Cell Sci 122, 3589–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2012. mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, Seong KM, Yu K, Min KJ, Jafari M, 2010. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res 13, 561–570. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Baulac M, Chiras J, Piérot L, Martin N, Pillon B, Deweer B, Dubois B, Marsault C, 1994. Amygdalohippocampal MR Volume Measurements in the Early Stages of Alzheimer Disease. AJNR Am J Neuroradiol. 5, 929–937. [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH, 2006. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440, 352–357. [DOI] [PubMed] [Google Scholar]
- Li J, Feng L, Xing Y, Wang Y, Du L, Xu C, Cao J, Wang Q, Fan S, Liu Q, Fan F, 2014a. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. International journal of molecular sciences 15, 5928–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kim SG, Blenis J, 2014b. Rapamycin: one drug, many effects. Cell Metab 19, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Valencia A, Sapp E, Masso N, Alexander J, Reeves P, Kegel KB, Aronin N, DiFiglia M, 2010. Aberrant Rab11-Dependent Trafficking of the Neuronal Glutamate Transporter EAAC1 Causes Oxidative Stress and Cell Death in Huntington's Disease. The Journal of Neuroscience 30, 4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XA,W-L; Alafuzzoff I; Soininen H; Winblad B; Pei J-J, 2004. Phorphorylated eukaryotic translation factor 4E is elevated in Alzheimer brain. NeuroReport 15, 2237–2240. [DOI] [PubMed] [Google Scholar]
- Liao VH, Yu CW, Chu YJ, Li WH, Hsieh YC, Wang TT, 2011. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev 132, 480–487. [DOI] [PubMed] [Google Scholar]
- Lim G, Chu T, Yang F, Beech W, Frautschy SA, Cole GM, 2001. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. The Journal of Neuroscience 21, 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Wu YC, Sun GC, Ho CY, Wong TY, Lin CH, Chen HH, Yeh TC, Li CJ, Tseng CJ, Cheng PW, 2018. Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease (dagger). Journal of clinical medicine 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HL, Chen Y, Cui GH, Zhou JF, 2005. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol Sin 26, 603–609. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhu D, Jiang P, Tang X, Lang Q, Yu Q, Zhang S, Che Y, Feng X, 2019. Resveratrol synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson disease in mice. Behavioural brain research 367, 10–18. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li PK, Lin J, Fuchs JR, Marcucci G, Li C, Chan KK, 2009. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett 19, 706–709. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Li ZH, Liu L, Tang WX, Wang Y, Dong MR, Xiao C, 2016. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front Pharmacol 7, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofrumento DD, Nicolardi G, Cianciulli A, De Nuccio F, La Pesa V, Carofiglio V, Dragone T, Calvello R, Panaro MA, 2014. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate immunity 20, 249–260. [DOI] [PubMed] [Google Scholar]
- Lu M, Su C, Qiao C, Bian Y, Ding J, Hu G, 2016. Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson’s Disease via Autophagy and Mitochondrial ROS Clearance. Int J Neuropsychopharmacol 19, pyw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S. i., Chen D, Su F, Shiloh A, Guarente L, Gu W, 2001. Negative Control of p53 by Sir2α Promotes Cell Survival under Stress. Cell 107, 137–148. [DOI] [PubMed] [Google Scholar]
- Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM, 2009. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 29, 9078–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL, 2011. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Human molecular genetics 20, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Richardson A, Strong R, Oddo S, 2011. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One 6, e25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA, 2010. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci 30, 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R, del Valle J, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Nunez O, Pallas M, Portero-Otin M, Osta R, Navarro X, 2014. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 11, 419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrioli J, D’Amico R, Zucchi E, Gessani A, Fini N, Fasano A, Caponnetto C, Chio A, Dalla Bella E, Lunetta C, Mazzini L, Marinou K, Soraru G, de Biasi S, Lo Tartaro D, Pinti M, Cossarizza A, group R.-A.i., 2018. Rapamycin treatment for amyotrophic lateral sclerosis: Protocol for a phase II randomized, double-blind, placebo-controlled, multicenter, clinical trial (RAP-ALS trial). Medicine (Baltimore) 97, e11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP, 1996. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506. [DOI] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB, 2000. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. Journal of immunology (Baltimore, Md. : 1950) 164, 6509–6519. [DOI] [PubMed] [Google Scholar]
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB, 2014. mTOR inhibition improves immune function in the elderly. Sci Transl Med 6, 268ra179. [DOI] [PubMed] [Google Scholar]
- Mannick JBM,M; Hockey HP; Roma G; Beibel M; Kulmatycki K; Watkins M; Shavlakadze T; Zhou W; Quinn D; Glass D; Klickstein, 2018. TORC1 Inhibition Enhances Immune Function and Reduces Infections in the Elderly. Sci Transl Med 10. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P, 2005. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. The Journal of biological chemistry 280, 37377–37382. [DOI] [PubMed] [Google Scholar]
- Marsh JL, Walker H, Theisen H, Zhu YZ, Fielder T, Purcell J, Thompson LM, 2000. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum Mol Genet 9, 13–25. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin M-J, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R, 2013. Metformin improves healthspan and lifespan in mice. Nat Commun 4, 2192–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini D, Bonito-Oliva A, Bertho M, Fisone G, 2018. Inhibition of mTORC1 Signaling Reverts Cognitive and Affective Deficits in a Mouse Model of Parkinson’s Disease. Front Neurol 9, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes F, Hettich MM, Schilling J, Flores-Dominguez D, Blank N, Wiglenda T, Buntru A, Wolf H, Weber S, Vorberg I, Dagane A, Dittmar G, Wanker E, Ehninger D, Krauss S, 2018. Inhibition of the MID1 protein complex: a novel approach targeting APP protein synthesis. Cell Death Discov 4, 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugeri A, Mazzone MG, Giuliano F, Vinciguerra M, Basile G, Barchitta M, Agodi A, 2018. Curcumin Modulates DNA Methyltransferase Functions in a Cellular Model of Diabetic Retinopathy. Oxid Med Cell Longev 2018, 5407482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C, Cherny R, Fraser F, Fuller S, Smith M, Beyreuther K, Bush A, Masters C, 1999. Soluble Pool of Aβ Amyloid as a Determinant of Severity of Neurodegeneration in Alzheimer’s Disease. Annals of Neurology 46, 860–866. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R, 2011. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, Wei X, Bazzan AJ, Zhong L, Bowen B, Intenzo CM, Iacovitti L, Newberg AB, 2016. N-Acetyl Cysteine May Support Dopamine Neurons in Parkinson’s Disease: Preliminary Clinical and Cell Line Data. PLoS One 11, e0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI, 2002. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99, 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS, Turner RS, 2017. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. Journal of neuroinflammation 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, Laco MN, Hayden MR, Oliveira CR, Rego AC, 2017. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Molecular neurobiology 54, 5385–5399. [DOI] [PubMed] [Google Scholar]
- Nassar SZ, Badae NM, Issa YA, 2018. Effect of amylin on memory and central insulin resistance in a rat model of Alzheimer’s disease. Arch Physiol Biochem, 1–9. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY, 2006. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 314, 130. [DOI] [PubMed] [Google Scholar]
- Ng C-H, Guan MSH, Koh C, Ouyang X, Yu F, Tan E-K, O’Neill SP, Zhang X, Chung J, Lim K-L, 2012. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci 32, 14311–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B, 2014. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41, 61–68. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Vuu MD, Huynh MA, Yamaguchi M, Tran LT, Dang TPT, 2018. Curcumin Effectively Rescued Parkinson’s Disease-Like Phenotypes in a Novel Drosophila melanogaster Model with dUCH Knockdown. Oxid Med Cell Longev 2018, 2038267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Cabecinha M, Tillmann A, Kerr F, Wong CT, Cardenes D, Vincent AJ, Bettedi L, Li L, Grönke S, Dols J, Partridge L, 2016. Increased Glucose Transport into Neurons Rescues Aβ Toxicity in Drosophila. Curr Biol 26, 2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, Aichour MTE, Akinyemi RO, Alahdab F, Asgedom SW, Awasthi A, Barker-Collo SL, Baune BT, Béjot Y, Belachew AB, Bennett DA, Biadgo B, Bijani A, Bin Sayeed MS, Brayne C, Carpenter DO, Carvalho F, Catalá-López F, Cerin E, Choi J-YJ, Dang AK, Degefa MG, Djalalinia S, Dubey M, Duken EE, Edvardsson D, Endres M, Eskandarieh S, Faro A, Farzadfar F, Fereshtehnejad S-M, Fernandes E, Filip I, Fischer F, Gebre AK, Geremew D, Ghasemi-Kasman M, Gnedovskaya EV, Gupta R, Hachinski V, Hagos TB, Hamidi S, Hankey GJ, Haro JM, Hay SI, Irvani SSN, Jha RP, Jonas JB, Kalani R, Karch A, Kasaeian A, Khader YS, Khalil IA, Khan EA, Khanna T, Khoja TAM, Khubchandani J, Kisa A, Kissimova-Skarbek K, Kivimäki M, Koyanagi A, Krohn KJ, Logroscino G, Lorkowski S, Majdan M, Malekzadeh R, März W, Massano J, Mengistu G, Meretoja A, Mohammadi M, Mohammadi-Khanaposhtani M, Mokdad AH, Mondello S, Moradi G, Nagel G, Naghavi M, Naik G, Nguyen LH, Nguyen TH, Nirayo YL, Nixon MR, Ofori-Asenso R, Ogbo FA, Olagunju AT, Owolabi MO, Panda-Jonas S, Passos V.M.d.A., Pereira DM, Pinilla-Monsalve GD, Piradov MA, Pond CD, Poustchi H, Qorbani M, Radfar A, Reiner RC Jr., Robinson SR, Roshandel G, Rostami A, Russ TC, Sachdev PS, Safari H, Safiri S, Sahathevan R, Salimi Y, Satpathy M, Sawhney M, Saylan M, Sepanlou SG, Shafieesabet A, Shaikh MA, Sahraian MA, Shigematsu M, Shiri R, Shiue I, Silva JP, Smith M, Sobhani S, Stein DJ, Tabarés-Seisdedos R, Tovani-Palone MR, Tran BX, Tran TT, Tsegay AT, Ullah I, Venketasubramanian N, Vlassov V, Wang Y-P, Weiss J, Westerman R, Wijeratne T, Wyper GMA, Yano Y, Yimer EM, Yonemoto N, Yousefifard M, Zaidi Z, Zare Z, Vos T, Feigin VL, Murray CJL, 2019. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 18, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niraula P, Kim MS, 2019. N-Acetylcysteine extends lifespan of Drosophila via modulating ROS scavenger gene expression. Biogerontology 20, 533–543. [DOI] [PubMed] [Google Scholar]
- Oh S-I, Park J-K, Park S-K, 2015. Lifespan extension and increased resistance to environmental stressors by N-acetyl-L-cysteine in Caenorhabditis elegans. Clinics 70, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M, 2010. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS one 5, e8758–e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M, 2004. Curcumin Has Potent Anti-Amyloidogenic Effects for Alzheimer’s β-Amyloid Fibrils In Vitro. Journal of Neuroscience Research 75, 742–750. [DOI] [PubMed] [Google Scholar]
- Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA, 2017. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 89, 1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, Huang J, Xu B, Long D, Li J, Li Q, Xu L, Xuan A, 2018. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69, 351–363. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K, 2010. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. The Journal of biological chemistry 285, 8340–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB, 2004. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600. [DOI] [PubMed] [Google Scholar]
- Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL, 2008. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Human molecular genetics 17, 3767–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Li H, Ma J, Tan Y, Xiao Q, Ding JQ, Chen SD, 2012. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Translational Neurodegeneration 1, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P, 2007. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 6, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, Jankovic J, 2008. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131, 1969–1978. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendia LE, Majeed M, Sahebkar A, 2016. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed Pharmacother 82, 578–582. [DOI] [PubMed] [Google Scholar]
- Pandareesh MD, Shrivash MK, Naveen Kumar HN, Misra K, Srinivas Bharath MM, 2016. Curcumin Monoglucoside Shows Improved Bioavailability and Mitigates Rotenone Induced Neurotoxicity in Cell and Drosophila Models of Parkinson’s Disease. Neurochem Res 41, 3113–3128. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT, 2002. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci 5, 731–736. [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C, 2005. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nature genetics 37, 349–350. [DOI] [PubMed] [Google Scholar]
- Parker JA, Connolly JB, Wellington C, Hayden M, Dausset J, Neri C, 2001. Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc Natl Acad Sci U S A 98, 13318–13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R, 2008. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei JJ, An WL, Zhou XW, Nishimura T, Norberg J, Benedikz E, Gotz J, Winblad B, 2006. P70 S6 kinase mediates tau phosphorylation and synthesis. FEBS Lett 580, 107–114. [DOI] [PubMed] [Google Scholar]
- Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ, 2019. Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis 70, S145–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Porquet D, Casadesús G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegrí C, Sanfeliu C, Camins A, Pallàs M, del Valle J, 2013. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. AGE 35, 1851–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Kalra JK, Kumar A, 2015. Neuroprotective effect of N-acetyl cysteine against streptozotocin-induced memory dysfunction and oxidative damage in rats. 26, 13. [DOI] [PubMed] [Google Scholar]
- Prasad A, Bharathi V, Sivalingam V, Girdhar A, Patel BK, 2019. Molecular Mechanisms of TDP-43 Misfolding and Pathology in Amyotrophic Lateral Sclerosis. Front Mol Neurosci 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price Nathan L., Gomes Ana P., Ling Alvin J.Y., Duarte Filipe V., Martin-Montalvo A, North Brian J., Agarwal B, Ye L, Ramadori G, Teodoro Joao S., Hubbard Basil P., Varela Ana T., Davis James G., Varamini B, Hafner A, Moaddel R, Rolo Anabela P., Coppari R, Palmeira Carlos M., de Cabo, R., Baur Joseph A., Sinclair David A., 2012. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab 15, 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WM, Biagioli M, Shahani N, Swarnkar S, Huang WC, Page DT, MacDonald ME, Subramaniam S, 2014. Huntingtin promotes mTORC1 signaling in the pathogenesis of Huntington’s disease. Sci Signal 7, ra103. [DOI] [PubMed] [Google Scholar]
- Qian W, Li H, Pan N, Zhang C, 2018. Curcumin Treatment is Associated with Increased Expression of the N-Methyl-D-Aspartate Receptor (NMDAR) Subunit, NR2A, in a Rat PC12 Cell Line Model of Alzheimer’s Disease Treated with the Acetyl Amyloid-beta Peptide, Abeta(25–35). Med Sci Monit 24, 2693–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi Y, 2014. Protective effects of curcumin against rotenone and salsolinol-induced toxicity: implications for Parkinson’s disease. Neurotox Res 25, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimmi A, Khosrobakhsh F, Izadpanah E, Moloudi MR, Hassanzadeh K, 2015. N-acetylcysteine prevents rotenone-induced Parkinson’s disease in rat: An investigation into the interaction of parkin and Drp1 proteins. Brain Research Bulletin 113, 34–40. [DOI] [PubMed] [Google Scholar]
- Rascón B, Hubbard BP, Sinclair DA, Amdam GV, 2012. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging (Albany NY) 4, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC, 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36, 585–595. [DOI] [PubMed] [Google Scholar]
- Ravikumar BD,R; Rubinsztein C, 2002. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Regitz C, Fitzenberger E, Mahn FL, Dußling LM, Wenzel U, 2016. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. European journal of nutrition 55, 741–747. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M, 1992. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet (London, England) 339, 1523–1526. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, Gylys KH, Badmaev V, Heath DD, Apostolova LG, Porter V, Vanek Z, Marshall GA, Hellemann G, Sugar C, Masterman DL, Montine TJ, Cummings JL, Cole GM, 2012. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther 4, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK, 2012. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell metabolism 15, 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M, 2015. Parkinson’s disease as a result of aging. Aging cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Cheng Z, Rozzo A, Palazzolo I, Kelley GR, Dong X, Krainc D, White MF, 2011. IRS2 increases mitochondrial dysfunction and oxidative stress in a mouse model of Huntington disease. The Journal of clinical investigation 121, 4070–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saewanee N, Praputpittaya T, Malaiwong N, Chalorak P, Meemon K, 2019. Neuroprotective effect of metformin on dopaminergic neurodegeneration and α-synuclein aggregation in C. elegans model of Parkinson’s disease. Neuroscience Research. [DOI] [PubMed] [Google Scholar]
- Sanchis A, García-Gimeno MA, Cañada-Martínez AJ, Sequedo MD, Millán JM, Sanz P, Vázquez-Manrique RP, 2019. Metformin treatment reduces motor and neuropsychiatric phenotypes in the zQ175 mouse model of Huntington disease. Exp Mol Med 51, 65–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Sood A, Mehrotra A, Kamboj SS, 2012. N-Acetylcysteine Reverses Mitochondrial Dysfunctions and Behavioral Abnormalities in 3-Nitropropionic Acid-Induced Huntington’s Disease. Neurodegenerative Diseases 9, 145–157. [DOI] [PubMed] [Google Scholar]
- Sang Q, Liu X, Wang L, Qi L, Sun W, Wang W, Sun Y, Zhang H, 2018. Curcumin Protects an SH-SY5Y Cell Model of Parkinson’s Disease Against Toxic Injury by Regulating HSP90. Cell Physiol Biochem 51, 681–691. [DOI] [PubMed] [Google Scholar]
- Satyal SH, Schmidt E, Kitagawa K, Sondheimer N, Lindquist S, Kramer JM, Morimoto RI, 2000. Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc Natl Acad Sci U S A 97, 5750–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill R, Schott J, Stevens J, Rossor M, Fox N, 2002. Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A 99, 4703–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR, 1999. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet 8, 397–407. [DOI] [PubMed] [Google Scholar]
- Schweiger S, Matthes F, Posey K, Kickstein E, Weber S, Hettich MM, Pfurtscheller S, Ehninger D, Schneider R, Krauß S, 2017. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Scientific reports 7, 13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ, 2009. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong KM, Yu M, Lee KS, Park S, Jin YW, Min KJ, 2015. Curcumin mitigates accelerated aging after irradiation in Drosophila by reducing oxidative stress. Biomed Res Int 2015, 425380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A, 2017. Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer’s disease in a rat model: A behavioral and electrophysiological study. Brain Res Bull 131, 142–149. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta YK, 2002. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life sciences 71, 2489–2498. [DOI] [PubMed] [Google Scholar]
- Sharma N, Nehru B, 2018. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 26, 349–360. [DOI] [PubMed] [Google Scholar]
- Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD, Meydani M, Ordovas JM, Li D, Lai CQ, 2013. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age (Dordr) 35, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Liu S, Fonseca VA, Thethi TK, Shi L, 2019. Effect of metformin on neurodegenerative disease among elderly adult US veterans with type 2 diabetes mellitus. BMJ Open 9, e024954–e024954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique YH, Naz F, Jyoti S, 2014. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. Biomed Res Int 2014, 606928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Aggarwal B, 1995. Activation of Transcription Factor NF-κB Is Suppressed by Curcumin (Diferulolylmethane). The Journal of Biological Chemistry 270, 24995–25000. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K, 2003. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L, 2012. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PloS one 7, e47699–e47699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR, 2003. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet 12, 1555–1567. [DOI] [PubMed] [Google Scholar]
- Soh JW, Marowsky N, Nichols TJ, Rahman AM, Miah T, Sarao P, Khasawneh R, Unnikrishnan A, Heydari AR, Silver RB, Arking R, 2013. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila. Exp Gerontol 48, 229–239. [DOI] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V, 2010. Inhibition of mTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-β Levels in a Mouse Model of Alzheimer’s Disease. PLOS ONE 5, e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats KAH,S; Schonefeldt S; Bento-Abreu A; Dooley J; Van Damme, P.; Liston A; Robberecht W; Van Den Bosch, L., 2013. Rapamycin increases survival in ALS mice lacking mature lymphocytes. Mol Neurodegener 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Matson WR, Ferrante RJ, 2008. Evidence of oxidant damage in Huntington’s disease: translational strategies using antioxidants. Annals of the New York Academy of Sciences 1147, 79–92. [DOI] [PubMed] [Google Scholar]
- Stan RM,MM; Cafferkey R; Johnson RK; Rosenberg M; Livi GP, 1994. Interaction between FKBP12-Rapamycin and TOR Involves a Conserved Serine Residue. The Journal of Biological Chemistry 269, 32027–32030. [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK, 2009. The TOR pathway comes of age. Biochim Biophys Acta 1790, 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE, 2016. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE, 2013. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 68, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB, 2009. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochemical and biophysical research communications 378, 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckow B, Suckow M, 2006. Lifespan Extension by the Antioxidant Curcumin in Drosophila Melanogaster. International Journal of Biomedical Science 2, 402–405. [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P, 2008. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A 105, 3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram JR, Poore CP, Sulaimee NHB, Pareek T, Cheong WF, Wenk MR, Pant HC, Frautschy SA, Low CM, Kesavapany S, 2017. Curcumin Ameliorates Neuroinflammation, Neurodegeneration, and Memory Deficits in p25 Transgenic Mouse Model that Bears Hallmarks of Alzheimer’s Disease. J Alzheimers Dis 60, 1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki P, Troulinaki K, Tavernarakis N, 2007. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature 445, 922–926. [DOI] [PubMed] [Google Scholar]
- Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ, 2009. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci 12, 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JM, Wong ES, Lim KL, 2009. Protein misfolding and aggregation in Parkinson’s disease. Antioxidants & redox signaling 11, 2119–2134. [DOI] [PubMed] [Google Scholar]
- Tapia E, Sanchez-Lozada LG, Garcia-Nino WR, Garcia E, Cerecedo A, Garcia-Arroyo FE, Osorio H, Arellano A, Cristobal-Garcia M, Loredo ML, Molina-Jijon E, Hernandez-Damian J, Negrette-Guzman M, Zazueta C, Huerta-Yepez S, Reyes JL, Madero M, Pedraza-Chaverri J, 2014. Curcumin prevents maleate-induced nephrotoxicity: relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Radic Res 48, 1342–1354. [DOI] [PubMed] [Google Scholar]
- Tardiolo G, Bramanti P, Mazzon E, 2018. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 23, 3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Brown RH Jr., Cleveland DW, 2016. Decoding ALS: from genes to mechanism. Nature 539, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, Graves M, Rogers E, Ortiz D, Shea TB, 2005. N-acteyl cysteine alleviates oxidative damage to central nervous system of ApoE-deficient mice following folate and vitamin E-deficiency. Journal of Alzheimer’s Disease 7, 135–138. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Baumer D, Parkinson NJ, Scaber J, Ansorge O, Talbot K, 2008. TDP-43 expression in mouse models of amyotrophic lateral sclerosis and spinal muscular atrophy. BMC Neurosci 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen PS, 2015. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKPDS, 1998. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865. [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A, 2007. Resveratrol increases vascular oxidative stress resistance. American journal of physiology. Heart and circulatory physiology 292, H2417–2424. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A, 2006. Resveratrol Prolongs Lifespan and Retards the Onset of Age-Related Markers in a Short-Lived Vertebrate. Current Biology 16, 296–300. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Robberecht W, Van Den Bosch L, 2017. Modelling amyotrophic lateral sclerosis: progress and possibilities. Disease Models & Mechanisms 10, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe C, van Dyk HC, Engelbrecht L, van der Westhuizen FH, Kinnear C, Loos B, Bardien S, 2017. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol Neurobiol 54, 2752–2762. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V, 2018. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol 314, H693–H703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Eaton EN, Imai S-I, Frye RA, Pandita TK, Guarente L, Weinberg RA, 2001. hSIR2SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell 107, 149–159. [DOI] [PubMed] [Google Scholar]
- Vázquez-Manrique RP, Farina F, Cambon K, Dolores Sequedo M, Parker AJ, Millán JM, Weiss A, Déglon N, Neri C, 2016. AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Hum Mol Genet 25, 1043–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P, 2010. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. The Journal of biological chemistry 285, 9100–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L, 2005. A Role for SIR-2.1 Regulation of ER Stress Response Genes in Determining C. elegans Life Span. Developmental Cell 9, 605–615. [DOI] [PubMed] [Google Scholar]
- Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Vescovi PP, Fogliano V, Marchelli R, 2005. Bioavailability of trans-resveratrol from red wine in humans. Molecular nutrition & food research 49, 495–504. [DOI] [PubMed] [Google Scholar]
- Wahlqvist ML, Lee M-S, Hsu C-C, Chuang S-Y, Lee J-T, Tsai H-N, 2012. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with Type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord 18, 753–758. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE Jr., Walle UK, 2004. High absorption but very low bioavailability of oral resveratrol in humans. Drug metabolism and disposition: the biological fate of chemicals 32, 1377–1382. [DOI] [PubMed] [Google Scholar]
- Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, Shen CK, 2012. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci U S A 109, 15024–15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kim J, Lee S, Kim Y, Jung M, Kwon H, Ahn Y, 2014. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer’s disease Drosophila models. BMC Complementary and Alternative Medicine 14, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL, Ju B, Zhang YZ, Yin HL, Liu YJ, Wang SS, Zeng ZL, Yang XP, Wang HT, Li JF, 2017. Protective Effect of Curcumin Against Oxidative Stress-Induced Injury in Rats with Parkinson’s Disease Through the Wnt/ beta-Catenin Signaling Pathway. Cell Physiol Biochem 43, 2226–2241. [DOI] [PubMed] [Google Scholar]
- Wang ZH, Zhang JL, Duan YL, Zhang QS, Li GF, Zheng DL, 2015. MicroRNA-214 participates in the neuroprotective effect of Resveratrol via inhibiting alpha-synuclein expression in MPTP-induced Parkinson’s disease mouse. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 74, 252–256. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan C-C, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA, 2012. Rapamycin slows aging in mice. Aging Cell 11, 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D, 2004. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689. [DOI] [PubMed] [Google Scholar]
- Wright DJ, Renoir T, Smith ZM, Frazier AE, Francis PS, Thorburn DR, McGee SL, Hannan AJ, Gray LJ, 2015. N-Acetylcysteine improves mitochondrial function and ameliorates behavioral deficits in the R6/1 mouse model of Huntington’s disease. Translational Psychiatry 5, e492–e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Boudreau DM, Park Y, Simonds NI, Freedman AN, 2014. Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opinion on Drug Safety 13, 1071–1099. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T, 2011. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neuro-Signals 19, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D, Sui R, Zhang Z, 2019. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. Journal of cellular biochemistry 120, 4942–4951. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hou XY, Liu Y, Zong YY, 2009. Different protection of K252a and N-acetyl-L-cysteine against amyloid-beta peptide-induced cortical neuron apoptosis involving inhibition of MLK3-MKK7-JNK3 signal cascades. J Neurosci Res 87, 918–927. [DOI] [PubMed] [Google Scholar]
- Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK, 2005. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol 27, 485–497. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM, 2005. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280, 5892–5901. [DOI] [PubMed] [Google Scholar]
- Yang W, Hekimi S, 2010. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS biology 8, e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA, 2005. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M, 2003. Molecular mechanisms of N-acetylcysteine actions. Cellular and Molecular Life Sciences CMLS 60, 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT, 2005. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39, 1119–1125. [DOI] [PubMed] [Google Scholar]
- Zhang C, Browne A, Child D, Tanzi RE, 2010a. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem 285, 28472–28480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, Lin SC, 2016. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab 24, 521–522. [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu J, Shi J-S, 2010b. Anti-inflammatory activities of resveratrol in the brain: Role of resveratrol in microglial activation. European journal of pharmacology 636, 1–7. [DOI] [PubMed] [Google Scholar]
- Zhang J, 2006. Resveratrol inhibits insulin responses in a SirT1-independent pathway. The Biochemical journal 397, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lin Y, Dai X, Fang W, Wu X, Chen X, 2019. Metformin treatment improves the spatial memory of aged mice in an APOE genotype-dependent manner. FASEB J 33, 7748–7757. [DOI] [PubMed] [Google Scholar]
- Zhang LF, Yu XL, Ji M, Liu SY, Wu XL, Wang YJ, Liu RT, 2018. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T alpha-synuclein mouse model of Parkinson’s disease. Food & function 9, 6414–6426. [DOI] [PubMed] [Google Scholar]
- Zhang S, Salemi J, Hou H, Zhu Y, Mori T, Giunta B, Obregon D, Tan J, 2010c. Rapamycin promotes beta-amyloid production via ADAM-10 inhibition. Biochem Biophys Res Commun 398, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, Chen S, Yang D, Wang Y, Zhang X, Wang Z, Le W, 2011. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy 7, 412–425. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K, 2014. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci 69, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HF, Li N, Wang Q, Cheng XJ, Li XM, Liu TT, 2015. Resveratrol decreases the insoluble Aβ1– 42 level in hippocampus and protects the integrity of the blood–brain barrier in AD rats. Neuroscience 310, 641–649. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Przedborski S, 2008. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Annals of the New York Academy of Sciences 1147, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE, 2001. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CW, Grossman H, Neugroschl J, Parker S, Burden A, Luo X, Sano M, 2018. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s & dementia (New York, N. Y.) 4, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T, 2004. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, 2011. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]


