Abstract
Deep brain stimulation (DBS) is a promising therapeutic modality for the treatment of drug craving and addiction. To date, the nucleus accumbens has received the most attention as a potential target region for examining the impact of DBS on cocaine seeking in preclinical models. The present study investigated the effects of DBS in brain regions that send major glutamatergic projections to the nucleus accumbens including the basolateral amygdala (BLA) and ventral hippocampus (vHipp) as well as subregions of the medial prefrontal cortex (mPFC) including the anterior cingulate, infralimbic and prelimbic cortices. The current results showed that DBS in the infralimbic cortex, but not the prelimbic or anterior cingulate cortices, selectively attenuated cocaine-primed reinstatement of drug seeking in rats. The present data also demonstrated that DBS of the BLA and vHipp attenuated the reinstatement of both cocaine and sucrose seeking. These results indicate that the infralimbic cortex may be a suitable target for DBS to prevent relapse of cocaine taking.
Keywords: Prefrontal Cortex, Anterior Cingulate, Prelimbic Cortex, Basolateral Amygdala, Ventral Hippocampus, Relapse
1. Introduction
Cocaine use disorder is a serious public health concern both in the United States and worldwide. Despite years of preclinical and clinical research, pharmacological therapies have met with limited success and there remain no FDA-approved pharmacotherapies for cocaine use disorder (Pierce et al., 2012). Recent evidence shows that deep brain stimulation (DBS), an FDA-approved treatment for movement disorders (Lozano and Lipsman, 2013), may be a viable therapeutic option in the treatment of intractable drug addiction (Muller et al., 2013; Pierce and Vassoler, 2013). To date, the majority of these studies have focused primarily on the nucleus accumbens, a limbic structure that plays a critical role in the reinforcing properties of drugs of abuse, including cocaine. Thus, DBS of the nucleus accumbens shell, but not the core subregion, attenuated both drug priming- and cue-induced reinstatement of cocaine seeking, animal models of relapse (Guercio et al., 2015; Vassoler et al., 2008; Vassoler et al., 2013). DBS of the accumbens shell also suppressed locomotor sensitization to cocaine (Creed et al., 2015), another behavioral task that reflects aspects of plasticity related to drug craving (Robinson and Berridge, 2001; Steketee and Kalivas, 2011).
The mechanisms underlying the ability of DBS to attenuate drug seeking remain unclear. The attenuation of cocaine reinstatement by nucleus accumbens shell DBS does not appear to be due to inactivation of the medium spiny neurons as intra-accumbal shell infusion of GABA receptor agonists, baclofen and muscimol, or the local anesthetic lidocaine did not mimic the effects seen with DBS (Vassoler et al., 2013). Instead the mechanisms of action of DBS appear to be quite complex. Electrophysiological experiments, for example, indicated that accumbens DBS antidromically stimulated cortical interneurons, which in turn inhibited the spontaneous activity of cortico-accumbal glutamatergic projection neurons (McCracken and Grace, 2007). Consistent with these findings, DBS of the accumbens shell activated the infralimbic cortex and pharmacological inactivation of this nucleus suppressed cocaine seeking (Vassoler et al., 2013).
The infralimbic cortex in the rat is a subnucleus of the mPFC, which also includes the prelimbic cortex and anterior cingulate, all of which have been implicated in aspects of cocaine-induced behavioral plasticity (Kalivas, 2009; Pierce and Wolf, 2013; Schmidt and Pierce, 2010). The vHipp and BLA also send rich glutamatergic projections to the nucleus accumbens (Friedman et al., 2002; Phillipson and Griffiths, 1985) and contribute to the reinstatement of cocaine seeking (Grimm and See, 2000; Luscher and Malenka, 2011; McFarland and Kalivas, 2001; Schmidt and Pierce, 2010; Sun and Rebec, 2003). In the current study, we assessed the effect of DBS in brain regions that send robust glutamatergic projections to the nucleus accumbens (i.e. anterior cingulate, infralimbic cortex, prelimbic cortex, BLA, and vHipp) on the reinstatement of cocaine seeking.
2. Results
2.1. DBS of the infralimbic cortex attenuates cocaine reinstatement
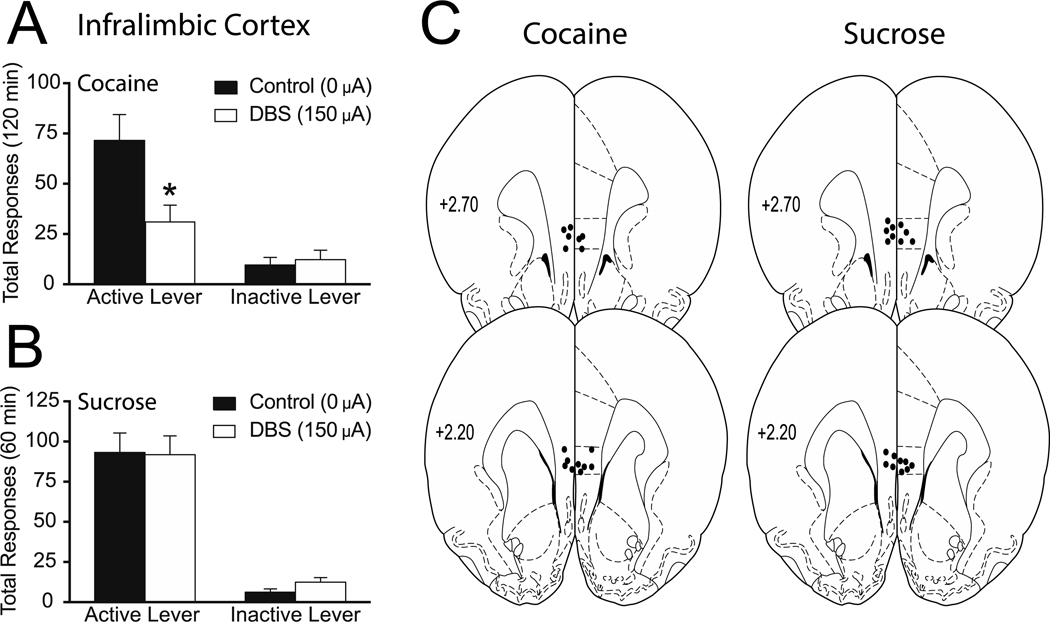
Rats reliably acquired cocaine self-administration; active lever responding in a representative cohort averaged 107.3 (± 10.3), 117.1 (± 10.8), and 109.9 (± 11.6) across the last three self-administration sessions. Responding in the extinction sessions immediately preceding reinstatement sessions was <15% of the level maintained during self-administration and was similar between reinstatement test sessions for all groups. Following cocaine self-administration and extinction, DBS was administered to the infralimbic cortex (0 μA or 150 μA) throughout a 2-hour cocaine-primed reinstatement session. For all experiments, DBS (150 μA) and control (0 μA) sessions were counterbalanced within-subjects and no order effect of stimulation was observed. Total active and inactive lever responding (mean ± SEM) from the reinstatement sessions are presented in Figure 1A. A two-way ANOVA revealed no effect of DBS treatment (F(1,7) = 2.593, p=0.1514), a significant main effect of active/inactive lever (F(1,7) = 37.10, p=0.0005) and a significant interaction between these two variables (F(1,7) = 7.876, p=0.0263). Subsequent pairwise analyses indicated that the total active lever responses between the 0 μA and 150 μA treatment were significantly different (Bonferroni, p<0.05).
Figure 1. Deep brain stimulation of the infralimbic mPFC attenuates cocaine, but not sucrose, reinstatement.
Mean (±SEM) active and inactive lever responses from cocaine (A) or sucrose (B) reinstatement sessions with 0 μA or 150 μA stimulation of the infralimbic cortex. (C) Electrode placements from the infralimbic cortex (dark circles). The values are in millimeters, relative to bregma. *p < 0.05 0 μA compared to 150 μA. There were 8–9 animals per group.
To determine if this effect of DBS was reinforcer-specific (or potentially due to motor impairment), we tested the effect of infralimbic cortex DBS on sucrose reinstatement. Total active and inactive lever responding (mean ± SEM) are presented in Figure 1B. A two-way ANOVA revealed no effect of DBS treatment (F(1,8) = 0.2339, p=0.6416), a significant main effect of active/inactive lever (F(1,8) = 70.74, p<0.0001) and no interaction between these two variables (F(1,8) = 0.4386, p=0.5264). The electrode placements for both cocaine and sucrose experiments are shown in Figure 1C.
2.2. DBS of the prelimbic cortex or anterior cingulate had no influence on cocaine reinstatement
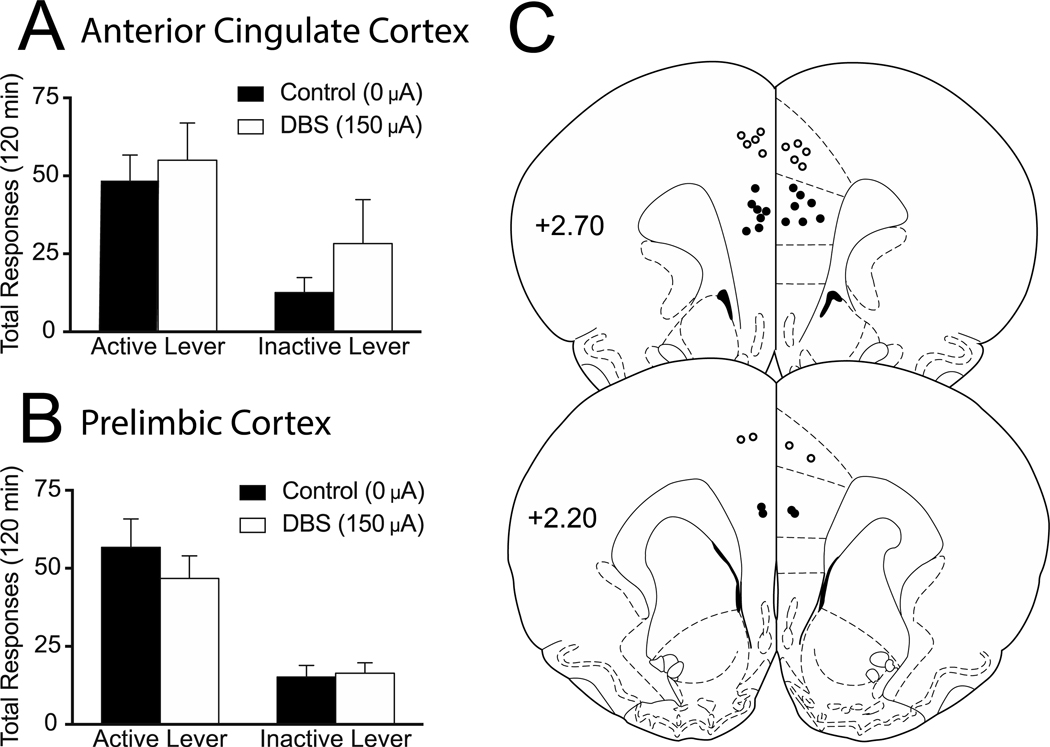
Total active and inactive lever responding (mean ± SEM) from the reinstatement sessions during which DBS was applied to the anterior cingulate or prelimbic cortex are presented in Figures 2A and 2B, respectively. The analysis of the anterior cingulate data (Fig 2B) revealed no effect of DBS treatment (F(1,6) = 1.181, p=0.3189), a significant main effect of active/inactive lever (F(1,6) = 31.87, p=0.0013) and no interaction between these two variables (F(1,6) = 1.523, p=0.2633). The prelimbic cortex analysis (Fig 2B) revealed no effect of DBS treatment (F(1,7) = 1.779, p=0.2240), a significant main effect of lever responding (F(1,7) = 35.09, p=0.0006), and no interaction between these two variables (F(1,7) = 0.7098, p=0.4274). The electrode placements for both regions are shown in Figure 2C.
Figure 2. Deep brain stimulation of the prelimbic or anterior cingulate cortices does not alter cocaine reinstatement.
Mean (±SEM) active and inactive lever responses from cocaine reinstatement sessions with 0 μA or 150 μA stimulation aimed at the anterior cingulate (A) or prelimbic cortex (B). (C) Electrode placements from the anterior cingulate (white filled circles) and prelimbic cortex (black filled circles). The values are in millimeters, relative to bregma. *p < 0.05 0 μA compared to 150 μA. There were 7–8 animals per group.
2.3. Deep brain stimulation of the BLA attenuates the reinstatement of both cocaine and sucrose seeking
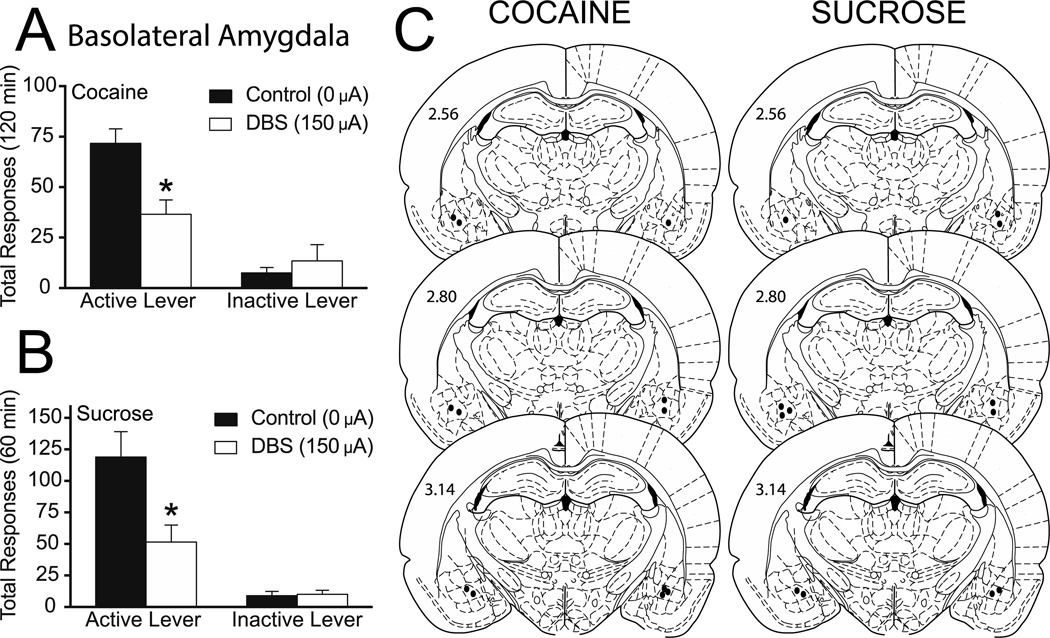
Total active and inactive lever responding (mean ± SEM) from the reinstatement sessions during which DBS was applied to the BLA are presented in Figure 3A. A two-way ANOVA revealed a strong trend of DBS treatment (F(1,6) = 5.171, p=0.0633), a significant main effect of active/inactive lever (F(1,6) = 88.07, p<0.0001) and a significant interaction between these two variables (F(1,6) = 7.76, p=0.0318). Subsequent pairwise analyses indicated that the total active lever responses between the 0 μA and 150 μA treatment were significantly different (Bonferroni, p<0.05).
Figure 3. Deep brain stimulation of the basolateral amygdala attenuates both cocaine and sucrose reinstatement.
Mean (±SEM) active and inactive lever responses from cocaine (A) or sucrose (B) reinstatement sessions with 0 μA or 150 μA stimulation of the BLA. (C) Electrode placements from the BLA (dark circles). The values are in millimeters, relative to bregma. *p < 0.05 0 μA compared to 150 μA. There were 7 animals per group.
To determine if the effects of DBS in the BLA were reinforcer-specific, we tested its effects on sucrose reinstatement. Total active and inactive lever responding (mean ± SEM) from the reinstatement session during which DBS was applied to the BLA are presented in Figure 3B. A two-way ANOVA revealed a significant main effect of DBS treatment (F(1,5) = 10.86, p=0.0216), a significant main effect of active/inactive lever responding (F(1,5) = 28.69, p=0.0030) and a significant interaction between these two variables (F(1,5) = 26.31, p=0.0037). Subsequent pairwise analyses indicated that the total active lever responses between the 0 μA and 150 μA treatment were significantly different (Bonferroni, p<0.05). The electrode placements are shown in Figure 3C.
2.4. Deep brain stimulation of the vHipp attenuates the reinstatement of both cocaine and sucrose seeking
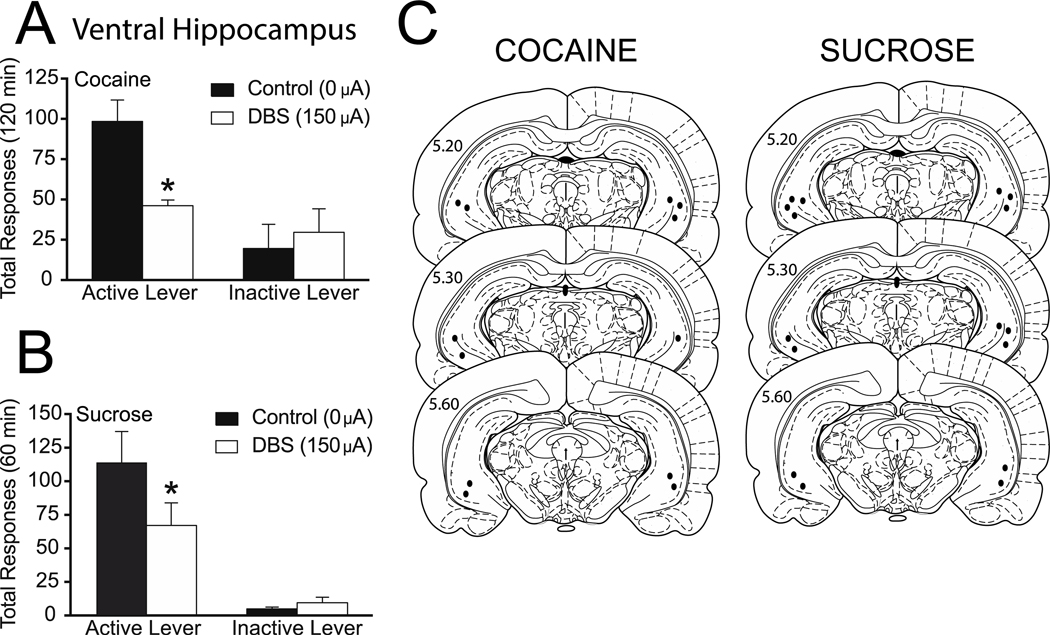
Total active and inactive lever responding (mean ± SEM) during reinstatement sessions when DBS was applied to the vHipp are presented in Figure 4A. A two-way ANOVA revealed a significant effect of DBS treatment (F(1,5) = 9.453, p=0.0276), a significant main effect of active/inactive lever responding (F(1,5) = 8.092, p=0.0360) as well as a significant interaction between these two variables (F(1,5) = 12.83, p=0.0158). Subsequent pairwise analyses indicated that the total active lever responses between the 0 μA and 150 μA treatment were significantly different (Bonferroni, p<0.05).
Figure 4. Deep brain stimulation of the ventral hippocampus attenuates both cocaine and sucrose reinstatement.
Mean (±SEM) active and inactive lever responses from cocaine (A) or sucrose (B) reinstatement sessions with 0 μA or 150 μA stimulation aimed at the vHipp. (C) Electrode placements from the vHipp (dark circles). The values are in millimeters, relative to bregma. *p < 0.05 0 μA compared to 150 μA. There were 6–8 animals per group.
We next assessed the effect of DBS in the vHipp on sucrose reinstatement. Total active and inactive lever responding (mean ± SEM) from the sucrose reinstatement session during which DBS was applied to the vHipp are presented in Figure 4B. A two-way ANOVA revealed no effect of DBS treatment (F(1,7) = 3.428, p=0.1066). There was a significant active/inactive lever effect (F(1,7) = 23.45, p=0.0019) as well as a significant interaction between these two variables (F(1,7) = 7.2, p=0.0314). Subsequent pairwise analyses indicated that the total active lever responses between the 0 μA and 150 μA treatment were significantly different (Bonferroni, p<0.05). The electrode placements are shown in Figure 4C.
3. Discussion
In this study, we examined whether cortical nuclei that send glutamatergic projections to the nucleus accumbens could serve as target regions for DBS in the treatment of cocaine craving and addiction. The present results indicate that high frequency DBS of the infralimbic cortex selectively attenuated the reinstatement of cocaine seeking. Further, we show that this effect is region-specific as DBS in the anterior cingulate or prelimbic cortex had no effect on cocaine reinstatement. DBS of the BLA or the vHipp attenuated the reinstatement of both cocaine and sucrose seeking, illustrating a more generalized disruption of reward seeking. Collectively, these results provide evidence that the infralimbic cortex may be a suitable target for DBS in the treatment of intractable drug addiction.
Our findings add to the complex literature focusing on the role of the mPFC in the reinstatement of cocaine seeking (Moorman et al., 2015). The mPFC subregions have differential glutamatergic projections to the nucleus accumbens. The dorsal mPFC (i.e. anterior cingulate and dorsal prelimbic cortices) projects mainly to the nucleus accumbens core, whereas the ventral mPFC (i.e. ventral prelimbic and infralimbic cortices) projects primarily to the nucleus accumbens shell (Berendse et al., 1992; Ding et al., 2001; Wright and Groenewegen, 1995). In contrast to the present findings, previous work clearly indicates that manipulations of the dorsal mPFC (including the prelimbic cortex) modulate cocaine seeking (Kalivas et al., 2005; Stefanik et al., 2012). High frequency electrical stimulation of the dorsal mPFC also was shown to suppress both cocaine- and sucrose-seeking behaviors (Levy et al., 2007) but, in sharp contrast to the present work, the stimulation was administered repeatedly with drug seeking assessed a day after the last administration (Levy et al., 2007). Although of interest, the mechanisms underlying this effect undoubtedly reflect DBS-induced neuronal plasticity as opposed to the acute effect of this manipulation studied here. It is becoming increasingly clear that the therapeutic effects of DBS are not due to any one single mechanism (McCracken and Grace, 2007; Vassoler et al., 2013). For example, pharmacological inactivation of the anterior cingulate, prelimbic, and infralimbic mPFC by local injection of the GABA agonists baclofen and muscimol similarly attenuated cocaine reinstatement (Vassoler et al., 2013). Although DBS-mediated inactivation may be consistent with pharmacological manipulations in other regions, including the BLA and vHipp, it is likely not the mechanism of DBS which selectively attenuates cocaine reinstatement in the infralimbic, but not prelimbic or anterior cingulate cortices. That said, previous work from our group indicated that DBS activates local neuronal cell bodies (Vassoler et al., 2013). This finding is particularly relevant to the present findings since activation of the infralimbic cortex suppressed cocaine seeking (Peters et al., 2008), although this effect may be due to either a direct influence on the accumbens or more circuit-wide effects on the mesoaccumbens dopamine system (LaLumiere et al., 2012). Although the precise mechanisms underlying the effects of DBS still remain to be illuminated, the current findings add to a growing literature that the infralimbic cortex plays a critical role in cocaine seeking (Kalivas et al., 2005).
The present results also showed that DBS of the BLA attenuated the reinstatement of both cocaine and sucrose seeking. The amygdala can be divided into many subnuclei, several of which play an important role in drug-seeking behavior, particularly cue-induced reinstatement of cocaine seeking (Fuchs et al., 2005; Grimm and See, 2000; Mashhoon et al., 2009; McFarland et al., 2004; Stefanik and Kalivas, 2013). The BLA is also implicated in drug priming-induced reinstatement of cocaine seeking. Thus, lesions of the BLA attenuated cocaine priming-induced reinstatement (Yun and Fields, 2003). Further, antagonism of D1-like and D2-like dopamine receptors in the BLA blocked cocaine priming-induced reinstatement (Alleweireldt et al., 2006; Di Ciano, 2008). In addition to reducing drug-seeking behavior, our results also show that DBS of the BLA also attenuates sucrose reinstatement. This is consistent with data suggesting that the BLA plays a complex role in reward-related behaviors which can depend on behavioral tasks and outcomes (Carelli et al., 2003; Tye et al., 2008; Tye et al., 2010). Specifically, lesions of the BLA blocked conditioned place preference for food (Everitt et al., 1991) as well as impaired approach to a conditioned stimulus predictive of sucrose reinforcement (Burns et al., 1993). However, other studies indicate that inactivation of the BLA has no effect on, or even potentiates reinstatement of food seeking (McLaughlin and Floresco, 2007).
Similar to the BLA, the current findings showed that DBS of the vHipp impaired both cocaine and sucrose reinstatement. The hippocampus can be broadly segregated into dorsal and ventral regions (Moser and Moser, 1998). The dorsal hippocampus is critical for spatial memory (Moser et al., 1995), whereas the ventral hippocampus plays an important role in regulating motivated behaviors (Henke, 1990). The vHipp is the major output region of the hippocampus (Groenewegen et al., 1987) with strong projections to the nucleus accumbens, particularly the shell subregion (Fanselow and Dong, 2010). Consistent with the current results, pharmacological inactivation of the vHipp attenuated cocaine priming-induced reinstatement of drug seeking (Rogers and See, 2007; Sun and Rebec, 2003). Perhaps not surprisingly, modulation of the vHipp can affect other reward-related behaviors. Specifically, stimulation of ghrelin receptors in the vHipp increased ad libitum food taking and operant responding for food reward (Kanoski et al., 2013). Moreover, stimulation of glucagon-like peptide-1 receptors in the vHipp attenuated both ad libitum food taking and operant responding for food reward (Hsu et al., 2015). These findings suggest that disrupting activity in the vHipp can impair non-drug reward processing, in line with the present observation that DBS of the vHipp attenuated sucrose seeking. Collectively, the nonspecific effects of BLA and vHipp DBS on cocaine and sucrose seeking suggest that these nuclei may not be appropriate targets for the treatment of cocaine addiction.
In conclusion, the current findings contribute to the growing literature indicating that DBS may be a viable therapeutic intervention in the treatment of cocaine addiction. It was previously suggested that the mPFC was a potentially effective target for DBS in the treatment of addiction (Luigjes et al., 2012; Wang et al., 2018) and the current findings refined this speculation with data focusing on the infralimbic subregion of the mPFC. The present work highlights the utility of examining in greater detail the circuit-wide influences of DBS in the limbic system in order to optimize DBS as a therapeutic strategy.
4. Experimental Procedures
4.1. Animals and housing:
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY). Rats were individually housed with food and water available ad libitum. A 12/12 hr light/dark cycle was used with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. All experimental procedures were consistent with the ethical guidelines of the US National Institutes of Health and were approved by the Perelman School of Medicine Institutional Animal Care and Use Committee at the University of Pennsylvania.
4.2. Drugs:
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse (Rockville, MD) and dissolved in bacteriostatic 0.9% saline.
4.3. Materials:
All experiments used Med-Associates (East Fairfield, VT) instrumentation enclosed within ventilated, sound attenuating chambers. Each operant conditioning chamber was equipped with response levers, stimulus lights, food pellet dispensers and injection pumps for injecting drugs intravenously.
4.4. Surgery:
Prior to surgery, rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine. For rats used in cocaine self-administration, an indwelling silastic catheter was inserted into the right jugular vein and sutured in place. The catheter was then threaded subcutaneously over the shoulder blade and was routed to a mesh backmount platform (CamCaths, Cambridge, UK/Strategic Applications Inc., Libertyville, Il) that was sutured below the skin between the shoulder blades. The catheters were sealed with plastic obturators when not in use. Following catheter implantation (for cocaine self-administration), rats were mounted in a stereotaxic apparatus (Kopf Instruments, CA); this was the first surgical step for rats predetermined for sucrose self-administration experiments. Bipolar stainless steel electrodes (Plastics One, Roanoke, VA) were trimmed and implanted into to the basolateral amygdala, ventral hippocampus, infralimbic medial prefrontal cortex, prelimbic medial prefrontal cortex, or anterior cingulate prefrontal cortex according to the following coordinates, relative to bregma (Paxinos and Watson, 1997): basolateral amygdala: −2.8 mm anteroposterior (A/P), ±5.0 mm mediolateral (M/L), −8.5 mm dorsoventral (D/V); ventral hippocampus: −5.5 mm A/P, ±5.0 mm M/L, −6.5 mm D/V; infralimbic prefrontal cortex: +2.5 mm A/P, ±2.0 mm M/L, −5.39 mm D/V, 21.78° angle; prelimbic prefrontal cortex: +2.5 mm A/P, ±2.0 mm M/L, −4.2 mm D/V, 19.5° angle; anterior cingulate prefrontal cortex: +2.5 mm A/P, ±2.0 mm M/L, −3.0 mm D/V, 27.8° angle. Electrodes were cemented in place by affixing dental acrylic to three stainless steel screws fastened to the skull. Rats recovered for seven days; catheters were flushed daily with 0.3 ml of an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline during the recovery period and after each daily self-administration session.
4.5. Cocaine self-administration, extinction, and reinstatement.
Following the recovery period, rats were placed in operant conditioning chambers and allowed to press a lever for intravenous cocaine infusions (0.254 mg of cocaine in 59 μL of saline, infused over 5 s). Rats initially were trained using a fixed ratio 1 (FR1) schedule of reinforcement. When the animals achieved stable responding with the FR1 schedule (i.e., <15% variation in total presses over 3 consecutive days), they were switched to an FR5 schedule. A 20 s timeout period during which active, drug-paired lever responses had no scheduled consequences followed each cocaine infusion. Each operant chamber was also equipped with an inactive lever. Responses made on the inactive lever had no scheduled consequences. The maximum number of cocaine infusions was capped at 30 per daily 2 h self-administration session. After 21–24 d of daily cocaine self-administration sessions (encompassing both FR1 and FR5 phases), rats underwent an extinction phase during which cocaine was replaced with 0.9% bacteriostatic saline. Daily 2 h extinction sessions were conducted until active lever responding was <15% of the responses averaged over the last 3 days of cocaine self-administration. Reinstatement of cocaine seeking was promoted by non-contingent administration of cocaine (10 mg/kg, i.p.) immediately prior to the initiation of the reinstatement session. Each reinstatement test day was followed by extinction sessions (typically only one or two) until responding was again <15% of the responses achieved during self-administration.
4.6. Sucrose self-administration, extinction, and reinstatement.
Following recovery from surgery, a separate group of rats was allowed to self-administer 45 mg Noyes sucrose pellets (Research Diets; New Brunswick, NJ) using the same equipment and procedures described above. After 21–27 d of daily 1 h sucrose-reinforced operant sessions, rats underwent an extinction phase during which active lever responding no longer resulted in sucrose delivery. Daily extinction sessions were continued until active lever responding was <15% of the responses averaged over the last 3 days of sucrose self-administration. Reinstatement of sucrose seeking was promoted by non-contingent administration of one sucrose pellet every 2 min during the first 10 min of the reinstatement test session. Each reinstatement test day was followed by extinction sessions (typically only one or two) until responding was again <15% of the responses achieved during self-administration.
4.7. Deep brain stimulation.
In most clinical and preclinical DBS experiments, many parameters are fixed and uniform across studies. We used alternating current with biphasic symmetrical pulses (60 μs pulse width and a 160 Hz frequency), parameters that are consistent with previous work from our lab and others (Chang et al., 2003; Mayberg et al., 2005; Vassoler et al., 2013). Stimulation intensities, in contrast, are often varied within and between studies, usually in the range of 50–200 μA (Benazzouz and Hallett, 2000; Chang et al., 2003; Mayberg et al., 2005). We previously reported that 150 μA of current is an effective stimulation intensity in our reinstatement paradigm (Vassoler et al., 2008). Concurrent with the start of a cocaine or sucrose reinstatement session, 0 or 150 μA current was delivered continuously to the bipolar electrodes. The stimulation continued for the duration of the reinstatement session, consistent with our previous studies (Guercio et al., 2015; Vassoler et al., 2008; Vassoler et al., 2013) and mirroring chronic stimulation typical for clinical DBS. Throughout the 0 μA condition, the stimulation tethers were attached in the exact same manner as the 150 μA condition but with no current delivered. The 0 μA and 150 μA currents were administered in a within-subjects counterbalanced fashion across two reinstatement test days with intervening extinction sessions.
4.8. Verification of electrode placements.
After the completion of all experiments, rats were given an overdose of pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline followed by 10% formalin. The brains were removed and coronal sections (100 μm) were collected after sectioning with a vibratome (Technical Products International; St. Louis, MO). Animals with electrode placements outside of the areas of interest, or with excessive mechanical damage, were excluded from subsequent data analysis.
4.9. Statistics.
Statistical analysis was performed in Prism 7.0 with alpha set at p<0.05. All reinstatement experiments were analyzed with two-way ANOVAs with the repeated measures and within-subjects factor of DBS treatment during reinstatement sessions (0 μA or 150 μA) and within-subjects factor of lever (active or inactive). Pairwise analyses were made with Bonferroni post-tests (p < 0.05).
Deep brain stimulation of the infralimbic cortex attenuated the reinstatement of cocaine seeking, an animal model of relapse.
This effect was relatively selective as deep brain stimulation of the prelimbic cortex and anterior cingulate had no effect on cocaine seeking.
Deep brain stimulation of the ventral hippocampus and basolateral amygdala blocked cocaine seeking but also attenuated sucrose seeking.
Acknowledgments:
This work was supported by the following grants from the National Institutes of Health: R01 DA15214 (RCP); K01 DA030445 and R01 DA037897 (HDS); T32 DA028874 (LAG, SESJ); F31 DA037748 (LAG); K01 DA39308 (MEW).
Abbreviations:
- BLA
basolateral amygdala
- DBS
deep brain stimulation
- mPFC
medial prefrontal cortex
- vHipp
ventral hippocampus
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL, 2006. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 31, 363–74. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Hallett M, 2000. Mechanism of action of deep brain stimulation. Neurology. 55, S13–6. [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ, 1992. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 316, 314–47. [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ, 1993. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 55, 167–83. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA, 2003. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci. 23, 8204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Shi LH, Luo F, Woodward DJ, 2003. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 983, 174–84. [DOI] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Luscher C, 2015. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 347, 659–64. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, 2008. Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci. 122, 129–39. [DOI] [PubMed] [Google Scholar]
- Ding DC, Gabbott PL, Totterdell S, 2001. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res. 917, 81–9. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW, 1991. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 42, 1–18. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC, 2002. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol. 450, 345–65. [DOI] [PubMed] [Google Scholar]
- Fuchs RA., Evans KA., Ledford CC., Parker MP., Case JM., Mehta RH., See RE., 2005. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 30, 296–309. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE, 2000. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 22, 473–9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP, 1987. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 23, 103–20. [DOI] [PubMed] [Google Scholar]
- Guercio LA, Schmidt HD, Pierce RC, 2015. Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of both cocaine and sucrose seeking in rats. Behav Brain Res. 281, 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke PG, 1990. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull. 25, 691–5. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE, 2015. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 40, 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J, 2005. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 45, 647–50. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, 2009. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 10, 561–72. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Ricks KM, Grill HJ, 2013. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 73, 915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW, 2012. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 35, 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A, 2007. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 27, 14179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N, 2013. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 77, 406–24. [DOI] [PubMed] [Google Scholar]
- Luigjes J., van den Brink W., Feenstra M., van den Munckhof P., Schuurman PR., Schippers R., Mazaheri A., De Vries TJ., Denys D., 2012. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 17, 572–83. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC, 2011. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 69, 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Tsikitas LA, Kantak KM, 2009. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 29, 1641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH, 2005. Deep brain stimulation for treatment-resistant depression. Neuron. 45, 651–60. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA, 2007. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 27, 12601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW, 2001. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 21, 8655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW, 2004. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 24, 1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Floresco SB, 2007. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience. 146, 1484–94. [DOI] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G, 2015. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 1628, 130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG, 1995. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 92, 9697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, 1998. Functional differentiation in the hippocampus. Hippocampus. 8, 608–19. [DOI] [PubMed] [Google Scholar]
- Muller UJ, Voges J, Steiner J, Galazky I, Heinze HJ, Moller M, Pisapia J, Halpern C, Caplan A, Bogerts B, Kuhn J, 2013. Deep brain stimulation of the nucleus accumbens for the treatment of addiction. Ann N Y Acad Sci. 1282, 119–28. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW, 2008. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 28, 6046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT., Griffiths AC., 1985. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 16, 275–96. [DOI] [PubMed] [Google Scholar]
- Pierce RC, O’Brien CP, Kenny PJ, Vanderschuren LJ, 2012. Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harb Perspect Med. 2, a012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Vassoler FM, 2013. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology (Berl). 229, 487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME, 2013. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. In Addiction. Vol., Pierce RC, Kenny PJ, ed.êds. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp. 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC, 2001. Incentive-sensitization and addiction. Addiction. 96, 103–14. [DOI] [PubMed] [Google Scholar]
- Rogers JL, See RE, 2007. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 87, 688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC, 2010. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 1187, 35–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT, 2012. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW, 2013. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 7, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW, 2011. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 63, 348–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV, 2003. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 23, 10258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH, 2008. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 453, 1253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH, 2010. Amygdala neural encoding of the absence of reward during extinction. J Neurosci. 30, 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM., Schmidt HD., Gerard ME., Famous KR., Ciraulo DA., Kornetsky C., Knapp CM., Pierce RC., 2008. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 28, 8735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, White SL, Hopkins TJ, Guercio LA, Espallergues J, Berton O, Schmidt HD, Pierce RC, 2013. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J Neurosci. 33, 14446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TR, Moosa S, Dallapiazza RF, Elias WJ, Lynch WJ, 2018. Deep brain stimulation for the treatment of drug addiction. Neurosurg Focus. 45, E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ, 1995. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 361, 383–403. [DOI] [PubMed] [Google Scholar]
- Yun IA, Fields HL, 2003. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 121, 747–57. [DOI] [PubMed] [Google Scholar]