Abstract
ETS transcription factors play an important role in the specification and differentiation of endothelial cells during vascular development. Despite previous studies, the role of the founding member of the ETS family, Ets1, in vascular development in vivo is only partially understood. Here, we generated a zebrafish ets1 mutant by TALEN genome editing and tested functional redundancy between Ets1 and a related ETS factor Etv2 / Etsrp / ER71. While zebrafish ets1−/− mutants have a normal functional vascular system, etv2−/−;ets1−/ embryos had more severe angiogenic defects and lower expression levels of kdr and kdrl, the two zebrafish homologs of the mammalian Vascular Endothelial Growth Factor Receptor 2 VEGFR2/Flk1, than etv2−/−embryos. Expression of constitutively active Mitogen-Activated Protein Kinase1 (MAP2K1) within endothelial cells partially rescued this angiogenic defect. Interestingly, ets1−/− embryos displayed extensive apoptosis within the trunk vasculature despite exhibiting normal vascular patterning. Loss of Ets1 combined with a partial knockdown of Etv2 function resulted in a decrease in endothelial cell numbers in the axial vasculature, which argues for a role of Ets1 in promoting vasculogenesis. We also demonstrate that although both Ets1 and Etv2 can induce ectopic vascular marker expression in zebrafish embryos, Ets1 activity is dependent on MAPK-mediated phosphorylation of its Thr30 and Ser33 residues, while Etv2 activity is not. Together, our results identify a novel function of Ets1 in regulating endothelial cell survival during vasculogenesis in vivo. Based on these findings, we propose a revised model of how Ets1 and Etv2 play unique and partially redundant roles to promote vascular development.
Keywords: Vasculogenesis, Angiogenesis, ETS transcription factors, Zebrafish, Vascular development
Introduction
Embryonic vascular development is characterized by two distinct phases known as vasculogenesis and angiogenesis. Vasculogenesis is defined as the de novo differentiation and assembly of the primary axial vessels from endothelial progenitor cells (EPCs), which arise in the lateral plate mesoderm (LPM) (Risau and Flamme, 1995; Zhong, 2005). In zebrafish embryos, EPCs are specified at early stages of gastrulation and migrate towards the midline in two waves to form the dorsal aorta (DA) and posterior cardinal vein (PCV)(Fouquet et al., 1997; Jin et al., 2005; Kohli et al., 2013). The subsequent coordinated sprouting, growth and branching of the initial primary vessels constitutes angiogenesis (Semenza, 2007). The morphogenetic plasticity of blood vessels is essential for normal physiological processes like wound healing and the oestrous cycle, but is also implicated in several diseases, including cancer, diabetic retinopathy and other vascular malformations (Carmeliet, 2005; Ferrara and Kerbel, 2005; Folkman, 1995).
Much progress has been made in elucidating the molecular mechanisms that govern the formation of an intricately patterned vascular system. In particular, the zebrafish has become a popular model organism to study vascular development owing to its high genetic amenability, rapid external development and optical transparency of its embryos which permits easy visualization of developmental events. High conservation of signaling pathways and gene function across vertebrates has allowed the extrapolation of findings in zebrafish to other higher vertebrate species like mice and even humans. Among some of these key findings, several transcription factors in the E-26 Transformation-Specific (ETS) family have emerged as key regulators of embryonic vascular development. Of the known ETS factors expressed in endothelial cells, Etv2/ER71/Etsrp is required for the formation of a functional vascular system and is, thus, considered a master-regulator of vascular development (Craig and Sumanas, 2016; Pham et al., 2007; Sumanas and Lin, 2006). Loss of Etv2 function in both zebrafish and mice results in severe vascular defects including reduced endothelial cell differentiation and endothelial gene expression, mis-patterned and non-lumenized intersegmental vessels and an absence of a circulation, leading to embryonic lethality (Craig et al., 2015; Ferdous et al., 2009; Lee et al., 2008; Pham et al., 2007; Sumanas and Lin, 2006). Being one of the earliest markers of endothelial progenitors, Etv2 plays a critical role in the initiation of transcriptional networks that drive the differentiation of endothelial progenitors into mature endothelial cells (De Val et al., 2008).
The expression of multiple ETS factors (with highly similar DNA-binding ‘ETS’ domains) within endothelial cells poses a question of the specific roles and functions adopted by these related proteins in mediating vascular development. While etv2−/− zebrafish embryos have severe vascular defects during early stages of development, there is partial recovery of the vasculature over time, suggesting that other factors may partially compensate for the loss of Etv2 function. We have shown in a previous study that a related ETS factor fli1b functions together with etv2 to drive vasculogenesis and angiogenesis (Craig et al., 2015). However, the extent of redundancy between other ETS factors in mediating vascular development is still poorly understood.
The founding member of the ETS family, Ets1, has been studied in several physiological processes including vascular development, immune cell function and tumor progression. While its function in the development of the immune system, specifically its involvement in T and B cell differentiation, has been well characterized (Bories et al., 1995; Muthusamy et al., 1995), its precise function in vascular development in vivo remains unclear. Previous in vitro studies have implicated Ets1 in mediating angiogenesis and apoptosis of endothelial cells (Chen et al., 1997; Sato et al., 2001; Teruyama et al., 2001; Wernert et al., 1999). Similarly, ets1 morpholino knockdown (morphant) zebrafish embryos were reported to have angiogenic defects (Pham et al., 2007). However, a previously reported zebrafish ets1um206 mutant displayed normal vascular patterning (Kok et al., 2015) and Ets1−/− mutant mice did not display any apparent vascular abnormalities (despite exhibiting immune defects) (Wei et al., 2009). In contrast, ets1−/−;ets2−/− mice displayed angiogenic defects and increased apoptosis of endothelial cells, demonstrating functional redundancy between Ets1 and Ets2 (Wei et al., 2009). Thus, the lack of a vascular phenotype in both Ets1−/− mice and zebrafish could be explained by the redundancy of Ets1 with other ETS factors expressed in endothelial cells. We hypothesized that Ets1 may play yet additional roles in vascular development which have not been previously recognized due to the redundancy between different ETS transcription factors. In the current study, we investigated potential redundancy between Ets1 and Etv2 during vascular development in vivo in zebrafish embryos.
Our results demonstrate that while ets1−/− fish have normal vasculature, loss of Ets1 function in etv2−/− embryos results in impairment of the partial recovery observed in etv2−/−embryos. We further demonstrate that ets1−/−; etv2−/−embryos have lower expression of the zebrafish Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) homologs kdr and kdrl and that an increase in downstream Mitogen-activated Protein Kinase (MAPK)/Extracellular signal-regulated kinase (ERK) signaling can partially compensate for the loss of Ets1 function. In contrast to the Etv2 protein, Ets1 contains an N-terminal pointed (PNT) domain which mediates phosphorylation of Thr30 and Ser33 sites by acting as a docking site for ERK2 (Hollenhorst et al., 2011; Seidel and Graves, 2002). We show that the transcriptional activity of Ets1 in inducing endothelial gene expression is dependent on the phosphorylation of these residues by the MAPK/ERK pathway. Together with the results of previous studies on related ETS factors fli1b and etv2, it is evident that different ETS factors, despite having overlapping functions, exhibit contrasting levels of activity and contribute to vascular development to varying degrees.
Materials and methods
2.1. Zebrafish strains and staining
The following zebrafish lines were used for experiments in this study unless otherwise noted: Tg(kdrl:EGFP)s843 (Jin et al., 2005), Tg(fli1a:EGFP)y1 (Lawson and Weinstein, 2002), Tg(kdrl:nls-mCherry) (Verma et al., 2010), etv2y11 (Pham et al., 2007). An ets1ci14 homozygous mutant (hereafter referred to as ets1−/−) containing a 5 bp deletion -TCTGG- at the intron1-exon2 boundary was generated in the Tg(kdrl:GFP) line using TALEN technology (Sander et al., 2011). TAL3070 (Addgene plasmid #41244; http://n2t.net/addgene:41244; RRID:Addgene_41244) and TAL3071(Addgene plasmid #41245; http://n2t.net/addgene:41245; RRID:Addgene_41245) were gifts from Keith Joung. The ets1−/−;Tg(kdrl:GFP) line was crossed into the Tg(kdrl:nls-mCherry) line to generate an ets1−/−;Tg(kdrl:GFP);Tg(kdrl:nls-mCherry) line which was used for cell counts. Embryonic staging was performed according to established criteria (Kimmel et al., 1995). Developmental delay in morphant or chemically treated embryos was accounted for by allowing embryos to develop until morphological staging criteria matched that of control embryos.
2.2. Morpholinos
A previously-validated etv2 morpholino – etv2 MO2 (5’- CACTGAGTCCTTATTTCACTATATC; Gene Tools, Inc.) (Sumanas and Lin, 2006) was injected into embryos at the 1–2 cell stage. For high dose injections (equivalent to the etv2−/− phenotype), 5 ng of etv2 MO2 was injected into each embryo. For low dose injections, either 0.125 ng or 0.25 ng was injected.
2.3. Chemical treatments
For SL327 experiments, embryos were treated with either 1% DMSO or 15 μM SL327 (Selleckchem #S1066) dissolved in DMSO. 25–50 embryos were transferred to a single well of a 6-well cell culture plate and either DMSO or SL327 was added to the well. The plate was placed on a rocking nutator in a temperature-regulated incubator. Treatment times varied based on the experiment and are further described in the Results section. To stop treatment, embryos were washed twice with embryo water for 5 minutes on the nutator and then left in embryo water until the required stage for analysis.
2.4. Real time quantitative PCR
Pools of 10–20 embryos were frozen on dry ice at the required developmental stage. Embryos were homogenized in Trizol (Invitrogen #15596026) using a 23-gauge needle, and extraction of RNA was carried out using the Zymo Direct-zol RNA Miniprep kit (Zymo #R2051). The purified RNA was quantified using the Nanodrop Spectrophotometer® (Thermofisher). cDNA synthesis was performed using the SuperScript® VILO cDNA Synthesis Kit (Invitrogen). Quantitative real-time PCR (qRT-PCR) was carried out using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems) in a StepOnePlus™ Real-Time PCR System. Reactions were performed in at least technical triplicates and the results represent at least biological duplicates. Fold changes were calculated either using the 2−ΔΔCt or relative standard curve method, and ef1α was used as a control. Primer sequences are listed below:
ef1α: F- 5’-TCACCCTGGGAGTGAAACAGC , R- 5’-ACTTGCAGGCGATGTGAGCAG
cdh5: F- 5’-GGTGCCTCCGACAAGGATGA , R - 5’-AACACTCTTTTGCTCTGGCGT
ets1: F - 5’-TGTGGGTCGCATTAGCAGAG , R- 5’-AGTCCCGTCCTATGACAGCT
etv2: F- 5’-GAGCTGTTGCACAAAGGTCA , R- 5’-CAGAGAGGGACGAGGTTCTG
fli1a: F-5’-GGCTCTCCAACAGTGGTCTC , R - 5’-TTGAACTCTCCGTTGGTTC
kdrl: F- 5’-CCATCATCCATTTGTGGAGG , R - 5’-GAGGATGAGGGTGTCACCGAC
kdr: F- 5’-TGATCGACCTTTAGACAGGC , R- 5’-ATCTCTCCGTTTGTCAGCGG
2.5. Overexpression of ets1, etv2 and actMAP2K1
Two methods were employed for overexpression of ets1 and etv2. For mRNA overexpression, the ets1 coding sequence (cds) was PCR-amplified from an ets1 probe template and ligated into a pT3TS vector (Hyatt and Ekker, 1998) between BglII and SpeI sites (ThermoFisher). The sequences of all constructs were verified by sequencing at the DNA Core at Cincinnati Children’s Hospital. In vitro transcription was performed using the mMessage T3 kit (Ambion) following linearization of the T3TS vector using BamHI (ThermoFisher). Purification of RNA was performed using an RNA Clean and Concentrator kit (Zymo Research). Approximately 50 pg of either ets1 or etv2 mRNA was injected into the yolk of embryos at the 1–2 cell stage. Since the injection of ets1 mRNA did not have any effect on embryonic vasculature, we cloned the ets1 cds into the pXeX vector, which contains a ubiquitous Xenopus ef1α promoter driving expression of the downstream gene in all cells (Johnson and Krieg, 1994). Using the pT3TS-ets1 plasmid as a template, the ets1 cds was PCR-amplified and ligated into the pXeX vector between the BamH1 and EcoRV sites (ThermoFisher). Sequencing was performed to verify the sequence of the pXeX:ets1 construct. Approximately 50 pg of either the XeX:ets1 or a previously generated XeX:etv2 construct (Sumanas et al., 2008) was injected into zebrafish embryos at the 1-cell stage.
For expression of constitutively active MAPK in endothelial cells, 25 pg of pTol2-fli1ep:mCherryactMAP2K1 construct + 20 pg of tol2 mRNA (Covassin et al., 2009) was injected into embryos at the one cell stage. Note: the same construct is also referred to as pTol-fli1epcherryactMEKK (Wythe et al., 2013).
2.6. Site-directed mutagenesis
To remove critical phosphorylation sites in Ets1 (which have been shown to be required for Ets1 function), T30A and S33A mutations were introduced using the QuikChange II Site-Directed Mutagenesis Kit (Agilent #200523). The following nucleotide substitutions were introduced:
Thr30: ACT →Ala30: GCT
Ser33: AGT →Ala33: GCT
The following primers containing the specific mutations were designed. Amplification was performed according to the manufacturer’s protocol.
XeX_Ets1_pMut_F: GCTGATGTTCCTCTTCTGGCTCCGGGAGCTAAGGAAATGATGTC
XeX_Ets1_pMut_R: GACATCATTTCCTTAGCTCCCGGAGCCAGAAGAGGAACATCAGC
2.7. Imaging and image analysis
For images captured with the AxioImager Z1 (Zeiss) compound microscope, whole embryos were mounted in 3% methylcellulose on glass slides. Z-stack images were obtained either with the 5× or 10× objective, using the ICC3 fluorescent camera. The Extended Focus module in the Axiovision 4.9 software was used to create maximum intensity projections from z-stacks. Measurements of fluorescent intensity in the axial vasculature of the trunk region was performed using the open source image editing software ImageJ.
For confocal imaging, embryos were mounted in 0.6% low-melting agarose and imaged using 4×, 10× or 20× objectives on either a Nikon A1 Inverted microscope or Nikon A1R LUN-V Inverted microscope at the CCHMC Confocal Imaging Core. For a given experiment, all embryo images were obtained using the same objective and zoom settings. Nikon Elements AR (5.20.0) Denoise.ai algorithm was used to remove noise from images with high background signal. Cell counts in the Tg(kdrl:nls-mCherry; kdrl:GFP) embryos and cleaved Caspase3 immuno-stained embryos were performed using Imaris 9.3 software (Oxford instruments). In order to define a size for the cells, the diameters of 5 cells of varying size were measured and an average size was calculated. The ‘Spots’ function was used to perform an automated count of the number of cells within the trunk axial vessels (defined using a fixed Region of Interest to exclude the Inter segmental vessels). Following the automated count, the 3D rendering was analyzed in detail to ensure that only true nuclear mCherry signals were detected. Cells that were not detected by the software due to a weaker signal were manually marked and rare signals which were falsely detected as cells were unselected.
2.8. Apoptosis assay
Embryos were fixed at the required time point in 4% PFA at 4 °C overnight. Following fixation, embryos were washed four times with PBST (PBS + 0.1% Tween-20) at room temperature and then blocked in 10% lamb serum for two hours at room temperature. Detection of cleaved Caspase3 was performed with purified rabbit anti-active Caspase3 antibody (1:100, Becton Dickinson catalog #559565) and goat anti-rabbit Alexa 594 (1:1000, Thermofisher catalog# A-11037). Vascular GFP was detected using a chicken anti-GFP antibody (1:500, Abcam catalog#13970) and goat anti-chicken Alexa 488 (1:1000, Thermofisher catalog# A-11039).
2.9. FACS analysis
Embryos (in a kdrl:GFP background) were collected at the desired timepoint and dissociated into a single cell suspension as previously described (Manoli and Driever, 2012). Wild-type GFP-negative embryos were used to gate GFP fluorescence. A ratio of the number of GFP+ single cells to the total number of cells was calculated for all samples. Analysis was performed on the MoFlo XDP Cell Sorter (Beckman Coulter) at the CCHMC FlowCore.
2.10. Statistical Analysis
All experiments were repeated at least twice, and the data obtained represents at least 2 biological replicates. For quantitative analysis of ISV number and cell counts, files were blinded before analysis. All graphs were plotted and statistical tests were performed in Graphpad Prism 8.0.2. Normality tests were performed to determine the distribution of the data: if the data were normally distributed, a Student’s t-test was performed in pair-wise manner to determine statistical significance between groups. If the data did not exhibit normal distribution, a Mann Whitney-U test was performed to test for statistical significance. A p-value of <0.05 was used as a cut-off.
Results
1. Ets1 functions redundantly with Etv2 to promote vascular development
To investigate the role of Ets1 in vascular development, an ets1ci14 mutant was generated using TALEN technology. Following the TALEN mRNA injection into Tg(kdrl:GFP) embryos, a mutant was isolated which had a 5 bp deletion in exon 2 of the ets1 gene, resulting in a premature stop codon (Suppl. Fig. S1A–C). The homozygous mutant is predicted to be a null as the truncated protein product would lack the DNA-binding ETS domain present at the C terminus of the protein. qPCR analysis at the 15-somite stage (16.5 hpf) and 24 hpf revealed no change in the expression levels of ets1 in the ets1ci14embryos (Suppl. Fig. S1D). This was confirmed by in situ hybridization, which also showed no qualitative change in the expression of ets1 in the ets1ci14 embryos (Suppl. Fig. S1E,F). Similar to the previously published ets1um206 mutant (Kok et al., 2015), ets1ci14;Tg(kdrl:GFP) mutant embryos (hereafter referred to as ets1−/− mutants) were morphologically normal and did not exhibit any observable vascular defects (Fig. 1A–B’). ets1−/− mutant adults were viable and also did not exhibit any apparent phenotype (data not shown). We then hypothesized that Ets1 may function redundantly with other ETS factors expressed in endothelial cells to promote vascular development. We decided to investigate potential functional redundancy between Ets1 and the ETS factor Etv2, which is one of the earliest markers of vascular endothelial progenitors and is considered to be a master-regulator of vasculogenesis (Craig et al., 2015; Craig and Sumanas, 2016; Pham et al., 2007; Sumanas and Lin, 2006). To this end, we generated an ets1−/−;etv2+/− mutant by crossing the ets1−/− mutant into the previously generated etv2y11 line (Pham et al., 2007). We and others have previously shown that the loss of Etv2 function results in severe vascular defects during development, resulting in embryonic lethality both in zebrafish and mice (Craig et al., 2015; Lee et al., 2008; Pham et al., 2007; Sumanas and Lin, 2006). Although the ets1−/−;etv2+/− embryos did not exhibit any developmental defects (data not shown), ets1−/−etv2−/− embryos displayed severe vascular defects (Fig. 1D–D’, H-H’), resulting in embryonic lethality by approximately five days post-fertilization. To determine whether Ets1 functions redundantly with Etv2, we compared the vascular phenotype of the ets1−/−;etv2−/− embryos to the etv2−/− embryos. While the etv2−/− embryos are known to have extremely severe vascular defects at early time points, over time, there appears to be a partial recovery of vascular patterning, with stunted and misguided ISVs beginning to emerge at around 32 hpf (Craig et al., 2015). Indeed, the double mutant ets1−/−;etv2−/− embryos had more severe angiogenic defects than the single mutant etv2−/−embryos (Fig. 1, Suppl. Fig. S2). The ets1−/−;etv2−/− embryos had a significantly lower number of ISVs, as well as a decrease in the number of full sprouts (Fig. 1I,J). These results suggest that Ets1 functions partially redundantly with Etv2 to promote vascular development.
Figure 1. Loss of Ets1 function exacerbates vascular defects in etv2−/−embryos.
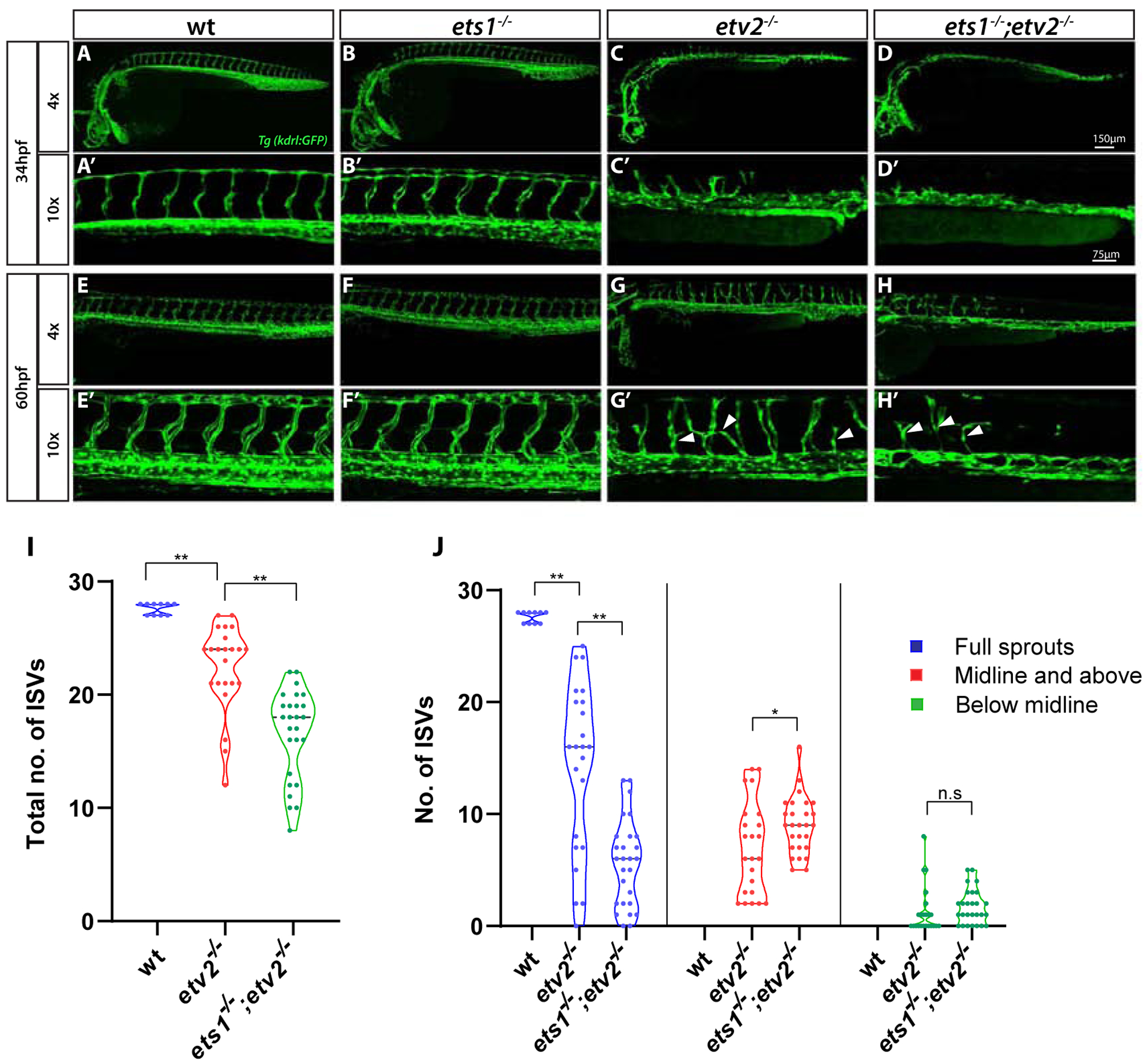
(A-H’) Confocal images of Tg(kdrl:GFP) expression in wt, ets1−/−, etv2−/−and ets1−/−; etv2−/−embryos at 34 hpf and 60 hpf using 4× and 10× objectives. ets1−/−embryos display normal vascular patterning (B-B’, F-F’), similar to wild type embryos (A-A’,E-E’). ISVs begin to emerge in etv2−/−embryos at ~34 hpf (C’) but are absent in ets1−/−; etv2−/− double mutant embryos (D’). At 60 hpf, ets1−/−; etv2−/−embryos have fewer ISVs and less recovery in axial vasculature than etv2−/− embryos (G-H’). (I-J) Quantitative analysis of total number of ISVs and ISV height at 60 hpf. Note the reduction in number of ISVs (I) and full sprouts (J) in ets1−/−; etv2−/−compared to etv2−/− embryos. *P<0.05, ** P <0.01; n.s – not significant. Horizontal bars within violin plots represent median. Arrowheads indicate mis-patterned and stunted ISVs.
2. Ets1 promotes vascular development through transcriptional regulation of VEGFR2 homologs
Members of the ETS family of transcription factors contain a common DNA binding ETS domain at the C-terminus of the protein which binds a consensus GGA(A/T) sequence, often resulting in functional redundancy between them (Garrett-Sinha, 2013; Hollenhorst et al., 2011). To test if Ets1 and Etv2 shared similar transcriptional targets, we performed qPCR for specific endothelial genes which are known to be regulated by Etv2 activity, in etv2−/− and ets1−/−;etv2−/− e1mbryos (Fig. 2A). There was no difference in the expression levels of pan-endothelial marker cdh5 between etv2−/− and ets1−/−;etv2−/− embryos at 32 hpf. However, transcript levels of VEGFR2 homologs kdrl and kdr were significantly lower in the ets1−/−;etv2−/− embryos compared to the etv2−/− embryos, suggesting that Ets1 is involved in the regulation of kdrl and kdr expression. This is consistent with previous studies that have demonstrated Ets1 to be a direct regulator of murine Vegfr2/Flk1 expression (Elvert et al., 2003; Kappel et al., 2000). The loss of flk1/kdrl function is known to lead to defects in angiogenesis in zebrafish embryos (Covassin et al., 2009; Shin et al., 2016). In endothelial cells, the MAPK/ERK pathway is activated downstream of VEGF signaling, leading to multiple physiological changes in cells, including their proliferation and migration (Koch and Claesson-Welsh, 2012). We tested if the activation of MAPK signaling would rescue the angiogenic defects observed in the ets1−/−;etv2−/− embryos. To achieve this, we injected a low dose (0.25 ng) of a previously validated etv2 morpholino (etv2 MO) (Sumanas and Lin, 2006) into wild-type and ets1−/− embryos and analyzed the sprouting phenotype at 28 hpf (Fig. 2B–E,G). It was evident that the loss of Ets1 function sensitized the embryos to the effect of the low dose of morpholino, resulting in a reduction in the number of ISVs in the etv2 MO; ets1−/− embryos. To determine if the overexpression of a constitutively active MAP2K1 in endothelial cells would ameliorate the severity of this sprouting defect, we injected a fli1ep:mCherry-actMAP2K1 construct together with the etv2 MO (0.25 ng) into ets1−/− embryos. Quantitative analysis of the phenotype revealed a statistically significant increase in the total number of ISVs in the ets1−/−; etv2 MO; fli1aep:mCh-actMAP2K1 embryos compared to the ets1−/−; etv2 MO embryos which did not contain the constitutively active MAP2K1 construct (Fig. 2G). Thus, the overexpression of constitutively active MAP2K1 was able to compensate for the loss of Ets1 and drive the formation of ISVs in these embryos. Together, these results suggest that Ets1 is involved in regulating the expression levels of VEGFR2 homologs kdr and kdrl which are upstream of the MAPK pathway and play an important role in angiogenesis.
Figure 2. Overexpression of a constitutively active MAP2K1 construct compensates for a reduction in kdrl and kdr expression and partially rescues angiogenic defects in ets1−/−; etv2 MO embryos.
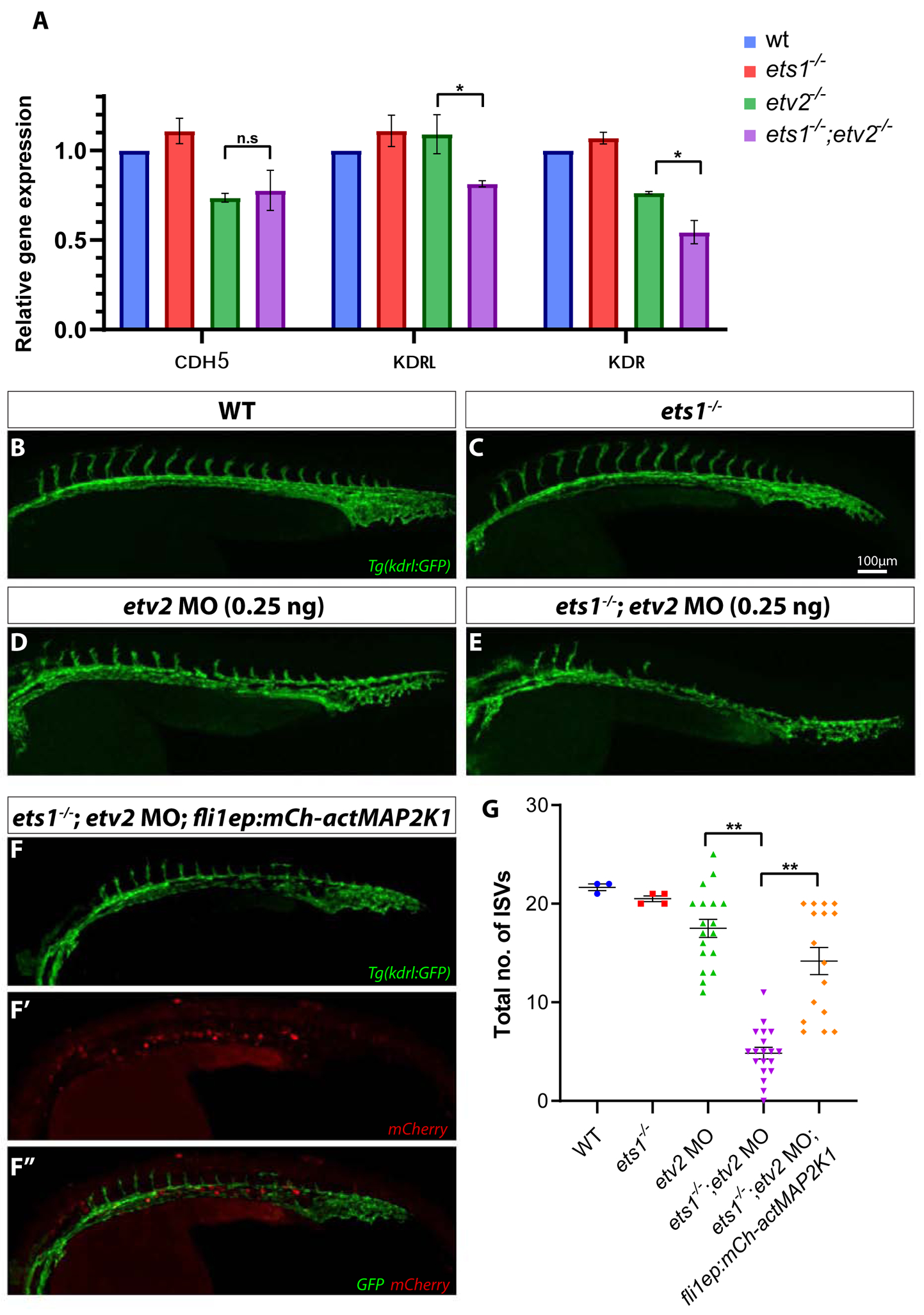
(A) qPCR analysis of endothelial genes cdh5, kdrl and kdr in whole embryos at 32 hpf. Note the reduction in kdrl and kdr expression in ets1−/−; etv2−/−embryos compared to etv2−/−embryos (B-F’’) Confocal images of 28 hpf Tg(kdrl:GFP) wildtype, ets1−/−, etv2 MO (0.25 ng), ets1−/−; etv2 MO (0.25 ng) and ets1−/−; etv2 MO; fli1aep:mCh-actMAP2K1 embryos. Note the mild angiogenic defects in etv2 MO (0.25 ng) embryos (D) and more severe defects in ets1−/−; etv2 MO embryos(E). (F-F’’) Split images showing vascular GFP expression (F), mCherry expression from the actMAP2K1 construct (F’) and a merged image with GFP and mCherry (F’’). (G) Quantification of ISVs in embryos from B-F. Note the increase in the number of ISVs in ets1−/−; etv2 MO; fli1aep:mCh-actMAP2K1 embryos compared to the ets1−/−; etv2 MO embryos. *P<0.05, ** P<0.01; n.s – not significant; columns and horizontal bars in dotplots represent mean, error bars represent ± SEM.
3. Ets1 influences endothelial cell number in axial vessels during vasculogenesis
The severe defects in sprouting angiogenesis observed in ets1−/−; etv2−/− embryos may be a consequence of early vasculogenic defects during the specification, differentiation and/or proliferation of vascular endothelial progenitor cells. To determine endothelial cell number in the axial vessels, we crossed ets1−/−mutants into a kdrl:nls-mCherry line which has nuclear mCherry expression in vascular endothelial cells (Verma et al., 2010). A low dose of etv2 MO (0.25 ng) was injected into Tg(kdrl:nls-mCherry); Tg(kdrl:GFP) and ets1−/−; Tg(kdrl:nls-mCherry); Tg(kdrl:GFP) embryos, and confocal images of the trunk region of the embryos were obtained at 28 hpf (Fig. 3A–D). Endothelial cell numbers were determined by counting cells in confocal images (performed using Imaris software) and FACS analysis of GFP+ cells. There was a statistically significant decrease in the cell numbers and percentage of GFP+ cells in the ets1−/−; etv2 MO embryos, compared to the etv2 MO embryos (Fig. 3E,F). Interestingly, a slight (but not statistically significant) decrease in cell number was also observed in ets1−/− embryos compared to wild-type kdrl:GFP; kdrl:nls-mCherry embryos. These results argue that Ets1 functions redundantly with Etv2 during vasculogenesis and suggest that the enhanced angiogenic defect observed in the ets1−/−; etv2−/− embryos could be, in part, due to a reduction in vascular endothelial cell numbers in the axial vessels during vasculogenesis.
Figure 3. Loss of Ets1 function impairs vasculogenesis in Etv2-deficient embryos.
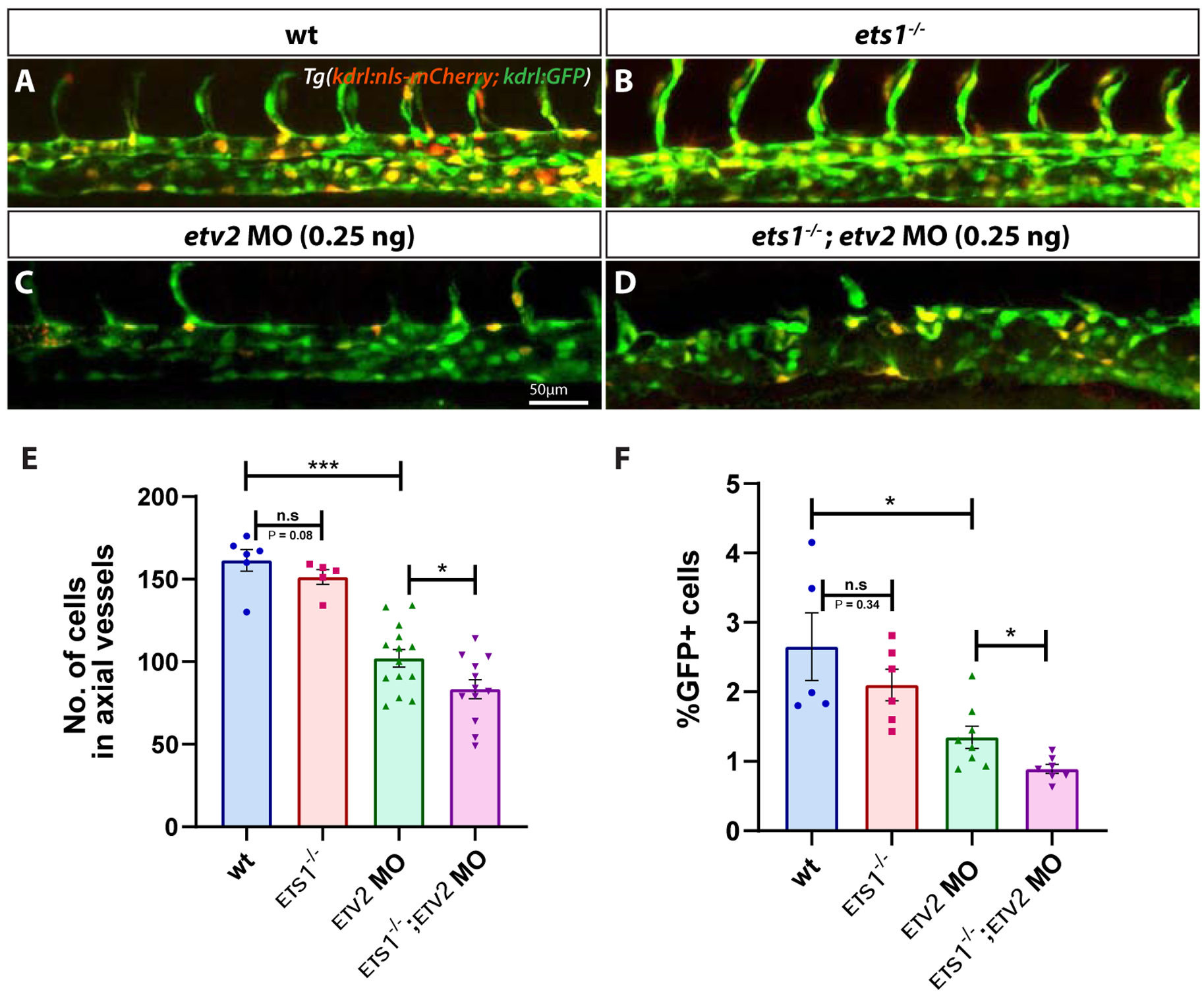
(A-D) Confocal micrographs of 28 hpf Tg(kdrl:GFP; kdrl:nls-mCherry) wild-type, ets1−/−, etv2 MO (0.25ng) and ets1−/−; etv2 MO (0.25 ng) zebrafish embryos (trunk region). (E) Quantitative analysis of endothelial cell number in the axial vessels of embryos in A-D by cell counts in Imaris. (F) FACS analysis of GFP+ endothelial cells in 28 hpf embryos. Note the decrease in endothelial cell number between etv2 MO and ets1−/−; etv2 MO embryos (E,F). Lateral views; anterior is to the left. *P<0.05, *** P<0.001; n.s – not significant; error bars represent ± SEM.
4. Loss of Ets1 results in an increase in apoptosis of endothelial cells
It has been previously demonstrated that the loss of Etv2 function results in the apoptosis of endothelial progenitor cells in the trunk (Craig et al., 2015; Pham et al., 2007). In addition, Ets1 has been previously implicated in promoting the survival of endothelial cells by preventing apoptosis in murine embryos (Wei et al., 2009). To determine if there was increased apoptosis of endothelial cells upon the loss of Ets1 function, we performed Cleaved Caspase3 immunofluorescent staining (together with immunofluorescent staining for GFP) on embryos at approximately 24 hpf. Indeed, the ets1−/− embryos showed increased apoptosis in the trunk region overlapping with the vasculature, although overall vascular patterning was not affected (Fig. 4A–B’’). As expected, there was a significant amount of cell death present in the region of the axial vasculature in both etv2−/− and ets1−/−;etv2−/−embryos (Fig. 4C–D’’). Quantification of apoptosis revealed a small, yet significant, increase in the number of apoptotic cells in the trunk vasculature in ets1−/−;etv2−/−embryos compared to etv2−/− embryos. This argues that zebrafish Ets1, similar to its mammalian homologs, functions to prevent vascular endothelial cell apoptosis. The lack of any apparent vascular defects despite the significant amount of apoptosis in the trunk axial vasculature of the ets1−/− embryos could be explained by the fact that etv2 and related ETS genes compensate for the loss of Ets1 function and are able to restore normal numbers of endothelial cells.
Figure 4. Ets1 promotes endothelial cell survival in axial vessels.
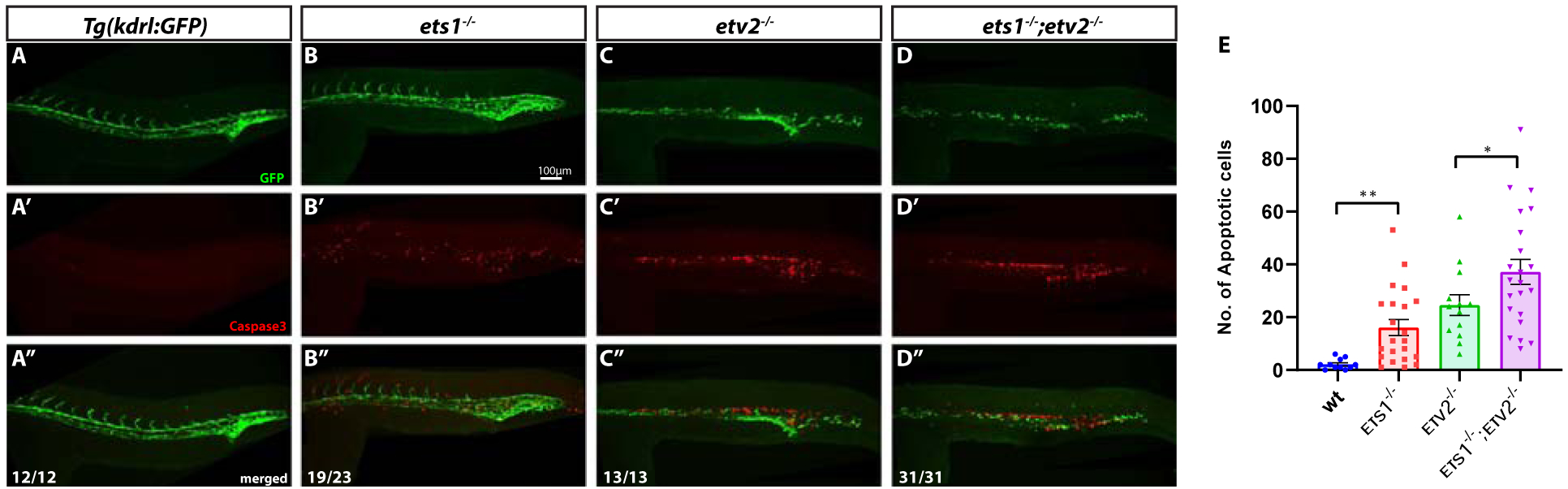
(A-D’’) Tg(kdrl:GFP), ets1−/−, etv2−/− and ets1−/−; etv2−/−embryos at 24 hpf immuno-stained for apoptosis marker Cleaved Caspase3 and vascular GFP. Note the normal vasculature in ets1−/−embryos (B) despite extensive Caspase 3 staining in the axial vessels (B’). Both etv2−/− (C-C’’) and ets1−/−; etv2−/−embryos (D-D’’) exhibit severe vascular defects and extensive apoptosis in the trunk region. (E) Quantification of apoptotic cells within trunk vasculature in 24 hpf embryos. Note the increase in apoptotic cells in the ets1−/−; etv2−/− embryos compared to etv2−/− embryos. * P<0.05, ** P<0.01; horizontal bars represent mean, error bars represent ± SEM.
5. Overexpression of Ets1 induces vascular markers at a later stage than Etv2
We had previously reported that the overexpression of zebrafish etv2 or fli1b mRNA or human Etv2 mRNA in zebrafish embryos results in greatly increased and precocious expression of multiple vascular endothelial markers (Craig et al., 2015; Sumanas et al., 2008; Sumanas and Lin, 2006). In contrast, overexpression of human Ets1 failed to induce ectopic markers in zebrafish embryos (Sumanas et al 2008). The reason for the differential activities of these ETS factors has not been previously investigated. To determine if zebrafish Ets1 could activate other endothelial genes, we overexpressed it by injecting ets1 mRNA into embryos. Unlike etv2 RNA, which can induce endothelial gene expression at very early stages, the ets1 mRNA-injected embryos did not exhibit any vascular phenotype at either early (Fig. 5A–C) or late stages of embryogenesis (data not shown). Because mRNA can be quickly degraded in the injected embryos, we then tested if ets1 expression from the injection of an XeX DNA construct, which utilizes a ubiquitous Xenopus EF1α promoter (Johnson and Krieg, 1994), could cause ectopic vascular marker expression at a later stage.
Figure 5. Ets1 exhibits delayed activity in inducing endothelial gene expression compared to Etv2.
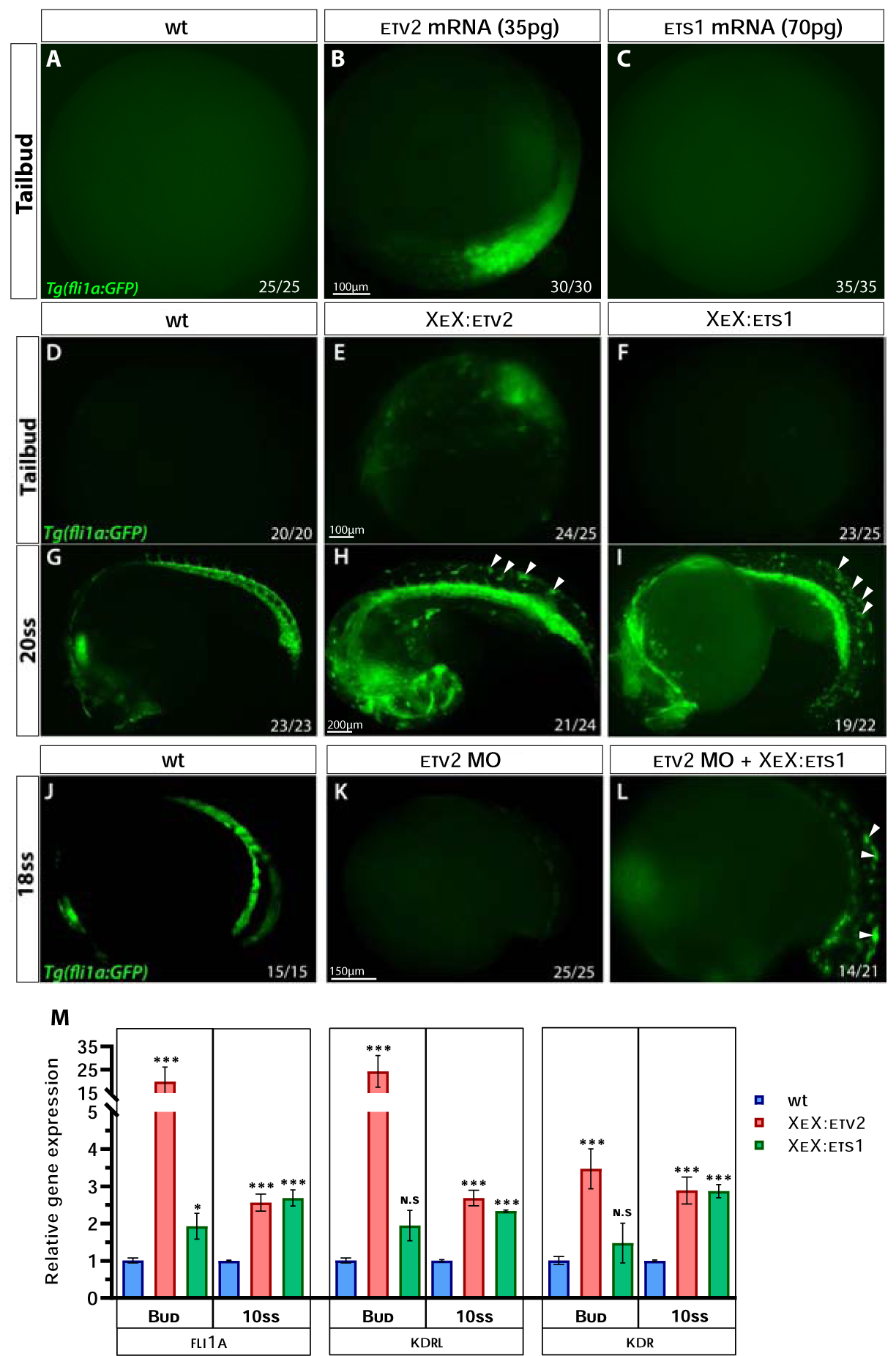
(A-C) Compound microscope images of GFP fluorescence in Tg(fli1a:GFP) wild-type, etv2 mRNA and ets1 mRNA-injected embryos at the tailbud (10 hpf) stage. Note the GFP fluorescence in etv2 mRNA-injected embryos (B) and the lack thereof in ets1 mRNA-injected embryos (C). (D-I) GFP fluorescence in Tg(fli1a:GFP) wild-type, XeX:etv2 and XeX:ets1-injected embryos at the tailbud and 20-somite stage (19 hpf). Note the absence of GFP fluorescence in XeX:ets1 embryos at the tailbud stage (F) but presence of ectopic GFP expression at 20-somite stage as indicated by white arrowheads (I). (J-L) GFP fluorescence in Tg(fli1a:GFP) wild-type , etv2 MO and etv2 MO; XeX:ets1 embryos at 18-somite stage (18 hpf). Note the GFP expression induced by XeX:ets1 even in the absence of Etv2 function (L). (M) qPCR of wildtype, XeX:etv2 and XeX:ets1 whole embryos at the tailbud and 10-somite stage. *P<0.05, **P<0.01 (P value was calculated between wt and injected embryos); n.s – not significant; error bars represent ± SEM.
While the injection of an XeX:etv2 DNA construct produced ectopic and precocious GFP expression in Tg(fli1a:GFP) embryos (similar to etv2 RNA injection), the XeX:ets1-injected embryos did not produce any phenotype at early stages (Fig. 5D–F). However, XeX:ets1-injected embryos displayed ectopic GFP expression at the 20-somite stage (19 hpf), similar to XeX:etv2-injected embryos (Fig. 5G–I), suggesting that Ets1 is able to induce the expression of endothelial genes in cells at a later timepoint than Etv2. Furthermore, ets1 overexpression under the XeX promoter was able to induce endothelial genes even in the absence of etv2 function (Fig. 5J–L). Quantitative analysis of gene expression levels by qPCR on whole embryos injected with either the XeX:ets1 or XeX:etv2 construct revealed that endothelial genes fli1a and kdrl were highly induced as early as the tailbud (10 hpf) stage in the XeX:etv2-injected embryos, while the induction of these genes in the XeX:ets1-injected embryos only occurred by around the 10-somite stage (14 hpf) (Fig. 5M). We postulated that the difference in timing of activity between ets1 and etv2 could be due to differences in their protein domain structure.
6. Inhibition of MAPK and removal of phosphorylation sites suppresses the activity of Ets1
One major difference in the protein structure between Ets1 and Etv2 is the presence of the Ets1 N-terminal Pointed (PNT) domain which acts as a docking site for ERK2 (Hollenhorst et al., 2011; Seidel and Graves, 2002). Previous studies have demonstrated that the phosphorylation of conserved Thr38 and Ser41 residues of Ets1 (adjacent to its Pointed/PNT domain) by the MAPK/ERK pathway is required for Ets1 activity (Chen et al., 2017; Piserchio et al., 2017). In contrast, the Etv2 protein does not have consensus ERK phosphorylation sites. To test the effect of MAPK activity on the function of Ets1 and Etv2, we injected Tg(fli1a:GFP) embryos with the XeX:ets1 or XeX:etv2 construct and then treated the embryos with 15 μM SL327, which is a selective chemical inhibitor of MEK (an upstream activator of ERK) in the MAPK signaling pathway (Hong et al., 2006; Shin et al., 2016), or DMSO (control). Embryos were treated starting at the 75% epiboly stage (8 hpf) and analyzed at approximately the 12-somite stage (~15 hpf) by confocal imaging. Treatment with SL327 did not have a significant effect on GFP fluorescence in the uninjected control embryos (Fig. 6A,D). As expected, the XeX:etv2 and XeX:ets1-injected embryos had significant ectopic GFP fluorescence (Fig. 6B,C). XeX:etv2-injected embryos treated with SL327 still had ectopic GFP fluorescence, arguing that inhibition of MAPK activity did not have a significant effect on Etv2 activity (Fig. 6B,E). In contrast, XeX:ets1-injected embryos treated with SL327 did not exhibit ectopic GFP fluorescence and their vasculature was similar to that of uninjected embryos treated with SL327, arguing that the activity of Ets1 was suppressed upon MAPK inhibition (Fig. 6C,F). Our findings indicate the requirement of MAPK activity for Ets1 but not Etv2 function in the induction of endothelial gene expression.
Figure 6. Ets1 activity depends on MAPK signaling and phosphorylation of conserved Thr30 and Ser33 residues.
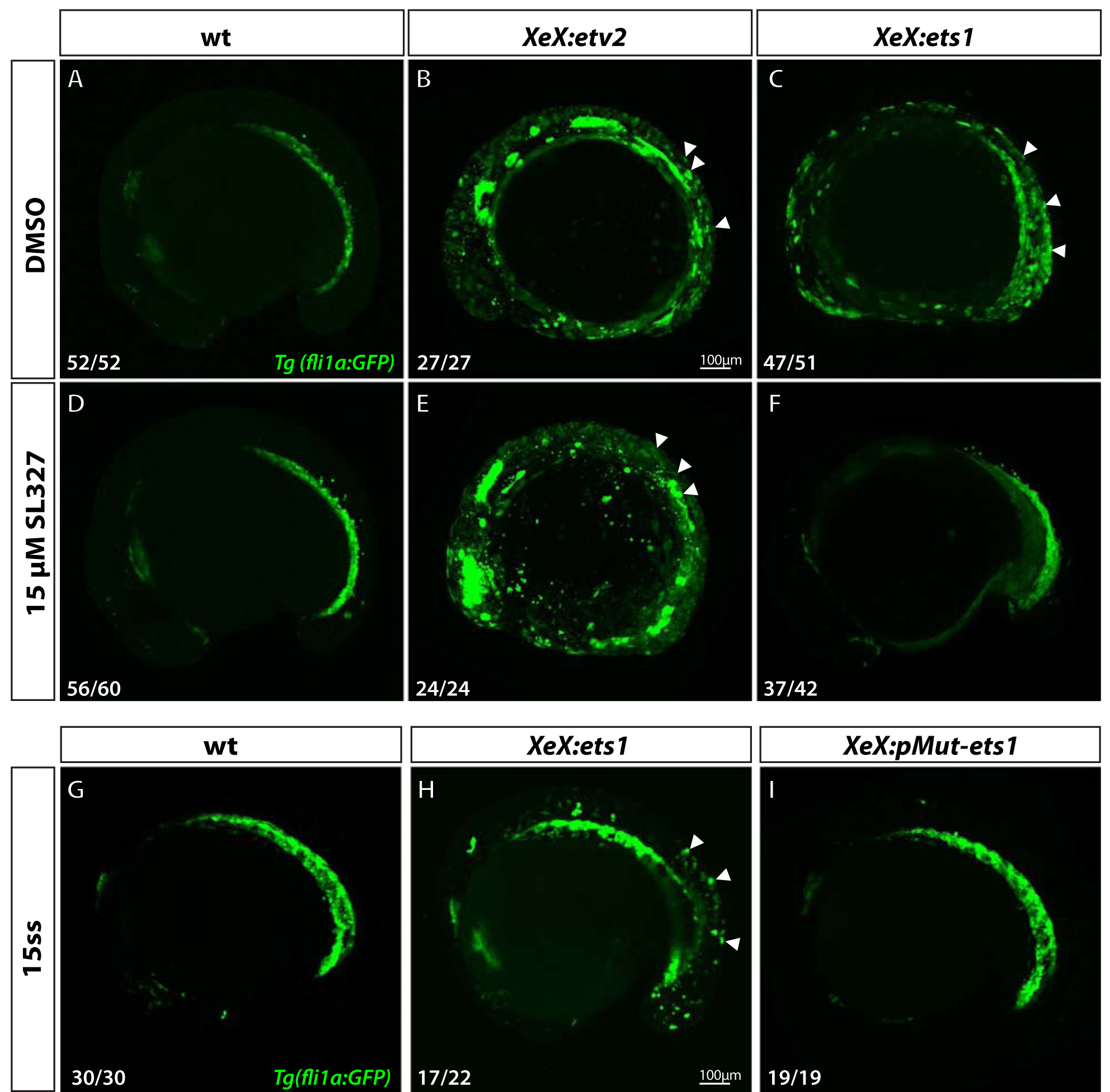
(A-F) Confocal micrographs of 12-somite stage Tg(fli1a:GFP) wild-type, XeX:etv2 and XeX:ets1- injected embryos treated with either DMSO (A-C) or 15 μM MAPK inhibitor SL327 (D-F) from 70% epiboly-12-somite stage. Note the absence of ectopic GFP fluorescence in XeX:ets1 embryos treated with SL327 (F) compared to those treated with DMSO (C). (G-I) Confocal micrographs of 15-somite stage Tg(fli1a:GFP) wild-type embryos, XeX:ets1 and XeX:pMut-ets1 (containing Thr30Ala and Ser33Ala mutations) embryos. Note the reduction of ectopic GFP expression in embryos injected with the XeX:pMut-ets1 construct containing mutated phosphorylation sites (I) compared to XeX:ets1-injected embryos (H). White arrowheads indicate ectopic GFP expression.
To determine if the conserved Thr30 and Ser33 residues within the zebrafish Ets1 protein (equivalent toThr38 and Ser41 residues of the murine Ets1) were necessary for endothelial gene induction in an in vivo zebrafish model, we used site-directed mutagenesis to replace the ERK phosphorylation sites in the XeX:ets1 construct by T30A and S33A substitutions (Suppl. Fig.3). Embryos were injected either with the wild-type XeX:ets1 construct or the phospho-mutant XeX:pMut-ets1 construct, and confocal imaging was performed at approximately the 15-somite stage. The XeX:pMut-ets1 embryos did not display any ectopic GFP fluorescence, as opposed to XeX:ets1-injected embryos. These results suggest that the phosphorylation of the Thr30 and Ser33 sites by MAPK is necessary for the induction of endothelial genes by Ets1 in vivo.
7. Inhibition of MAPK activity during vasculogenesis exacerbates angiogenic defects in etv2-deficient embryos
If MAPK signaling is required for Ets1 activity, then it is expected that the inhibition of MAPK signaling in etv2 mutants would result in similar angiogenic defects to the defects observed in etv2−/−; ets1−/− mutant embryos. To test that, we injected embryos with 5 ng of etv2 morpholino (which results in a phenotype similar to etv2−/− mutants) and then treated them with either DMSO (control) or 15 μM SL327 from the tailbud stage to the 20-somite stage (Fig. 7A–D). Etv2 MO-injected embryos treated with SL327 had a significantly lower number of ISVs and a lower percentage of full sprouts at 48 hpf compared to their DMSO-treated counterparts (Fig. 7E,F). These results resemble those obtained from the comparative ISV analysis of the etv2−/− and ets1−/−;etv2−/− embryos. Though we cannot rule out the effect of MAPK inhibition on other downstream transcription factors and signaling pathways, these results suggest that MAPK-mediated phosphorylation of Ets1 during vasculogenesis is, at least in part, responsible for the partial recovery of angiogenesis observed in Etv2-deficient embryos.
Figure 7. Inhibition of MAPK signaling during vasculogenesis exacerbates angiogenic defects in Etv2- deficient embryos.
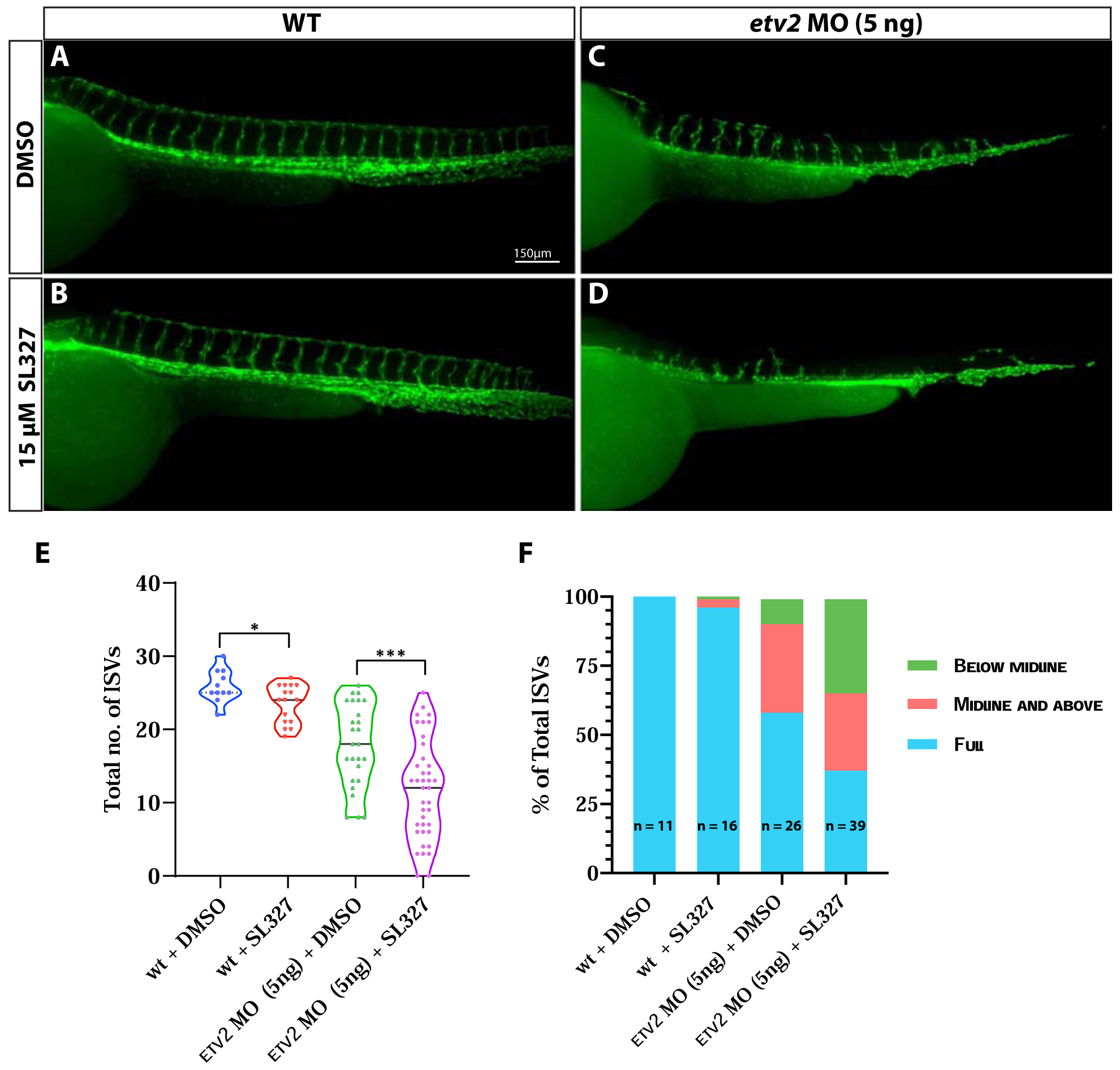
(A-D) Compound microscope images of 48 hpf Tg(kdrl:GFP) wild-type and etv2 MO (5ng)-injected embryos treated either with DMSO or 15μM MAPK inhibitor SL327. Note the severe ISV sprouting defects in etv2 MO + SL327 embryos (D) compared to etv2 MO + DMSO embryos (C). (E,F) Quantitative analysis of ISV number and height. Note the decrease in total number of ISVs (E) and percentage of full sprouts (F) in etv2 MO+SL327 embryos compared to etv2 MO + DMSO embryos. *P<0.05, *** P <0.001. Horizontal bar within violin plots represents median.
Discussion
Despite numerous prior studies on the function of Ets1 in cell culture, its role in vascular development in vivo has been only partially understood. In the current study, we demonstrate that Ets1 functions partially redundantly with a related ETS factor Etv2, to regulate the expression of key VEGF receptor genes kdr and kdrl and to promote endothelial cell survival during vasculogenesis in zebrafish embryos. We also describe a difference in the timing of activity of Ets1 compared to Etv2, which we attribute to differences in functional domains between the two proteins.
Although a previous study demonstrated angiogenic defects in zebrafish embryos upon the knockdown of Ets1 using a morpholino (Pham et al., 2007) a subsequent ets1um206 zebrafish mutant was reported to exhibit normal vasculature (Kok et al., 2015). Similarly, Ets1−/−mice did not exhibit vascular defects, despite having severe immune defects (Wei et al., 2009). The 5 bp deletion present in zebrafish ets1ci14 mutants is predicted to lead to a frameshift and a premature Stop codon, resulting in a severely truncated 22aa protein lacking the ETS DNA-binding domain and PNT domain and is, thus, predicted to be a null. The ets1ci14;Tg(kdrl:GFP) mutant zebrafish display normal vascular patterning and exhibit normal development, consistent with the published ets1um206 mutant (Kok et al., 2015). The expression of ets1 persisted in the ets1ci14 embryos, suggesting that the possibility of genetic compensation is unlikely (El-Brolosy et al., 2019). Our results show that Ets1 functions partially redundantly with Etv2. However, additional redundancies between Ets1 and other ETS transcription factors are possible and were not investigated in the current study. We have previously demonstrated a similar redundancy between the ETS factors fli1b and etv2 (Craig et al., 2015). The loss of Fli1b function alone had no impact on vascular formation and function, but the combined loss of fli1b and etv2 function resulted in severe vascular defects, with no recovery being observed in fli1b−/−; etv2 MO embryos (Craig et al., 2015). Thus the severity of the phenotype observed in the fli1b−/−; etv2−/− embryos appeared to be greater than that of the ets1−/−; etv2−/− embryos, suggesting that the degree of redundancy may vary between different ETS factors. All the proteins in the ETS family contain a conserved DNA-binding ETS domain (Hollenhorst et al., 2011). However, differences in other structural domains, such as the Pointed (PNT) domain could lead to different levels of protein activity. Differences in cooperative binding partners may also explain variations in binding targets and overall function.
The earliest difference in ISV sprouting between ets1−/−; etv2−/− and etv2−/− embryos is observed at approximately 32 hpf, at which point ISVs begin to sprout in the trunk in the etv2−/− embryos, but are absent in the ets1−/−;etv2−/− embryos. qPCR analysis revealed a reduction in the expression of VEGFR2 homologs kdr and kdrl in the ets1−/−; etv2−/− embryos compared to etv2−/−embryos at this stage (Fig. 2A). Previous studies have implicated kdrl in acting synergistically with kdr in binding VegfA ligands, to promote normal vascular patterning in zebrafish embryos (Bahary et al., 2007; Covassin et al., 2006). Consequently, a reduction in both kdr and kdrl levels would lead to defects in vascular development (Bahary et al., 2007). Our results point to the regulation of both kdrl and kdr expression by Ets1, which supports a previous study describing Ets1-mediated expression of Flk1 (VEGFR2) in mice (Elvert et al., 2003). While it is likely that Ets1 directly regulates the expression of multiple endothelial genes, the angiogenic phenotype observed in the ets1−/−; etv2−/− embryos resembles sprouting defects associated with a reduction in VEGF signaling, which is likely due to a reduction in kdrl and kdr expression (Shin et al., 2016). The MAPK/ERK pathway has been shown to be the downstream effector of VEGF signaling which drives angiogenesis (Shin et al., 2016; Wythe et al., 2013). Indeed, overexpression of constitutively active MAP2K1 in endothelial cells of ets1−/−;etv2 MO (low) embryos resulted in a significant increase in the number of ISVs compared to ets1−/−;etv2 MO (low) embryos without the actMAP2K1 construct. The expression of actMAP2K1 results in the activation of multiple downstream effectors including Ets1. We posit that in the absence of Ets1 function, the rescue of ISV sprouting occurs through these other transcription factors and pathways downstream of VEGF signaling (Fig. 8B). Although high levels of mCherry are observed in only a few endothelial cells, some additional endothelial cells exhibit low levels of mCherry. It is also likely that mCherry expression is higher at earlier developmental stages and then decreases over time. This partial rescue in sprouting corroborates previous findings which have demonstrated the requirement of the VEGF/MAPK/ERK signaling axis to drive angiogenesis (Shin et al., 2016).
Figure 8. Model of Ets1 activity and timing.
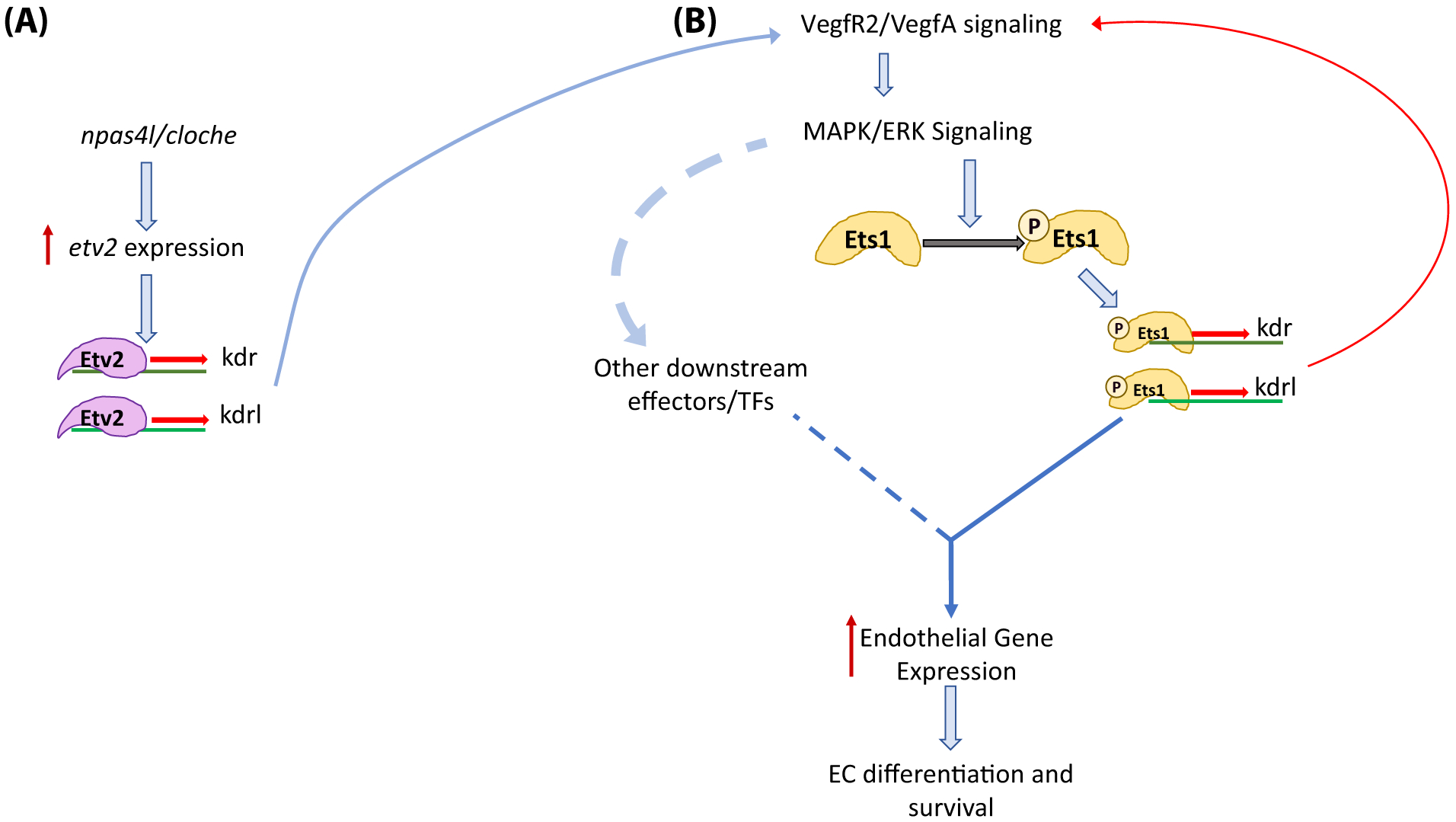
(A) Early induction of etv2 expression in lateral plate mesodermal cells by npas4l/cloche. Etv2 initiates the VEGFR2/VEGFA signaling cascade by inducing the expression of kdr and kdrl. (B) Binding of VEGFA ligands to VEGFR2 leads to an increase in downstream MAPK/ERK signaling resulting in the phosphorylation of Ets1. Ets1p (phosphorylated Ets1) induces the expression of kdr and kdrl which creates a positive feedback loop to promote overall endothelial gene expression, endothelial cell differentiation and survival.
Intriguingly, ets1−/−; etv2 MO embryos had a lower number of endothelial cells in the axial vasculature than etv2 MO embryos, suggesting that Ets1 plays a role during vasculogenesis. It is possible that the increase in severity of the angiogenic phenotype in the ets1−/−; etv2−/−embryos could be, at least in part, due to a reduction in the number of endothelial cells in the axial vessels formed during vasculogenesis. Indeed, our data reveal that the loss of Ets1 function in etv2−/− embryos (ets1−/−; etv2−/−) results in an increase in apoptosis of endothelial cells compared to etv2−/−embryos. It was striking, though, that despite a high amount of endothelial cell apoptosis, the ets1−/−embryos still displayed normal vascular patterning. This is the first vascular phenotype observed in ets1−/−embryos and it suggests that Ets1 functions in promoting endothelial cell survival during vasculogenesis, although its function is not essential for normal vascular patterning. This also corroborates findings from a previous study carried out in mice which revealed the regulation of antiapoptotic genes by both Ets1 and Ets2 (Wei et al., 2009). Although Ets1−/−mice lacked vascular defects, double mutant Ets1−/−;Ets2−/−mice displayed angiogenic defects and were shown to have increased apoptosis of endothelial cells (Wei et al., 2009). Similarly, cultured aortic ECs from Ets1−/−;Ets2fl/fl(+Cre) mice had high levels of apoptosis. Interestingly, Ets1−/−;Ets2fl/fl(−Cre) cells also exhibited apoptosis; i.e the loss of Ets1 function alone was sufficient to induce apoptosis in endothelial cells. We propose that the lack of a vascular phenotype in the ets1−/−embryos, despite significant endothelial cell apoptosis, is due to the compensatory action of Etv2, Fli1b and other ETS factors expressed in endothelial cells, which mediate normal vascular patterning even in the absence of Ets1 function possibly through increased endothelial cell proliferation. Collectively, our results reveal a role for Ets1 in promoting endothelial cell survival during embryonic vasculogenesis.
We demonstrate a dramatic change in the activity of Ets1 over time during development using overexpression of Ets1. At the tailbud stage, there was no (or very little) induction of endothelial genes by Ets1, but by the 10-somite stage, the activities of Ets1 and Etv2 were comparable. This distinct difference in the timing of activity between Ets1 and Etv2 is likely due to the structural differences between the two related proteins. The Ets1 protein contains a PNT domain and phosphorylation sites adjacent to it, which Etv2 lacks (Hollenhorst et al., 2011). The ~80 residue conserved PNT domain is present in approximately one-third of ETS factors and plays important roles in mediating the phosphorylation of specific residues and the recruitment of coactivators such as Creb-binding protein (CBP) (Hollenhorst et al., 2011). The PNT domain acts as a docking site for ERK2 which phosphorylates Thr38 and Ser41 (Hollenhorst et al., 2011; Seidel and Graves, 2002). Prior biochemical studies have demonstrated a requirement of the phosphorylation of conserved Thr38 and Ser41 sites by MAPK/ERK2 for the activity of Ets1 (Nelson et al., 2010; Piserchio et al., 2017). Inhibition of MAPK/ERK signaling by SL327 treatment and the substitution of the conserved phosphorylation sites at Thr30 and Ser33 within the zebrafish Ets1 protein resulted in a loss of ectopic GFP induction in embryos. Additionally, we show that Etv2 activity is MAPK-independent, supporting results from a previous study which demonstrated VEGF -independent expression of etv2 (Casie Chetty et al., 2017). We also show that the inhibition of MAPK signaling during vasculogenesis exacerbates angiogenic defects in Etv2 deficient embryos, similar to the phenotype observed in ets1−/−;etv2−/−embryos. While we cannot rule out the effects of MAPK inhibition on other downstream molecules and signaling pathways, it is likely that insufficient phosphorylation of Ets1 during vasculogenesis in MAPK-treated embryos contributes to the enhanced angiogenic defect observed in the etv2 MO + SL327 embryos. Collectively, these results demonstrate the requirement of MAPK signaling and the phosphorylation of the T30 and S33 residues for the transcriptional activity of Ets1 in vascular development. The requirement for MAPK-mediated phosphorylation would also explain the delay in Ets1-mediated induction of endothelial genes, compared to Etv2. Interestingly, our results also demonstrate that overexpression of ets1 induces endothelial genes even in the absence of Etv2 function. The loss of Etv2 function would dampen MAPK signaling within endothelial progenitors, leading to a reduction in endothelial cell differentiation. However, other ETS factors such as Fli1a, Fli1b and Erg (which are also expressed in endothelial cells) could subsequently promote VEGF signaling by inducing VEGF receptors and compensate for the loss of Etv2 function. This, in turn, would induce phosphorylation of Ets1 at a later time point. Ectopic induction of endothelial genes by XeX:ets1 in the absence of Etv2 function could also be explained by the activation of MAPK signaling through alternate pathways.
Based on these and previous data, we propose the following model for the function of Ets1 in vascular development (Fig. 8). At early developmental stages, Etv2 expression is induced in endothelial progenitors within the lateral plate mesoderm by upstream regulators that include the npas4l transcription factor, as well as other signaling pathways which are still poorly understood (Lee et al., 2008; Reischauer et al., 2016). Since Etv2 is not dependent on MAPK-mediated phosphorylation for its activity, it initiates the expression of endothelial genes including VEGFR2 genes kdr and kdrl. The expression of VEGF receptors in endothelial progenitors allows cells to respond to VEGFA ligands and increase VEGF/MAPK signaling within the cells. This increase in MAPK signaling leads to the phosphorylation and activation of Ets1 (and other downstream effectors). Ets1P (phosphorylated Ets1) is now able to induce the expression of endothelial genes, including kdr and kdrl, which in return causes in an increase in VEGF/MAPK signaling within the cell. This positive feedback loop drives endothelial cell differentiation and survival, and is critical for the formation of a functional vascular system.
In summary, it is clear that different ETS factors, despite having overlapping functions, exhibit contrasting levels of activity and contribute to vascular development to varying degrees. The same ETS transcription factors including Ets1 and Etv2 have also been implicated in pathological angiogenesis which includes tumor angiogenesis, as well as regenerative angiogenesis after ischemic injury (Baltrunaite et al., 2017; Park et al., 2016). Therefore, elucidating the functional redundancy between different ETS factors expressed in endothelial cells will provide us better insight into the transcriptional regulation during embryonic vascular development as well as pathological and regenerative angiogenesis.
Supplementary Material
HIGHLIGHTS.
Zebrafish Ets1 functions redundantly with Etv2 to promote vasculogenesis and angiogenesis
Active MEKK partially rescues angiogenic defects in ets1−/−;etv2−/− zebrafish embryos
Zebrafish ets1 mutants show increased endothelial cell apoptosis
Phosphorylation of Ets1 is required to induce endothelial genes in zebrafish embryos
Acknowledgements
This research was supported by NIH R01 HL107369 and NIH R21 AI128445 awards to S.S. and by Cincinnati Children’s Research Foundation. We would like to thank Allison Lubert for helping to generate the ets1ci14 zebrafish line and Kristina Baltrunaite and Emily Thomas for assisting with experiments. We thank the CCHMC aquatics staff for excellent fish care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahary N, Goishi K, Stuckenholz C, Weber G, LeBlanc J, Schafer CA, Berman SS, Klagsbrun M, Zon LI, 2007. Duplicate VegfA genes and orthologues of the KDR receptor tyrosine kinase family mediate vascular development in the zebrafish. Blood 110, 3627–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltrunaite K, Craig MP, Palencia Desai S, Chaturvedi P, Pandey RN, Hegde RS, Sumanas S, 2017. ETS transcription factors Etv2 and Fli1b are required for tumor angiogenesis. Angiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bories J-C, Willerford DM, Grévin D, Davidson L, Camus A, Martin P, Stéhelin D, Alt FW, 1995. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature 377, 635. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, 2005. Angiogenesis in life, disease and medicine. Nature 438, 932. [DOI] [PubMed] [Google Scholar]
- Casie Chetty S, Rost MS, Enriquez JR, Schumacher JA, Baltrunaite K, Rossi A, Stainier DY, Sumanas S, 2017. Vegf signaling promotes vascular endothelial differentiation by modulating etv2 expression. Developmental Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fu Y, Day DS, Sun Y, Wang S, Liang X, Gu F, Zhang F, Stevens SM, Zhou P, Li K, Zhang Y, Lin RZ, Smith LEH, Zhang J, Sun K, Melero-Martin JM, Han Z, Park PJ, Zhang B, Pu WT, 2017. VEGF amplifies transcription through ETS1 acetylation to enable angiogenesis. Nat Commun 8, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fisher RJ, Riggs CW, Rhim JS, Lautenberger JA, 1997. Inhibition of vascular endothelial growth factor-induced endothelial cell migration by ETS1 antisense oligonucleotides. Cancer Res 57, 2013–2019. [PubMed] [Google Scholar]
- Covassin LD, Siekmann AF, Kacergis MC, Laver E, Moore JC, Villefranc JA, Weinstein BM, Lawson ND, 2009. A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcg1 signaling during artery development. Developmental biology 329, 212–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND, 2006. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proceedings of the National Academy of Sciences 103, 6554–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MP, Grajevskaja V, Liao H-K, Balciuniene J, Ekker SC, Park J-S, Essner JJ, Balciunas D, Sumanas S, 2015. Etv2 and fli1b function together as key regulators of vasculogenesis and angiogenesis. Arteriosclerosis, thrombosis, and vascular biology 35, 865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MP, Sumanas S, 2016. ETS transcription factors in embryonic vascular development. Angiogenesis 19, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL, 2008. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 135, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Brolosy MA, Kontarakis Z, Rossi A, Kuenne C, Guenther S, Fukuda N, Kikhi K, Boezio GL, Takacs CM, Lai S-L, 2019. Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, 2003. Cooperative interaction of hypoxia-inducible factor-2α (HIF-2α) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1). Journal of Biological Chemistry 278, 7520–7530. [DOI] [PubMed] [Google Scholar]
- Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, 2009. Nkx2–5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proceedings of the National Academy of Sciences 106, 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS, 2005. Angiogenesis as a therapeutic target. Nature 438, 967. [DOI] [PubMed] [Google Scholar]
- Folkman J, 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine 1, 27. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Weinstein BM, Serluca FC, Fishman MC, 1997. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol 183, 37–48. [DOI] [PubMed] [Google Scholar]
- Garrett-Sinha LA, 2013. Review of Ets1 structure, function, and roles in immunity. Cellular and molecular life sciences 70, 3375–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenhorst PC, McIntosh LP, Graves BJ, 2011. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem 80, 437–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT, 2006. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol 16, 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt TM, Ekker SC, 1998. Vectors and techniques for ectopic gene expression in zebrafish, Methods in cell biology. Elsevier, pp. 117–126. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY, 2005. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Krieg PA, 1994. pXeX, a vector for efficient expression of cloned sequences in Xenopus embryos. Gene 147, 223–226. [DOI] [PubMed] [Google Scholar]
- Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G, 2000. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood 96, 3078–3085. [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, 1995. Stages of embryonic development of the zebrafish. Developmental dynamics 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Koch S, Claesson-Welsh L, 2012. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harbor perspectives in medicine 2, a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli V, Schumacher JA, Desai SP, Rehn K, Sumanas S, 2013. Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev Cell 25, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND, 2015. Reverse Genetic Screening Reveals Poor Correlation between Morpholino-Induced and Mutant Phenotypes in Zebrafish. Developmental Cell 32, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM, 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology 248, 307–318. [DOI] [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K, 2008. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli M, Driever W, 2012. Fluorescence-activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harb Protoc 2012. [DOI] [PubMed] [Google Scholar]
- Muthusamy N, Barton K, Leiden JM, 1995. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature 377, 639–642. [DOI] [PubMed] [Google Scholar]
- Nelson ML, Kang H-S, Lee GM, Blaszczak AG, Lau DK, McIntosh LP, Graves BJ, 2010. Ras signaling requires dynamic properties of Ets1 for phosphorylation-enhanced binding to coactivator CBP. Proceedings of the National Academy of Sciences 107, 10026–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Lee T-J, Bhang SH, Liu F, Nakamura R, Oladipupo SS, Pitha-Rowe I, Capoccia B, Choi HS, Kim TM, 2016. Injury-mediated vascular regeneration requires endothelial ER71/ETV2. Arteriosclerosis, thrombosis, and vascular biology 36, 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM, 2007. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol 303, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piserchio A, Warthaka M, Kaoud TS, Callaway K, Dalby KN, Ghose R, 2017. Local destabilization, rigid body, and fuzzy docking facilitate the phosphorylation of the transcription factor Ets-1 by the mitogen-activated protein kinase ERK2. Proceedings of the National Academy of Sciences 114, E6287–E6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischauer S, Stone OA, Villasenor A, Chi N, Jin SW, Martin M, Lee MT, Fukuda N, Marass M, Witty A, Fiddes I, Kuo T, Chung WS, Salek S, Lerrigo R, Alsio J, Luo S, Tworus D, Augustine SM, Mucenieks S, Nystedt B, Giraldez AJ, Schroth GP, Andersson O, Stainier DY, 2016. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 535, 294–298. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I, 1995. Vasculogenesis. Annual review of cell and developmental biology 11, 73–91. [DOI] [PubMed] [Google Scholar]
- Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh J-RJ, 2011. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nature biotechnology 29, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Teruyama K, Nakano T, Oda N, Abe M, Tanaka K, IWASAKA-YAGI C, 2001. Role of transcription factors in angiogenesis: Ets-1 promotes angiogenesis as well as endothelial apoptosis. Annals of the New York Academy of Sciences 947, 117–123. [PubMed] [Google Scholar]
- Seidel JJ, Graves BJ, 2002. An ERK2 docking site in the Pointed domain distinguishes a subset of ETS transcription factors. Genes Dev 16, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, 2007. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. Journal of cellular biochemistry 102, 840–847. [DOI] [PubMed] [Google Scholar]
- Shin M, Beane TJ, Quillien A, Male I, Zhu LJ, Lawson ND, 2016. Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development 143, 3796–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S, 2008. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood 111, 4500–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Lin S, 2006. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol 4, e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruyama K, Abe M, Nakano T, Iwasaka-Yagi C, Takahashi S, Yamada S, Sato Y, 2001. Role of transcription factor Ets-1 in the apoptosis of human vascular endothelial cells. Journal of cellular physiology 188, 243–252. [DOI] [PubMed] [Google Scholar]
- Verma A, Bhattacharya R, Remadevi I, Li K, Pramanik K, Samant GV, Horswill M, Chun CZ, Zhao B, Wang E, Miao RQ, Mukhopadhyay D, Ramchandran R, Wilkinson GA, 2010. Endothelial cell-specific chemotaxis receptor (ecscr) promotes angioblast migration during vasculogenesis and enhances VEGF receptor sensitivity. Blood 115, 4614–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Srinivasan R, Cantemir-Stone CZ, Sharma SM, Santhanam R, Weinstein M, Muthusamy N, Man AK, Oshima RG, Leone G, Ostrowski MC, 2009. Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood 114, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernert N, Stanjek A, Kiriakidis S, Hügel A, Jha HC, Mazitschek R, Giannis A, 1999. Inhibition of Angiogenesis In Vivo by ets-1 Antisense Oligonucleotides—Inhibition of Ets-1 Transcription Factor Expression by the Antibiotic Fumagillin. Angewandte Chemie International Edition 38, 3228–3231. [DOI] [PubMed] [Google Scholar]
- Wythe JD, Dang LT, Devine WP, Boudreau E, Artap ST, He D, Schachterle W, Stainier DY, Oettgen P, Black BL, Bruneau BG, Fish JE, 2013. ETS factors regulate Vegf-dependent arterial specification. Dev Cell 26, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong TP, 2005. Zebrafish genetics and formation of embryonic vasculature. Current topics in developmental biology 71, 53–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


