Abstract
CLN2 neuronal ceroid lipofuscinosis is a hereditary neurodegenerative disorder characterized by progressive vision loss, neurological decline, and seizures. CLN2 disease results from mutations in TPP1 that encodes the lysosomal enzyme tripeptidyl peptidase-1 (TPP1). Children with CLN2 neuronal ceroid lipofuscinosis experience ocular disease, characterized by progressive retinal degeneration associated with impaired retinal function and gradual vision loss culminating in total blindness. A similar progressive loss of retinal function is also observed in a dog CLN2 model with a TPP1 null mutation. A study was conducted to evaluate the efficacy of periodic intravitreal injections of recombinant human (rh) TPP1 in inhibiting retinal degeneration and preserving retinal function in the canine model. TPP1 null dogs received periodic intravitreal injections of rhTPP1 in one eye and vehicle in the other eye beginning at approximately 12 weeks of age. Ophthalmic exams, in vivo ocular imaging, and electroretinography (ERG) were repeated regularly to monitor retinal structure and function. Retinal histology was evaluated in eyes collected from these dogs when they were euthanized at end-stage neurological disease (43–46 weeks of age). Intravitreal rhTPP1 dosing prevented disease-related declines in ERG amplitudes in the TPP1-treated eyes. At end-stage neurologic disease, TPP1-treated eyes retained normal morphology while the contralateral vehicle-treated eyes exhibited loss of inner retinal neurons and photoreceptor disorganization typical of CLN2 disease. The treatment also prevented the development of disease-related focal retinal detachments observed in the control eyes. Uveitis occurred secondary to the administration of the rhTPP1 but did not hinder the therapeutic benefits. These finding demonstrate that periodic intravitreal injection of rhTPP1 preserves retinal structure and function in canine CLN2 disease.
Keywords: Intravitreal drug delivery, electroretinogram, genetic diseases, retina, retinal degeneration, uveitis
1. Introduction
The neuronal ceroid lipofuscinoses (NCLs) are a group of rare lysosomal storage disorders characterized by intracellular accumulation of autofluorescent storage bodies in many tissues. The clinical disease is manifested by progressive retinal degeneration and vision loss as well as widespread neurodegeneration accompanied by progressive brain atrophy, severe decline in cognitive and motor functions, seizures, and premature death (Mole et al., 2011). The CLN2 form of NCL is caused by mutations in the TPP1 gene that result in deficiencies in functional tripeptidyl peptidase-1 (TPP1) (Liu et al., 1998; Mole and Cotman, 2015; Sleat et al., 1997). TPP1 is a soluble lysosomal enzyme involved in the normal degradation of proteins and peptides in cells throughout the body. Like other soluble lysosomal enzymes, TPP1 released from the cell surface can be taken up by other cells via cell-surface mannose-6-phosphate receptors and transported to lysosomes via normal endosomal trafficking (Brooks, 2009; Chang et al., 2008; Grubb et al., 2010; Guhaniyogi et al., 2009; Wong et al., 2010). This uptake process is the basis for the efficacy of enzyme replacement therapy in treating lysosomal storage disorders (Grubb et al., 2010; Markham, 2017; Schulz et al., 2017; Vuillemenot et al., 2015; Whiting et al., 2014). In the canine model of CLN2 disease, periodic infusion of recombinant human (rh) TPP1 into the cerebrospinal fluid (CSF) significantly delayed the onset and progression of most neurologic signs and significantly extended lifespan (Whiting et al., 2014). However, this treatment had no effect on the disease-related progressive decline in retinal function or retinal degeneration (Whiting et al., 2014). It is hypothesized that intravitreal administration of TPP1 may be effective in treating CLN2 disease-related retinal degeneration.
In dogs with a null variant in the TPP1 ortholog, retinal degeneration is characterized by progressive loss of inner retinal function, indicated by gradual reduction of b-wave amplitudes beginning at approximately 5 months of age (Katz et al., 2008; Whiting et al., 2013). For both scotopic and photopic responses, a-wave amplitudes decline in untreated dogs late in the disease progression (Katz et al., 2008; Whiting et al., 2013). In most affected dogs the retinas develop focal retinal detachments that increase in number and size as the disease progresses (Whiting et al., 2015). When these lesions are present in a dog, the severity is symmetrical between the two eyes (Whiting et al., 2015). Morphometric analyses of eyes collected immediately after euthanasia revealed loss of retina inner nuclear layer nuclei and disorganization of photoreceptor inner and outer segments (Katz et al., 2008). When lifespan is extended by CSF TPP1 enzyme infusion (Whiting et al., 2014) or by CNS direct gene therapy (Whiting et al., 2015), the a-wave amplitudes continue to decline progressively, in addition to abolition of the b-wave, and the ERG eventually becomes nonrecordable for all responses (Whiting et al., 2016). In addition, with sufficient lifespan extension achieved by CNS TPP1 gene therapy, there is a loss of most retinal cell types but selective preservation of ganglion cells (Whiting et al., 2016).
Periodic intravitreal injection is commonly used for delivery of therapeutic molecules targeted to the inner retina and has proved therapeutic for chronic diseases such as age-related macular degeneration and diabetic retinopathy (Ba et al., 2015; Harkins et al., 2016; Martin, 2018; Radhakrishnan et al., 2017; Villegas et al., 2017). Previous studies have found injected proteins to have half-lives of approximately 6–10 days in the vitreous (depending on protein molecular weight) with significant transport from the vitreous to the retina (Awwad et al., 2017; Edington et al., 2017; Patel et al., 2015; Radhakrishnan et al., 2017). Given the history of success with intravitreal therapeutic protein and peptide delivery for treating retinal disease, we hypothesized that periodic intravitreal injection of rhTPP1 could inhibit retinal degeneration and loss of retinal function associated with CLN2 disease. Therefore, studies were conducted in the canine CLN2 disease model to test the efficacy of periodic intravitreal injections of rhTPP1 enzyme in preserving retinal function and structure.
2. Materials and methods
2.1. Animals
The study employed Dachshunds (n=4) that were homozygous for a recessive null mutation in TPP1 (Awano et al., 2006) from a research colony maintained in AALAC-accredited facilities at the University of Missouri. The affected dogs utilized in this study were generated by breeding healthy dogs that were heterozygous for the disease allele and shared no common ancestor for at least the two previous generations. Puppies were genotyped within several weeks of birth at the TPP1 c.325 locus using an allelic discrimination assay that distinguishes the normal and disease alleles (Awano et al., 2006). Dogs were maintained on a 12:12 daily light cycle and were socialized outside of their kennels by lab staff daily in addition to receiving routine husbandry and veterinary care. All studies were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Missouri Animal Care and Use Committee.
2.2. Ophthalmic Examinations
All dogs underwent a thorough ophthalmic examination by a board-certified veterinary ophthalmologist (JWP) at 10 weeks of age prior to inclusion in the study. No clinical signs of CLN2 disease are apparent at this age. Only dogs with normal ocular findings at this age were included in the study. Exams were performed 1 day and 1 week following each treatment and at additional times if ocular complications related to the treatments were observed. Examination included visually-mediated behavioral assessment and slit lamp biomicroscopy (SL14; Kowa Co. Ltd., Tokyo, Japan). Indirect ophthalmoscopy was also performed after dilating the pupils with a short-acting mydriatic (tropicamide 1%; Alcon, Fort Worth, TX).
2.3. Treatments
For each treatment, 100 μL of rhTPP1 enzyme (1 mg/ml or 3 mg/ml) in 10 mM sodium phosphate, 10% trehalose, pH 6.5 (Vuillemenot et al., 2015; Whiting et al., 2014) was injected into the vitreous of the left eye (OS) of each dog, and an equivalent volume of vehicle (10 mM sodium phosphate, 10% trehalose, pH 6.5) was injected into the vitreous of the right eye (OD). The amount of rhTPP1 in each dose is indicated in Table 1. Prior to each injection, the dog was sedated with intramuscular administration of buprenorphine (0.015mg/kg) and dexmedetomidine (20mcg/kg). The ocular surface was rinsed with dilute povidone-iodine solution, and 1 drop each of 0.3% ofloxacin and 0.5% proparacaine hydrochloride was administered to each eye. For each injection, the dorsolateral bulbar conjunctiva was grasped with forceps and the globe rotated ventromedially. The attached needle of an insulin syringe (0.5”, 29G) was inserted 5–7mm posterior to the limbus directed posterior to the lens, and the scheduled solution was injected into the superior-nasal quadrant of the vitreous. Intraocular pressure (IOP) measurements for each eye were performed prior to and 20 minutes following each injection; if necessary, measurements were repeated until pressures returned to normal.
Table 1:
Treatment schedules for dogs A, B, C and D.
| Treatment Schedule | |||||||
|---|---|---|---|---|---|---|---|
| Dog A | Dog B | Dog C | Dog D | ||||
| Week | Dose | Week | Dose | Week | Dose | Week | Dose |
| 1 | 0.1 mg | 1 | 0.1 mg | 1 | 0.1 mg | 1 | 0.1 mg |
| 3 | 0.1 mg | 3 | 0.1 mg | 3 | 0.1 mg | 5 | 0.1 mg |
| 5 | 0.3 mg | 5 | 0.1 mg | 5 | 0.1 mg | 11 | 0.1 mg |
| 11 | 0.1 mg | 7 | 0.1 mg | 7 | 0.1 mg | 17 | 0.1 mg |
| 9 | 0.1 mg | 9 | 0.1 mg | 23 | 0.1 mg | ||
| 11 | 0.1 mg | 12 | 0.1 mg | ||||
| 14 | 0.1 mg | 15 | 0.1 mg | ||||
| 17 | 0.1 mg | 18 | 0.1 mg | ||||
| 20 | 0.1 mg | 24 | 0.1 mg | ||||
| 23 | 0.1 mg | ||||||
| 26 | 0.1 mg | ||||||
Enrollment in the study was staggered as affected dogs became available. This facilitated making alterations to the treatment protocol for subsequent dogs based on preliminary results obtained from the dogs that were previously treated. Based on our prior studies with rhTPP1 infusions into the CSF (Vuillemenot et al., 2015; Whiting et al., 2014), the initial protocol for this study entailed administration of intravitreal rhTPP1 once every 2 weeks with a starting dose of 0.1 mg and a planned gradual dose escalation. For the first dog enrolled (Dog A), after escalating from 0.1 mg to a single 0.3 mg dose, the treated eye exhibited severe ocular inflammation (see Results for details), so all subsequent doses for Dogs A, B, C, and D were 0.1 mg. The frequency of dosing was also adjusted during the study based on whether adverse effects were observed during follow-up ophthalmic exams and electroretinography recordings (see Results). The actual dosing schedule for each dog is shown in Table 1. Dosing began for each dog at approximately 12 weeks of age (designated as week one of treatment).
2.4. Immunosuppression and inflammation control
In previous studies in which TPP1-null Dachshunds had received rhTPP1 either via infusion into the CSF or via CNS gene therapy, exposure to the protein elicited immune reactions ranging from CNS inflammation to acute anaphylactoid reactions (Katz et al., 2014; Katz et al., 2015; Vuillemenot et al., 2015). Therefore, the dogs in this study were maintained on low levels of immunosuppressants. For dogs A, B and C twice-daily (BID) oral administration of 25 mg cyclosporine began one week prior to the first intravitreal injection (25 mg capsules USP modified, TEVA Pharmaceuticals, North Wales, PA). After each dog reached a body weight of 4 kg, the dose was adjusted to 35 mg BID and maintained throughout the study period. Leflunomide (2.5 mg/kg oral once daily for 2 months, then reduced to every other day) was added mid-study for additional immunosuppression. Leflunomide dosing began in week 21, week 19, and week 17 for dogs A, B and C respectively. For dog D, cyclosporine and leflunomide dosing began 3 weeks prior to the first intravitreal injection and was continued throughout the study period (for dog D, leflunomide dosing was administered daily until euthanasia).
Topical corticosteroid (0.05% difluprednate, Durezol, Alcon; or 1% prednisolone acetate) and non-steroidal anti-inflammatory (0.1% nepafenac, Nevanac, Alcon; or 0.03% flurbiprofen sodium) drops were administered to the cornea of each eye beginning 24 hours prior to each injection and for a period following each injection: Durezol (4 times daily) and Nevanac (3 times daily) were used for at least 3 days following each injection or until significant inflammation had resolved, at which time Durezol and Nevanac were replaced with prednisolone and flurbiprofen, respectively. Administration frequency of these medications was tapered as inflammation resolved, and their use was discontinued once all signs of inflammation had resolved. Immediately following every other intravitreal rhTPP1 injection, a sub-tenon injection of 2 mg of triamcinolone acetonide (Kenalog-10, Bristol-Myers Squibb, Princeton, NJ) was administered to each eye.
2.5. In vivo retinal imaging
For all dogs, in vivo imaging of each eye was performed prior to initiating treatment and monthly thereafter. Imaging of the vitreous and retina was performed with the dog under general anesthesia using a combined confocal scanning laser ophthalmoscope (SLO) and spectral-domain optical coherence tomography (OCT) instrument (Spectralis HRA/OCT, Heidelberg, Germany) as previously described (Whiting et al., 2015).
2.6. Electroretinography
Bilateral electroretinogram (ERG) evaluations were performed for all dogs prior to initiating treatment (12 weeks of age) and monthly thereafter as described previously.(Whiting et al., 2013) No ERG recording was performed sooner than 1 week after an intravitreal injection. Each ERG session consisted of scotopic and photopic recordings (Ekesten et al., 2013) elicited bilaterally and recorded simultaneously with a commercial instrument (HMsERG model 2000; RetVet Corp., Columbia, MO). During 20 minutes of dark adaptation, a set of low intensity flashes (0.01 cd.s/m2; 10 flashes administered at 2 second intervals) was delivered every 4 minutes and the average response recorded after each 4 minute interval to assess rod function. In order to assess mixed rod and cone function, stimulus intensity was increased to 3 cd.s/m2 (4 flashes at 10 second intervals) and then to 10 cd.s/m2 (four flashes at 20 second intervals), and the average was recorded for each light intensity. Photopic responses were recorded after 10 minutes of light adaptation with a background luminance of 30 cd/m2. To evaluate cone function, individual flash (32 flashes at 0.5 second intervals) and 30Hz flicker stimuli were presented with an intensity of 3 cd.s/m2. ERG waveforms in all recordings were evaluated, and the amplitudes for the a- and b-waves were measured as previously described (Whiting et al., 2013).
2.7. Retinal morphology
All four dogs were maintained until they reached neurological end-stage disease, defined by the progression of neurological signs to the point that the dogs were no longer able to eat without assistance and suffered from such severe ataxia that they were in danger of injuring themselves from falling or bumping into obstacles. At this point, when the dogs were between 43 and 46 weeks of age, final ERG and ocular imaging data were obtained. After imaging, the dogs were euthanized by intravenous administration of pentobarbital.
Immediately after euthanasia, the eyes were enucleated and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.4 (Tracy et al., 2016). The fixed tissues were stored in the fixative at 4°C until further dissection could be performed. For histological analyses, the eyecup was dissected into 10 regions as illustrated in Fig. 1.
Fig. 1.

Diagram illustrating locations of areas of the eyes from which sections were obtained for microscopic examination and analysis. The black annulus represents the ciliary body, the white circle represents the retina, and the black central dot represents the optic nerve head. The rectangular regions labeled with letters and numbers were dissected from each eye and sections for light and electron microscopy were obtained from the locations indicated by red lines. The regions that were analyzed were central-posterior retina (regions A and D), mid-superior central retina (regions B), superior-ciliary body (regions C), and mid-inferior central retina (regions E).
Samples from selected regions were washed in 0.17 M sodium cacodylate buffer (pH 7.4), post-fixed with osmium tetroxide, and embedded in epoxy resin for light and electron microscopy. Cross-sections of the retina or ciliary body from each region were cut in the orientation shown in Fig. 1 on a Reichert Ultracut S ultramicrotome at a thickness of 0.8 μm, mounted on glass slides and stained with toluidine blue. Images of each of regions A1, B1, C1, and E1 representing 400 μm of retinal length or 1460 μm of ciliary body length were obtained with a Leica AF6000 microscope with a computer-controlled motorized stage using a 40 X objective and Leica Application Suite X software to create a composite image of each sample. For regions A, B and E the number of nuclei in the outer and inner nuclear layers were counted using Photoshop and ImageJ software (https://imagej.nih.gov/ij/) (Katz et al., 2017).
For electron microscopy, the blocks used to obtain the sections for light microscopic analysis were trimmed for ultrathin sectioning. Sections of each of these blocks were cut at thickness of 70 to 80 nm and mounted on 200 mesh copper grids. The sections were stained with lead citrate and uranyl acetate and were examined with a JEOL JEM-1400 transmission electron microscope equipped with a Gatan digital camera.
2.8. Statistical Analyses
All statistical tests were performed using SigmaPlot (Systat Software Inc., San Jose, CA). Data were subjected to the Shapiro-Wilk test to confirm normal distribution. Repeated measures 2-way ANOVA was used to compare ERG data from CLN2-affected eyes treated with intravitreal rhTPP1 to those from vehicle treated eyes to determine if the treatment was able to prevent deficits in ERG b-wave amplitude related to CLN2 disease progression. Follow-up pairwise comparisons were performed with the Holm-Sidak correction (α=0.05) to control family-wise error rate. Statistical comparison of the retinal cell counts between the rhTT1 and vehicle treated eyes was performed separately for each retinal region using Student’s t-test.
3. Results
3.1. Ocular health
All dogs exhibited intraocular inflammation to varying degrees following all but the first injections of rhTPP1. No intraocular inflammation was observed at any time in the control eyes that received vehicle injections. The inflammation in the rhTPP1-treated eyes was typically characterized by transient anterior and posterior uveitis that was controlled with topical anti-inflammatory medications (see Methods for ‘Treatments’). Abnormal debris in the vitreous was observed on OCT images during active inflammation, particularly near the optic nerve head (Figure 2). Sub-tenon injection of triamcinolone at the time of the rhTPP1 administration decreased the severity of the post-treatment inflammation. While active inflammation resolved over time, areas of vitreal opacity persisted long-term and were apparent with in vivo imaging. While the vitreal debris present during active inflammation was not observed long-term once the inflammation resolved, persisting areas of vitreal opacity did partially obscure the underlying retina on OCT (Fig. 2). More severe inflammatory reactions did occur approximately 1 week after specific injections: after a greater dose of 0.3 mg in dog A and after dose 2 in dog D. These reactions were characterized by anterior and posterior uveitis, localized retinal hemorrhage, and inflammation of the optic nerve head. In dog A, the inflammation persisted for several weeks and resulted in a marked reduction in ERG amplitudes (Fig. 3). Based on these symptoms, the next scheduled injection was delayed and the dose was reduced to 0.1 mg. Clinical signs of inflammation largely resolved within 1 month after this injection, but dog A received no further intravitreal rhTPP1 injections for the remainder of the study. For dog D, in order to allow the treatment-related inflammation to resolve completely, the dosing interval was increased to 6 weeks and this interval was used for the remainder of the study period. No adverse systemic effects of the treatments were observed clinically or detected with periodic complete blood counts and blood chemistry analyses.
Fig. 2.

SLO fundus images and corresponding OCT images from the center of the optic nerve head from all dogs throughout the study. Inflammation that occurred early in the study in each dog resulted in long-term vitreal opacity OS for Dogs A, B, and C despite resolution of all active inflammation. This inflammatory response prompted an end to dosing in dog A and reduction in the dosing frequency for the remainder of the study period in dogs B and C. Dosing frequency was further reduced in Dog D, which allowed inflammation to fully resolve between doses and minimized long term vitreal opacity in this dog. Many large focal retinal detachments were present in the control eye of dog C at 10 months of age.
Fig. 3.

ERG response amplitudes for dog A. Short-term intravitreal rhTPP1 treatment partially preserved retinal function. In this dog, b-wave amplitudes in the rhTPP1 treated eye (OS, blue traces) were greater than those in the contralateral control eye (OD, red traces) for all stimulus conditions including rod responses (A), cone responses (B), and mixed rod/cone response (C); b-wave to a-wave amplitude ratios for the mixed rod/cone response were also greater in the treated eye (D). Dog A exhibited a marked decrease in ERG amplitudes in the rhTPP1 treated eye at 4 months of age following a severe inflammatory reaction to a 0.3mg TPP1 injection (see ‘Ocular Health’ section of Results); ERG amplitudes recovered once the clinically observable inflammation resolved. Treatment benefits waned toward the end of the study since dog A received its final intravitreal injection early in the study period at approximately 5 months of age. Black and gray traces represent data from normal (n=7) and CLN2-affected (n=6) untreated dogs previously obtained from Dachshunds from the same research colony (Whiting et al., 2013).
3.2. Intravitreal injection of rhTPP1 preserves retinal function
All dogs exhibited preservation of retinal function as assessed by ERG in the eye treated with intravitreal rhTPP1 relative to the control contralateral eye for all light stimulus conditions (Figs. 3–6). While dog A exhibited partial preservation of the b-wave after short term treatment (Fig. 3), greater preservation of function was observed in dogs B, C, and D that received longer treatment courses (Figs. 4–6). Significantly greater b-wave amplitudes were observed in the treated eyes of dogs B, C and D for pure rod (Fig. 4), mixed rod/cone (Fig. 5) and pure cone (Fig. 6) responses relative to the vehicle-treated eyes. Dog B exhibited a marked increase in rod (Figs. 4, 7) and mixed rod/cone b-wave amplitudes (Figs. 5, 7) in the latter half of the study resulting in amplitudes that were greater than the range observed in normal dogs for several of the recordings. rhTPP1 treatment had no effect on the a-wave amplitudes as no consistent differences were noted between the treated eye and the control eye of any of the dogs (data not shown). The ratio of b-wave to a-wave amplitudes in the treated eye was partially preserved for dog A (Fig. 3D), and fully preserved within the normal range for dogs B, C, and D throughout the study period (Fig. 8), while this ratio progressively declined in the control eyes.
Fig. 6.

ERG cone response amplitudes for dogs B, C, and D. Intravitreal rhTPP1 preserved retinal cone responses in CLN2-affected dogs. The average values for all dogs receiving long-term treatment are shown in panel A and individual results for dogs 2, 3 and 4 are shown in panels B, C, and D respectively. Cone response b-wave amplitudes in the rhTPP1 treated eye (OS, blue traces) were greater than the contralateral control eye (OD, red traces) (†p<0.01, ‡p<0.001). Even at end-stage disease, amplitudes in the treated eye for all dogs were normal while the response from the control eye was non-recordable at this stage. Data from normal (n=7) and affected (n=6) untreated dogs are historical data obtained from Dachshunds from the same research colony (Whiting et al., 2013).
Fig. 4.

ERG rod response amplitudes for dogs B, C, and D. Intravitreal rhTPP1 preserved retinal rod responses in these CLN2-affected dogs. The mean values for all dogs receiving long-term treatment are shown in panel A and individual results for dogs B, C, and D are shown in panels B, C, and D respectively. Mean rod response b-wave amplitudes in the rhTPP1 treated eyes (OS, blue traces) were greater than in the contralateral control eyes (OD, red traces) (*p<0.05). Even at end-stage disease, amplitudes in the treated eye for dog B were greater than normal and for dogs C and D were near normal. This is particularly remarkable since responses in the control eye of each dog were non-recordable by this stage. Data from normal (n=7) and CLN2-affected (n=6) untreated dogs are historical data obtained from Dachshunds from the same research colony (Whiting et al., 2013).
Fig. 5.
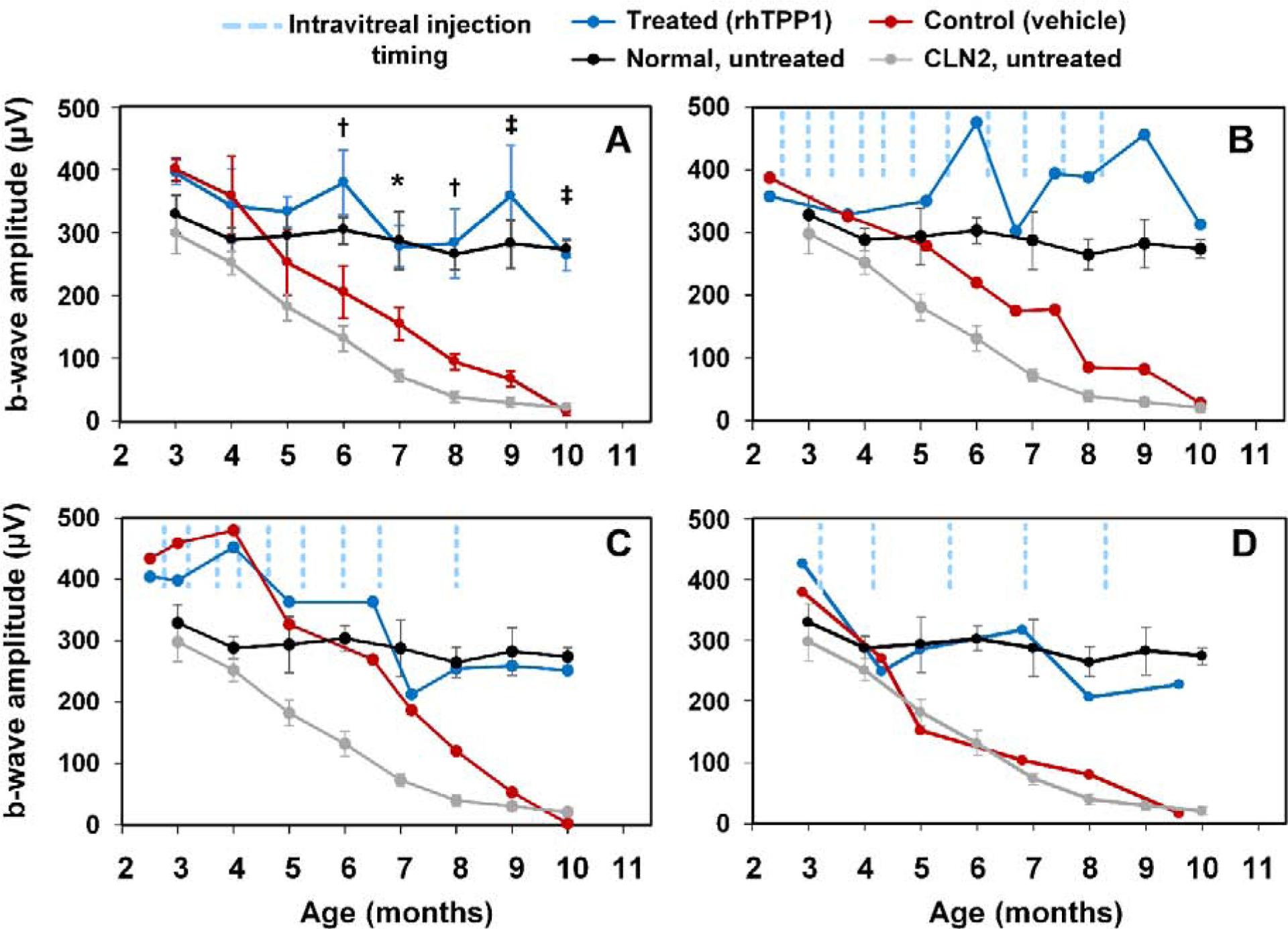
ERG mixed rod and cone response amplitudes for dogs B, C, and D. Intravitreal rhTPP1 preserved retinal mixed rod and cone responses in CLN2-affected dogs. The average values for all dogs receiving long-term treatment are shown in panel A and individual results for dogs B, C, and D are shown in panels B, C, and D respectively. Mean mixed response b-wave amplitudes in the rhTPP1 treated eye (OS, blue traces) were greater than the contralateral control eye (OD, red traces) (*p<0.05, †p<0.01, ‡p<0.001). Even at end-stage disease, amplitudes in the treated eye for all dogs were normal or greater than normal while the responses from the control eyes were non-recordable at this stage. Data from normal (n=7) and CLN2-affected (n=6) untreated dogs are historical data obtained from Dachshunds from the same research colony (Whiting et al., 2013).
Fig. 7.

ERG waveforms from dog B at 3, 6, and 9 months of age. Retinal rod and cone responses from the rhTPP1 treated eye (OS, blue traces) remained normal or greater than normal throughout the study period, while the response from the vehicle treated control eye (OD, red traces) declined dramatically with age, as is typical for untreated CLN2-affected dogs.
Fig. 8.

Intravitreal rhTPP1 preserved ERG b-wave to a-wave amplitude ratios in CLN2-affected dogs. The average values for all dogs receiving long-term treatment are shown in panel A and individual results for dogs B, C, and D are shown in panels B, C, and D respectively. Amplitude ratios from the mixed rod and cone response in the rhTPP1 treated eye (OS, blue traces) were greater than the contralateral control eye (OD, red traces) (*p<0.05, ‡p<0.001). Even at end-stage disease, amplitude ratios in the treated eye for all dogs were normal while the ratios in the control eyes were drastically reduced at this stage. Data from normal (n=7) and affected (n=6) untreated dogs are historical data obtained from Dachshunds from the same research colony.
3.3. Intravitreal injection of rhTPP1 preserves retinal morphology
In vivo imaging of the retina with SLO and OCT clearly demonstrated the development of localized retinal detachments in some of the control eyes (Fig.2), but did not have the resolution to enable visualization of differences in retinal structure between the rhTPP1-treated and control eyes. To determine whether such differences were present, morphological evaluations of the retinas were performed on the eyes collected at the time of euthanasia. As reported in previous studies, there is a thinning of both the inner and outer retina in untreated TPP1-null Dachshunds, with the thinning being more pronounced in the inner than outer retina (Katz et al., 2008). Quantitative analyses of the numbers of cell nuclei in the inner nuclear layer (INL) from 3 different retinal regions demonstrated a significant inhibition of disease-related loss of cells with nuclei located in the INL of rhTPP1-treated eyes relative to the contralateral vehicle-treated eyes (Fig. 9A). The number of photoreceptor nuclei in the same 3 regions of the retina was similar between the two eyes of each dog, with trends toward lower nuclear densities in the vehicle-treated eyes that were not statistically significant (Fig. 9B). The difference in numbers of cell nuclei in the inner nuclear layer between the treated and untreated eyes of dog B is apparent in the light micrographs in Fig. 10. In addition, in the TPP1-treated eyes the photoreceptor outer segments retained their normal straight and parallel morphology, whereas in the vehicle-treated eyes of each of the dogs, the outer segments appeared to be irregular in shape and orientation (Fig. 10). Intraocular inflammation that was evident by SLO to varying degrees following administration of rhTPP1 was microscopically characterized by perivascular to interstitial infiltrate of predominantly lymphocytes and plasma cells in the ciliary body and retina. Inflammatory cells were not present in the vehicle treated eyes.
Fig. 9.

Total number of cell nuclei in the (A) inner nuclear layer (INL) and the (B) outer nuclear layer (ONL) of the retinas from dogs B, C and D in which one eye was treated with periodic injections of rhTPP1 (Treated) and the other eye was injected at the same times with vehicle (Untreated). Nuclei counts were done on areas along the horizontal mid-line of the eye within 500 μm of the optic nerve head (Central), or along the superior-inferior mid-line half-way between the optic nerve head and the superior (Superior) or inferior (Inferior) ora serrata. In all INL regions examined, there were significantly fewer nuclei in the untreated than in the treatedeye at the time of euthanasia (p<0.05, t-test). There were no significant differences between the numbers of ONL nuclei in any of the regions examined. Error bars represent SEM.
Fig. 10.

Light micrographs of sections from the central posterior retinas of the rhTPP1 treated (A) and vehicle-treated (B) eyes of dog B, euthanized at end-stage disease at approximately 11 months of age. In the untreated eye there were fewer nuclei in the inner nuclear layer (inl), whereas the numbers of nuclei in the outer nuclear layer (onl) were similar. The photoreceptor outer segments in the vehicle-treated eye were more irregular in shape and orientation than those in the treated eye. Bar in (B) indicates the magnification of both micrographs.
Electron microscopic examination of the rod outer segments indicated that normal morphology was retained at the bases of the outer segments of both the rhTPP1- and vehicle-treated eyes (Fig. 11 A and C). However, at the distal ends of the outer segments, the disc membranes from the rhTPP1-treated eyes remained tightly packed together, straight, and parallel to one another (Fig. 11B). In contrast, in the vehicle-treated eyes, the rod outer segment discs were more loosely packed and less straight, and irregular gaps between the discs were present in areas of the retina where the retina remained closely apposed to the underlying retinal pigment epithelium (Fig. 11D). These differences were observed in each of the dogs evaluated in this study.
Fig. 11.

Electron micrographs of the base (A) and the apical end (B) of rod outer segments from the eye posterior pole of dog B that received intravitreal injections of rhTPP1, and electron micrographs of the base (C) and apical end (D) of rod outer segments from the vehicle-treated eye of the same dog. The dog was euthanized at approximately 11 months of age. The outer segment discs are more tightly packed in the rods from the rhTPP1-treated eye, particularly at the apical ends of the outer segments. Bar in (A) indicates the magnification of all 4 micrographs.
Upon ultrastructural examination of the outer plexiform layer and flanking inner and outer nuclear layers of rhTPP1-treated and vehicle-treated eyes, the only consistent difference between the eyes was that in those that were treated with rhTPP1, axons extending from the inner plexiform layer into the outer nuclear layer (arrows in Fig. 12A) were quite common, but were rare in the vehicle-treated eyes (Fig. 12B). In the vehicle-treated eyes, axons extending in the direction of the outer nuclear layer in most cases terminated before the inner boundary of this layer (arrow in Fig 12B).
Fig. 12.

Electron micrographs of the retinal outer plexiform layer (opl) and adjacent portions of the inner nuclear layer (inl) and outer nuclear layer (onl) from the rhTPP1-treated (A) and vehicle-treated (B) eyes of dog B euthanized at approximately 11 months of age. The major difference between these areas of the retinas was that axons from inl cells frequently extended into the onl of the treated eye (arrows in A), whereas most axons in this region of the untreated eye terminated near the inner edge of the onl.
In the rhTPP1-treated eyes, the ciliary bodies were compact and tightly adherent to the underlying sclera (Fig. 13A). In contrast, in the vehicle-treated eyes, the ciliary bodies were as much as 3 times as thick as those in the rhTPP1-treated eyes, and the stroma was much more loosely organized with many acellular gaps in the tissue (Fig. 13B). In addition, except in small areas, the ciliary body was completely separated from the sclera in the vehicle treated eyes (Fig. 13B).
Fig. 13.

Light micrographs of the ciliary bodies (cb) and adjacent portions of the scleras (s) from the rhTPP1-treated (A) and vehicle-treated (B) eyes of dog B euthanized at approximately 11 months of age. The ciliary body of the sham-treated eye is dramatically distended and includes unstained gaps relative to the much more compact ciliary body of the rhTPP1-treated eye. In the rhTPP1-treated eyes, the ciliary body was tightly adhered to the underlying sclera (s), whereas the ciliary body was separated from the sclera in the sham-treated eyes.
Electron microscopic examination of the ciliary body revealed a dramatic decrease in the density of the collagen layers in this tissue in the vehicle-treated relative to the rhTPP1-treated eyes (Fig. 14). The melanocytes appeared similar in both eyes of each dog. However, most of the stromal cells (s) of the sham-treated eye were very thin compared to stromal cells of the rhTPP1-treated eyes (Fig. 14).
Fig. 14.

Electron micrographs of areas of the ciliary bodies from the TPP1-treated (A) and vehicle-treated (B) eyes of dog B illustrating the tight packing between the cells and extracellular matrix in the treated eye and the presence of large gaps within the tissue of the vehicle-treated eye associated with a decrease in collagen (c) density, and a thinning of the stromal cell bodies (s). No obvious pathological changes were apparent in the melanocytes (m).
4. Discussion
This study demonstrates that repeated intravitreal injections of rhTPP1 are effective in inhibiting retinal degeneration and preserving retinal function in canine CLN2 disease. In dogs B and C, injections performed once every 3 weeks provided adequate TPP1 delivery to maintain normal or above normal ERG amplitudes. However, dosing every 3 weeks resulted in recurrent inflammatory reactions. To determine whether less frequent dosing could achieve a similar therapeutic benefit with less intraocular inflammation, dog D was treated at 6 week intervals rather than at 3 week intervals. The less frequent dosing was just as effective at preserving retinal function, while substantially reducing the severity and duration of post-injection intraocular inflammation. It is possible that even less frequent dosing would be sufficient to preserve retinal function and eliminate the treatment-related inflammation completely. Indeed, data from dog A demonstrate that while short-term dosing early in the disease course was not sufficient to completely preserve normal retinal function, the eye that received a few early doses of rhTPP1 exhibited ERG amplitudes that were greater than those of the vehicle-treated eye for 6 months after the rhTPP1 injections were discontinued. The overall results indicate that optimization of the dosing regimen should be possible by determining the minimum dosing frequency and amount of rhTPP1 per dose necessary to maintain normal ERG amplitudes while minimizing or eliminating inflammatory responses.
In this study rhTPP1 dosing was initiated before any signs of retinal degeneration or loss of function were detectable. In addition, it is important to assess whether rhTPP1 administration could halt or reverse disease-related retinal pathology if treatment is initiated after partial declines in visual function have already occurred. This would indicate whether continued decline in retinal function later in the disease process is due to lack of TPP1.
Inflammation following intravitreal injection of rhTPP1 was not unexpected since rhTPP1 is a foreign protein for dogs with a null mutation in the TPP1 gene. While the eye is widely considered to be an immune-privileged site, other studies have demonstrated that there are limits to this privilege (Caspi, 2010; Caspi et al., 2008; Dick, 2017; Egwuagu et al., 2015; Lee et al., 2014; Marticorena et al., 2012; Trivizki et al., 2018). In addition, dog eyes may be more susceptible to developing immune-mediated uveitis than eyes of other species (Bistner, 1994; Paulsen et al., 1986; Wilcock and Peiffer, 1987). In addition, since therapeutic efficacy was retained even at the lowest doses and dosing frequency tested, our data suggest that even in the dog model, it may be possible to develop a dosing regimen that is effective therapeutically but does not result in ocular inflammation. The experience of contract research companies that have performed numerous preclinical studies in dogs for pharmaceuticals that have subsequently undergone human clinical trials indicates that treatment-related inflammatory responses that occur in dogs are seldom recapitulated in human subjects (E. Adams and R. Reed, personal communications, 2014). Inflammatory changes observed in preclinical studies using heterologous protein are well known (Krzystolik et al., 2002). These inflammatory findings have not translated to humans with a variety of approved drugs administered via intravitreal injection (Wakshull et al., 2017), suggesting that IVT injection of rhTPP1 in children with CLN2 disease is unlikely to result in significant intraocular inflammation.
Not only are human subjects less prone to exhibit intraocular inflammation in response to IVT administration of “foreign” proteins, but it may be possible to induce immune tolerance prior to the IVT treatments. In a previous study with the dog model, we demonstrated that immune tolerance to rhTPP1 could be induced by repeated ICV infusion of this protein (Katz et al., 2014). Further study will be necessary to determine whether such induction of systemic immune tolerance would prevent intraocular inflammation associated with IVT administration of rhTPP1. As we have shown, however, local immunosuppressive treatment can attenuate the inflammation associated with the IVT TPP1 treatments. Indeed, IVT administration of other therapeutic proteins can result in intraocular inflammation that has been effectively treated with immunosuppressants (Bae and Lee, 2010; Bakri et al., 2008; Wickremasinghe et al., 2008).
IVT administration of rhTPP1 was clearly effective in preserving retinal function and structure in the canine CLN2 disease model. Although some of the morphological differences between the retinas of the treated and control eyes were not evaluated quantitatively, they were observed consistently among the dogs evaluated in this study. The morphological differences were consistent with the differences in ERG responses. Quantitative assessment of differences such as photoreceptor outer segment morphology awaits studies in which multiple dogs receive the same optimal treatment.
The efficacy of IVT administration of rhTPP1 in preserving retinal structure and function that was observed in this study supports the importance of evaluating IVT administration of rhTPP1 in human patients. Indeed, in a previous study it was demonstrated that periodic infusion of rhTPP1 into the CSF of TPP1-null dogs significantly delayed the onset and progression of neurological disease signs (Katz et al., 2014). This treatment is now being used to successfully treat children with CLN2 disease (Schulz et al., 2018). In this disorder, the onset of neurological signs typically precedes significant ocular disease. Diagnosing the disorder after the initial neurological signs are observed but prior to significant visual impairment creates an opportunity to initiate treatment that could delay or prevent disease-related retinal degeneration. In the dog model CLN2 disease results from a complete absence of TPP1. In human patients, on the other hand, the disorder can result from a variety of deleterious TPP1 variants, including null and missense variants. Further study will be necessary to determine whether IVT rhTPP1 is effective in treating ocular disease associated with other types of TPP1 variants.
In a previous study it was found that IVT injection of autologous mesenchymal stem cells (MSCs) programmed to overexpress and secrete human TPP1 was also effective in preserving retinal structure and function in the Dachshund CLN2 disease model (Tracy et al., 2016). Unlike periodic IVT administration of the recombinant protein, the stem cell treatment was not accompanied by significant intraocular inflammation. Possible reasons for this include the known anti-inflammatory properties of MSCs (Jiang and Xu, 2020; Regmi et al., 2019) and the likelihood that peak intravitreal and blood concentrations of TPP1 were much lower in the MSC-treated dogs than in dogs that received bolus IVT injections of rhTPP1. Unfortunately, there has been little interest to date in conducting human clinical trials for the MSC treatment approach for CLN2 disease. Preliminary experiments have been conducted to assess the safety and efficacy of IVT injection of AAV-TPP1 gene therapy vectors in the Dachshund model. To date the therapeutic efficacy of this treatment has been variable and the treatment has resulted in significant intraocular inflammation (unpublished findings).
CLN2 is an example of a disease that will require targeting therapies to multiple organ systems to effectively treat the entire disorder. Although rhTPP1 enzyme replacement administered via periodic infusion into the CSF is already used to treat the neurologic symptoms of CLN2 disease in children (Schulz et al., 2018), our previous studies with the dog model have demonstrated that direct targeting of therapeutics to the retina will probably be necessary in order to preserve vision (Whiting et al., 2016). The data presented here support further investigation to evaluate intravitreal enzyme replacement as a potential treatment to preserve vision in CLN2 disease.
Highlights.
Progressive retinal degeneration occurs in CLN2 neuronal ceroid lipofuscinosis.
Retinal degeneration results from deficiency in lysosomal enzyme TPP1.
Periodic intravitreal injection of recombinant TPP1 preserves retinal structure and function.
Acknowledgments
Thanks to those who contributed to the care and health of the dogs and colony management, Drs. Dawna Voelkl and Dietrich Volkmann who performed and organized the dog breeding procedures, Cheryl A. Jensen for assistance with microscopy, Jeffrey Student for technical assistance, and the students that socialize the dogs daily and assist with procedures. John Sinclair, Sundeep Chandra, Annalisa Nguyen, and Charles A. O’Neill were employed by and had a financial interest in BioMarin Pharmaceutical at the time this study was performed. Supported primarily by funds from BioMarin and also in part by U.S. National Institutes of Health grant EY023968.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awano T, Katz ML, O’Brien DP, Sohar I, Lobel P, Coates JR, Khan S, Johnson GC, Giger U, Johnson GS, 2006. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol Genet Metab 89, 254–260. [DOI] [PubMed] [Google Scholar]
- Awwad S, Mohamed Ahmed AHA, Sharma G, Heng JS, Khaw PT, Brocchini S, Lockwood A, 2017. Principles of pharmacology in the eye. Br J Pharmacol 174, 4205–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba J, Peng RS, Xu D, Li YH, Shi H, Wang Q, Yu J, 2015. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug design, development & therapy 9, 5397–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Lee SC, 2010. Bilateral intraocular inflammation after intravitreal bevacizumab in Behcet’s disease. Eye 24, 735. [DOI] [PubMed] [Google Scholar]
- Bakri SJ, Larson TA, Edwards AO, 2008. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol 246, 779–781. [DOI] [PubMed] [Google Scholar]
- Bistner S, 1994. Allergic- and immunologic-mediated diseases of the eye and adnexae. Vet Clin North Am Small Anim Pract 24, 711–734. [DOI] [PubMed] [Google Scholar]
- Brooks DA, 2009. The endosomal network. International Journal of Clinical Pharmacology & Therapeutics 47 Suppl 1, S9–17. [DOI] [PubMed] [Google Scholar]
- Caspi RR, 2010. A look at autoimmunity and inflammation in the eye. J Clin Invest 120, 3073–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G, Mattapallil MJ, Chan CC, 2008. Mouse models of experimental autoimmune uveitis. Ophthalmic Res 40, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA, Lobel P, Davidson BL, 2008. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Molecular Therapy: the Journal of the American Society of Gene Therapy 16, 649–656. [DOI] [PubMed] [Google Scholar]
- Dick AD, 2017. Doyne lecture 2016: intraocular health and the many faces of inflammation. Eye 31, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington M, Connolly J, Chong NV, 2017. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opinion On Drug Metabolism & Toxicology 13, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Egwuagu CE, Sun L, Kim SH, Dambuza IM, 2015. Ocular Inflammatory Diseases: Molecular Pathogenesis and Immunotherapy. Current Molecular Medicine 15, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekesten B, Komaromy AM, Ofri R, Petersen-Jones SM, Narfstrom K, 2013. Guidelines for clinical electroretinography in the dog: 2012 update. Doc Ophthalmol 127, 79–87. [DOI] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Sly WS, 2010. New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Research 13, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhaniyogi J, Sohar I, Das K, Stock AM, Lobel P, 2009. Crystal structure and autoactivation pathway of the precursor form of human tripeptidyl-peptidase 1, the enzyme deficient in late infantile ceroid lipofuscinosis. J Biol Chem 284, 3985–3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins KA, Haschke M, Do DV, 2016. Aflibercept for the treatment of diabetic macular edema. Immunotherapy 8, 503–510. [DOI] [PubMed] [Google Scholar]
- Jiang W, Xu J, 2020. Immune modulation by mesenchymal stem cells. Cell Prolif 53, e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Cooper JJ, O’Brien DP, Jeong M, Narfstrom K, 2008. Retinal pathology in a canine model of late infantile neuronal ceroid lipofuscinosis. Invest Ophthalmol Vis Sci 49, 2686–2695. [DOI] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Sibigtroth CM, Taylor JD, Carpentier M, Young WM, Wininger FA, Kennedy D, Vuillemenot BR, O’Neill CA, 2014. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease). J Neurosci Res 92, 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Jensen CA, Student JT, Johnson GC, Coates JR, 2017. Cervical spinal cord and motor unit pathology in a canine model of SOD1-associated amyotrophic lateral sclerosis. J Neurol Sci 378, 193–203. [DOI] [PubMed] [Google Scholar]
- Katz ML, Tecedor L, Chen Y, Williamson BG, Lysenko E, Wininger FA, Young WM, Johnson GC, Whiting RE, Coates JR, Davidson BL, 2015. AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Sci Transl Med 7, 313ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, Li W, Connolly E, O’Neill CA, Miller JW, 2002. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol 120, 338–346. [DOI] [PubMed] [Google Scholar]
- Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, Dick AD, 2014. Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol 36, 581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CG, Sleat DE, Donnelly RJ, Lobel P, 1998. Structural organization and sequence of CLN2, the defective gene in classical late infantile neuronal ceroid lipofuscinosis. Genomics 50, 206–212. [DOI] [PubMed] [Google Scholar]
- Markham A, 2017. Cerliponase Alfa: First Global Approval. Drugs 77, 1247–1249. [DOI] [PubMed] [Google Scholar]
- Marticorena J, Romano V, Gomez-Ulla F, 2012. Sterile endophthalmitis after intravitreal injections. Mediators of Inflammation 2012, 928123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DF, 2018. Evolution of Intravitreal Therapy for Retinal Diseases-From CMV to CNV: The LXXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole SE, Cotman SL, 2015. Genetics of the neuronal ceroid lipofuscinoses (Batten disease). Biochim Biophys Acta 1852, 2237–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole SE, Williams R, Goeble HH, 2011. The Neuronal Ceroid Lipofuscinoses (Batten Disease), 2nd ed Oxford University Press. [Google Scholar]
- Patel S, Muller G, Stracke JO, Altenburger U, Mahler HC, Jere D, 2015. Evaluation of protein drug stability with vitreous humor in a novel ex-vivo intraocular model. Eur J Pharm Biopharm 95, 407–417. [DOI] [PubMed] [Google Scholar]
- Paulsen ME, Lavach JD, Severin GA, Eichenbaum JD, 1986. The effect of lens-induced uveitis on the success of extracapsular cataract extraction: A retrospective study of 65 lens removals in the dog. Journal of the American Animal Hospital Association 22, 49–55. [Google Scholar]
- Radhakrishnan K, Sonali N, Moreno M, Nirmal J, Fernandez AA, Venkatraman S, Agrawal R, 2017. Protein delivery to the back of the eye: barriers, carriers and stability of anti-VEGF proteins. Drug Discov Today 22, 416–423. [DOI] [PubMed] [Google Scholar]
- Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH, 2019. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur J Cell Biol 98, 151041. [DOI] [PubMed] [Google Scholar]
- Schulz A, Ajayi T, Specchio N, de Los Reyes E, Gissen P, Ballon D, Dyke JP, Cahan H, Slasor P, Jacoby D, Kohlschutter A, Group CLNS, 2018. Study of Intraventricular Cerliponase Alfa for CLN2 Disease. New England Journal of Medicine 378, 1898–1907. [DOI] [PubMed] [Google Scholar]
- Schulz A, Specchio N, Gissen P, de los Reyes E, Cahan H, Slasor P, Ajayi T, Jacoby D, 2017. Intracerebroventricular cerliponase alfa for children with CLN2 disease: Interim results from an ongoing phase 2 extension study. European Journal of Paediatric Neurology 21. [Google Scholar]
- Sleat DE, Donnelly RJ, Lackland H, Liu CG, Sohar I, Pullarkat RK, Lobel P, 1997. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science 277, 1802–1805. [DOI] [PubMed] [Google Scholar]
- Tracy CJ, Whiting RE, Pearce JW, Williamson BG, Vansteenkiste DP, Gillespie LE, Castaner LJ, Bryan JN, Coates JR, Jensen CA, Katz ML, 2016. Intravitreal implantation of TPP1-transduced stem cells delays retinal degeneration in canine CLN2 neuronal ceroid lipofuscinosis. Exp Eye Res 152, 77–87. [DOI] [PubMed] [Google Scholar]
- Trivizki O, Schwartz S, Negri N, Loewenstein A, Rabina G, Shulman S, 2018. Noninfectious Inflammatory Response following Intravitreal Bevacizumab Injections: Description of a Cluster of Cases in Two Centers and a Review of the Literature. Ophthalmologica 240, 163–166. [DOI] [PubMed] [Google Scholar]
- Villegas VM, Aranguren LA, Kovach JL, Schwartz SG, Flynn HW Jr., 2017. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opinion on Drug Delivery 14, 273–282. [DOI] [PubMed] [Google Scholar]
- Vuillemenot BR, Kennedy D, Cooper JD, Wong AM, Sri S, Doeleman T, Katz ML, Coates JR, Johnson GC, Reed RP, Adams EL, Butt MT, Musson DG, Henshaw J, Keve S, Cahayag R, Tsuruda LS, O’Neill CA, 2015. Nonclinical evaluation of CNS-administered TPP1 enzyme replacement in canine CLN2 neuronal ceroid lipofuscinosis. Mol Genet Metab 114, 281–293. [DOI] [PubMed] [Google Scholar]
- Wakshull E, Quarmby V, Mahler HC, Rivers H, Jere D, Ramos M, Szczesny P, Bechtold-Peters K, Masli S, Gupta S, 2017. Advancements in Understanding Immunogenicity of Biotherapeutics in the Intraocular Space. Aaps J 19, 1656–1668. [DOI] [PubMed] [Google Scholar]
- Whiting RE, Jensen CA, Pearce JW, Gillespie LE, Bristow DE, Katz ML, 2016. Intracerebroventricular gene therapy that delays neurological disease progression is associated with selective preservation of retinal ganglion cells in a canine model of CLN2 disease. Exp Eye Res 146, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting RE, Narfstrom K, Yao G, Pearce JW, Coates JR, Castaner LJ, Jensen CA, Dougherty BN, Vuillemenot BR, Kennedy D, O’Neill CA, Katz ML, 2014. Enzyme replacement therapy delays pupillary light reflex deficits in a canine model of late infantile neuronal ceroid lipofuscinosis. Exp Eye Res 125, 164–172. [DOI] [PubMed] [Google Scholar]
- Whiting RE, Narfstrom K, Yao G, Pearce JW, Coates JR, Castaner LJ, Katz ML, 2013. Pupillary light reflex deficits in a canine model of late infantile neuronal ceroid lipofuscinosis. Exp Eye Res 116, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting RE, Pearce JW, Castaner LJ, Jensen CA, Katz RJ, Gilliam DH, Katz ML, 2015. Multifocal retinopathy in Dachshunds with CLN2 neuronal ceroid lipofuscinosis. Exp Eye Res 134, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickremasinghe SS, Michalova K, Gilhotra J, Guymer RH, Harper CA, Wong TY, Qureshi S, 2008. Acute intraocular inflammation after intravitreous injections of bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology 115, 1911–1915. [DOI] [PubMed] [Google Scholar]
- Wilcock BP, Peiffer J, R L, 1987. The Pathology of Lens-induced Uveitis in Dogs. Veterinary Pathology 24, 549–553. [DOI] [PubMed] [Google Scholar]
- Wong AM, Rahim AA, Waddington SN, Cooper JD, 2010. Current therapies for the soluble lysosomal forms of neuronal ceroid lipofuscinosis. Biochemical Society Transactions 38, 1484–1488. [DOI] [PubMed] [Google Scholar]


