Abstract
Background and Purpose:
Enlarged perivascular spaces (EPVS) have been associated with aging, increased stroke risk, decreased cognitive function and vascular dementia. However, the relationship of EPVS with age-related neuropathologies is not well understood. Therefore, the purpose of this study was to assess the neuropathologic correlates of EPVS in a large community-based cohort of older adults. The cognitive correlates of EPVS over and beyond those of other pathologies were also assessed.
Methods:
This study included 654 older deceased and autopsied participants of three longitudinal community-based studies of aging that had available data on cognition, ex-vivo brain MRI, and detailed neuropathologic examination. EPVS seen on ex-vivo MRI were histologically validated. Experienced observers rated EPVS burden in ex-vivo MRI using a semiquantitative four-level scale. Elastic-net regularized ordinal logistic regression was used to investigate associations of EPVS burden with age-related neuropathologies. Mixed effects models of cognition controlling for neuropathologies, demographics, and clinical factors, were used to determine whether EPVS burden has additional contributions to cognitive decline.
Results:
EPVS burden in the whole group was associated with gross infarcts (OR=1.67, p=0.0017) and diabetes (OR=1.73, p=0.004). When considering only non-demented participants (with mild or no cognitive impairment), EPVS burden was associated with gross infarcts (OR=1.74, p=0.016) and microscopic infarcts (OR=1.79, p=0.013). EPVS burden was associated with faster decline in visuospatial abilities (estimate=−0.009, p=0.028), in the whole group, as well as lower levels of semantic memory (estimate=−0.13, p=0.048) and visuospatial abilities (estimate=−0.11, p=0.016) at the time of death.
Conclusions:
EPVS and infarcts may share similar neurobiological pathways regardless of dementia status. EPVS burden is linked to diabetes independently of neuropathologies, extending recent findings in animal studies implicating diabetes in impairment of the glymphatic system. Finally, EPVS burden may reflect additional brain tissue injury that may contribute to cognitive decline, not captured with traditional neuropathologic measures.
Keywords: magnetic resonance imaging, MRI, neuropathology, cognition, perivascular spaces
Subject Terms: Aging, Magnetic Resonance Imaging, Cerebrovascular Disease/Stroke
Introduction
Perivascular spaces (PVS), also known as Virchow-Robin spaces, are fluid-filled spaces surrounding blood vessels as they course from the subarachnoid space through the brain parenchyma. The current body of literature suggests that PVS form a network that facilitates fluid transport between cerebrospinal and interstitial fluid, enables clearance of waste products from the brain, and is vital for maintaining brain homeostasis1. Additionally, PVS are a key part of the blood-brain barrier and have an important role during inflammatory processes2. Abnormal enlargement of perivascular spaces is common in older adults3. The literature has linked an increased burden of enlarged perivascular spaces (EPVS) to an increased risk of stroke4–7, lower cognitive function8, and vascular dementia9. Risk factors associated with EPVS include advanced age4,5,7, male sex5, hypertension4,5, features of small vessel disease4–7,10, and brain atrophy7. However, the neuropathologic correlates of EPVS in the brain of older adults are not well understood.
Only a few magnetic resonance imaging (MRI)-pathology studies have investigated the neuropathologic correlates of EPVS. An early investigation on 19 older adult patients, nine with EPVS, found an association with arteriolosclerosis11. A study on 14 older adult patients with cerebral amyloid angiopathy (CAA) and 10 middle-aged patients with intracerebral hemorrhage but no evidence of CAA pathology, showed that severe central semiovale EPVS were more frequent in patients with CAA12. Another study on 5 older adults with Alzheimer’s and CAA pathologies showed that more severe dilation of juxtacortical PVS was associated with higher cortical CAA score13. A study on a hospital-based cohort of 63 middle-aged and older adults, and a separate cohort of 54 young adult and middle-aged carriers and noncarriers of the hereditary cerebral hemorrhage with amyloidosis-Dutch type, showed that persons with CAA had significantly higher EPVS burden in the central semiovale compared to persons without14. Although the last three studies are in general agreement, there are discrepancies with previous work. Furthermore, a few important considerations must be taken into account when attempting to interpret the above results. First, all previous MRI-pathology investigations were conducted in small hospital-based cohorts, limiting statistical power and generalizability of findings. Second, only one or few pathologies were considered in each study and the effects of comorbid pathologies were not controlled for. This is problematic due to the fact that mixed pathologies are common in the brain of older adults15.
The purpose of this study was to investigate the neuropathologic correlates of EPVS burden by combining ex-vivo brain MRI and pathology (from autopsy) in a large community-based cohort of older adults. MRI was conducted ex-vivo to capture brain characteristics at the same condition of the brain as pathologic examination and to allow imaging of older adults independent of frailty level. Detailed pathologic examination was conducted to assess gross and microscopic infarcts, atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, amyloid-β plaques, neurofibrillary tangles, Lewy bodies, hippocampal sclerosis, and transactive response DNA binding protein of 43 kDa (TDP-43) pathology, and the association of EPVS burden with this comprehensive array of age-related neuropathologies was tested. Cognition was assessed longitudinally, and the independent association of EPVS burden with cognition and cognitive decline above and beyond the effects of neuropathologies and demographics was tested.
Materials and methods
Data availability
The data used in this work can be accessed by submitting a request to www.radc.rush.edu.
Participants
Older adults participating in three longitudinal, epidemiologic, clinical-pathologic cohort studies of aging, the Rush Memory and Aging Project, the Religious Orders Study16, and the Minority Aging Research Study17, were included in this work. All three studies were approved by the Institutional Review Board of Rush University Medical Center. Participants provided written informed consent and signed an anatomical gift act. All participants underwent annual uniform structured clinical evaluations, including cognitive function testing, medical history, and neurologic examination18. At the time of these analyses, 4566 participants of the parent projects had completed the baseline clinical evaluation. Of these, 586 died and 71 withdrew from the studies before the ex-vivo MRI sub-study began. Of the remaining 3909 persons, 1333 died, 1030 were autopsied (the remaining 303 were not autopsied because the death was not made known to study staff, or occurred out of town), and 830 had ex-vivo MRI and pathology data (the remaining 200 did not have ex-vivo MRI because the death occurred out of town). The first 654 consecutive participants with ex-vivo MRI and pathology data that passed quality tests were considered in this work (Table 1) (from the remaining 176, 19 did not pass quality tests and 157 had not been quality-tested).
Table 1.
Demographic and clinical characteristics of the participants.
| Characteristics | No Dementia | Dementia | Combined |
|---|---|---|---|
| N | 353 | 301 | 654 |
| Age at death, years (SD) | 89.3 (6.71) | 91.1 (6.52) | 90.1 (6.67) |
| Male, n (%) | 99 (28) | 81 (27) | 180 (28) |
| Education, years (SD) | 15.7 (3.7) | 15.6 (3.5) | 15.7 (3.6) |
| Median length of follow-up, years | 7.9 | 8.0 | 8.0 |
| Median time between last clinical evaluation and death, years | 0.75 | 0.57 | 0.67 |
| Mini-mental State Examination (MMSE), mean (SD) | 26.7 (2.6) | 11.4 (8.4) | 19.8 (9.7) |
| Heart disease, n (%) | 81 (23) | 45 (15) | 126 (19) |
| Hypertension, n (%) | 251 (71) | 211 (70) | 462 (71) |
| Diabetes, n (%) | 85 (24) | 60 (20) | 145 (22) |
| Smoking, n (%) | 124 (35) | 90 (30) | 214 (33) |
| Systolic blood pressure, mm Hg (SD) | 128 (20) | 126 (22) | 127 (21) |
| Diastolic blood pressure, mm Hg (SD) | 71 (11) | 71 (11) | 71 (11) |
| At least one copy of the ε4 allele, n (%) | 78 (22) | 106 (35) | 184 (28) |
| Right hemisphere, n (%) | 159 (45) | 126 (42) | 285 (44) |
| Postmortem Interval to Fixation, h (SD) | 9.8 (7.2) | 8.7 (5.9) | 9.3 (6.7) |
| Postmortem Interval to Imaging, d (SD) | 38.5 (20.1) | 39.5 (18.3) | 38.9 (19.2) |
| 3T Siemens Verio | 127 (36) | 102 (34) | 229 (35) |
Cognitive function testing and clinical diagnosis
A battery of 17 cognitive tests was used annually to assess performance in five cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability16. Composite scores for each domain as well as for global cognition were constructed by averaging z-scores from different tests within each cognitive domain, as well as over all tests. In addition, the Mini-Mental State Examination was used only for descriptive purposes and the Complex Ideational Material was used only for diagnostic purposes.
Alzheimer’s dementia was diagnosed based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association19. A summary final diagnostic opinion was rendered by a neurologist at the time of death, blinded to all postmortem data. Two groups of participants in descending order of average cognitive impairment were defined based on the final diagnosis: the whole group (including demented and non-demented participants), and the subgroup of non-demented participants. The reason for studying these two groups was that any resulting associations of EPVS burden with age-related neuropathologies and cognitive decline would indicate a potential role of EPVS in biomarkers which would be of importance for older adults in general, and for those without dementia in particular.
Brain hemisphere preparation
At autopsy, the brain was removed and the hemisphere with more visible pathology was selected for ex-vivo MRI and pathologic examination, while the contralateral hemisphere was frozen and stored. The selected hemisphere was immersed in phosphate-buffered 4% paraformaldehyde solution and refrigerated at 4°C within 30 min after removal from the skull. Prior to ex-vivo MRI, the hemisphere was positioned in a container filled with 4% paraformaldehyde solution with its medial aspect facing the bottom of the container, and returned to room temperature20,21. Gross examination of the hemisphere was performed within 2 weeks after ex-vivo MRI, followed by histopathologic diagnostic examination21.
Ex-vivo MRI: data acquisition and pre-processing
Ex-vivo MRI data were collected approximately 30 days postmortem using 3 Tesla MRI scanners. Due to the long study duration and scanner upgrades, four MRI scanners were used in this work. Nevertheless, two dimensional multi-echo spin-echo sequences with 0.6×0.6×1.5 mm3 resolution and similar values for other parameters were used on all scanners (see Supplementary Table I), and only T2-weighted images collected at echo times between 49.5–55 ms were used in the analysis to maintain consistency in image contrast across scanners. All data were collected sagittally. The data from different participants were first bias-field-corrected and then intensity-normalized by means of z-scores to standardize the dynamic range used for display when rating EPVS burden. Only intensities within the 2.5–97.5 percentile range were considered in the calculation of the mean and standard deviation used in the conversion of raw image intensities to z-scores.
EPVS burden rating
An experienced observer blinded to all pathologic and clinical data rated EPVS burden in the brain hemisphere of each participant based on the intensity-normalized T2-weighted ex-vivo MRI data, using a semiquantitative four-level scale (none=0, mild=1, moderate=2, severe=3) (Fig.1). EPVS were defined as hyperintense, tubular, three-dimensional structures. The four levels of the rating scale were defined as follows. None: no EPVS; mild: fewer than 10 EPVS; moderate: more than 10 EPVS, but not widespread; severe: widespread EPVS (Fig.1).
Figure 1.

Examples of ex-vivo EPVS burden rating for twelve participants.
Intra-rater and inter-rater reliability were assessed using Cohen’s kappa coefficient (κ). For intra-rater reliability assessment, the experienced observer repeated the rating in 266 of the 654 participants. For inter-rater reliability assessment, the experienced observer trained a second observer to rate EPVS burden, and the second observer rated 30 of the participants.
Neuropathologic evaluation
Following ex-vivo MRI, hemispheres underwent detailed neuropathologic examination by a board-certified neuropathologist blinded to all clinical and imaging data. A detailed description of the well-established procedures for neuropathologic examination can be found in reference21. In brief, hemispheres were first sectioned into 1 cm thick coronal slabs and were macroscopically evaluated. Selected tissue blocks were then dissected, embedded in paraffin, cut into sections, and mounted on glass slides. Gross infarcts of any age were rated as present or absent. Microscopic infarcts of any age were detected in a minimum of nine regions and were also rated as present or absent. Atherosclerosis at the circle of Willis was rated as none, mild, moderate, or severe. Arteriolosclerosis was assessed in one section of anterior basal ganglia and was rated as none, mild, moderate, or severe. Cerebral amyloid angiopathy was assessed in four regions and was rated as none, mild, moderate, or severe. Amyloid-β plaques and neurofibrillary tangles were assessed in eight brain regions, and separate composite measures for β-amyloid burden and paired helical filaments tau tangle density were constructed. Lewy bodies were detected in six regions and were rated as present or absent. Hippocampal sclerosis was rated as present or absent. TDP-43 pathology was rated on four levels: no inclusions; inclusions in amygdala only; inclusions in amygdala and entorhinal cortex or hippocampus CA1; inclusions in amygdala, entorhinal cortex or hippocampus CA1, and neocortex (Table 2).
Table 2.
EPVS burden and neuropathologic findings.
| Mild or None | Moderate | Severe | |
|---|---|---|---|
| N (%) | 311 (48) | 170 (26) | 173 (26) |
| Gross infarcts, n (%) | 109 (35) | 83 (49) | 85 (49) |
| Microscopic infarcts, n (%) | 97 (31) | 65 (38) | 72 (41) |
| Mild | 166 (53) | 79 (47) | 92 (53) |
| Mild | 113 (36) | 73 (43) | 76 (44) |
| Mild | 137 (44) | 85 (50) | 69 (40) |
| High or intermediate | 218 (70) | 124 (73) | 121 (70) |
| Amyloid plaques, mean (SD) | 0.86 (0.66) | 0.84 (0.63) | 0.85 (0.62) |
| Neurofibrilary tangles, mean (SD) | 0.80 (0.87) | 0.78 (0.89) | 0.70 (0.84) |
| Lewy Bodies, n (%) | 90 (29) | 45 (27) | 54 (31) |
| Hippocampal sclerosis, n (%) | 46 (15) | 18 (11) | 13 (8) |
| Inclusions in amygdala only | 51 (16) | 40 (24) | 25 (14) |
Histopathologic validation of EPVS
Twelve single EPVS from six participants (two participants with mild EPVS burden rating, two with moderate and two with severe rating; two EPVS per participant) were first identified on ex-vivo MRI scans and then evaluated neuropathologically. EPVS selection involved reviewing ex-vivo MRI scans from a small number of randomly selected participants with different ratings until two examples of hyperintense, tubular, three-dimensional structures were identified in each of six participants. No preference was given to any particular brain region. The twelve single EPVS identified in ex-vivo MRI were spatially matched with pre-existing and photographed 1cm slabs of postmortem brain tissues. Representative blocks were taken, embedded in paraffin wax, and underwent 6-micron serial sectioning throughout the block. Every 10th tissue section was stained with hematoxylin and eosin and evaluated by experienced neuropathologists.
Statistical analysis
Differences in experimental variables across groups of participants were identified using one-way analysis of variance (ANOVA) and chi-squared tests, and relationships of EPVS burden with demographic and clinical variables were investigated using Spearman’s correlations. Elastic-net regularized ordinal logistic regression was conducted to investigate the association of EPVS burden (dependent variable) with the presence of gross and microscopic infarcts, the severity of atherosclerosis, arteriolosclerosis and cerebral amyloid angiopathy, the amyloid-β plaques score and neurofibrillary tangles score, the presence of Lewy bodies, and the level of TDP-43 (hippocampal sclerosis was not included in the analyses due to very low numbers of participants with this pathology for at least one level of EPVS burden). The same elastic-net regularized ordinal logistic regression also considered parameters for age at death, sex, years of education, clinical variables that were shown to be correlated with EPVS burden, postmortem interval to fixation, postmortem interval to imaging, and MRI scanner used. The hyperparameters α and λ of the elastic-net were selected empirically and by 10-fold cross-validation, respectively. The above analysis was first conducted in the whole group of 654 participants and then repeated in the subgroup of 353 non-demented participants.
Linear mixed-effects models were used to investigate the independent association of EPVS burden with the rate of cognitive decline above and beyond what was explained by neuropathologies and demographics. The longitudinal dependent variable was a composite cognitive score (for global cognition or each of the five cognitive domains) and the independent variables were the EPVS burden, all the neuropathologies, demographics and covariates listed in the previous paragraph, as well as the interaction of each of these variables with the time before death. Associations of EPVS burden with the rate of cognitive decline were considered significant when the interaction term of EPVS burden with time before death was statistically significant (p<0.05). This analysis was first conducted in the whole group and then repeated in the subgroup of non-demented participants.
Results
Demographic and clinical characteristics, and EPVS burden
Among the 654 participants, 353 were not demented and 301 were demented at the last evaluation (Table 1). Demented participants were on average 1.8 years older at the time of death (ANOVA, p=0.046), had less time between the last clinical evaluation and death (ANOVA, p=0.03), lower Mini-Mental State Examination score (ANOVA, p<0.0001), and higher frequency of at least one copy of the ε4 allele (chi-squared test, p=0.04) compared to non-demented participants.
EPVS burden levels of 0 and 1 were combined due to the small number of participants with a burden of 0 (N=7). Intra-rater reliability for EPVS rating was substantial (κ=0.73, 95% confidence interval (CI)=[0.67,0.78]) and inter-rater reliability (κ=0.58, 95% CI=[0.37,0.75]) was moderate. EPVS burden in the whole group was positively correlated with diabetes (chi-squared test, p=0.002), differed across scanners used for ex-vivo MRI (chi-squared test, p<0.0001), and was positively correlated with postmortem interval to imaging (Spearman, p=0.0002) (however, it was determined that the relation to postmortem interval to imaging was only a scanner effect; see Supplementary Materials). EPVS burden was not associated with age, sex, years of education, dementia, history of hypertension, heart disease, smoking, systolic blood pressure, diastolic blood pressure, APOE genotype, hemisphere side, or postmortem interval to fixation.
Histopathologic validation of EPVS
Neuropathologic evaluation confirmed ten out of twelve single EPVS identified on ex-vivo MRI scans from six participants. Neuropathologically-confirmed EPVS had distinctive characteristics including a dilated space around the vessel, presence of tissue rarefaction, perivascular hemosiderin macrophages, and some, but not all, had moderate-to-severe arteriolosclerosis of small vessels within the PVS (Fig.2). One EPVS was a possible confirmation as there was only the presence of tissue rarefaction around the blood vessel and moderate arteriolosclerosis of small vessels, but no dilated perivascular space. Lastly, one EPVS could not be validated due to the tissue having been distributed to a different study.
Figure 2.
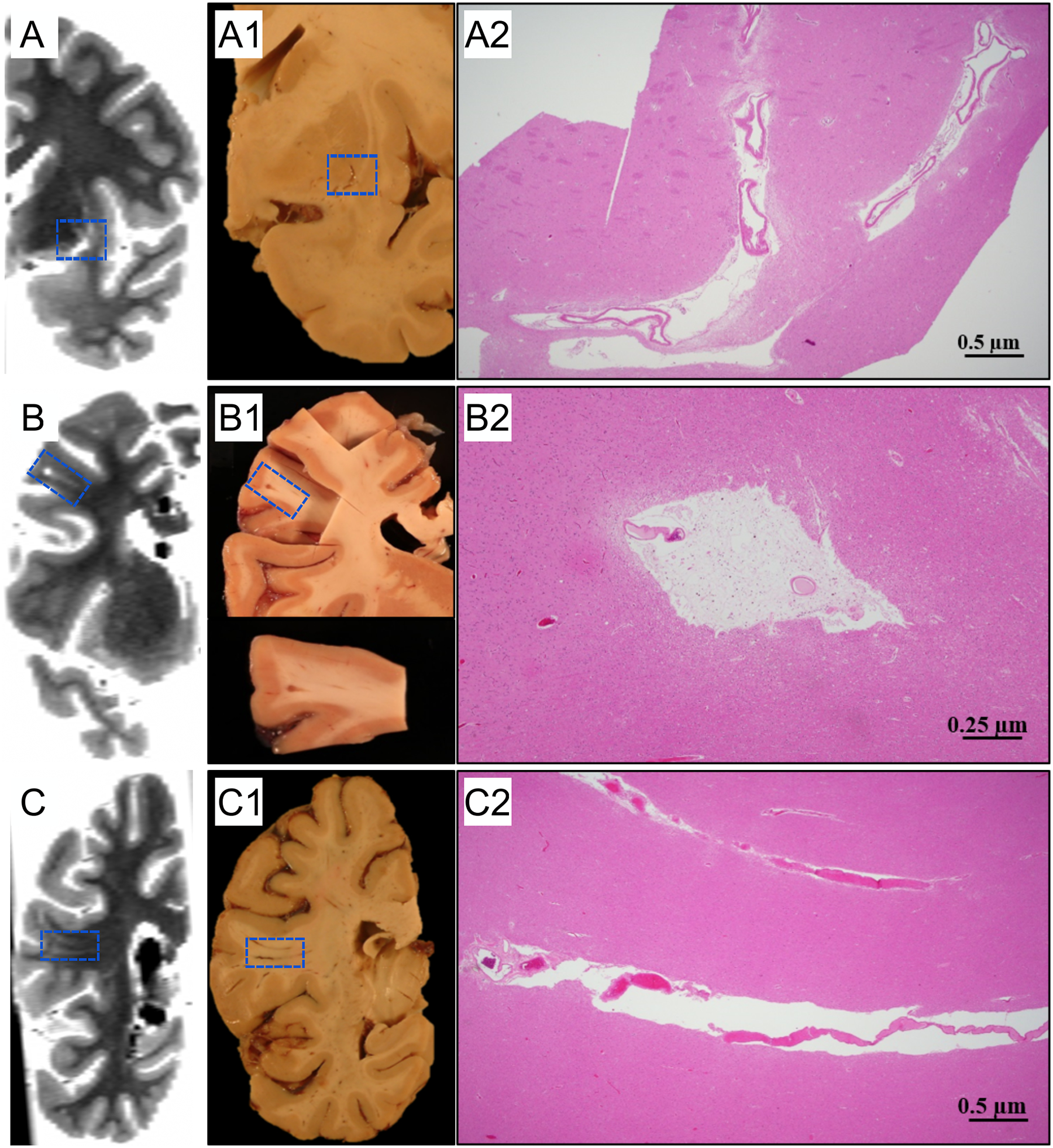
Examples of histopathologic validation of EPVS. Three participants with mild (A), moderate (B), and severe (C) EPVS burden are shown. The first column (A,B,C) shows ex-vivo MR images that have been reformatted to match the plane shown in the pictures of the tissue slabs (A1,B1,C1). Histological findings show dilated PVS (A2, B2, C2), tissue rarefaction around blood vessels (A2), hemosiderin-laden perivascular macrophages, and severe hyaline thickening of small arterioles (B2).
Association of EPVS burden with neuropathologies
Neuropathologic characteristics of the participants at different levels of EPVS burden are shown in Table 2. When considering the whole group of 654 participants, elastic-net regularized ordinal logistic regression (α=0.25, λ=0.17) showed that EPVS burden was associated with gross infarcts (odds ratio (OR)=1.67, 95% CI=[1.21,2.30], p=0.0017) and diabetes (OR=1.73, 95% CI=[1.19,2.50], p=0.004). When considering only non-demented participants (N=353), elastic-net regularized ordinal logistic regression (α=0.9, λ=0.09) showed that EPVS burden was associated with gross infarcts (OR=1.74, 95% CI=[1.11,2.73], p=0.016) and microscopic infarcts (OR=1.79, 95% CI=[1.13,2.83], p=0.013).
Association of EPVS burden with cognitive decline
A linear mixed effects model controlling for neuropathologies, demographics and other covariates (including diabetes), showed that EPVS burden was associated with faster decline in visuospatial abilities (estimate=−0.009, standard error (SE)=0.004, p=0.028), in the whole group. EPVS burden was not associated with decline in other cognitive domains or global cognition. In addition, EPVS burden in the whole group was associated with lower levels of semantic memory (estimate=−0.13, SE=0.06, p=0.048) and visuospatial abilities (estimate=−0.11, SE=0.04, p=0.016) at the time of death. When considering only non-demented participants, EPVS burden was not associated with decline or level of cognition. Correction for multiple comparisons (analyses for six cognitive measures in two groups, i.e. 12 analyses in total) was not conducted.
Discussion
The present study investigated the neuropathologic correlates of EPVS burden by combining ex-vivo brain MRI and detailed neuropathologic examination in a large community-based cohort of older adults. Infarcts were associated with higher EPVS burden in the whole group as well as in the subgroup of non-demented participants. This finding suggests that EPVS and infarcts may share similar neurobiological pathways that are present in both demented and non-demented older adults. EPVS burden was also associated with diabetes independently of neuropathologies, which adds important new evidence to recent findings in animal studies implicating diabetes in impairment of the glymphatic system. In addition, based on longitudinal cognitive evaluation, EPVS burden had small contributions to cognitive decline above and beyond the effects of neuropathologies and demographics, suggesting that EPVS burden may capture some additional brain tissue injury.
In-vivo studies have linked EPVS burden to lacunar infarction4–7 and increased risk of stroke7, however no information on comorbid neuropathologies was available. Mixed pathologies are common in older adults and therefore considering a wide array of age-related neuropathologies is important in determining the neuropathologic correlates of EPVS burden. The present study extends previously published work and provides robust evidence that EPVS burden is associated with infarcts independent of other neuropathologies.
Although the exact mechanism underlying the observed association is not fully understood, EPVS and infarcts may share similar neurobiological pathways. The various etiologies for PVS expansion that have been proposed5–7,10,12–14, including accumulation of beta-amyloid in the vessel wall of arterioles, hypertension, or blood-brain barrier failure, may also lead to vessel wall damage, inflammation, impaired autoregulation, and/or luminal narrowing and occlusion, precipitating focal brain parenchymal ischemia and infarction1,22. EPVS have also been associated with cerebral microbleeds10, and a separate study observed that acutely extravasated blood and hemosiderin migrated through EPVS propagating an inflammatory reaction, which may contribute to the formation of lacunar infarcts23. Most recently, it was demonstrated in rodents that within minutes of an ischemic insult, spreading depolarizations followed by vasoconstriction enlarged the PVS24. Further research is required to disentangle the exact mechanisms behind the observed association of EPVS burden with infarcts.
The few previous MRI-pathology studies on the neuropathologic correlates of EPVS generated mixed findings and implicated arteriolosclerosis11 and CAA12–14. This discrepancy with the present work may be due to some important differences in the methodology used. Previous MRI-pathology investigations involved hospital-based cohorts, while the present work was conducted in a large community-based cohort. Furthermore, previous studies considered only one or few pathologies and did not control for the effects of comorbid pathologies, while the present work performed detailed neuropathologic examination and controlled for comorbid neuropathologies. Finally, differences in the method used for assessing EPVS burden (e.g. visual rating, or in-slice count, or individual vessel histology) and the brain regions under investigation (e.g. whole brain vs. basal ganglia, or centrum semiovale, or juxtacortical), may be additional causes for the discrepancy between this and previous MRI-pathology studies.
EPVS burden was also associated with diabetes independently of neuropathologies. Since perivascular spaces are thought to play a major role in the glymphatic system where they function as the main channels for clearance of interstitial solutes from the brain25, this is an important new lead extending recent findings in animal models implicating diabetes in impairment of the glymphatic system26. More specifically, recent evidence in a chemically-induced diabetes model in middle-aged rats suggested that diabetes was associated with reduced glymphatic drainage in the hippocampus and hypothalamus27. The results of the present work in a community-based cohort showing that EPVS burden is associated with diabetes independently of neuropathologies, provides additional evidence suggesting involvement of diabetes in impairment of the glymphatic system.
When considering both demented and non-demented participants, EPVS burden was associated with faster decline in visuospatial abilities, as well as lower levels of semantic memory and visuospatial abilities, above and beyond the effects of neuropathologies, demographics and other covariates (including diabetes). However, the associations were borderline significant and correction for multiple comparisons (12 analyses) may render them non-significant. This study extends previous work on the cognitive profile of EPVS in community cohorts8,28–30 and suggests that EPVS burden may capture some additional brain tissue injury that has small contributions to cognitive decline above and beyond those of other measures included in the model (i.e. neuropathologies, demographics, diabetes). When considering only non-demented participants, no association of EPVS burden with decline or level of cognition was demonstrated, a finding that is in agreement with recent reports in community cohorts31,32. This result may suggest that the link between EPVS and cognition is detectable only after substantial impairment, or may simply be due to lower statistical power in the subgroup of non-demented participants.
The present work has important strengths and also few weaknesses. The strengths include a) studying a large community-based cohort of older adults, b) conducting detailed neuropathologic examination and controlling for the effects of comorbid pathologies in models of EPVS burden, and c) using ex-vivo MRI to image independent of frailty level and ensure that imaging and neuropathologic examination capture the same brain condition. One weakness of the present study is the use of a rating scale for assessing EPVS burden instead of measuring the total number or volume of EPVS. However, the latter requires EPVS segmentation software33,34 that is currently not publicly available. Another weakness is the lack of in-vivo translation. However, the results of histopathologic validation of EPVS provides confidence in the present findings. Finally, a minor weakness is that laterality was not studied, since only one cerebral hemisphere was imaged per participant.
Conclusion
The present study showed that EPVS burden was associated with infarcts, independent of comorbid neuropathologies, in the whole group as well as in the subgroup of non-demented participants. This suggests that EPVS and infarcts may share similar neurobiological pathways regardless of dementia status. EPVS burden was also associated with diabetes independently of neuropathologies, which adds important new evidence to recent findings in animal studies implicating diabetes in impairment of the glymphatic system. Finally, EPVS burden showed borderline significant associations with faster cognitive decline above and beyond the effects of neuropathologies and demographics, suggesting that EPVS burden may reflect additional brain tissue injury that has small contributions to cognitive decline not captured with traditional neuropathologic measures.
Supplementary Material
Acknowledgments
The authors would like to thank the participants and staff of the Rush Memory and Aging Project, Religious Orders Study, and Minority Aging Research Study.
Sources of Funding
This study was supported by National Institutes of Health grants P30AG010161, UH2NS100599, UH3NS100599, R01AG015819, R01AG064233, RF1AG022018, R01AG056405, R01AG042210, R01AG17917, K08NS089830.
Non-standard Abbreviations and Acronyms
- PVS
Perivascular spaces
- EPVS
enlarged perivascular spaces
- CAA
cerebral amyloid angiopathy
- MRI
magnetic resonance imaging
- TDP-43
transactive response DNA binding protein of 43 kDa
- ANOVA
analysis of variance
- CI
confidence interval
- OR
odds ratio
Footnotes
Disclosures
The authors have no conflict of interest to report.
Supplemental Materials
Expanded Materials & Methods
Supplementary Tables I-II
Supplementary Figure I
References
- 1.Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res. 2018;114:1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechmann I, Galea I, Perry VH. What is the blood–brain barrier (not)? Trends Immunol. 2007;28:5–11. [DOI] [PubMed] [Google Scholar]
- 3.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large Virchow-Robin spaces: MR-clinical correlation. Am J Neuroradiol. 1989;10:929–936. [PMC free article] [PubMed] [Google Scholar]
- 4.Rouhl RPW, van Oostenbrugge RJ, Knottnerus ILH, Staals JEA, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008;255:692–696. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y-C, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of Dilated Virchow-Robin Spaces Is Associated With Age, Blood Pressure, and MRI Markers of Small Vessel Disease: A Population-Based Study. Stroke. 2010;41:2483–2490. [DOI] [PubMed] [Google Scholar]
- 6.Doubal FN, MacLullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke. 2010;41:450–454. [DOI] [PubMed] [Google Scholar]
- 7.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM. Enlarged Perivascular Spaces and Cerebral Small Vessel Disease. Int J Stroke. 2015;10:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLullich AMJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin Space Is a Sensitive Indicator of Cerebral Microvascular Disease: Study in Elderly Patients with Dementia. AJNR Am J Neuroradiol. 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 10.Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, Ayres A, Schwab KM, Martinez-Ramirez S, Goldstein JN, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular Lesions in the White Matter on Magnetic Resonance Imaging in the Elderly. A Morphometric Correlation With Arteriolosclerosis and Dilated Perivascular Spaces. Brain. 1991;114:761–774. [DOI] [PubMed] [Google Scholar]
- 12.Charidimou A, Jaunmuktane Z, Baron J-C, Burnell M, Varlet P, Peeters A, Xuereb J, Jäger R, Brandner S, Werring DJ. White matter perivascular spaces: An MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, Macklin EA, Rozemuller AJ, Gurol ME, Greenberg SM, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab. 2016;36:576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Ramirez S, van Rooden S, Charidimou A, van Opstal AM, Wermer M, Gurol ME, Terwindt G, van der Grond J, Greenberg SM, van Buchem M, et al. Perivascular Spaces Volume in Sporadic and Hereditary (Dutch-Type) Cerebral Amyloid Angiopathy. Stroke. 2018;49:1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis JAD. 2018;64:S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: Ongoing Efforts to Obtain Brain Donation in African Americans without Dementia. Curr Alzheimer Res. 2012;9:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, et al. Decision Rules Guiding the Clinical Diagnosis of Alzheimer’s Disease in Two Community-Based Cohort Studies Compared to Standard Practice in a Clinic-Based Cohort Study. Neuroepidemiology. 2006;27:169–176. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group Under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 20.Arfanakis K, Evia AM, Leurgans SE, Cardoso LFC, Kulkarni A, Alqam N, Lopes LF, Vieira D, Bennett DA, Schneider JA. Neuropathologic Correlates of White Matter Hyperintensities in a Community-Based Cohort of Older Adults. J Alzheimers Dis. 2020;73:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotrotsou A, Schneider JA, Bennett DA, Leurgans SE, Dawe RJ, Boyle PA, Golak T, Arfanakis K. Neuropathologic correlates of regional brain volumes in a community cohort of older adults. Neurobiol Aging. 2015;36:2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrag M, McAuley G, Pomakian J, Jiffry A, Tung S, Mueller C, Vinters HV, Haacke EM, Holshouser B, Kido D, et al. Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathol. 2010;119:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, Mortensen KN, Stæger FF, Bork PAR, Bashford L, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367:eaax7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliff JJ, Nedergaard M. Is There a Cerebral Lymphatic System? Stroke. 2013;44 (6 Suppl 1):S93–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Chopp M, Jiang Q, Zhang Z. Role of the glymphatic system in ageing and diabetes mellitus impaired cognitive function. Stroke Vasc Neurol. 2019;4:90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37:1326–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passiak BS, Liu D, Kresge HA, Cambronero FE, Pechman KR, Osborn KE, Gifford KA, Hohman TJ, Schrag MS, Davis LT, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology. 2019;92:e1309–e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y-C, Dufouil C, Soumaré A, Mazoyer B, Chabriat H, Tzourio C. High Degree of Dilated Virchow-Robin Spaces on MRI is Associated with Increased Risk of Dementia. J Alzheimers Dis. 2010;22:663–672. [DOI] [PubMed] [Google Scholar]
- 30.Ding J, Sigurðsson S, Jónsson PV, Eiriksdottir G, Charidimou A, Lopez OL, van Buchem MA, Guðnason V, Launer LJ. Large Perivascular Spaces Visible on Magnetic Resonance Imaging, Cerebral Small Vessel Disease Progression, and Risk of Dementia: The Age, Gene/Environment Susceptibility–Reykjavik Study. JAMA Neurol. 2017;74:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilal S, Tan CS, Adams HHH, Habes M, Mok V, Venketasubramanian N, Hofer E, Ikram MK, Abrigo J, Vernooij MW, et al. Enlarged perivascular spaces and cognition: A meta-analysis of 5 population-based studies. Neurology. 2018;91:e832–e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valdés Hernández M del C, Ballerini L, Glatz A, Muñoz Maniega S, Gow AJ, Bastin ME, Starr JM, Deary IJ, Wardlaw JM. Perivascular spaces in the centrum semiovale at the beginning of the 8th decade of life: effect on cognition and associations with mineral deposition. [published online June 27, 2019] Brain Imaging Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Gao Y, Park SH, Zong X, Lin W, Shen D. Structured Learning for 3-D Perivascular Space Segmentation Using Vascular Features. IEEE Trans Biomed Eng. 2017;64:2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballerini L, Lovreglio R, Valdés Hernández M del C, Ramirez J, MacIntosh BJ, Black SE, Wardlaw JM. Perivascular Spaces Segmentation in Brain MRI Using Optimal 3D Filtering. Sci Rep. 2018;8:2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this work can be accessed by submitting a request to www.radc.rush.edu.


