Abstract
Social interaction can be seen as a dynamic feedback loop that couples action, reaction, and internal cognitive processes across individual agents. A fuller understanding of the social brain requires a description of how the neural dynamics across coupled brains are linked and how they co-evolve over time. Here, we elaborate this multi-brain framework, which considers social interaction as an integrated network of neural systems that dynamically shapes behavior, shared cognitive states, and social relationships. We describe key findings from multi-brain experiments in humans and animal models which shed new light on the function of social circuits in health and disease. Finally, we discuss recent progress in elucidating the cellular-level mechanisms underlying inter-brain neural dynamics and outline key areas for future research.
Keywords: Social interaction, inter-brain synchrony, multi-brain recording, hyperscanning
Social interaction as a feedback loop in a multi-brain system
As we navigate the world around us, we are continuously making decisions to secure our health, well-being, and ultimately survival. These decisions are based on sensory information from the environment, but they are also shaped by feedback from the world as we act upon it, creating a dynamic loop between action and reaction (Fig. 1). As part of this process, animals have evolved to form predictions about their environment and how it will respond to their actions in order to make more adaptive choices. Predictive models are formed based on learned statistics of the natural world, past experiences, and intrinsic knowledge built into the structure of the brain. Adaptive action thus depends on well-formed priors that capture the features of the environment and are built upon experience in a stable, and relatively predictable, external world.
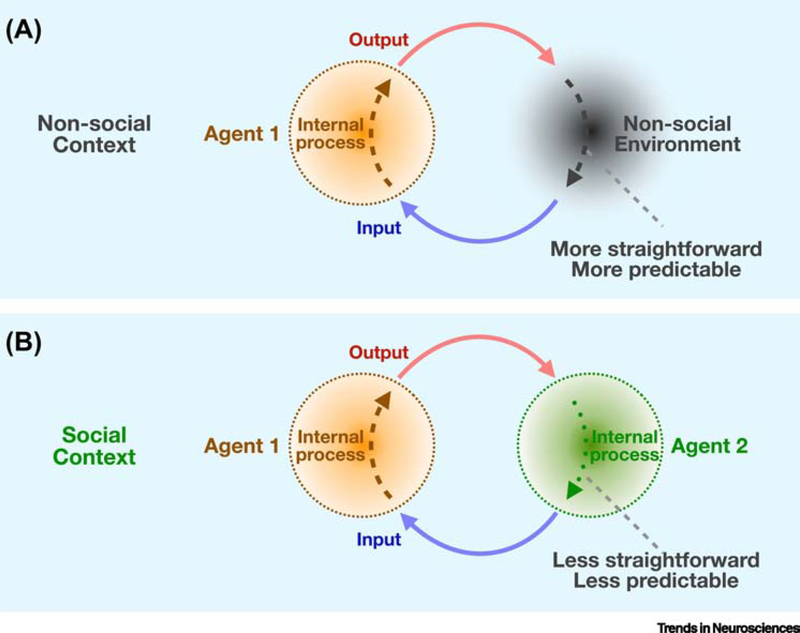
Figure 1. The decision-making process as a dynamic feedback loop.
Illustration of the decision-making process of an agent in a non-social (A) vs. social (B) context. For simplicity, the social context is illustrated here for the case of a two-agent situation. The agent makes choices based on a sensorimotor transformation that maps an input space to specific behavioral outputs. In the non-social context, the agent uses feedback from the environment which is relatively stable and predictable. In contrast, the social context couples the agent directly to another agent whose internal state (e.g. goals, beliefs, etc.) are hidden. Behavior and behavioral responses from the interacting partner are highly unpredictable, creating a more complex decision-making processes that engages mentalization, dynamic prediction, and other cognitive processes.
In contrast to acting in a predictable environment, maneuvering a social environment engages the brain in a fundamentally different way. Interacting agents are not isolated, and their behavioral decisions are intimately linked as they act and react directly to one another [1,2]. Social agents must flexibly adjust their decision-making schema in response to others’ behavior, anticipate the responses of others, and model their goals and internal processes [3–5] in order to behave adaptively, communicate, and coordinate in pursuit of goals. Sometimes individuals interact indirectly through the environment towards shared goals (e.g. during group hunting), and/or over an extended period of time (e.g. when individual decisions collectively cause and are influenced by climate change). Even in these scenarios, individuals anticipate and react to the actions of others in the short or long term within a shared social context. This reciprocal exchange of behavior is substantially more complicated and unpredictable than non-social action, and it increases the complexity of the decision-making process dramatically [6].
These unique features of social interaction suggest a conceptual framework that considers social agents not as isolated actors, but as embedded in an integrated system of interactors. Such a framework focuses attention not just on single brains, but on the emergent neural properties of multi-brain systems [7–9]. Owing to these considerations, researchers have increasingly focused on exploring the brain during naturalistic interaction [7,10], and on applying techniques to simultaneously measure neural activity in multiple interacting agents [9]. In this review, we elaborate this multi-individual framework and provide an overview of key results from multi-brain studies, illustrating how this approach has furthered our understanding of the social brain. We discuss important considerations in the design and interpretation of multi-brain studies as well as recent developments in understanding the cellular-level neural mechanisms underlying inter-brain dynamics.
A multi-brain framework to study neural systems in interaction
An integrated system of interactors
Abstractly, social exchange can be thought of as an interaction between two or more neural systems that are coupled to one another through sensory inputs and behavior. Yet because of their physical separation, interaction between brains has to be mediated by channels of expressible communication (e.g. movement, vocalization, touch, and/or via the physical environment) (Fig. 2A). There are physical and biological limits to how much information can be communicated at a given time, and not all processes relevant for social interaction can be or are communicated (i.e. expressed and/or perceived). For example, while internal states such as fear may shape expressed behavior, they may also be hidden from external view. Thus, as part of the information is lost in external communication, the amount of information communicated is a fraction (and likely a lower dimensional representation) of the total information in the neural processes within each system (Fig. 2A).
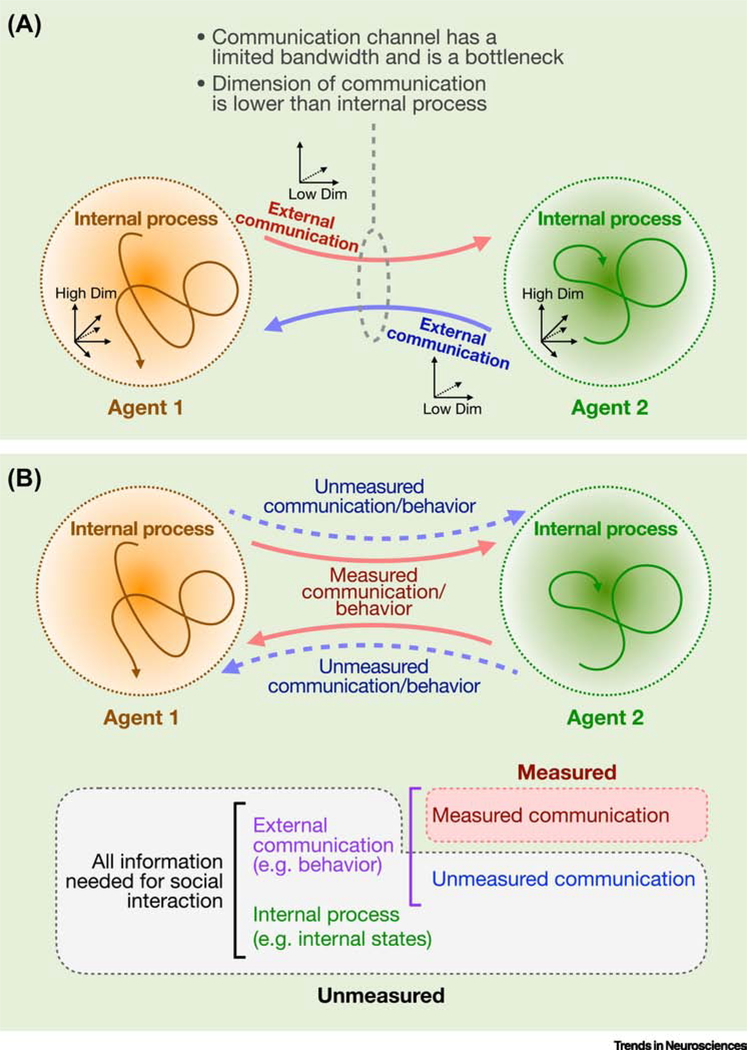
Figure 2. Neural systems are communicated through a behavioral bottleneck.
Illustration of social communication between two interacting agents. (A) As the behavioral outputs of each agent form part of the other’s input space, interacting agents become coupled in an integrated system (Fig. 1). The full range of neural processes that shape behavioral decisions span a higher dimensional space than that of expressed behavior, so communication between agents is limited by a channel with limited bandwidth (the communication bottleneck). (B) From a third-person perspective, observation of only a limited part of external communication (explicit behavior measured by experimenters) provides an impoverished view that lacks information about the underlying neural processes. Direct measure of the neural processes and their dynamical relationships across agents may provide additional information about unmeasured variables and the interaction itself.
Still, despite this communication bottleneck, animals and humans are often able to infer some internal processes of others through external (behavioral) cues. Humans routinely infer nonexplicit intentions in others based on their speech and body language, and animals such as mice may share stress states and fear associations through observational learning [11,12]. Such internal processes—whether or not they are expressed—play an important role in shaping social interaction.
Classically, neuroscientists have aimed to discover neural computations in single individuals that convert sets of inputs into behavioral outputs. Applying this approach to social interaction has yielded insight into the neural processes underlying social behavior [13,14]. However, despite ongoing effort to apply sophisticated machine learning methods to improve behavioral tracking and classification [15–17], we, as experimental observers, still have limited access to the full communication space of individuals. Many variables relevant to understanding social interaction cannot be precisely measured (Fig. 2B). For example, the full repertoire of odor cues shared between individuals is not feasibly measurable, and the true dynamics of internal states such as attention are often not accessible. As a complete description of any decision process requires knowing the full input and output space as well as internal variables, observing only a fraction of external variables gives an impoverished view that does not capture the internal processes, and their relationships across individuals, which may be most informative.
The multi-brain approach
An alternative approach to studying social interaction is to frame interacting agents as coupled within a single integrated system [6,7] (Fig. 3). Under this framework, one can define and measure properties that arise at the level of the system, such as dual/multi-agent behavioral properties or interbrain neural dynamics, and study how they are related (Fig. 4). Individual’s actions may give rise to emergent behavior at the dyadic or group level, and the underlying neural processes may exhibit shared dynamics that reflect alignment of behavior or internal states. As neural dynamics carry information that may not be available from behavioral analysis (Fig. 2), patterns of shared activity across individuals may also provide novel information about the interaction itself or the relationship between agents. This approach may also reveal fundamental mechanisms by which coordinated behavior is orchestrated by integrated neural systems.
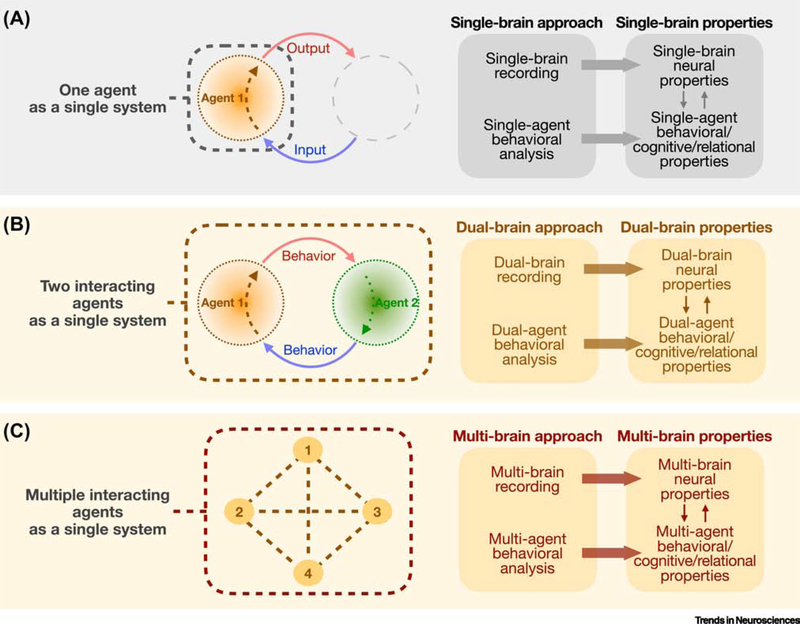
Figure 3. A multi-brain approach to study interacting agents as a single system.
Different conceptual frameworks to study social interaction, considering one or multiple interacting agents as a single system. Various neural and behavioral properties at the level of single, dual, or multiple individuals may be studied using single-, dual-, or multi-brain approaches, respectively. For example, recording neural dynamics in two or more individuals simultaneously provides access to measures of inter-brain neural properties such as synchrony that may correlate with dual/multi-agent behavioral or cognitive variables. The multi-brain approach can be applied to study two agents in interaction, or to study the inter-brain dynamics of multiple individuals in a group interaction.
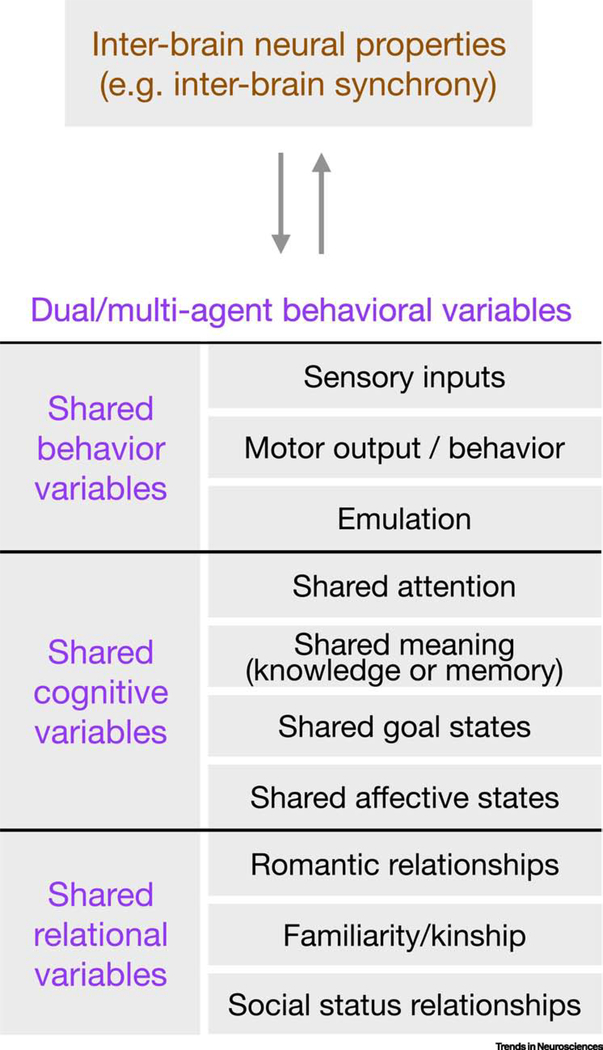
Figure 4. Shared social variables and inter-brain dynamics.
Inter-brain dynamics measured across two or more interacting agents may provide information about shared social variables, ranging from behavioral variables to internal cognitive and relational variables. Behavior variables include shared sensory inputs, synchronized behavior, or coordinated behavior that is not precisely aligned (e.g. music production in a duet where each individual plays a distinct voice). Cognitive variables include neural processes that are internal but may shape behavioral outputs, including shared attention, semantic constructs, goal states, affective states, and other processes such as mentalization and simulation. Relational variables include those that describe social relationships across individuals such as status and familial relationships.
Growing appreciation for these possibilities has spurred a tremendous effort in recent years to explore the emergent neural properties that arise across multiple brains in interaction (Fig. 3). The development of techniques to record neural dynamics simultaneously from two or more subjects has opened the door for a rigorous investigation into how inter-brain neural dynamics may provide a substrate for interaction, communication, coordination, and collective behavior [8–10,18].
Inter-brain dynamics as neural correlates of shared social variables
Since its introduction nearly two decades ago [9], the multi-brain approach has been applied to study people in interaction and their inter-brain neural dynamics across a wide range of social contexts. Collectively, these studies have identified patterns of inter-brain neural dynamics, including inter-brain synchronization, which correlate with social variables such as behavioral coordination, shared cognitive states, and social relationships (Fig. 4). Inter-brain dynamics have also been observed in non-human primates [19] and other animals [20,21], suggesting that emergent patterns of activity across interacting agents may be conserved across social species.
Techniques and task designs for multi-brain research in humans
A repertoire of non-invasive recording approaches, collectively referred to as hyperscanning [9], has been employed to explore neural dynamics during human social interaction. These mainly include functional magnetic resonance imaging (fMRI), functional near-infrared spectroscopy (fNIRS), and electroencephalography (EEG). These methods measure hemodynamic or electrical activity in the brain and can be applied to multiple subjects to record their neural activity simultaneously.
Using these methods, researchers have measured inter-brain dynamics across different task structures and social contexts. One dimension along which task structure may vary is in the degree of real-time interactivity that subjects are engaged in [22]. While some tasks engage individuals in structured turn-based games where decisions are made on discrete trials [23–26], other tasks allow for real-time interaction, as in face-to-face communication [27] or music production [28–30]. These distinct task designs allow researchers to probe inter-brain correlates of different types of social variables. For example, turn-based games may reveal neural correlates of internal processes such as mentalization and strategy formation, while interactive tasks can explore correlates of active coordination. Task designs can also vary the social context to structure interactions by providing specific goals for participants to pursue. Varying goal structure, for example by specifying either shared or antagonistic goals in games, allows researchers to probe differences in inter-brain dynamics that correlate with cooperative or competitive behavior [23–26,31–34].
Initial studies that explored inter-brain dynamics during turn-based economic games [9,23,26] and vocal, gestural, or affective communication [35–37] employed dual fMRI recordings. While this approach provides brain-wide access to neural signals, the technical constraints of fMRI limit tasks to those that do not require direct contact, physical interaction, or naturalistic settings. In order to explore inter-brain dynamics during more interactive contexts, fNIRS [34,38] and EEG [24,28] have also been used extensively. Because fNIRS only requires attachment of lightweight spectrometers to a subject’s head, recordings of cortical hemodynamic activity can be made in multiple individuals during interaction without restraining natural movement. Similarly, EEG allows recordings of cortical activity at high temporal resolution during unconstrained interaction, allowing exploration of high frequency dynamics and network patterns.
Inter-brain dynamics related to shared behavioral variables
The application of these various techniques and tasks has allowed examination of how inter-brain dynamics relate to multiple layers of shared social variables, including behavioral variables (e.g. coordination of external behavior), cognitive variables (internal states such as attention), and relational variables (such as romantic states). One set of tasks uses interpersonal coordination to explore inter-brain neural correlates of shared behavior. During games in which subjects must synchronize timing of a button press, inter-brain synchrony predicts successful coordination [34,38,39]. During behavior alignment tasks using rhythmic finger tapping [40,41], body movement [42], and gestural imitation [43], inter-brain synchrony has also been observed to predict coordinated behavior. Of note, however, in many situations, such as during emulation, similar patterns of activity may arise from common sensory inputs and/or concurrent behavior. Such synchrony may not necessarily reflect the social component of interaction, as synchrony may be observed across non-interacting individuals receiving the same sensory inputs or engaged in the same actions. Thus, in order to isolate the true contribution of social interaction, it is important to exclude the effects of common sensory inputs and identical actions.
Interestingly, when interactors do not perceive the same sensory inputs and perform identical actions, inter-brain synchrony still occurs. Musicians playing duets [28,29] show synchronized brain activity, and when playing in quartets [44], exhibit functionally connected brain networks even when playing different notes. Moreover, many behavior coordination studies report higher inter-brain synchrony during interaction than during control conditions where subjects’ are recorded outside of the interactive context [40–43]. These lines of evidence suggest that inter-brain synchrony does not simply reflect the identical sensory or motor signals correlated by task structure, but arises in part from the underlying processes in each brain as it is engaged in a social context. This includes the coordination of behavior and perception during interaction, as well as the affective and cognitive processes that shape it.
Inter-brain dynamics related to shared cognitive variables
In some studies, the alignment of internal cognitive variables such as attention has been explored more directly. For example, face-to-face contact during social interaction and vocal communication [27,45,46] have been associated with synchronization of neural circuits involved in attention, and mutual eye contact dynamically couples a distributed neural network across subjects [47,48]. Attention directed toward common goal pursuits has also been associated with increased synchrony [49,50], and joint attention may be mediated by social signals that elicit shared neural dynamics such as gaze or gestural cues [51]. Intriguingly however, inter-brain dynamics have also been linked to alignment of abstract cognitive variables such as semantic constructs and psychological states [52,53]. For example, when individuals listen to spoken stories, hemodynamic or neural signals are correlated across speaker and listener in circuits underlying language comprehension, abstract thought and mentalization [37,54–56]. The degree of inter-brain correlation predicts language comprehension [37], supporting the idea that shared neural representations of semantic and cognitive constructs may be related to narrative understanding [57]. Consistent with this, synchrony predicts language comprehension between individuals in a noisy environment [58] and expert teachers show greater inter-brain synchrony with students than novice teachers during collaboration [59].
Inter-brain dynamics related to shared relational variables
Shared neural dynamics have also been found to correlate with relational social variables, including romantic relationships, kinship, and leader-follower relationships. While inter-brain synchrony in romantic couples [60,61] and parent-child dyads [62–64] predicts their success in cooperative games, the brains of strangers in the same task (and stranger parent-child dyads) do not display the same degree of synchrony. And during free interaction [65], musical production [66], strategic card games [24], and coordinated finger tapping [40], inter-brain dynamics also distinguish between leaders and followers, suggesting that they encode information about social relationships as they evolve. In these social relationships, how biological sex and/or gender identity contribute to inter-brain dynamics remains an interesting point for future investigation [39,67]. Collectively, these studies demonstrate that inter-brain neural dynamics encode various social variables ranging from shared behavior and attention to shared cognitive and relational states.
Inter-brain dynamics as observed in animal studies
Although many studies have focused on human social interaction, effort has also been made to explore inter-brain neural dynamics in non-human primates and other animal species. Because animals cannot engage in highly complex interactions such as playing card games or producing music, social contexts for probing inter-brain dynamics in animals are more limited than in human subjects. Nevertheless, using simple observational and unconstrained behavior tasks, studies in animals have also uncovered shared neural dynamics that predict social interaction and relational variables. Electrophysiological recordings of single neurons in monkey premotor cortex show inter-brain synchronization while one animal observes the other completing a rewarded task [19]. In mice, large-scale calcium imaging of populations of single neurons have revealed inter-brain synchrony in the prefrontal cortex during natural behavior [21]. And in bats, recordings of local field potentials in the prefrontal cortex reveal inter-brain synchrony during social interaction [20]. A consistent conclusion from studies of mice and bats is that inter-brain synchrony predicts social interaction, but does not arise simply from concurrent movements or behavior. Further, inter-brain synchrony in mice also predicts the development of social dominance relationships between animals [21]. This echoes reports from human studies which show that inter-brain synchrony can predict leader-follower relationships [40,65,68] and captures information about cognitive processes during social competition [69,70]. Taken together, these results demonstrate that inter-brain synchrony is a general phenomenon that extends across species, and that synchrony in non-human animals can similarly encode diverse social variables such as coordinated behavior and relational states.
Considerations in measuring inter-brain dynamics
Careful consideration of experimental design and analytical methodology is necessary in order to isolate relevant measures of inter-brain dynamics, and to control for contributions to neural signals that may result spuriously from rhythmic activity or structurally correlated task variables [71]. Inter-brain dynamics have typically been examined using measures of synchrony such as correlation and coherence, measures of directional interaction such as Granger causality and partial directed coherence (PDC), and statistical modeling approaches. Measures of time-series correlation (of EEG power or hemodynamic response) provide a simple measure of shared inter-brain dynamics but may miss synchronous relationships that are phase shifted. Measures of directional interaction (e.g. Granger causality) may provide additional information about asymmetric communication between brains [35]. For example, in leader-follower settings, brain processes in the leader may precede those in the follower [30]—measures such as PDC account for such asymmetries and reveal how directional influence correlates with task variable or social relationships. At the same time, some measures of coherence, including PDC, may not be robust to spurious inter-brain dynamics that arise due to rhythmic activity inherent to neural systems. These may be avoided using more direct measures of the covariance in phase relationships, such as the circular correlation coefficient [71], and by using analytical controls such as phase randomized signals. Importantly, other measures of network level functional relationships across brains (e.g. graph theoretic measures of connectivity [29,44,72]) may also be informative. Development of new theoretical and computational frameworks to analyze the evolution of inter-brain neural dynamics, especially across more than two individuals, may represent a fruitful focal point for future research.
Considerations in disentangling inter-individual variables
During natural social exchange, many levels of external and internal variables may be aligned across individuals, and there are likely many distinct inter-brain neural correlates of social variables present simultaneously [52,73]. These components may be separable and separately interpretable in highly controlled experimental settings. However, in many cases, these components may be highly intermixed, creating a challenge in interpretation of how they reflect shared social variables. While it is possible to examine relationships between inter-brain dynamics and measurable behavioral variables, linking neural activity to cognitive or relational variables presents a further challenge. Because variables such as attention or encoding of goal states cannot be explicitly measured, interpreting their relationship with inter-brain dynamics depends on controlled task design in order to rule out contributions from confounding factors. Specifically, contributions from activity due to sensory stimuli or behavior, which may be correlated due to task structure, must be controlled or factored out, experimentally or computationally. Lastly, selection of metrics that are robust to detecting spurious synchrony will increase interpretability [71] of inter-brain dynamics, especially when experimental controls are limited.
Sensory modalities involved in inter-brain dynamics
One important question regarding the mechanisms underlying inter-brain dynamics is whether specific sensory modalities are involved, and if so, which are particularly important. So far, studies across both humans and animal models suggest that inter-brain dynamics are not modality specific. Indeed, in humans, inter-brain synchrony has been observed during tactile stimulation [74], linguistic communication [37,54] (with no visual cues), and gestural communication [35] (with no auditory cues), indicating that neither visual nor auditory information are fully necessary. While work in animals has not explored tasks that limit specific sensory inputs, the observation of synchrony in mice and bats [20,21], which rely on largely distinct sensory modalities for social communication, suggests that the general phenomenon (across species) is not specific to any particular modality. Still, future research may shed light on how inter-brain dynamics in certain contexts depend on specific communication modalities.
The neural basis of inter-brain dynamics
While it is now clear that inter-brain activity patterns provide correlates of shared social variables, there is still relatively little known about the neural mechanisms that support inter-brain dynamics. It is not yet known to what degree inter-brain dynamics are driven by well-defined neural components, such as circuits that perform specific computations or subpopulations of molecularly defined cells. Partly, this is because noninvasive techniques such as fMRI and EEG cannot resolve neural dynamics at the single-cell level, precluding the possibility of linking region- and brain-wide activity with microcircuit computations. In light of this, application of multi-brain approaches in animals, where neural dynamics can be recorded from single cells [75–77] and molecularly defined ensembles [78,79], may be fruitful.
Systems and circuits involved in inter-brain dynamics
Evidence from human hyperscanning studies points to various neural networks that appear to play an important role in inter-brain dynamics. One intriguing finding is that inter-brain dynamics are not localized in the brain but are instead observed across many distinct brain regions depending on social context and task. During coordinated finger tapping [41,80] and music production [29,30], synchronization of motor and premotor areas correlates with behavioral alignment, while synchrony across frontal and parietal areas may relate to shared attention. Similarly, synchrony in frontal circuits during cooperative interaction [24,25,34,38] may be interpreted as a correlate of conceptual or cognitive alignment, as these structures are implicated in the control of goal states and mental models.
In human multi-brain studies, regions associated with the mentalization network [5], including the medial prefrontal cortex (mPFC), anterior cingulate, superior temporal junction, and temporoparietal junction, have been routinely identified as components that contribute to inter-brain dynamics. Similarly, regions involved in the mirror neuron system [81,82] have also been implicated, including the frontal gyrus, premotor cortex, and posterior parietal cortex. Broadly, the involvement of circuits in the mentalization and mirror neuron networks suggests important roles for complex cognitive processes including theory of mind, mental modeling, emulation, and simulation of behavioral and affective states. Interestingly, while regions of the PFC have been consistently identified in human studies [24,28,35,38], work in non-human animals has also implicated the mPFC in neural synchrony [20,21], suggesting overlap in some of the core underlying processes across species.
As the wide range of implicated circuits suggests a diversity of underlying processes, it remains an important open question whether and to what degree inter-brain dynamics vary across different brain regions. A strength of EEG and fMRI recordings in humans is the ability to systematically explore regional differences in inter-brain dynamics during complex tasks, as well as spatiotemporal patterns of activity in brain-wide networks across individuals [37]. In complement to this, examining the cellular-level components that underlie inter-brain dynamics may shed light on how they vary regionally and how they are engaged in different social contexts.
Frequency components and time scales of inter-brain dynamics
Many EEG recordings have explored the diversity of inter-brain dynamics within the temporal domain by examining oscillatory neural components across different frequency bands. Several sub-second frequency bands, ranging from slower theta and alpha bands to high-frequency gamma, have been implicated in inter-brain dynamics. In particular, inter-brain synchronization has predominantly been observed in slower frequency ranges including theta (4–7 Hz) [28,29,42,44,83] and alpha-mu (8–13 Hz) [25,30,62,84,85], while some studies implicate power coherence in the beta range (14–30 Hz) [30,42,55,84]. Interestingly, some research also implicates higher frequency gamma oscillations (30–60 Hz) [84,86–88] in inter-brain dynamics, suggesting a role for mechanisms reflected in gamma activity such as attentional control [89,90]. The diversity of synchronous activity in different frequency bands may reflect differences across brain regions, recruitment of specific sets of neurons that exhibit distinct electrophysiological properties, and specific behavioral contexts [91].
Although EEG allows researchers to measure cortical activity at high temporal resolution, recordings of local field potentials (LFP) and single neuron spiking activity in interacting humans has been difficult due to the invasive nature of these methods. However, extracellular electrode recordings in bats [20] have identified inter-brain correlations of LFP power and spiking activity in PFC during social interaction that predict the onset of future interaction. Interestingly, inter-brain correlations of LFP power were most prominent in high frequency bands (30–150 Hz), consistent with some human multi-brain studies that identify power coherence in the gamma band [84,86–88]. The observation of inter-brain synchrony across different frequency bands again suggests involvement of multiple neural processes beyond those that directly generate behavior.
Interestingly, modulation of power in these sub-second frequency bands often occurs over a slower time scale of seconds to minutes [20,30,83,86]. This slow time scale seems to reflect the low-frequency nature of behavioral and internal state dynamics between individuals that takes place over the course of interaction. Similarly, recordings of calcium dynamics that reflect region-wide neural activity also show strong inter-brain correlations over seconds to minutes [21]. While individual neurons and circuits are capable of producing high-frequency (sub-second) activity patterns within a single brain [91], it is likely that the communication bottleneck and neuromodulatory changes of internal states constrain power modulations in high-frequency bands to slower dynamics.
Cellular substrates of inter-brain dynamics
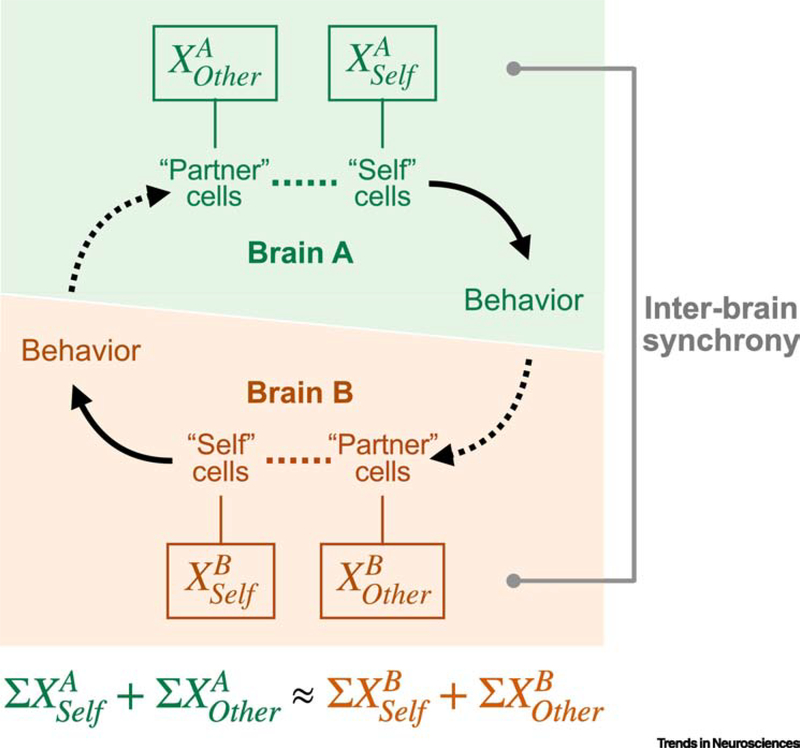
While electrode recordings of LFP provide a good measure of regional activity with high temporal resolution, optical recordings using genetically encoded calcium indicators provide access to hundreds of single units simultaneously [76], which can be labeled based on expression of genetic markers or connectivity [79]. This technology offers access to the cellular mechanisms underlying inter-brain dynamics. Based on this approach, miniature head-mounted microscopes were used to record single cell calcium dynamics from mPFC neurons during free social interaction in mice [21]. When mice were engaged with each other, the overall activity of mPFC neurons across animals was highly correlated, yet this inter-brain synchrony was diminished when a barrier was introduced to disrupt interaction. Interestingly, analysis of single cell contributions showed that inter-brain synchrony depends on two subsets of neurons that separately encode specific behaviors of the subject animal and those of its social partner. Although these two neural components are largely non-overlapping, they collectively represent behavior of both animals, such that synchronization of region-wide activity emerges during interaction (Fig. 5). As many brain regions have been implicated in tracking variables of self and partner [92–96], this may represent a common neural basis underlying inter-brain dynamics across different neural systems.
Figure 5. Single-cell neural components give rise to inter-brain synchrony.
Schematic illustration of the neural basis for inter-brain synchrony in the mouse mPFC during social interaction. Single neurons that encode behavioral decisions of the subject (subject cells) or the interaction partner (partner cells) collectively respond to the shared behavior repertoire of both individuals. Because of the specific behavioral variables that they encode, activity in these largely non-overlapping neural ensembles becomes synchronized across individuals during interaction. (Adapted from [21])
During a competitive encounter, the degree of inter-brain synchrony across dyads of mice predicts future interaction as well as the development of social dominance relationships [21]. Importantly, this correlate of relational state depended specifically on these behavior-encoding cells, indicating that neural synchrony reflects engagement of specific computational circuits as opposed to non-specific regional activity. Consistent with this, prefrontal and parietal regions [24,28,35,38,96] involved in self and partner perception in humans are strongly implicated in inter-brain dynamics. As each region contains many distinct neuronal subpopulations, this raises the question of whether particular cell types or circuits contribute preferentially to inter-brain dynamics. The availability of molecular and genetic tools in mice [79] presents a unique opportunity for a more precise interrogation of the cellular-level components that underlie inter-brain neural dynamics.
Neural components encoding hidden variables
One common finding across multi-brain studies is that inter-brain dynamics often predict or correlate with social interaction—defined using behavioral measures—yet cannot be explained by structurally correlated behavior or sensory inputs. In mice, inter-brain synchrony was observed even when contributions from all observable behaviors were discounted using a multivariate statistical model [21], and in bats, neural synchrony exceeded the degree of behavioral correlation across animals [20]. In other words, while neural components that encode sensory cues and behavior contribute to inter-brain dynamics, other neural processes, hidden from external observation, also play an important role. In addition to encoding self and partner behavior, neurons in the mPFC may also encode specific behavior sequences, plans, decision rules and contingencies, or internal states such as attention. Involvement of the mentalization and mirror neuron networks in humans lends strong support to this idea. While it is not yet known whether such neural computations contribute to inter-brain dynamics in animals, these questions can be addressed using molecular and genetic tools. For example, recording activity of specific circuit components or neuromodulatory signals that are involved in processes such as social attention [89,97–99] may shed light on whether and to what degree internal state variables contribute to inter-brain dynamics.
Computational models of multi-brain interactions
The striking temporal and regional heterogeneity of inter-brain dynamics points to a confluence of neural processes that span behavioral, cognitive, affective and relational domains. Development of computational models may shed light on how activity in single cells gives rise to regional and network-level dynamics, and how these dynamics may be shaped by biophysical or anatomical constraints [100]. Artificial systems such as adversarial neural networks can also be used to explore how behavior of interacting agents is related to computational processes, internal states (such as attention or memory), and shared dynamics. Such approaches hold promise to move us toward a more unified theoretical framework of the mechanisms underlying inter-brain dynamics.
Inter-brain dynamics at the group level
Beyond dyadic interactions, individuals may also come together into larger groups, where relatively simple local interactions can generate highly complex behavior at the group level [101,102]. While most studies have explored inter-brain dynamics across dyads, there has been recent effort to generalize the multi-brain framework beyond dyadic interaction. One study found that during quartet guitar playing, neural dynamics in four individuals’ brains are recruited into a hyper-brain network that evolves throughout the interaction [44]. Remarkably, the connectivity of the hyper-brain network correlated with different phases of the musical piece, and also reflected directional relationships between guitarists’ roles. In a classroom of 12 students, inter-brain synchrony between students could predict class engagement [103], suggesting that coherence across individuals in a group may provide a neural correlate of group attention and cognitive alignment. Shared group neural dynamics in the prefrontal cortex also predicted group cohesion and intergroup hostility [104,105], suggesting that multi-brain dynamics may be a substrate for shaping group interactions. These studies break new ground by demonstrating the feasibility of group recordings and the potential for discovery of multi-brain neural dynamics at the group level. Development of novel approaches to measure functional connectivity across multiple brains will be an important agenda for future research, as analysis of pairwise synchronization may provide a limited view in group settings. In complement, development in recording technologies [77,106] that enable monitoring of neural dynamics in groups of freely behaving animals will open the door to study underlying mechanisms with unprecedented precision and cellular resolution.
Inter-brain dynamics as biomarkers for social disruption
As inter-brain dynamics correlate with social variables such as cooperativity and shared affective states, disruptions in inter-brain dynamics may also signal deficits in social interaction. In line with this, several studies have begun to investigate how inter-brain dynamics may be altered in mental and neurodevelopmental disorders that affect social interaction. Frontal inter-brain synchrony is reduced in adults with autism spectrum disorder (ASD) compared to non-ASD controls [107]. Disruptions in interbrain dynamics have also been observed in interactions between ASD children and their mothers, and these disruptions correlated with the severity of ASD symptoms [108]. During interactions between healthy individuals and those with Borderline Personality Disorder, inter-brain synchrony was reduced compared to synchrony between two healthy people [109]. These findings suggest that social deficits, at least in some disorders, may be linked to the misalignment of specific internal processes. Moreover, the fact that inter-brain dynamics correlated with particular symptoms in ASD raises the possibility that individual variability in inter-brain dynamics may be related to disease heterogeneity [107,108]. Deeper exploration of how inter-brain dynamics vary within and across patient populations can strengthen our understanding of the mechanisms underlying specific symptoms. This may shed new light on core deficits common across different disorders, as well as the heterogeneity of presentations within patient groups, possibly leading to more effective therapies and diagnostic tools.
Inter-brain dynamics as a mechanism for social coordination
Beyond providing a neural correlate of shared social variables, inter-brain dynamics such a synchrony may also reflect a biological mechanism to coordinate behavior, cognitive/affective states, or social relationships [52]. Testing a causal role for inter-brain synchrony requires simultaneous manipulation of neural activity in interacting partners. While the idea of inter-brain synchrony as a casual mechanism in neural systems has been discussed in the past, demonstrating a causal role in shaping neural or behavioral processes remains a substantial challenge (see Outstanding Questions). Still, recent efforts have begun to test the causal role of inter-brain dynamics in humans using transcranial alternating current stimulation (tACS). Researchers found that in-phase (but not anti-phase) stimulation of the motor cortex in two participants increased synchronized tapping, suggesting that inter-brain synchrony during the preparatory period may promote action coordination [110]. In another study, researchers found that simultaneous stimulation of frontal and parietal regions disrupts behavioral synchronization during drumming [111]. The opposing effects on behavioral synchronization raise questions about the regional and temporal variability of inter-brain dynamics and how to more precisely manipulate specific patterns of neural activity.
Outstanding Questions.
Inter-brain neural dynamics emerge from brain regions and networks that play a role in behavior and cognitive functions, yet the underlying cellular-level mechanisms are not clear. Do inter-brain dynamics such as neural synchrony arise from specific anatomically or molecularly defined neural circuits or cell subpopulations? If so, what computational functions do these circuits play, and how are they engaged during interaction in different social contexts?
How are inter-brain dynamics related to social dysfunction in psychiatric and neurodevelopmental disorders, and can alterations in inter-brain dynamics provide an informative endophenotype or biomarker for disease states?
How do inter-brain dynamics across multiple individuals during social interaction relate to the organization of group-level behavioral/relational properties and collective behavior?
The synchronization of neural processes across individuals is associated with shared behavioral, cognitive, and relational variables, yet a functional role for inter-brain synchrony in coordinating social interaction has not been demonstrated. Are inter-brain dynamics purely correlative, or does the synchronization of neural processes across individuals play a causal role in shaping social interaction?
Despite these findings, it is still unclear at the conceptual level how inter-brain dynamics could exert causal influence on neural or behavioral processes, as explicit knowledge of inter-brain dynamics requires measuring activity in two or more individuals. However, while no individual has physical access to the internal processes of another, individuals can make inferences about other’s internal states based on behavioral cues [1,2,5]. In principal, this mentalization process may support an estimate of the synchronization of internal state variables across individuals which can inform decisions. For instance, a subject’s attentional state may be compared with the estimated attentional state of an interacting partner by some circuit. By computing the synchronization of self and inferred attentional states across individuals, such a circuit could shape behavior based on estimated synchrony of their attentional states. While such a mechanism has not been tested, it is possible to determine whether any neural components encode the inter-brain synchronization of specific neural processes. The identification of circuits that encode inter-brain dynamics can provide a steppingstone toward further investigation of their potential role in shaping interaction. Moreover, testing a causal role for inter-brain dynamics may require precise knowledge of the cellular-level circuits or neural subpopulations involved. Such specificity is feasible using animal models where precise manipulation of well-defined circuits can be achieved using implanted electrodes or optogenetics [112].
Concluding Remarks
The multi-brain framework expounded in this review moves beyond treating individuals as isolated actors, instead considering interacting agents as an integrated network of neural systems. Through this lens, researchers have interrogated the emergent neural properties of multi-individual systems and explored how they relate to social interaction. Insights into inter-brain dynamics and their underlying mechanisms will continue to transform our understanding of the social brain in health and in disease. More broadly, a deeper understanding of inter-brain dynamics may provide unique insight into the neural basis of collective behavior which gives rise to a broad range of economic, political, and socio-cultural activities that shape society. As we expand our knowledge of the brain and our capacity to engage directly with it, this line of research can facilitate advances in brain-to-brain interface technology that may offer direct, high-bandwidth connection between brains, bypassing the bottleneck of external communication. Ultimately, all these developments will transform how we see ourselves, how we interact with each other, and how we collectively shape our shared future.
Highlights.
Social interaction engages individuals directly with one another, coupling them in a dynamic feedback loop of action and reaction. A new conceptual framework, which views interacting agents as embedded in an integrated system, focuses attention on the emergent neural properties of multiple brains as they coordinate across individuals during social interaction.
Inter-brain neural dynamics that arise across brains of interacting individuals provide neural correlates of shared social variables, including coordinated behavior, shared cognitive or affective states, and relational states such as dominance or familial relationships.
Recent work extends the view of inter-brain dynamics to include their study in animal model systems, revealing the existence of inter-brain synchronization across diverse species. Invasive recording techniques and molecular tools available in animal models shed new light on the specific circuit-level mechanisms underlying inter-brain dynamics.
Acknowledgements
We thank D. Wei and X. Cui for insightful comments. This work was supported in part by NIH F31-MH117966 and R01-NS113124, a Searle Scholars Award, a Klingenstein-Simons Fellowship, a Packard Foundation Fellowship, and a McKnight Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rilling JK and Sanfey AG (2011) The Neuroscience of Social Decision-Making. Annu. Rev. Psychol. 62, 23–48 [DOI] [PubMed] [Google Scholar]
- 2.Sanfey AG (2007) Social Decision-Making: Insights from Game Theory and Neuroscience. Science (80-. ). 318, 598–602 [DOI] [PubMed] [Google Scholar]
- 3.Kliemann D and Adolphs R The social neuroscience of mentalizing: challenges and recommendations., Current Opinion in Psychology, 24 01-December-(2018), Elsevier B.V., 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spunt RP and Adolphs R The neuroscience of understanding the emotions of others., Neuroscience Letters, 693 06-February-(2019), Elsevier Ireland Ltd, 44–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frith CD and Frith U The Neural Basis of Mentalizing., Neuron, 50 18-May-(2006), Cell Press, 531–534 [DOI] [PubMed] [Google Scholar]
- 6.Chen P and Hong W (2018) Neural Circuit Mechanisms of Social Behavior. Neuron 98, 16–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schilbach L et al. (2013) Toward a second-person neuroscience. Behav. Brain Sci. 36, 393–414 [DOI] [PubMed] [Google Scholar]
- 8.Hasson U et al. Brain-to-brain coupling: A mechanism for creating and sharing a social world., Trends in Cognitive Sciences, 16 February-(2012), NIH Public Access, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montague PR et al. (2002) Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–64 [DOI] [PubMed] [Google Scholar]
- 10.Redcay E and Schilbach L (2019) Using second-person neuroscience to elucidate the mechanisms of social interaction. Nat. Rev. Neurosci. 20, 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allsop SA and Wichmann R (2018) Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 173, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panksepp J and Panksepp JB (2013) Toward a cross-species understanding of empathy. Trends Neurosci. 36, 489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolphs R (2009) The Social Brain: Neural Basis of Social Knowledge. Annu. Rev. Psychol. 60, 693–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spunt RP and Adolphs R A new look at domain specificity: Insights from social neuroscience., Nature Reviews Neuroscience, 18 21-August-(2017), Nature Publishing Group, 559–567 [DOI] [PubMed] [Google Scholar]
- 15.Hong W et al. (2015) Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl. Acad. Sci. 112, E5351–E5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DJ and Perona P (2014) Toward a Science of Computational Ethology. Neuron 84, 18–31 [DOI] [PubMed] [Google Scholar]
- 17.Mathis A et al. (2018) DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 [DOI] [PubMed] [Google Scholar]
- 18.Liu T and Pelowski M (2014) A new research trend in social neuroscience: Towards an interactive-brain neuroscience. PsyCh J. 3, 177–188 [DOI] [PubMed] [Google Scholar]
- 19.Tseng P-H et al. (2018) Interbrain cortical synchronization encodes multiple aspects of social interactions in monkey pairs. Sci. Rep. 8, 4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W and Yartsev MM (2019) Correlated Neural Activity across the Brains of Socially Interacting Bats. Cell 178, 413–428.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsbury L et al. (2019) Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell 178, 429–446.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T and Pelowski M (2014) Clarifying the interaction types in two-person neuroscience research. Front. Hum. Neurosci. 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King-Casas B et al. (2005) Getting to Know You: Reputation and Trust in a Two-Person Economic Exchange. Science (80-. ). 308, 78–83 [DOI] [PubMed] [Google Scholar]
- 24.Babiloni F et al. (2006), Hypermethods for EEG hyperscanning., in 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 3666–3669 [DOI] [PubMed] [Google Scholar]
- 25.De Vico Fallani F et al. (2010) Defecting or Not Defecting: How to “Read” Human Behavior during Cooperative Games by EEG Measurements. PLoS One 5, e14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krueger F et al. (2007) Neural correlates of trust. Proc. Natl. Acad. Sci. U. S. A. 104, 20084–20089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang J et al. (2012) Neural Synchronization during Face-to-Face Communication. J. Neurosci. 32, 16064–16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindenberger U et al. (2009) Brains swinging in concert: Cortical phase synchronization while playing guitar. BMC Neurosci. 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sänger J et al. (2012) Intra- and interbrain synchronization and network properties when playing guitar in duets. Front. Hum. Neurosci. 6, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sänger J et al. (2013) Directionality in hyperbrain networks discriminates between leaders and followers in guitar duets. Front. Hum. Neurosci 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balconi M and Vanutelli ME Cooperation and competition with hyperscanning methods: Review and future application to emotion domain., Frontiers in Computational Neuroscience, 11 29-September-(2017), Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nozawa T et al. (2016) Interpersonal frontopolar neural synchronization in group communication: An exploration toward fNIRS hyperscanning of natural interactions. Neuroimage 133, 484–497 [DOI] [PubMed] [Google Scholar]
- 33.Liu N et al. (2016) Nirs-based hyperscanning reveals inter-brain neural synchronization during cooperative jenga game with face-to-face communication. Front. Hum. Neurosci. 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui X et al. (2012) NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage 59, 2430–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schippers MB et al. (2010) Mapping the information flow from one brain to another during gestural communication. Proc. Natl. Acad. Sci. U. S. A. 107, 9388–9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anders S et al. (2011) Flow of affective information between communicating brains. Neuroimage 54, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens GJ et al. (2010) Speaker-listener neural coupling underlies successful communication. Proc. Natl. Acad. Sci. U. S. A. 107, 14425–14430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funane T et al. (2011) Synchronous activity of two people’s prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. J. Biomed. Opt. 16, 077011. [DOI] [PubMed] [Google Scholar]
- 39.Cheng X et al. (2015) Synchronous brain activity during cooperative exchange depends on gender of partner: A fNIRS-based hyperscanning study. Hum. Brain Mapp. 36, 2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konvalinka I et al. (2014) Frontal alpha oscillations distinguish leaders from followers: Multivariate decoding of mutually interacting brains. Neuroimage 94, 79–88 [DOI] [PubMed] [Google Scholar]
- 41.Holper L et al. (2012) Between-brain connectivity during imitation measured by fNIRS. Neuroimage 63, 212–222 [DOI] [PubMed] [Google Scholar]
- 42.Yun K et al. (2012) Interpersonal body and neural synchronization as a marker of implicit social interaction. Sci. Rep. 2, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumas G et al. (2010) Inter-Brain Synchronization during Social Interaction. PLoS One 5, e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller V et al. (2018) Hyperbrain network properties of guitarists playing in quartet. Ann. N. Y. Acad. Sci. 1423, 198–210 [DOI] [PubMed] [Google Scholar]
- 45.Tang H et al. (2015) Interpersonal brain synchronization in the right temporo-parietal junction during face-to-face economic exchange. Soc. Cogn. Affect. Neurosci. 11, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirsch J et al. (2018) A cross-brain neural mechanism for human-to-human verbal communication. Soc. Cogn. Affect. Neurosci. 13, 907–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch J et al. (2017) Frontal temporal and parietal systems synchronize within and across brains during live eye-to-eye contact. Neuroimage 157, 314–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koike T et al. (2016) Neural substrates of shared attention as social memory: A hyperscanning functional magnetic resonance imaging study. Neuroimage 125, 401–412 [DOI] [PubMed] [Google Scholar]
- 49.Koike T et al. (2019) Role of the right anterior insular cortex in joint attention-related identification with a partner. Soc. Cogn. Affect. Neurosci. 14, 1131–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szymanski C et al. (2017) Teams on the same wavelength perform better: Inter-brain phase synchronization constitutes a neural substrate for social facilitation. Neuroimage 152, 425–436 [DOI] [PubMed] [Google Scholar]
- 51.Saito DN et al. (2010) Stay tuned: Inter-individual neural synchronization during mutual gaze and joint attention. Front. Integr. Neurosci. 4, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasson U and Frith CD (2016) Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 371, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stolk A et al. (2014) Cerebral coherence between communicators marks the emergence of meaning. Proc. Natl. Acad. Sci. U. S. A. 111, 18183–18188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiegelhalder K et al. (2014) Interindividual synchronization of brain activity during live verbal communication. Behav. Brain Res. 258, 75–79 [DOI] [PubMed] [Google Scholar]
- 55.Pérez A et al. (2017) Brain-To-brain entrainment: EEG interbrain synchronization while speaking and listening. Sci. Rep. 7, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y et al. (2017) Measuring speaker-listener neural coupling with functional near infrared spectroscopy. Sci. Rep. 7, 43293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen M et al. (2019) Shared understanding of narratives is correlated with shared neural responses. Neuroimage 184, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai B et al. (2018) Neural mechanisms for selectively tuning in to the target speaker in a naturalistic noisy situation. Nat. Commun. 9, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun B et al. (2020) Behavioral and brain synchronization differences between expert and novice teachers when collaborating with students. Brain Cogn. 139, 105513. [DOI] [PubMed] [Google Scholar]
- 60.Kinreich S et al. (2017) Brain-to-Brain Synchrony during Naturalistic Social Interactions. Sci. Rep. 7, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan Y et al. (2017) Cooperation in lovers: An fNIRS-based hyperscanning study. Hum. Brain Mapp. 38, 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leong V et al. (2017) Speaker gaze increases information coupling between infant and adult brains. Proc. Natl. Acad. Sci. U. S. A. 114, 13290–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reindl V et al. (2018) Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. Neuroimage 178, 493–502 [DOI] [PubMed] [Google Scholar]
- 64.Nguyen T et al. (2020) The effects of interaction quality on neural synchrony during mother-child problem solving. Cortex 124, 235–249 [DOI] [PubMed] [Google Scholar]
- 65.Jiang J et al. (2015) Leader emergence through interpersonal neural synchronization. Proc. Natl. Acad. Sci. U. S. A. 112, 4274–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sänger J et al. (2013) Directionality in hyperbrain networks discriminates between leaders and followers in guitar duets. Front. Hum. Neurosci. 7, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker JM et al. (2016) Sex differences in neural and behavioral signatures of cooperation revealed by fNIRS hyperscanning. Sci. Rep. 6, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sänger J et al. (2012) Intra- and interbrain synchronization and network properties when playing guitar in duets. Front. Hum. Neurosci. 6, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu T et al. (2017) Inter-brain network underlying turn-based cooperation and competition: A hyperscanning study using near-infrared spectroscopy. Sci. Rep. 7, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Špiláková B et al. (2019) Dissecting social interaction: dual-fMRI reveals patterns of interpersonal brain-behavior relationships that dissociate among dimensions of social exchange. Soc. Cogn. Affect. Neurosci. 14, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burgess AP (2013) On the interpretation of synchronization in EEG hyperscanning studies: A cautionary note. Front. Hum. Neurosci. 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciaramidaro A et al. (2018) Multiple-Brain Connectivity during Third Party Punishment: An EEG Hyperscanning Study Sci. Rep. 8, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fairhurst M and Dumas G (2019), PsyArXiv Preprints | Reciprocity and alignment: quantifying coupling in dynamic interactions.. [Online]. Available: https://psyarxiv.com/nmg4x/ [Accessed: 07-Jun-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldstein P et al. (2018) Brain-to-brain coupling during handholding is associated with pain reduction. Proc. Natl. Acad. Sci. U. S. A. 115, E2528–E2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buzsáki G Large-scale recording of neuronal ensembles., Nature Neuroscience, 7 May-(2004), 446–451 [DOI] [PubMed] [Google Scholar]
- 76.Yang W and Yuste R (2017) In vivo imaging of neural activity. Nat. Methods 14, 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh KK et al. (2011) Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buzsáki G et al. Tools for probing local circuits: High-density silicon probes combined with optogenetics., Neuron, 86 08-April-(2015), Cell Press, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo L et al. (2008) Genetic dissection of neural circuits. Neuron 57, 634–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Konvalinka I et al. (2014) Frontal alpha oscillations distinguish leaders from followers: Multivariate decoding of mutually interacting brains. Neuroimage 94, 79–88 [DOI] [PubMed] [Google Scholar]
- 81.Rizzolatti G and Craighero L (2004) THE MIRROR-NEURON SYSTEM. Annu. Rev. Neurosci. 27, 169–192 [DOI] [PubMed] [Google Scholar]
- 82.Iacoboni M and Dapretto M The mirror neuron system and the consequences of its dysfunction., Nature Reviews Neuroscience, 7 December-(2006), 942–951 [DOI] [PubMed] [Google Scholar]
- 83.Toppi J et al. (2016) Investigating Cooperative Behavior in Ecological Settings: An EEG Hyperscanning Study. PLoS One 11, e0154236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dumas G et al. (2010) Inter-Brain Synchronization during Social Interaction. PLoS One 5, e12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konvalinka I et al. (2014) Frontal alpha oscillations distinguish leaders from followers: Multivariate decoding of mutually interacting brains. Neuroimage 94, 79–88 [DOI] [PubMed] [Google Scholar]
- 86.Kinreich S et al. (2017) Brain-to-Brain Synchrony during Naturalistic Social Interactions. Sci. Rep. 7, 17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mu Y et al. (2017) The role of gamma interbrain synchrony in social coordination when humans face territorial threats. Soc. Cogn. Affect. Neurosci. 12, 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahn S et al. (2018) Interbrain phase synchronization during turn-taking verbal interaction—a hyperscanning study using simultaneous EEG/MEG. Hum. Brain Mapp. 39, 171–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim H et al. (2016) Prefrontal Parvalbumin Neurons in Control of Attention. Cell 164, 208–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clayton MS et al. The roles of cortical oscillations in sustained attention., Trends in Cognitive Sciences, 19 01-April-(2015), Elsevier Ltd, 188–195 [DOI] [PubMed] [Google Scholar]
- 91.Buzsáki G et al. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms., Neuron, 80 30-October-(2013), Neuron, 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piva M et al. (2019) The dorsomedial prefrontal cortex computes task-invariant relative subjective value for self and other. Elife 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Danjo T et al. (2018) Spatial representations of self and other in the hippocampus. Science 359, 213–218 [DOI] [PubMed] [Google Scholar]
- 94.Omer DB et al. (2018) Social place-cells in the bat hippocampus. Science 359, 218–224 [DOI] [PubMed] [Google Scholar]
- 95.Haroush K and Williams ZM (2015) Neuronal Prediction of Opponent’s Behavior during Cooperative Social Interchange in Primates. Cell 160, 1233–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dumas G et al. (2020) The Human Dynamic Clamp Reveals the Fronto-Parietal Network Linking Real-Time Social Coordination and Cognition. Cereb. Cortex 30, 3271–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller EK and Buschman TJ (2013) Cortical circuits for the control of attention. Curr. Opin. Neurobiol. 23, 216–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein JT et al. (2009) Social attention and the brain. Curr. Biol. 19, R958–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mu Y et al. (2016) Oxytocin enhances inter-brain synchrony during social coordination in male adults. Soc. Cogn. Affect. Neurosci. 11, 1882–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dumas G et al. (2012) Anatomical connectivity influences both intra- and inter-brain synchronizations. PLoS One 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Couzin ID and Krause J (2003) Self-Organization and Collective Behavior in Vertebrates,
- 102.Couzin ID Collective cognition in animal groups., Trends in Cognitive Sciences, 13 January-(2009), 36–43 [DOI] [PubMed] [Google Scholar]
- 103.Dikker S et al. (2017) Brain-to-Brain Synchrony Tracks Real-World Dynamic Group Interactions in the Classroom. Curr. Biol. 27, 1375–1380 [DOI] [PubMed] [Google Scholar]
- 104.Yang J et al. (2020) Within-group synchronization in the prefrontal cortex associates with intergroup conflict. Nat. Neurosci. 23, 754–760 [DOI] [PubMed] [Google Scholar]
- 105.Ikeda S et al. (2017) Steady Beat Sound Facilitates both Coordinated Group Walking and Inter-Subject Neural Synchrony. Front. Hum. Neurosci. 11, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aharoni D and Hoogland TM Circuit investigations with open-source miniaturized microscopes: Past, present and future., Frontiers in Cellular Neuroscience, 13 29-January-(2019), Frontiers Media S.A., 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanabe HC et al. (2012) Hard to “tune in”: Neural mechanisms of live face-to-face interaction with high-functioning autistic spectrum disorder. Front. Hum. Neurosci. 6, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Q et al. (2020) Autism Symptoms Modulate Interpersonal Neural Synchronization in Children with Autism Spectrum Disorder in Cooperative Interactions. Brain Topogr. 33, 112–122 [DOI] [PubMed] [Google Scholar]
- 109.Bilek E et al. (2017) State-dependent cross-brain information flow in borderline personality disorder. JAMA Psychiatry 74, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Novembre G et al. Interpersonal synchrony enhanced through 20 Hz phase-coupled dual brain stimulation. DOI: 10.1093/scan/nsw172 [DOI] [PMC free article] [PubMed]
- 111.Szymanski C et al. (2017) Hyper-transcranial alternating current stimulation: Experimental manipulation of inter-brain synchrony. Front. Hum. Neurosci. 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yizhar O et al. (2011) Optogenetics in Neural Systems. Neuron 71, 9–34 [DOI] [PubMed] [Google Scholar]