Abstract
Despite the outstanding clinical results of immune checkpoint blockade (ICB) in melanoma and other cancers, clinical trials in breast cancer have reported low responses to these therapies. Current efforts are now focused on improving the treatment efficacy of ICB in breast cancer using new combination designs such as molecularly targeted agents, including histone deacetylase inhibitors (HDACi). These epigenetic drugs have been widely described as potent cytotoxic agents for cancer cells. In this work, we report new non-canonical regulatory properties of ultra-selective HDAC6i over the expression and function of epithelial-mesenchymal transition pathways and the invasiveness potential of breast cancer. These unexplored roles position HDAC6i as attractive options to potentiate ongoing immunotherapeutic approaches. These new functional activities of HDAC6i involved regulation of the E-cadherin/STAT3 axis. Pre-treatment of tumors with HDAC6i induced critical changes in the tumor microenvironment, resulting in improved effectiveness of ICB and preventing dissemination of cancer cells to secondary niches. Our results demonstrate for the first time that HDAC6i can both improve ICB antitumor immune responses and diminish the invasiveness of BC with minimal cytotoxic effects, thus departing from the cytotoxicity-centric paradigm previously assigned to HDACi.
Keywords: Histone Deacetylases, PD-L1, Breast Cancer, metastasis, Nexturastat A, Immunotherapy, E-Cadherin
Introduction:
Among breast cancer (BC) subtypes, triple-negative breast cancer (TNBC) is characterized by the lack of expression of estrogen receptor type α (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) (1). This subtype presents a significant challenge to targeted therapy and is more likely to metastasize, versus other BC subtypes (2). Although the infiltration and composition of immune cells may vary across different BC subtypes (3), it has been shown that TNBC exhibits a high degree of stromal and tumor-infiltrating lymphocytes (TILs) (4), including immunosuppressive regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAMs). In addition to altering the endogenous properties of tumor cells, reprogramming the inflammatory properties and cellular composition of the tumor microenvironment (TME) could be a viable choice to overcome resistance to immunotherapy, as seen in the TONIC clinical trial (5) and other studies comparing the TME between responders and non-responders to specific anticancer therapies (6). Within the realm of immunotherapy, reprogramming the T-cell activity by overcoming the co-inhibitory pathways with neutralizing antibody (Immune checkpoint blockade-mechanism) has taken up a prime role (7).
Mechanisms underlying resistance to immune checkpoint blockade (ICB) are still not fully understood. Although several studies have highlighted acquired resistance mechanisms in T cells (8), primary and adaptive pathways in other components of the TME have not been explored in detail. Modalities regulating the epithelial-mesenchymal transition (EMT) have been proposed as promising targets to overcome ICB resistance (9). Specifically, recent studies indicate that cancer patients refractory to the established ICB treatment, such as anti-programmed cell death protein 1 (αPD-1 therapy), have altered expression of genes involved in EMT and cell adhesion, including down-regulation of E-Cadherin (E-Cad) (10). Importantly, TAMs are negative regulators of E-Cad expression in cancer cells (11). However, it is not clear how decreased E-Cad leads to ICB resistance. Even less is known about whether a pharmacologic approach could restore E-Cad expression and thus sensitize tumors to αPD-1 treatment. Epigenetic modulators have been explored as a potential class of molecules to overcome the ICB resistance pathways, due to their ability to affect multiple cellular processes (12).
Histone deacetylases (HDACs), a family of 11 zinc-dependent iso-enzymes described initially as histone modifiers, modify various proteins involved in diverse cellular processes unrelated to the chromatin environment. The role of specific HDACs in cell proliferation and survival have been extensively studied (13). However, their involvement in the regulation of immune-related pathways is not entirely understood (14). Pan-HDAC inhibitors (HDACis) have been used as anticancer agents in clinical settings. But critical limitations, including low activity in solid tumors and cardiac toxicity (15), have limited their use in combination therapies. For example, vorinostat is a pan-HDACi that activates multiple apoptotic pathways in both cancer and normal cells, thus limiting its use for extended periods (16). The non-selective nature of current HDACis is likely a significant cause of HDACi-associated clinical toxicities. On the other hand, ricolinostat, which targets HDAC6 and class I-HDACs, showed relatively lower levels of adverse effects, well-tolerability, and increased efficacy in initial clinical trial, combined with anticancer drug lenalidomide and steroid drug dexamethasone (17). It remains underexplored whether selective HDACis could achieve equal efficacy with reduced side-effects than pan-HDACi. The findings from phase 1b trial with ricolinostat provide additional rationales to investigate HDAC-specific inhibitors featuring selectivity and lower toxicity.
We used a highly specific HDAC6i, Nexturastat A (NextA), which has been used in multiple in vitro and in vivo models (18–21). NextA was screened against all 11 isozymes. Within the similar Class 1 and Class 4 isozymes, NextA displayed low micromolar activity compared to the low nanomolar activity against HDAC6 as well as high levels of selective inhibition against members of the related Class 2 HDAC isozymes, reaching >1000-fold selectivity in some cases. The functional specificity of NextA was confirmed by its ability to induce hyperacetylation of α-tubulin, a hallmark of HDAC6 inhibition (21). This next-generation HDAC6i has minimal cytotoxic effects, and mainly affects immune-related functions in tumor and immune cells (18). Examples include regulation of macrophage-phenotype and tumoral expression of immunosuppressive molecules, e.g., PD-L1, PD-L2, B7-H3, and B7-H4 (22). Also, HDAC6 regulates tubulin and cortactin, which are important modulators of cellular shape, motility, and cytoskeletal structure (23), making it a critical factor in metastasis. Overexpression of HDAC6 increased chemotactic cell motility, whereas selective inhibition of HDAC6 leading to tubulin-hyperacetylation, inhibited the invasion and motility of fibroblasts (24). Moreover, HDAC6 is associated with anchorage-independent growth in BC, such as patient-derived adenocarcinoma SKBR3 and hormonal receptor-positive MCF7 (25).
Given the central role of HDAC6 in determining tumoral characteristics, we tested the hypothesis that pharmacological and genetic inhibition of HDAC6 confers benefits at multiple levels, such as primary and metastatic tumor growth. Our results indicated that NextA potentiates the anti-tumor effect of ICB by inducing noticeable changes within TME, including diminished infiltration of immunosuppressive cells and upregulation of adhesion molecules such as E-Cad. These findings support the novel concept of HDAC6i as immunomodulatory agents and differentiate them from the canonical cytotoxic anti-cancer role previously assigned to HDACis.
Materials and methods:
Tumor implantation:
Experiments involving mice were executed in accordance with approved protocols by the Institutional Care and Use Committee (IACUC) at George Washington University, along with supervision from IACUC Research Compliance team. Briefly, four-six weeks old female BALB/c mice were obtained from the Charles River Laboratories (Wilmington, MA). For in vivo tumor studies, mice were subcutaneously injected into the fat-pad of shaved fourth mammary glands with 0.5× 105 mammary carcinoma 4T1 cells, suspended in 100 μL 1X sterile phosphate-buffered saline (PBS) (Corning, 21–040-CV, Corning, NY). For singular treatment experiments, treatment began when the tumors were palpable (approx. 3×3mm3). For pre-treatment combination experiments, mice were injected with 25mg/kg NextA four days post-tumor implantation, followed by αPD-1 antibody at specified concentration of 3 mg/kg when the control group reached a size of 3×3mm3. For combination experiments, mice were injected with vehicle control, single treatments of 25 mg/kg NextA, 3mg/kg anti-mouse PD-1 (BioXCell, BE0146, West Lebanon, NH), or the combination of both. Control mice were injected with 100 μl 1X PBS (Corning, 21–040-CV, Corning, NY) as vehicle. Mice were injected six days a week until tumors in control group reached maximum size according to the IACUC guidelines (2000 mm3). The overall health of the mice and tumor sizes were monitored twice a week. For the genetic knockdown cell lines, non-target control and shHDAC6–4T1 cells were injected parallelly in six-week-old female mice adhering to the same cell number and location of injection. The progression of tumor was recorded every 3 days.
At the terminal stage, mice were euthanized with CO2 asphyxiation and cervical dislocation. Mice were dissected to harvest primary tumors and lungs (main location for metastatic events). Other major organs (heart, liver, bone marrow) were checked for metastatic nodules, despite limited occurrence.
Imaging for real-time metastatic progression:
To observe the progression of metastatic disease in absence of primary tumor, weekly bioluminescence measurements were performed. Soluble Luciferin (XenoLight D-Luciferin - K+ Salt) (Perkin Elmer, 122799, Boston, MA) was injected intraperitoneally, and bioluminescence was measured using IVIS Lumina In Vivo Imaging System (Perkin Elmer, Boston, MA).
In vivo tumor passaging and in vitro culturing/storing:
4T1 cell line, a model for triple-negative murine carcinoma, was a generous gift from Dr. Scott Abrams’ laboratory at RPCI, Buffalo, NY (authenticated by IDEXX through STR-profiling: details provided as supplemental document). Cells were passaged once in vivo, and tumor clones were grown from the tumor explants, which were selected for optimal and consistent growth and frozen down for further in vivo experimentations. When preparing cells for further tumor injection, mice were euthanized for tumor extraction and processed under sterile conditions. Cell concentration adjusted for appropriate amount per mouse (0.5 × 105/100 μL) and immediately injected into animals. To generate in vivo metastatic clone of 4T1, tumor nodules harvested from lung of primary tumor-bearing mice were digested and cultured in the presence of 6-thioguanine (Sigma-Aldrich, A4660–2, Washington DC) in RPMI media, as described previously (26). Cells for storing purposes were immediately frozen in 90% Fetal Bovine Serum (FBS) (Gibco, 16–140-071, Gaithersburg, MD) with 10% Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, D2650, Washington DC) and stored in vapor phase of liquid nitrogen.
siRNA transfection:
To abrogate the expression of E-Cadherin (E-Cad) in 4T1 cells, murine siRNA for E-Cad (Santa Cruz, sc-35243, Dallas, TX) was used. Reverse transfection was performed using lipofectamine 3000 (ThermoFisher Scientific, L3000015, Waltham, MA). Knockdown was assessed after 48 hours of transfection by qRT-PCR and Western blotting.
shRNA transfection:
To abrogate the expression of HDAC6 in 4T1 cells, murine shRNA for HDAC6 (Sigma-Aldrich, SHCLNG-NM_010413 MISSION® shRNA Bacterial Glycerol Stock; Washington DC) was packaged into lentiviral particles and virally transfected into 4T1 along with a non-target control vector. The knockdown strain was cultured under antibiotic selection and segregated in single clones with serial dilution. Knockdown was verified with western blot.
Luciferase-expression:
4T1 cells were transfected with a luciferase-expressing plasmid containing a puromycin selection marker (Promega, 6741, Madison, WI). 4T1 cells were assessed for puromycin sensitivity and determined to be sensitive at a dose of 8 μg/ml or higher. Lipofectamine 2000 (ThermoFisher Scientific, 11668019, Waltham, MA) was used for the transfection. Antibiotic selected cells were serially diluted to single colonies from which the colony expressing highest luciferase luminescence was used for in vivo experiments.
Tumor/T cell co-culture:
4T1 mammary carcinoma cells were cultured in a six-well plate to form a monolayer attaining 75–80% confluency. Whole spleens from BALB/c mice, either non-tumor bearing or 4T1 tumor-bearing, were harvested and homogenized. CD3+ cells were isolated by EasySep magnetic kit (Stemcell Technologies, 19851, 18000, Vancouver, BC) through negative selection. Cells were checked for viability to >90%. CD3+ splenocytes were stimulated with CD3/28 magnetic dynabeads (ThermoFisher Scientific, 11456D, Waltham, MA) overnight in complete RPMI. Stimulated T cells were then layered onto the 4T1-monolayer for 24 hours. The αPD-1 antibody was then added to the culture to a final concentration of 1ug/ml and cultured for another 24 hours. An anti-mouse interferon-gamma neutralizing antibody (PBL assay Science, Rabbit serum 22500–1; Piscataway, NJ) was then added to the culture. In a separate well, NextA was added to a final concentration of 2.5 μM or 5 μM. Tumor cells were collected after 24 hours of incubation for the detection of PD-L1 expression (Qiagen, 74104, Germantown, MD) through qRT-PCR.
Small Molecule inhibitors:
The HDAC6 selective inhibitor NextA and SS-2–08 (27) (used for control experiment alone) were purchased/obtained from Dr. Alan Kozikowski (Starwise Pharmaceuticals, Madison, Wisconsin, USA). NextA was kept at a stock solution of 10 mg/mL and diluted with 1X PBS (Corning, 21–040-CV, Corning, NY) to 25 mg/kg immediately before injection. The pan-HDACi LBH589 (ThermoFisher Scientific, 50–148-338, Waltham, MA) was dissolved in DMSO and further diluted in PBS for in vitro use. Entinostat (Selleckchem, S1053, Houston, TX) was dissolved in DMSO, diluted in PBS, and used for control experiments. The STAT3 inhibitor Stattic was purchased from Selleckchem (S7024).
Flow Cytometry:
Mice were euthanized, and tumors were immediately processed for analysis by flow cytometry. The tumor was rendered into a single-cell suspension by manual chopping and washing. Cells were stained for co-stimulatory molecules and tumor infiltrated cells (marker-list provided in supplemental methods). After staining for 30 min at room temperature, cells were washed, fixed and re-suspended in FACS buffer (1X PBS + 0.1% BSA).
All flow cytometry panels included a LIVE/DEAD® Fixable Aqua Dead Cell Stain (ThermoFisher Scientific, L34957, Waltham, MA) to determine viability. At least 50,000 events were collected using a BD Celesta Cell Analyzer and analyzed using FlowJo software 10.4.
Quantitative Real-time PCR (qRT-PCR):
Total RNA was extracted from cells using RNeasy Mini Kit (Qiagen, 74104, Germantown, MD) or the Trizol reagent for in vivo tumor samples (ThermoFisher Scientific, 15596018, Waltham, MA). Samples were processed immediately or stored at −80°C. RNA was quantified by NanoDrop Spectrophotometer (NanoDrop Technologies, ND-1000, Wilmington, DE). The 260/280 ratios were routinely monitored to be over 1.8 for subsequent use. The cDNA was synthesized using iScript kit (Bio-Rad, 1708891, Hercules, CA). Target mRNA was quantified using MyIQ single-color real-time PCR detection system from Bio-Rad (Hercules, CA) and iQ SYBR green Supermix (Bio-Rad, 1708882, Hercules, CA). Primers targeting PD-L1, IFNγ, E-Cad, cMYC, Vimentin, MMP9, Twist, VCAM1, and GAPDH for qRT-PCR were designed from Origene (Rockville, MD) and customized from Invitrogen (Waltham, MA) or purchased from Qiagen (Table 1. Supplementary Figure 7). Cycling conditions were used as per manufacturer’s instructions. Single product amplification was confirmed by melting curve analysis, and primer efficiency was near 100% in all the experiments performed. Target mRNA levels were normalized to GAPDH expression using the method described by Pfaffl et al. (28).
Immunoblotting:
Cells were lysed in RIPA buffer (ThermoFisher Scientific Pierce, 89900, Waltham, MA) supplemented with 1X protease and phosphatase inhibitor (ThermoFisher Scientific Pierce, A32961, Waltham, MA). Lysates were sonicated at 4°C for 8 minutes (8 cycles of 30s on, 30s off). Protein concentration was determined using a BCA protein assay (Thermo Fisher Scientific Pierce, 23225, Waltham, MA) according to the manufacturer’s protocol. Samples were mixed with NuPAGE LDS 4x loading gel (ThermoFisher Scientific, NP0007, Waltham, MA) and NuPAGE 10x reducing agent (ThermoFisher Scientific, NP0009, Waltham, MA), and boiled at 95°C. Next, samples were loaded on to 4–20% (Bio-Rad, 4561093, Hercules, CA) or 10% gels (Bio-Rad, 4561033, Hercules, CA), transferred to PVDF membranes and blocked with Odyssey Blocking Buffer (LI-COR Biosciences, 927–40100, Lincoln, NE). Bands were detected using an Azure Biosystems Imaging System c600 (Dublin, CA). The antibodies used for immunoblotting included: PD-L1 (ProSci, 4059, Poway, CA), Acetylated H3 (Cell signaling, 9677, Danvers, MA), STAT3 (Cell signaling, 9145), E-Cad (Cell Signaling, 3195S), Tubulin (Cell signaling, 2144), and acetylated tubulin (Cell signaling, 5335).
Wound Closure Assay and microscopic quantification:
4T1 cells were cultured into the glass bottom plates (Cellvis, D35–14-1-N, Mountain View, CA) within a 14mm central area to a confluency of 70–80%. A diagonal scratch was introduced with a sterile 1ml pipette tip. Floating cells were washed out of the lanes with PBS and treated with 5 uM NextA. Petri dishes, with or without treatment, were placed on a stage-mounted incubation system for imaging (Oko Lab) with the temperature set at 37°C, CO2 at 5%, and humidity at 95%.
Time-lapse images from a large stitched area, including the majority of the wound, were captured on a Leica DMi8 microscope with a piezo stage (Pecon). Leica LAS software was used to control the stage, microscope, and the camera. The software module – stage navigator was used to select the area for imaging, with a continuous adaptive autofocus control to correct for drift over the long period of acquisition. Images were taken using phase-contrast mode with 20x/0.4 dry objective (Leica HC PL Fuotar) using Hamamatsu Flash-4 camera at a pixel resolution of 0.84 μm. The total imaged area was approximately 4.5×3.3 mm, after stitching of the adjacent fields of the images. The individual images comprising the sets were captured at 10% overlap to ensure proper cross-correlation for stitching and registration as individual images. Fields from control and treated samples were taken every 30 minutes for 16 hours. The rate of cell migration and wound closure was measured by Image J (NIH).
Statistical Analysis:
At least three biological repeats were included in all molecular experiments. We used only female mice for the in vivo studies. The sample size for all in vivo studies was determined by power analysis, using α = 0.05, power = 0.9. Most in vivo experiments had similar endpoints. Antitumor responses were assessed by comparing tumor growth rate and mice survival. Three different approaches were used: 1) separate analyses of variance at different points in time; 2) fitting growth curves to individual mice to obtain estimates of growth rates that can be compared between groups; and 3) defining a critical tumor size, determining the time required to reach that size for each mouse, and comparing these times between groups using a multiple t-tests. In vitro analysis for comparing treatment, groups were performed by using column statistics of one-way ANOVA (Tukey’s multiple comparison test) and t-test. All in vivo and in vitro, statistical analysis were conducted using GraphPad Prism 7.
Results:
Selective HDAC6 inhibitors inhibit primary and secondary metastatic growth of murine mammary carcinoma in a dose-dependent manner
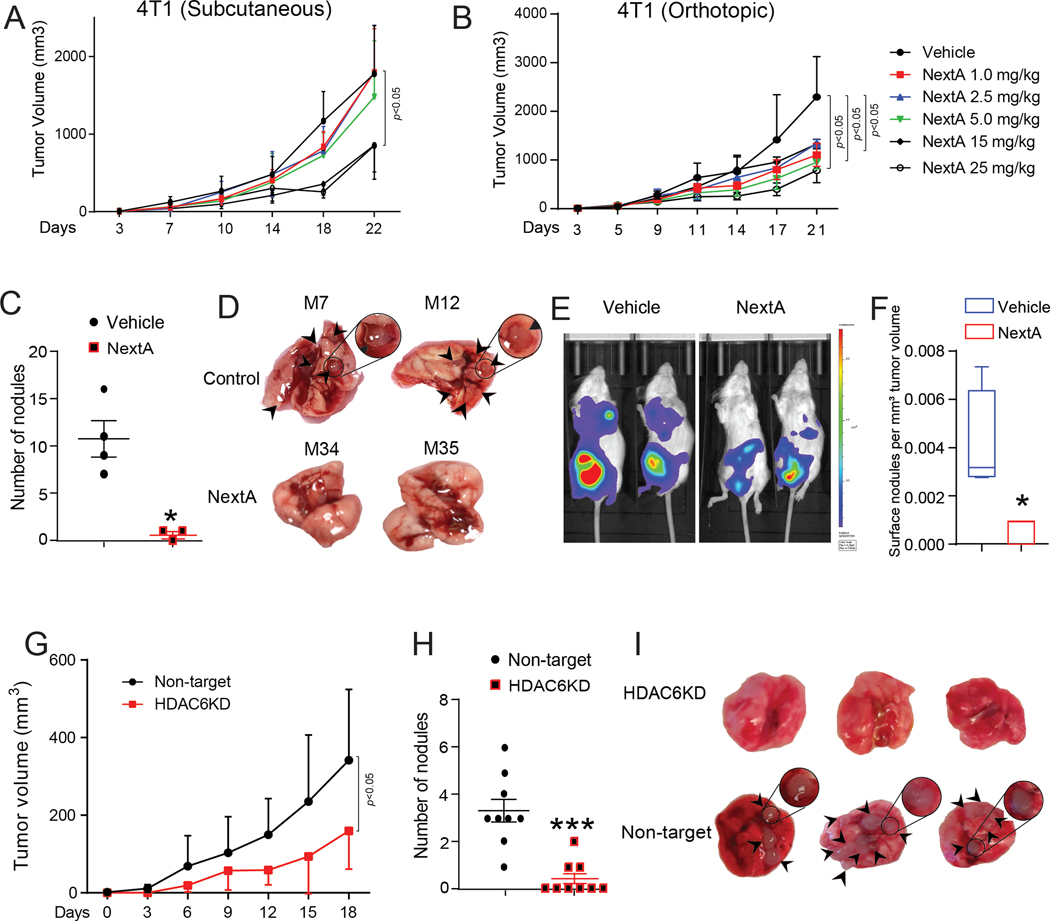
HDAC6 inhibitors, such as WT161 and its analogs, have been effective in suppressing multiple myeloma by overriding proteasome inhibition (29), and BC cells by inhibition of growth hormone receptors (30). We have described the effect of pharmacological inhibition of HDAC6 through the small-molecule inhibitor NextA in melanoma (18,19,31). To extrapolate its effect on BC in a preclinical model, we performed an in vivo dose-titration of NextA. We implanted the in vivo passaged 4T1 cells in separate cohorts of mice subcutaneously (in the right flank); (Fig. 1A) and orthotopically in the fourth mammary gland; (Fig. 1B). We treated mice with palpable tumors (3×3 mm3) with a range of NextA doses, i.e., 1, 2.5, 5, 15, 25 mg/kg, along with vehicle control. NextA was able to reduce tumor growth at the highest concentration of 25 mg/kg, compared to the lesser doses, at both sites. Since 4T1 is capable of spontaneously metastasizing to the distant organs from its orthotopic site, we also examined the effect of NextA on the lung-tumor burden, the main metastatic niche for 4T1 cells. Most notably, the highest dose of NextA was able to demonstrate a significant reduction in tumor nodule quantitation (Fig. 1C), gross physiology of lungs (Fig. 1D), and overall tumor burden by in vivo bioluminescence imaging (Fig. 1E). Quantifying the occurrence of distant metastasis per unit of primary tumor size, we found a similar trend of reduction in metastasis with NextA treatment (Fig. 1F). Also, we compared NextA-induced toxicity in vitro for apoptosis, cytotoxicity, and viability with broad-spectrum HDACi Panobinostat (LBH589, a pan-HDAC inhibitor) (32), class I inhibitor Entinostat; both utilized in clinical studies for its anti-cancer properties (33), and a second HDAC6 inhibitor SS-2–08 (27) via a triplex assay Apotox. We found that compared to the broad-spectrum HDAC inhibitors, NextA and SS-2–08 conferred significantly less apoptosis and cytotoxicity while maintaining cell viability even at higher doses (Suppl. Fig. 1A, B, C). This observation suggests that HDAC6 inhibitors function by altering the anti-tumor and anti-metastatic properties of the tumor, with minimal toxicity incurred upon the tumor cells.
Fig. 1: Selective HDAC6i inhibits primary and secondary metastatic growth 4T1 in a dose-dependent manner.
(A) Growth kinetics of 4T1 implanted subcutaneously to the flank of female BALB/c mice, treated with different doses of NextA (1.0 to 25mg/kg), 5X/week. (B) Growth kinetics of 4T1 implanted orthotopically in 4th mammary gland, treated with different doses of NextA, 5X/week. (C) Quantification of lung nodules in vector control vs. 25mg/kg NextA treated mice, 5X/week. (D) Pictorial representation of lung nodules. (E) Tumor burden measured by Luciferin K-salt injected bioluminescent mice. (F) Quantification of surface nodules as counted per cubic mm of tumor volume. (G) Growth kinetics of non-target vs. HDAC6KD-4T1 tumors implanted orthotopically into mice. (H) Quantification of lung nodules from non-target vs. HDAC6KD-4T1 tumor-bearing host. (I) Pictorial representation of lung nodules.
To investigate if the modulation in tumor aggressiveness is dependent on HDAC6 or stems from a non-specific function of NextA, we knocked out HDAC6 in the 4T1 cells to evaluate the effect of genetic abrogation of HDAC6 on tumor growth and appearance of metastatic nodules in lungs. The HDAC6KD-4T1 tumors showed impaired growth compared to the non-target control tumors (Fig. 1G). Additionally, we observed a significant reduction in the tumor nodule formation in the lung, as shown by the tumor nodule count (Fig. 1H) and gross appearance of the lung (Fig. 1I). Notably, we did not observe any difference in cellular proliferation between control vs. HDAC6KD 4T1 cells while culturing in vitro. Similarly, we evaluated if the effect of HDAC6i could also reduce the formation of metastatic nodules in other cancer models. Although the effect of NextA on primary tumor growth in the metastatic melanoma model B16F10 was not as pronounced (only significant at day 21) as 4T1 (Suppl. Fig. 2A), we observed a significant reduction in lung nodules in the treated group (25 mg/kg) (Suppl. Fig. 2B). Therefore, we conclude that the effect of HDAC6i is not restricted to BC and could modulate the metastatic formation in other cancer types.
HDAC6 inhibition modulates the expression of epithelial-mesenchymal transition and invasion-related genes in BC.
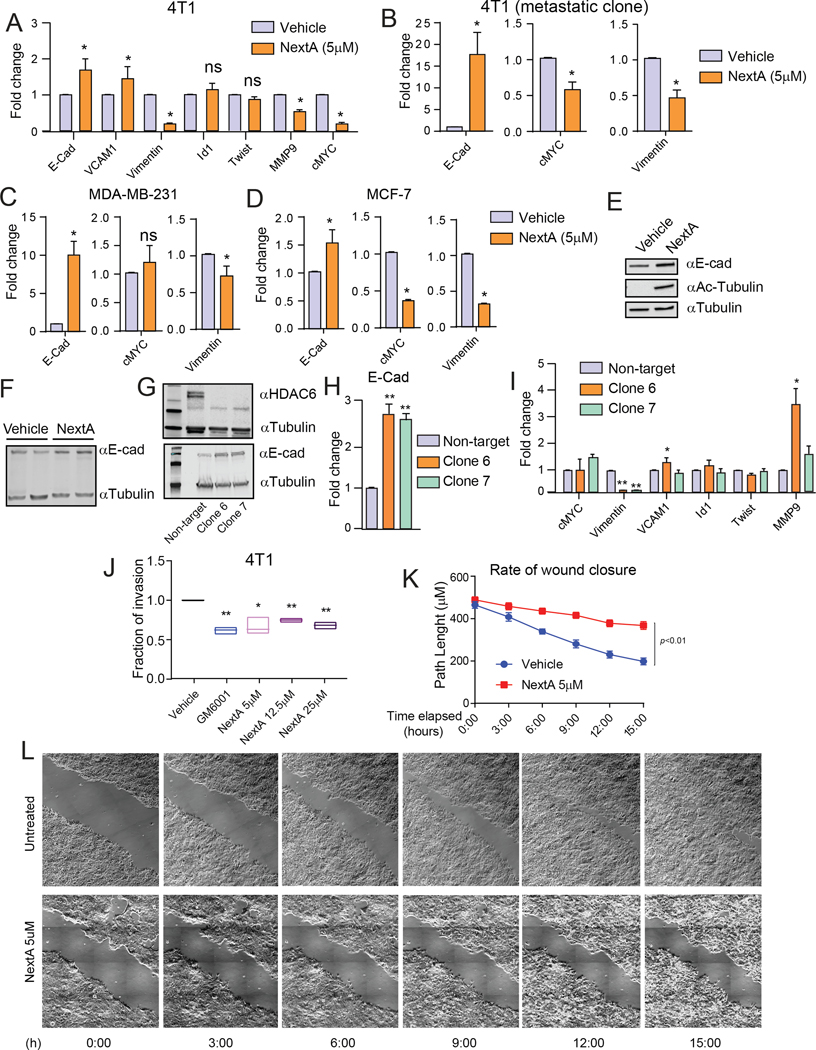
Since NextA had a notable effect on visible metastatic nodule formation (Fig. 1C–E), we investigated whether HDAC6i could elicit a systemic change in the expression of EMT-related genes across different tumor types. When examining the expression of prominent EMT pathway genes in the NextA treated 4T1 cells, we found significant downregulation of cMYC, Vimentin, and MMP9 (Fig. 2A). Importantly, the same outcome was observed in the isolated metastatic clone 4T1-met, obtained from the metastasized tissue in the lung (Fig. 2B) through 6-thioguanin selection. To test if the modulation of the EMT pathways is limited to the murine TNBC model alone, we further tested cell lines related to murine HR+ as well as human TNBC models. Firstly, we used MC4L2, a murine carcinoma model with an HR+ status (34) (Suppl. Fig. 3A). Secondly, we used human TNBC model MDA-MB 231 (Fig 2C), and the human HR+ cell line MCF7 (Fig. 2D). Although not all genes responded to the same extent, E-Cadherin (E-Cad) was consistently upregulated upon NextA treatment. The increased expression of E-Cad was also confirmed by immunoblot in 4T1 cells treated with NextA at 5 μM (Fig. 2E). Additionally, in vivo explants of 25 mg/kg NextA-treated 4T1 showed a noticeable enhancement in E-Cad (Fig. 2F). Multiple HDAC6KD clones were tested for the endogenous expression of E-Cad. We observed a noticeable increase in E-Cad expression at both protein (Fig. 2G) and RNA (Fig. 2H) levels compared to the non-target control. E-Cad primarily functions as an inhibitor of EMT and promotes apoptosis in tumor cells (35). Our data suggested that HDAC6 inhibition could affect the process of EMT via E-Cad. We tested LBH589 as a broad-spectrum epigenetic modulator to compare with HDAC6i (Suppl. Fig. 3B-C). While LBH589 also enhanced E-Cad expression, it also enhanced some of the EMT-promoting genes in the different cell lines. The knockdown clones were also tested for their expression of several EMT genes. Vim was observed to decrease noticeably with HDAC6 knockdown, while the other genes were increased or modulated minimally (Fig. 2I).
Fig. 2: HDAC6 inhibition modulates the expression of epithelial-mesenchymal transition and invasion-related genes in BC.
(A) Gene expression of EMT-molecules with or without in vitro NextA treatment (5μM) on 4T1 cells by qPCR. (B) Expression of EMT-genes with or without in vitro NextA (5μM) on metastatic 4T1 lung-isolated clones by qPCR. (C) Expression of EMT-genes after in vitro NextA treatment (5μM) on MDA-MB-231 cells by qPCR. (D) Expression of EMT-genes after in vitro NextA treatment (5μM) on MCF7 cells by qPCR. (E) Evaluation of E-Cad levels in 4T1 cells after in vitro NextA treatment (5μM) by western blot. Evaluation of acetylated tubulin as an internal indicator for NextA mediated acetylation function. (F) Detection of the endogenous levels of E-Cad by western blot from in vivo tumor samples treated with NextA (25mg/kg). (G) Endogenous expression of HDAC6 and E-Cad protein in non-target vs. HDAC6KD-4T1 clones. (H) Corresponding E-Cad RNA levels in non-target vs. HDAC6KD-4T1 clones.. (I) Expression of EMT-genes in non-target and HDAC6KD-4T1 cells. (J) NextA dose-dependent invasion by 4T1 cells measured in 2D invasion chamber. GM-6001, a broad-spectrum MMP-inhibitor, is used as a positive control. (K) Quantification of wound closure of 4T1 cells throughout 16h, with or without NextA, as analyzed by ImageJ. (L) Snapshots of wound closure of 4T1 cells throughout 16h, with or without NextA, captured on Leica DMi8 microscope with a piezo stage (Pecon) with phase-contrast.
Next, we examined if the effect on EMT pathways could be translated into the invasive potential of the tumor cells. In a two-dimensional invasion chamber, a dose-dependent application of NextA reduced the invasiveness of both 4T1 (Fig. 2J) and MC4L2 (Suppl. Fig. 3D). In conjunction, we evaluated the effect of NextA on tumor cell migration using a time-lapsed wound closure microscopic assay. As expected, untreated 4T1 cells almost completely closed the wound after 16 hours of an injury (i.e., a scratch). However, 4T1 cells treated with 5 μM NextA only partially filled the scratch path within the same period (Fig. 2K–L) of observation (Video recording shown as supplemental material). HDAC6KD-4T1 cells also showed a decrease in wound closure abilities (Suppl. Fig. 3E).
Inhibition of HDAC6 modulates the PD-1/PD-L1 axis in BC tumors
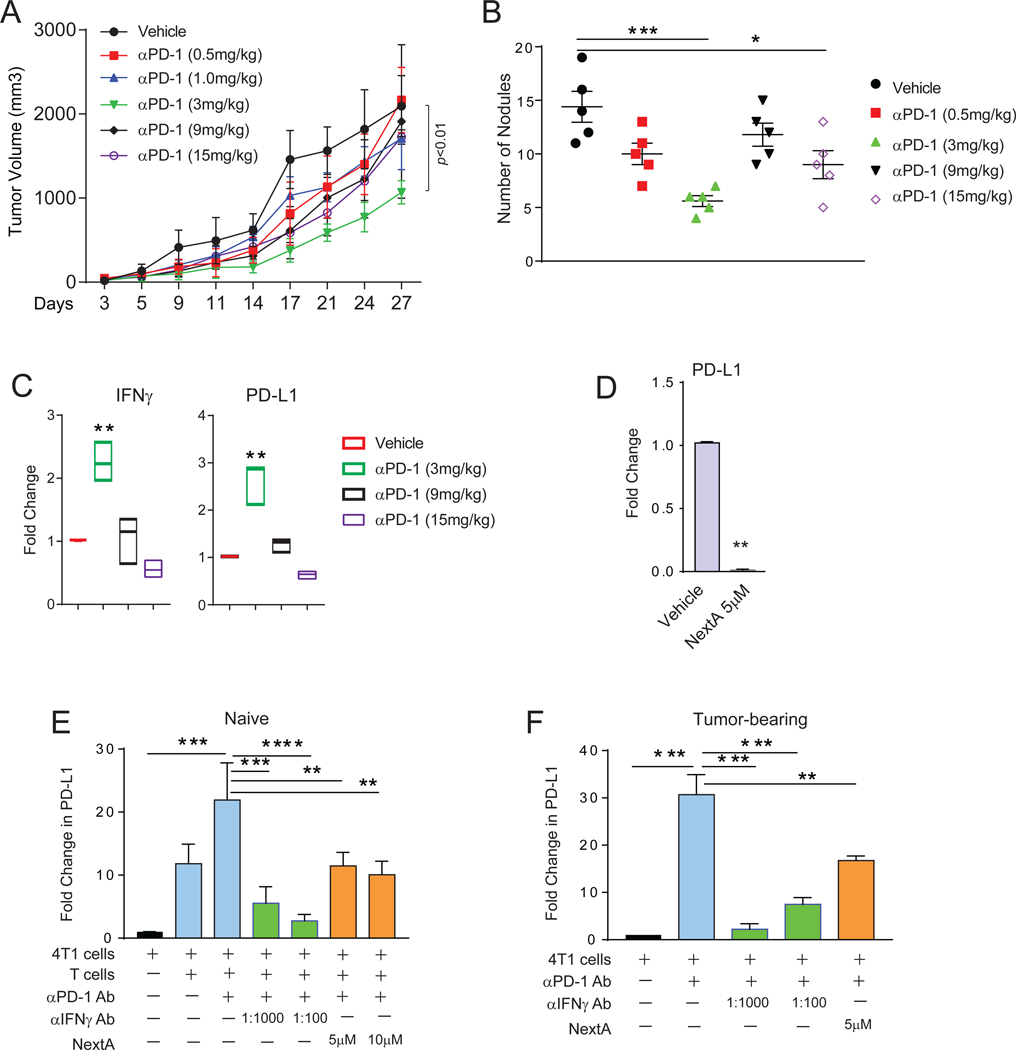
The TME provides critical immune contexture to therapeutic response in cancer. Enhanced infiltration of immune cells has been described as a predictive biomarker for the success of immunotherapy (36). BC presents considerable heterogeneity in this respect (37). TNBC is characterized by infiltration of stromal and intra-tumoral lymphocytes (TILs), facilitating ICB-mediated immunotherapy (38). Also, αPD-1 antibody was shown to enhance central and effector memory T cells and the TH1 response in metastatic melanoma (39). In order to evaluate the strength of this treatment arm, we tested the efficacy of αPD-1 antibody against 4T1 with a range of 0.5, 1, 3, 9 and 15 mg/kg and observed a notable difference in primary (Fig. 3A) and secondary (Fig. 3B) tumor growth at a dose of 3 mg/kg and no further advantage at higher doses. We observed a noticeable declining health status in mice receiving higher doses, including weight loss, ruffled fur, and restricted movement. We found a significant increase in intra-tumoral IFNγ levels (Fig. 3C), accompanied by an enhancement in the expression of PD-L1 (Fig. 3C). Interestingly, this was most evident at the dosage of 3 mg/kg. An in vitro treatment of 4T1 cells with IFNγ recapitulated the overexpression of PD-L1 (Suppl. Fig 4A), confirming our in vivo observation.
Fig. 3: αPD-1 blockade and HDAC6is have opposite effects on the expression of PD-L1 in BC.
(A) Growth kinetics of 4T1 tumors implanted in the 4th mammary gland of female BALB/c mice, treated with different doses of αPD-1 antibody (0.5 to 15mg/kg). (B) Quantification of lung nodules in vehicle control vs. αPD-1 treated mice. (C) Gene expression of IFNγ and PD-L1 in the in vivo tumors collected from the mice with or without αPD-1 treatment measured by qPCR. (D) Expression of PD-L1 gene in 4T1 cells treated in vitro with 5μM of NextA measured by qPCR. (E) Expression of PD-L1 on tumor cells co-cultured with CD3+ splenocytes from non-tumor bearing mice and αPD-1 antibody, with or without treatment with NextA or IFNγ-neutralizing antibody, measured by qPCR. (F) Expression of PD-L1 on tumor cells co-cultured with CD3+ splenocytes from 4T1 tumor-bearing mice and αPD-1 antibody, with or without treatment with NextA or IFNγ-neutralizing antibody, measured by qPCR.
We previously described that HDAC6 modulates PD-L1 expression and function via STAT3-pathway in melanoma (40). We wanted to evaluate if this was mirrored in BC. In an initial screening on 4T1 cells, we observed that PD-L1 RNA expression was significantly diminished with NextA treatment (Fig. 3D and Suppl. Fig 4B) and HDAC6-KD (Suppl. Fig. 4D). Reports indicated that pan-HDACis have the opposite effect on PD-L1, increasing the expression of this immunosuppressive mediator (19). We also observed an increase in PD-L1 in BC cells treated with LBH589 (Suppl. Fig. 4C). In order to show that the up-regulation of PD-L1 is a direct effect of IFNγ present in the TME, we treated 4T1 cells in vitro with NextA or vehicle and co-cultured them with CD3/CD28 activated T cells +/− IFNγ blocking antibody (1:1000 and 1:100 dilutions). As shown in Fig. 3E, the expression of PD-L1 in 4T1 was upregulated when co-cultured with activated T cells. However, it was diminished after adding IFNγ blocking antibody. A similar down-regulation of PD-L1 was found when adding NextA to the co-cultured cells. As described previously, 4T1 tumor is unique with its higher accumulation of MDSC (41). These cells not only populate the primary tumor but also migrate to the spleen and reprogram the T cells through their immunosuppressive functions. Therefore, we harvested CD3+ splenocytes from 4T1 tumor-bearing BALB/c mice, cultured in the presence of αPD-1 antibody, and subjected them to NextA treatment +/− IFNγ-neutralizing antibody. As shown in Fig. 3F, NextA was still capable of decreasing PD-L1 expression within the tumor-reprogrammed T cells. Moreover, these observations suggested a rationale for combining the treatment of NextA with αPD-1-mediated immunotherapy to mitigate its pro-tumorigenic effect via upregulation of PD-L1.
Priming the TME with HDAC6i improves the anti-tumor activity of ICB.
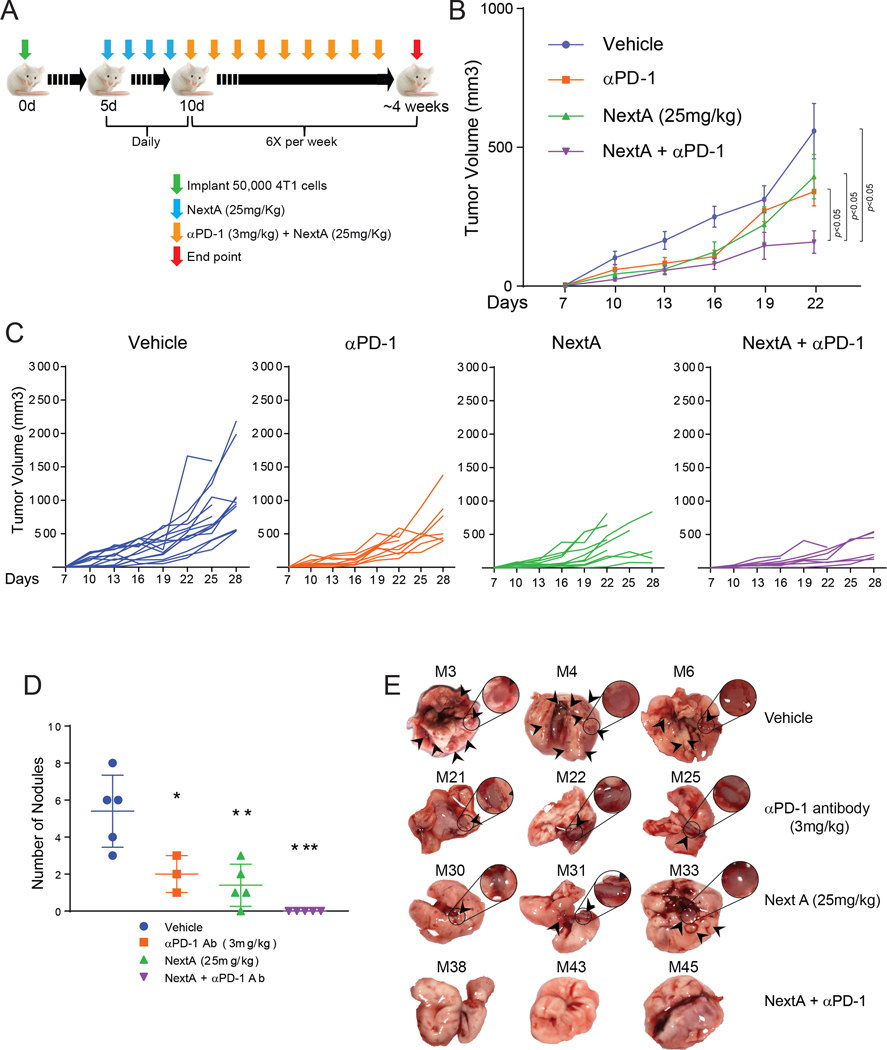
Supported by our previous observation in melanoma (18,22) and our initial results in BC cells, we hypothesized that NextA could improve the anti-tumor effect of αPD-1 blockade by decreasing the expression of immunosuppressive pathways and altering the invasive properties in tumor. Keeping the aggressive nature of 4T1 in perspective, we chose the doses that showed maximum efficacy in their respective dose titration experiments (3 mg/kg for αPD-1 and 25 mg/kg for NextA, Fig. 1 and 3). To preserve the effect of hormonal milieu on tumor-growth and metastatic potential of the primary tumor, we implanted the primary tumor orthotopically. Our preliminary attempt was to begin both treatments at the same time when the primary tumor reached 3×3 mm3. Although showing a slight reduction in tumor growth (Suppl. Fig. 5), this attempt failed to demonstrate noticeable benefits. Therefore, in order to pre-educate the immune system throughout the initial neoplastic events, we began to treat the mice with NextA at the determined dose (25mg/kg) before the tumors became palpable, i.e., 4 days post-tumor implantation. Sequentially, we started the αPD-1 treatment (3mg/kg) when the primary tumors within the untreated groups reached a size of 3×3 mm3, as shown in the schema (Fig. 4A). This pre-treatment approach with NextA demonstrated significant retardation in tumor growth (Fig. 4B and 4C) while the single treatments with NextA (25 mg/kg) and αPD-1 (3 mg/kg) alone maintained similar kinetics observed in previous independent treatments. Endpoint lung morphology also showed a significant reduction in secondary tumor burden, as indicated by nodule quantification (Fig. 4D) and gross morphology (Fig. 4E). The data, therefore, indicates a superior advantage of pre-treatment approach over conventional simultaneous treatment.
Fig. 4: Priming the TME with HDAC6i improves the antitumor activity of ICB.
(A) Schematic representation of combination treatment. (B) Growth kinetics of 4T1 tumors implanted in the 4th mammary gland of female BALB/c mice, treated with the predetermined doses of NextA and αPD-1; 6X/week. (C) Growth kinetics of the individual tumors in each treatment group. (D) Quantification of lung nodules from each treatment group. (E) Pictorial representation of lung nodules from each group.
ICB and HDAC6i combination treatment modified the TME by altering effector molecules
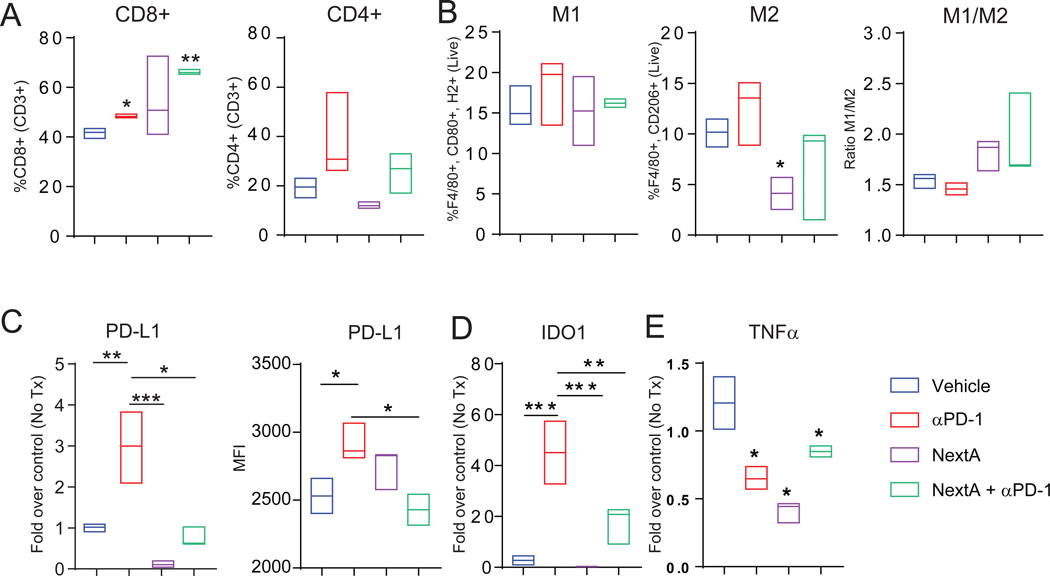
To gain an insight into the alteration of TME after treatment, we performed an immunophenotyping of tumors post-treatment. As described previously, αPD-1 treatment enhanced the CD8+ T cell infiltration without a significant change to CD4+ cells (Fig. 5A). This trend was maintained with the combination treatment. Notably, we observed that NextA reduce the M2-like population (Fig. 5B), which suggests that the inflammatory microenvironment in tumor can be modified with HDAC6-inhibition. However, the treatment alone or in combination with αPD-1 did not change the M1/M2 ratio significantly, despite a downward trend. To further investigate the TME components, we evaluated central and effector memory cells within CD4+ and CD8+ subpopulations with no conclusive difference. The NK, NKT, and MDSC also did not show a significant trend, while Treg population was enhanced in NextA and combination groups (Suppl. Fig. 6A–D).
Fig. 5: ICB and HDAC6i combination treatment modified the TME by altering effector molecules.
Flow cytometry quantification of total effector and helper T cells (A) and M1 and M2 macrophage and their ratio (B) in primary tumors harvested from the treated mice. (C) Quantification of PD-L1 gene (left, by qPCR) and surface expression (right, by flow cytometry). qPCR quantification of IDO1 (D) and TNFα (E) gene expression in primary tumors harvested from the treated mice.
As shown in previous work (18,20), HDAC6 inhibition reduces the expression of PD-L1 in cancer cells. To validate it in the combination treatment regimen, we looked at the surface (Fig. 5C, right) and intra-tumoral RNA expression (Fig. 5C, left) of PD-L1, both of which showed an increase in the PD-L1 expression within the αPD-1 alone group. However, PD-L1 was diminished in the NextA and combination groups. We also evaluated the expression levels of several other costimulatory/inhibitory molecules that belong to the B7 family and determine the T cell activation status, such as, PD-L2, B7-H3, B7-H4 and OX40L in response to the treatment. However, we did not observe any discernible changes (Suppl. Fig. 6E–H), suggesting the effect of NextA to be specific towards PD-L1. Another molecule that followed a similar trend as PD-L1 was indoleamine 2,3-dioxygenase (IDO1) (Fig. 5D), also reported being directly regulated by IFNγ (42). IDO1, as described previously, is a marker for poor prognosis, immune escape, and metastasis in TNBC (43). Decreasing the functional level of IDO1 in tumor may be an additional mechanism for the reduction in tumor growth and metastasis. We probed pro-inflammatory cytokines, such as IL1β and IL6, to investigate the inflammatory environment of the tumor. While IL1β was not modulated significantly in the combination group, the IL6 expression was least abundant in αPD-1 and combination groups (Suppl. Fig. 6I–J). The overall decrease in the expression of IL6 may indicate a reduction in the chronic inflammation within the tumor tissue, also partly explaining the reduction in metastasis, since IL6 in TNBC, either tumor-derived or macrophage/adipocyte-derived, is correlated with invasion and malignancy (44). Moreover, we observed a discernible decrease in TNFα under all the tested conditions (Fig. 5E). Given the tumor-promoting roles of TNFα by exacerbating chronic inflammation, the treatment regimens indicated an effective mechanism in reducing tumor pathogenesis (45).
Combination treatment modified the TME by altering EMT-related gene expression.
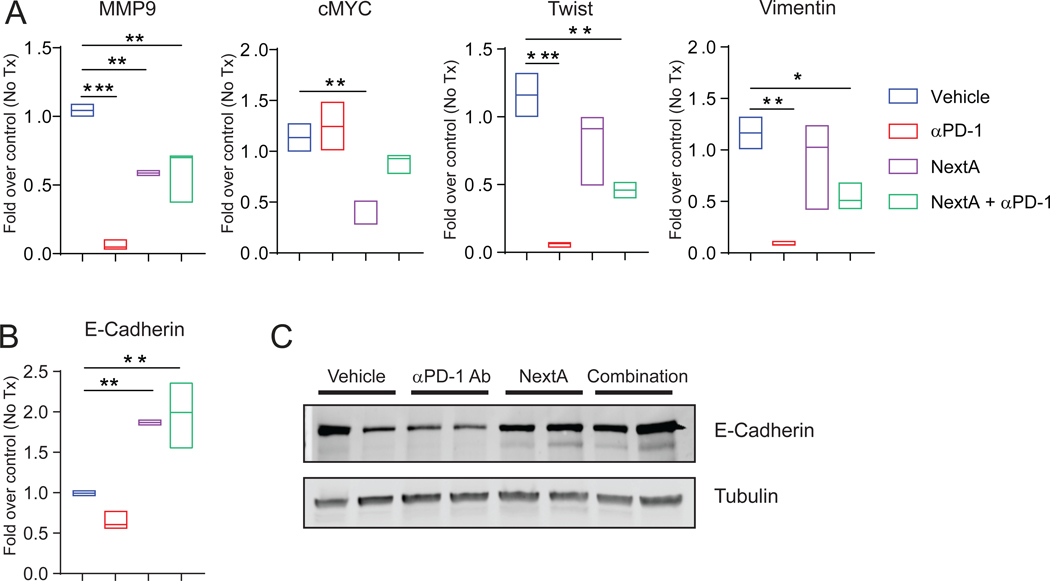
To investigate if our previous effect of NextA on EMT-genes in 4T1 monolayer (Fig. 2G) could be reproduced in the combination therapy, we measured a panel of invasion/metastasis-related genes in tumor samples. Multiple pro-metastatic genes, such as MMP9 (46), cMYC (47), Twist (48), and Vimentin (49), were found to be significantly reduced in monotherapy groups (Fig. 6A). The expression pattern of E-Cad demonstrated a recapitulation of the in vitro data. As shown in Fig. 6B and 6C, NextA alone increased E-Cad levels in both single and combination groups. Therefore, we hypothesize that by regulating several EMT effector molecules, NextA alone or in combination with αPD-1 induces intrinsic changes to the tumor cells that may be independent of the immune system.
Fig. 6: Combination treatment modified the TME by altering EMT-related gene expression.
(A) Quantification of various EMT-related gene expressions in the tumor mass harvested from the treated mice, measured by qPCR. (B) Quantification of E-Cad gene expression in the tumor mass harvested from the treated mice, measured by qPCR. (C) Measurement of E-Cad protein expression in the tumor mass harvested from the treated mice, detected by immunoblot.
The anti-invasive properties of NextA are mediated through its regulation on E-Cad.
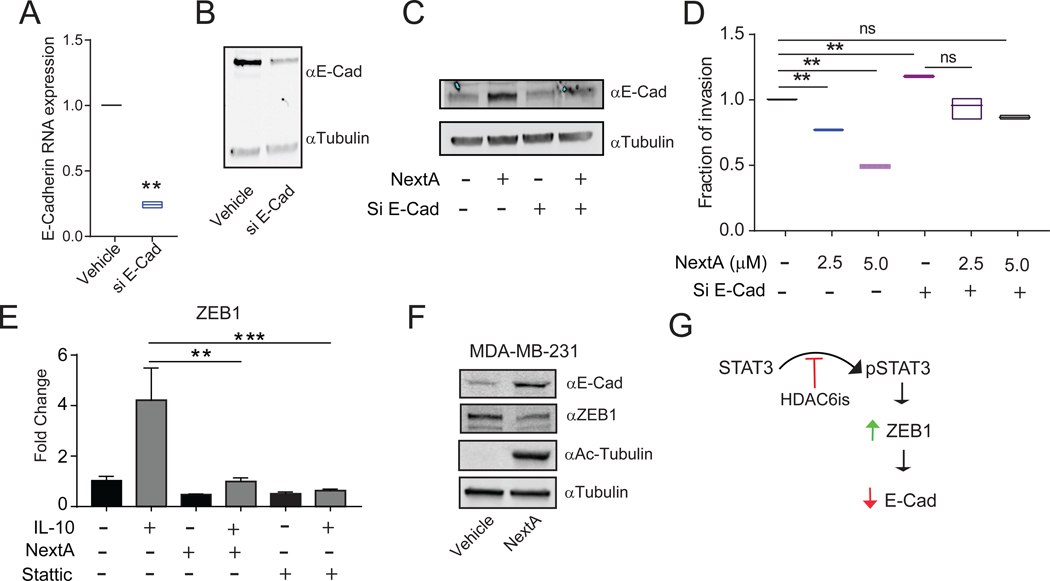
One of the anti-metastatic molecules consistently upregulated in the presence of NextA was E-Cad. Therefore, we hypothesized that the anti-invasive property of NextA is partly mediated through E-Cad regulation. To investigate this, we partially ablated E-Cad expression in 4T1 cells, as detected by qRT-PCR (Fig. 7A) and western blot (Fig. 7B). Importantly, the extent of E-Cad induction was reduced in the presence of NextA (Fig. 7C). We also evaluated the invasive capabilities of the vehicle vs. E-Cad siRNA 4T1 cells after NextA treatment. The E-Cad knock-down alone increased the invasion of 4T1 cells. However, treatment with NextA did not reduce the invasion of the siE-Cad tumor cells to a significant level, as observed under NextA alone (Fig. 7D). On the other hand, treatment with NextA on non-transfected cells demonstrated a significant reduction in the in vivo/vitro invasive properties of 4T1 (Fig. 1C–E, 2G). This indicated that the activity of NextA on BC invasive properties demands the presence of an intact level of E-Cad. We then sought to identify a common regulator of E-Cad that could potentially be regulated by HDAC6. As the previous work from our group has identified, HDAC6 acts as a potent regulator of STAT3 by maintaining its phosphorylation status (50). Additionally, as described previously, STAT3 plays a critical role in downregulating E-Cad by promoting the expression of the E-Cad transcriptional repressor ZEB1 (51). The localization of HDAC6 in nucleus and its ability to regulate STAT3 are supportive evidence of this observation (50,52).
Fig. 7: Anti-invasive properties of NextA is mediated through its regulation on E-Cad.
(A) Measurement of E-Cad gene expression by qPCR in 4T1 cells in vitro treated with E-Cad siRNA. (B) Measurement of E-Cad protein expression by Western blot in 4T1 cells in vitro treated with E-Cad siRNA. (C) Measurement of E-Cad protein expression by Western blot in 4T1 cells in vitro treated with E-Cad SiRNA and NextA (5μM). (D) Invasion capability of E-Cad silenced 4T1 cells at different concentrations of NextA (2.5 and 5μM) measured in 2D invasion chamber. (E) Expression of ZEB1 in the 4T1 cells after NextA (5μM) and Stattic (10μM) treatment, measured by qPCR. (F) MDA-MB-231 cells were treated with NextA (5μM), and the expression of E-Cad, ZEB1, and tubulin, evaluated by immunoblot. (G) A regulatory model is proposing HDAC6is as modulators of the STAT3/ZEB1 axis and subsequent regulation of the expression of E-Cadherin.
Supported by these observations, we hypothesized that the effect of HDAC6 on E-Cad might be mediated through the axis STAT3/ZEB1. To test this hypothesis, we evaluated the expression of ZEB1 in 4T1 cells treated with either NextA or the STAT3 inhibitor Stattic by qRT-PCR +/− STAT3 activator IL-10 (53). The expression of ZEB1 was significantly reduced in the presence of NextA and Stattic after stimulation of the STAT3 pathway (Fig. 7E), thus demonstrating the direct participation of HDAC6 in the regulation of the STAT3/ZEB1 axis. Unfortunately, we were unable to detect the ZEB1 protein in murine cell lines. However, the protein expression of ZEB1 was diminished in the human BC cell line MDA-MB-231 after treatment with NextA (Fig. 7F). Taking the above results into consideration, we propose that STAT3 inhibits E-Cad by associating with ZEB1 (51). HDAC6 promotes this pathway by facilitating the phosphorylation of STAT3 (19). However, the HDAC6i NextA inhibits the phosphorylation of STAT3, thereby abrogating this process (Fig. 7G).
Discussion
Epigenetic modulators, including HDACi, have emerged as a newer therapeutic modality and gained popularity due to their effect on inflammation, cell differentiation, and tumoricidal effects (54), leading to testing and approval of >20 HDACi by the FDA (12,55). However, most broad-spectrum HDACi tend to exhibit toxicity profiles as well, e.g., thrombocytopenia, neutropenia, nausea, vomiting, diarrhea, and fatigue (12). Therefore, it is imperative to leverage the benefits of HDACi with a milder toxicity profile. Our approach using selective HDAC6i has shown minimal cytotoxic effects while influencing immune-functions within TME (18,31,40). HDAC6i regulates macrophage phenotype, antigen presentation, and expression of key molecules involved in immunosuppressive pathways, e.g., PD-L1, PD-L2, B7-H3, and B7-H4 (20). Interestingly, HDAC6i may show different modalities of tumor inhibition. In the case of myeloma, as shown in patient-derived cells and cell lines, HDAC6i WT161 increased the accumulation of polyubiquitinated proteins and activation of the stress-activated protein kinase JNK that enhanced endoplasmic reticulum (ER) stress and unfolded protein response (UPR). WT161 also triggered the expression of CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) and its Activating transcription factor 4 (ATF4). There was significant down-regulation of ER stress sensor proteins inositol-requiring enzyme 1α (IRE1α) and protein kinase R (PRKR)-like ER kinase (PERK) with WT161. Moreover, the antiapoptotic protein X-linked inhibitor of apoptosis (XIAP) was down-regulated with WT161 treatment. Cumulatively, WT161 treatment enhanced apoptosis in tumor. This was further leveraged by combining it with the proteasome inhibitor bortezomib (BTZ) (29). On ERα+ MCF7 cells, WT161 triggered caspase-7 and PARP cleavage in a dose-dependent fashion and induced XIAP downregulation. WT161 also decreased the expressions of EGFR, its downstream effector molecule phospho-ERK, and ERα in MCF7 cells independent of caspase (30).
NextA, however, does not alter apoptosis/proliferation in the in vitro cultured cells. The difference in growth is observed in vivo: the invasive growth of tumor cells within normal tissue. This, in combination with our data on invasion and wound healing dynamics, suggests that NextA targets the pathways specifically involved in cellular motility and invasion, critical in tumor progression. As molecular support of this notion, we have provided the panel of EMT genes. Moreover, we hypothesize that in monolayer culture conditions without the presence of any stress, cells may not be particularly dependent on HDAC6. Therefore, the application of HDAC6i may not show a discernible effect on cell survival or proliferation. However, under the chronic inflammatory condition in cancerous tissues in vivo, HDAC6 expression and function are heightened (56). We hypothesize that at this stage, tumor cells get particularly dependent on HDAC6 for cellular motility, invasiveness, and wound healing from the host immunosurveillance. Therefore, effect of HDAC6-abrogation is manifested more prominently in vivo. Unlike the other HDAC6i, e.g., WT161, NextA possibly does not work through the apoptotic/cell stress pathways operative in cell monolayers.
Our observations indicated an involvement of HDAC6 in metastasis, verified at the molecular level. NextA was found to make tumors less susceptible to undergo EMT, a preliminary step in the process of metastasis. Interestingly, some of these genes show a broader spectrum of activities outside EMT and metastasis. For example, MMP9 may regulate cell growth, inflammation, angiogenesis (57). cMYC, an oncogene in several tumor types, can modulate cancer cell metabolism and proliferation (58). Vimentin inhibits autophagy through binding to 14–3-3 and Beclin 1 (59). E-Cad is an integral component of the Adherens Junctions (AJs) and a principal promoter of epithelial phenotype and contact inhibition. It acts as a tumor suppressor by regulating tumor-promoting pathways, e.g., Hippo, Wnt, TGFβ, NF-κB, RTK, and Src family kinase (60). Thus, direct or indirect regulation of HDAC6 on E-Cad can be instrumental in determining the metastatic outcome of tumor cells.
The potential effect of HDAC6 on E-Cad has been briefly described previously in esophageal squamous cell carcinoma (61) and lung-inflammation (62). Inhibition of HDAC6 is important in reversing the TGF-β mediated induction of EMT, a readout of which was E-Cad (63). E-Cad is downregulated by STAT3 via ZEB1 in colorectal cancer (51). HDAC6 promotes STAT3 phosphorylation and ectopic function in nucleus without changing its acetylation status or other proteins required for pSTAT3 homeostasis (e.g., PP2A, Shp-2, and JAK2 proteins) (19). Previous work from our group has shown nuclear localization of HDAC6 to regulate IL10 expression in APC (52). Our data indicate an additional avenue for HDAC6 to regulate E-Cad via STAT3, bolstering the role of NextA in modifying the EMT-capabilities of epithelial tumors.
While ICB may confer anti-tumoral advantages, e.g., T-cell activation and immunological memory, it may activate immunosuppressive pathways dampening T-cell function and reducing the potency of the treatment. Current treatments to prevent disease recurrence include radiation, hormonal therapy, targeted therapy, etc. (10). However, some therapies may pose serious side-effects, e.g., 1) ischemic heart attack, pneumonitis, lymphedema, and secondary malignancy from radiation (11, 12); 2) blood clot, stroke, fatigue, pain, hot flashes, bone loss, mood swing and depression from hormonal therapies and aromatase inhibitors (13); 3) resistant tumor, high blood pressure, skin problems (14). To investigate if we can reap the benefits of αPD-1 antibody while avoiding the side-effects, we performed a dose-titration. Uniquely, the beneficial effects were most prominent in the lower to medium ranges, while the higher doses failed to provide a meaningful difference in tumor growth and metastasis. We hypothesize that this may have been caused by excessive adverse immune reactions and inflammation at the higher doses, which may have resulted in a cytokine storm, organ-specific immune reactions, and overall toxicity (64). Other groups and we have reported αPD-1 antibody to induce an enhancement in IFNγ expression (18). However, while IFNγ indicates activated status of effector T cells, it also engages the STAT1/IRF1 pathway (65). IRF1 (interferon regulatory factor 1) binds to the PD-L1 promoter to act as a transcriptional activator (66). We observed a similar trend in BC.
To circumvent the drawbacks of the single treatment, we pre-treated tumors with NextA to reduce the pro-tumoral and immunosuppressive mediators in the TME. This “epigenetic priming” approach enhanced the efficacy of the treatment while changing the molecular properties of tumor. Epigenetic priming was first observed via two subsequent clinical studies, where a subset of patients from the first unsuccessful NSCLC trial (dual application of entinostat and azacytidine) went on to a second ICB trial (nivolumab) and showed remarkable progression-free survival (12). Multiple mechanisms may constitute the events of priming by epigenetic drugs, such as upregulation of tumor antigens (TAA), co-stimulatory molecules, stress-induced ligands, and death-inducing receptors and checkpoint ligands on tumor cells (67). Significant improvement of antitumor immune responses when combing αPD-1 and ultra-selective HDAC6i was identified in a recent pre-clinical study (18).
To summarize, our work indicates the previously underappreciated impact of HDAC6i on breast TME, including modulation of several immune and non-immune components. We also found that HDAC6i is more effective as a pre-treatment agent in immunotherapeutic combinations versus simultaneous treatment. This “priming” activity of HDAC6i suggests that HDAC6 causes substantial changes in cellular composition and inflammatory status, beginning at the neoplastic state. Furthermore, because many HDAC6i-affected immune-related processes are deregulated in ICB-resistant tumors, our approach may generate a foundation for overcoming ICB resistance in BC to investigate in the future.
Supplementary Material
Statement of significance.
Ultra-selective HDAC6 inhibitors can reduce tumor growth and invasiveness of breast cancer by non-canonical mechanisms unrelated to the previously cytotoxic properties attributed to HDAC inhibitors.
Acknowledgments
Funded by NIH R21 CA184612-01 and Melanoma Research Foundation CDA Grant Award (A.V.), and NIH R01CA206529 (R.L.). We would like to acknowledge the important technical contributions and the advice of Kimberlyn Acklin, MS, SCYM(ASCP), at The George Washington University Flow Cytometry Core Facility, and Bethany Rentz, RVT, at The George Washington University Office of Animal Research. In addition, we would like to acknowledge the following graduate and undergraduate students in the laboratory for their kind participation and assistance: Prathima Vembu, Sophiya John Ephrame, Sarthak Shah, and Ifeoma Ikwuemesi.
Footnotes
Disclosures: The authors have no financial or non-financial conflict of interest.
References
- 1.de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol 2011;137:183–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz Bessone MI, Gattas MJ, Laporte T, Tanaka M, Simian M. The Tumor Microenvironment as a Regulator of Endocrine Resistance in Breast Cancer. Front Endocrinol (Lausanne) 2019;10:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrero-Vicent C, Guerrero A, Gavila J, Gozalbo F, Hernandez A, Sandiego S, et al. Predictive and prognostic impact of tumour-infiltrating lymphocytes in triple-negative breast cancer treated with neoadjuvant chemotherapy. Ecancermedicalscience 2017;11:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019 [DOI] [PubMed] [Google Scholar]
- 6.Choi J, Gyamfi J, Jang H, Koo JS. The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol 2018;33:133–45 [DOI] [PubMed] [Google Scholar]
- 7.Azoury SC, Straughan DM, Shukla V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr Cancer Drug Targets 2015;15:452–62 [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Saci A, Szabo PM, Chasalow SD, Castillo-Martin M, Domingo-Domenech J, et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun 2018;9:3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields BD, Koss B, Taylor EM, Storey AJ, West KL, Byrum SD, et al. Loss of E-Cadherin Inhibits CD103 Antitumor Activity and Reduces Checkpoint Blockade Responsiveness in Melanoma. Cancer Res 2019;79:1113–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Wang H, Li G, Song Y, Wang S, Zhu F, et al. Activated macrophages down-regulate expression of E-cadherin in hepatocellular carcinoma cells via NF-kappaB/Slug pathway. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2014;35:8893–901 [DOI] [PubMed] [Google Scholar]
- 12.Banik D, Moufarrij S, Villagra A. Immunoepigenetics Combination Therapies: An Overview of the Role of HDACs in Cancer Immunotherapy. Int J Mol Sci 2019;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichert N, Choukrallah MA, Matthias P. Multiple roles of class I HDACs in proliferation, differentiation, and development. Cell Mol Life Sci 2012;69:2173–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woan KV, Sahakian E, Sotomayor EM, Seto E, Villagra A. Modulation of antigen-presenting cells by HDAC inhibitors: implications in autoimmunity and cancer. Immunol Cell Biol 2012;90:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gryder BE, Sodji QH, Oyelere AK. Targeted cancer therapy: giving histone deacetylase inhibitors all they need to succeed. Future Med Chem 2012;4:505–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun WJ, Huang H, He B, Hu DH, Li PH, Yu YJ, et al. Romidepsin induces G2/M phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis induction through JNK/c-Jun/caspase3 pathway in hepatocellular carcinoma cells. Biochem Pharmacol 2017;127:90–100 [DOI] [PubMed] [Google Scholar]
- 17.Yee AJ, Bensinger WI, Supko JG, Voorhees PM, Berdeja JG, Richardson PG, et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial. Lancet Oncol 2016;17:1569–78 [DOI] [PubMed] [Google Scholar]
- 18.Knox T, Sahakian E, Banik D, Hadley M, Palmer E, Noonepalle S, et al. Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci Rep 2019;9:6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lienlaf M, Perez-Villarroel P, Knox T, Pabon M, Sahakian E, Powers J, et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Molecular oncology 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woan KV, Lienlaf M, Perez-Villaroel P, Lee C, Cheng F, Knox T, et al. Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation. Molecular oncology 2015;9:1447–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman JA, Woan K, Perez-Villarroel P, Villagra A, Sotomayor EM, Kozikowski AP. Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth. J Med Chem 2012;55:9891–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woan KV, Lienlaf M, Perez-Villaroel P, Lee C, Cheng F, Knox T, et al. Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation. Mol Oncol 2015;9:1447–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol 2011;2011:875824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A 2003;100:4389–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, et al. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 2008;68:7561–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, et al. Synthetic analogues of migrastatin that inhibit mammary tumor metastasis in mice. Proc Natl Acad Sci U S A 2005;102:3772–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen S, Hadley M, Ustinova K, Pavlicek J, Knox T, Noonepalle S, et al. Discovery of a New Isoxazole-3-hydroxamate-Based Histone Deacetylase 6 Inhibitor SS-208 with Antitumor Activity in Syngeneic Melanoma Mouse Models. J Med Chem 2019;62:8557–77 [DOI] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hideshima T, Qi J, Paranal RM, Tang W, Greenberg E, West N, et al. Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma. Proc Natl Acad Sci U S A 2016;113:13162–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hideshima T, Mazitschek R, Qi J, Mimura N, Tseng JC, Kung AL, et al. HDAC6 inhibitor WT161 downregulates growth factor receptors in breast cancer. Oncotarget 2017;8:80109–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavares MT, Shen S, Knox T, Hadley M, Kutil Z, Barinka C, et al. Synthesis and Pharmacological Evaluation of Selective Histone Deacetylase 6 Inhibitors in Melanoma Models. ACS Med Chem Lett 2017;8:1031–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atadja P Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 2009;280:233–41 [DOI] [PubMed] [Google Scholar]
- 33.Connolly RM, Rudek MA, Piekarz R. Entinostat: a promising treatment option for patients with advanced breast cancer. Future Oncol 2017;13:1137–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanari C, Luthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, et al. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro effects of estrogens and progestins. Cancer Res 2001;61:293–302 [PubMed] [Google Scholar]
- 35.Pecina-Slaus N Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 2003;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing X, Guo J, Wen X, Ding G, Li B, Dong B, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology 2018;7:e1356144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vikas P, Borcherding N, Zhang W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag Res 2018;10:6823–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi K, Mishima K, Ohmura H, Hanamura F, Ito M, Nakano M, et al. Activation of central/effector memory T cells and T-helper 1 polarization in malignant melanoma patients treated with anti-programmed death-1 antibody. Cancer science 2018;109:3032–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M L PPV, T K MP, E S JP, et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Mol Oncol 2016;10:735–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun 2017;8:14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991;5:2516–22 [PubMed] [Google Scholar]
- 43.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2012;2:722–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmad N, Ammar A, Storr SJ, Green AR, Rakha E, Ellis IO, et al. IL-6 and IL-10 are associated with good prognosis in early stage invasive breast cancer patients. Cancer immunology, immunotherapy : CII 2018;67:537–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ham B, Fernandez MC, D’Costa Z, Brodt P. The diverse roles of the TNF axis in cancer progression and metastasis. Trends Cancer Res 2016;11:1–27 [PMC free article] [PubMed] [Google Scholar]
- 46.Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014;5:2736–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao DJ, Dickson RB. c-Myc in breast cancer. Endocr Relat Cancer 2000;7:143–64 [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927–39 [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Yi M, Zhang R, Li J, Chen S, Cai J, et al. Vimentin is a crucial target for anti-metastasis therapy of nasopharyngeal carcinoma. Mol Cell Biochem 2018;438:47–57 [DOI] [PubMed] [Google Scholar]
- 50.Cheng F, Lienlaf M, Wang HW, Perez-Villarroel P, Lee C, Woan K, et al. A Novel Role for Histone Deacetylase 6 in the Regulation of the Tolerogenic STAT3/IL-10 Pathway in APCs. J Immunol 2014;193:2850–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong H, Hong J, Du W, Lin YW, Ren LL, Wang YC, et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem 2012;287:5819–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng F, Lienlaf M, Perez-Villarroel P, Wang HW, Lee C, Woan K, et al. Divergent roles of histone deacetylase 6 (HDAC6) and histone deacetylase 11 (HDAC11) on the transcriptional regulation of IL10 in antigen presenting cells. Mol Immunol 2014;60:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018;24:563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel) 2010;3:2751–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suraweera A, O’Byrne KJ, Richard DJ. Combination Therapy With Histone Deacetylase Inhibitors (HDACi) for the Treatment of Cancer: Achieving the Full Therapeutic Potential of HDACi. Frontiers in oncology 2018;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li C, Cao L, Xu C, Liu F, Xiang G, Liu X, et al. The immunohistochemical expression and potential prognostic value of HDAC6 and AR in invasive breast cancer. Human pathology 2018;75:16–25 [DOI] [PubMed] [Google Scholar]
- 57.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res 2012;18:5546–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 2014;50:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendonsa AM, Na TY, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene 2018;37:4769–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li N, Tie XJ, Liu PJ, Zhang Y, Ren HZ, Gao X, et al. Effects of down-regulation of HDAC6 expression on proliferation, cell cycling and migration of esophageal squamous cell carcinoma cells and related molecular mechanisms. Asian Pacific journal of cancer prevention : APJCP 2013;14:685–9 [DOI] [PubMed] [Google Scholar]
- 62.Liu L, Zhou X, Shetty S, Hou G, Wang Q, Fu J. HDAC6 inhibition blocks inflammatory signaling and caspase-1 activation in LPS-induced acute lung injury. Toxicology and applied pharmacology 2019;370:178–83 [DOI] [PubMed] [Google Scholar]
- 63.Xu L, Liu N, Gu H, Wang H, Shi Y, Ma X, et al. Histone deacetylase 6 inhibition counteracts the epithelial-mesenchymal transition of peritoneal mesothelial cells and prevents peritoneal fibrosis. Oncotarget 2017;8:88730–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep 2019;29:3766. [DOI] [PubMed] [Google Scholar]
- 66.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 2006;580:755–62 [DOI] [PubMed] [Google Scholar]
- 67.Dunn J, Rao S. Epigenetics and immunotherapy: The current state of play. Mol Immunol 2017;87:227–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.